This observational study assesses the capability of intraoperative molecular imaging to localize visually occult, nonpalpable tumors and quantify margin distances during resection.
Key Points
Question
Can intraoperative molecular imaging localize nonpalpable, visually occult tumors and accurately predict margin adequacy during resection?
Findings
In this nonrandomized open-label trial of 40 adults with a T1 pulmonary lesion radiographically suspicious for a nonpalpable cancer, 83% of nonpalpable, nonvisible lesions were identified by intraoperative molecular imaging and intraoperative molecular imaging–calculated margins were nearly identical to those reported on final pathology.
Meaning
By identifying occult tumors and cancer-positive margins that may have been missed by conventional surgical methods, intraoperative molecular imaging can improve surgical management of nonpalpable tumors across the field of surgical oncology.
Abstract
Importance
Complete (R0) resection is the dominant prognostic factor for survival across solid tumor types. Achieving adequate tumor clearance with appropriate margins is particularly difficult in nonpalpable tumors or in situ disease. Previous methods to address this problem have proven time consumptive, impractical, or ineffective.
Objective
To assess the capability of intraoperative molecular imaging (IMI), a novel technology using a fluorescent tracer targeted to malignant cells, to localize visually occult, nonpalpable tumors and quantify margin distances during resection.
Design, Setting, and Participants
This nonrandomized open-label trial of IMI using a folate receptor-targeted fluorescent tracer enrolled patients between May 2017 and June 2020 at a single referral center. Eligible patients included those with a small (T1) lung lesion suspicious for malignant neoplasms and with radiographic features suggestive of a nonpalpable lesion.
Interventions
Patients were preoperatively infused with a folate receptor-targeted near-infrared tracer. Intraoperatively, surgeons used thoracoscopic visualization and palpation to identify lesions. IMI was performed to detect the lesion in situ, and lesions were imaged ex vivo. Margins were assessed by IMI before comparison with those reported on final histopathologic analysis.
Main Outcomes and Measures
The main outcomes were whether IMI could (1) localize nonpalpable lung lesions in situ and (2) quantify margin distance with comparison with final pathology as the criterion standard. Patient demographic information and lesion characteristics were prospectively recorded.
Results
Of 40 patients, 26 (65%) were female, and the median (interquartile range) age was 66.5 (62-72) years. Conventional surgical methods localized 22 of 40 lesions (55%), while IMI localized 36 of 40 (90%). Of 18 nonpalpable lesions, 15 (83.3%) were identified by IMI. Both palpable and nonpalpable lesions demonstrated mean signal-to-background ratio more than 2. An IMI margin was able to be calculated for 39 of 40 patients (95%). IMI margins were nearly identical to margins reported on final pathology (R2 = 0.9593), with median (interquartile range) difference of 1.3 (0.7-2.0) mm. IMI detected 2 margins in nonpalpable tumors that were clinically unacceptable and would have had a high probability of recurrence.
Conclusions and Relevance
To our knowledge, this study presents the first clinical use of IMI for nonpalpable tumors and provides proof of principle for the utility of IMI across the field of surgical oncology in identifying occult disease and tumor-positive margins.
Introduction
Surgery remains the mainstay of therapy for solid tumors, and complete (R0) resection is the dominant prognostic factor for survival across tumor types.1 Achieving adequate disease clearance with appropriate margins is particularly difficult in cancers marked by subtle parenchymal changes, including early-stage breast, thyroid, hepatic, and pulmonary malignant neoplasms.2,3,4 In such cases, visual inspection, palpation of the operative field, and excised specimen analysis are often insufficient to localize tumors or identify involved margins, leading to early locoregional recurrence.1 Previous methods to improve localization of these malignant neoplasms, including preoperative hook wire placement, radiotracer guidance, and various optical spectroscopic techniques, have proven time consumptive, expensive, impractical, or ineffective.5 Furthermore, there are no existing methods of real-time, intraoperative margin evaluation.
Here, we harness the emerging field of intraoperative molecular imaging (IMI) using receptor-targeted fluorescent tracers to determine whether nonpalpable, visually occult tumors can be identified in vivo and for pathologic margin assessment. IMI is the clinical application of real-time optical imaging in the near-infrared spectrum, using fluorescently tagged, tumor-targeted probes.6,7 As a pilot study, we conducted a nonrandomized trial (NCT02602119) of IMI in 40 patients with a small (T1) lung lesion suspicious for malignant neoplasms and with radiographic features suggestive of a nonpalpable lesion. We hypothesized that IMI could provide real-time localization of nonpalpable lung lesions and quantify margin distances nearly identical to those calculated by formal pathologic analysis. To our knowledge, this study presents the first clinical use of IMI for nonpalpable tumors and provides proof of principle for the clinical use of IMI across the field of surgical oncology.
Methods
Summary of Study Design
The study received approval from the University of Pennsylvania institutional review board. Forty patients with a small (T1) lung lesion suspicious for malignant neoplasm and with radiographic features suggestive of a nonpalpable lesion (ground glass opacity with or without solid component) were enrolled between May 2017 and June 2020. Exclusion criteria included people younger than 18 years, people unable to give informed consent, people who spoke languages other than English, and patients with prior chest surgery. FA-S0456 (intravenous, 0.025 mg/kg) was administered 6 to 24 hours prior to resection. During minimally invasive video-assisted thoracic surgery, surgeons first used thoracoscopic visualization and finger palpation to identify known lesions. Next, a standard clinical grade optical imaging device with a λ for excitation laser of 785 nm, and λ for emission band-pass filter of 800 to 835 nm (VisionSense) was used to capture signal from the fluorescent probe that had localized to the lesion of interest. After resection, specimens were imaged ex vivo and margins were assessed by IMI as described below. If tumor-positive or close margins (defined as <0.5 cm) were identified, additional parenchyma was removed and specimens were then submitted for pathologic analysis. This cutoff value was selected to be consistent with the standards used in previous trials of IMI in thoracic surgery.8,9
Study Drug
FA-S0456 (C61H63N9Na4O17S4; molecular weight: 1414.42) is a folate analog conjugated to the near-infrared dye, S0456. FA-S0456 excites at 774 nm to 776 nm and emits at 794 nm to 796 nm. The tracer targets the folate receptor alpha, which is overexpressed 90% of pulmonary adenocarcinomas and 70% of squamous cell carcinomas and has been shown to identify solitary pulmonary nodules as small as 2 mm. This drug was provided by On Target Laboratories.10,11
Video-Assisted Thoracic Surgery and Back Table Imaging Device
In situ, real-time fluorescent imaging was performed during video-assisted thoracic surgery using a 5-mm, 0° thoracoscope (VisionSense) optimized for detection of FA-S0456. This device is a high-definition, dual-band (white-light and near-infrared) camera system capable of near-infrared emission and detection. A 785-nm excitation source was used, and fluorescence was detected using a band-pass filter ranging from 800 nm to 835 nm. For ex vivo evaluation, a free-standing exoscope with similar optical imaging settings was used.
Post Hoc Image Analysis
Post hoc image analysis was conducted with ImageJ.12 Mean fluorescence intensity (MFI) of the lesion was obtained by analyzing monochromatic near-infrared images and measuring the region of interest that correlated with the lesion. Background fluorescence (0.5-1.0 cm from margin) was also obtained. Calculations were repeated in triplicate, and signal-to-background fluorescence ratios (SBR) were calculated using the equation MFI of the lesion/MFI of the background. Mean SBR assessments were calculated, and a mean SBR more than 2.0 was considered fluorescent.
Statistical Analysis
Owing to the exploratory nature of this study, logistic regression was used to determine patient and histopathologic variables associated with in situ lesion fluorescence. Comparisons were made using Stata version 14 (StataCorp). A P value less than .05 was considered statistically significant.
Results
Patient Characteristics
Of the 40 enrolled patients, the mean (interquartile range [IQR]) patient age was 66.5 (62-72) years. Race and ethnicity data were self-reported by study participants. Within the study cohort, 7 patients (17.5%) self-identified as non-Hispanic Black, and 32 (80%) self-identified as non-Hispanic White. The majority of patients were female (26 [65%]) and formerly smoked (30 [75%]; 28.6 mean pack-years). The mean (IQR) lesion size was 1.52 (1.10-1.60) cm, and the mean (IQR) depth was 0.43 (0-0.625) cm from the pleural surface. By histopathologic analysis, the majority of tumors were invasive adenocarcinomas (27 [67.5%]). The other lesions were adenocarcinomas in situ (6 [15%]), squamous cell carcinoma of the lung (2 [5%]), small cell lung cancer (2 [5%]), or benign lesions (3 [7.5%]). Twenty-two lesions were palpable by conventional thoracoscopic methods and 18 were nonpalpable (Figure 1A for representative images). Nonpalpable lesions (Figure 1B for representative images) were smaller (1.41 vs 1.6 cm) and deeper from the pleural surface (0.57 vs 0.32 cm) than palpable lesions (eFigure 1 in the Supplement), although these trends did not reach statistical significance. All patients received the entire infusion of FA-S0456 and there were no adverse events associated with infusion of the study drug. A full summary of patient and lesion characteristics is provided in the Table.
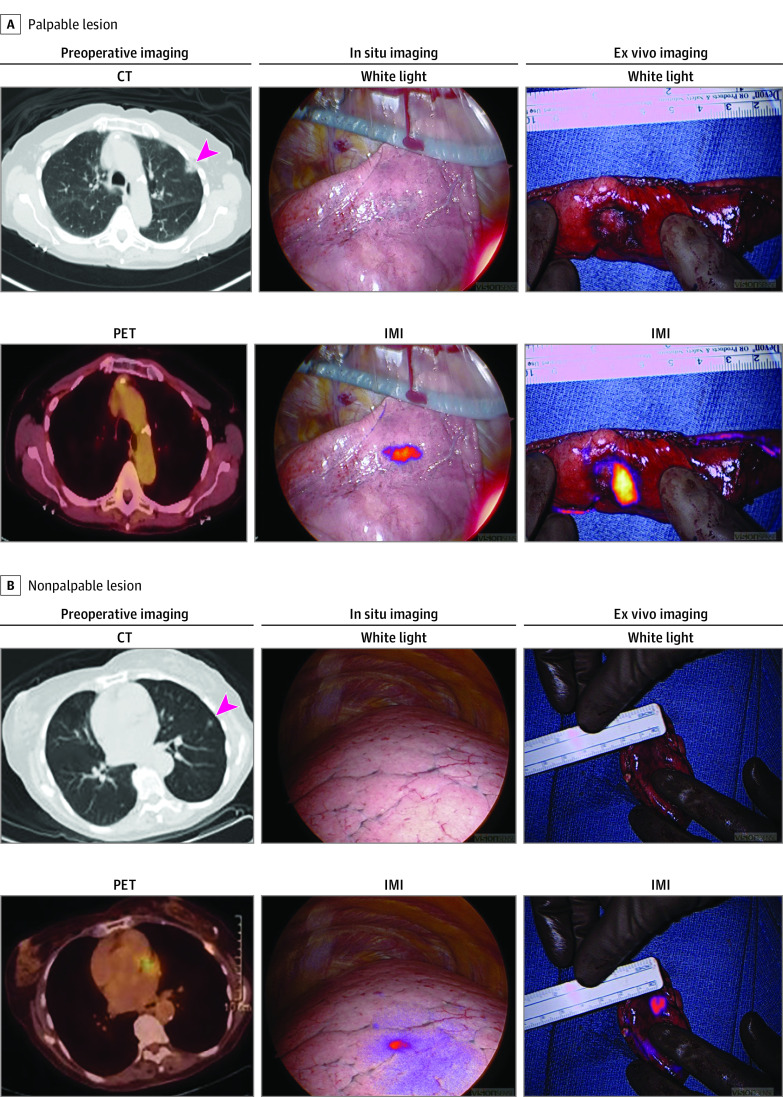
Figure 1. Intraoperative Molecular Imaging (IMI) Localizes Palpable and Nonpalpable Pulmonary Lesions.
A, Representative images of a patient with a palpable lesion including preoperative computed tomography (CT) scan with arrowhead marking the lesion, preoperative positron emission tomography (PET) scan, white-light thoracoscopic images and intraoperative molecular imaging localization with near-infrared (NIR) tracer both in situ and on the back table. B, An identical set of images from a representative patient with a nonpalpable lesion.
Table. Patient Demographics and Lesion Characteristics.
| Characteristic | No. (%) |
|---|---|
| Sex | |
| Male | 14 (35) |
| Female | 26 (65) |
| Age, mean (IQR), y | 66.5 (62-72) |
| Former smoker | 31 (77.5) |
| Pack-years, mean (IQR) | 28.6 (15.75-45) |
| Lesion characteristics, mean (IQR) | |
| PET SUV | 2.15 (0.94-3.03) |
| Size of lesion, cm | 1.52 (1.10-1.60) |
| Depth of lesion, cm | 0.43 (0-0.625) |
| Tumor location | |
| RUL | 12 (30) |
| RML | 3 (7.5) |
| RLL | 8 (20) |
| LUL | 11 (27.5) |
| LLL | 6 (15) |
| Final pathology | |
| Invasive adenocarcinoma | 27 (67.5) |
| Adenocarcinoma in situ | 6 (15) |
| Squamous cell carcinoma | 2 (5) |
| Small cell lung cancer | 2 (5) |
| Benign lesion | 3 (7.5) |
| Tumor differentiation | |
| Well differentiated | 11 (27.5) |
| Moderately differentiated | 17 (42.5) |
| Poorly differentiated | 7 (17.5) |
| Not reported | 5 (12.5) |
Abbreviations: IQR, interquartile range; LLL, left lower lobe; LUL, left upper lobe; PET, positron emission tomography; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe; SUV, standardized uptake value.
Palpable and Nonpalpable Pulmonary Lesions
Figure 2A denotes lesion location, palpability, and fluorescence. Conventional surgical methods localized 22 of 40 lesions (55%), while IMI localized 36 of 40 (90%). Of 22 palpable lesions, 21 (95.4%) were fluorescent in vivo. Of 18 lesions that were nonpalpable and could not be located by traditional thoracoscopic methods, 15 (83.3%) were fluorescent. The remaining 3 lesions required standard-of-care methods of generous wedge resection (n = 1) or formal lobectomy (n = 2) given inability to localize the lesion. On back-table analysis, 35 of 40 lesions (87.5%) were palpable by traditional surgical methods, and 39 of 40 lesions (97.5%) were fluorescent by IMI. One lesion was not able to be definitively identified in vivo or ex vivo by either traditional methods or IMI and necessitated repeated sectioning by frozen section pathology to identify the lesion, a small (1 cm) adenocarcinoma that was 2.5 cm deep to the pleural surface.
Figure 2. Synoptic Summary of Lesion Location and Fluorescence.
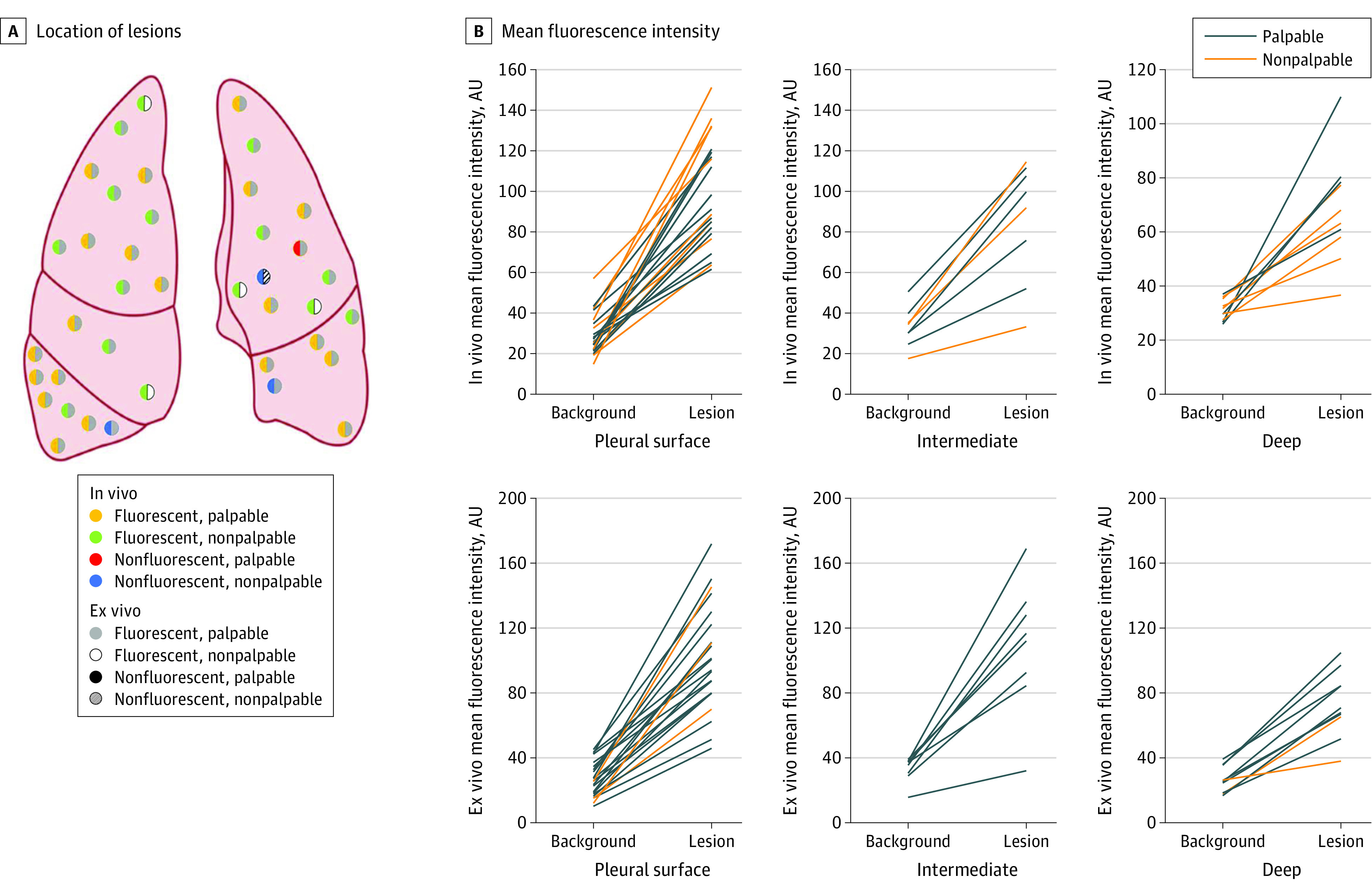
A, Location of lesions with color schema describing palpability and fluorescence. The color in the left hemisphere of each nodule denotes in vivo properties, and the color in the right hemisphere denotes ex vivo properties. B, Mean fluorescence intensity of lesion and background measurements, stratified by depth (surface, intermediate depth [0.01-0.99 cm], and deep lesions [≥1 cm]). Each line links lesion and background measurements from the same individual. AU indicates arbitrary unit.
MFI of lesions decreased as a function of lesion depth from the pleural surface (Figure 2B). Surface lesions displayed significantly greater MFI compared with deep (≥1 cm) lesions, both in vivo (98.5 vs 68.2 arbitrary units; P = .003) and ex vivo (101.28 vs 73.2 arbitrary units; P = .02). Both palpable and nonpalpable lesions demonstrated mean SBR more than 2, and there were no significant differences in SBR when stratified by palpability (eFigure 2A in the Supplement). Receiver operating characteristic curve evaluation (eFigure 2B in the Supplement) showed the area under the curve obtained for in vivo MFI was 0.986 (95% CI, 0.965-1.00) and the area under the curve obtained for ex vivo MFI was 0.985 (95% CI, 0.964-1.000).
Margin Distance
To assess whether IMI could quantify margin distance for nonpalpable disease, we imaged all resected specimens with a ruler for scale to yield white-light and near-infrared images (eFigure 3A in the Supplement). Images were then analyzed using ImageJ to quantify margin distance by first calibrating the distance of a centimeter in pixels and then measuring from the edge of the fluorescent signal to the staple line (eFigure 3B in the Supplement). Margin distance calculated by IMI was compared with the margin reported on final histopathologic analysis several days postoperatively (Figure 3A).
Figure 3. Near Real-Time Quantification of Margin Distance Using Intraoperative Molecular Imaging (IMI).
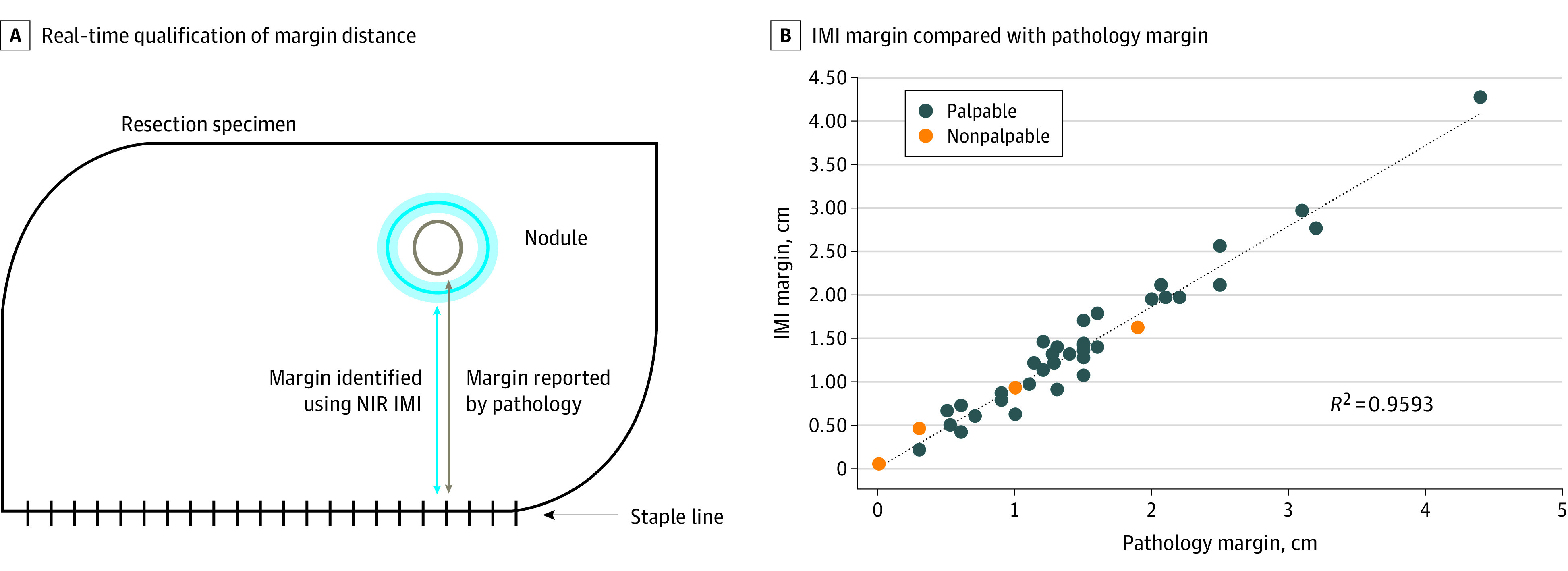
A, Association between margin calculated by IMI and margin measured by pathology. B, IMI margin compared with pathology margin for all lesions in the study. Orange points denote lesions that were not palpable on the back table. NIR indicates near infrared.
An IMI margin was able to be calculated for 39 of 40 patients (95%), given that a single patient’s lesion was nonfluorescent on ex vivo imaging. The margin distance measured by IMI ranged from 0.5 mm to 42.7 mm. IMI margins were nearly identical to margins reported on final pathology, with coefficient of determination (R2) of 0.9593 (Figure 3B). IMI slightly underpredicted margin distance (trend line equation: y = 0.934x + 0.0072), but the median (IQR) difference in margins was 1.3 (0.7-2.0) mm. IMI detected 2 margins that were clinically unacceptable and would have led to a high probability of recurrence for these patients. These margins were not detectable by visualization or palpation and would have been missed by traditional methods.
Clinical Management of Nonpalpable Tumors
In total, 15 of 40 lesions (37.5%) were localized by IMI alone (Figure 4A shows a representative patient). These patients underwent minimally invasive wedge resection and were spared the morbidity of opening the chest or an extended resection such as a segmentectomy or lobectomy, which would have been pursued given failure of traditional methods to localize the tumors. IMI also allowed for rapid margin assessment by ex vivo evaluation. This was most useful in patients 5 (Figure 4B) and 10, whose tumors were not palpable but IMI detected tumor-positive and close margins, respectively. This near real-time feedback allowed immediate reresection, as opposed to frozen section pathology, which would have necessitated additional time under anesthesia and its attendant morbidity.
Figure 4. Intraoperative Molecular Imaging (IMI) Alters Surgical Management of Nonpalpable Lesions.
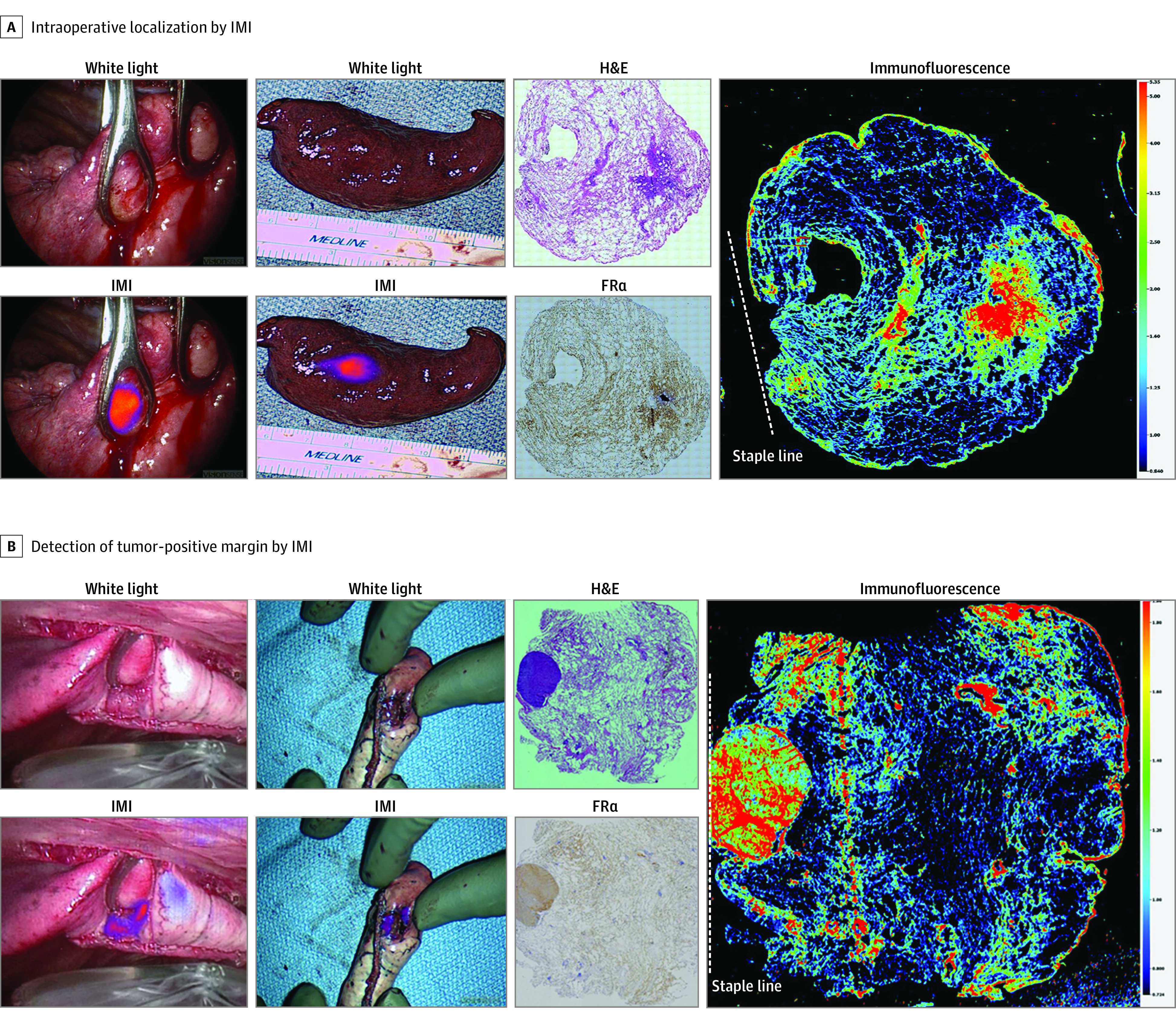
A, Representative images from a tumor that was not able to be detected by traditional surgical methods of visual inspection and palpation. IMI clearly identifies the lesion of interest both in situ and ex vivo. Histologic evaluation using hematoxylin-eosin (H&E) staining, folate receptor alpha (FRα) staining, and immunofluorescence confirm that fluorescence is concentrated in the area of tumor, which overexpressed FRα and was far from the staple line (dashed line). B, Identical images of a nonpalpable tumor with a tumor-positive margin as indicated by fluorescence on the staple line on ex vivo imaging and confirmed by immunofluorescence microscopy.
Discussion
Reliable localization and margin assessment during resection of nonpalpable tumors are formidable challenges for the field of surgical oncology, especially in early-stage disease, or in malignant neoplasms marked by subtle parenchymal distortions, including hepatic, thyroid, breast, and lung cancers.2,3,4,5 Here, we demonstrate 2 novel innovations, the use of optical imaging with a fluorescently labeled probe for in vivo localization of nonpalpable tumors and for quantification of margin distance, both of which altered the surgical management of many patients in this trial.
Previous attempts to localize nonpalpable tumors in oncologic surgery have shown limited efficacy with a well-documented adverse effect profile including bleeding, device dislodgement, and additional radiation exposure.5 Furthermore, these techniques primarily assist with in vivo localization but do not offer adjunctive margin assessment and lack resolution below 1 cm. Current margin assessment relies on frozen section pathology, a resource-intensive method that prolongs time under anesthesia and often requires repeated sectioning to identify nonpalpable lesions.13,14
Optical imaging is ideal for localization and margin assessment in nonpalpable tumors owing to its safety, accuracy, and rapidity. Previous work by our group and others has demonstrated the utility of optical imaging in the resection of palpable solid tumors, but the technology has not been extended to nonpalpable lesions.6,7,8,15,16,17,18,19,20 Furthermore, prior attempts to use optical imaging for margin assessment have concerned a dichotomous assessment of whether or not a margin is tumor positive.17,21 We demonstrate a novel technique of assessing the exact distance between the margin and the tumor, an innovation that allows surgeons to make a rapid determination of whether the margin distance is adequate in accordance with national guidelines.
Most notable in our study was the finding that nearly 40% of the patients with nonpalpable tumors in this trial had alterations in their clinical management based on IMI findings. In many of these cases, IMI localized tumors or quantified margins in lesions that were indistinguishable from normal lung parenchyma by visual inspection or palpation. IMI facilitated removal of the lesion by a small wedge resection, rather than a lobectomy, which would have involved resection of a large amount of normal lung parenchyma in patients who often have compromised baseline pulmonary function. We specifically recruited patients with small, clinical stage-T1 tumors to study the ability of IMI to address the pressing clinical problem of resection adequacy in nonpalpable disease. However, we predict that the technology will also provide a useful adjunct in resection of larger tumors.
With the rise of minimally invasive thoracic surgery, we anticipate that IMI will continue to gain prominence as an adjunct to the surgeon’s ability to localize pulmonary lesions. The increasing use of video-assisted thoracic surgery has been an important innovation in reducing operative morbidity for patients and decreasing length of stay. However, these benefits have come at the cost of the removal or distortion of haptic feedback to the surgeon. The specific camera used in this study is a wavelength-specific thoracoscope designed to detect FA-S0456. This specialized camera is an additional cost to video-assisted thoracic surgery, but we anticipate that this cost will be less relevant as wavelength-tunable scopes are developed and standardized for minimally invasive thoracic surgery.
Limitations
This trial uses a near-infrared probe, FA-S0456, that specifically targets malignant cells that overexpress folate receptor alpha. Thus, it is not useful in tumors that lack this expression profile. We anticipate that the clinical utility of IMI will expand as new targeted tracers are developed for different malignant neoplasms. We acknowledge that this exploratory study was not randomized because of the inability to determine a priori whether or not a lesion is palpable. Furthermore, the study cohort was small, and additional trials at other centers and in other tumor types are the subject of ongoing studies to confirm efficacy and justify further development. IMI itself is limited in depth of lesion detection, which is reflected in our study.
Conclusions
This trial demonstrates that IMI can improve the surgical management of patients with nonpalpable pulmonary lesions by identifying occult tumors and cancer-positive margins that may have been missed by conventional surgical methods. The potential of this work is far reaching and offers proof of principle for the use of IMI in guiding surgical management of nonpalpable tumors across the field of surgical oncology.
eFigure 1. Lesion characteristics stratified by intraoperative palpability of the lesion
eFigure 2. Lesion fluorescence and ROC evaluation
eFigure 3. Method of quantifying resection margins using IMI
References
- 1.Aliperti LA, Predina JD, Vachani A, Singhal S. Local and systemic recurrence is the Achilles heel of cancer surgery. Ann Surg Oncol. 2011;18(3):603-607. doi: 10.1245/s10434-010-1442-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed M, Rubio IT, Klaase JM, Douek M. Surgical treatment of nonpalpable primary invasive and in situ breast cancer. Nat Rev Clin Oncol. 2015;12(11):645-663. doi: 10.1038/nrclinonc.2015.161 [DOI] [PubMed] [Google Scholar]
- 3.Papini E. The dilemma of non-palpable thyroid nodules. J Endocrinol Invest. 2003;26(1):3-4. doi: 10.1007/BF03345115 [DOI] [PubMed] [Google Scholar]
- 4.Inoue Y, Takahashi M, Arita J, et al. Intra-operative freehand real-time elastography for small focal liver lesions: “visual palpation” for non-palpable tumors. Surgery. 2010;148(5):1000-1011. doi: 10.1016/j.surg.2010.02.009 [DOI] [PubMed] [Google Scholar]
- 5.Keating J, Singhal S. Novel methods of intraoperative localization and margin assessment of pulmonary nodules. Semin Thorac Cardiovasc Surg. 2016;28(1):127-136. doi: 10.1053/j.semtcvs.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 6.Tipirneni KE, Warram JM, Moore LS, et al. Oncologic procedures amenable to fluorescence-guided surgery. Ann Surg. 2017;266(1):36-47. doi: 10.1097/SLA.0000000000002127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernot S, van Manen L, Debie P, Mieog JSD, Vahrmeijer AL. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol. 2019;20(7):e354-e367. doi: 10.1016/S1470-2045(19)30317-1 [DOI] [PubMed] [Google Scholar]
- 8.Low PS, Henne WA, Doorneweerd DD. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc Chem Res. 2008;41(1):120-129. doi: 10.1021/ar7000815 [DOI] [PubMed] [Google Scholar]
- 9.Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem. 2005;338(2):284-293. doi: 10.1016/j.ab.2004.12.026 [DOI] [PubMed] [Google Scholar]
- 10.Sienko A, Allen TC, Zander DS, Cagle PT. Frozen section of lung specimens. Arch Pathol Lab Med. 2005;129(12):1602-1609. doi: 10.5858/2005-129-1602-FSOLS [DOI] [PubMed] [Google Scholar]
- 11.Walts AE, Marchevsky AM. Root cause analysis of problems in the frozen section diagnosis of in situ, minimally invasive, and invasive adenocarcinoma of the lung. Arch Pathol Lab Med. 2012;136(12):1515-1521. doi: 10.5858/arpa.2012-0042-OA [DOI] [PubMed] [Google Scholar]
- 12.ImageJ. Accessed July 22, 2021. https://imagej.nih.gov/ij/
- 13.Hoogstins CES, Tummers QR, Gaarenstroom KN, et al. A novel tumor-specific agent for intraoperative near-infrared fluorescence imaging: a translational study in healthy volunteers and patients with ovarian cancer. Clin Cancer Res. 2016;22(12):2929-2938. doi: 10.1158/1078-0432.CCR-15-2640 [DOI] [PubMed] [Google Scholar]
- 14.Kennedy GT, Okusanya OT, Keating JJ, et al. The optical biopsy: a novel technique for rapid intraoperative diagnosis of primary pulmonary adenocarcinomas. Ann Surg. 2015;262(4):602-609. doi: 10.1097/SLA.0000000000001452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu G, van den Berg NS, Martin BA, et al. Tumour-specific fluorescence-guided surgery for pancreatic cancer using panitumumab-IRDye800CW: a phase 1 single-centre, open-label, single-arm, dose-escalation study. Lancet Gastroenterol Hepatol. 2020;5(8):753-764. doi: 10.1016/S2468-1253(20)30088-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishio N, van den Berg NS, van Keulen S, et al. Optical molecular imaging can differentiate metastatic from benign lymph nodes in head and neck cancer. Nat Commun. 2019;10(1):5044. doi: 10.1038/s41467-019-13076-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Predina JD, Newton AD, Keating J, et al. A phase I clinical trial of targeted intraoperative molecular imaging for pulmonary adenocarcinomas. Ann Thorac Surg. 2018;105(3):901-908. doi: 10.1016/j.athoracsur.2017.08.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Dam GM, Themelis G, Crane LMA, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nat Med. 2011;17(10):1315-1319. doi: 10.1038/nm.2472 [DOI] [PubMed] [Google Scholar]
- 19.Keating J, Newton A, Venegas O, et al. Near-infrared intraoperative molecular imaging can locate metastases to the lung. Ann Thorac Surg. 2017;103(2):390-398. doi: 10.1016/j.athoracsur.2016.08.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Keulen S, van den Berg NS, Nishio N, et al. Rapid, non-invasive fluorescence margin assessment: optical specimen mapping in oral squamous cell carcinoma. Oral Oncol. 2019;88:58-65. doi: 10.1016/j.oraloncology.2018.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Keulen S, Nishio N, Birkeland A, et al. The sentinel margin: Intraoperative ex vivo specimen mapping using relative fluorescence intensity. Clin Cancer Res. 2019;25(15):4656-4662. doi: 10.1158/1078-0432.CCR-19-0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Lesion characteristics stratified by intraoperative palpability of the lesion
eFigure 2. Lesion fluorescence and ROC evaluation
eFigure 3. Method of quantifying resection margins using IMI