Abstract
Background:
There is lack of uniformity in the reflectance confocal microscopy (RCM) terminology for melanocytic lesions.
Objective:
To review published RCM terms for melanocytic lesions and identify redundant, synonymous terms.
Methods:
Systematic review of original research articles adhering to PRISMA guidelines was conducted until August 15, 2018. Two investigators gathered all published RCM terms used to describe melanoma and melanocytic nevi. Synonymous terms were grouped based on similarity in definition and in histopathological correlation.
Results:
Out of 156 full-text screened articles, 59 studies met the inclusion criteria. We identified 209 terms; 191 (91.4%) corresponding to ‘high-magnification/cellular level’ terms and 18 (8.6%) corresponding to ‘low-magnification/architectural patterns’ terms. The overall average use frequency of RCM terms was 3.1 times (range 1 – 31). By grouping of individual RCM terms based on ‘likely-synonymous’ definitions and by eliminating terms lacking clear definition, the total number of RCM terms could be potentially reduced from 209 to 40 terms (80.8% reduction).
Limitations:
Non-English and non-peer reviewed articles were excluded.
Conclusions:
This systematic review of published RCM terms identified significant terminology redundancy. It provides the basis for subsequent terminology consensus on melanocytic neoplasms.
Keywords: reflectance confocal microscopy, melanoma, nevus, melanocytic, diagnosis, non-invasive, systematic review
INTRODUCTION
Reflectance confocal microscopy (RCM) allows for non-invasive in vivo visualization of the skin at a “quasi-histological” resolution.1 In the 1990s, the histological correlates of RCM attributes of skin were first described.2, 3 Since, numerous publications have shown the utility of RCM in the diagnosis of melanoma4–7 and non-melanoma skin cancer,8–10 in monitoring of non-invasive skin treatments11, 12 and in guiding dermatological surgery.13–16
As demonstrated in a previous systematic review, RCM terminology in the literature lacks consistency and contains many redundant terms.17 We identified a total of 139 RCM terms used to describe non-melanocytic lesions (NMLs); by grouping these terms for synonymy, we were able to further shorten the list of terms by more than 50%.17 For melanocytic lesions, a consensus study of RCM terminology was published in 2007 by Scope et al;18 however, a multitude of new RCM terms, describing melanocytic neoplasms, has been published since.
Recently, category I Current Procedural Terminology (CPT) reimbursement codes have been allotted to RCM imaging in the U.S;1, 19 we anticipate a consequent rise in the integration of RCM into clinical practice.20 To that end, there is a pressing need for standardization of RCM terminology of melanocytic lesions17, 21 – to enable structured reporting of RCM examinations and to facilitate RCM teaching to novices. Herein, we performed a systematic review of the terms used to describe the RCM features of melanocytic neoplasms and identified redundant, synonymous terms.
METHODS
The results of this systematic review were obtained according to the guidelines for reporting systematic reviews as published in the PRISMA Statement (available in www.prisma-statement.org). All images used for illustrating RCM terms were acquired using a commercial RCM system (Vivascope 1500 or Vivascope 3000, Caliber ID, Rochester, NY), under an IRB protocol (#17–083). The principles of RCM imaging have been previously described.1
Eligibility criteria
We included all original, peer-reviewed RCM articles published between 1995 and 2018 that contained the diagnosis of nevi, including congenital and Spitz nevi, and cutaneous melanoma. So-called “borderline” melanocytic lesions, such as atypical Spitzoid tumors, were also included. We excluded articles describing RCM features of collision tumors and melanocytic lesions of special anatomic sites, such as genitalia, nails and eyelids. Literature reviews, single case-reports, conference abstracts, animal studies, and publications lacking full-text were excluded; due to the lack of peer-review process, we also excluded book chapters.
Information sources, search and Study selection
Systematic literature searches were conducted (August 15, 2018) in four databases with no specified date, age, sex, or language restrictions. The databases searched were: (1) MEDLINE (via PubMed); (2) Embase; (3) The Cochrane Library (Cochrane); and (4) Web of Science (WoS). In an effort to be comprehensive and include grey literature publications into the data set of citations, conference proceedings and abstracts were retrieved from Embase and WoS utilizing broad and inclusive publication-type filters. Search results were combined in a bibliographic management tool (EndNote, Clarivate Analytics) and duplicates were eliminated both electronically and manually to ensure an efficient de-duplication process. The search strategy employed the Medical Subject Headings (MeSH) phrases: [“Microscopy, Confocal”] AND [“Skin Neoplasms” OR “Dermatology” OR “melanoma” OR “nevus” AND “in vivo”] AND [“Terminology” OR “Current Procedural Terminology” OR “Terminology as Topic” OR “Dictionaries as Topic” OR “Data Accuracy” OR “Algorithms” OR “Reproducibility of Results” OR “Classification”]. Bibliographies within retrieved articles were also reviewed to identify additional studies. For this specific systematic review, we included only RCM terms pertinent for melanocytic lesions and excluded terms related to non-melanocytic neoplasms (e.g., basal cell carcinoma, squamous cell carcinoma and lichen planus-like keratosis). Two authors (C.N-D. and K.L.) independently screened all relevant titles and abstracts for eligibility. If necessary, full-text articles were screened. Differences in judgment were resolved with a third reviewer (M.J.) until consensus was achieved (Figure 1).
Figure 1:
PRISMA diagram.
Data collection and extraction process
Two authors (C.N-D. and K.L.) extracted data from the included studies independently. Disagreements were resolved by consensus; if agreement could not be reached, a third author (M.J.) was used as referee. The following information was extracted from each study: the RCM term, its definition, diagnosis associated with the term, the histopathological correlates associated with that term (when reported), and whether the term was used in an algorithm. All extracted RCM terms were recorded as published in the literature, chronologically, in an Excel spreadsheet (Microsoft, Redmond, WA). To weight the use-frequency of RCM terms, we recorded the number of studies that utilized each term. Lastly, five authors (C.N-D., K.L., J.M., S.A., M.J.) grouped all terms into ‘likely-synonymous’ terms based on their RCM definitions and on similar histopathologic correlates (e.g. “pagetosis’, “pagetoid infiltration”, and “atypical cells in the epidermis”). Next, the ‘likely-synonymous’ RCM terms were organized by the skin layer to which they had been ascribed: (1) epidermis, (2) dermo-epidermal junction (DEJ), and (3) dermis. Finally, we categorized all ‘likely-synonymous’ RCM terms into two categories: (a) high-magnification, cellular-level terms (based on optical sections, typically 0.5×0.5μm to 1×1mm in area) (e.g. atypical cells, inflammatory infiltrate), and (b) low-magnification patterns (based on mosaic RCM images, typically >1×1mm to 8×8mm in area) (e.g. ringed, meshwork or clod pattern). RCM terms lacking definition in the main text, tables, or figures of an article (or where the definition could not be clearly inferred by the name of the term), were listed as ‘definition not available (N/A)’. Additionally, following previously-published approach for simplifying terminology,22 we extracted basic terminology units from composite term (e.g. the term “Large roundish cells in the epidermis” contains the basic units “cells”, “large”, roundish” and “epidermis”). ‘Composite’ terms were defined as those that contained two or more basic units, while ‘simple’ terms were those with a single unit. We then categorized the basic elements into: ‘cells / structure’ (the cell type could be explicit or implied by the term, e.g., “cells”–in the aforementioned example implies melanocytes); ‘morphological descriptors / modifiers’ (e.g., “large” and “roundish”); and ‘anatomic location / distribution (e.g. “epidermis”).
Summary measures and statistical analysis
Descriptive statistics were used to categorize the number of RCM terms by diagnosis and by anatomic layer. ‘Use frequency’ was based on the number of papers describing each RCM term. ‘Average use frequency’ described the proportion between ‘use frequency’ for RCM terms (as individual terms or as subgroups of likely-synonymous terms) divided by the total ‘use frequency’ for all terms describing that diagnosis.
RESULTS
After screening 156 full-text articles, 59 studies were identified that met the inclusion criteria (Figure 1).4–6, 23–78 In total, 209 RCM terms were identified: 191 (91.4%) ‘high-magnification/cellular-level’ terms, and 18 (8.6%) ‘low-magnification/architectural pattern’ terms. The use frequency of each individual RCM term and ‘likely-synonymous’ groups of RCM terms are shown in Tables I–II. Representative images for each category are shown (Figures 2&3).
Table I:
Reflectance confocal microscopy terms used to describe melanocytic lesions at high magnification, cellular-level resolution
| RCM term | Use frequency of RCM term, n | Definition | Part of an algorithm | Histopathologic correlates |
|---|---|---|---|---|
| EPIDERMIS (n=68; 35.4%) | ||||
| - Terms describing presence of cells in the epidermis, utilizing ‘pagetoid’ without a specified cell shape | ||||
| Pagetoid cells4, 6, 23, 28–30, 35, 39, 41, 42, 48, 49, 52, 55, 58, 61, 63–68, 72, 75–78 | 27 | Large nucleated cells, twice the size of keratinocytes (>20 um), with a dark nucleus and bright cytoplasm. | Presence of melanocytes in suprabasal layers of the epidermis | |
| Pagetoid spread25, 32, 33, 56–58, 60, 62, 75 | 9 | Borsari 201825 | ||
| Widespread pagetoid infiltration4, 6, 36, 37, 43, 53, 58, 59,68 | 9 | Pellacani 200768 | ||
| Pagetoid melanocytes44, 54 | 2 | |||
| Cells in pagetoid pattern70 | 1 | |||
| Pagetoid spread of atypical melanocytes27 | 1 | |||
| Irregular intraepidermal growth of melanocytes at the periphery of the lesion32 | 1 | |||
| -Terms describing presence of cells in the epidermis, specifying ‘pleomorphic (round and dendritic) shape | ||||
| Polymorphic melanocytic cells45–47 | 3 | Variability of the aspect of pagetoid cells (round and dendritic) | ||
| Pleomorphic pagetoid infiltration68 | 1 | |||
| Pleomorphic pagetoid cells23 | 1 | |||
| Pleomorphic pagetoid shape56 | 1 | |||
| Pleomorphism (in the epidermis)72 | 1 | |||
| Bright dendrites/Dendritic/Round cells within the epidermis64 | 1 | |||
| Striking pleomorphism49 | 1 | |||
| -Terms describing presence of cells in the epidermis, specifying ‘round’ shape | ||||
| Roundish pagetoid cells36, 37, 49, 56, 59, 68, 72, 74, 75 | 9 | Large roundish nucleated cells, with a dark nucleus and bright cytoplasm, within suprabasal layers | Pellacani 2007,68 Segura 200923 | |
| Round pagetoid cells4, 6, 52, 53, 57, 58 | 6 | Guitera 20104 | ||
| Round cells5, 38, 57 | 3 | |||
| Large roundish cells in the epidermis34 | 1 | |||
| Round pagetoid cells with halo4* | 1 | |||
| -Terms describing presence of cells in the epidermis, specifying ‘dendritic’ shape | ||||
| Dendritic pagetoid cells4, 6, 49, 50, 52, 56, 58, 68, 72, 74, 75 | 11 | Bright nucleated cells with dendritic-like branches within suprabasal layers | ||
| Dendritic cells5, 38, 55, 57 | 4 | -- | ||
| Large dendritic cells in the epidermis34 | 1 | -- | ||
| -Terms describing presence of cells in the epidermis, specifying ‘low refractivity’ | ||||
| Hyporeflective pagetoid cells77, 78 | 2 | Round dark structures (similar to ‘holes’) within the epidermis | ||
| Dark pagetoid cells52* | 1 | -- | ||
| -Terms describing presence of ‘atypical’/’irregular’ cells in the epidermis without a specific shape | ||||
| Cell atypia6, 52, 58 | 3 | -- | ||
| Atypical cells24, 69 | 2 | -- | ||
| Enlarged atypical melanocytes32 | 1 | -- | ||
| Irregularly bright melanocytic cells46 | 1 | -- | ||
| Weighted subtotal | 105 | |||
| -Terms describing the presence of dendrites in the epidermis | ||||
| Composite branching dendrites37, 46, 47, 55 | 4 | Numerous bright tangled lines within the epidermal layers, originating from dendritic cells with not always visible cell body | Dendritic projections of melanocytes or Langerhans cells in the epidermis | |
| Simple branching dendrites46, 47 | 2 | |||
| Bright dendrites/Tangled lines/dendritic cells64 | 1 | |||
| Dendritic structures26 | 1 | |||
| Dendritic processes26 | 1 | |||
| Frequent, coarse, branching dendrites54 | 1 | |||
| Weighted subtotal | 10 | |||
| -Terms describing the presence of melanocyte infiltration of hair follicles | ||||
| Follicular localization of pagetoid cells and/or atypical cells4, 29, 34, 61 | 4 | Infiltration of dendritic/roundish cells in the inner portion of the hair follicle or elongated protrusions around the entire perimeter of the follicle | Guitera 20104 | Infiltration of hair follicles and adnexal structures by atypical melanocytes. Typically seen in melanoma of the lentigo maligna type |
| Pagetoid cells around follicular opening6, 52, 58 | 3 | |||
| Folliculotropism24, 64 | 2 | |||
| Atypical melanocytes surrounding | 1 | |||
| adnexal openings26 | ||||
| Infiltration of adnexal structures27 | 1 | |||
| Irregular aggregates of bright perifollicular cells31 | 1 | |||
| Atypical cells infiltrating follicular structures62 | 1 | |||
| Weighted subtotal | 13 | |||
| -Terms describing the presence of a normal/regular spinous and granular layer: | ||||
| Honeycombed pattern (typical/regular)4, 23, 38, 39, 43, 49, 55–57, 63, 65, 66, 68, 75 | 14 | Well-demarcated cellular outlines of keratinocytes forming a grid-like structure resembling a ‘honeycomb’ in the spinous and granular layers. | Normal epidermal keratinocytes | |
| Readily detected keratinocyte cell borders45–47 | 3 | |||
| Regular epidermal pattern67 | 1 | |||
| -Terms describing the presence of a normal/regular basal layer: | ||||
| Cobblestone pattern4, 23, 38, 39, 42, 43, 49, 57, 58, 63, 65, 66, 68, 69, 72, 74, 75, 77 | 18 | In the basal layer aggregates of small, nonnucleated polygonal cells and bright cytoplasm can be seen creating a ‘cobblestone’ pattern. | ||
| Monomorphic melanocytic cells45–47 | 3 | |||
| Cobblestone with small nucleated cells72, 74 | 2 | |||
| Typical cells in the basal layer28, 36 | 2 | |||
| Homogeneous bright melanocytic cells46 | 1 | |||
| Monomorphic reflactile cells55 | 1 | |||
| Typical basal cells23 | 1 | Segura 200923 | ||
| Weighted subtotal | 46 | |||
| -Terms related to acanthosis of the epidermis | ||||
| Broadened honeycomb pattern4, 23, 58, 61, 69, 72, 74, 75 | 7 | Prominent bright epidermis intermingled with papillae | Acanthosis of the epidermis | |
| Acanthosis49 | 1 | |||
| Weighted subtotal | 8 | |||
| -Terms describing with the presence of atypical keratinocytes/ disarrangement of keratinocytes | ||||
| Epidermal disarray4, 6, 23, 27, 50, 52, 55, 58, 68 | 9 | Irregularly shaped keratinocytes with poorly defined or absent borders | Atypical keratinocytes with variation in size, shape, and crowding of the nuclei | |
| Atypical honeycomb58, 68, 69, 72, 74, 77 | 6 | |||
| Disarranged epidermis57, 67, 69, 72, 75 | 5 | |||
| Disarray38, 56, 65, 66 | 4 | |||
| Disarranged epidermal pattern43, 63, 67 | 3 | |||
| Poorly defined or absent keratinocyte cell borders45–47 | 3 | |||
| Irregular epidermal pattern67 | 1 | |||
| Honeycomb atypical and disarray52 | 1 | |||
| Irregularly shaped keratinocytes43 | 1 | |||
| Poorly defined keratinocyte cell border54 | 1 | |||
| Loss of keratinocyte cellular borders54 | 1 | |||
| Honeycombed and irregular keratinocytes64 | 1 | |||
| Epidermal disruption49 | 1 | |||
| Weighted subtotal | 37 | |||
| -Terms related to a ‘speckled’ appearance in the epidermis | ||||
| Grainy image54, 55, 68, 72 | 4 | Bright granular dust-like particles barely discernible as individual granules at the level of epidermis | Extracellular melanin granules in the epidermis | |
| Epidermal granularity57 | 1 | |||
| Weighted subtotal | 5 | |||
| DERMOEPIDERMAL JUNCTION (n=46; 23.9%) | ||||
| -Terms associated with an irregular papillary contours | ||||
| Non-edged papillae4, 6, 23, 29, 31, 33, 36, 37, 39–41, 43, 48, 49, 52, 53, 57–59, 61, 63, 65, 67–70, 72, 74–77 | 31 | Dermal papillae without a demarcating bright rim of cells; the inter-papillary areas of the epidermis are widened and often show large atypical melanocytes | Pellacani 200768 and Guitera 20104 | Disarranged rete ridges with a disorderly proliferation of melanocytes not confined to the sides and tips of the rete ridges. Loss of the papillary contour is associated with flattening of the DEJ. |
| Irregular ringed30 | 1 | |||
| -Terms associated with loss of papillae contours | ||||
| Non-visible dermal papillae4, 50, 58, 75 | 4 | |||
| Irregular / disarrayed dermal epidermal junction39, 44, 64 | 3 | |||
| Non-visible papillary contours40, 69 | 2 | |||
| Loss of dermal papillae (flat DEJ)31 | 1 | |||
| Poorly visualized dermal papillae54 | 1 | |||
| DEJ disarray57 | 1 | |||
| Disarrangement at the DEJ77 | 1 | |||
| Absence of papillae77 | 1 | |||
| Weighted subtotal | 46 | |||
| -Terms associated with regular dermal papilla | ||||
| Edged papillae4, 6, 23, 28, 36, 48–50, 52, 57, 58, 63, 65, 67–72, 74, 77 | 22 | Dermal papillae with clearly outlined contours. They may result from single cells forming rims around the papillae (‘rings’), or by ‘junctional nests’ constituted by compact melanocytic aggregates with sharp borders | Segura 200923 | Pigmented basal keratinocytes and melanocytes along the sides of the rete ridges |
| Rings64 | 1 | |||
| Weighted subtotal | 23 | |||
| -Terms describing the presence of ‘atypical’ cells at the DEJ | ||||
| Atypical cells at the DEJ29, 33, 39, 42, 48, 49, 67, 70, 76, 78 | 10 | Presence of bright round or dendritic nucleated cells that are abnormally large in size (>50 um), display unusual contour (e.g. Triangular, starshaped) or have large and eccentric nuclei at the DEJ | Proliferation of atypical melanocytes as solitary units along the DEJ | |
| Cytological atypia25, 30, 41, 53, 56, 59, 60, 63, 65 | 9 | Borsari 201825 | ||
| Marked atypia of basal cells6, 23, 52, 58, 72 | 5 | |||
| Atypical cells40, 68 | 2 | |||
| More than 3 atypical cells at the junction in five images4 | 1 | Guitera 20104 | ||
| Marked cell atypia in the basal layer75 | 1 | |||
| Bright nucleated cells26 | 1 | |||
| Scattered bright structures31 | 1 | |||
| Predominance of single cells over nests32 | 1 | |||
| Large dendritic or round cells at the epithelial-stromal junction and/or in the stroma35 | 1 | |||
| Atypical nucleated cells at the DEJ36 | 1 | |||
| Cellular atypia in the DEJ37 | 1 | |||
| Atypical cells at basal layer43 | 1 | |||
| Mild and marked cellular atypia4 | 1 | |||
| Atypical and pleomorphic refractile cells55 | 1 | |||
| Bright, highly refractile particles55 | 1 | |||
| Presence of large visible cells68 | 1 | |||
| -Terms describing atypical cells with other shapes at the DEJ | ||||
| Spindled cells49, 57, 69** | 3 | |||
| -Terms describing presence of ‘atypical’ cells with additional features | ||||
| Focal increase of atypical melanocytes and nests27 | 1 | |||
| Weighted subtotal | 43 | |||
| -Terms describing the presence of round-shaped junctional aggregates, regular | ||||
| Junctional nests5, 40, 48, 49, 55–58, 67–69, 72, 74, 77, 78 | 15 | Elongated and branching tubular structures with heterogeneous reflectance harboring aggregates and individual polymorphous nucleated cells at the DEJ. They often bulge into dermal papillae. | Junctional nested proliferation of melanocytes. Elongated junctional nests of melanocytes may bridge between adjacent rete ridges | |
| Junctional clusters4, 23, 58, 63, 65, 68, 71, 72 | 8 | |||
| -Terms describing the presence of elongated, tubular junctional aggregates | ||||
| Junctional thickening4, 26, 43, 58, 63, 65, 68, 69, 71, 72, 74 | 11 | |||
| Cord-like rete ridges27 | 1 | |||
| Nonhomogeneous cellularity67 | 1 | |||
| - Terms describing the presence of short/bridging tubular junctional aggregates | ||||
| Mitochondria-like structures77 | 1 | |||
| Short interconnections67 | 1 | |||
| Weighted subtotal | 38 | |||
| -Terms describing sheet-like proliferation of melanocytes at the DEJ | ||||
| Sheet-like structures5, 23, 24, 43, 49, 56, 67–69, 72, 74, 77 | 12 | Cells distributed at the transition of the DEJ showing loss of dermal papillae not aggregated in clusters but closely distributed in the same plane with the loss of dermal papillae | Florid lentiginous proliferation of atypical melanocytes along the DEJ; mostly seen in melanomas in sun-damaged skin | |
| Sheet of cells4, 6, 30, 52, 58 | 5 | |||
| Sheet-like25 | 1 | |||
| Sheets of round to dendritic nucleated cells26 | 1 | |||
| Tangled filaments/dendrites crossing the papillae41 | 1 | |||
| Sheets of mainly dendritic atypical cells27 | 1 | |||
| Weighted subtotal | 21 | |||
| -Terms describing elongated structures bulging from the hair follicles | ||||
| Medusa head-like24, 64 | 2 | Small protrusions or elongated protrusions from hair follicles. They correspond to the “medusa head-like” structure when distributed around the entire perimeter of the follicle | N/A | |
| Bulging around hair follicle64 | 1 | |||
| Weighted subtotal | 3 | |||
| SUPERFICIAL DERMIS (n=68; 32.8%) | ||||
| -Terms describing the presence of solitary melanocytes in the papillary dermis | ||||
| Nucleated cells within the dermal papillae4, 6, 28, 29, 36, 37, 52, 59, 61, 68, 76 | 11 | Round to oval or triangular cells with welldemarcated bright cytoplasm and well demarcated dark nucleus infiltrating dermal papillae | Guitera 20104 | Presence of atypical melanocytes in the superficial (papillary) dermis |
| Nucleated cells in the dermis58, 69, 72, 75 | 4 | |||
| Isolated cells in the papilla72, 74 | 2 | |||
| Dermal infiltration of roundish cells64 | 1 | |||
| Atypical melanocytes within the upper dermis27 | 1 | |||
| Atypical cells in the dermal papilla33 | 1 | |||
| Dermal nucleated cells57 | 1 | |||
| Isolated cells within the upper dermis63 | 1 | |||
| Isolated cells within the papillary dermis65 | 1 | |||
| Atypical nucleated cells in dermis67 | 1 | |||
| Atypical nucleated dermal cells23 | 1 | Segura 200923 | ||
| Atypical cells infiltrating papillary dermis78 | 1 | |||
| Spindle cells in superficial dermis58*** | 1 | |||
| Weighted subtotal | 27 | |||
| -Terms describing the presence of cohesive nests of melanocytes in the papillary dermis | ||||
| Dense nests4, 25, 48, 49, 56, 58, 60, 67–72, 77 | 14 | Compact aggregates with sharp margins and monomorphous cells in which it is difficult to discern individual cell borders | Borsari 201825 | Round to oval junctional or dermal nests of melanocytes |
| Dermal nest5, 23, 40, 43, 48, 55, 57, 67, 78 | 9 | |||
| Melanocytic cell nests45–47 | 3 | |||
| Dense clusters63, 65, 74 | 3 | |||
| Dense regular nests23, 75 | 2 | |||
| Confluence of nests69** | 1 | |||
| Dermal cell clusters72 | 1 | |||
| Nesting25 | 1 | |||
| Weighted subtotal | 34 | |||
| -Terms describing the presence of discohesive/irregular nests of melanocytes in the papillary dermis | ||||
| Dense and sparse nests25, 30, 48, 49, 56, 60, 67, 70 | 8 | Clusters of somewhat loosely aggregated cells in which some of cells have clearly defined cell borders and little dark space can be seen inbetween some, but not all, cells | Irregular or discohesive nests of melanocytes | |
| Dishomogeneous nest4, 6, 52, 58, 69, 72, 74 | 7 | |||
| Sparse nest58, 68, 69, 72, 74 | 5 | |||
| Non-homogeneous nests23, 68 | 2 | |||
| Irregular clods30 | 1 | |||
| Atypical nucleated cells arranged in nests41 | 1 | |||
| Aggregates of atypical cells62 | 1 | |||
| Dishomogeneous clusters63 | 1 | |||
| Sparse cell clusters65 | 1 | |||
| Marked pleomorphism within nests49 | 1 | |||
| Discohesive junctional nests77 | 1 | |||
| Dense irregular nests75 | 1 | |||
| Dense dishomogeneous clusters75 | 1 | |||
| Weighted subtotal | 31 | |||
| -Terms describing the presence of ‘cerebriform’ aggregates in the dermis | ||||
| Cerebriform nest5, 6, 23, 30, 36, 37, 40, 48, 52, 53, 56, 58, 68, 69, 72, 74–76 | 18 | Nodular aggregates of atypical melanocytes in melanoma with dermal component | ||
| Cerebriform clusters49, 59, 63, 65, 75 | 5 | |||
| Weighted subtotal | 23 | |||
| -Terms describing the presence of melanophages in the papillary dermis | ||||
| Plump cells23, 33, 56–58, 63, 69, 70, 72–75, 77 | 13 | Irregularly shaped bright cells with ill-defined borders and usually no visible nucleus | Melanophages | |
| Melanophages25, 26, 30, 50, 51, 60, 64, 65 | 8 | Borsari 201825 | ||
| Plump-bright cells4, 40, 68 | 3 | |||
| Bright, round-to-triangular, nonnucleated cells26 | 1 | |||
| Weighted subtotal | 25 | |||
| -Terms describing the presence of inflammation (other than melanophages) in the papillary dermis | ||||
| Inflammation38, 49 | 2 | Bright spots and small bright particles in the dermis | Inflammatory cells (other than melanophages) | |
| Bright spots56, 69 | 2 | |||
| Inflammatory infiltrate48, 67 | 2 | |||
| Dermal inflammation24 | 1 | |||
| Dermal bright cells51 | 1 | |||
| Bright small cells and/or hyperreflective spots68 | 1 | |||
| Small bright cells and particles72 | 1 | |||
| Bright particles73 | 1 | |||
| Bright hyperreflecting spots23 | 1 | |||
| Bright dots77 | 1 | |||
| Weighted subtotal | 13 | |||
| -Terms describing patterns of collagen in the papillary dermis | ||||
| Bundled collagen72, 74, 77 | 3 | Elongated fibrillar structures (1 – 5 um) without cellular component distributed side by side through the dermis | Various correlates ranging from normal collagen scar-like collagen, to solar elastosis. | |
| Reticulated collagen72, 74, 77 | 3 | |||
| Coarse collagen24, 70 | 2 | |||
| Bright fibrillar structures75 | 1 | |||
| Thickened collagen / curled fibers (elastosis)64 | 1 | |||
| Stromal fibre (‘collagen’) morphology48 | 1 | |||
| Highly reflecting fibers50 | 1 | |||
| Curled highly refractive collagen fibers4 | 1 | |||
| Fibroplasia67 | 1 | |||
| Reticulated fibers68 | 1 | |||
| Broadened reticulated fibers68 | 1 | |||
| Thick cordons68 | 1 | |||
| Weighted subtotal | 17 | |||
| -Terms describing the presence of prominent blood vessels in the papillary dermis | ||||
| Enlarged vessels23, 50, 75 | 3 | Blood vessels appear as dark tubular structures in the dermis in which movement of bright round cells (white blood cells) is seen. Irregular vessels refer to blood vessels with abnormal diameter, density, or orientation compared to normal skin | Dilated or increased vascularity in the superficial dermis | |
| Tortuous morphology of vessels33 | 1 | |||
| Irregular vessels40 | 1 | |||
| Prominent vascularity56 | 1 | |||
| Horizontal vessels58 | 1 | |||
| Atypical vessels59 | 1 | |||
| Weighted subtotal | 8 | |||
| TERMS WITH DEFINITION NOT AVAILABLE (N=9; 4.7%) | ||||
| Nests (NOS)4, 24, 58, 64, 69 | 5 | -- | -- | |
| Disarray of melanocytic architecture45–47 | 3 | -- | -- | |
| Ill-defined follicle contour64 | 1 | Aspect of the external border of the hair follicle, defined according to its outline | -- | |
| Atypical cells with dark halo4* | 1 | -- | -- | |
| Oval cells57 | 1 | -- | -- | |
| Elongated cells57 | 1 | -- | -- | |
| Triangular cells57 | 1 | -- | -- | |
| Cord-like structures62 | 1 | Pseudonetwork on dermoscopy | -- | |
| DEJ atypia57 | 1 | |||
| N/A TOTAL | 15 | |||
| TOTAL TERMS (N=191; 100%) | ||||
Described in amelanotic/hypomelanocitc melanoma.
Described in Spitzoid lesions.
Described in desmoplastic melanoma.
Table 2:
Reflectance confocal microscopy terms used to describe the patterns of melanocytic lesions at low magnification.
| RCM term | Use frequency of RCM term, n | Definition | Histopathologic correlates |
|---|---|---|---|
| TERMS WITH DEFINITION (N=15) | |||
| Ringed pattern5, 24, 25, 38–42, 48, 49, 56, 62, 67, 70, 71 | 15 | A pattern consisting of edged-papillae presenting a demarcated rim of bright basal cells forming ‘rings’ | Lentiginous or small-nested junctional proliferation of melanocytes |
| Weighted subtotal | 15 | ||
| Meshwork pattern5, 24, 25, 30, 38–42, 48, 56, 62, 67, 70, 71, 77 | 16 | Enlarged interpapillary spaces predominantly constituted by junctional thickenings and/or non-edged papillae | Nested junctional proliferation of melanocytes with bridging between adjacent rete ridges |
| Weighted subtotal | 16 | ||
| Clod pattern25, 39–42, 48, 56, 67, 70, 71 | 10 | A low-magnification pattern composed of predominance of dense compact nests/clusters of melanocytes within the superficial dermis | Dermal nested proliferation of melanocytes |
| Small clods (<150 μm)70 | 1 | ||
| Large clods (>150 μm)70 | 1 | ||
| Weighted subtotal | 12 | ||
| Mixed pattern70 | 1 | Combination of any of the DEJ patterns (i.e. ringed, meshwork or clod pattern). | Melanocytic neoplasms with a junctional and dermal components |
| Weighted subtotal | 1 | ||
| Non-specific25, 30, 39, 41, 48, 56, 67 | 7 | Lack of a recognizable pattern at low-magnification mosaic view of the DEJ (i.e. absence of ringed, meshwork or clod pattern). Usually associated with abrupt or vague epidermadermal transition | Melanocytic proliferation with a flattened DEJ or marked attenuation of the undulation DEJ pattern |
| Structureless area38 | 1 | ||
| Aspecific pattern60 | 1 | ||
| Weighted subtotal | 9 | ||
| Asymmetry47 | 1 | The overall distribution of confocal structures in one half of the lesion does not mirror those in the other half | Asymmetry |
| Architectural disorder41 | 1 | ||
| Weighted subtotal | 2 | ||
| Peripheral rim of nests48 | 1 | Presence of clusters of cells (nests) detectable along the entire perimeter of the lesion | Presence of junctional or dermal nests of melanocytes at the periphery of the lesion |
| Peripheral melanocytic nests62 | 1 | ||
| Rim of nests at the periphery69 | 1 | ||
| Weighted subtotal | 3 | ||
| Sharp border cut-off69 | 1 | Clear demarcation between lesional and peripheral skin. Seen in Spitz nevus. | Sharp demarcation |
| Weighted subtotal | 1 | ||
| TERMS WITH DEFINITION NOT AVAILABLE (N=3; 10.3%) | |||
| Uneven pattern24 | 1 | -- | |
| Undefined epidermal pattern43 | 1 | -- | |
| Specific pattern60 | 1 | -- | |
| Weighted subtotal | 3 | ||
| TOTAL FOR PATTERNS TERMS (N=18) | 62 |
Figure 2:
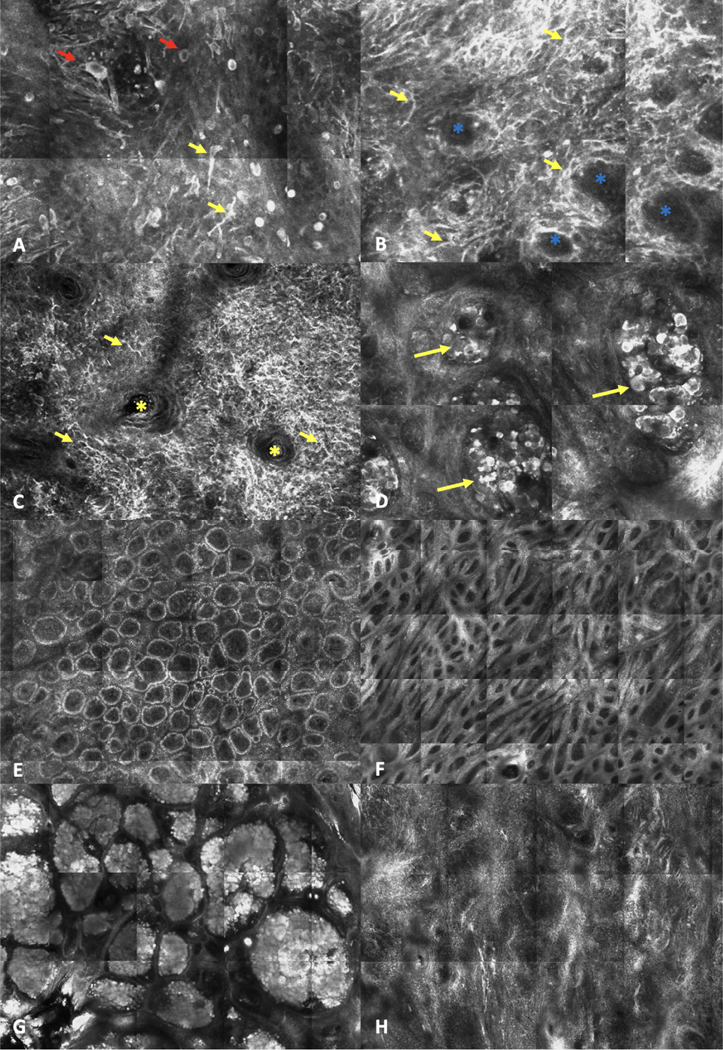
Examples of reflectance confocal microscopy terms. A-D. ‘High-magnification’/cellular details examples of melanoma features. A. Round (red arrows) and dendritic (yellow arrows) pagetoid cells in the epidermis (2.0 × 2.0 mm). B. Atypical cells at the dermoepidermal junction (DEJ) (yellow arrows) and non-edged papillae (blue asterisks) (2.0 × 2.0 mm). C. Bright, large, dendritic nucleated cells (yellow arrows) with folliculotropism (yellow asterisk shows a hair follicle) in a lentigo maligna melanoma (0.75 × 0.75 mm). D. Dishomogeneous (‘dense and sparse’) nest (yellow arrows) at the dermis level (1.0 × 1.0 mm). E-H. ‘Low-magnification’ / patterns examples used to describe melanocytic lesions. E. Ringed pattern (2.0 × 1.0 mm). F. Meshwork pattern (2.7 × 2.0 mm). G. Clod pattern (2.5 × 3.5 mm). H. Non-specific pattern.
The overall average use-frequency of the RCM terms was 3.1 times (653 mentions of 210 terms; range 1–31 mentions). For ‘high-magnification’ terms, average use-frequency was 3.0 times (591 mentions of 191 terms; range 1–31), while for ‘low-magnification’ terms, average use-frequency was 3.4 times (62 mentions of 18 terms; range 1–18). The most-commonly used high magnification terms were ‘non-edged papillae’ (use frequency, n=31), ‘pagetoid cells’ (n=27), and ‘edged papillae’ (n=22). Only 14 (7.3%) were used in an algorithm. (Table I). Among low-magnification terms, most frequently used were ‘meshwork pattern’ (n=16), ‘ringed pattern’ (n=15), and ‘clod pattern’ (n=10) (Table II).
We grouped terms on the basis of similarity in definitions and found 40 ‘likely-synonymous’ clusters. By further excluding 12 terms lacking a clear definition, the total number terms could be reduced from 209 to 40 (80.8% reduction) (Table I).
High magnification / cellular-level resolution terms
Among 191 high-magnification terms, 182 terms had an identifiable definition and could be grouped into 32 ‘likely-synonymous’ clusters. Nine additional terms (6.6%) lacked a clear definition and were excluded from the likely-synonymous’ clustering and from further analysis. When stratifying the 182 individual high-magnification terms by their anatomic distribution, 68 terms mapped to the epidermis (37.4%), 46 terms to DEJ (23.9%), and 68 terms to the dermis (32.8%). When stratifying high-magnification terms by ‘likely-synonymous groups’, 13 terms (40.6%) mapped to the epidermis, 11 (34.3%) to the DEJ, and 8 (25.0%) to the superficial dermis.
Of 182 terms, 52 (28.5%) were simple terms and 130 (71.4%) were composite terms. We extracted 125 basic terminology units, 57 labeling the ‘cell / structure’ type, 51 describing the morphological attributes of these cells, and 17 relating to their anatomic distribution. We found that the 55 cells / structures could be categorized into eight recurring themes, namely basic terminology units describing: melanocytes as individual cells, dendritic processes without visible cell body, aggregated melanocytes, keratinocytes, DEJ structures, inflammatory cells, collagen structures and blood vessels (Table III).
Table 3:
Breakdown of reflectance confocal microscopy terms for melanocytic neoplasms into basic terminology units. Abbreviations: DEJ = dermo epidermal junction.
| Cells / structures | Morphology modifiers/Descriptors | Anatomic location /Distribution |
|---|---|---|
| Relates to melanocytes as individual cells | ||
| Atypical cells | Dendritic | Pagetoid spread |
| Atypical melanocytes | Enlarged | Pagetoid pattern |
| Bright nucleated cells | Hyporeflective/dark | Pagetoid infiltration |
| Cell atypia | Irregularly bright | Widespread |
| Cells (NOS**) | Large | Focal |
| Cellular atypia | Pleomorphic | Folliculotropism |
| Cytological atypia | Round | Infiltrating adnexal structures |
| Melanocytic cells | Spindled | Non-specified |
| Melanocytes | With halo | In the dermal papillae |
| Nucleated cells | At the DEJ | |
| Pagetoid cells | Dermal infiltration | |
| Infiltrating papillary dermis | ||
| At the periphery | ||
| Relates to dendritic processes without visible cell body | ||
| Branching dendrites * | Composite dendrites | |
| Dendritic processes * | Simple dendrites | |
| Tangled lines * | Coarse dendrites | |
| Relates to aggregated melanocytes | ||
| Aggregates of atypical cells | Cerebriform | Around the hair follicle |
| Bulging | Dense | Junctional |
| Cells clusters | Dense and sparse | Dermal |
| Clods | Dense dishomogeneous | |
| Clusters | Dense irregular | |
| Junctional thickening | Discohesive | |
| Medusa head-like | Dishomogeneous | |
| Melanocytic cell nests | Irregular | |
| Mitochondria-like structures | Marked pleomorphism (within nest) | |
| Nesting | Non-homogeneous | |
| Nests | Pleomorphism (within nest) | |
| Sheet-like | Sparse | |
| Related to keratinocytes | ||
| Acanthosis | Atypical | |
| Basal cells | Broadened (Honeycomb) | |
| Cobblestone pattern | Irregular | |
| Epidermal disarray | Monomorphic | |
| Epidermal pattern | Poorly-defined | |
| Honeycomb pattern | Readily detected | |
| Keratinocyte cell borders | Regular | |
| Refractile cells | Typical | |
| Relates to DEJ structures/shape | ||
| DEJ disarray | Absent | |
| Dermal papillae | Edged | |
| Papillae | Loss of (papilla / papillary contour) | |
| Papillary contours | Non-edged | |
| Non-visible | ||
| Poorly visualized | ||
| Ringed | ||
| Relates to stromal structures | ||
| Inflammatory cells | ||
| Bright dots | Round-to-triangular | Dermal |
| Bright non-nucleated cells | ||
| Bright particles | ||
| Bright small cells | ||
| Bright spots | ||
| Dermal bright cells | ||
| Inflammatory infiltrate | ||
| Inflammation | ||
| Melanophages | ||
| Plump cells | ||
| Stromal fibers | ||
| Bright fibrillar structures | Broadened | |
| Collagen | Bundled | |
| Curled fibers | Coarse | |
| Fibroplasia | Reticulated | |
| Highly reflecting fibers | Thickened | |
| Stromal fibre | ||
| Thick cordons | ||
| Blood vessels | ||
| Vascularity | Atypical | |
| Vessels | Enlarged | |
| Horizontal | ||
| Irregular | ||
| Prominent | ||
| Tortuous morphology | ||
Dendritic structures is a descriptive term that can pertain to dendritic processes emanating from either melanocytes or from Langerhans cells
NOS – not otherwise specified; the context of the original term implies that “cells” refer to melanocytes.
‘Grainy image’ and ‘epidermal granularity’ are not included in the table.
Low magnification / architectural patterns terms
By categorizing low-magnification terms into ‘likely-synonymous’ clusters, we reduced 18 individual terms to 8 ‘likely-synonymous’ group terms (55.5% reduction) (Table II). Three additional terms (16.6%) lacked a clear definition. When stratifying by anatomic level, all terms were ascribed to the DEJ and/or superficial dermis level and none to the epidermis.
DISCUSSION
In the present systematic review, describing the RCM terminology of melanocytic lesions, we extracted 209 terms from 59 studies. We grouped the individual RCM terms into ‘likely-synonymous’ clusters, based on similarity in definition and/or histopathology correlates, and excluded terms lacking a clear definition, potentially distilling 40 non-redundant terms from the 209 (80.8% reduction).
In a systematic review of RCM terminology for describing NMLs, we found that the average number of uses per term was only 1.6 times. Herein, we found that RCM terms from melanocytic neoplasms were used with an average frequency of 3.1. A higher average use frequency for melanocytic neoplasms, compared to NMLs, might be attributed to the previous consensus on melanocytic terminology in 2007.18 We also found in the present study a higher average use frequency for low-magnification terms, compared to high-magnification terms (average use of 3.4 vs 3.0, respectively). This may suggest higher consistency in the use of low-magnification RCM terms for melanocytic lesions.
We also found that RCM melanocytic terms are often composite (71.4%). This complexity may emanate from the fact that multiple RCM attributes need to be weighed to differentiate melanoma from challenging nevi – such as the shape, size and anatomic distribution of individual or aggregated melanocytes. Towards simplifying RCM terminology, we extracted 124 ‘basic terminology units’ from the 191 ‘high-magnification’ RCM terms, and categorized them into individual units describing ‘cells’ or ‘structures’ type, followed by the morphological attributes of these cells/structures and their anatomic distribution. This is akin to extracting basic words from complex sentences. This lays the groundwork for an expert agreement study that will eliminate redundancy and identify from the list the best basic descriptors (words) and composite criteria (sentences).
Creating a concise list of pertinent RCM terms for the diagnosis of melanocytic lesions is an important step towards widespread adaptation RCM. Along similar lines, Pellacani et al. recently published an expert consensus study, in which the experts identified 18 principal RCM terms for diagnosis of melanocytic and non-melanocytic lesions. These terms were then further clustered into two melanoma-specific key features (atypical cells (at any level) and DEJ disarray), one basal cell carcinoma-specific key features (basaloid cords/islands), and one squamous cell carcinoma-specific key feature (keratinocyte disarray). Identification of one of these four key features by novices was associated with a skin cancer diagnostic sensitivity of 91% and specificity of 57%.21 This study highlighted that a simplified shortlist of skin cancer ‘key-criteria’ could be easily learned and successfully utilized by novice RCM readers. A similar approach for triaging lesions has been proposed by simplified dermoscopy algorithms.79. However, the RCM diagnosis of challenging melanocytic cases by experts requires a more elaborate set of criteria.
Limitations:
We included only full-text articles in English to allow direct comparisons of terms without translation bias. RCM terms published in non-English papers may have been missed. Non-peer reviewed articles (e.g. book chapters) were excluded and some terms used by the experts could have been missed. The weightage attributed to RCM terms may be influenced by the frequency of publications of individual researchers or research groups. Also, some terms were grouped together but further studies are needed to determine if more granularity is relevant or needed (e.g. collagen and elastosis). Finally, literature search was conducted on August, 2018, thereby, some recent key terms might have been overlooked.
Conclusion:
We propose a more concise glossary of RCM terms for the diagnosis of melanocytic lesions. By grouping the RCM terms, based on ‘likely-synonymous’ definitions, and by eliminating terms lacking a clear definition, the list could be reduced by over 80%. This systematic review lays the ground for an expert Delphi consensus for melanocytic lesions terminology. Furthermore, a concise glossary of RCM terms can facilitate standardizing of RCM diagnosis reports, teaching of novices, and communicating of research findings.
Capsule summary:
Reflectance confocal microscopy (RCM) melanocytic neoplasms terminology is inconsistently used. Via systematic review, we identified redundant terms, and categorized 209 synonymous terms into 40 groups (80.8% reduction).
The proposed shortened list of RCM-terms for melanocytic neoplasms may be easier to teach to novices and provides the basis for subsequent terminology consensus.
Acknowledgments
Founding source: This research is funded in part by a grant from the National Cancer Institute / National Institutes of Health (P30-CA008748) made to the Memorial Sloan Kettering Cancer Center. Alon Scope’s RCM research is funded by the Israel Science Foundation (ISF-1546-16).
Footnotes
Conflict of interest: Halpern and Marghoob have conflicts of interest to declare. See attached COI forms.
Consent for publication: The authors consent the publication of this submission (manuscript and figures).
Prior presentation: none; IRB status: N/A; Manuscript word count: 2126/2500
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rajadhyaksha M, Marghoob A, Rossi A, Halpern AC, Nehal KS. Reflectance confocal microscopy of skin in vivo: From bench to bedside. Lasers Surg Med 2017;49:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajadhyaksha M, Grossman M, Esterowitz D, Webb RH, Anderson RR. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol 1995;104:946–52. [DOI] [PubMed] [Google Scholar]
- 3.Rajadhyaksha M, Gonzalez S, Zavislan JM, Anderson RR, Webb RH. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison with histology. J Invest Dermatol 1999;113:293–303. [DOI] [PubMed] [Google Scholar]
- 4.Guitera P, Pellacani G, Crotty KA, Scolyer RA, Li LX, Bassoli S et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol 2010;130:2080–91. [DOI] [PubMed] [Google Scholar]
- 5.Pellacani G, De Pace B, Reggiani C, Cesinaro AM, Argenziano G, Zalaudek I et al. Distinct melanoma types based on reflectance confocal microscopy. Exp Dermatol 2014;23:414–8. [DOI] [PubMed] [Google Scholar]
- 6.Guitera P, Menzies SW, Longo C, Cesinaro AM, Scolyer RA, Pellacani G. In vivo confocal microscopy for diagnosis of melanoma and basal cell carcinoma using a two-step method: analysis of 710 consecutive clinically equivocal cases. J Invest Dermatol 2012;132:2386–94. [DOI] [PubMed] [Google Scholar]
- 7.Navarrete-Dechent C, Cordova M, Liopyris K, Rishpon A, Aleissa S, Rossi AM et al. Reflectance confocal microscopy and dermoscopy aid in evaluating repigmentation within or adjacent to lentigo maligna melanoma surgical scars. J Eur Acad Dermatol Venereol 2020;34:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez S, Tannous Z. Real-time, in vivo confocal reflectance microscopy of basal cell carcinoma. J Am Acad Dermatol 2002;47:869–74. [DOI] [PubMed] [Google Scholar]
- 9.Nori S, Rius-Diaz F, Cuevas J, Goldgeier M, Jaen P, Torres A et al. Sensitivity and specificity of reflectance-mode confocal microscopy for in vivo diagnosis of basal cell carcinoma: a multicenter study. J Am Acad Dermatol 2004;51:923–30. [DOI] [PubMed] [Google Scholar]
- 10.Rishpon A, Kim N, Scope A, Porges L, Oliviero MC, Braun RP et al. Reflectance confocal microscopy criteria for squamous cell carcinomas and actinic keratoses. Arch Dermatol 2009;145:766–72. [DOI] [PubMed] [Google Scholar]
- 11.Navarrete-Dechent C, Cordova M, Liopyris K, Yelamos O, Aleissa S, Hibler B et al. Reflectance confocal microscopy-guided carbon dioxide laser ablation of low-risk basal cell carcinomas: A prospective study. J Am Acad Dermatol 2019;81:984–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navarrete-Dechent C, Cordova M, Postow MA, Pulitzer M, Lezcano C, Halpern AC et al. Evaluation of the Response of Unresectable Primary Cutaneous Melanoma to Immunotherapy Visualized With Reflectance Confocal Microscopy: A Report of 2 Cases. JAMA Dermatol 2019;155:347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navarrete-Dechent C, Mori S, Cordova M, Nehal KS. Reflectance confocal microscopy as a novel tool for presurgical identification of basal cell carcinoma biopsy site. J Am Acad Dermatol 2019;80:e7–e8. [DOI] [PubMed] [Google Scholar]
- 14.Navarrete-Dechent C, Cordova M, Aleissa S, Liopyris K, Dusza SW, Phillips W et al. Reflectance confocal microscopy confirms residual basal cell carcinoma on clinically negative biopsy sites before Mohs micrographic surgery: A prospective study. J Am Acad Dermatol 2019;81:417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navarrete-Dechent C, Cordova M, Aleissa S, Liopyris K, Dusza SW, Kose K et al. Lentigo maligna melanoma mapping using reflectance confocal microscopy correlates with staged excision: A prospective study. J Am Acad Dermatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navarrete-Dechent C, Aleissa S, Cordova M, Liopyris K, Lee EH, Rossi AM et al. Incompletely excised lentigo maligna melanoma is associated with unpredictable residual disease: clinical features and the emerging role of reflectance confocal microscopy. J Eur Acad Dermatol Venereol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navarrete-Dechent C, DeRosa AP, Longo C, Liopyris K, Oliviero M, Rabinovitz H et al. Reflectance confocal microscopy terminology glossary for nonmelanocytic skin lesions: A systematic review. J Am Acad Dermatol 2019;80:1414–27 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scope A, Benvenuto-Andrade C, Agero AL, Malvehy J, Puig S, Rajadhyaksha M et al. In vivo reflectance confocal microscopy imaging of melanocytic skin lesions: consensus terminology glossary and illustrative images. J Am Acad Dermatol 2007;57:644–58. [DOI] [PubMed] [Google Scholar]
- 19.Current Procedural Terminology, Professional Edition. Chicago IL: American Medical Association; 2016. [Google Scholar]
- 20.Jain M, Pulijal SV, Rajadhyaksha M, Halpern AC, Gonzalez S. Evaluation of Bedside Diagnostic Accuracy, Learning Curve, and Challenges for a Novice Reflectance Confocal Microscopy Reader for Skin Cancer Detection In Vivo. JAMA Dermatol 2018;154:962–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pellacani G, Scope A, Gonzalez S, Guitera P, Farnetani F, Malvehy J et al. Reflectance confocal microscopy made easy: The 4 must-know key features for the diagnosis of melanoma and nonmelanoma skin cancers. J Am Acad Dermatol 2019;81:520–6. [DOI] [PubMed] [Google Scholar]
- 22.Errichetti E, Zalaudek I, Kittler H, Apalla Z, Argenziano G, Bakos R et al. Standardization of dermoscopic terminology and basic dermoscopic parameters to evaluate in general dermatology (non-neoplastic dermatoses): an expert consensus on behalf of the International Dermoscopy Society. Br J Dermatol 2020;182:454–67. [DOI] [PubMed] [Google Scholar]
- 23.Segura S, Puig S, Carrera C, Palou J, Malvehy J. Development of a two-step method for the diagnosis of melanoma by reflectance confocal microscopy. J Am Acad Dermatol 2009;61:216–29. [DOI] [PubMed] [Google Scholar]
- 24.de Carvalho N, Farnetani F, Ciardo S, Ruini C, Witkowski AM, Longo C et al. Reflectance confocal microscopy correlates of dermoscopic patterns of facial lesions help to discriminate lentigo maligna from pigmented nonmelanocytic macules. Br J Dermatol 2015;173:128–33. [DOI] [PubMed] [Google Scholar]
- 25.Borsari S, Pampena R, Benati E, Bombonato C, Kyrgidis A, Moscarella E et al. In vivo dermoscopic and confocal microscopy multistep algorithm to detect in situ melanomas. Br J Dermatol 2018;179:163–72. [DOI] [PubMed] [Google Scholar]
- 26.Ahlgrimm-Siess V, Hofmann-Wellenhof R, Cao T, Oliviero M, Scope A, Rabinovitz HS. Reflectance confocal microscopy in the daily practice. Semin Cutan Med Surg 2009;28:180–9. [DOI] [PubMed] [Google Scholar]
- 27.Ahlgrimm-Siess V, Massone C, Scope A, Fink-Puches R, Richtig E, Wolf IH et al. Reflectance confocal microscopy of facial lentigo maligna and lentigo maligna melanoma: a preliminary study. Br J Dermatol 2009;161:1307–16. [DOI] [PubMed] [Google Scholar]
- 28.Alarcon I, Carrera C, Palou J, Alos L, Malvehy J, Puig S. Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. Br J Dermatol 2014;170:802–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alarcon I, Carrera C, Alos L, Palou J, Malvehy J, Puig S. In vivo reflectance confocal microscopy to monitor the response of lentigo maligna to imiquimod. J Am Acad Dermatol 2014;71:49–55. [DOI] [PubMed] [Google Scholar]
- 30.Bombonato C, Ribero S, Pozzobon FC, Puig-Butille JA, Badenas C, Carrera C et al. Association between dermoscopic and reflectance confocal microscopy features of cutaneous melanoma with BRAF mutational status. J Eur Acad Dermatol Venereol 2017;31:643–9. [DOI] [PubMed] [Google Scholar]
- 31.Braga JC, Scope A, Klaz I, Mecca P, Gonzalez S, Rabinovitz H et al. The significance of reflectance confocal microscopy in the assessment of solitary pink skin lesions. J Am Acad Dermatol 2009;61:230–41. [DOI] [PubMed] [Google Scholar]
- 32.Busam KJ, Charles C, Lohmann CM, Marghoob A, Goldgeier M, Halpern AC. Detection of intraepidermal malignant melanoma in vivo by confocal scanning laser microscopy. Melanoma Res 2002;12:349–55. [DOI] [PubMed] [Google Scholar]
- 33.Carrera C, Palou J, Malvehy J, Segura S, Aguilera P, Salerni G et al. Early stages of melanoma on the limbs of high-risk patients: clinical, dermoscopic, reflectance confocal microscopy and histopathological characterization for improved recognition. Acta Derm Venereol 2011;91:137–46. [DOI] [PubMed] [Google Scholar]
- 34.Cinotti E, Labeille B, Debarbieux S, Carrera C, Lacarrubba F, Witkowski AM et al. Dermoscopy vs. reflectance confocal microscopy for the diagnosis of lentigo maligna. J Eur Acad Dermatol Venereol 2018. [DOI] [PubMed] [Google Scholar]
- 35.Cinotti E, Singer A, Labeille B, Grivet D, Rubegni P, Douchet C et al. Handheld In Vivo Reflectance Confocal Microscopy for the Diagnosis of Eyelid Margin and Conjunctival Tumors. JAMA Ophthalmol 2017;135:845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colombino M, Paliogiannis P, Pagliarello C, Cossu A, Lissia A, Satta R et al. Dermoscopy and confocal microscopy for metachronous multiple melanomas: morphological, clinical, and molecular correlations. Eur J Dermatol 2018;28:149–56. [DOI] [PubMed] [Google Scholar]
- 37.Curchin CE, Wurm EM, Lambie D, Longo C, Pellacani G, Soyer HP. First experiences using reflectance confocal microscopy on equivocal skin lesions in Queensland. Australas J Dermatol 2011;52:89–97. [DOI] [PubMed] [Google Scholar]
- 38.De Pace B, Farnetani F, Losi A, Ciardo S, De Carvalho N, Cesinaro AM et al. Reinterpreting dermoscopic pigment network with reflectance confocal microscopy for identification of melanoma-specific features. J Eur Acad Dermatol Venereol 2018;32:947–55. [DOI] [PubMed] [Google Scholar]
- 39.Debarbieux S, Depaepe L, Poulalhon N, Balme B, Dalle S, Thomas L. Reflectance confocal microscopy accurately discriminates between benign and malignant melanocytic lesions exhibiting a ‘dermoscopic island’. J Eur Acad Dermatol Venereol 2013;27:e159–65. [DOI] [PubMed] [Google Scholar]
- 40.Farnetani F, Scope A, Braun RP, Gonzalez S, Guitera P, Malvehy J et al. Skin Cancer Diagnosis With Reflectance Confocal Microscopy: Reproducibility of Feature Recognition and Accuracy of Diagnosis. JAMA Dermatol 2015;151:1075–80. [DOI] [PubMed] [Google Scholar]
- 41.Ferrari B, Pupelli G, Farnetani F, De Carvalho NT, Longo C, Reggiani C et al. Dermoscopic difficult lesions: an objective evaluation of reflectance confocal microscopy impact for accurate diagnosis. J Eur Acad Dermatol Venereol 2015;29:1135–40. [DOI] [PubMed] [Google Scholar]
- 42.Figueroa-Silva O, Cinotti E, de Almeida Silva T, Moscarella E, Lallas A, Ciardo S et al. Diagnostic accuracy of reflectance confocal microscopy for lesions typified by dermoscopic island. J Eur Acad Dermatol Venereol 2016;30:1594–8. [DOI] [PubMed] [Google Scholar]
- 43.Floristan Muruzabal U, Gamo Villegas R, Pampin Franco A, Pinedo Moraleda F, Perez Fernandez E, Lopez-Estebaranz JL. Combined in vivo reflectance confocal microscopy and digital dermoscopy for follow up of patients at high risk of malignant melanoma: A prospective case series study. J Dermatol 2017;44:681–9. [DOI] [PubMed] [Google Scholar]
- 44.Gareau D, Hennessy R, Wan E, Pellacani G, Jacques SL. Automated detection of malignant features in confocal microscopy on superficial spreading melanoma versus nevi. J Biomed Opt 2010;15:061713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerger A, Hofmann-Wellenhof R, Langsenlehner U, Richtig E, Koller S, Weger W et al. In vivo confocal laser scanning microscopy of melanocytic skin tumours: diagnostic applicability using unselected tumour images. Br J Dermatol 2008;158:329–33. [DOI] [PubMed] [Google Scholar]
- 46.Gerger A, Koller S, Kern T, Massone C, Steiger K, Richtig E et al. Diagnostic applicability of in vivo confocal laser scanning microscopy in melanocytic skin tumors. J Invest Dermatol 2005;124:493–8. [DOI] [PubMed] [Google Scholar]
- 47.Gerger A, Koller S, Weger W, Richtig E, Kerl H, Samonigg H et al. Sensitivity and specificity of confocal laser-scanning microscopy for in vivo diagnosis of malignant skin tumors. Cancer 2006;107:193–200. [DOI] [PubMed] [Google Scholar]
- 48.Gill M, Longo C, Farnetani F, Cesinaro AM, Gonzalez S, Pellacani G. Non-invasive in vivo dermatopathology: identification of reflectance confocal microscopic correlates to specific histological features seen in melanocytic neoplasms. J Eur Acad Dermatol Venereol 2014;28:1069–78. [DOI] [PubMed] [Google Scholar]
- 49.Guida S, Pellacani G, Cesinaro AM, Moscarella E, Argenziano G, Farnetani F et al. Spitz naevi and melanomas with similar dermoscopic patterns: can confocal microscopy differentiate? Br J Dermatol 2016;174:610–6. [DOI] [PubMed] [Google Scholar]
- 50.Guitera P, Haydu LE, Menzies SW, Scolyer RA, Hong A, Fogarty GB et al. Surveillance for treatment failure of lentigo maligna with dermoscopy and in vivo confocal microscopy: new descriptors. Br J Dermatol 2014;170:1305–12. [DOI] [PubMed] [Google Scholar]
- 51.Guitera P, Li LX, Scolyer RA, Menzies SW. Morphologic features of melanophages under in vivo reflectance confocal microscopy. Arch Dermatol 2010;146:492–8. [DOI] [PubMed] [Google Scholar]
- 52.Guitera P, Menzies SW, Argenziano G, Longo C, Losi A, Drummond M et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for diagnosis of difficult amelanotic and light-coloured skin lesions. Br J Dermatol 2016;175:1311–9. [DOI] [PubMed] [Google Scholar]
- 53.Guitera P, Pellacani G, Longo C, Seidenari S, Avramidis M, Menzies SW. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol 2009;129:131–8. [DOI] [PubMed] [Google Scholar]
- 54.Langley RG, Burton E, Walsh N, Propperova I, Murray SJ. In vivo confocal scanning laser microscopy of benign lentigines: comparison to conventional histology and in vivo characteristics of lentigo maligna. J Am Acad Dermatol 2006;55:88–97. [DOI] [PubMed] [Google Scholar]
- 55.Langley RG, Walsh N, Sutherland AE, Propperova I, Delaney L, Morris SF et al. The diagnostic accuracy of in vivo confocal scanning laser microscopy compared to dermoscopy of benign and malignant melanocytic lesions: a prospective study. Dermatology 2007;215:365–72. [DOI] [PubMed] [Google Scholar]
- 56.Longo C, Farnetani F, Ciardo S, Cesinaro AM, Moscarella E, Ponti G et al. Is confocal microscopy a valuable tool in diagnosing nodular lesions? A study of 140 cases. Br J Dermatol 2013;169:58–67. [DOI] [PubMed] [Google Scholar]
- 57.Lovatto L, Carrera C, Salerni G, Alos L, Malvehy J, Puig S. In vivo reflectance confocal microscopy of equivocal melanocytic lesions detected by digital dermoscopy follow-up. J Eur Acad Dermatol Venereol 2015;29:1918–25. [DOI] [PubMed] [Google Scholar]
- 58.Maher NG, Solinas A, Scolyer RA, Puig S, Pellacani G, Guitera P. Detection of desmoplastic melanoma with dermoscopy and reflectance confocal microscopy. J Eur Acad Dermatol Venereol 2017;31:2016–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maier T, Sattler EC, Braun-Falco M, Korting HC, Ruzicka T, Berking C. Reflectance confocal microscopy in the diagnosis of partially and completely amelanotic melanoma: report on seven cases. J Eur Acad Dermatol Venereol 2013;27:e42–52. [DOI] [PubMed] [Google Scholar]
- 60.Mandel VD, Bombonato C, Pampena R, Kyrgidis A, Borsari S, Benati E et al. Integration of dermoscopy and reflectance confocal microscopy for distinguishing melanomas from nevi of the breast area. J Eur Acad Dermatol Venereol 2018;32:940–6. [DOI] [PubMed] [Google Scholar]
- 61.Menge TD, Hibler BP, Cordova MA, Nehal KS, Rossi AM. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): A prospective study. J Am Acad Dermatol 2016;74:1114–20. [DOI] [PubMed] [Google Scholar]
- 62.Moscarella E, Rabinovitz H, Oliviero MC, Brown L, Longo C, Zalaudek I et al. The role of reflectance confocal microscopy as an aid in the diagnosis of collision tumors. Dermatology 2013;227:109–17. [DOI] [PubMed] [Google Scholar]
- 63.Pellacani G, Bassoli S, Longo C, Cesinaro AM, Seidenari S. Diving into the blue: in vivo microscopic characterization of the dermoscopic blue hue. J Am Acad Dermatol 2007;57:96–104. [DOI] [PubMed] [Google Scholar]
- 64.Persechino F, De Carvalho N, Ciardo S, De Pace B, Casari A, Chester J et al. Folliculotropism in pigmented facial macules: Differential diagnosis with reflectance confocal microscopy. Exp Dermatol 2018;27:227–32. [DOI] [PubMed] [Google Scholar]
- 65.Pellacani G, Cesinaro AM, Seidenari S. Reflectance-mode confocal microscopy of pigmented skin lesions--improvement in melanoma diagnostic specificity. J Am Acad Dermatol 2005;53:979–85. [DOI] [PubMed] [Google Scholar]
- 66.Pellacani G, Cesinaro AM, Seidenari S. Reflectance-mode confocal microscopy for the in vivo characterization of pagetoid melanocytosis in melanomas and nevi. J Invest Dermatol 2005;125:532–7. [DOI] [PubMed] [Google Scholar]
- 67.Pellacani G, Farnetani F, Gonzalez S, Longo C, Cesinaro AM, Casari A et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol 2012;66:e109–21. [DOI] [PubMed] [Google Scholar]
- 68.Pellacani G, Guitera P, Longo C, Avramidis M, Seidenari S, Menzies S. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol 2007;127:2759–65. [DOI] [PubMed] [Google Scholar]
- 69.Pellacani G, Longo C, Ferrara G, Cesinaro AM, Bassoli S, Guitera P et al. Spitz nevi: In vivo confocal microscopic features, dermatoscopic aspects, histopathologic correlates, and diagnostic significance. J Am Acad Dermatol 2009;60:236–47. [DOI] [PubMed] [Google Scholar]
- 70.Pellacani G, Scope A, Farnetani F, Casaretta G, Zalaudek I, Moscarella E et al. Towards an in vivo morphologic classification of melanocytic nevi. J Eur Acad Dermatol Venereol 2014;28:864–72. [DOI] [PubMed] [Google Scholar]
- 71.Pellacani G, Scope A, Ferrari B, Pupelli G, Bassoli S, Longo C et al. New insights into nevogenesis: in vivo characterization and follow-up of melanocytic nevi by reflectance confocal microscopy. J Am Acad Dermatol 2009;61:1001–13. [DOI] [PubMed] [Google Scholar]
- 72.Pellacani G, Vinceti M, Bassoli S, Braun R, Gonzalez S, Guitera P et al. Reflectance confocal microscopy and features of melanocytic lesions: an internet-based study of the reproducibility of terminology. Arch Dermatol 2009;145:1137–43. [DOI] [PubMed] [Google Scholar]
- 73.Ruini C, Manfredini M, Pellacani G, Mandel VD, Tomasi A, Ponti G. Confocal microscopy characterization of BRAFV600E mutated melanomas. Melanoma Res 2015;25:367–71. [DOI] [PubMed] [Google Scholar]
- 74.Schwartz RJ, Vera K, Navarrete N, Lobos P. In vivo reflectance confocal microscopy of halo nevus. J Cutan Med Surg 2013;17:33–8. [DOI] [PubMed] [Google Scholar]
- 75.Segura S, Pellacani G, Puig S, Longo C, Bassoli S, Guitera P et al. In vivo microscopic features of nodular melanomas: dermoscopy, confocal microscopy, and histopathologic correlates. Arch Dermatol 2008;144:1311–20. [DOI] [PubMed] [Google Scholar]
- 76.Stanganelli I, Longo C, Mazzoni L, Magi S, Medri M, Lanzanova G et al. Integration of reflectance confocal microscopy in sequential dermoscopy follow-up improves melanoma detection accuracy. Br J Dermatol 2015;172:365–71. [DOI] [PubMed] [Google Scholar]
- 77.Urvanegia AC, Tavoloni Braga JC, Shitara D, Fregnani JH, Neves JI, Pinto CA et al. Reflectance confocal microscopy features of BRAF V600E mutated thin melanomas detected by immunohistochemistry. PLoS One 2017;12:e0179745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Losi A, Longo C, Cesinaro AM, Benati E, Witkowski A, Guitera P et al. Hyporeflective pagetoid cells: a new clue for amelanotic melanoma diagnosis by reflectance confocal microscopy. Br J Dermatol 2014;171:48–54. [DOI] [PubMed] [Google Scholar]
- 79.Jaimes N, Marghoob AA. Triage amalgamated dermoscopic algorithm. J Am Acad Dermatol 2020. [DOI] [PubMed] [Google Scholar]