Abstract
Crude polysaccharides from Spirulina platensis (SP) were isolated by maceration with a hot alkali solution and further fractionated by DEAE-52 cellulose and Sephadex G-100 chromatography into two purified fractions PSP-1 and PSP-2. The monosaccharide composition analysis indicated that SP was mainly composed of rhamnose and glucose, while PSP-1 and PSP-2 were composed only of glucose. The composition analysis of PSP-1 and PSP-2 by HPLC, FT-IR, and NMR showed that PSP-1 and PSP-2 were branching dextran, and their structures were (1 → 4)-linked-α-D-Glcp as the main chain, and C-6 replaced the single α-D-Glcp as the linear structure of the branch chain. The glucans (SP/PSP-1/PSP-2) can significantly improve the phagocytic ability of macrophages, enhance iNOS activity, promote NO production, and increase IL-6 mRNA expression, so they may possess certain immunomodulatory activity.
1. Introduction
Spirulina platensis (S. platensis) is an ancient lower prokaryotic plant that belongs to the Cyanophyta category, Cyanophycean, and Oscillatoria.1S. platensis, which is rich in protein, polysaccharides, polyunsaturated fatty acids, vitamins, and phenols, has a great application value in the nutritious diet.2−4 In recent years, S. platensis has provided an important source for biomedicine.2,5 According to research reports, S. platensis-related products exhibit excellent biological activity and pharmaceutical properties, such as anti-inflammatory, antidiabetic, antioxidant, antitumor, and hepatoprotective activities, having broad prospects in improving physical fitness and disease prevention.6−9
Polysaccharides are a general term for natural macromolecular carbohydrates and their derivatives, usually composed of monosaccharide units bonded together by glycosidic linkages.10 The number of these monosaccharide units is at least greater than 10, and their molecular weight usually ranges from tens of thousands to millions. As we all know that the structure usually determines the character, and the structure of polysaccharides also determines their properties and functions. The types of polysaccharide monomers and their polymerization mode, as well as their molecular weight and much more, all affect their physical, chemical, and structural characteristics and further affect how they function. Since the cultivation methods of raw materials, the source, and the extraction methods of polysaccharides are different, the structure, as well as molecular weight of the obtained polysaccharides, is also different, which ultimately contributes to their function.11 Modern pharmacological studies have shown that polysaccharides have multiple biological activities, such as antiviral activity against herpes simplex virus type 1 (HSV-1),12 inhibitory effects on corneal neovascularization,13 increased immunomodulating activity,14 and also anticoagulant activity mediated by heparin cofactor II.15 As a result, it is essential to clarify the precise structures of polysaccharides. Although there are few studies on the specific structure and function of S. platensis polysaccharides, many studies are still worthy of attention because of the different structures and functions.
Therefore, in this study, we aimed to better understand and elucidate the structural characteristics and immunomodulatory activities of the polysaccharides from S. platensis. We use isoelectric precipitation combined with an anion exchange column as a new purification method. Three polysaccharides designed as SP, PSP-1, and PSP-2 were isolated and purified from S. platensis. We investigated monosaccharide composition through HPLC, and further, high-performance gel permeation chromatography (HPGPC), Fourier transform infrared spectroscopy (FT-IR), and nuclear magnetic resonance (NMR) analysis were used to demonstrate the conformation and characteristics of the polysaccharides. Finally, we measured the effects of the polysaccharides on RAW 264.7 cell proliferation, NO production, and related cytokine expression to assess their immunomodulatory activity. This work will provide useful information on better isolating the polysaccharides from S. platensis and analyzing their advanced structural characteristics and will also help to further study the relationship between the structure and biological activity.
2. Results and Discussion
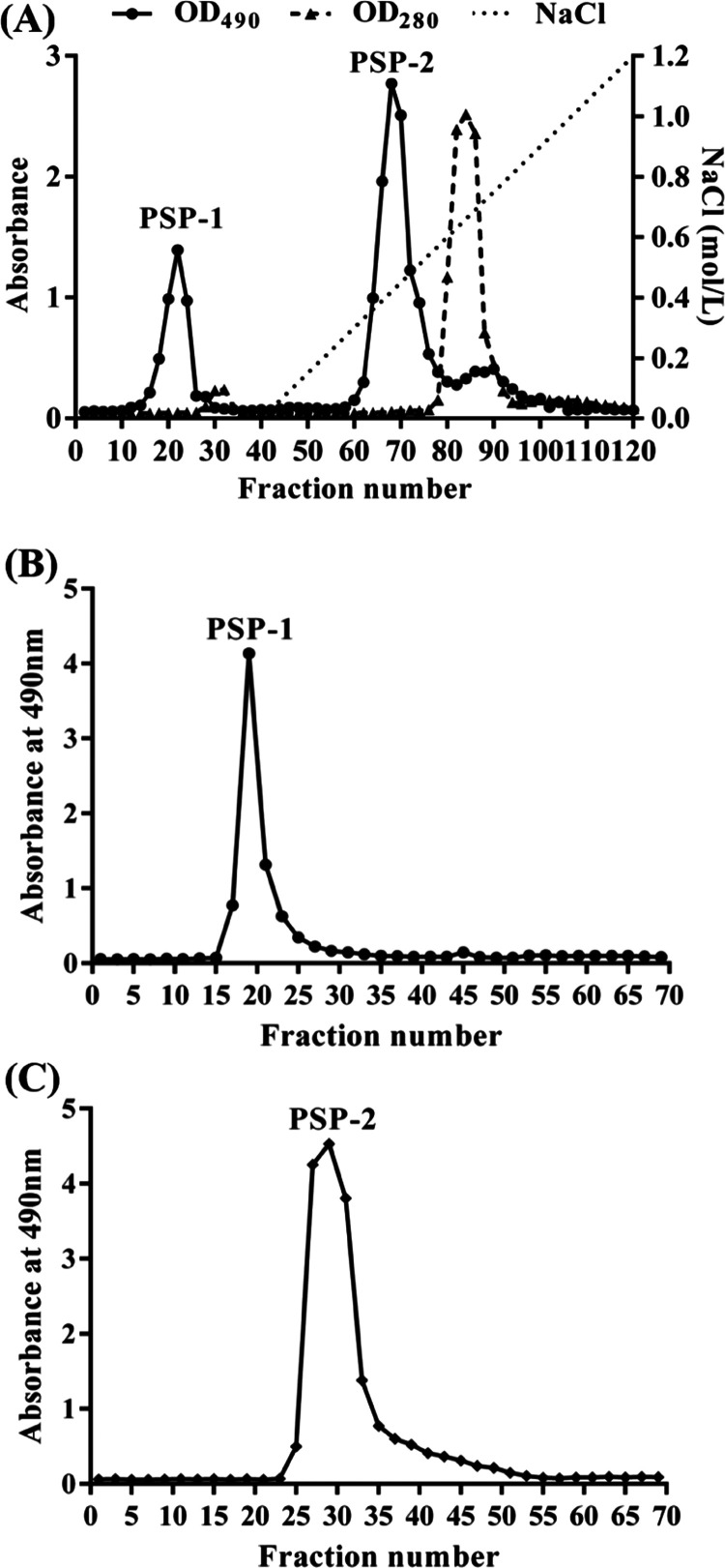
2.1. Isolation, Purification, and Composition Analysis of SP, PSP-1, and PSP-2
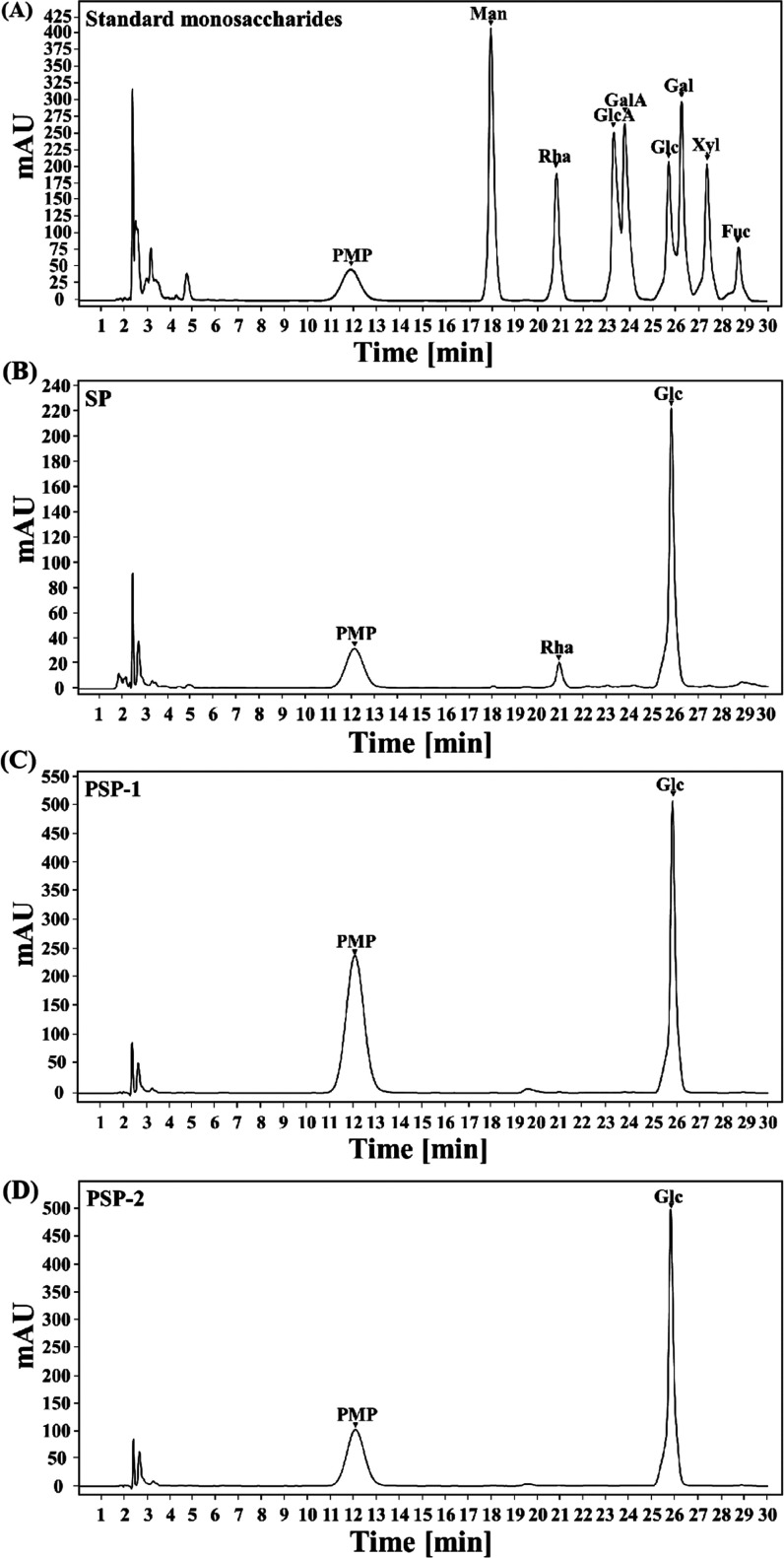
The crude polysaccharide SP was obtained from S. platensis by maceration with a hot alkali solution, deproteinization with a cation exchange chromatography column, and precipitation with ethanol, with a yield of 7.55% (w/w) based on the dried powder of S. platensis. SP was fractionated on a DEAE-52 cellulose anion exchange column, and the profile eluted by deionized water and a NaCl solution is presented in Figure 1A. After fractionation, two fractions (PSP-1 and PSP-2) were obtained from an aqueous NaCl gradient (0–1 M). After desalting, lyophilizing, and redissolving the two fractions, they were further eluted on a Sephadex G-100 column severally with deionized water. As shown in Figure 1B,C, the independent elution profiles of both PSP-1 and PSP-2 have only one symmetrical peak, indicating that they are relatively pure single polymers and have uniform molecular weights. However, there was a difference in the order of the collection tubes of PSP-1 and PSP-2. On the one hand, it may indicate that the molecular weights of the two fractions are different, which needed to be explained by the determination of weight determination. On the other hand, it is possible that the elution of PSP-1 and PSP-2 was carried out as two independent experiments, and the loading quantity of each sample was also different, so it may cause differences in the collection order. Two fractions were collected separately, enriched, and freeze-dried. The polysaccharide content was detected using the phenol–sulfuric acid method. The general physicochemical properties of SP, PSP-1, and PSP-2 are shown in Table 1. The contents of total carbohydrate, protein, and sulfate in SP were 73.80, 1.93, and 2.07%, respectively. The contents of total carbohydrate, protein, and sulfate in PSP-1 were 96.54, 0.17, and 0.57%, respectively. The contents of total carbohydrate, protein, and sulfate in PSP-1 were 95.10, 0.38, 1.05%, respectively. The content analysis showed that there are more impurities in the crude SP samples, and the purity of the polysaccharides in the samples PSP-1 and PSP-2 is higher. Compared with other studies, the polysaccharides we obtained were of relatively high quality. Monosaccharide composition analysis is shown in Figure 2. Figure 2A shows the HPLC result of each monosaccharide standard. By comparing with the HPLC of standard compounds, it was found that SP (Figure 2B) was mainly composed of rhamnose and glucose, while PSP-1 (Figure 2C) and PSP-2 (Figure 2D) were composed only of glucose, yet none of the components contained uronic acid. These results suggested that PSP-1 and PSP-2 were highly purified, water-soluble neutral glucans. However, in the previous reports, the Chaiklahan team had reported the presence of glucose, ribose, galactose, mannose, and rhamnose in the polysaccharides from S. platensis.(16) On the other hand, Periyannan Rajasekara et al. used high-temperature immersion in the sodium hydroxide solution to extract polysaccharides from S. platensis and found that the polysaccharides were composed of glucose, xylose, rhamnose, mannose, galactose, and fucose.17 It seemed that the different extraction methods of polysaccharides and their origins may cause diversity in their monosaccharide composition and molecular weight.
Figure 1.
(A) Elution profile of SP on the DEAE-52 chromatography column with a gradient of NaCl solution (0–1 M); (B) elution profile of PSP-1 on the Sephadex G-100 gel chromatography column with distilled water; and (C) elution profile of PSP-2 on the Sephadex G-100 gel chromatography column with distilled water.
Table 1. Basic Physical and Chemical Properties of S. platensis SP, PSP-1, and PSP-2a.
| content
(%) |
|||
|---|---|---|---|
| components | SP | PSP-1 | PSP-2 |
| carbohydrate | 73.80 ± 0.98 | 96.54 ± 0.18 | 95.10 ± 0.23 |
| protein | 1.93 ± 0.021 | 0.17 ± 0.015 | 0.38 ± 0.026 |
| sulfate | 2.07 ± 0.31 | 0.57 ± 0.14 | 1.05 ± 0.19 |
Results are expressed as mean ± standard error (n = 3).
Figure 2.
HPLC chromatograms of PMP derivatives of 10 standard monosaccharides (A) and component monosaccharides of the purified fractions: SP (B), PSP-1 (C), and PSP-2(D). Peaks: mannose (Man), rhamnose (Rha), glucuronic acid (GluA), galacturonic acid (GalA), glucose (Glc), galactose (Gal), xylose (Xyl), and fucose (Fuc).
2.2. Determination of Molecular Weight
The homogeneity and molecular weight of the three purified polysaccharides were analyzed by HPGPC. As shown in Figure 3A,B, each purified polysaccharide had a single and symmetrical peak in the HPGPC elution profile, indicating that all of them were homogeneous polysaccharides. The average molecular weights of PSP-1 and PSP-2 were 93.856 and 113.736 kDa, respectively.
Figure 3.
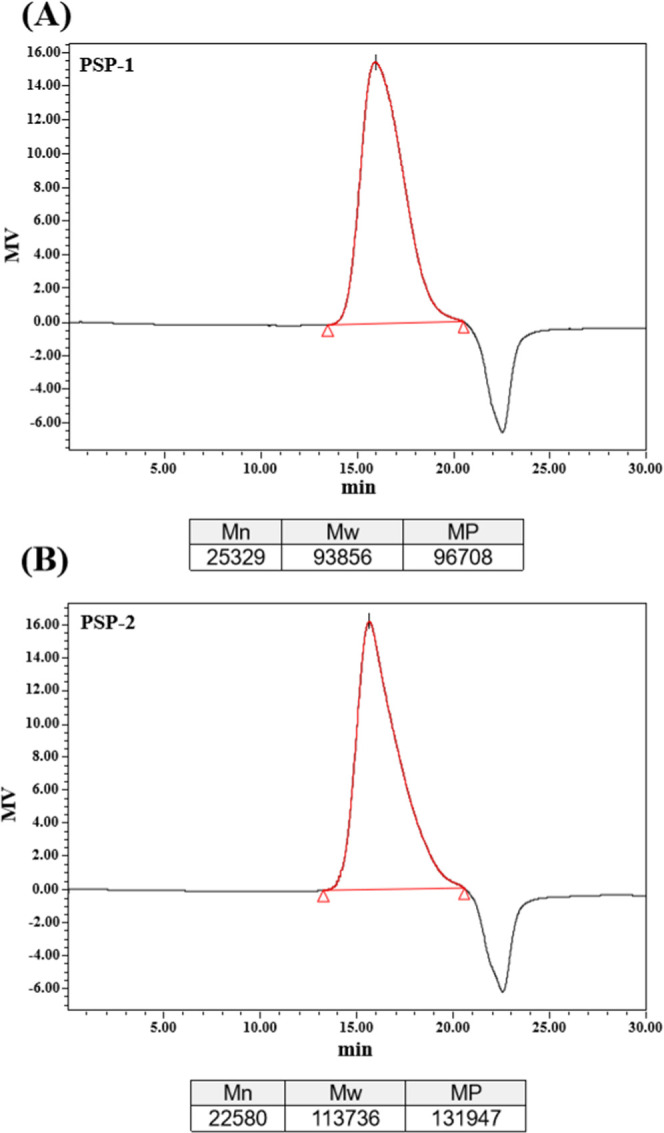
Molecular weight distributions of PSP-1 (A) and PSP-2 (B).
2.3. FT-IR Spectra Analysis of PSP-1 and PSP-2
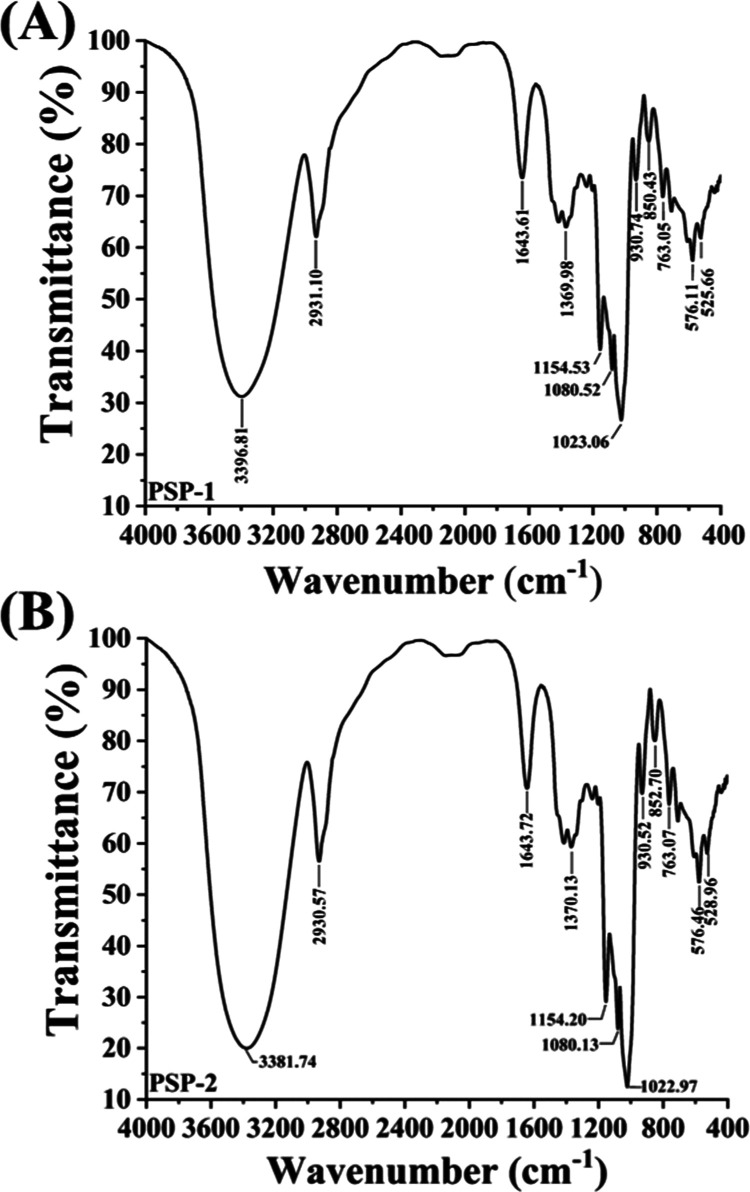
The FT-IR spectra of pure PSP-1 and PSP-2 fractions are shown in Figure 4A,B. As can be seen, PSP-1 and PSP-2 have greater absorption at 3396.81 and 3391.74 cm–1, which was due to the stretching vibration of O–H.18 The absorption peaks at 2931.10 and 2930.57 cm–1 were caused by the stretching vibration of the C–H bond.19 The absorption peaks at 1600–1650 cm–1 are due to the bound water.20 Relatively high absorbance values were at around 1200–1000 cm–1, which were mainly caused by the C–O stretching vibration, C–C stretching vibration, and C–OH bending vibration of polysaccharides. Bands of this region (1200–1000 cm–1) are typical of polysaccharides. The bands in this region (1200–1000 cm–1) are typical for polysaccharides.21 The peak near 850 cm–1 indicates the presence of α-glycosidic bonds in each component of the spirulina polysaccharide.22 The absorption near 763 cm–1 may be due to the presence of the D-glucopyranose ring.23 The absence of the peak between 1700 and 1775 cm–1 suggests that neither glucuronic acid nor diacetyl ester was present.24 Therefore, by combining the analysis results of infrared spectroscopy and the determination results of monosaccharide composition above about the polysaccharide structure of PSP-1 and PSP-2, we can preliminarily confirm that PSP-1 and PSP-2 were D-glucans connected by the α-glycosidic bond.
Figure 4.
FI-IR spectra of PSP-1 (A) and PSP-2 (B).
2.4. 1H NMR, 13C NMR, and 2D NMR Analysis
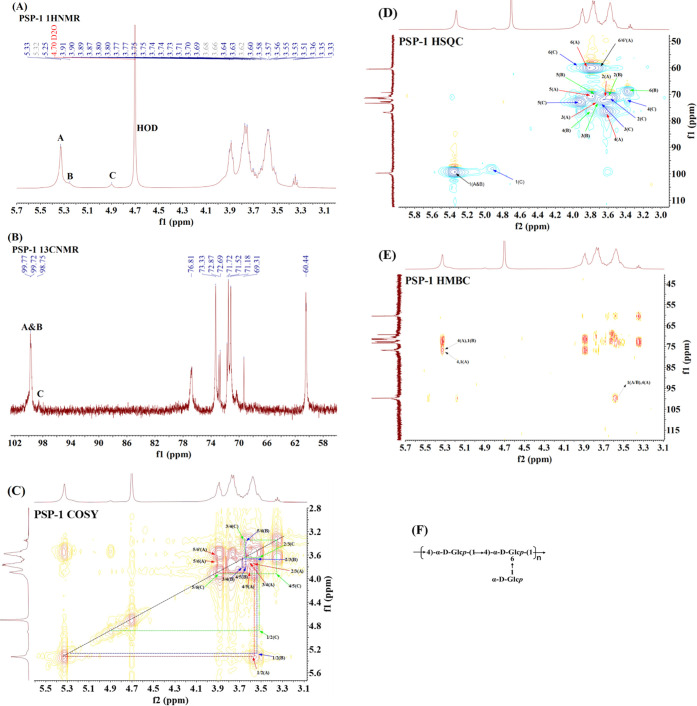
The NMR spectra of PSP-1 and PSP-2 are shown in Figures 5 and S1. Because PSP-1 and PSP-2 have similar spectral behaviors, PSP-1 was used as an example for structural analysis. As shown in Figure 5A, there are three main peaks in the anomeric hydrogen region (4.4–5.6 ppm), which indicates that there are mainly three types of glucose residues in PSP-1, labeled as A, B, and C. The chemical shifts of the anomeric protons of residues A, B, and C are 5.33, 5.25, and 4.90 ppm, respectively. In the anomalous region of the HSQC spectrum, the corresponding resonances of the 13C signals of residues A, B, and C are 99.77, 99.72, and 98.75 ppm, respectively, based on the C-1/H-1 crossover peak (Figure 5D). In 13C NMR (Figure 5B), the chemical shifts of the anomeric carbon of the three residues were marked. The detailed NMR analysis of A, B, and C residues is as follows, and the major chemical shifts of 1H and 13C signals of PSP-1 and PSP-2 are shown in Table 2.
Figure 5.
1H spectrum (A); 13C NMR spectrum (B); COSY spectrum (C); HSQC spectrum (D); and HMBC spectrum (E) of PSP-1. (F) The predicted structural repeating unit of the polysaccharides.
Table 2. Chemical Shifts of the Main Residues of Polysaccharides from S. platensis.
| |
chemical
shifts, δ (ppm) |
||||||
|---|---|---|---|---|---|---|---|
| glycosidic linkage | H-1/C-1 | H-2/C-2 | H-3/C-3 | H-4/C-4 | H-5/C-5 | H-6/C-6 | |
| A | →4)-α-D-Glcp-(1→ | 5.33 | 3.56 | 3.74 | 3.58 | 3.89 | 3.77/3.63 |
| 99.77 | 71.72 | 72.87 | 76.81 | 71.72 | 60.44 | ||
| B | →4-6)-α-D-Glcp-(1→ | 5.25 | 3.55 | 3.69 | 3.80 | 3.60 | 3.36 |
| 99.72 | 71.69 | 72.69 | 77.78 | 72.69 | 69.31 | ||
| C | α-D-Glcp-(1→ | 4.90 | 3.53 | 3.64 | 3.35 | 3.91 | 3.89 |
| 98.75 | 72.87 | 73.33 | 71.52 | 72.69 | 60.12 | ||
For residue A, because of the cross peaks of H-1/H-2, H-2/H-3, H-3/H-4, H-4/H-5, and H-5/H-6 in the COSY spectrum (Figure 5C), the chemical shifts of H-2, H-3, H-4, H-5, H-6, and H-6′ are 3.56, 3.74, 3.58, 3.89, 3.77, and 3.63 ppm, respectively. According to the correlation of C/H in the nonheterotopic region of the HSQC spectrum, the corresponding resonance of 13C of C-2, C-3, C-4, C-5, and C-6 of residue A was 71.72, 72.87, 76.81, 71.72, and 60.44 ppm, respectively. The downfield chemical shift of C-4 at 76.81 ppm proved the presence of (1 → 4) linkages. These assignments were also supported by previous reports.25−28 Therefore, residue A is considered to be →4)-α-D-Glcp-(1→.
Likewise, for residue B, H-2 was related to H-1 (5.28 ppm) in the COSY spectrum (Figure 5C), so the resonance of H-2 was 3.55 ppm. Based on the C-2/H-2 crossover peak at 71.69/3.55, the chemical shift of C-2 was derived from the HSQC spectrum (Figure 5D) at 71.69 ppm. The chemical shifts of 1H NMR of residues B were determined by COSY and HSQC spectra of H-3, H-4, H-5, H-6, and 13C NMR of C-3, C-4, C-5, and C-6, respectively. Because of the downfield spin system of both C-4 (77.78 ppm) and C-6 (69.31 ppm), residue B was assigned to →4-6)-α-D-Glcp-(1→. This result is consistent with relevant literature reports.27,28
In the case of residue C, the same derivation and distribution method as residues A and B were used to obtain C-1/H-1(98.75/4.90 ppm), C-2/H-2(72.87/3.53), C-3/H-3(73.33/3.64), C-4/H-4(71.52/3.35 ppm), C-5/H-5 (72.69/3.91 ppm), and C-6/H-6 (60.12/3.89 ppm) from the COSY (Figure 5C) and HSQC spectra (Figure 5D). This indicated that residue C was assigned to α-D-Glcp-(1→ by comparing the proton and carbon chemical shifts with the literature reports.26,27,29
The HMBC experiment was carried out to enable us to identify glycosidic linkages between sugar residues, as shown in Figure 5E. Examining the cross peaks of both anomeric 1H and 13C of each sugar residue could help to identify the sequence of residues in the polysaccharide. Cross peaks between H-1 (5.33 ppm) of residue A and C-4 (76.81 ppm) of residue A, H-4 (3.58 ppm) of residue A and C-1 (99.77 ppm) of residue A, and H-1 (5.25 ppm) of residue B and C-4 (76.81 ppm) of residue A were observed, indicating that →4)-α-D-Glcp-(1→ and →4,6)-α-D-Glcp-(1→ were linked to each other through 1,4-O-glycosidic bonds as the main chain of the polysaccharide.
Based on our analysis, it can be inferred that PSP-1 and PSP-2 are glucans composed mainly of (1→4)-linked-α-D-Glcp as the linear backbone attached by single α-D-Glcp as a branched chain through (1→6) glycosidic bonds. The structural repeating unit of the polysaccharides is shown in Figure 5F.
Based on the results of NMR analysis such as COSY, HSQC, etc., we inferred the monomer components of the polysaccharides and the structure of the polysaccharides and compared them with different literature studies to confirm our conclusions. However, this article does still have certain shortcomings. If we can use the methylation of the polysaccharides combined with GC-MS analysis to determine the glycosidic bond position, connection mode, and corresponding percentages of the monosaccharide residues, it will help us determine their structure more accurately. The research of Wang et al.27 and Shi et al.28 used hot water to extract polysaccharides from Cordyceps sinensis and Dictyophora echinovolvata, respectively. They used methylation and GC-MS analysis as well as NMR to determine the structure of polysaccharides. However, we used a hot alkali solution to extract spirulina polysaccharides. Although there were differences in materials and processes, similar polysaccharides structures were obtained. Our COSY, HSQC, and other NMR results were compared with these studies, which overcame the shortcomings of our lack of methylation experiments and strongly supported our conclusions on the structure of polysaccharides PSP-1 and PSP-2.
2.5. Immunomodulatory Effects of S. platensis Polysaccharides
2.5.1. Effects on Macrophage Viability
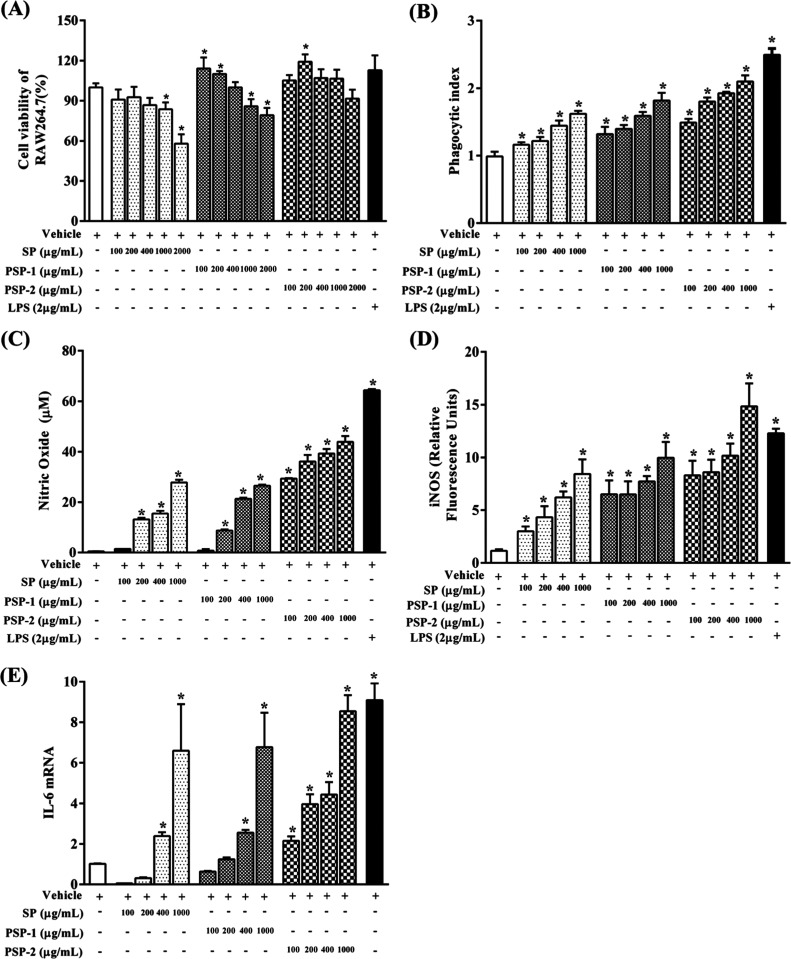
Macrophages were widely distributed in almost all tissues and have been regarded as an important part of host resistance to microbial invaders and malignant tumors.30 In addition, macrophages can not only respond to endogenous stimuli resulting from injury or infection but also respond to signals generated by antigen-specific immune cells.31 Therefore, to characterize the immunomodulatory effects of various components of S. platensis polysaccharides on the macrophage model in vitro, we first studied the effects of different concentrations of the polysaccharides on cell viability. As shown in Figure 6A, the cell viability decreased gradually with the increasing concentration of the polysaccharides SP and PSP-1 of S. platensis. Compared with the control group, SP and PSP-1 significantly reduced the vitality of RAW 264.7 cells under ambient concentrations of 1000–2000 μg/mL (p < 0.05), while PSP-2 had no effect on the vitality of RAW 264.7 cells. Generally, when cell viability is maintained at more than 80%, the substance is not considered cytotoxic. The results show that the action concentration for the follow-up experiments can be taken in the range of 100–1000 μg/mL.
Figure 6.
Effect of SP, PSP-1, and PSP-2 on RAW 264.7 cells. Effects of SP, PSP-1, and PSP-2 treatment cells at different concentrations (100, 200, 400, 1000, and 2000 μg/mL) on the viability of RAW 264.7 cells (A). Phagocytic capacity of RAW 264.7 cells pretreated with SP, PSP-1, and PSP-2 (B). NO production of RAW 264.7 cells pretreated with SP, PSP-1, and PSP-2 (C). Relative expression of iNOS in RAW 264.7 cells treated with SP, PSP-1, and PSP-2 (D). Gene expression levels of IL-6 cytokines in RAW 264.7 cells treated with SP, PSP-1, and PSP-2 (E). Cells were cultured with different concentrations (100, 200, 400, and 1000 mg/mL) of SP, PSP-1, and PSP-2 for 24 h. LPS (2 μg/mL) was used as a positive control. *p < 0.05 and **p < 0.01 compared with the control group (0 mg/mL), n = 6.
2.5.2. Effects on Macrophage Phagocytosis
Macrophages are specialized phagocytes, so phagocytosis is one of the most significant characteristics of macrophage activation.32 In fact, phagocytosis is the first and most critical process that threatens pathogens in the immune response.33 As shown in Figure 6B, macrophages were treated with different concentrations (100–1000 μg/mL) of SP, PSP-1, and PSP-2, with the increasing of substrate concentration, the neutral red uptake as well as the phagocytic index increased significantly (p < 0.05) compared with the control group, indicating that polysaccharides activated the phagocytic capacity of macrophages. The order of the maximum phagocytic index is PSP-2 (2.09) > PSP-1 (1.80) > SP (1.49), but all are smaller than that of LPS (2.49), indicating that each component of spirulina polysaccharides has certain immunomodulatory activity. Furthermore, PSP -2 seemed to be the most effective component in improving the phagocytosis of RAW 264.7 cells at this concentration range.
2.5.3. Effect on NO Production in Macrophages
NO is one of the main effector molecules that activate macrophages to destroy tumor cells and participate in various molecular and biological pathways, such as regulating apoptosis and controlling the host’s defense against infectious pathogens.21 To further evaluate the effect of spirulina polysaccharides on the macrophage function, the Griess method was used to measure the effects of SP, PSP-1, and PSP-2 at different concentrations (100–1000 μg/mL) on the production of NO by macrophages. As shown in Figure 6C, SP, PSP-1, and PSP-2 stimulated NO production by macrophages in a dose-dependent manner. LPS-mediated stimulation of RAW 264.7 cells significantly promoted the secretion of NO; similarly, the NO secretion levels of cells treated with SP, PSP-1, and PSP-2 were significantly higher than those of the control group (p < 0.05), except for the concentration of SP and PSP-1 at 100 μg/mL. At the maximum working concentration (1000 μg/mL), PSP-2 stimulated RAW 264.7 cells to produce NO at 43.91 μM content greater than those of SP (27.82 μM) and PSP-1 (26.52 μM), but all of them were less than that of positive control LPS. The results showed that spirulina polysaccharides SP, PSP-1, and PSP-2 can promote the secretion of NO by activating RAW 264.7 cells.
2.5.4. Effect on iNOS Enzymatic Activity
iNOS is responsible for controlling the synthesis of NO. Therefore, changes in the expression of iNOS will lead to downstream changes in various physiological events, including immune regulation.34 The enhancing effect of SP, PSP-1, and PSP-2 on iNOS enzyme activity was examined. As shown in Figure 6D, compared with the control group, SP, PSP-1, and PSP-2 significantly increased the relative expression of iNOS (p < 0.05) in a dose-dependent manner. In addition, at the maximum working dose (1000 μg/mL), the relative expression of iNOS (14.84) of PSP-2 was greater than that of RAW 264.7 macrophages stimulated by LPS (12.28). Importantly, the amount of NO secretion was consistent with the enzyme activity of iNOS, and these results show that SP, SP-1, and PSP-2 have a certain immunomodulatory effect.
2.5.5. Effects on IL-6 mRNA Expression
It is well known that the activated macrophages secrete various cytokines, chemokines, and growth factors to regulate their own functions and promote adaptive immunity.35 IL-6, as a cytokine, plays a key role in the regulation of the cell function, including the proliferation and differentiation of T and B cells, as well as changes in the phenotypic function of macrophages.34 To further confirm the changes in cytokine and NO secretion, quantitative RT-PCR was performed to detect the expression of genes related to NO or cytokine production. As shown in Figure 6E, similar to the trend of NO secretion, compared with the control group, SP, PSP-1, and PSP-2 upregulated the expression of IL-6 mRNA in a dose-dependent manner. PSP-2 showed the strongest stimulating activity on IL-6 mRNA expression at all concentrations compared to SP and PSP-1. However, under the maximum of its working concentration (1000 μg/mL), the IL-6 mRNA expression level was still lower than that of macrophages stimulated by LPS in the positive control group. In summary, these results indicate that spirulina polysaccharides (especially PSP-2) can activate macrophages by upregulating IL-6 gene expression to regulate autoimmunity.
We found that PSP-2 was more effective against macrophages than PSP-1. Considering that the biological activity of the polysaccharides is affected by the structure and physicochemical properties, from the above, we could know that the main difference between PSP-1 and PSP-2 lay in their molecular weights and sulfate content. Based on the results of the immunomodulatory activity study, the larger the molecular weight of polysaccharides, the better the immunostimulatory effect on macrophages. According to the relevant literature,36,37 we found that this may be due to the different molecular weights of PSP-1 and PSP-2, leading to different effects on macrophages. Studies have reported that Lycium barbarum polysaccharides with larger molecular weights had stronger immunomodulatory activity.38 In addition, studies have shown that polysaccharides with higher sulfate content have stronger anticancer ability.39 The reason for these results may be that polysaccharides with larger molecular weights are more conducive to the production of active polymer structures, which sulfate groups are also helpful to.40 The structure determines the properties and thus the function of the polysaccharides. However, the biological activity of polysaccharides in vitro does not fully reflect their biological activity in vivo,41 especially for polysaccharides with large molecular weights, as it is difficult for them to enter the human body to directly exert their activity. Therefore, such polysaccharides may first exert local immune regulation and anti-inflammatory effects in the intestine or other parts and then affect the overall body’s immune-inflammatory state.42
3. Conclusions
In the present study, crude polysaccharides of S. platensis (SP) were isolated by maceration with a hot alkali solution and further fractionated by DEAE-52 cellulose and Sephadex G-100 chromatography into two purified fractions, named PSP-1 and PSP-2. The structural characterization and in vitro immunomodulatory activity of SP, PSP-1, and PSP-2 fractions were investigated. The results showed that the polysaccharide purity values of SP, PSP-1, and PSP-2 were 73.80, 96.54, and 95.10%, respectively, and the crude SP contained a small amount of protein (1.93 ± 0.021%), while there was barely any protein in PSP-1 and PSP-2. Meanwhile, SP, PSP-1, and PSP-2 had small amounts of sulfate, which were 2.07 ± 0.31, 0.57 ± 0.14, and 1.05 ± 0.19%, respectively. The composition analysis of PSP-1 and PSP-2 by HPLC, FI-IR, and NMR showed that PSP-1 and PSP-2 were branching dextran, and their structures were (1 → 4)-linked-α-D-Glcp as the main chain, and C-6 was replaced by a single α-D-Glcp as the linear structure of the branch chain. Moreover, it demonstrated that SP, PSP-1, and PSP-2 had effective immunomodulatory activity through the macrophage phagocytic and NO secretion ability, iNOS activity, and cytokine IL-6 mRNA expression. When the concentration was at 100–1000 μg/mL, the polysaccharides can significantly improve the phagocytic ability of macrophage RAW 264.7 cells and stimulate the macrophages to produce NO, as well as the secretion of iNOS and IL-6 expression.
Our results demonstrated that S. platensis polysaccharides have the potential for immunomodulation and can be applied to the development and utilization of new functional foods, which laid a certain theoretical foundation for the comprehensive application of S. platensis.
4. Materials and Methods
4.1. Materials
S. platensis was purchased from Fujian Shenliu Health Food Co. (Fujian, China). A strong cation exchange chromatography column (Uni SP 50 xs) was purchased from Suzhou Nanomicro Technology Co. (Suzhou, China). DEAE-52 cellulose, Sephadex G-100, neutral red solution (0.33%, sterile-filtered, suitable for cell culture), and dialysis membranes (1000 D) were purchased from Beijing Solarbio Science & Technology Co. (Beijing, China). Monosaccharide standards (xylose, rhamnose, fucose, mannose, glucose, galactose, glucuronic acid, and galacturonic acid) were purchased from Shanghai Macklin Biochemical Co. 1-Phenyl-3-methyl-5-pyrazolone (PMP), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and lipopolysaccharide (LPS) were purchased from Sigma-Aldrich Co. (MO). Mouse leukemia cells of monocytes and macrophages (RAW 264.7) were obtained from the Chinese Academy of Sciences Cell Collection Center (Shanghai, China). A nitric oxide synthase assay kit was purchased from Beyotime Biochemical Co. (Shanghai, China). All other chemicals used were of analytical grade.
4.2. Preparation of S. platensis Crude Polysaccharide
The S. platensis powder (20 g) was mixed with 400 mL of a 1.25% (w/v) NaOH solution of pH 12.0 for 3 h at 80 °C. The pH of the extract was adjusted to 4. After stirring at 25 °C for 15 h, the extract was centrifuged at 8000 rpm for 10 min and the insoluble precipitate was discarded. The extraction solution was then neutralized, concentrated, and dialyzed with a 1000 Da MWCO dialysis bag for 48 h at 4 °C. The protein in the solution was removed using a Uni SP 50 xs strong cation exchange chromatography column (Φ 2.6 × 30 cm2) and precipitated with 75% ethanol at 4 °C for 24 h. The precipitate was centrifuged and redissolved in distilled water and lyophilized to obtain S. platensis crude polysaccharide (SP).
4.3. Isolation and Purification of Polysaccharides PSP-1 and PSP-2
SP was dissolved in distilled water and centrifuged. The supernatants were applied to an anion exchange column (Φ 2.6 × 40 cm2) with DEAE-52 cellulose and were sequentially eluted with 0–1 M sodium chloride at 1 mL/min. Each fraction (3.0 mL) was collected and separately detected by the phenol–sulfuric acid method at 490 nm43 and the spectrophotometer method at 280 nm. As a result, two relevant fractions were obtained, concentrated, dialyzed against ultrapure water, further purified on a Sephadex G-100 column (Φ 2.6 × 100 cm2), and then eluted with deionized water at a flow rate of 1 mL/min. The fractions obtained were combined and monitored by the phenol–sulfuric acid method. Each fraction generated a single elution peak representing PSP-1 and PSP-2. Finally, two purified fractions (PSP-1 and PSP-2) with high polysaccharide contents were collected, concentrated, dialyzed, and lyophilized for further study.
4.4. Characterization of S. platensis Polysaccharides
4.4.1. General Physicochemical Property Analysis
The total carbohydrate content and protein content of SP, PSP-1, and PSP-2 were determined by the phenol–sulfuric acid method using glucose as a standard and the Bradford method using bovine serum albumin (BSA) as a standard, respectively.43,44 The sulfate content of the polysaccharides was determined by the BaCl2 gelatin method using K2SO4 as a standard.45
4.4.2. Determination of Molecular Weight
The homogeneity and molecular weight were determined by high-performance gel permeation chromatography (HPGPC, Waters Corporation) using a Waters 1525 HPLC instrument equipped with a refractive index detector and an ultrahydrogel linear column (300 × 7.8 mm2, Waters). The column was eluted with the mobile phase of a 0.1 mol/L NaNO3 solution at a flow rate of 0.9 mL/min and the column oven temperature was set at 25 °C. T-series Dextran as a standard was used for the calibration curve.46
4.4.3. Monosaccharide Composition Analysis
Monosaccharide composition was determined by the PMP-HPLC method.47,48 In brief, the polysaccharide sample (∼1 mg) was hydrolyzed with 4 M TFA at 110 °C in a nitrogen atmosphere for 6 h. The hydrolysate was dried under vacuum and then derivatized by adding 450 μL of a 1-phenyl-3-methyl-5-pyrazolone (PMP) solution (0.5 M, in methanol) and 450 μL of 0.3 M NaOH and incubated at 70 °C for 30 min. The reaction was stopped by neutralization with 450 μL of 0.3 M HCl, followed by chloroform extraction (1 mL, three times). The extract solution was analyzed by HPLC on an Agilent DEAE203170 instrument with a 693970-902 Agilent TC-C18 column (4 μm, 4.6 × 150 mm2) at 35 °C with UV detection at 245 nm. The mobile phase was composed of 10 mM 70% ammonium acetate (solvent A) and 30% acetonitrile (solvent B) at a flow rate of 0.8 mL/min. Glucose (Glc), glucuronic acid (GlcA), galactose (Gal), galacturonic acid (GalA), mannose (Man), rhamnose (Rha), xylose (Xyl), and fucose (Fuc) were used as monosaccharide standards for the identification and quantification of the corresponding peaks.
4.4.4. Infrared Spectroscopy Analysis
FT-IR spectroscopy was performed using an IS50 FT-IR spectrometer (Thermo Scientific) in the range of 4000–400 cm–1 with KBr pellets. The samples were subjected to 32 scans. OMNIC software was used to analyze the results.49
4.4.5. Nuclear Magnetic Resonance Spectroscopy
PSP-1 and PSP-2 (30 mg) were dissolved in D2O and then freeze-dried. This procedure was repeated two times to completely exchange H2O with D2O, and the polysaccharides were finally dissolved in 1 mL of D2O at room temperature for 3 h before NMR analysis. Both 1H and 13C spectra were recorded on a Bruker AV-800 MHz NMR spectrometer (Germany) at 295 K. They were further subjected to 2D NMR spectroscopy, including homonuclear 1H/1H correlation (COSY), heteronuclear single quantum coherence (HSQC), and heteronuclear multiple bond correlation (HMBC) experiments through the standard Bruker pulse sequence.50
4.5. Macrophage Immune-Modulating Activity
4.5.1. Cell Culture
Mouse leukemia cells of monocytes and macrophages (RAW 264.7) were obtained from the Chinese Academy of Sciences Cell Collection Center (Shanghai, China). The cells were cultured at 37 °C in DMEM supplemented with a 10% FBS and 1% penicillin–streptomycin antibiotic mixture in a humidified atmosphere with 5% CO2 and 95% air.
4.5.2. Cytotoxicity Assay
The cytotoxic effects of SP, PSP-1, and PSP-2 on RAW 264.7 cells were measured by the MTT assay. In brief, adherent RAW 264.7 cells in 96-well plates (1 × 104 cells/well) were cultured at different concentrations of SP, PSP-1, and PSP-2 (100–2000 μg/mL) prepared in DMEM (10% FBS). LPS (2 μg/mL) was used as a positive control.51 The treated cells were incubated for 24 h to estimate the viability. MTT (5 mg/mL) was prepared and 15 μL of MTT was added to each well and incubated for 4 h. After incubation, the observed purple color of crystals was dissolved with 150 μL of DMSO for 10 min. The absorbance was detected at 490 nm.
4.5.3. Neutral Red Uptake Assay
Phagocytic activity was determined with the neutral red uptake assay.52 In brief, adherent RAW 264.7 cells in 96-well plates (1 × 105 cells/well) were treated at different concentrations of SP, PSP-1, and PSP-2 (100–1000 μg/mL) and LPS (2 μg/mL) for 48 h, and then the culture medium was abandoned. One hundred microliters of a 0.11% (w/v) neutral red solution was added to each well and cultured for a further 4 h. After cultivation, the pretreated macrophage was washed three times with phosphate-buffered saline (PBS), and 100 μL of cell lysis buffer (acetic acid/ethanol; 1:1) was added and incubated at 4 °C for 2 h. The absorbance was determined at 540 nm using a microplate ELISA reader.
4.5.4. Nitric Oxide Analysis
RAW 264.7 cells were seeded at a density of 2 × 106 cells/mL onto 96-well plates and preincubated for 12 h, followed by stimulation at varying concentrations of polysaccharides for 24 h. NO accumulation in the culture medium was measured using the Griess reagent. Briefly, the media were collected and then mixed with an equal volume of the Griess reagent (1% sulfanilamide in dd H2O and 0.1% N-(1-naphthyl)ethylenediamine in 5% phosphoric acid). After 10 min, the absorbance at 540 nm was measured using a microplate reader. NaNO2 was used to generate a standard curve.19
4.5.5. iNOS Enzymatic Activity Assay
iNOS enzymatic activity was determined using a nitric oxide synthase assay kit according to the manufacturer’s recommendations. In brief, the culture supernatant was removed and 100 μL of the NOS assay buffer (1 ×) solution was added to each well. After that, 100 μL of the NOS assay reaction solution (50% NOS assay buffer, 39.8% MilliQ water, 5% l-arginine solution, 5% 0.1 mM NADPH, and 0.2% DAF-FMDA) was added to each well and the plate was incubated for 40 min at 37 °C. Fluorescence was then measured with a fluorescence microplate ELISA reader at an excitation of 485 nm and an emission of 528 nm.53
4.5.6. Real-Time Quantitative PCR
To evaluate the mRNA expression levels of IL-6, total RNA from LPS and SP-, PSP-1-, and PSP-2-treated RAW 264.7 cells were prepared by the TRIZOL method (Beyotime, China), according to the manufacturer’s instructions. cDNA was synthesized with 1 μg of total RNA using the BeyoRT II First Strand cDNA Synthesis Kit with gDNA Eraser (Beyotime, code No. D7170M). RT-PCR was carried out using a two-step RT-qPCR system kit (Takara, China) with the following primer sequences: 5′-TACTCGGCAAACCTAGTGCG-3′ (forward) and 5′-GTGTCCCAACATTCATATTGTCAGT-3′ (reverse) for mouse IL-6, 5′-TTTGTCAAGCTCATTTCCTGGTATG-3′ (forward) and 5′-TGGGATAGGGCCTCTCTTGC-3′ (reverse) for mouse Gapdh. The Gapdh primer was used as an internal control. RT-qPCR was performed in a 7300 Real-Time PCR Detection System (ABI) with the SYBR Green Realtime PCR Master Mix (Toyobo, Osaka, Japan) in a 20 μL reaction volume. The changes in gene expression were determined relative to that of Gapdh using the 2–ΔΔ Ct method.
4.6. Statistical Analysis
Each experiment was performed in triplicate, and the data are demonstrated as mean ± standard deviation (SD). Statistical analysis was performed by one-way analysis of variance (ANOVA). Values of p < 0.05 denoted the presence of a statistically significant difference. Statistical analyses were performed using the statistical package for Social Sciences (SPSS) version 17.0 (SPSS Inc., Chicago, IL).
Acknowledgments
This work was supported by the Major Applied Agricultural Technology Innovation Projects of Shandong Province (SD2019ZZ009), the National Key R&D Program of China (2018YFD0901004), the opening project of Fujian Provincial Engineering Technology Research Center of Marine Functional Food (Nos. 2900/Z820235 and 2900/Z820237).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c02175.
NMR spectra of PSP-2 including 1H NMR, 13C NMR, COSY, HSQC, and HMBC experiments (PDF)
Author Contributions
J.L., Y.Z., S.Y., G.L., J.L., D.W., and L.W.: conceived and designed the experiments; J.L., Y.Z., G.L., and D.W.: performed the experiments and analyzed the data; J.L., G.L., and J.L.: contributed the reagents, materials, and analysis tools; J.L., Y.Z., D.W., B.Z., and L.W.: wrote the paper.
The authors declare no competing financial interest.
Supplementary Material
References
- Kulshreshtha A.; Zacharia A. J.; Jarouliya U.; Bhadauriya P.; Prasad G. B.; Bisen P. S. Spirulina in health care management. Curr. Pharm. Biotechnol. 2008, 9, 400–405. 10.2174/138920108785915111. [DOI] [PubMed] [Google Scholar]
- Abu-Taweel G. M.; Mohsen G. A.; Antonisamy P.; Arokiyaraj S.; Kim H. J.; Kim S. J.; Park K. H.; Kim Y. O. Spirulina consumption effectively reduces anti-inflammatory and pain related infectious diseases. J. Infect. Public Heal. 2019, 12, 777–782. 10.1016/j.jiph.2019.04.014. [DOI] [PubMed] [Google Scholar]
- Çelekli A.; Yavuzatmaca M. Predictive modeling of biomass production by Spirulina platensis as function of nitrate and NaCl concentrations. Bioresour. Technol. 2009, 100, 1847–1851. 10.1016/j.biortech.2008.09.042. [DOI] [PubMed] [Google Scholar]
- da Silva S. C.; Fernandes I. P.; Barros L.; Fernandes Â.; José Alves M.; Calhelha R. C.; Pereira C.; Barreira J. C. M.; Manrique Y.; Colla E.; Ferreira I. C. F. R.; Filomena Barreiro M. Spray-dried Spirulina platensis as an effective ingredient to improve yogurt formulations: Testing different encapsulating solutions. J. Funct. Foods 2019, 60, 103427 10.1016/j.jff.2019.103427. [DOI] [Google Scholar]
- Winarni Agustini T.; Farid Ma’ruf W.; Widayat W.; Suzery M.; Hadiyanto H.; Benjakul S. Application of Spirulina platensis on ice cream and soft cheese with respect to their nutritional and sensory perspectives. Jurnal Teknologi 2016, 78, 4–2. 10.11113/jt.v78.8216. [DOI] [Google Scholar]
- Aissaoui O.; Amiali M.; Bouzid N.; Belkacemi K.; Bitam A. Effect of Spirulina platensis ingestion on the abnormal biochemical and oxidative stress parameters in the pancreas and liver of alloxan-induced diabetic rats. Pharm. Biol. 2017, 55, 1304–1312. 10.1080/13880209.2017.1300820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabana E. F.; Gabr M. A.; Moussa H. R.; El-Shaer E. A.; Ismaiel M. M. S. Biochemical composition and antioxidant activities of Arthrospira (Spirulina) platensis in response to gamma irradiation. Food Chem. 2017, 214, 550–555. 10.1016/j.foodchem.2016.07.109. [DOI] [PubMed] [Google Scholar]
- Abdel-Daim M. M.; Farouk S. M.; Madkour F. F.; Azab S. S. Anti-inflammatory and immunomodulatory effects of Spirulina platensis in comparison to Dunaliella salinain acetic acid-induced rat experimental colitis. Immunopharmacol. Immunotoxicol. 2015, 37, 126–139. 10.3109/08923973.2014.998368. [DOI] [PubMed] [Google Scholar]
- Gargouri M.; Magne C.; El Feki A. Hyperglycemia, oxidative stress, liver damage and dysfunction in alloxan-induced diabetic rat are prevented by Spirulina supplementation. Nutr. Res. 2016, 36, 1255–1268. 10.1016/j.nutres.2016.09.011. [DOI] [PubMed] [Google Scholar]
- Wang M.; Yang X. B.; Zhao J. W.; Lu C. J.; Zhu W. Structural characterization and macrophage immunomodulatory activity of a novel polysaccharide from Smilax glabra Roxb. Carbohydr. Polym. 2017, 156, 390–402. 10.1016/j.carbpol.2016.09.033. [DOI] [PubMed] [Google Scholar]
- Chen X.; Wu G.; Huang Z. Structural analysis and antioxidant activities of polysaccharides from cultured Cordyceps militaris. Int. J. Biol. Macromol. 2013, 58, 18–22. 10.1016/j.ijbiomac.2013.03.041. [DOI] [PubMed] [Google Scholar]
- Chirasuwan N. C. R.; Ruengjitchatchawalya M. Anti HSV-1 activity of Spirulina platensis polysaccharide. Kasetsart J.: Nat. Sci. 2007, 41, 311–318. [Google Scholar]
- Yang L.; Wang Y.; Zhou Q.; Chen P.; Wang Y.; Wang Y.; Liu T.; Xie L. Inhibitory effects of polysaccharide extract from Spirulina platensis on corneal neovascularization. Mol. Vis. 2009, 15, 1951. [PMC free article] [PubMed] [Google Scholar]
- Pugh N.; Ross S. A.; ElSohly H. N.; ElSohly M. A.; Pasco D. S. Isolation of three high molecular weight polysaccharide preparations with potent immunostimulatory activity from Spirulina platensis, aphanizomenon flos-aquae and Chlorella pyrenoidosa. Planta Med. 2001, 67, 737–42. 10.1055/s-2001-18358. [DOI] [PubMed] [Google Scholar]
- Majdoub H.; Ben Mansour M.; Chaubet F.; Roudesli M. S.; Maaroufi R. M. Anticoagulant activity of a sulfated polysaccharide from the green alga Arthrospira platensis. Biochim. Biophys. Acta, Biomembr. 2009, 1790, 1377–81. 10.1016/j.bbagen.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Chaiklahan R.; Chirasuwan N.; Triratana P.; Loha V.; Tia S.; Bunnag B. Polysaccharide extraction from Spirulina sp. and its antioxidant capacity. Int. J. Biol. Macromol. 2013, 58, 73–78. 10.1016/j.ijbiomac.2013.03.046. [DOI] [PubMed] [Google Scholar]
- Rajasekar P.; Palanisamy S.; Anjali R.; Vinosha M.; Elakkiya M.; Marudhupandi T.; Tabarsa M.; You S.; Prabhu N. M. Isolation and structural characterization of sulfated polysaccharide from Spirulina platensis and its bioactive potential: In vitro antioxidant, antibacterial activity and Zebrafish growth and reproductive performance. Int. J. Biol. Macromol. 2019, 141, 809–821. 10.1016/j.ijbiomac.2019.09.024. [DOI] [PubMed] [Google Scholar]
- Wang X.-Y.; Yin J. Y.; Nie S. P.; Xie M. Y. Isolation, purification and physicochemical properties of polysaccharide from fruiting body of Hericium erinaceus and its effect on colonic health of mice. Int. J. Biol. Macromol. 2018, 107, 1310–1319. 10.1016/j.ijbiomac.2017.09.112. [DOI] [PubMed] [Google Scholar]
- Yu X.-H.; Liu Y.; Wu X. L.; Liu L. Z.; Fu W.; Song D. D. Isolation, purification, characterization and immunostimulatory activity of polysaccharides derived from American ginseng. Carbohydr. Polym. 2017, 156, 9–18. 10.1016/j.carbpol.2016.08.092. [DOI] [PubMed] [Google Scholar]
- Velazquez G.; Herrera-Gomez A.; Martin-Polo M. O. Identification of bound water through infrared spectroscopy in methylcellulose. J. Food Eng. 2003, 59, 79–84. 10.1016/S0260-8774(02)00428-4. [DOI] [Google Scholar]
- Nie C.; Zhu P.; Ma S.; Wang M.; Hu Y. Purification, characterization and immunomodulatory activity of polysaccharides from stem lettuce. Carbohydr. Polym. 2018, 188, 236–242. 10.1016/j.carbpol.2018.02.009. [DOI] [PubMed] [Google Scholar]
- Rout D.; Mondal S.; Chakraborty I.; Pramanik M.; Islam S. S. Chemical analysis of a new (1-->3)-, (1-->6)-branched glucan from an edible mushroom, Pleurotus florida. Carbohydr. Res. 2005, 340, 2533–2539. 10.1016/j.carres.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Shen C.-Y.; Zhang W.-L.; Jiang J.-G. Immune-enhancing activity of polysaccharides from Hibiscus sabdariffa Linn. via MAPK and NF-kB signaling pathways in RAW264.7 cells. J. Funct. Foods 2017, 34, 118–129. 10.1016/j.jff.2017.03.060. [DOI] [Google Scholar]
- Heperkan Z. D.; Bolluk M.; Bulbul S. Structural analysis and properties of dextran produced by Weissella confusa and the effect of different cereals on its rheological characteristics. Int. J. Biol. Macromol. 2020, 143, 305–313. 10.1016/j.ijbiomac.2019.12.036. [DOI] [PubMed] [Google Scholar]
- Niu Y.; Yan W.; Lv J.; Yao W.; Yu L. L. Characterization of a novel polysaccharide from tetraploid Gynostemma pentaphyllum makino. J. Agric. Food Chem. 2013, 61, 4882–4889. 10.1021/jf400236x. [DOI] [PubMed] [Google Scholar]
- Petersen B. O.; Motawie M. S.; Moller B. L.; Hindsgaul O.; Meier S. NMR characterization of chemically synthesized branched alpha-dextrin model compounds. Carbohydr. Res. 2015, 403, 149–156. 10.1016/j.carres.2014.05.011. [DOI] [PubMed] [Google Scholar]
- Wang J.; Nie S. P.; Cui S. W.; Wang Z. J.; Phillips A. O.; Phillips G. O.; Li Y. J.; Xie M. Y. Structural characterization and immunostimulatory activity of a glucan from natural Cordyceps sinensis. Food Hydrocolloids 2017, 67, 139–147. 10.1016/j.foodhyd.2017.01.010. [DOI] [Google Scholar]
- Shi X.-d.; Li O. Y.; Yin J. Y.; Nie S. P. Structure identification of alpha-glucans from Dictyophora echinovolvata by methylation and 1D/2D NMR spectroscopy. Food Chem. 2019, 271, 338–344. 10.1016/j.foodchem.2018.07.160. [DOI] [PubMed] [Google Scholar]
- Zhao C.; Li M.; Luo Y.; Wu W. Isolation and structural characterization of an immunostimulating polysaccharide from fuzi, Aconitum carmichaeli. Carbohydr. Res. 2006, 341, 485–491. 10.1016/j.carres.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Tucci H. T.; Martins J.; Sposito Gde C.; Camarini P. M.; de Oliveira A. S. Closed Kinetic Chain Upper Extremity Stability test (CKCUES test): a reliability study in persons with and without shoulder impingement syndrome. BMC Musculoskeletal Disord. 2014, 15, 1 10.1186/1471-2474-15-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser D. M.; Edwards J. P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–69. 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.; Zhao S.; Zhu P.; Nie C.; Ma S.; Wang N.; Du X.; Zhou Y. Purification, characterization and immunomodulatory activity of water extractable polysaccharides from the swollen culms of Zizania latifolia. Int. J. Biol. Macromol. 2018, 107, 882–890. 10.1016/j.ijbiomac.2017.09.062. [DOI] [PubMed] [Google Scholar]
- Qi J.; Kim S. M. Characterization and immunomodulatory activities of polysaccharides extracted from green alga Chlorella ellipsoidea. Int. J. Biol. Macromol. 2017, 95, 106–114. 10.1016/j.ijbiomac.2016.11.039. [DOI] [PubMed] [Google Scholar]
- Lee J. S.; Synytsya A.; Kim H. B.; Choi D. J.; Lee S.; Lee J.; Kim W. J.; Jang S.; Park Y. I. Purification, characterization and immunomodulating activity of a pectic polysaccharide isolated from Korean mulberry fruit Oddi (Morus alba L.). Int. Immunopharmacol. 2013, 17, 858–866. 10.1016/j.intimp.2013.09.019. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Liu D.; Fang L.; Zhao X.; Zhou A.; Xie J. A galactomannoglucan derived from Agaricus brasiliensis: Purification, characterization and macrophage activation via MAPK and IkappaB/NFkappaB pathways. Food Chem. 2018, 239, 603–611. 10.1016/j.foodchem.2017.06.152. [DOI] [PubMed] [Google Scholar]
- Huang L. H.; Liu H.; Chen J. Y.; Sun X. Y.; Yao Z. H.; Han J.; Ouyang J. M. Seaweed Porphyra yezoensis polysaccharides with different molecular weights inhibit hydroxyapatite damage and osteoblast differentiation of A7R5 cells. Food Funct. 2020, 11, 3393–3409. 10.1039/C9FO01732A. [DOI] [PubMed] [Google Scholar]
- Dou Z.; Chen C.; Fu X. Digestive Property and Bioactivity of Blackberry Polysaccharides with Different Molecular Weights. J. Agric. Food Chem. 2019, 67, 12428–12440. 10.1021/acs.jafc.9b03505. [DOI] [PubMed] [Google Scholar]
- Feng L.; Xiao X.; Liu J.; Wang J.; Zhang N.; Bing T.; Liu X.; Zhang Z.; Shangguan D. Immunomodulatory Effects of Lycium barbarum Polysaccharide Extract and Its Uptake Behaviors at the Cellular Level. Molecules 2020, 25, 1351 10.3390/molecules25061351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M. L.; Lee B. Y.; You S. G. Relationship between oversulfation and conformation of low and high molecular weight fucoidans and evaluation of their in vitro anticancer activity. Molecules 2010, 16, 291–297. 10.3390/molecules16010291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y.; Gong G.; Guo Y.; Wang Z.; Song S.; Zhu B.; Zhao L.; Jiang J. Purification, structural features and immunostimulatory activity of novel polysaccharides from Caulerpa lentillifera. Int. J. Biol. Macromol. 2018, 108, 314–323. 10.1016/j.ijbiomac.2017.12.016. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Xu Y.; Lv J.; Cheng M.; Wu Y.; Cao K.; Zhang X.; Mou X.; Fan Q. New utilization of Polygonum multiflorum polysaccharide as macromolecular carrier of 5-fluorouracil for controlled release and immunoprotection. Int. J. Biol. Macromol. 2018, 116, 1310–1316. 10.1016/j.ijbiomac.2018.02.052. [DOI] [PubMed] [Google Scholar]
- Xu X.; Yang J.; Luo Z.; Zhang X. Lentinula edodes-derived polysaccharide enhances systemic and mucosal immunity by spatial modulation of intestinal gene expression in mice. Food Funct. 2015, 6, 2068–80. 10.1039/C4FO01192A. [DOI] [PubMed] [Google Scholar]
- DuBois M.; Gilles K. A.; Hamilton J. K.; Rebers P. A.; Smith F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. 10.1021/ac60111a017. [DOI] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–54. 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Zhu Z.-Y.; Luo Y.; Dong G. L.; Ren Y. Y.; Chen L. J.; Guo M. Z.; Wang X. T.; Yang X. Y.; Zhang Y. Effects of the ultra-high pressure on structure and alpha-glucosidase inhibition of polysaccharide from Astragalus. Int. J. Biol. Macromol. 2016, 87, 570–576. 10.1016/j.ijbiomac.2016.03.024. [DOI] [PubMed] [Google Scholar]
- Yan J. K.; Wang Y. Y.; Qiu W. Y.; Wu L. X.; Ding Z. C.; Cai W. D. Purification, structural characterization and bioactivity evaluation of a novel proteoglycan produced by Corbicula fluminea. Carbohydr. Polym. 2017, 176, 11–18. 10.1016/j.carbpol.2017.08.063. [DOI] [PubMed] [Google Scholar]
- Strydom D. J. Chromatographic separation of 1-phenyl-3-methyl-5-pyrazolone-derivatized neutral, acidic and basic aldoses. J. Chromatogr. A 1994, 678, 17–23. 10.1016/0021-9673(94)87069-1. [DOI] [Google Scholar]
- Chen S.; Siu K. C.; Wang W. Q.; Liu X. X.; Wu J. Y. Structure and antioxidant activity of a novel poly-N-acetylhexosamine produced by a medicinal fungus. Carbohydr. Polym. 2013, 94, 332–338. 10.1016/j.carbpol.2012.12.067. [DOI] [PubMed] [Google Scholar]
- Athmouni K.; Belhaj D.; Chawech R.; Jarraya R.; El Feki A.; Ayadi H. Characterization of polysaccharides isolated from Periploca angustifolia and its antioxidant activity and renoprotective potential against cadmium induced toxicity in HEK293 cells and rat kidney. Int. J. Biol. Macromol. 2019, 125, 730–742. 10.1016/j.ijbiomac.2018.12.046. [DOI] [PubMed] [Google Scholar]
- Jiang J.; Kong F.; Li N.; Zhang D.; Yan C.; Lv H. Purification, structural characterization and in vitro antioxidant activity of a novel polysaccharide from Boshuzhi. Carbohydr. Polym. 2016, 147, 365–371. 10.1016/j.carbpol.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Song Y.-R.; Han A. R.; Lim T. G.; Lee E. J.; Hong H. D. Isolation, purification, and characterization of novel polysaccharides from lotus (Nelumbo nucifera) leaves and their immunostimulatory effects. Int. J. Biol. Macromol. 2019, 128, 546–555. 10.1016/j.ijbiomac.2019.01.131. [DOI] [PubMed] [Google Scholar]
- Wu C.-H.; Yang M.-Y.; Lee Y.-J.; Wang C.-J. Nelumbo nucifera leaf polyphenol extract inhibits breast cancer cells metastasis in vitro and in vivo through PKCα targeting. J. Funct. Foods 2017, 37, 480–490. 10.1016/j.jff.2017.08.021. [DOI] [Google Scholar]
- Shi S.-H.; Yang W. T.; Huang K. Y.; Jiang Y. L.; Yang G. L.; Wang C. F.; Li Y. beta-glucans from Coriolus versicolor protect mice against S. typhimurium challenge by activation of macrophages. Int. J. Biol. Macromol. 2016, 86, 352–361. 10.1016/j.ijbiomac.2016.01.058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.