This cohort study outlines the associations and interactions between interleukin 6, chronic kidney disease, and the risk of major cardiovascular outcomes in patients with chronic coronary syndrome.
Key Points
Question
What are the associations between interleukin 6 (IL-6) and cardiovascular outcomes in patients with chronic coronary syndrome in association with kidney function?
Findings
In this cohort study of 14 611 patients with chronic coronary syndrome, IL-6 and estimated glomerular filtration rates were obtained. Elevated IL-6 was in continuous models associated with higher risk of major adverse cardiovascular events in all chronic kidney disease strata and in categorical analysis (IL-6 ≥2.0 ng/L vs <2.0 ng/L) associated with major adverse cardiovascular events in all chronic kidney disease strata.
Meaning
IL-6 and chronic kidney disease stage may help to identify patients with chronic coronary syndrome for anti-inflammatory treatment.
Abstract
Importance
Inflammation promotes cardiovascular disease and anti-inflammatory treatment reduces cardiovascular events in patients with chronic coronary syndrome. Chronic kidney disease (CKD) is a risk factor for cardiovascular disease. It is unclear how inflammation mediated by interleukin 6 (IL-6) in patients with CKD is linked to cardiovascular disease.
Objective
To investigate associations between IL-6 and cardiovascular outcomes in patients with chronic coronary syndrome in association with kidney function.
Design, Setting, and Participants
This multicenter cohort study included patients enrolled at 663 centers in 39 countries with chronic coronary syndrome who were included in the Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy (STABILITY) trial. Patients were enrolled between December 2008 and April 2010 and were followed up for a median length of 3.7 years. Analysis in this substudy began September 2020.
Exposures
Exposures were IL-6 and creatinine estimated glomerular filtration rates (eGFR), which were collected at baseline. Associations between continuous and categorical levels (<2.0 ng/L vs ≥2.0 ng/L) of IL-6 and cardiovascular outcomes were tested in association with eGFR cutoffs (normal eGFR level [≥90 mL/min/1.73 m2], mildly decreased eGFR level [60-90 mL/min/1.73 m2], and moderately to severely decreased eGFR level [<60 mL/min/1.73 m2]).
Main Outcomes and Measures
Main outcome was major adverse cardiovascular events (MACE), a composite of cardiovascular death, myocardial infarction, and stroke.
Results
This substudy of the STABILITY trial included 14 611 patients with available IL-6 levels at baseline. The median (interquartile range) age was 65 (59-71) years, and 2700 (18.5%) were female. During follow-up, MACE occurred in 1459 individuals (10.0%). Higher levels of IL-6 were in continuous models independently associated with risk of MACE (P < .001) in all CKD strata. Using predefined strata, elevated IL-6 level (≥2.0 vs <2.0 ng/L) was associated with increased risk of MACE at normal kidney function (2.9% vs 1.9% events/y [hazard ratio, 1.35; 95% CI, 1.02-1.78]), mild CKD (3.3% vs 1.9% [hazard ratio, 1.57; 95% CI, 1.35-1.83]), and moderate to severe CKD (5.0% vs 2.9% [hazard ratio, 1.60; 95% CI, 1.28-1.99]).
Conclusions and Relevance
In patients with chronic coronary syndrome, elevated levels of IL-6 were associated with risk of MACE in all CKD strata. Thus, IL-6 and CKD stage may help when identifying patients with chronic coronary syndrome for anti-inflammatory treatment.
Introduction
Interleukin 1β (IL-1β) and IL-6 are cytokines that play an important role in the inflammatory response seen during the process of atherosclerosis.1 Based on these observations, we and others have shown that increasing levels of IL-6 are associated with cardiovascular events.2,3 Similarly, chronic kidney disease (CKD) is associated with cardiovascular disease (CVD).4 The underlying mechanism connecting CKD and CVD remains not fully understood, with inflammation proposed as a potential link.4 Still, it is unknown whether inflammatory activity as reflected by biomarkers are associated with cardiovascular outcomes across the range of kidney function.
Anti-inflammatory drugs including colchicine and canakinumab have been shown to reduce the risk of cardiovascular events in patients with acute and chronic coronary syndromes.5,6,7 Hence, attenuation of the inflammatory pathways might have cardioprotective effects. Recently, novel IL-6 inhibitors have been shown to reduce inflammatory activity and are entering phase 2 to 3 clinical trials, and there is an urgent need to better identify target populations that may benefit the most from anti-inflammatory treatment.8,9 Thus, the aim of this study was to outline the associations and interactions between IL-6, CKD, and the risk of major cardiovascular outcomes in patients with chronic coronary syndrome.
Methods
The Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy (STABILITY) trial randomized 15 828 patients in 39 countries with chronic coronary syndrome to darapladib (inhibitor of lipoprotein-associated phospholipase A2) or placebo, added to optimal medical therapy. Patients were enrolled between December 2008 and April 2010 and were followed up for a median length of 3.7 years. No treatment effects were observed with darapladib on major adverse cardiovascular events (MACE).10 The study design, inclusion and exclusion criteria, end points, and outcomes have previously been published10 and are detailed in the eAppendix in Supplement 1. The trial was approved by national regulatory authorities and ethics committee and/or institutional review boards of each participating country, and all patients provided written informed consent.
Venous blood samples were obtained at randomization, and all patients with available high-sensitivity IL-6 measurements were included. Details about biomarker analysis is provided in the eAppendix in Supplement 1.
Exposures were IL-6 and estimated glomerular filtration rates (eGFR), defined as continuous and categorical variables. For illustrative purposes, CKD was categorized into CKD stages, normal (eGFR ≥90 mL/min/1.73 m2), mildly decreased (eGFR 60-90mL/min/1.73 m2), and moderately to severely decreased (eGFR <60 mL/min/1.73 m2). For categorical analysis, IL-6 was categorized into less than 2.0 ng/L or 2.0 ng/L or more because this level was close to the study median of 2.1 ng/L. Primary end point was MACE (cardiovascular death, myocardial infarction [MI], or stroke). Secondary end points included all-cause death, cardiovascular death, MI, stroke, and hospitalization for heart failure. End points were assessed from randomization to first occurrence and adjudicated by a blinded events committee.10
Patient characteristics were assessed as medians for continuous variables and frequencies with percentages for categorical variables. χ2 and Kruskal-Wallis tests were performed for categorical and continuous variables, respectively. For analyses of continuous IL-6, natural log transformation was applied before entering IL-6 into the models. Associations between continuous IL-6 levels, CKD strata, and outcome were assessed in restricted cubic splines with randomized treatment and the interaction between eGFR and the linear part of the spline representation of IL-6. A global test of nonlinearity was performed and if the test was far from statistically significant, linearity was assumed for the biomarkers. In secondary analyses, associations between IL-6 and outcome were assessed by including continuous eGFR into the model. Associations between IL-6 and outcomes were tested in Cox regression models including CKD strata. For categorical analyses, Cox proportional hazard ratios (HRs) with 95% CIs were estimated with IL-6 levels less than 2.0 ng/L as reference. Two models were fitted accounting for potential confounders as detailed in the eAppendix in Supplement 1. All analyses were performed on complete observations. All tests were 2-sided, and P value less than .05 implied statistical significance. Analysis in this substudy began September 2020.
Results
A total of 14 611 patients were included in this substudy; their baseline characteristics were similar to the total STABILITY cohort (eTable 1 in Supplement 1). Baseline characteristics are presented in the Table. The median (interquartile range) age was 65 (59-71) years, and 2700 (18.5%) were female. Compared with patients with normal kidney function, patients with CKD tended to be older and female and more often had comorbidities including hypertension, multivessel CHD, polyvascular disease, or prior percutaneous coronary intervention/coronary artery bypass graft surgery.
Table. Baseline Characteristics Associated With eGFR Levels at Randomizationa.
| Characteristic | Baseline eGFR, mL/min/1.73 m2 | P value | ||
|---|---|---|---|---|
| eGFR ≥90 (n = 2722) | eGFR 60-90 (n = 8578) | eGFR <60 (n = 3311) | ||
| Demographics and risk factors | ||||
| Age, y, median (IQR) | 57.0 (51-62) | 65.0 (60-71) | 71.0 (66-76) | <.001 |
| Female, No. (%) | 389 (14.3) | 1470 (17.1) | 841 (25.4) | <.001 |
| Male, No. (%) | 2333 (85.7) | 7108 (82.9) | 2470 (74.6) | <.001 |
| BMI, median (IQR) | 28.4 (25-32) | 28.4 (26-32) | 28.4 (26-32) | .09 |
| Current smoker, No. (%) | 884 (32.5) | 1480 (17.3) | 289 (8.7) | <.001 |
| Geographic region, No. (%) | ||||
| Asia/Pacific | 639 (23.5) | 1441 (16.8) | 501 (15.1) | <.001 |
| Eastern Europe | 673 (24.7) | 2123 (24.7) | 654 (19.8) | |
| North America | 516 (19.0) | 2205 (25.7) | 1118 (33.8) | |
| South America | 135 (5.0) | 509 (5.9) | 231 (7.0) | |
| Western Europe | 759 (27.9) | 2300 (26.8) | 807 (24.4) | |
| Medical history, No. (%) | ||||
| Hypertension | 1781 (65.4) | 6057 (70.6) | 2614 (78.9) | <.001 |
| Diabetes | 1144 (42.0) | 3097 (36.1) | 1410 (42.6) | <.001 |
| Multivessel CHD | 333 (12.2) | 1222 (14.2) | 519 (15.7) | .001 |
| Prior MI | 1753 (64.4) | 5093 (59.4) | 1805 (54.5) | <.001 |
| Prior PCI or CABG | 1958 (71.9) | 6407 (74.7) | 2529 (76.4) | <.001 |
| Polyvascular disease | 335 (12.3) | 1241 (14.5) | 649 (19.6) | <.001 |
| Biochemical analyses, median (IQR) | ||||
| hs-IL-6, ng/L | 1.9 (1-3) | 2.0 (1-3) | 2.5 (2-4) | <.001 |
| hs-CRP, mg/dL | 0.14 (0.10-0.30) | 0.13 (0.10-0.30) | 0.16 (0.10-0.40) | <.001 |
| No. (total n = 13 907) | 2600 | 8165 | 3142 | NA |
| White blood cell count, /µL | 6800 (6000-8000) | 6500 (6000-8000) | 6600 (6000-8000) | <.001 |
| No. (total n = 14 145) | 2642 | 8297 | 3206 | NA |
| Hemoglobin, g/dL | 14.6 (13.8-15.3) | 14.5 (13.6-15.3) | 13.8 (12.8-14.8) | <.001 |
| No. (total n = 14 162) | 2645 | 8306 | 3211 | NA |
| Cystatin C, ng/L | 0.8 (1-1) | 1.0 (1-1) | 1.3 (1-2) | <.001 |
| No. (total n = 14 376) | 2678 | 8441 | 3257 | NA |
| Lp-PLA2 activity, μmol/min/L | 173.5 (143-206) | 172.3 (143-203) | 171.9 (143-206) | <.001 |
| No. (total n = 14 349) | 2671 | 8424 | 3254 | NA |
| hs-Troponin T, ng/mL | 0.007 (0.005-0.010) | 0.009 (0.006-0.013) | 0.014 (0.009-0.020) | <.001 |
| No. (total n = 14 331) | 2670 | 8416 | 3245 | NA |
| NT-proBNP, ng/L | 109.0 (54-229) | 161.0 (81-334) | 303.0 (148-679) | <.001 |
| No. (total n = 14 366) | 2677 | 8432 | 3257 | NA |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CABG, coronary artery bypass graft surgery; CHD, coronary heart disease; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; hs, high-sensitivity; IL-6, interleukin 6; IQR, interquartile range; Lp-PLA2, lipoprotein-associated phospholipase A2; MI, myocardial infarction; NA, not applicable; NT-proBNP, N-terminal pro–B-type natriuretic peptide; PCI, percutaneous coronary intervention.
SI conversion factors: To convert CRP to mg/L, multiply by 10; hemoglobin to g/L, multiply by 10; troponin T to µg/L, multiply by 1; white blood cell count to ×109/L, multiply by 0.001.
eGFR levels are based on the Chronic Kidney Disease Epidemiology Collaboration equation.
Median (interquartile range) IL-6 level was 2.1 (1-3) ng/L. Patients with CKD vs patients with normal kidney function were more likely to have higher levels of IL-6, C-reactive protein, troponin T, and N-terminal pro–B-type natriuretic peptide (Table). Patients with IL-6 levels 2.0 ng/L or higher vs less than 2.0 ng/L were older, more often female, and had higher prevalence of comorbidities (eTable 2 in Supplement 1).
Primary End Point
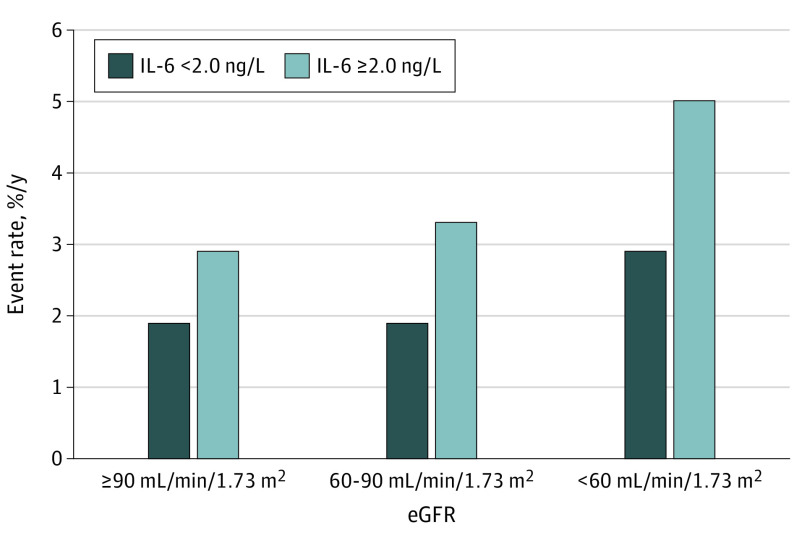
During a median follow-up of 3.7 years, the primary end point of MACE occurred in 1459 patients (10.0%). When fitting continuous levels of IL-6 into restricted cubic splines, elevated IL-6 levels were associated with higher risk of MACE across the range of eGFR and in all CKD strata (Figure 1 and eFigure 1 in Supplement 1). Accordingly, higher vs lower levels of IL-6 (≥2.0 ng/L vs <2.0 ng/L) were associated with increased event rates of MACE across all CKD strata in unadjusted analyses (eFigure 2 in Supplement 1). After adjustments the associations remained, and higher IL-6 levels were associated with increased risk of MACE in patients with normal kidney function (HR, 1.35; 95% CI, 1.02-1.78), in patients with mild CKD (HR, 1.57; 95% CI, 1.35-1.83), and in patients with moderate to severe CKD (HR, 1.60; 95% CI, 1.28-1.99) (Figure 2 and eFigure 3 in Supplement 1).
Figure 1. Associations Between Continuous IL-6 Concentrations and Outcome Across CKD Strata.

Estimated event rates of outcomes across chronic kidney disease (CKD) strata (estimated glomerular filtration rate [eGFR] ≥90, 60-90, and <60 mL/min/1.73 m2) associated with continuous baseline concentrations of interleukin 6 (IL-6) fitted as restricted cubic splines. The P values correspond with the test of the association between outcome and IL-6 (PIL-6), eGFR (PCKD), and the multiplicative eGFR × IL-6 interaction (PInt). The x-axis is presented on a logarithmic scale. Vertical dashed lines represent the 3 sample quartiles. CV indicates cardiovascular; MACE, major adverse cardiovascular events; MI, myocardial infarction.
Figure 2. Event Rates for Major Adverse Cardiovascular Events by Chronic Kidney Disease Strata.
Event rates for major adverse cardiovascular events (cardiovascular death, myocardial infarction, and stroke) associated with baseline concentrations of interleukin 6 (IL-6) (≥2.0 ng/L and <2.0 ng/L) by chronic kidney disease strata (estimated glomerular filtration rate [eGFR] ≥90, 60-90, and <60 mL/min/1.73 m2).
Secondary End Points
Higher levels of IL-6 were in continuous models associated with increased risk of all-cause death, cardiovascular death, MI, stroke, and heart failure, especially among patients with CKD (Figure 1 and eFigure 1 in Supplement 1). Similarly, higher vs lower levels of IL-6 were associated with increased risk of cardiovascular outcomes among patients regardless of CKD strata in unadjusted analysis (eFigure 2 in Supplement 1). When adjusting for potential confounders several associations remained, and higher vs lower IL-6 levels were associated with increased risk of all-cause death, cardiovascular death, and heart failure across all CKD strata and for MI and stroke only among patients with mild CKD (eFigure 3 in Supplement 1).
Discussion
We showed that higher levels of IL-6 in patients with chronic coronary syndrome were independently associated with increased risk of MACE across the range of eGFR and within commonly used CKD strata. Also, the magnitude of net risk with elevated levels of IL-6 increased with higher CKD burden.
In line with previous observations, this study has shown that higher levels of IL-6 are associated with cardiovascular outcomes in patients with chronic coronary syndrome.2,11 Similar associations between inflammatory biomarkers and subsequent cardiovascular outcomes have been reported in the general population.3 The present study builds on these observations and establishes that higher levels of IL-6 are associated with cardiovascular events in patients with chronic coronary syndrome and CKD, with the absolute largest risk among patients with higher burden of CKD.
CKD is a well-established risk factor for CVD, and elevated levels of inflammatory biomarkers is a common finding among patients with CKD.4,12 The cause explaining increased risk of CVD among patients with CKD is not completely known but may in part be explained by inflammation linking CKD and CVD. In support of this theory, experimental studies have shown that kidney injury activates the NOD-like receptor protein 3 (NLRP3) inflammasome, which subsequently activates inflammatory responses mediated through IL-1β and IL-6.12 Thus, elevated levels of IL-6 in patients with higher burden of CKD might in part explain our findings.
Although there is evidence that chronic inflammation plays a pivotal role in the development of CVD, no anti-inflammatory drug is routinely used in patients with chronic coronary syndrome. Several anti-inflammatory drugs including colchicine and canakinumab have been shown to reduce the risk of cardiovascular events in patients with acute and chronic coronary syndrome.5,6,7 With canakinumab, the reduction of cardiovascular events was especially observed among patients with moderate CKD and in patients with reduced on-treatment levels of IL-6 and C-reactive protein.13,14 In contrast, anti-inflammatory drugs not directly targeting the IL-6 pathway (methotrexate and darapladib) have not been associated with reduction of cardiovascular events.10,15 Recently, novel IL-6 inhibitors have been shown to reduce inflammatory activity and are entering phase 2 to 3 clinical trials.8,9 Therefore, there is an urgent need to identify target populations that might gain most benefits from anti-inflammatory treatment. Our findings provide a target population at high-risk who might benefit from IL-6 inhibition, ie, patients with chronic coronary syndrome with elevated IL-6 levels and CKD.
Limitations
This is one of the largest studies analyzing the association between IL-6 level and outcome in patients with chronic coronary syndrome and CKD. Still, this study is not without limitations. Owing to the observational nature of this study, the associations cannot be interpreted as causal and residual confounding cannot be excluded. Also, patients in this study were included in a randomized clinical trial, which might not be generalizable to common practice. For example, patients with eGFR level less than 30 mL/min/1.73 m2 were excluded in the STABILITY trial, and therefore, the findings cannot be extrapolated to patients with severe CKD.
Conclusions
In patients with chronic coronary syndrome, higher levels of IL-6 were associated with increased risk of cardiovascular outcomes across the range of eGFR and within CKD strata, with the absolute largest risk among patients with more severe CKD. Thus, IL-6 level and CKD status should be useful as decision support for selection of patients with chronic coronary syndrome who may derive benefit from anti-inflammatory treatment with general and specific IL-6 inhibition.
eAppendix.
eTable 1. Baseline characteristics in the STABILITY trial cohort and in the biomarker substudy
eTable 2. Baseline characteristics in relation to IL-6 levels at randomization
eFigure 1. Associations between continuous IL-6 and eGFR concentrations and outcome
eFigure 2. Associations between IL-6 concentrations and outcome across CKD stages in unadjusted analysis
eFigure 3. Associations between IL-6 concentrations and outcome across CKD stages in adjusted analysis
Nonauthor Collaborators. The STABILITY Investigators.
References
- 1.Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ Res. 2016;118(1):145-156. doi: 10.1161/CIRCRESAHA.115.306656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Held C, White HD, Stewart RAH, et al. ; STABILITY Investigators . Inflammatory biomarkers interleukin-6 and c-reactive protein and outcomes in stable coronary heart disease: experiences from the STABILITY (stabilization of atherosclerotic plaque by initiation of darapladib therapy) trial. J Am Heart Assoc. 2017;6(10):e005077. doi: 10.1161/JAHA.116.005077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101(15):1767-1772. doi: 10.1161/01.CIR.101.15.1767 [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296-1305. doi: 10.1056/NEJMoa041031 [DOI] [PubMed] [Google Scholar]
- 5.Nidorf SM, Fiolet ATL, Mosterd A, et al. ; LoDoCo2 Trial Investigators . Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383(19):1838-1847. doi: 10.1056/NEJMoa2021372 [DOI] [PubMed] [Google Scholar]
- 6.Tardif J-C, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381(26):2497-2505. doi: 10.1056/NEJMoa1912388 [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Everett BM, Thuren T, et al. ; CANTOS Trial Group . Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119-1131. doi: 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 8.Kleveland O, Kunszt G, Bratlie M, et al. Effect of a single dose of the interleukin-6 receptor antagonist tocilizumab on inflammation and troponin T release in patients with non-ST-elevation myocardial infarction: a double-blind, randomized, placebo-controlled phase 2 trial. Eur Heart J. 2016;37(30):2406-2413. doi: 10.1093/eurheartj/ehw171 [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Devalaraja M, Baeres FMM, et al. ; RESCUE Investigators . IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2021;397(10289):2060-2069. doi: 10.1016/S0140-6736(21)00520-1 [DOI] [PubMed] [Google Scholar]
- 10.White HD, Held C, Stewart R, et al. ; STABILITY Investigators . Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med. 2014;370(18):1702-1711. doi: 10.1056/NEJMoa1315878 [DOI] [PubMed] [Google Scholar]
- 11.Kaptoge S, Seshasai SRK, Gao P, et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J. 2014;35(9):578-589. doi: 10.1093/eurheartj/eht367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutton HL, Ooi JD, Holdsworth SR, Kitching AR. The NLRP3 inflammasome in kidney disease and autoimmunity. Nephrology (Carlton). 2016;21(9):736-744. doi: 10.1111/nep.12785 [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, MacFadyen JG, Glynn RJ, et al. Inhibition of interleukin-1β by canakinumab and cardiovascular outcomes in patients with chronic kidney disease. J Am Coll Cardiol. 2018;71(21):2405-2414. doi: 10.1016/j.jacc.2018.03.490 [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ; CANTOS Trial Group . Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391(10118):319-328. doi: 10.1016/S0140-6736(17)32814-3 [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Everett BM, Pradhan A, et al. ; CIRT Investigators . Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med. 2019;380(8):752-762. doi: 10.1056/NEJMoa1809798 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix.
eTable 1. Baseline characteristics in the STABILITY trial cohort and in the biomarker substudy
eTable 2. Baseline characteristics in relation to IL-6 levels at randomization
eFigure 1. Associations between continuous IL-6 and eGFR concentrations and outcome
eFigure 2. Associations between IL-6 concentrations and outcome across CKD stages in unadjusted analysis
eFigure 3. Associations between IL-6 concentrations and outcome across CKD stages in adjusted analysis
Nonauthor Collaborators. The STABILITY Investigators.