Abstract
Sphingomyelin synthase related protein (SMSr) has no SM synthase activity but has ceramide phosphorylethanolamine (CPE) synthase activity in vitro. Although SMSr is ubiquitously expressed in all tested tissues, the CPE levels in most mammalian tissues or cells are extremely low or undetectable. Therefore, SMSr seems not to be a functional CPE synthase in vivo and its real biological function needs to be elucidated. In this study, we utilized purified recombinant SMSr and adenovirus-mediated SMSr in vivo expression to show that SMSr has phosphatidylethanolamine phospholipases C (PE-PLC) activity, i.e., it can generate DAG through PE hydrolysis in the absence of ceramide. Further, we found that SMSr has no phosphatidylcholine (PC)-PLC, phosphatidylserine (PS)-PLC, phosphatidylglycerol (PG)-PLC, and phosphatidic phosphatase (PAP) activities, indicating that SMSr-mediated PE-PLC activity has specificity. We conclude that SMSr is a mammalian PE-PLC. Importantly, SMSr can regulate steady state levels of PE in vivo, and it should be a new tool for PE-related biological study.
Keywords: Sphingomyelin synthase related protein (SMSr), Phospholipase C (PLC), Phosphatidylethanolamine PLC (PE-PLC), CPE synthase, SM synthase, SMS family
1. Introduction
Unlike sphingomyelin synthase 1 and 2 (SMS1 and SMS2), SMS related protein (SMSr) has no SM synthase activity [1,2]. SMSr is conserved throughout the animal kingdom, whereas SMS1 and SMS2 are only expressed in mammalian tissues [1,2]. It was shown that SMSr can transfer the phosphorylethanolamine from PE to ceramide, thereby forming ceramide phosphorylethanolamine (CPE) [3], in vitro. Although SMSr is ubiquitously expressed in all tested tissues [4], CPE levels in the liver and plasma are extremely low [4,5] and in most of mammalian tissues or cells, CPE is undetectable (unpublished observation). Moreover, depletion of SMSr does not influence plasma, liver, and macrophage CPE levels (extremely low though) [4]. Furthermore, SMSr is not a critical regulator of ceramide homeostasis in mice and SMSr-mediated CPE biosynthesis is dispensable for mouse development [5]. Therefore, SMSr seems not to be a functional CPE synthase in vivo and its real biological function needs to be determined.
While we were doing SMSr study, a most recent paper has been published [6]. It claimed that SMSr generates diacylglycerol via the hydrolysis of glycerophospholipids in the absence of ceramide [6]. Although we reached same conclusion that SMSr can act as a PLC [6], however, we showed that SMSr has enzyme specificity and it can only hydrolyze PE but not phosphatidylcholine (PC), phosphatidylserine (PS), phosphatidylglycerol (PG), and phosphatidic acid (PA). Importantly, SMSr activity regulates steady state levels of PE and DAG in vivo.
2. Materials and methods
2.1. Mice
We previously prepared hepatocyte-specific Sms1 KO/global Sms2 double KO (dKO) mice [7]. We crossed them with our global Smsr KO mice [4] and obtained hepatocyte-specific Sms1 KO/global Sms2/global Smsr triple KO (tKO) mice. All animals are bred on a C57BL/6 genetic background. Experiments involving animals were conducted with the approval of State University of New York Downstate Medical Center Institutional Animal Care and Use Committee. The procedures followed were in accordance with institutional guidelines.
2.2. Tissue culture and transfection
Cos7 cells were maintained in growth medium (Dulbecco's modified Eagle's medium (DMEM) with 10% FBS, 2 mM L-glutamine, 1 mM sodium pyruvate, 100 units/ml penicillin and 100 μg/ml streptomycin) at 37 °C in 5% CO2. Cos7 cells overexpressing SMSs were established by transfection using Lipofectamine 2000 (Invitrogen) with p3xflags-cmv-13 vectors (Sigma) containing SMSr. All cells were cultured at 37 °C in 5% CO2. The harvested cells were stored at −80 °C until use.
2.3. SMSr-flag immunoprecipitate preparation
The SMSr-flag transfected cells were homogenized in buffer (5% Sucrose, 50 mM Tris-HCl, 1 mM EDTA) containing a protease inhibitor cocktail (Roche Diagnostics). Supernatants were transferred to new tubes after centrifugation at 1000g for 10 min at 4 °C and stored at −80 °C until use. Protein concentrations of the homogenates were determined with a BCA Protein assay (Pierce) using BSA as standard. Different from liver homogenates, the supernatants from cells (~1000 μg protein) were further incubated with 20 μl of resuspended volume of protein A/G plus-agarose (Santa Cruz) at 4 °C for 30 min. After spinning at 8200 g for 30 s at 4 °C, the supernatants were then incubated with 50 μl of resuspended volume of EZview™ red anti-flag® M2 affinity gel for overnight at 4 °C. Finally, extracted SMSr was isolated by centrifugation at 8200g for 30 s at 4 °C for enzyme activity assays.
2.4. Recombinant SMSr preparation
The cDNA for human SMSr was cloned into an expression vector modified from pFastBac Dual, with a Strep tag II amino acid sequence (Trp-Ser-His-Pro-Gln-Phe-Glu-Lys) and a PreScission Protease recognition sequence added to the N terminus of cDNA of SMSr for affinity chromatography. The SMSr protein was expressed using a baculovirus/Spodoptera frugiperda (SF9) cell system and purified with the procedure similar to rSMS1 and rSMS2 preparation [8].
2.5. Enzyme activity assays
For SMS and CPE assays, the procedure was as reported [4]. For PLC assays, SMSr-flag purified by immunoprecipitation or rSMSr (150 ng) was incubated with reaction buffer (50 mM Tris-HCl, pH 7.4, 140 mM NaCl, 10 mM dimethylglutarate, with or without 2 mM CaCl2) and NBD-PC or NBD-PE or NBD-PS or NBD-PG (2 μg, 16:0–06:0, Avanti Polar Lipids) in total volume of 500 μl as well as replacing NBD-substrates to [14C]-PC/[14C]-PE (0.3 μCi, American Radiolabeled Chemical) for incubation. The reactions were incubated at 37 °C water-bath for 1 h, and then stopped by adding 500 μl of chloroform: methanol (2:1 v/v) with vigorous vortex. The lipids were then extracted and dried. For analysis, the dried lipid phase was re-dissolved in a 20 μl of chloroform and applied to a TLC plate (Silica gel, Whatman). NBD- or [14C]-substrates and generated NBD- or [14C]-DAG were separated using a solvent (chloroform: methanol, 15:1, v/v). After thoroughly drying the TLC plate, the NBD-lipid species were visualized under the UV light, while 14C-lipid species were visualized by an exposure to an autoradiography film (HyBlot CL). For PI-PLC activity assay, TopFluor-PI (2 μg, 16:0–06:0, Avanti Polar Lipids) was used and the rest procedure was similar to other PLC assays.
2.6. Lipid measurements
PE, DAG, SM, and PC were measured by a PE Assay Kit (Biovision, Cat# K499), a DAG Assay Kit (Cell Biolabs, Cat# MET-5028), a SM Assay Kit (Biovision, Cat# K600), and a PC Assay Kit (Biovision, Cat# K576), following the corresponding protocols, respectively. Ceramide and CPE were measured by LC/MS/MS at University of Taxes San Antonio, on the fee-for-service basis.
2.7. Adenovirus (AdV)-SMSr administration
AdV-SMSr was prepared by (ViraQuest Inc.). Wild type or Sms1/Sms2/Smsr triple KO mice were injected (i.v.) with AdV-SMSr (1 × 1011 viral particles/mouse), respectively. AdV-null was used as the control. At the day 4 after the injection, mouse livers and plasma were collected for enzyme and lipid analyses.
2.8. Statistical analysis
Each experiment was conducted at least two times. Data were expressed as the mean ± standard deviation (SD). Differences between two groups were analyzed by the unpaired, two-tailed Studenťs t-test. P-values < 0.05 were considered significant.
3. Results
3.1. Purified recombinant SMSr has PE-PLC activity in vitro
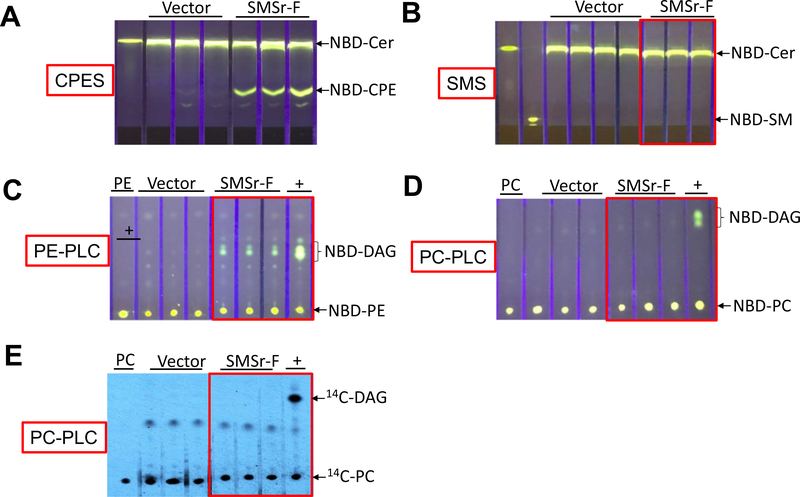
To examine whether SMSr is a PE-PLC, we expressed SMSr-flag in Cos7 cells through expression vector transfection. To avoid the noise from endogenous SMSr, we immunoprecipitated flagged SMSr using a flag antibody and then utilizing the precipitate to perform CPE synthase, SMS, PE-PLC, and PC-PLC activity assays, respectively. We found that SMSr-flag had CPE synthase but had no SMS activity in the test tube (Fig. 1A and B). Next, we utilized the same precipitate to measure PE-PLC activity, using NBD-PE as a substrate (no ceramide was added). We found that SMSr-flag mediated NBD-diglyceride (DAG) formation (Fig. 1C), indicating that SMSr can hydrolyze PE without ceramide. We also utilized NBD-PC and 14C-PC to perform PC-PLC activity and found that SMSr-flag did not hydrolyze both PCs (Fig. 1D and E).
Fig. 1.
CPE synthase, SMS, PE-PLC, and PC-PLC activity assays, using SMSr-flag immunoprecipitate and exogenous substrates. Cos7 cells were transfected with SMSr-flag expression or empty vector. SMSr-flag (SMSr-F) was immunoprecipitated and the precipitate was used to perform CPE synthase, SMS, PE-PLC, and PC-PLC activity assays, as described in “Materials and Methods”. The products were separated by thin layer chromatography. (A) CPE synthase activity assay, using NBD-Cer and PE as two substrates. (B) SMS activity assay, using NBD-Cer and PC as two substrates. (C) PE-PLC activity assay, using NBD-PE as a substrate. (D) PC-PLC activity assay, using NBD-PC as a substrate. (E) PC-PLC activity assay, using 14C-PC as a substrate. +, Bacterial PLC was used as a positive control. The development solvent for (A) and (B) was Chloroform:Methanol:NH4OH: 14:6:1 (v/v/v); The development solvent for (C)-(E) was Chloroform:Methanol: 15:1 (v/v). The results were the representative of three independent experiments.
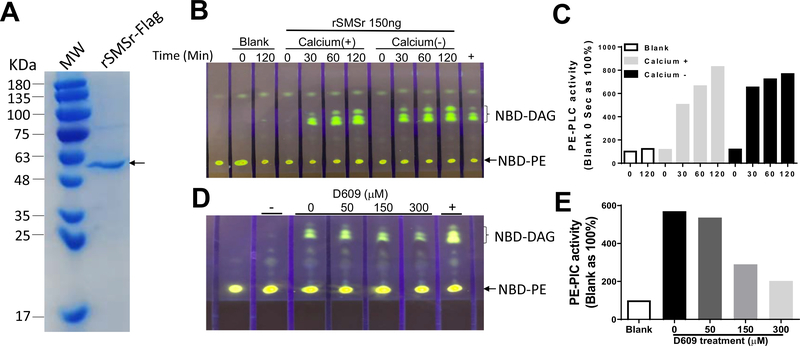
To more precisely show that SMSr is a PE-PLC. We expressed recombinant rSMSr-flag in baculovirus/Spodoptera frugiperda (SF9) cell system and then purified it, using flag affinity column chromatography. As shown in Fig. 2A, the purity of rSMSr-flag was about 90%. We then utilized the rSMSr for the following study. First of all, we measured PE-PLC activity with difference incubation time with or without calcium. We observed a rSMSr-mediated PE-PLC activity, which was time dependent and calcium independent (Fig. 2B and C). We then chose one hour as an incubation time for the rest of the study. It is known that D609 inhibited both PC-PLC and SM synthase activities [9]. We evaluated whether D609 can also inhibit rSMSr-mediated PE-PLC activity. Indeed, D609 inhibited the PE-PLC activity in a dose dependent fashion (Fig. 2D and E). It was reported that IC50 of D609 for PLC inhibition was about 130 μM [10] which was close to what we have observed (150 μM) (Fig. 2E).
Fig. 2.
Recombinant SMSr-flag purity examination and SMSr-mediated PE-PLC activity. (A) SDS-PAGE. The gel was stained by Coomassie Brilliant Blue. (B–C) Time course of SMSr-mediated PE-PLC activity and the effect of calcium on PE-PLC activity. (D–E) Effect of D609 on rSMSr. The development solvent was Chloroform: Methanol: 15:1 (v/v). The results were the representative of two independent experiments.
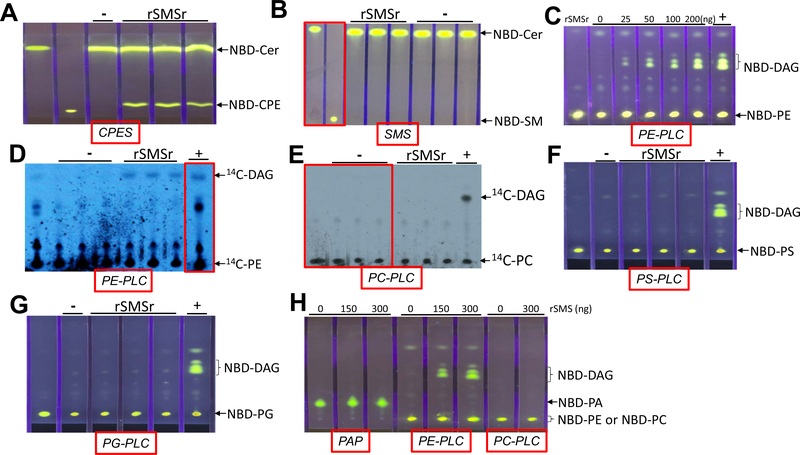
We next sought to use the rSMSr to measure CPE synthase, SMS, PE-PLC, PC-PLC, PS-PLC, PG-PLC, and PAP activities, in the test tubes, respectively. Indeed, rSMSr produced NBD-CPE (Fig. 3A) but not NBD-SM (Fig. 3B). Importantly, rSMSr produced NBD-DAG in a dose fashion when NBD-PE was used as a substrate (Fig. 3C) and produced 14C-DAG when 14C-PE was used (Fig. 3D). In contrast, rSMSr could not hydrolyze 14C-PC and NBD-PC (Fig. 3E and H) to produce respective DAGs. Moreover, rSMS has no ability to hydrolyze NBD-PS (Fig. 3F), NBD-PG (Fig. 3G), and NBD-PA (Fig. 3H) to produce NBD-DAG. Thus, SMSr has PE-PLC specificity.
Fig. 3.
CPE synthase, SMS, PE-PLC, PC-PLC, PS-PLC, PG-PLC, and PAP activity assays, using recombinant SMSr-flag (rSMSr) and exogenous substrates. Purified rSMSr (150 ng) was used for enzyme assays. (A) CPE synthase activity assay, using NBD-Cer and PE as two substrates. (B) SMS activity assay, using NBD-Cer and PC as two substrates. (C) PE-PLC activity assay, using NBD-PE as a substrate. Different amount of rSMSr were used. (D) PE-PLC activity assay, using 14C-PE as a substrate. (E) PC-PLC activity assay, using 14C-PC as a substrate. (F) PS-PLC activity assay, using NBD-PS as a substrate. (G) PG-PLC activity assay, using NBD-GC as a substrate. (H) PAP, PE-PLC, and PC-PLC activity assays, using NBD-PA, NBD-PE, and NBD-PC as a substrate, respectively. −, no protein was added. +, Bacterial PLC was used as a positive control. The development solvent for (A) and (B) was Chloroform:Methanol:NH4OH: 14:6:1 (v/v/v); The development solvent for (C)–(G) was Chloroform: Methanol: 15:1 (v/v). The results were the representative of three independent experiments.
3.2. SMSr is a PE-PLC in vivo
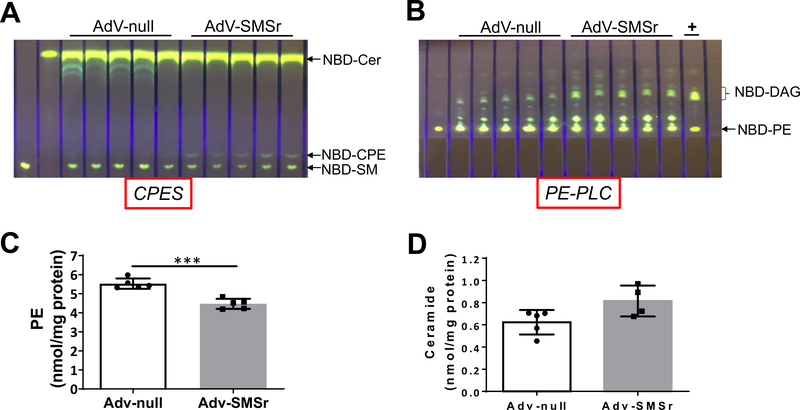
To have further evidence that SMSr is a PE-PLC in vivo, we injected AdV-SMSr or AdV-null into wild type mice. Since AdV is mainly expressed in the liver, AdV treatment has a liver expression specificity [11]. We isolated mouse liver at day 4 after the AdV injection and measured enzyme activities using liver homogenates. We found that AdV-SMSr treatment increased CPE synthase activity when exogenous NBD-Cer and PE were used as two substrates (Fig. 4A). We also noticed the formation of NBD-SM (Fig. 4A), which was related with endogenous SM synthase activity. There was no increase of NBD-SM (Fig. 4A), again, indicating that SMSr is not a SM synthase. Importantly, AdV-SMSr dramatically increased liver PE-PLC activity when only exogenous NBD-PE was used (Fig. 4B). More importantly, AdV-SMSr treatment significantly reduced liver PE (Fig. 4C) and induced instead of reduced ceramide levels (CPE synthase consume ceramide), although it was not statistically significant (Fig. 4D), suggesting that SMSr is a PE-PLC but not a CPE synthase in vivo.
Fig. 4.
The effect of AdV-SMSr on wild type mice. Wild type mice were injected (i.v.) with AdV-SMSr and AdV-null (1 × 1011 viral particles/mouse), respectively. Four days after the injection, mouse liver homogenates were prepared and used for enzyme assays and lipid analysis. (A) CPE synthase activity assay, using NBD-Cer and PE as two substrates. (B) PE-PLC activity assay, using NBD-PE as a substrate. +, bacterial PLC was used as a positive control. (C) liver PE measurement. (D) liver ceramide measurement. The development solvent for (A) was Chloroform:Methanol:NH4OH: 14:6:1 (v/v/v); The development solvent for (B) was Chloroform: Methanol: 15:1 (v/v). Values represent the mean ± SD, n = 4–5, ***P < 0.001.
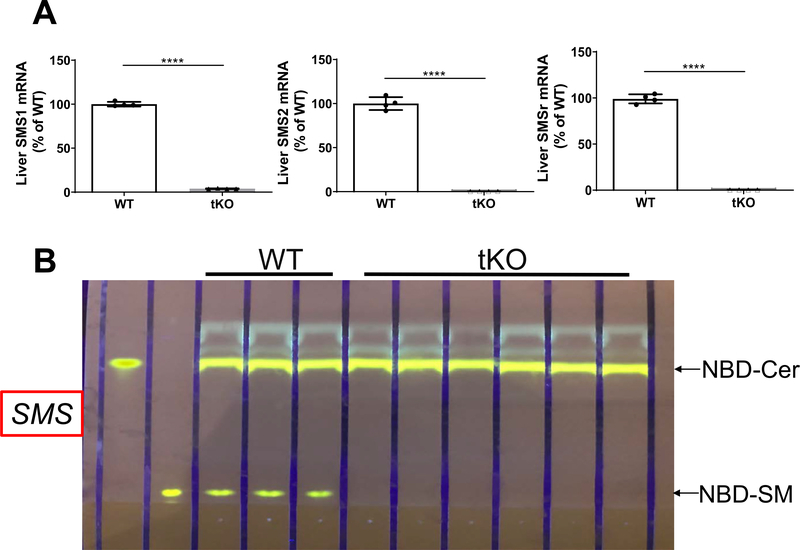
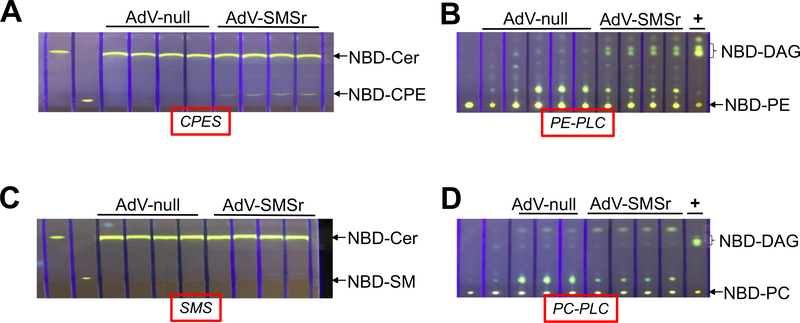
Previously, we found that all SMS family members have certain CPE synthase activity in vitro [12]. To avoid the noise from endogenous PE-PLC activity, potentially mediated by SMS family members, we prepared liver Sms1/global Sms2/global Smsr triple KO mice which have no SMS family expression (Fig. 5A) and no SM synthase activity in the liver (Fig. 5B). We then injected AdV-SMSr and AdV-null into 2-month-old tKO mice, respectively. At day 4 after the injection, we isolated the liver from the mice and prepared liver homogenates. By using different exogenous substrates, we measure CPE synthase, PE-PLC, SMS, and PC-PLC activity, respectively. We found that AdV-SMSr administration had a weak CPE synthase activity (NBD-ceramide and PE were used as two substrates) and a strong PE-PLC activity (only NBD-PE was used) (Fig. 6A and B). AdV-SMSr had no influence on SMS activity (NBD-ceramide and PC were used as two substrates) (Fig. 6C) and PC-PLC (NBD-PC was used as a substrate) (Fig. 6D). These results, again, indicated that SMSr can generate DAG through phosphatidylethanolamine (PE) but not phosphatidylcholine (PC) hydrolysis in the absence of ceramide. Thus, SMSr is a PE-PLC.
Fig. 5.
Liver mRNA levels of SMS family members and SMS activity in hepatocyte-specific Sms1/global Sms2/global Smsr triple KO mice. (A) Liver SMSr, SMS1, and SMS2 mRNA levels were measured by real-time PCR. (B) Liver SMS activity analysis, using NBD-Cer and PC as two substrates. The development solvent for was Chloroform:Methanol:NH4OH: 14:6:1 (v/v/v). Values represent the mean ± SD, n = 4, ****P < 0.0001.
Fig. 6.
The effect of AdV-SMSr on hepatocyte-specific Sms1/global Sms2/global Smsr triple KO mouse liver CPE synthase, PE-PLC, SMS, and PC-PLC activities. The triple KO mice (2-month-old) were injected (i.v.) with AdV-SMSr and AdV-null (1 × 1011 viral particles/mouse), respectively. Four days after the injection, mouse liver homogenates were prepared and used for enzyme assays. (A) CPE synthase activity assay, using NBD-Cer and PE as two substrates. (B) PE-PLC activity assay, using NBD-PE as a substrate. (C) SMS activity assay, using NBD-Cer and PC as two substrates. (D) PC-PLC activity assay, using NBD-PC as a substrate. +, bacterial PLC was used as a positive control. The development solvent for (A) and (C) was Chloroform:Methanol:NH4OH: 14:6:1 (v/v/v); The development solvent for (B) and (D) was Chloroform:Methanol: 15:1 (v/v).
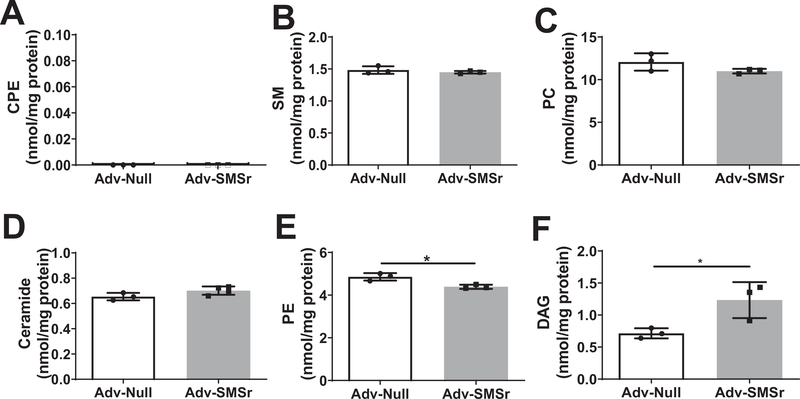
Next, we sought to measure liver CPE, SM, ceramide, PE, DAG, and PC levels in AdV-null and AdV-SMSr treated tKO mice, respectively. We found that liver CPE was undetectable in both AdV-null and AdV-SMSr groups (Fig. 7A). Also, AdV-SMSr did not influence SM (Fig. 7B) and PC levels (Fig. 7C). There was an increase of ceramide levels (Fig. 7D), however, it was not statistic significant. Importantly, AdV-SMSr significantly decreased PE (Fig. 7E) and increased DAG levels (Fig. 7F). Collectively, SMSr is not a CPE synthase but a PE-PLC, in vivo, which regulates PE and DAG in the liver.
Fig. 7.
Lipid analysis in the triple KO mouse liver after AdV-SMSr treatment. (A) CPE. (B) SM. (C) PC. (D) Ceramide. (E) PE. (F) DAG. Values represent the mean ± SD, n = 3, *P < 0.05.
4. Discussion
In current study, we utilized in vitro and in vivo approaches to show that SMSr can generate DAG through PE hydrolysis in the absence of ceramide. Moreover, we demonstrated that SMSr expression in mouse liver can influence PE and DAG, but not ceramide and PC levels.
Bacterial PE-PLC has been cloned [13], however, whether there is a mammalian PE-PLC is still unknown so far. SMSr has no ability to use PC and ceramide to produce SM, i.e., SM synthase activity. However, in vitro, SMSr can utilize PE and ceramide to produce DAG and a SM-like molecule, CPE [3], i.e., CPE synthase activity. It is noticed that SMSr, like SMS1 and SMS2, has a catalytic domain to break phosphodiester bond [1]. Thus, SMSr could potentially be a PE-PLC. We found that both immunoprecipitated flag-tagged SMSr (Fig. 1C) and rSMSr generate DAG (Fig. 3C and D) through their PE-PLC activity in the absence of ceramide. Moreover, like its inhibitory effect on PC-PLC, D609 also can inhibit rSMSr mediated PE-PLC activity in a dose dependent manner (Fig. 2C). Importantly, we expressed SMSr in SMS1/SMS2/SMSr triple deficient mice through adenovirus and we found that AdV-SMSr significantly increases liver PE-PLC activity (Fig. 6B).
In test tubes, all SMS family members have CPE synthase activity [12]. We confirmed this SMSr-mediated CPE synthase activity in current study (Figs. 1A, 4A, and 6A). However, this CPE synthase activity measurement did not reflect the real function of SMSr in vivo, since exogenous substrates, i.e., NBD-ceramide and PE, were used. As a matter of fact, although SMSr is expressed in all tested mammalian tissues and cells, CPE levels in them are extremely low [4,5] or undetectable (unpublished observation). Also, although SMSr is evolutionarily conservative, bulk production of CPE in insect cells is mediated by a different enzyme [3]. It has been suggested that SMSr, as a CPE synthase, controls ceramide homeostasis in the ER of Hela cells [3]. However, a later study indicated that SMSr-catalyzed CPE production is not sufficient to suppress ceramide-induced cell death [14]. We prepared global Smsr KO mice and we found that the CPE levels are not only extremely low in the plasma, liver, and macrophages but also were not different between wild type and the KO mice [12]. Similarly, SMSr expression (Fig. 7D) or deficiency [12] has no impact on ceramide levels suggests that SMSr/CPE synthase per se also is not a critical regulator of ceramide levels in vivo. It could be possible that the trace amount of CPE in the circulation and in mouse liver (due to residue blood) could come from microbiota which contains high levels of CPE [15] and is located in the lumen of intestine. Thus, it is a long-time puzzle for us, i.e., what is the real biological activity of SMSr [12]? We found an answer from current study, i. e., PE-PLC is a real biological function for SMSr.
PE plays essential roles in autophagy, cell division and protein folding, and represents a precursor for the synthesis of several protein modifications [16,17]. Abnormal high or low cellular PE/PC molar ratios in various tissues can influence energy metabolism and have been linked to disease progression [18]. It has been known for a long time that plasma PE level is a better indicator in the predictability of human atherosclerotic complications [19]. Certain species of PEs are closely associated with coronary heart diseases [20,21]. Very low-density lipoprotein (VLDL) is a well-known atherogenic particle in the circulation. PE content of newly secreted VLDL particles [22], and that of VLDL isolated from the lumen of the Golgi [23], were several-fold higher than that of circulating VLDL, suggesting that PE could play a role in VLDL assembly and/or secretion. Changes in the hepatic PE/PC molar ratio also have been linked to development of non-alcoholic fatty liver [24]. However, how to precisely regulate steady-state PE levels and what is the mechanism linking PE with metabolic diseases are not quite understood. The discovery that SMSr is a PE-PLC bringing a new direction of the PE study.
While we are doing SMSr study, a most recent paper has been published [6]. We are glad that the authors reached the same conclusion as ours, i.e., SMSr is a PLC. However, there is a difference between our results and theirs. The authors indicated that SMSr has a PC-PLC activity [6] and we found that SMSr has no such an activity (Figs. 1D, E, 3E, F, and 6D). As we knew, from CPE synthase reaction, CPE formation can be separated into two steps: 1) PE-PLC step, where PE is hydrolyzed into phosphorylethanolamine (P-ethanolamine) and DAG; and 2) CPE formation step, where P-ethanolamine is added onto ceramide. Similarly, for the SM synthase reaction, SM formation also can be separated into two steps: 1) PC-PLC step, where PC is hydrolyzed into phosphorylcholine (P-choline) and DAG; and 2) SM formation step, where P-choline is added onto ceramide. Given the fact that SMSr has CPE synthase (Figs. 1A, 3A, and 6A) but has no SM synthase activity (Figs. 1B, 3B, and 6C) [1,2] in vitro, it is not likely that SMSr can hydrolyze PC.
Moreover, Murakami and Sakane [6] showed that SMSr can also hydrolyze PA, but we did not find such as activity too (Fig. 3H). PA does not contain phosphodiester bone as PE and PC, thus, the rationale that SMSr has PAP activity (which is different from PLC activity) is not that obvious. Moreover, we showed that SMSr has no ability to hydrolyze PS and PG (Fig. 3F and G). Since NBD-PI is not commercially available, we used TopFluor-PI (a fluorescent PI) to perform PI-PLC activity. As shown in Supplement Fig. 1, after one hour incubation at 37 °C, there was an automatic hydrolysis of TopFluor-PI molecule (-, blank). Although we could not explain this phenomenon, no difference was observed after the treatment of different dose of rSMSr. Given the fact that mammals express six families of PI-PLCs, with at least 13 members [25], and SMSr has no PS-PLC, PG-PLC, PC-PLC, and PAP activities, it is not likely that it has PI-PLC activity. Thus, we believe that SMSr is a specific PE-PLC but not a pan-PLC and its specificity is an important property of SMSr.
We conclude that our study together with the most recent study [6] clearly solved a long-time puzzle, i.e., what is the real activity of SMSr in vivo? SMSr is a mammalian PE-PLC, and its biological and pathological functions deserve further investigation.
Supplementary Material
Acknowledgments
We thank Dr. Xianlin Han, from University of Taxes San Antonio, for his group's effort to measure ceramide and ceramide phosphorylethanolamine (CPE). This work was supported by grants VA Merit 000900-01, NIH HL1395821, and HL149730 to XCJ.
Abbreviations:
- SMSr
sphingomyelin synthase related protein
- PE
phosphatidylethanolamine
- PC
phosphatidylcholine
- PS
phosphatidylserine
- PG
phosphatidylglycerol
- PLC
phospholipase C
- KO
gene knockout
- AdV
adenovirus
- CPE
ceramide phosphorylethanolamine
- DAG
diglyceride
- PAP
phosphatidic phosphatase
Footnotes
Declaration of competing interest
The authors declare that they have no conflicts of interest with the contents of this article.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbalip.2021.159017.
References
- [1].Huitema K, van den Dikkenberg J, Brouwers JF, Holthuis JC, Identification of a family of animal sphingomyelin synthases, EMBO J. 23 (2004) 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tafesse FG, Ternes P, Holthuis JC, The multigenic sphingomyelin synthase family, J. Biol. Chem. 281 (2006) 29421–29425. [DOI] [PubMed] [Google Scholar]
- [3].Vacaru AM, Tafesse FG, Ternes P, Kondylis V, Hermansson M, Brouwers JF, Somerharju P, Rabouille C, Holthuis JC, Sphingomyelin synthase-related protein SMSr controls ceramide homeostasis in the ER, J. Cell Biol. 185 (2009) 1013–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ding T, Kabir I, Li Y, Lou C, Yazdanyar A, Xu J, Dong J, Zhou H, Park T, Boutjdir M, Li Z, Jiang XC, All members in the sphingomyelin synthase gene family have ceramide phosphoethanolamine synthase activity, J. Lipid Res. 56 (2015) 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bickert A, Ginkel C, Kol M, vom Dorp K, Jastrow H, Degen J, Jacobs RL, Vance DE, Winterhager E, Jiang XC, Dormann P, Somerharju P, Holthuis JC, Willecke K, Functional characterization of enzymes catalyzing ceramide phosphoethanolamine biosynthesis in mice, J. Lipid Res. 56 (2015) 821–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Murakami C, Sakane F, Sphingomyelin synthase-related protein generates diacylglycerol via the hydrolysis of glycerophospholipids in the absence of ceramide, J. Biol. Chem. 296 (2021), 100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li Z, Chiang YP, He M, Zhang K, Zheng J, Wu W, Cai J, Chen Y, Chen G, Chen Y, Dong J, Worgall TS, Jiang XC, Effect of liver total sphingomyelin synthase deficiency on plasma lipid metabolism, Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1866. (2021), 158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lou B, Liu Q, Hou J, Kabir I, Liu P, Ding T, Dong J, Mo M, Ye D, Chen Y, Bui HH, Roth K, Cao Y, Jiang XC, 2-Hydroxy-oleic acid does not activate sphingomyelin synthase activity, J. Biol. Chem. 293 (2018) 18328–18336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Adibhatla RM, Hatcher JF, Gusain A, Tricyclodecan-9-yl-xanthogenate (D609) mechanism of actions: a mini-review of literature, Neurochem. Res. 37 (2012) 671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Unno T, Beech DJ, Komori S, Ohashi H, Inhibitors of spasmogen-induced Ca2+ channel suppression in smooth muscle cells from small intestine, Br. J. Pharmacol. 125 (1998) 667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shayakhmetov DM, Li ZY, Ni S, Lieber A, Analysis of adenovirus sequestration in the liver, transduction of hepatic cells, and innate toxicity after injection of fiber-modified vectors, J. Virol. 78 (2004) 5368–5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ding T, Kabir I, Li Y, Lou C, Yazdanyar A, Xu J, Dong J, Zhou H, Park T, Boutjdir M, Li Z, Jiang XC, All members in the sphingomyelin synthase gene family have ceramide phosphoethanolamine synthase activity, J. Lipid Res. 56 (2015) 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Barker AP, Vasil AI, Filloux A, Ball G, Wilderman PJ, Vasil ML, A novel extracellular phospholipase C of Pseudomonas aeruginosa is required for phospholipid chemotaxis, Mol. Microbiol. 53 (2004) 1089–1098. [DOI] [PubMed] [Google Scholar]
- [14].Tafesse FG, Vacaru AM, Bosma EF, Hermansson M, Jain A, Hilderink A, Somerharju P, Holthuis JC, Sphingomyelin synthase-related protein SMSr is a suppressor of ceramide-induced mitochondrial apoptosis, J. Cell Sci. 127 (2014) 445–454. [DOI] [PubMed] [Google Scholar]
- [15].Panevska A, Skocaj M, Krizaj I, Macek P, Sepcic K, Ceramide phosphoethanolamine, an enigmatic cellular membrane sphingolipid, Biochim. Biophys. Acta Biomembr. 1861 (2019) 1284–1292. [DOI] [PubMed] [Google Scholar]
- [16].Vance JE, Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids, J. Lipid Res. 49 (2008) 1377–1387. [DOI] [PubMed] [Google Scholar]
- [17].Farine L, Butikofer P, The ins and outs of phosphatidylethanolamine synthesis in Trypanosoma brucei, Biochim. Biophys. Acta 1831 (2013) 533–542. [DOI] [PubMed] [Google Scholar]
- [18].van der Veen JN, Kennelly JP, Wan S, Vance JE, Vance DE, Jacobs RL, The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease, Biochim. Biophys. Acta Biomembr. 1859 (2017) 1558–1572. [DOI] [PubMed] [Google Scholar]
- [19].Kunz F, Stummvoll W, Plasma phosphatidylethanolamine–a better indicator in the predictability of atherosclerotic complications? Atherosclerosis 13 (1971) 413–425. [DOI] [PubMed] [Google Scholar]
- [20].Kunz F, Pechlaner C, Erhart R, Fend F, Muhlberger V, HDL and plasma phospholipids in coronary artery disease, Arterioscler. Thromb. 14 (1994) 1146–1150. [DOI] [PubMed] [Google Scholar]
- [21].Huang T, Zeleznik OA, Poole EM, Clish CB, Deik AA, Scott JM, Vetter C, Schernhammer ES, Brunner R, Hale L, Manson JE, Hu FB, Redline S, Tworoger SS, Rexrode KM, Habitual sleep quality, plasma metabolites and risk of coronary heart disease in post-menopausal women, Int. J. Epidemiol. 48 (2019) 1262–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Skipski VP, Barclay M, Barclay RK, Fetzer VA, Good JJ, Archibald FM, Lipid composition of human serum lipoproteins, Biochem. J. 104 (1967) 340–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hamilton RL, Fielding PE, Nascent very low density lipoproteins from rat hepatocytic Golgi fractions are enriched in phosphatidylethanolamine, Biochem. Biophys. Res. Commun. 160 (1989) 162–173. [DOI] [PubMed] [Google Scholar]
- [24].Li Z, Agellon LB, Allen TM, Umeda M, Jewell L, Mason A, Vance DE, The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis, Cell Metab. 3 (2006) 321–331. [DOI] [PubMed] [Google Scholar]
- [25].Kadamur G, Ross EM, Mammalian phospholipase C, Annu. Rev. Physiol. 75 (2013) 127–154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.