Abstract
Synthesis, FTIR spectral study, and X-ray diffraction analysis of single crystals of (CH3)4N[UO2(mba)3] (I), (CH3)4N[NpO2(mba)2(NO3)] (II), (CH3)4N[PuO2(mba)2(NO3)] (III), and (CH3)4N[NpO2(mba)(NO3)2] (IV), where mba is a monobromoacetate ion (CH2BrCOO–), were conducted. The main structural units of crystals I–IV are mononuclear anionic complexes of the [AnO2(mba)3]−, [AnO2(mba)2(NO3)]−, or [AnO2(mba)(NO3)2]− composition. All these complex units are characterized with the same crystal-chemical formula AB013 (A = AnO22+ and B01 = CH2BrCOO– or NO3–). Using the method of molecular Voronoi–Dirichlet polyhedra, the contributions of various types of noncovalent interactions into the formation of supramolecular structures of the obtained complexes were characterized. The analysis of coordination modes of all monobromoacetate-containing compounds from the Cambridge Structural Database was accomplished. Actinide contraction in the studied compounds is discussed.
1. Introduction
A lot of attention currently is given to hybrid organo-inorganic compounds of hexavalent uranium, which frequently incorporate the [UO2(L)3]− anionic complex units (L, anions of saturated or unsaturated monocarboxylic acids) in their crystal structures. The prevalence of the tricarboxylatouranilate complex group has been shown by the example of the previously studied acetate-, propionate-, butyrate-, valerate-, acrylate-, methacrylate-, and crotonate-containing compounds.1−12 The recently synthesized tribromopropionate-containing compounds of actinides are also constructed of the same [AnO2(L)3]− units (An, actinide).13 However, the known compounds with a trichloroacetic acid anion as a ligand feature complex units of different compositions, [AnO2(L)5]3–, in which trichloroacetate ions are coordinated to the AnO22+ cation in a monodentate mode.14 For this reason, the investigation of the interaction between other halogen-substituted saturated and unsaturated monocarboxylate anions and hexavalent uranium and transuranic elements (neptunium and plutonium) seems to be valuable. This work is devoted to FTIR spectroscopic and X-ray diffraction analyses of the actinyl coordination compounds with monobromoacetate anions and tetramethylammonium: (CH3)4N[UO2(mba)3] (I), (CH3)4N[NpO2(mba)2(NO3)] (II), (CH3)4N[PuO2(mba)2(NO3)] (III), and (CH3)4N[NpO2(mba)(NO3)2] (IV).
2. Results and Discussion
2.1. Description of Crystal Structures
The structures of I–IV contain one crystallographic type of actinide atom. The coordination polyhedra of actinide atoms in the structures of all synthesized compounds are hexagonal bipyramids AnO8 (An = U, Np, or Pu) with oxygen atoms of the AnO22+ cation in axial positions (Figure 1a). In the equatorial plane, the uranyl, neptunyl, and plutonyl ions coordinate three monobromoacetate ions in I (Figure 1c), two monobromoacetate ions and one nitrate ion in isostructural II and III (Figure 1d), or two nitrate ions and one monobromoacetate ion in IV (Figure 1e). All ligands implement the B01-4-(O2) coordination mode. The designations of coordination modes are given in accordance with ref (14). The geometric parameters of coordination polyhedra of actinide atoms in the structures of I–IV are provided in the Supporting Information. The Voronoi–Dirichlet polyhedra (VDP) of U, Np, and Pu atoms are hexagonal prisms (Figure 1b). Their volumes (see the Supporting Information) are in good agreement with average values of 9.2(2), 9.2(2), and 9.20(9) Å3 established for U(VI), Np(VI), and Pu(VI) atoms, respectively, surrounded by oxygen atoms.15−17
Figure 1.
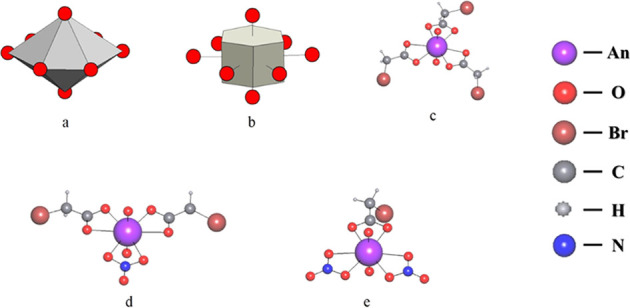
Coordination polyhedron (a), Voronoi–Dirichlet polyhedron (b), and the environment of actinide atoms (c–e) in crystal structures of I–IV.
The AnO22+ ions in structures of I–IV are almost linear and equilateral (see the Supporting Information).18 Actinide-containing structural units are the mononuclear complexes [UO2(CH2BrCOO)3]− in I, [AnO2(CH2BrCOO)2(NO3)]− (An = Np or Pu) in II and III, and [NpO2(CH2BrCOO)(NO3)2]− in IV. The actinide-containing complex anions in the structures of all synthesized compounds are represented with a crystal chemical formula AB013 (A = AnO22+ and B01 = CH2BrCOO– or NO3–). Due to the linkage of anionic complex units with outer-sphere cations with a developed network of noncovalent interactions (see below), the formation of supramolecular three-dimensional frameworks occurs in all the four structures.
2.2. Intermolecular Interactions
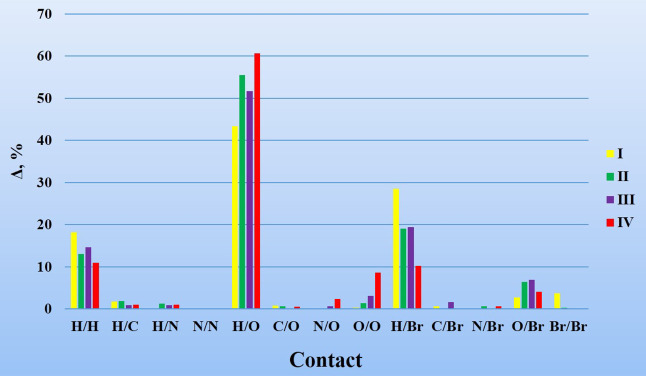
Anionic actinide-containing units in structures of I–IV are bound to tetramethylammonium cations into a three-dimensional supramolecular framework through electrostatic and intermolecular interactions. The existence of atoms of six chemical elements in the composition of each of the investigated compounds determines the theoretically possible presence of 21 types of pairwise noncovalent contacts. All intra- and intermolecular noncovalent interactions in the structures of crystalline substances can be reliably evaluated with the method of molecular Voronoi–Dirichlet polyhedra.19,20 The calculation of main parameters of noncovalent interactions was done with the program Intermol of the TOPOS program package.21,22 The results of the calculation are given in the form of a diagram in Figure 2. The vertical axis of the diagram reflects the parameter Δ (%), the partial contribution of certain types of noncovalent interactions to the total surface area of all faces of Voronoi–Dirichlet polyhedra.
Figure 2.
Partial contributions (Δ, %) of intermolecular noncovalent interactions in crystal structures of (CH3)4N[UO2(mba)3] (I), (CH3)4N[NpO2(mba)2(NO3)] (II), (CH3)4N[PuO2(mba)2(NO3)] (III), and (CH3)4N[NpO2(mba)(NO3)2] (IV).
According to the derived data, the most considerable contribution into the formation of supramolecular frameworks of all synthesized compounds is provided by the H/O, H/Br, and H/H contacts, the total partial contribution of which is in the range of 82–90%. The characteristics of discovered hydrogen bonds in the examined structures are provided in Table 1. According to the classification proposed by Steiner,23 all the mentioned hydrogen bonds can be attributed to medium or weak in strength.
Table 1. Characteristics of Hydrogen Bonds in Crystal Structures of I–IVa.
| bond C–H···A | Nb | d(C···A) (Å) | d(C–H) (Å) | d(H···A) (Å) | angle (C–H···A) (deg) | Ω(H···A) (%) |
|---|---|---|---|---|---|---|
| C10H18Br3NO8U, I | ||||||
| C–H···Br | 2 | 3.732–3.917 | 0.97 | 2.82–2.99 | 157.3–158.9 | 15.53–16.32 |
| C–H···O | 9 | 3.392–3.808 | 0.96–0.97 | 2.55–2.93 | 137.7–154.8 | 10.27–15.30 |
| C8H16Br2N2NpO9, II | ||||||
| C–H···O | 8 | 3.251–3.826 | 0.96–0.97 | 2.41–2.92 | 131.6–159.5 | 11.21–15.75 |
| C8H16Br2N2O9Pu, III | ||||||
| C–H···O | 7 | 3.267–3.753 | 0.96–0.97 | 2.37–2.87 | 148.0–164.3 | 10.41–17.42 |
| C6H14BrN3NpO10, IV | ||||||
| C–H···O | 8 | 3.173–3.683 | 0.96–0.97 | 2.40–2.77 | 137.4–164.0 | 10.01–17.04 |
Allowance was made for contacts with C–H···A angle > 130°, d(H···A) < 3 Å, and Ω(H···A) > 10%.
N is the number of contacts of a specified type.
A significant contribution of the Br/Br type of contacts was found for compound I with ΔBrBr = 3.67%, while for the other compounds, ΔBrBr does not exceed 1% (Figure 2). A more detailed study demonstrated the presence of a type II halogen bond24 in the structure of I (Figure 3). The existence of such a halogen bond determines the deviation of one monobromoacetate ion from the equatorial plane of the uranyl group.
Figure 3.
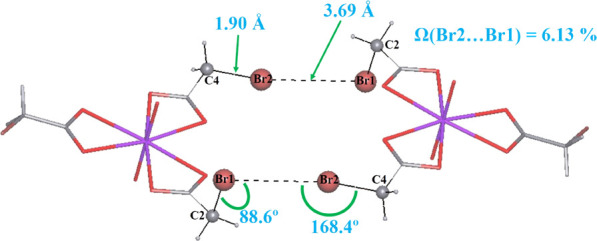
Halogen bond in the structure of I.
2.3. Coordination Modes
To summarize the available information on the structure and coordination modes of monobromoacetate ions, a crystal-chemical analysis of all monobromoacetate-containing compounds contained in the Cambridge Structural Database (CSD) was performed. The following requirements were imposed on the crystal structures for the preparation of the initial data set:
-
1)
The crystal structure contains monobromoacetate ions bound to a metal atom.
-
2)
The value of bond valence (S = v/CN, where v is the valence of the metal and CN is its coordination number in the structure) of the metal-oxygen bonds with monobromoacetate ions is ≥0.25.
-
3)
The crystal structure does not possess disorder.
-
4)
The crystal structure is determined with a nonzero R1 factor that does not exceed 0.1.
The stated requirements resulted in the structures of 17 compounds containing 30 crystallographically independent monobromoacetate ions. Bi, Ce, Cu, Fe, Mn, Sb, Ti, and V served as metal atoms in the observed complexes. In the investigated compounds, monobromoacetate ions exhibit four different coordination modes (Figure 4). The most widespread mode is B2 (10 ions). The identified coordination modes show that monobromoacetate ions are able to act as terminal (M1 and B01-4) and bridging (B2 and M2) ligands and are able to coordinate without chelating effects (M2, M1, and B2) or to form four-membered cycles with metal atoms (B01-4). In all the studied cases, the donor atoms of ligands were exclusively oxygen atoms and no covalent bonding between Br and metal atoms was observed as opposed to the available information on other halogen-substituted carboxylate complexes.14,25
Figure 4.
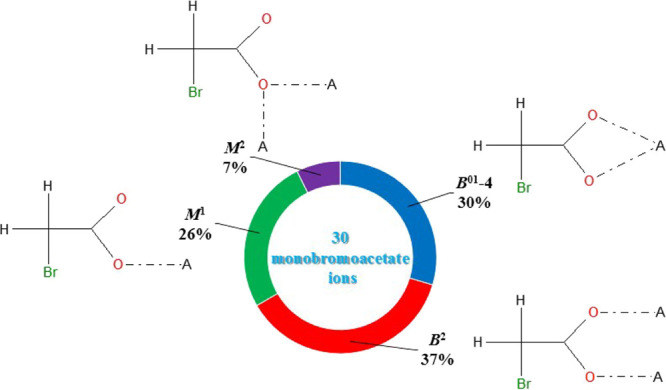
Four coordination modes of monobromoacetate ions in the crystal structures of all available coordination compounds in the CSD.
2.4. Actinide Contraction
The concept of actinide contraction represents the phenomenon of a regular decrease in the size of actinides with an increase in the atomic number. This effect is due to an increase in the effective charge of the atomic nucleus when filling the internal 5f electron shells. The existence of 5f contraction is confirmed by crystal-chemical estimates and quantum-chemical calculations of various levels.26−30 In An(VI) structures, actinide atoms are contained in the form of dioxocations AnO22+ with two short covalent An=O bonds, and actinide contraction is usually accompanied by a decrease in the lengths of the An=O bonds, while the average length of the equatorial An–O bonds changes insignificantly.31−33
The obtained results on the structures of I–IV confirm the opinion29−31 that the average An=O distances in the actinyl group decrease with increasing actinide atomic number: 1.765(5) Å for U in I, 1.744(4) Å for Np in II and IV, and 1.709(26) Å for Pu in III. At the same time, the average An–O distances in the equatorial plane in the U–Np–Pu series almost do not change and coincide within the standard errors of the bond length estimation (2.459(4) Å approximately for all the four structures). These data also agree with the results of FTIR spectroscopy (see below): the asymmetric stretching vibrations of actinyl ions appear at 915, 922, and 948 cm–1 for An = U, Np, and Pu, respectively, implying a gradual decrease in the An=O distances in the sequence.
The implementation of Voronoi–Dirichlet polyhedra allows one to quantitatively characterize all atoms with the assistance of the VVDP parameter (the volume of the Voronoi–Dirichlet polyhedron of an atom) or its one-dimensional analogue RSD (the radius of a sphere with the volume equal to VVDP). The volume of the Voronoi–Dirichlet polyhedron and, as a consequence, the radius of the spherical domain as integral characteristics depend on the length of all bonds of the central atom with its nearest neighbors in the crystal structure. In monobromoacetates I–IV, the radii of spherical domains of An(VI) atoms in the U–Np–Pu series are 1.31 Å (I), 1.30 Å (II and IV), and 1.29 Å (III) and are referred to as 1:0.996:0.987 to one another. A decrease in the distances in the actinyl group leads to an increase in the degree of nonsphericity of the Voronoi–Dirichlet polyhedron. According to the stereoatomic model of crystal structures,34 the dimensionless second moment of inertia of the Voronoi–Dirichlet polyhedron (G3) is a quantitative estimate of the uniformity of environment distribution. The minimum value of G3 = 0.077 corresponds to a sphere, and the more non-uniform the environment is, the higher the value of this parameter. In the synthesized compounds I–IV, the G3 parameter in the U–Np–Pu series increases sequentially: 0.0834 for I, 0.0837 for II and IV, and 0.0840 for III. These data are in good agreement with the previous results.35 The possibility of reliable determination of the actinide contraction phenomenon in the synthesized compounds proves the undeniable value of the methods of analysis within the stereoatomic model of crystal structures.
3. Experimental Section
3.1. Synthesis
Solutions of tetramethylammonium hydroxide (1 mol/L) and monobromoacetic acid (0.58 mol/L) were added to the water solutions of hexavalent actinide nitrates (UO2(NO3)2 (0.24 mol/L), NpO2(NO3)2 (0.18 mol/L), and PuO2(NO3)2 (0.21 mol/L)). The initial molar ratio of the reagents was AnO2(NO3)2:(CH3)4NOH:CH2BrCOOH = 1:1:5. The obtained transparent solutions were left for slow crystallization at a temperature of approximately 4–6 °C. The crystals, suitable for the X-ray diffraction experiment, were isolated after 4 (compounds II and IV), 5–7 (compound III), and 14 (compound I) days.
3.2. X-ray Diffraction Analysis
The data were collected on a Bruker KAPPA APEX II diffractometer with a CCD area detector using graphite monochromated MoKα (λ = 0.71073 Å) radiation at T = 298 K. The SAINT program was used to integrate the diffraction profiles, and the data were corrected for Lorentz and polarization effects as well as for absorption using SADABS.36 The crystal structures were solved by direct methods and refined by full-matrix least-squares refinement against F2 using SHELXTL software.37 All nonhydrogen atoms were readily located, and their positions were refined anisotropically. The hydrogen atoms of the tetramethylammonium cations and monobromoacetate anions were placed in geometrically calculated positions and refined with UH = 1.2Ueq(N,C).
The coordinates of basis atoms and the values of thermal parameters of the crystal structures were deposited at the Cambridge Crystallographic Data Centre38 under CCDC nos. 2078740, 2078743, 2078741, and 2078742 for I–IV, respectively. The parameters of the experiment and the final values of uncertainty factors for I–IV are given in Table 2. The main geometric parameters of synthesized compounds are provided in the Supporting Information.
Table 2. Crystallographic Data, Experimental Parameters, and Results of Refinement of (CH3)4N[UO2(mba)3] (I), (CH3)4N[NpO2(mba)2(NO3)] (II), (CH3)4N[PuO2(mba)2(NO3)] (III), and (CH3)4N[NpO2(mba)(NO3)2] (IV).
| compound | I | II | III | IV |
|---|---|---|---|---|
| chemical formula | C10H18Br3NO8U | C8H16Br2N2O9Np | C8H16Br2N2O9Pu | C6H14BrN3O10Np |
| syngony, space group, Z | triclinic, P1, 2 | monoclinic, P21/n, 4 | monoclinic, P21/n, 4 | triclinic, P1, 2 |
| a (Å) | 10.2846(5) | 12.8188(3) | 14.0745(4) | 9.4165(2) |
| b (Å) | 10.4846(5) | 10.7726(2) | 8.8946(2) | 9.6346(2) |
| c (Å) | 11.2692(6) | 13.0405(2) | 14.3877(4) | 10.1237(2) |
| α (deg) | 63.8330(10) | 90 | 90 | 76.0690(10) |
| β (deg) | 75.9080(10) | 91.8340(10) | 99.116(2) | 64.8660(10) |
| γ (deg) | 67.0570(10) | 90 | 90 | 84.1920(10) |
| V (Å3) | 1000.57(9) | 1799.86(6) | 1778.40(8) | 807.04(3) |
| Dx (g/cm3) | 2.516 | 2.513 | 2.562 | 2.490 |
| radiation type | (λ; Å) MoKα; 0.71073 | |||
| μ (mm–1) | 14.133 | 10.253 | 8.240 | 8.961 |
| temperature | (K) 296(2) | |||
| crystal dimensions (mm) | 0.17 × 0.22 × 0.26 | 0.08 × 0.12 × 0.15 | 0.10 × 0.14 × 0.17 | 0.18 × 0.18 × 0.20 |
| θmax (deg) | 29.993 | 30.000 | 24.594 | 29.994 |
| hkl range | –14 ≤ h ≤ 14 | –18 ≤ h ≤ 17 | –16 ≤ h ≤ 16 | –13 ≤ h ≤ 13 |
| –14 ≤ k ≤ 14 | –15 ≤ k ≤ 14 | –10 ≤ k ≤ 10 | –13 ≤ k ≤ 13 | |
| –15 ≤ l ≤ 15 | –18 ≤ l ≤ 18 | –16 ≤ l ≤ 16 | –14 ≤ l ≤ 14 | |
| reflection number: collected/unique (N1); Rint/with I > 2σ(I) (N2) | 19,675/5824; 0.0265/5038 | 21,838/5240; 0.0390/3811 | 23,512/2984; 0.0554/2115 | 19,192/4697; 0.0232/4268 |
| parameters refined | 208 | 199 | 199 | 190 |
| uncertainty values | ||||
| wR2 on N1 | 0.0879 | 0.0558 | 0.0683 | 0.0771 |
| R1 on N2 | 0.0335 | 0.0284 | 0.0307 | 0.0273 |
| S | 1.023 | 1.006 | 1.022 | 1.057 |
| Δρmin/Δρmax (e/Å3) | –1.813/1.805 | –0.899/1.151 | –0.844/1.070 | –1.447/1.987 |
3.3. FTIR Spectroscopy
FTIR spectra of compounds I–IV were measured in the range of 500–4000 cm–1 on an FTIR spectrometer (Shimadzu IR Prestige-21). The samples were prepared by careful grinding with melted NaCl. The assignment of absorption bands (Table 3) was preformed according to the literature data.39−41
Table 3. Assignment of Absorption Bands in FTIR Spectra of I–IV.
| wavenumber
(cm–1)a |
||||
|---|---|---|---|---|
| I | II | III | IV | assignment |
| 1536 v.s. | 1562 s. | 1488 v.s. | 1562 s. | νas(COO) |
| 1485 v.s. | 1487 s. | 1420 v.s. | 1487 s. | νas(COO) |
| 1448 m. | 1448 m. | νas(COO) | ||
| 1384 s. | 1384 s. | ν(NO3–) | ||
| 1255 m. | 1278 m. | 1278 m. | νs(COO) | |
| 1080 m. | 1218 w. | 1218 w. | 1218 w. | ν(−C–N−) |
| 1090 m. | ν(−C–N−) | |||
| 915 v.s. | 922 s. | 948 v.s. | 922 s. | νas(AnO22+) |
| 898 v.w. | δ(C–H) | |||
| 836 m. | 836 m. | δ(CH2) | ||
| 748 s. | 748 s. | δ(CH2) | ||
| 692 m. | 668 w. | 692 m. | ν(C–Br) | |
| 570 m. | δ(CH2) | |||
Band intensities: v.s.: very strong; s.: strong; m.: medium; w.: weak; v.w.: very weak.
Acknowledgments
The study was funded by a grant of the Russian Science Foundation (project number 20-73-10250). The authors acknowledge the Center for the Collective Use of Physical Methods of Investigation, A. N. Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c02296.
Geometric parameters of coordination polyhedra of actinide atoms in crystal structures of I–IV (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Navaza A.; Charpin P.; Vigner D.; Heger G. Single-crystal neutron diffraction: structure of sodium tris(acetato)-dioxouranate(1−). Acta Crystallogr., Sect. C: Struct. Chem. 1991, 47C, 1842–1845. 10.1107/S0108270191003165. [DOI] [Google Scholar]
- Burkov V. I.; Mistryukov V. E.; Mikhailov Y. N.; Chuklanova E. B. Chiroptical properties and structures of gyrotropic crystals of uranyl propionates. Russ. J. Inorg. Chem. 1997, 42, 327–331. [Google Scholar]
- Serezhkina L. B.; Vologzhanina A. V.; Klepov V. V.; Serezhkin V. N. Synthesis and X-ray diffraction study of (Cs0.5Ba0.25)[UO2(CH3COO)3] and Ba0.5[UO2(CH3COO)3]. Crystallogr. Rep. 2011, 56, 265–269. 10.1134/S1063774511010214. [DOI] [Google Scholar]
- Rojas R. M.; Herrero M. P.; Benetollo F.; Bombieri G. An X-ray and thermogravimetric study of the lanthanum(III) uranyl propionate-acetate systems. J. Less-Common Met. 1990, 162, 105–116. 10.1016/0022-5088(90)90463-T. [DOI] [Google Scholar]
- Serezhkin V. N.; Grigoriev M. S.; Abdulmyanov A. R.; Fedoseev A. M.; Savchenkov A. V.; Stefanovich S. Y.; Serezhkina L. B. Syntheses, crystal structures, and nonlinear optical activity of Cs2Ba[An(C2H5COO)3]4 (An = U, Np, Pu) and unprecedented octanuclear complex units in KR2(H2O)8[UO2(C2H5COO)3]5 (R = Sr, Ba). Inorg. Chem. 2017, 56, 7151–7160. 10.1021/acs.inorgchem.7b00809. [DOI] [PubMed] [Google Scholar]
- Klepov V. V.; Serezhkina L. B.; Vologzhanina A. V.; Pushkin D. V.; Sergeeva O. A.; Stefanovich S. Y.; Serezhkin V. N. Tris(acrylato)uranylates as a scaffold for NLO materials. Inorg. Chem. Commun. 2014, 46, 5–8. 10.1016/j.inoche.2014.04.024. [DOI] [Google Scholar]
- Pushkin D. V.; Vologzhanina A. V.; Serezhkina L. B.; Savchenkov A. V.; Korlyukov A. A.; Serezhkin V. N. Synthesis, crystal structure, and IR spectral study of Na[(UO2)(C3H7COO)3]·0.25H2O and K[(UO2)(C3H7COO)3]. Russ. J. Inorg. Chem. 2012, 57, 939–944. 10.1134/S0036023612070169. [DOI] [Google Scholar]
- Savchenkov A. V.; Vologzhanina A. V.; Serezhkina L. B.; Pushkin D. V.; Stefanovich S. Y.; Serezhkin V. N. Synthesis, structure, and nonlinear optical activity of K, Rb, and Cs Tris(crotonato)uranylates(VI). Z. Anorg. Allg. Chem. 2015, 641, 1182–1187. 10.1002/zaac.201400575. [DOI] [Google Scholar]
- Klepov V. V.; Serezhkina L. B.; Grigoriev M. S.; Shimin N. A.; Stefanovich S. Y.; Serezhkin V. N. Morphotropy in alkaline uranyl methacrylate complexes. Polyhedron 2017, 133, 40–47. 10.1016/j.poly.2017.04.041. [DOI] [Google Scholar]
- Savchenkov A. V.; Vologzhanina A. V.; Serezhkina L. B.; Pushkin D. V.; Serezhkin V. N. The first uranyl complexes with valerate ions. Acta Crystallogr., Sect. C: Struct. Chem. 2013, 69C, 721–726. 10.1107/S0108270113014832. [DOI] [PubMed] [Google Scholar]
- Savchenkov A. V.; Vologzhanina A. V.; Serezhkina L. B.; Pushkin D. V.; Serezhkin V. N. Synthesis and structure of AUO2(n-C3H7COO)3 and RbUO2(n-C4H9COO)3. Polyhedron 2015, 91, 68–72. 10.1016/j.poly.2015.02.035. [DOI] [Google Scholar]
- Serezhkin V. N.; Grigoriev M. S.; Abdulmyanov A. R.; Fedoseev A. M.; Savchenkov A. V.; Serezhkina L. B. Synthesis and X-ray crystallography of Mg(H2O)6AnO2(C2H5COO)32An = U, Np, or Pu. Inorg. Chem. 2016, 55, 7688–7693. 10.1021/acs.inorgchem.6b01154. [DOI] [PubMed] [Google Scholar]
- Serezhkin V. N.; Savchenkov A. V.; Serezhkina L. B.; Grigoriev M. S.; Budantseva N. A.; Fedoseev A. M. Peculiarities of the supramolecular assembly of tetraethylammonium and 3-brompropionate ions in uranyl, neptunyl, and plutonyl coordination compounds. Inorg. Chem. 2019, 58, 14577–14585. 10.1021/acs.inorgchem.9b02235. [DOI] [PubMed] [Google Scholar]
- Savchenkov A. V.; Uhanov A. S.; Grigoriev M. S.; Fedoseev A. M.; Pushkin D. V.; Serezhkina L. B.; Serezhkin V. N. Halogen bonding in uranyl and neptunyl trichloroacetates with alkali metals and improved crystal chemical formulae for coordination compounds. Dalton Trans. 2021, 50, 4210–4218. 10.1039/D0DT04083E. [DOI] [PubMed] [Google Scholar]
- Serezhkin V. N.; Savchenkov A. V.; Pushkin D. V.; Serezhkina L. B. Stereochemistry of uranium in oxygen-containing compounds. Appl. Solid State Chem. 2018, 2, 2–16. 10.18572/2619-0141-2018-2-3-2-16. [DOI] [Google Scholar]
- Serezhkin V. N.; Serezhkina L. B. Stereochemistry of neptunium in oxygen-containing compounds. Radiochemistry 2018, 60, 1–12. 10.1134/S1066362218010010. [DOI] [Google Scholar]
- Serezhkin V. N.; Pushkin D. V.; Serezhkina L. B. Stereochemistry of plutonium in oxygen-containing compounds. Radiochemistry 2018, 60, 221–232. 10.1134/S1066362218030013. [DOI] [Google Scholar]
- Serezhkin V. N.; Karasev M. O.; Serezhkina L. B. Causes of uranyl ion nonlinearity in crystal structures. Radiochemistry 2013, 55, 137–146. 10.1134/S106636221302001X. [DOI] [Google Scholar]
- Serezhkin V. N.; Savchenkov A. V. Application of the method of molecular Voronoi-Dirichlet polyhedra for analysis of noncovalent interactions in aripiprazole polymorphs. Cryst. Growth Des. 2020, 20, 1997–2003. 10.1021/acs.cgd.9b01645. [DOI] [Google Scholar]
- Serezhkin V. N.; Savchenkov A. V. Advancing the use of Voronoi-Dirichlet polyhedra to describe interactions in organic molecular crystal structures by the example of galunisertib polymorphs. CrystEngComm 2021, 23, 562–568. 10.1039/D0CE01535K. [DOI] [Google Scholar]
- Serezhkin V. N.; Serezhkina L. B.; Shevchenko A. P.; Pushkin D. V. A new method for analyzing intermolecular interactions in the structure of crystals: saturated hydrocarbons. Russ. J. Phys. Chem. 2005, 79, 918–928. [Google Scholar]
- Serezhkin V. N.; Shevchenko A. P.; Serezhkina L. B. New method of analysis of intermolecular contacts in the crystal structure: π-complexes. Russ. J. Coord. Chem. 2005, 31, 467–476. 10.1007/s11173-005-0121-3. [DOI] [Google Scholar]
- Steiner T. The hydrogen bond in the solid state. Angew. Chem., Int. Ed. 2002, 41, 48–76. . [DOI] [PubMed] [Google Scholar]
- Cavallo G.; Metrangolo P.; Milani R.; Pilati T.; Priimagi A.; Resnati G.; Terraneo G. The halogen bond. Chem. Rev. 2016, 116, 2478–2601. 10.1021/acs.chemrev.5b00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaj M.; Capter K. P.; Savchenkov A. V.; Pyrch M. M.; Cahill C. L. Syntheses, structures, and comparisons of heterometallic uranyl iodobenzoates with monovalent cations. Inorg. Chem. 2017, 56, 9156–9168. 10.1021/acs.inorgchem.7b01208. [DOI] [PubMed] [Google Scholar]
- Cotton F.; Wilkinson G.; Murillo C.; Bochmann M.. Advanced Inorganic Chemistry; 6th ed., Wiley: UK, 1999; pp. 1–1376. [Google Scholar]
- Kuchle W.; Dolg M.; Stoll H. Ab initio study of the lanthanide and actinide contraction. J. Phys. Chem. A. 1997, 101, 7128–7133. 10.1021/jp970920c. [DOI] [Google Scholar]
- Pyykkö P. Dirac-Fock one-centre calculations. Phys. Scr. 1979, 20, 647–651. 10.1088/0031-8949/20/5-6/016. [DOI] [Google Scholar]
- Laerdahl J.; Fœgri K.; Visscher L.; Saue T. A fully relativistic Dirac-Hartree-Fock and second-order Moller-Plesset study of the lanthanide and actinide contraction. J. Chem. Phys. 1998, 109, 10806–10817. 10.1063/1.477686. [DOI] [Google Scholar]
- Kovács A.; Konings R. J. M.; Szieberth D.; Krámos B. Study of the An-Cl bond contraction in actinide trichlorides. Struct. Chem. 2014, 25, 991–996. 10.1007/s11224-014-0406-6. [DOI] [Google Scholar]
- Grigor’ev M. S.; Krot N. N. Synthesis and single crystal X-ray diffraction study of U(VI), Np(VI), and Pu(VI) perchlorate hydrates. Radiochemistry 2010, 52, 375–381. 10.1134/S1066362210040090. [DOI] [Google Scholar]
- Fedoseev A. M.; Gogolev A. V.; Charushnikova I. A.; Shilov V. P. Tricarbonate complex of hexavalent Am with guanidinium: synthesis and structural characterization of [C(NH2)3]4[AmO2(CO3)3]·2H2O, comparison with [C(NH2)3]4[AmO2(CO3)3] (An = U, Np, Pu). Radiochim. Acta 2011, 99, 679–686. 10.1524/ract.2011.1875. [DOI] [Google Scholar]
- Yusov A. B.; Mishkevich V. I.; Fedoseev A. M.; Grigor’ev M. S. Complexation of An(VI) (An = U, Np, Pu, Am) with 2,6-pyridinedicarboxylic acid in aqueous solutions. Synthesis and structures of new crystalline compounds of U(VI), Np(VI), and Pu(VI). Radiochemistry 2013, 55, 269–278. 10.1134/S1066362213030053. [DOI] [Google Scholar]
- Krivovichev S.; Burns P.; Tananaev I.. Structural Chemistry of Inorganic Actinide Compounds; Elsiever Science, 2007; pp. 31–65. [Google Scholar]
- Serezhkin V. N.; Savchenkov A. V.; Serezhkina L. B.; Sidorenko G. V. Actinide contraction in oxygen-containing An(VI) compounds. Radiochemistry 2019, 61, 408–419. 10.1134/S1066362219040039. [DOI] [Google Scholar]
- SADABS; Bruker AXS Inc.: Madison, Wisconsin, 2001. [Google Scholar]
- Sheldrick G. M. Crystal structure refinement with SHELXL. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71C, 3–8. 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groom C. R.; Bruno I. J.; Lightfoot M. P.; Ward S. C. The Cambridge Structural Database. Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater. 2016, 72B, 171–179. 10.1107/S2052520616003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretsch E.; Bühlmann P.; Affolter C.. Structure Determination of Organic Compounds: Tables of Spectral Data; Springer-Verlag: Berlin Heidelberg, 2000; pp. 1–421. [Google Scholar]
- Nakamoto K.Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part A: Theory and Applications in Inorganic Chemistry; sixth ed., John Wiley & Sons, Inc.: 2009; pp. 1–426. [Google Scholar]
- Nakamoto K.Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry; sixth ed., John Wiley & Sons, Inc.: 2009; pp. 1–416. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.