Abstract
Purpose:
This work characterizes the contrast sensitivity function (CSF) in patients with successful repair of macula-off rhegmatogenous retinal detachment (RD) using an adaptive computerized contrast testing device.
Methods:
CSF was prospectively measured in patients with macula-off RD following successful repair and age-matched controls at W.K. Kellogg Eye Center and Massachusetts Eye and Ear using Adaptive Sensory Technology’s Manifold Contrast Vision Meter. Outcome measures included average area under the CSF curve, contrast-sensitivity thresholds (1-18 cycles per degree), and best-corrected visual acuity (BCVA) in RD eyes, fellow eyes, and controls. A subanalysis was performed in eyes with BCVA of 20/30 or better.
Results:
Twenty-three macula-off RD eyes following repair, fellow healthy eyes, and 45 age-matched control eyes underwent CSF testing. Mean BCVA of the 23 RD eyes was 0.250 logMAR, which was, significantly reduced compared with fellow eyes 0.032 (P < .001) and controls 0.026 (P < .001). There was a statistically significant reduction in average area under the CSF curve in RD eyes compared with fellow eyes (P < .0001) and age-matched controls (z score –0.90, P < .0001) and CSF reduction across all spatial frequencies. In the 15 RD eyes with BCVA of 20/30 or better, the mean CSF was significantly reduced vs fellow eyes (P = .02) and controls (P = .045).
Conclusions:
CSF in macula-off RD eyes following repair was significantly reduced compared with fellow eyes and age-matched controls. CSF may be a promising visual function end point with applications in clinical practice and future clinical trials.
Keywords: contrast sensitivity function, macula-off retinal detachment, qCSF, visual function, visual function end points
Introduction
While anatomic success for retinal reattachment after macula-off rhegmatogenous retinal detachment (RRD) has been reported to be near or greater than 90%, 1 there is a wide range of functional outcomes as measured by visual acuity (VA). Most large series have demonstrated that approximately 40% of patients will attain VA of 20/50 or better. 2 -4 Yet VA does not always accurately reflect the patient’s subjective assessment of visual function. 5 -8 To better assess visual function, various measures have been suggested and proven to be especially useful in macular pathologies, including multifocal electroretinography, 9 microperimetry, 10 dark adaptation, 11 and contrast sensitivity (CS). 12
Compared with VA, CS function (CSF) seems to correlate better with real-world everyday activities 13 -19 and subjectively perceived visual impairment 20 -22 and may potentially be diminished earlier in the course of neurodegenerative ocular pathologies. 7,23 Despite its promising role in visual function assessment, the inherent imperfections in sensitivity and/or precision of most CS testing methods have prevented wider adoption of CSF in clinical practice and clinical trials so far. Conventional laboratory CSF measurement that tests all possible combinations of spatial frequency and contrast is too time-consuming, 24 whereas older CS tests such as the Pelli-Robson chart 25 use coarse quantization and sampling that operates in only 1 spatial frequency. 8,26,27 Current clinically available CSF tests that evaluate both spatial frequency and contrast are typically preprinted letter charts, such as the Functional Acuity Contrast Test or the Vistech test, that exhibit poor range and resolution for sampling target contrast and frequency 28 -30 and poor test–retest reliability. 29,31,32
In 2010, Lesmes et al 33 used a Bayesian algorithm that maximized information extraction over a large set of possible stimuli, reducing the number of trials to reliably estimate the CSF from several hundreds that used traditional methods to several dozens. The respective time for test completion was reduced to 2 to 5 minutes, and thus Lesmes et al introduced the quick CSF (qCSF) method. 33 This active learning method evaluates CS across multiple different spatial frequencies 34 with both high sensitivity in detecting changes of visual functions and great test–retest reliability. 35
The qCSF seems to be a promising visual function end point that can provide the sensitive and precise signals required to track RD visual rehabilitation over time in clinical practice and emerges as a potential end point for future clinical trials that evaluate experimental RD treatment options, including neuroprotective agents. To our knowledge, there are currently no studies that investigate CSF with the qCSF method in RD eyes. We herein present an initial 2-center, prospective, observational study that uses the novel qCSF method on the Adaptive Sensory Technology platform to compare CSF in macula-off RRD eyes with fellow eyes and age-matched controls.
Methods
Patients were recruited from retina clinics between November 2016 and May 2017. Eligible participants met the following inclusion criteria: aged 18 years or older and 1 eye with successfully repaired macula-off RD. Patients with media opacities in the fellow eye such as vitreous hemorrhage or lens opacification greater than 1+ were excluded.
All participants underwent a dilated comprehensive ophthalmologic examination, spectral-domain optical coherence tomography, and CS testing using the qCSF method. 33 Medical records were reviewed for all patients who completed a CSF test to record baseline demographic and ocular characteristics, such as participant age and sex, as well as VA and lens status in the study eye.
Inclusion criteria included Snellen or equivalent best-corrected VA (BCVA) better than or equal to 20/200, no more than 1 one RD repair, no RD repair involving silicone oil, RD repair within 2 weeks of initial symptoms, trace to 1+ cataract or pseudophakia, no systemic disease that could affect vision, and no other macular ocular pathology in either eye, including macular edema or subretinal fluid detected on spectral-domain optical coherence tomography after RD repair.
CSF Testing Protocol
Study participants completed an in-clinic CSF test both binocularly and monocularly following measurement of BCVA using the computerized Adaptive Sensory Technology platform, which consisted of a light-emitting diode screen with a luminance of 95.4 candela/m2 and resolution of 1920 × 1080 pixels. The platform used 10 filtered Sloan letters with 128 possible contrasts (evenly distributed in log space from 0.002 to 1) and 19 possible spatial frequencies (evenly distributed in log space from 1.19 to 30.95 cycles per degree [cpd]). An adaptive Bayesian algorithm selected optimal stimuli of contrast and spatial frequency to maximize information gain. 33 The built-in active learning system made it possible for data collected at 1 spatial frequency to improve sensitivity estimates across all frequencies with a high test–retest reliability. The test–retest reliability of the qCSF method has been extensively studied and reported to be greater than 92.4%. 35 Hence, only 25 stimuli trials needed to be presented for each eye to estimate the broad metric provided by the area under the logarithm of CSF (AULCSF).
Patients verbally reported the letters to the examiner, who recorded whether their answers were “correct,” “incorrect,” or “no response.” The duration for each test was approximately 2 minutes. The data from the 25 trials constructed a CSF curve along a spatial frequency range of 1 to 18 cpd. This yielded the AULCSF curve, which represented the total measure of spatial vision. 33 Outcome measures used for statistical analysis included AULCSF and CS thresholds at 1, 1.5, 3, 6, 12, and 18 cpd.
Statistical Analysis
Descriptive analyses included means and SDs of VA and CS. A P value of less than .05 was considered statistically significant. In addition to comparing with the healthy fellow eye, the AULCSF of study participants was compared with the AULCSF for age-matched controls, as represented by the z score. BCVA as measured by Snellen was converted to its logMAR for analysis. A χ2 test was used to compare categorical demographic information (ratio of male to female and phakic status), and an independent t test was used to compare age between study eyes and controls. A linear mixed model with random intercept was used to compare CS outcome measures in the study eye and the fellow eye, and an independent t test was used to compare study eyes with control eyes.
Analyses were completed for each respective group then further analyzed in patients with BCVA better than or equal to 20/50 and 20/30 to delineate vision-related difficulties in patients whose BCVA after surgery was above average. Multivariable linear regression analysis was performed to assess the association between CS outcome measures and VA, adjusting for age, sex, and lens status. Statistical analysis was performed with Microsoft Excel, version 15.39, 2017, and SAS, version 9.4, 2017.
Results
Twenty-three eyes of 23 patients after repair of a macula-off RD, fellow healthy eyes, and 45 eyes of 45 age-matched controls were included in this study. The mean age for patients with RD was 59 years (range, 45-75 years); 14 were men and 9 were women. The median time from RD repair to testing was 349 days (range, 100-647 days). The mean (SD) BCVA of the 23 RD eyes was 0.250 (0.25) logMAR (approximate Snellen equivalent 20/35; range, 20/20-20/150). Nineteen RD eyes were pseudophakic, and 4 were phakic (≤1+ nuclear sclerosis). Mean (SD) BCVA for fellow eyes was 0.032 (0.10) (approximate Snellen, 20/21; range, 20/15-20/40). In the control group, the mean age was 61 years (range, 50-76 years), and there were 24 men and 21 women. There were no statistically significant differences in age or sex among study patients and controls. The mean (SD) BCVA of the controls was 0.026 (0.06) logMAR (approximate Snellen, 20/21; range, 20/20-20/30), and 32 eyes were phakic and 13 pseudophakic. The mean BCVA of the 23 RD eyes was found to be statistically significantly reduced compared with fellow eyes (P < .001) and controls (P < .001). Baseline characteristics are presented in Table 1.
Table 1.
Baseline characteristics.
| Baseline characteristics | Study participants | Control | P |
|---|---|---|---|
| (n = 23) | (n = 45) | ||
| Age, y | |||
| Mean | 59 | 61 | .33 |
| Range | 45-75 | 50-76 | |
| Age group, y, No. (%) | |||
| 20-49 | 1 (4) | 0 (0) | |
| 50-79 | 22 (96) | 45 (100) | |
| Sex, No. (%) | |||
| Male | 14 (61) | 24 (53) | .56 |
| Female | 9 (39) | 21 (47) | |
| Visual acuity of qualification eyea | |||
| Snellen equivalent, mean | 20/35 | 20/21 | < .001b |
| Phakic status, No. (%) | |||
| Phakic | 4 (17) | 32 (71) | < .001b |
| Pseudophakic | 19 (83) | 13 (29) | |
a Visual acuity was measured with habitual correction and Early Treatment Diabetic Retinopathy Study charts. Snellen equivalents were recorded.
b Indicates statistical significance.
Overall AULCSF of Study Eyes vs Fellow Eyes and Controls
In the 23 RD eyes, there was a statistically significant reduction in contrast function with a mean (SD) AULCSF of 0.896 (0.34) compared with 1.200 (0.25) in the age-matched controls, representing a z score of –0.901 (0.96) (P < .001) (Tables 2 and 3). There was also a statistically significant reduction in mean (SD) AULCSF compared with the fellow eyes, which had a mean AUCLSF of 1.247 (0.29) (P < .001) (see Table 2).
Table 2.
CS Function of All Eyes.
| Study eye (n = 23) | Fellow eye (n = 23) | P | |
|---|---|---|---|
| Mean AULCSF ± SD | 0.896 ± 0.34 | 1.247 ± 0.29 | < .001a |
| Mean 1.5 cpd ± SD | 1.348 ± 0.22 | 1.485 ± 0.23 | .005a |
| Mean 3.0 cpd ± SD | 1.245 ± 0.35 | 1.474 ± 0.26 | .004a |
| Mean 6.0 cpd ± SD | 0.887 ± 0.43 | 1.249 ± 0.33 | .001a |
| Mean 12.0 cpd ± SD | 0.285 ± 0.31 | 0.637 ± 0.44 | .003a |
| Mean 18.0 cpd ± SD | 0.044 ± 0.12 | 0.241 ± 0.33 | .003a |
| Study eye (n = 23) | Control (n = 45) | P | |
| Mean AULCSF ± SD | 0.896 ± 0.34 | 1.200 ± 0.25 | < .001a |
| Mean 1.5 cpd ± SD | 1.348 ± 0.22 | 1.460 ± 0.16 | .02a |
| Mean 3.0 cpd ± SD | 1.245 ± 0.35 | 1.472 ± 0.21 | .005a |
| Mean 6.0 cpd ± SD | 0.887 ± 0.43 | 1.215 ± 0.28 | < .001a |
| Mean 12.0 cpd ± SD | 0.285 ± 0.31 | 0.561 ± 0.34 | < .001a |
| Mean 18.0 cpd ± SD | 0.044 ± 0.12 | 0.174 ± 0.22 | .002a |
Abbreviations: AULCSF, area under the CS function curve; cpd, cycles per degree.
a Indicates statistical significance.
Table 3.
CS Function z Scores of All Eyes.
| Compared with control | Compared with fellow eye | |
|---|---|---|
| Z score (SD) | Z score (SD) | |
| Study eye | –0.901 (0.96) | –1.207 (1.17) |
| Study eye with VA ≥ to 20/50 | –0.637 (0.86) | –0.775 (0.89) |
| Study eye with VA ≥ to 20/30 | –0.513 (0.85) | –0.568 (0.80) |
Abbreviation: VA, visual acuity.
The mean (SD) AULCSF for the 4 study eyes that were phakic was 0.705 (0.14) compared with 0.936 (0.79) for the 19 study eyes that were pseudophakic. This difference was not statistically significant (P = .22).
AULCSF of Study vs Fellow Eyes at Individual Spatial Frequencies
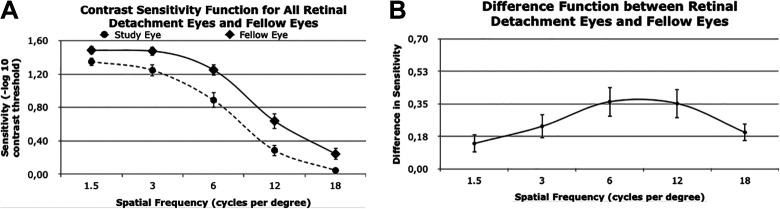
For both the study and fellow eye data, the CSF curve trended down with increased spatial frequency. At each individual spatial frequency value (1.5, 3, 6, 12, and 18 cpd), the CS of the study eye was lower than that of the fellow eye (Figure 1A). The largest difference in CSF was 0.36, measured at a spatial frequency of 6 cpd, which indicated 6 cpd was associated with the greatest decrease in CS from study eye to the fellow eye (Figure 1B).
Figure 1.
(A) CS function values for study eyes (ie, eyes with macula-off rhegmatogenous retinal detachment) and the corresponding fellow eyes for specific spatial frequency values. (B) Difference function between retinal detachment eyes and fellow eyes. Error bars indicate SE. Specific values are also provided in Table 2.
The multivariable regression analysis of CS on BCVA as logMAR was completed for overall AULCSF and for each spatial frequency. There was a significant decrease in AULCSF and in CS with increasing logMAR BCVA for all of the spatial frequencies except the highest spatial frequency, 18.0 cpd.
AULCSF of Study Eyes With VA 20/50 or Better vs Fellow Eyes and Controls
In the 18 study eyes with VA better than or equal to 20/50, the postoperative mean BCVA for the study eye was 0.142 logMAR (approximate Snellen, 20/28). Further analysis of AULCSF in patients with RD with VA better than or equal to 20/50 also demonstrated a statistically significant reduction in mean AULCSF between study and fellow eyes (P < .001) and between the study eyes and the control group (P = .007, Table 4) The mean (SD) AULCSF of study eyes with VA better than or equal to 20/50 was 0.997 (0.28) and of their fellow eyes was 1.244 (0.32). The z score compared with age-matched controls was –0.637 (0.86) for study eyes (see Table 3).
Table 4.
CS Function of Study Eyes With Visual Acuity Better Than or Equal to 20/50.
| Study eye (n = 18) | Fellow eye (n = 18) | P | |
|---|---|---|---|
| Mean AULCSF ± SD | 0.997 ± 0.28 | 1.244 ± 0.32 | .0018a |
| Mean 1.5 cpd ± SD | 1.385 ± 0.21 | 1.495 ± 0.24 | .0361a |
| Mean 3.0 cpd ± SD | 1.359 ± 0.22 | 1.471 ± 0.28 | .0468a |
| Mean 6.0 cpd ± SD | 1.052 ± 0.28 | 1.243 ± 0.37 | .0321a |
| Mean 12.0 cpd ± SD | 0.36 ± 0.31 | 0.618 ± 0.47 | .0464a |
| Mean 18.0 cpd ± SD | 0.056 ± 0.14 | 0.248 ± 0.36 | .0159a |
| Study eye (n = 18) | Control (n = 45) | P | |
| Mean AULCSF ± SD | 0.997 ± 0.28 | 1.200 ± 0.25 | .0072a |
| Mean 1.5 cpd ± SD | 1.385 ± 0.21 | 1.460 ± 0.16 | .1048 |
| Mean 3.0 cpd ± SD | 1.359 ± 0.22 | 1.472 ± 0.21 | .0309a |
| Mean 6.0 cpd ± SD | 1.052 ± 0.28 | 1.215 ± 0.28 | .0085a |
| Mean 12.0 cpd ± SD | 0.36 ± 0.31 | 0.561 ± 0.34 | .0409a |
| Mean 18.0 cpd ± SD | 0.056 ± 0.14 | 0.174 ± 0.22 | .0116a |
Abbreviations: AULCSF, area under the CS function curve; cpd, cycles per degree.
a Indicates statistical significance.
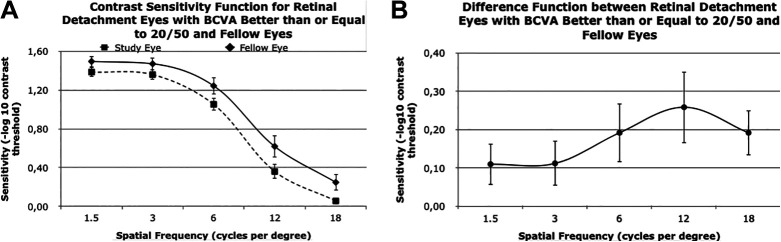
There was still a downward trend in CS vs spatial frequency both in the study and fellow eyes for participants with VA better than or equal to 20/50. Similarly, the CSF at each individual spatial frequency was lower for the study eye than the fellow eye, as it was with individuals whose BCVA was worse than 20/50 (Figure 2A). The largest decrease in sensitivity in the study eye compared with the fellow eye was at a spatial frequency of 12 cpd with a difference of 0.26 (Figure 2B).
Figure 2.
(A) CS function values for study eyes (ie, eyes with macula-off rhegmatogenous retinal detachment ) with best-corrected visual acuity (BCVA) of 20/50 or better and the corresponding fellow eyes for specific spatial frequency values. (B) Difference function between retinal detachment eyes and fellow eyes. Error bars indicate SE. Specific values are also provided in Table 4. (B) Difference between CS values in study eyes with BCVA of 20/50 or better and corresponding fellow eyes. Difference in values at 1.5, 3, 6, 12, and 18 cycles per degree were 0.11, 0.11, 0.19, 0.26, 0.19, respectively.
AULCSF of Study Eyes With VA 20/30 or Better vs Fellow Eyes and Controls
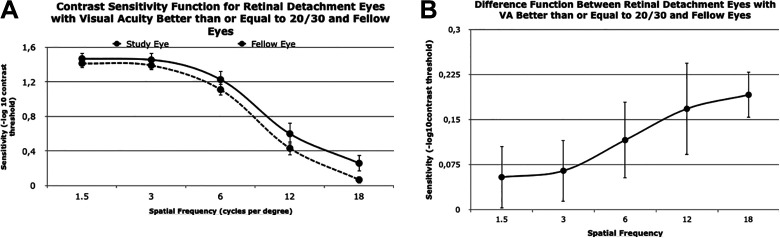
In the 15 study eyes with VA better than or equal to 20/30, the postoperative mean BCVA for the study eye was 0.050 (approximate Snellen, 20/22). The mean AULCSF was statistically different between study and fellow eyes (P = .02) and study and control eyes (P = .045). The z score for study eyes compared with age-matched controls was –0.513 (0.85). Similar to the 20/50 cutoff comparison, the CS values downtrended both for study and fellow eyes by spatial frequency for VA better than 20/30. The log CSF for the study eye was lower than the fellow eye for each spatial frequency (Figure 3A). The largest difference was 0.19 at a spatial frequency of 18 cpd (Figure 3B).
Figure 3.
(A) CS function values for study eyes (ie, eyes with macula-off rhegmatogenous retinal detachment) with a best-corrected visual acuity (BCVA) of 20/30 or better and corresponding fellow eyes for specific spatial frequency values. Error bars indicate SE. (B) Difference between CS values in study eyes with a best-corrected VA of 20/30 or better and corresponding fellow eyes. Difference in values at 1.5, 3, 6, 12, and 18 cycles per degree were 0.05, 0.06, 0.12, 0.17, and 0.19, respectively.
Comparing Contrast Curves Across All Groups
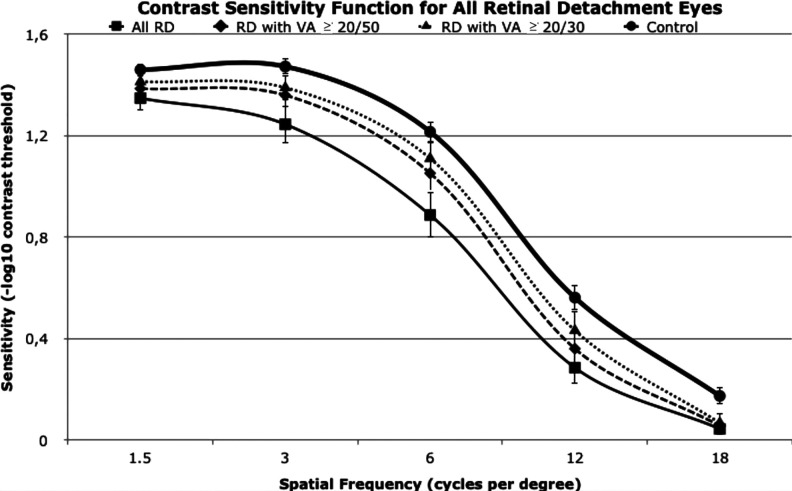
The CSF plots for all eyes and study eyes granularized by VA are presented together in Figure 4. The control eyes had the highest sensitivities per spatial frequency, followed by RD with VA 20/30 or better, RD with VA 20/50 or better, and all patients with RD combined. All plots follow a sinusoidal pattern.
Figure 4.
CS function values for all study eyes (ie, eyes with macula-off rhegmatogenous retinal detachment). RD indicates retinal detachment; VA, visual acuity.
Conclusions
We present an initial 2-center, prospective, observational study that used the qCSF active learning method to investigate CSF in macula-off RRD eyes in a sensitive and precise way that is applicable in our clinical practice. In our cohort we found that eyes with macula-off RRD after repair to have statistically significant CSF reduction across all spatial frequencies and the broad outcome measure, AULCSF, compared with healthy fellow eyes and age-matched controls. We suspect that CSF deficits occurred as a result of disruption of photoreceptor–retinal pigment epithelium interactions caused by outer retinal ischemia and mechanical photoreceptor loss during the detachment of the macula and/or during the surgical repair of the RRD.
Unlike older CS tests such as the Pelli-Robson chart 25 that use coarse quantization and sampling operating in only 1 spatial frequency, 26 the qCSF active learning method measures the CSF in many different spatial frequencies, 34 allowing for identification of disproportionate reductions at specific spatial frequencies or global changes specific to the type of retinal disease.
Multivariable linear regression analysis adjusted for age, sex, and lens status revealed a significant decrease in AULCSF and in CS with increasing logMAR VA across all spatial frequencies except the highest spatial frequency (18.0 cpd). Compared with the fellow healthy eyes, CS in macula-off RRD eyes was found to be reduced in all spatial frequencies, with the largest difference detected at 6 cpd. Of note, the CS threshold at 6 cpd has been found to be the best predictor of road sign and object detection and identification. 33 This builds on prior reports that have suggested CS to be better correlated with real-world everyday activities and subjective perception of visual function. 13 -22
Compared with VA, CSF seems to correlate better with real-world everyday activities including mobility, 13 target and face identification, 14 driving, 15,16 walking, 17 and reading, 18,19 as well as subjectively perceived visual impairment. 20 -22 Further, CS has been shown to be impaired earlier in the course of neurodegenerative ocular pathologies when VA is still unaffected, 7,21 the latter often underestimating the onset and/or severity of visual impairment. 36 -38
Using the 25-item National Eye Institute Visual Function Questionnaire in patients with RRD, 39 Okamoto et al reported vision-related quality of life to be significantly correlated with postoperative CS measured by preprinted letter charts, yet no correlation was found between vision-related quality of life and postoperative VA. 40
Several previous reports have shown reduced CS in patients with RRD using preprinted letter charts. 41 -43 Despite measuring CS using methods with poor range and resolution for sampling target contrast and frequency 28 -30 and poor test–retest reliability, 29,31,32 those reports demonstrated the detrimental effect of RD on CS.
Using preprinted letter charts, Okamoto and colleagues showed that in macula-on RD eyes, CS decreased significantly from preoperative values following surgery despite the BCVA remaining stable. 41 Using similar methods, Ozgür and Esgin reviewed 9 patients following–scleral buckle RD repair with BCVA better than log MAR 0.8 and found CS in RD eyes to be reduced compared with the healthy fellow eyes, yet to a nonstatistically significant degree. 43
Kawamura et al demonstrated lower CS in 36 macula-off RRD eyes with a postoperative BCVA of greater than 1.0 compared with macula-on RRD eyes following surgery. 42 The latter group used a Takagi glare tester CGT-2000 to measure CS, which has been shown to have relatively poor accuracy and repeatability. 44
In clinical practice, a visual function metric more sensitive to subtle changes than VA would be particularly valuable in detecting subtle subjective visual impairment noted by the patient and subsequently better guide our clinical judgment on initiating and evaluating our therapeutic interventions. CS has been shown to be impaired earlier in the course of neurodegenerative ocular pathologies when VA is still unaffected, 7,23 whereas VA has been shown to often underestimate the onset and/or severity of visual impairment. 36 -38 In our subgroup analysis of the 15 macula-off RRD eyes with BCVA better than or equal to 20/30, the mean CSF was significantly reduced compared with fellow eyes (P = .02) and controls (P = .045).
VA, although consistently the predominant visual function outcome in clinical trials, may not be the ideal functional end point. Future RRD trials for novel therapeutic agents, such as neuroprotective agents, may benefit from CSF as a primary or secondary functional end point. An end point with reduced test–retest variability will allow for detection of smaller critical differences between treatment arms and recruitment of smaller sample sizes. CSF measured with the active learning qCSF method demonstrates high sensitivity and precision at the same time, 35 emerging as a promising visual function end point.
The qCSF has been used so far to measure CSF in several clinical populations, including those with amblyopia, 45,46 multiple sclerosis, 22 glaucoma, 47 dry age-related macular degeneration, 48 central serous chorioretinopathy, 49 early diabetic retinopathy, 50 retinal vein occlusion, 51 and aging. 52 The present study is the first to our knowledge to report on qCSF in macula-off RRD.
The strengths of this study include the prospective enrollment of patients who met the eligibility criteria within 2 university-based practices with a standardized protocol for in-clinic CS testing. A limitation of this study, besides the relatively small sample size, is that even with a standardized testing protocol, not all patients were tested at the same interval post RD repair. An additional longitudinal study with standardized follow-up time points may identify more subtle fluctuations of the CSF over the course of recovery from RD.
In conclusion, in our initial report on qCSF in macula-off RRD eyes, CSF was found to be significantly reduced across all spatial frequencies even in the subselection of eyes with VA better or equal to 20/30. CSF measured with the qCSF active learning method seems to be a promising visual function end point that can provide the sensitive and precise signals required to assess visual function in patients with macula-off RRD in clinical practice and emerges as a potential novel end point for future RD studies. Further studies with larger samples and longitudinal follow-up of RD eyes are warranted to validate our results.
Footnotes
Authors’ Note: This work was presented in part at the 2017 American Society of Retina Specialists annual meeting August 11 to 15, 2017, in Boston, Massachusetts, USA.
Ethical Approval: This study is part of an institutional review board–approved protocol compliant with the Health Insurance Portability and Accountability Act (HIPAA) at the University of Michigan W.K. Kellogg Eye Center and Massachusetts Eye and Ear, and it adhered to the tenets of the Declaration of Helsinki.
Statement of Informed Consent: Written informed consent was obtained from all patients before participation in the study.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J.B.M. has served as a consultant for Alcon, Allergan, Zeiss, Heidelberg, and Genentech. The other authors have nothing to declare.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Vitreoretinal Surgery Foundation Research Award (to M.T.).
ORCID iD: Eun Y. Choi, MD  https://orcid.org/0000-0002-8774-2706
https://orcid.org/0000-0002-8774-2706
References
- 1. Schaal S, Sherman MP, Barr CC, Kaplan HJ. Primary retinal detachment repair: comparison of 1-year outcomes of four surgical techniques. Retina. 2011;31(8):1500–1504. doi:10.1097/IAE.0b013e31820d3f55 [DOI] [PubMed] [Google Scholar]
- 2. Wilkinson CP, Bradford RH, Jr. Complications of draining subretinal fluid. Retina. 1984;4(1):1–4. doi:10.1097/00006982-198400410-00001 [DOI] [PubMed] [Google Scholar]
- 3. Burton TC. Recovery of visual acuity after retinal detachment involving the macula. Trans Am Ophthalmol Soc. 1982;80:475–497. [PMC free article] [PubMed] [Google Scholar]
- 4. Ross WH. Visual recovery after macula-off retinal detachment. Eye (Lond). 2002;16(4):440–446. doi:10.1038/sj.eye.6700192 [DOI] [PubMed] [Google Scholar]
- 5. Arden GB. The importance of measuring contrast sensitivity in cases of visual disturbance. Br J Ophthalmol. 1978;62(4):198–209. doi:10.1136/bjo.62.4.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marmor MF. Contrast sensitivity versus visual acuity in retinal disease. Br J Ophthalmol. 1986;70(7):553–559. doi:10.1136/bjo.70.7.553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jindra LF, Zemon V. Contrast sensitivity testing: a more complete assessment of vision. J Cataract Refract Surg. 1989;15(2):141–148. doi:10.1016/s0886-3350(89)80002-1 [DOI] [PubMed] [Google Scholar]
- 8. Ginsburg AP. Contrast sensitivity and functional vision. Int Ophthalmol Clin. 2003;43:15. doi:10.1097/00004397-200343020-00004 [DOI] [PubMed] [Google Scholar]
- 9. Moschos MM, Nitoda E. The role of mf-ERG in the diagnosis and treatment of age-related macular degeneration: electrophysiological features of AMD. Semin Ophthalmol. 2018;33(4):461–469. doi:10.1080/08820538.2017.1301496 [DOI] [PubMed] [Google Scholar]
- 10. Roh M, Laíns I, Shin HJ, et al. Microperimetry in age-related macular degeneration: association with macular morphology assessed by optical coherence tomography. Br J Ophthalmol. 2019;103(12):1769–1776. doi:10.1136/bjophthalmol-2018-313316 [DOI] [PubMed] [Google Scholar]
- 11. Laíns I, Miller JB, Park DH, et al. Structural changes associated with delayed dark adaptation in age-related macular degeneration. Ophthalmology. 2017;124(9):1340–1352. doi:10.1016/j.ophtha.2017.03.061 [DOI] [PubMed] [Google Scholar]
- 12. Keane PA, Patel PJ, Ouyang Y, et al. Effects of retinal morphology on contrast sensitivity and reading ability in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51(11):5431–5437. doi:10.1167/iovs.09-4846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marron JA, Bailey IL. Visual factors and orientation-mobility performance. Am J Optom. Physiol Opt. 1982;59(5):413–426. doi:10.1097/00006324-198205000-00009 [DOI] [PubMed] [Google Scholar]
- 14. Owsley C, Sloane ME. Contrast sensitivity, acuity, and the perception of ‘real-world’ targets. Br J Ophthalmol. 1987;71(10):791–796. doi:10.1136/bjo.71.10.791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Freeman EE, Muñoz B, Turano KA, West SK. Measures of visual function and time to driving cessation in older adults. Optom Vis Sci. 2005;82(8):765–773. doi:10.1097/01.opx.0000175008.88427.05 [DOI] [PubMed] [Google Scholar]
- 16. Owsley C, McGwin G. Vision and driving. Vision Res. 2010;50(23):2348–2361. doi:10.1016/j.visres.2010.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Geruschat DR, Turano KA, Stahl JW. Traditional measures of mobility performance and retinitis pigmentosa. Optom Vis Sci. 1998;75(7):525–537. doi:10.1097/00006324-199807000-00022 [DOI] [PubMed] [Google Scholar]
- 18. Owsley C. Contrast sensitivity. Ophthalmol Clin North Am. 2003;16(2):171–178. doi:10.1016/s0896-1549(03)00003-8 [DOI] [PubMed] [Google Scholar]
- 19. Brown B. Reading performance in low vision patients: relation to contrast and contrast sensitivity. Am J Optom Physiol Opt. 1981;58(3):218–226. doi:10.1097/00006324-198103000-00006 [DOI] [PubMed] [Google Scholar]
- 20. Lennerstrand G, Ahlström CO. Contrast sensitivity in macular degeneration and the relation to subjective visual impairment. Acta Ophthalmol. 1989;67(3):225–233. doi:10.1111/j.1755-3768.1989.tb01863.x [DOI] [PubMed] [Google Scholar]
- 21. West SK, Rubin GS, Broman AT, et al. How does visual impairment affect performance on tasks of everyday life? The SEE project. Arch Ophthalmol. 2002;120(6):774–780. doi:10.1001/archopht.120.6.774 [DOI] [PubMed] [Google Scholar]
- 22. Stellmann JP, Young KL, Poüttgen J, Dorr M, Heesen C. Introducing a new method to assess vision: computer-adaptive contrast-sensitivity testing predicts visual functioning better than charts in multiple sclerosis patients. Mult Scler J Exp Transl Clin. 2015;1:2055217315596184. doi:10.1177/2055217315596184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Woods RL, Wood JM. The role of contrast sensitivity charts and contrast letter charts in clinical practice. Clin Exp Optom. 1995;78(2):43–57. doi.org/10.1111/j.1444-0938.1995.tb00787.x [Google Scholar]
- 24. Kelly DH, Savoie RE. A study of sine-wave contrast sensitivity by two psychophysical methods. Percept Psychophys. 1973;14(2):313–318. doi:10.3758/BF03212397 [Google Scholar]
- 25. Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clin Vis Sci. 1988;2(3):187–199. [Google Scholar]
- 26. Thayaparan K, Crossland MD, Rubin GS. Clinical assessment of two new contrast sensitivity charts. Br J Ophthalmol. 2007;91(6):749–752. doi:10.1136/bjo.2006.109280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alexander KR, Barnes CS, Fishman GA. Characteristics of contrast processing deficits in X-linked retinoschisis. Vis Res. 2005;45(16):2095–2107. doi:10.1016/j.visres.2005.01.037 [DOI] [PubMed] [Google Scholar]
- 28. Richman J, Spaeth GL, Wirostko B. Contrast sensitivity basics and a critique of currently available tests. J Cataract Refract Surg. 2013;39(7):1100–1106. doi:10.1016/j.jcrs.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 29. Pesudovs K, Hazel CA, Doran RML, Elliott DB. The usefulness of Vistech and FACT contrast sensitivity charts for cataract and refractive surgery outcomes research. Br J Ophthalmol. 2004;88(1):11–16. doi:10.1136/bjo.88.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bhren J, Terzi E, Bach M, Wesemann W, Kohnen T. Measuring contrast sensitivity under different lighting conditions: comparison of three tests. Optom Vis Sci. 2006;83(5):290–298. doi:10.1097/01.opx.0000216100.93302.2d [DOI] [PubMed] [Google Scholar]
- 31. Elliott DB, Bullimore MA. Assessing the reliability, discriminative ability, and validity of disability glare tests. Invest Ophthalmol Vis Sci. 1993;34(1):108–119. [PubMed] [Google Scholar]
- 32. Kelly SA, Pang Y, Klemencic S. Reliability of the CSV-1000 in adults and children. Optom Vis Sci. 2012;89(8):1172–1181. doi:10.1097/OPX.0b013e318264097b [DOI] [PubMed] [Google Scholar]
- 33. Lesmes LA, Lu ZL, Baek J, Albright TD. Bayesian adaptive estimation of the contrast sensitivity function: the quick CSF method. J Vis. 2010;10(3):1–21. doi:10.1167/10.3.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dorr M, Wille M, Viulet T, Sanchez E, Bex PJ, Lu Z. Next-generation vision testing: the quick CSF. Curr Dir Biomed Eng. 2015;1(1):1–4. doi:10.1515/cdbme-2015-0034 [Google Scholar]
- 35. Hou F, Lesmes LA, Kim W, et al. Evaluating the performance of the quick CSF method in detecting contrast sensitivity function changes. J Vis. 2016;16(6):18. doi:10.1167/16.6.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Preti RC, Ramirez LMV, Pimentel SLG, et al. Effect of a single intravitreal bevacizumab injection on contrast sensitivity and macular thickness in eyes with macular edema from central retinal vein occlusion: a prospective, nonrandomized, three-month follow-up study. Ophthalmic Res. 2014;51(3):140–145. doi:10.1159/000357737 [DOI] [PubMed] [Google Scholar]
- 37. Murugappan M, Vayalil J, Bade A, Bittner AK. Reliability of quick contrast sensitivity function testing in adults without ocular disease and patients with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 57(12):616–616. [Google Scholar]
- 38. Ramulu PY, Dave P, Friedman DS. Precision of contrast sensitivity testing in glaucoma. Invest Ophthalmol Vis Sci. 56(7):2225–2225. [Google Scholar]
- 39. Mangione CM, Lee PP, Gutierrez PR, Spitzer K, Berry S, Hays RD; National Eye Institute Visual Function Questionnaire Field Test Investigators. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119(7):1050–1058. doi:10.1001/archopht.119.7.1050 [DOI] [PubMed] [Google Scholar]
- 40. Okamoto F, Okamoto Y, Hiraoka T, Oshika T. Vision-related quality of life and visual function after retinal detachment surgery. Am J Ophthalmol. 2008;146(1):85–90. doi:10.1016/j.ajo.2008.02.011 [DOI] [PubMed] [Google Scholar]
- 41. Okamoto F, Sugiura Y, Okamoto Y, et al. Changes in contrast sensitivity after surgery for macula-on rhegmatogenous retinal detachment. Am J Ophthalmol. 2013;156(4):667–672. doi:10.1016/j.ajo.2013.05.017 [DOI] [PubMed] [Google Scholar]
- 42. Kawamura H, Fujikawa M, Sawada O, Sawada T, Saishin Y, Ohji M. Contrast sensitivity after pars plana vitrectomy: comparison between macula-on and macula-off rhegmatogenous retinal detachment. Ophthalmic Res. 2016;56(2):74–78. doi:10.1159/000445210 [DOI] [PubMed] [Google Scholar]
- 43. Ozgür S, Esgin H. Functional assessment of reattached macula in nine cases with excellent Snellen acuities. Eye (Lond). 2007;21(4):503–505. doi:10.1038/sj.eye.6702241 [DOI] [PubMed] [Google Scholar]
- 44. Konrad P. Takagi glare tester CGT1000 for contrast sensitivity and glare testing in normal individuals and cataract patients. J Refract Surg. 2007;23(5):492–498. doi:10.3928/1081-597X-20070501-13 [DOI] [PubMed] [Google Scholar]
- 45. Hou F, Huang CB, Lesmes L, et al. qCSF in clinical application: efficient characterization and classification of contrast sensitivity functions in amblyopia. Invest Ophthalmol Vis Sci. 2010;51(10): 5365–5377. doi:10.1167/iovs.10-5468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jia W, Zhou J, Lu ZL, Lesmes LA, Huang CB. Discriminating anisometropic amblyopia from myopia based on interocular inhibition. Vision Res. 2015;114:135–141. doi:10.1016/j.visres.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lin S, Mihailovic A, West SK, et al. Predicting visual disability in glaucoma with combinations of vision measures. Transl Vis Sci Technol. 2018;7(2):22–22. doi:10.1167/tvst.7.2.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lesmes LA, Jackson ML, Bex P. Visual function endpoints to enable dry AMD clinical trials. Drug Discov Today. 2013;10(1):e43–e50. doi:10.1016/j.ddstr.2012.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Marmalidou A, Kim EL, Silverman R, et al. A novel contrast sensitivity test as a new measure of visual function in central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2018;59(9):3126. [Google Scholar]
- 50. Joltikov KA, de Castro VM, Davila JR, et al. Multidimensional functional and structural evaluation reveals neuroretinal impairment in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2017;58(6):BIO277–BIO290. doi:10.1167/iovs.17-21863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Silverman RF, Kasetty M, Vingopoulos F, et al. Measuring contrast sensitivity function with active learning in retinal vein occlusion: a new endpoint of visual function. Ophthalmic Sure Lasers Imaging Retina. 2020;51(7):392–400. doi:10.3928/23258160-20200702-04 [DOI] [PubMed] [Google Scholar]
- 52. Yan FF, Hou F, Lu ZL, Hu X, Huang CB. Efficient characterization and classification of contrast sensitivity functions in aging. Sci Rep. 2017;7(1):5045. doi:10.1038/s41598-017-05294-0 [DOI] [PMC free article] [PubMed] [Google Scholar]