Abstract
BACKGROUND
Inadequate pain reduction during anesthetic injection is a significant medical and surgical problem. Vibratory distraction reduces this pain; however, there are minimal data identifying those who respond best.
OBJECTIVE
To quantify analgesia from vibration before anesthetic injection.
MATERIALS AND METHODS
In this partially blinded, single-institution trial, adult participants were randomized to intervention (vibratory anesthetic device, VAD ON) or placebo (VAD OFF). Pain was assessed using the 11-point numeric rating scale (NRS). Relative reduction in NRS between VAD OFF and ON was used to identify minimum clinically important and substantially clinically important difference in pain.
RESULTS
One hundred one tested sites from 87 subjects were assessed. Sixty-three percent were men with a median age of 66 years. From univariate analysis, males, subjects aged <60, and head and neck (HN) treated subjects had a significant reduction in NRS (p < .05). Multivariate analysis identified NRS reductions in females <60 (p = .012), males ≥70 (p = .002), females and males treated on HN (p = .048 and p = .035, respectively), and males ≥70 treated on HN (p = .012). Substantially clinically important difference (≥57% NRS reduction) included subjects <60, females <70, HN treatment aged 60 to 69, males ≥70, and females treated on HN.
CONCLUSION
Vibratory anesthetic device reduces pain during anesthetic injection, primarily for HN treatments and older male subjects.
Pain is a significant surgical problem; 75% of surgical patients experience pain before, during, or after procedures.1 Approximately 12 million dermatologic procedures are performed annually, including Mohs micrographic surgery (MMS), excisions, laser therapy, body sculpting, and injectable cosmetics.2 One-third of MMS patients experience significant pain intraoperatively, and over 50% experience pain postoperatively.3–5
Local anesthetics control pain in the dermatologic setting.6 However, the injection and infiltration of anesthetics causes significant patient discomfort. Pain of anesthetic injection can be reduced by tactile distraction with vibration.7–11 Vibration has been successfully used in several clinical settings, including dermatologic surgery, pediatrics, phlebotomy, dentistry, and injectable cosmetics.7,12–27 This has led to significant reduction in patient pain and anxiety.28
Pain perception and response to analgesia has significant variability among age, sex, and treatment site. Little is known about the effects of vibration analgesia with regards to these 3 variables.29–33 Thus, there is a knowledge gap in identifying the patient subgroups undergoing dermatological procedures which benefit most from vibration. Furthermore, clinical applicability has not been explicitly reported in the dermatologic literature, reflecting another knowledge gap for dermatologic surgeons.
In this study, we performed a large, randomized clinical trial of vibration analgesia in dermatologic surgery patients. To build upon previous studies, we developed a rigorous training and pain assessment algorithm, which minimized the influenceof potential confounders, such as previous vibration analgesia or local anesthetic (Figure 1). By studying a larger subject pool than has been previously tested, we present a well-powered, multivariate analysis of 3 subject variables: age, sex, and treatment site. Novel to this study, we quantify the clinical impact of change in the pain score, in addition to statistical changes in the pain score (see Methods).34,35 By reporting both clinical impact and statistical change in the pain score, we provide greater confidence in clinical applicability of vibratory analgesia.
Figure 1.
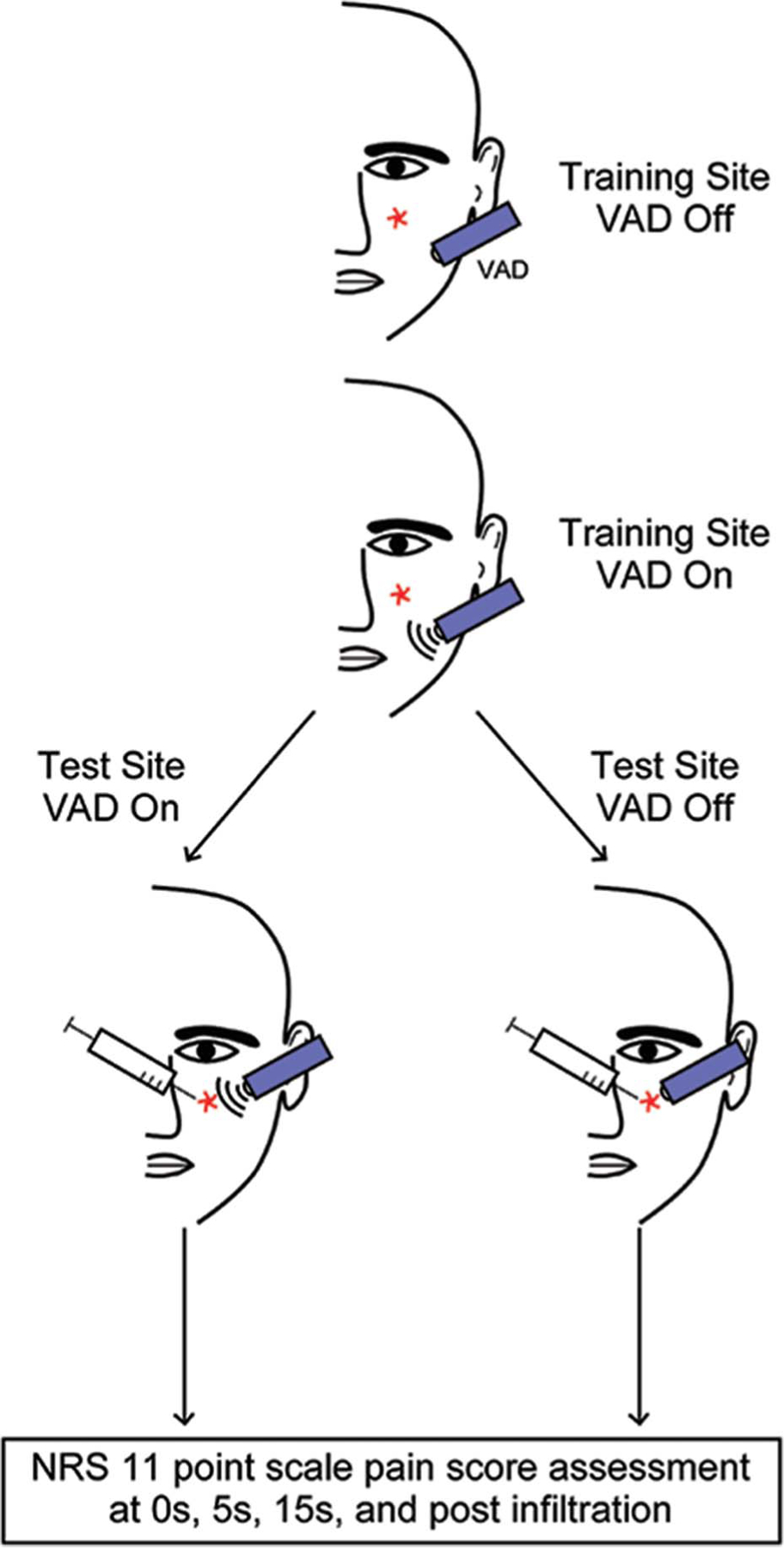
Algorithm for pain assessment with and without VAD. All subjects were trained to the VAD OFF and VAD ON states at a site approximately 5 cm from the test site (indicated by red star). Subjects were then blindly randomized to minimize premediated biases. The treatment was then applied to the injection site, and pain scores were assessed at 0, 5, 15 seconds, and after injection. All injections were conducted at the 15-second time point. VAD, vibratory anesthetic device.
Methods
Study Design
A randomized, partially blinded, parallel-group clinical trial of perception and interpretation of a vibration stimulus compared with placebo (vibratory device in “off” mode) during syringe-mediated cutaneous infiltration of anesthetic was performed. The protocol was approved by the University of Pittsburgh Institutional Review Board (PRO17110134) and conforms to the ethical guidelines of the Declaration of Helsinki.
Participants
All adults (18+) presenting for MMS, surgical excision, and/or other cutaneous cancer removal surgery at Falk Dermatologic Surgery Clinic affiliated with the University of Pittsburgh Medical Center were eligible for inclusion. Subject enrollment occurred during a 90-day period from April to June 2018. Eligible subjects were informed of the study 24 to 72 hours before treatment via phone call.
Patients with expressed interest in the study were given more information on the day of the study, including the risks and benefits of participating. Any subsequent questions were answered at this time. All participants were given a preprocedural questionnaire and provided written informed consent. A follow-up phone call was conducted after the procedure.
Participant Training
Participants underwent a training session to answer trial questions (“What is your pain level?”) using the 11-point numeric rating scale (NRS) as well as descriptive adjectives of sensation (“Can you describe the sensation you felt on your skin?”). The vibratory anesthetic device (VAD) was placed on a “training site” approximately 5 cm away from their treatment site in the OFF mode for 15 seconds (Figure 1). Participants were then asked to describe the sensation they felt on their skin as well as their NRS pain level. This process was repeated with the VAD in the ON mode.
During this time, patients who were unable to discern between the VAD ON and OFF modes, expressed NRS >0, could not communicate effectively, had a language barrier, or were unable to follow instructions were excluded (Figure 2).
Figure 2.
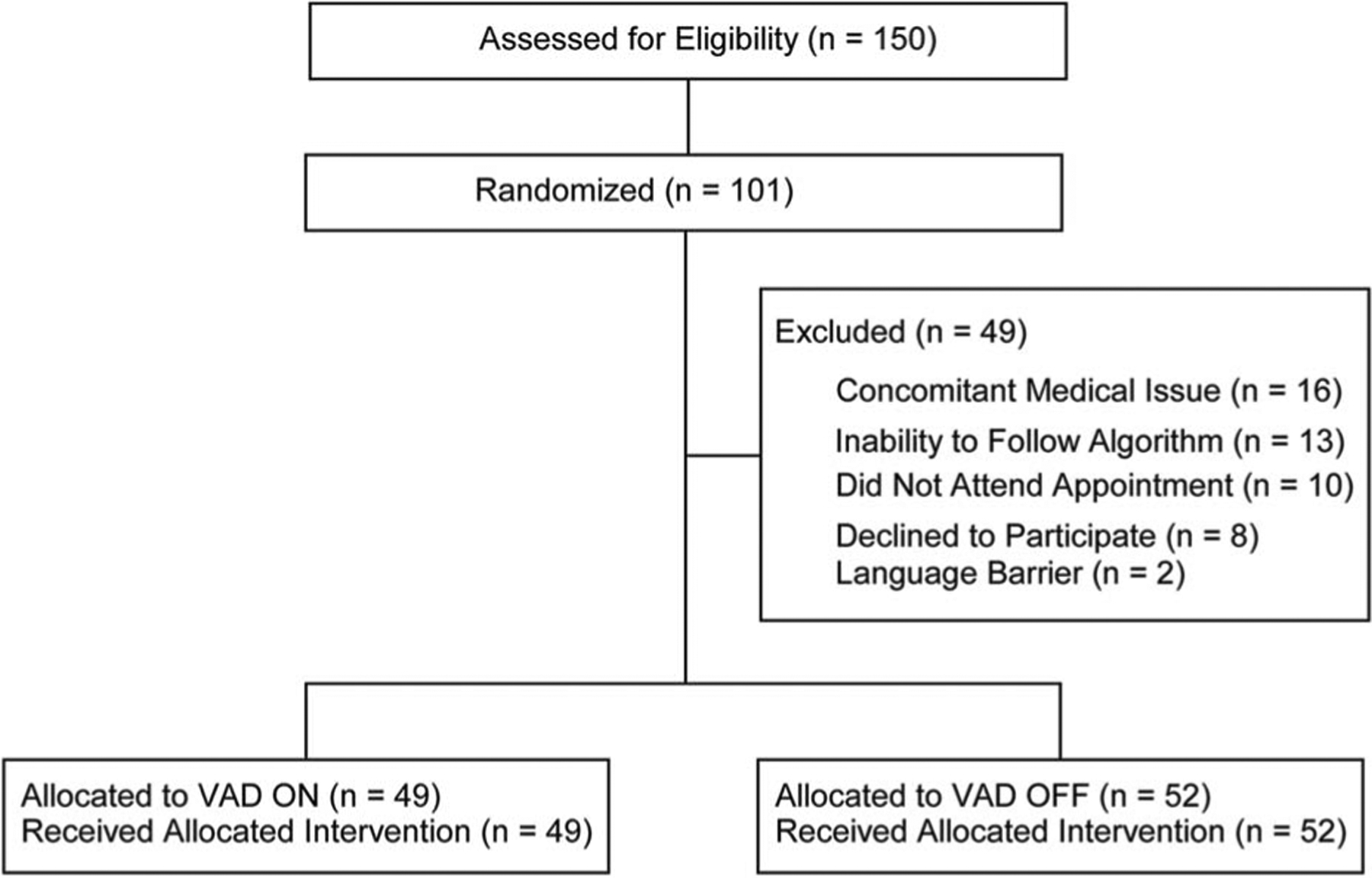
CONSORT 2010 flow diagram. Flow diagram of patient recruitment and exclusion through the study. Note that n represents the number of sites tested (n = 101, of 87 total subjects). Inclusion criteria: Adults (18+) scheduled for cutaneous cancer removal surgery. Exclusion criteria: Procedure cancellation due to a concomitant medical issue (n = 16), inability to complete the training phase (n = 13), did not attend their appointment (n = 10), declined to participate (n = 8), or were non-English speakers (n = 2). One hundred one tested sites were randomly allocated into 2 separate cohorts: VAD ON (n = 49) and VAD OFF (n = 52). All participants who were allocated were included in the final analysis. VAD, vibratory anesthetic device.
Intervention
The VAD is a 10-cm, handheld, battery-operated device by Finever. When ON, the device oscillates at a continuous frequency of 100 Hz. It is nonexperimental and available for purchase over-the-counter. To reduce the pressure from the device, a cotton ball was placed at the tip with subsequent placement into a nitrile glove.18
The VAD, anesthetic injection and infiltration, and surgical procedure were all performed by a single, board-certified dermatologic surgeon (BTC). The anesthetic used in all cases was lidocaine hydrochloride 1% with epinephrine 1:100,000 and buffered with 8.4% bicarbonate 1:10 (Hospira Inc., Lake Forest, IL). The anesthetic was prepared on the day of the procedure and administered in a 3-mL syringe with a 30-gauge needle.
Outcome Measures
Subjects’ pain was assessed using the validated 11-point NRS (0 = no pain, 10 = worst pain imaginable) and recorded by P. Govas or R.M. Slaugenhaupt.36 The pain level was evaluated at 4 timepoints: preanesthetic injection with a stimulus (VAD ON or OFF) at 0 second and 5 seconds; the timepoint of anesthetic injection at 15 seconds (NRS) with a stimulus (VAD ON or OFF); and 5-second postinfiltration with no stimulus present. Patients communicated their NRS at the above timepoints.
Relative change in NRS was calculated with the below:
A comprehensive meta-analysis performed by Olsen and colleagues identified how objective changes in the pain score correlate with subjective, patient-reported assessments of pain. From this study, the team defined minimally clinically important difference (MCID) and substantially clinically important difference (SCID) in the pain score as a relative reduction of 22% to 56% and ≥57% in NRS, respectively.34 We use these parameters when discussing MCID and SCID.
Sample Size
As per Ferreira-Valente and colleagues and Fix and colleagues, parameters of power = 0.80, alpha = 0.05, a mean control NRS of 2.6, and SD of 1 were defined, and a single cohort sample size of 5 was calculated to detect differences in NRS.17,37 Sample size was calculated using ClinCalc (ClinCalc LLC, Arlington Heights, IL).38 Categorical age subgroups (<60, 60–70, and >70 years) were defined to evaluate the cohorts below and above the average age of our subject group. This distribution well approximates the distribution of patients undergoing Mohs surgery.2,39 Our subgroups also maximize the statistical power of each subgroup. Any group that was underpowered from this standard was excluded.
Randomization
All subjects were randomly assigned to the treatment group (VAD ON) or control/placebo group (VAD OFF) using a Gaussian random number generator (https://www.google.com/search?q=random+number). The randomization sequence and assignment of participants was performed by 1 researcher (P.G.).
Blinding
Participants and the surgeon (B.T.C.) were blinded to which arm of the study participants were enrolled until after the “training” portion and immediately before the anesthetic injection.
Statistical Methods
The unpaired 2-tailed Student t-test was used for univariate analysis of all variables for comparison of NRS means. A threshold for significance was set at p < .05. All values collected were analyzed to report mean values ± SE from the mean.
Multivariate linear regression analysis was used to compare categorical variables (age, sex, and treatment site). Multivariate analysis of the overall cohort identified p < .2 for age, sex, and treatment site. All further subgroup analysis was performed using multivariate analysis with a threshold for significance set at p < .05. All statistical analyses were calculated by R. Kazi with Igor Pro (Wavemetrics, version 8) and STATA (StataCorp, version 13) software and confirmed with a consulting statistician (K.M.R.).
Results
Study Population
One hundred fifty surgical sites across 136 subjects were initially eligible to participate. Ultimately, 101 sites across 87 consented subjects were studied (Figure 2). Participants were excluded because of a concomitant medical issue making them ineligible for surgery, inability to follow the treatment algorithm, missed appointment, declined participation, and language barriers. Fifty-two tested sites were randomized into the VAD OFF group and 49 into the VAD ON group (Figure 1). All randomized subjects completed the trial without complication. The majority of participants was men (n = 64, 63%). The average age of subjects was 66 ± 1.1 years (range: 32–89). Baseline demographics were not statistically different between the VAD OFF and VAD ON groups (Table 1).
TABLE 1.
Subject Demographics
| Total (n = 101) | VAD OFF (n = 52) | VAD ON (n = 49) | |
|---|---|---|---|
| Age, mean (range) | 66 (32–89) | 67.4 (43–88) | 64.5 (32–89) |
| <60, n (%) | 25 (24.8) | 11 (21.1) | 14 (28.6) |
| 60–69 | 40 (39.6) | 20 (38.5) | 20 (40.8) |
| ≥70 | 36 (35.6) | 21 (40.3) | 15 (30.6) |
| Sex, n (%) | |||
| Female | 37 (36.6) | 20 (54) | 17 (46) |
| Male | 64 (63.4) | 32 (50) | 32 (50) |
| Treatment site, n (%) | |||
| HN | 72 (71.2) | 38 (52.8) | 34 (47.2) |
| TE | 29 (28.8) | 14 (48.3) | 15 (51.7) |
HN, head and neck; TE, trunk and extremities; VAD, vibratory anesthetic device.
Outcomes
All randomized subjects described the sensation of vibration as “vibration” or “buzzing.” Across all tested sites, there was a 40% reduction in reported NRS between the VAD ON group (2.0 ± 0.3) and the VAD OFF group (1.2 ± 0.2), which achieved statistical significance (p = .016). Because statistical significance does not always correlate with clinical significance, that is, what the patient truly perceives, we performed analyses to determine the clinical relevance of change in the pain score. As determined by Olsen and colleagues,34 a 22% to 56% reduction in NRS represents MCID, whereas a reduction ≥57% represents SCID. Across all tested sites, the reduction in NRS achieved MCID (see Supplemental Digital Content 1, Table S1, http://links.lww.com/DSS/A211). Analysis by age found that those younger than 60 years achieved a statistically significant reduction in NRS (p = .045) and SCID. Those aged 60 to 69 years and 70 years and older achieved MCID without statistically significant reductions in NRS (see Supplemental Digital Content 1, Table S1, http://links.lww.com/DSS/A211). By sex, male and female (n = 37) subgroups both achieved MCID; however, only male subjects had a statistically significant reduction in NRS (p = .048) (see Supplemental Digital Content 1, Table S1, http://links.lww.com/DSS/A211). Anatomically, the head and neck (HN) cohort achieved MCID as well as statistically significant reduction in NRS (p = .007), whereas no change was noted in the trunk and extremity (TE) group (see Supplemental Digital Content 1, Table S1, http://links.lww.com/DSS/A211).
Clinical efficacy and multivariate subgroup analysis were performed for age, sex, and treatment site. Subgroups that achieved SCID included women younger than 60 years, women aged 60 to 69, men 70 years and older, HN treated subjects aged 60 to 69 years, and female HN subjects(see Supplemental Digital Content 2, Table S2, http://links.lww.com/DSS/A212). Multivariate analysis, when subdivided by age, identified statistical significance for female subjects younger than 60 years (p = .012), men 70 years and older (p = .002), and HN treatment in subjects 70 years and older (p = .021). When subdivided by the treatment site, both female (p = .049) and male (p = .035) HN subjects were statistically significant. Finally, when assessing all 3 parameters, only men older than 70 years treated on HN showed statistical significance (p = .012) (see Supplemental Digital Content 2, Table S2, http://links.lww.com/DSS/A212).
Discussion
Pain during dermatological procedures, including the injection of anesthetic, is a major contributor to patient discomfort and has resulted in avoidance of medical care.40 Recently, physicians have used vibration during injection to successfully reduce pain.7,13,16,17,41 In this study, VAD reduced NRS across all subjects by 40% during needle injection, paralleling results of the previous literature (see Supplemental Digital Content 1, Table S1, http://links.lww.com/DSS/A211).7,12,13,15–19. However, there remains a fundamental lack of detail regarding 3 factors: (1) which subgroups most benefit from vibration; (2); how the statistical change in the pain score translates to clinical efficacy, and (3) which subgroups show minimal response to vibration. By using a large subject cohort and a standardized approach to testing all subjects, as well as connecting quantitative changes to clinical impact through the use of minimal and substantial clinically important differences, we have begun addressing these gaps.
The role of age in pain and analgesia is poorly understood. In general, adolescent patients report higher pain scores with injections, but among adults, age is not a factor in pain perception.42,43 Furthermore, many small cohort studies found that age was not a factor in patient response to vibration analgesia.17,24,44 In the intraoperative MMS setting, younger patients are more likely to report pain.3 However, postoperatively, there are conflicting findings; Limthongkul and colleagues showed no correlation between age and pain, whereas Firoz and colleagues reported increased pain in younger patients.4,5 In our study, age was found to influence vibration efficacy. Subjects younger than 60 years were the only cohort to achieve both SCID and statistically significant efficacy in response to VAD (see Supplemental Digital Content 1, Table S1, http://links.lww.com/DSS/A211). However, our subgroup analysis revealed that SCID could beachieved in other age groups depending on parameters such as treatment site and sex (see Supplemental Digital Content 2, Table S2, http://links.lww.com/DSS/A212). Therefore, although VAD may be most efficacious in younger patients, all age groups under specific circumstances may benefit from the application of VAD during anesthetic injection.
Sex also influences pain and analgesia in patients. There are conflicting reports of pain perception during injections between males and females as some studies show no sex-based differences, whereas others show reports of higher pain scores in female subjects.43,45–47 The impact of VAD analgesia during injections has not shown differences between sexes in the dermatological and nondermatological literature.17,48 Among MMS patients, there are no reported differences in pain as a function of sex, although male subjects were more likely to require post-MMS opioid analgesia.3–5,49 Although both male and female subjects achieved MCID in our study, only male subjects had the additional statistically significant reduction in NRS due to VAD (see Supplemental Digital Content 1, Table S1, http://links.lww.com/DSS/A211). As with age, different male and female subgroups achieved SCID and statistical significance. Thus, there is heterogeneity in vibratory efficacy between sex and age cohorts that would benefit from future study.
Finally, pain from injections varies depending on the anatomical site. Previous studies have shown that greater pain is perceived from HN injections compared with TE.32,33 Intraoperative and post-MMS pain were more commonly reported on HN treated sites.3,4,50 Fix andcolleagues17 found no difference when VAD was applied to any anatomic site; however, only the back, nasofacial sulcus, and forehead were assessed. Our analyses showed MCID and statistically significant reduction in NRS for HN sites, whereas TE sites revealed neither clinical nor statistical differences (see Supplemental Digital Content 1, Table S1, http://links.lww.com/DSS/A211).
Furthermore, HN-treated subgroups separated by age and sex showed SCID and statistical differences (see Supplemental Digital Content 2, Table S2, http://links.lww.com/DSS/A212). In the dermatological and nondermatological setting, some studies have demonstrated efficacy of vibration at sites on TE.17,51–53 For example, use of vibration has eased pain of venipuncture and nail procedures in children.16,41 Several reasons may account for the differences in the results of our study and those in the literature. Although all used vibration, there are likely differences in the configuration and geometry of the vibration device, differences in the operator technique, as well as the application of other simultaneous distractors (i.e., cold temperature).52,53 Anecdotally, we have found that treatment of peripheral sites requires longer durations of VAD to achieve analgesia. Therefore, VAD is likely efficacious at TE, but further studies regarding how best to used VAD remain.
Limitations
This study lacks complete blinding for both the subject and operator. Given the nature of vibration, it is practically difficult to implement complete blinding. To overcome this, all subjects were blinded until the exact moment in which vibration or placebo was implemented. Furthermore, the sole operator (B.T.C.) was blinded until the time of injection.
Several studies have used split-site algorithms to test vibratory analgesia.17,54,55 We initially tested our hypothesis using such an approach; however, we encountered several confounders. In a subset of 40 treated sites using a split-site approach, 24 had improved analgesia; however, several subjects reported higher NRS in the VAD OFF mode than in VAD ON. Ultimately, it was not possible to confidently interpret a reduced NRS as being the result of VAD or due to the activity of local anesthetic. Therefore, to minimize this potential confounding, a split-site study was not performed.
This study used a significance criterion of p < .05. If a criterion of p < .01, then significance is seen in HN subjects (p = .007) and M ≥70 (p = .005). Although these groups achieve statistical significance, our conclusions regarding MCID and SCID remain applicable for practitioners.
Finally, this study used a single operator at a single center, which may limit generalizability. This was done to prevent operator bias in these analyses. Further assessment of this approach using a multicenter, multioperator approach is needed for generalizability.
Conclusions
This study draws 3 main conclusions:
Vibration analgesia is a clinically and statistically effective modality in reducing the discomfort of anesthetic injection during dermatologic procedures across the majority of patients.
By testing multiple variables, several patient subgroups who received the most benefit from vibratory anesthesia have been identified.
Trunk and extremity–treated sites showed minimal efficacy because of VAD, and further study is necessary to determine parameters to achieve noninvasive analgesia at these sites.
Supplementary Material
Acknowledgments
The authors thank Dr. Kristine M. Ruppert, DrPH, for aiding in the statistical analysis of this manuscript.
The project described was supported by the National Institutes of Health through Grant Number UL1TR001857. The authors have indicated no significant interest with commercial supporters. R. Kazi and P. Govas contributed equally. IRB Approval status: Reviewed and approved by the University of Pittsburgh; approval PRO17110134; Clinicaltrials.gov listing: NCT03467685.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.dermatologicsurgery.org).
References
- 1.Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg 2003;97:534–40, table of contents. [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim O, Berson DS, Rohrer TE. Trends in dermatologic surgery: results of the American society for dermatologic surgery procedure and consumer surveys. Dermatol Surg 2019;45:303–6. [DOI] [PubMed] [Google Scholar]
- 3.Connolly KL, Nehal KS, Dusza SW, Rossi AM, et al. Assessment of intraoperative pain during Mohs micrographic surgery (MMS): an opportunity for improved patient care. J Am Acad Dermatol 2016;75:590–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Limthongkul B, Samie F, Humphreys TR. Assessment of postoperative pain after Mohs micrographic surgery. Dermatol Surg 2013;39:857–63. [DOI] [PubMed] [Google Scholar]
- 5.Firoz BF, Goldberg LH, Arnon O, Mamelak AJ. An analysis of pain and analgesia after Mohs micrographic surgery. J Am Acad Dermatol 2010;63:79–86. [DOI] [PubMed] [Google Scholar]
- 6.Glass JS, Hardy CL, Meeks NM, Carroll BT. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol 2015;73:543–60; quiz 61–2. [DOI] [PubMed] [Google Scholar]
- 7.Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg 2013;132:675–84. [DOI] [PubMed] [Google Scholar]
- 8.Hanna MN, Elhassan A, Veloso PM, Lesley M, et al. Efficacy of bicarbonate in decreasing pain on intradermal injection of local anesthetics: a meta-analysis. Reg Anesth Pain Med 2009;34:122–5. [DOI] [PubMed] [Google Scholar]
- 9.Bartfield JM, Crisafulli KM, Raccio-Robak N, Salluzzo RF. The effects of warming and buffering on pain of infiltration of lidocaine. Acad Emerg Med 1995;2:254–8. [DOI] [PubMed] [Google Scholar]
- 10.Palmon SC, Lloyd AT, Kirsch JR. The effect of needle gauge and lidocaine pH on pain during intradermal injection. Anesth Analg 1998;86:379–81. [DOI] [PubMed] [Google Scholar]
- 11.Martires KJ, Malbasa CL, Bordeaux JS. A randomized controlled crossover trial: lidocaine injected at a 90-degree angle causes less pain than lidocaine injected at a 45-degree angle. J Am Acad Dermatol 2011;65:1231–3. [DOI] [PubMed] [Google Scholar]
- 12.Nanitsos E, Vartuli R, Forte A, Dennison PJ, et al. The effect of vibration on pain during local anaesthesia injections. Aust Dent J 2009;54:94–100. [DOI] [PubMed] [Google Scholar]
- 13.Park KY, Lee Y, Hong JY, Chung WS, et al. Vibration anesthesia for pain reduction during intralesional steroid injection for keloid treatment. Dermatol Surg 2017;43:724–7. [DOI] [PubMed] [Google Scholar]
- 14.Nasehi A, Bhardwaj S, Kamath AT, Gadicherla S, et al. Clinical pain evaluation with intraoral vibration device during local anesthetic injections. J Clin Exp Dent 2015;7:e23–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuwahara H, Ogawa R. Using a vibration device to ease pain during facial needling and injection. Eplasty 2016;16:e9. [PMC free article] [PubMed] [Google Scholar]
- 16.Smith KC, Comite SL, Balasubramanian S, Carver A, et al. Vibration anesthesia: a noninvasive method of reducing discomfort before dermatologic procedures. Dermatol Online J 2004;10:1. [PubMed] [Google Scholar]
- 17.Fix WC, Chiesa-Fuxench ZC, Shin T, Etzkorn J, et al. Use of a vibrating kinetic anesthesia device reduces the pain of lidocaine injections: a randomized split-body trial. J Am Acad Dermatol 2019;80:58–9. [DOI] [PubMed] [Google Scholar]
- 18.Gresham KA, Carroll BT. A simple elastomer-pad vibratory dampener to maximize pain control of injections in patient’s undergoing dermatological surgery. Dermatol Surg 2016;42:788–90. [DOI] [PubMed] [Google Scholar]
- 19.Mally P, Czyz CN, Chan NJ, Wulc AE. Vibration anesthesia for the reduction of pain with facial dermal filler injections. Aesthet Plast Surg 2014;38:413–8. [DOI] [PubMed] [Google Scholar]
- 20.Duplisea MJ, Flores K. Buzzing away the pain: using an electric toothbrush for vibration anesthesia during painful procedures. Pediatr Dermatol 2019;36:414–5. [DOI] [PubMed] [Google Scholar]
- 21.Veneva E, Cholakova R, Raycheva R, Belcheva A. Efficacy of vibrotactile device DentalVibe in reducing injection pain and anxiety during local anaesthesia in paediatric dental patients: a study protocol for a randomised controlled clinical trial. BMJ Open 2019;9:e029460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chorney SR, Villwock JA, Suryadevara AC. Vibration versus ice to reduce cosmetic botulinum toxin injection pain-A randomized controlled trial. Ear Nose Throat J 2019;98:351–5. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Dong W, Wang M, Xu N. Investigation of the efficacy and safety of topical vibration anesthesia to reduce pain from cosmetic botulinum toxin A injections in Chinese patients: a multicenter, randomized, self-controlled study. Dermatol Surg 2017;43:S329–35. [DOI] [PubMed] [Google Scholar]
- 24.Shaefer JR, Lee SJ, Anderson NK. A vibration device to control injection discomfort. Compend Contin Educ Dent 2017;38:e5–e8. [PubMed] [Google Scholar]
- 25.Hegde KM RN, Srinivasan I, D R MK, et al. Effect of vibration during local anesthesia administration on pain, anxiety, and behavior of pediatric patients aged 6–11 years: a crossover split-mouth study. J Dent Anesth Pain Med 2019;19:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tung J, Carillo C, Udin R, Wilson M, et al. Clinical performance of the DentalVibe(R) injection system on pain perception during local anesthesia in children. J Dent Child (Chic) 2018;85:51–7. [PubMed] [Google Scholar]
- 27.Raslan N, Masri R. A randomized clinical trial to compare pain levels during three types of oral anesthetic injections and the effect of Dentalvibe((R)) on injection pain in children. Int J Paediatr Dent 2018;28:102–10. [DOI] [PubMed] [Google Scholar]
- 28.Momin MA, Hashimoto K, Honda K, Yosue T. The effects of vibration on pain and anxiety during local anesthesia administration. JSM Dent 2004;2:1022. [Google Scholar]
- 29.Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 2013;111:52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci 2012;13:859–66. [DOI] [PubMed] [Google Scholar]
- 31.Packiasabapathy S, Sadhasivam S. Gender, genetics, and analgesia: understanding the differences in response to pain relief. J Pain Res 2018;11:2729–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meeks NM, Glass JS, Carroll BT. Acute pain management in dermatology: mechanisms and pathways. J Am Acad Dermatol 2015;73:533–40; quiz 41–2. [DOI] [PubMed] [Google Scholar]
- 33.Alam M, Geisler A, Sadhwani D, Goyal A, et al. Effect of needle size on pain perception in patients treated with botulinum toxin type A injections: a randomized clinical trial. JAMA Dermatol 2015;151:1194–9. [DOI] [PubMed] [Google Scholar]
- 34.Olsen MF, Bjerre E, Hansen MD, Hilden J, et al. Pain relief that matters to patients: systematic review of empirical studies assessing the minimum clinically important difference in acute pain. BMC Med 2017;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94:149–58. [DOI] [PubMed] [Google Scholar]
- 36.Thong ISK, Jensen MP, Miro J, Tan G. The validity of pain intensity measures: what do the NRS, VAS, VRS, and FPS-R measure? Scand J Pain 2018;18:99–107. [DOI] [PubMed] [Google Scholar]
- 37.Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain 2011;152:2399–404. [DOI] [PubMed] [Google Scholar]
- 38.Rosner B Fundamentals of Biostatistics (7th ed). Boston, MA: Brooks/Cole, Cengage Learning; 2011. [Google Scholar]
- 39.Reeder VJ, Gustafson CJ, Mireku K, Davis SA, et al. Trends in Mohs surgery from 1995 to 2010: an analysis of nationally representative data. Dermatol Surg 2015;41:397–403. [DOI] [PubMed] [Google Scholar]
- 40.Sokolowski CJ, Giovannitti JA Jr, Boynes SG. Needle phobia: etiology, adverse consequences, and patient management. Dent Clin North Am 2010;54:731–44. [DOI] [PubMed] [Google Scholar]
- 41.Secil A, Fatih C, Gokhan A, Alpaslan GF, et al. Efficacy of vibration on venipuncture pain scores in a pediatric emergency department. Pediatr Emerg Care 2014;30:686–8. [DOI] [PubMed] [Google Scholar]
- 42.Nakashima Y, Harada M, Okayama M, Kajii E. Analgesia for pain during subcutaneous injection: effectiveness of manual pressure application before injection. Int J Gen Med 2013;6:817–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nahm FS, Lee PB, Park SY, Kim YC, et al. Pain from intramuscular vaccine injection in adults. Rev Med Chil 2012;140:192–7. [DOI] [PubMed] [Google Scholar]
- 44.Eichhorn MG, Karadsheh MJ, Krebiehl JR, Ford DM, et al. Vibration for pain reduction in a plastic surgery clinic. Plast Surg Nurs 2016;36:63–8. [DOI] [PubMed] [Google Scholar]
- 45.Loram L, Horwitz E, Bentley A. Gender and site of injection do not influence intensity of hypertonic saline-induced muscle pain in healthy volunteers. Man Ther 2009;14:526–30. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell JR, Whitney FW. The effect of injection speed on the perception of intramuscular injection pain. A clinical update. AAOHN J 2001;49:286–92. [PubMed] [Google Scholar]
- 47.Ge HY, Madeleine P, Arendt-Nielsen L. Gender differences in pain modulation evoked by repeated injections of glutamate into the human trapezius muscle. Pain 2005;113:134–40. [DOI] [PubMed] [Google Scholar]
- 48.Alanazi KJ, Pani S, AlGhanim N. Efficacy of external cold and a vibrating device in reducing discomfort of dental injections in children: a split mouth randomised crossover study. Eur Arch Paediatr Dent 2019;20:79–84. [DOI] [PubMed] [Google Scholar]
- 49.Harris K, Curtis J, Larsen B, Calder S, et al. Opioid pain medication use after dermatologic surgery: a prospective observational study of 212 dermatologic surgery patients. JAMA Dermatol 2013;149:317–21. [DOI] [PubMed] [Google Scholar]
- 50.Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg 2011;37:1007–13. [DOI] [PubMed] [Google Scholar]
- 51.Inal S, Kelleci M. Relief of pain during blood specimen collection in pediatric patients. MCN Am J Matern Child Nurs 2012;37:339–45. [DOI] [PubMed] [Google Scholar]
- 52.Baxter AL, Cohen LL, McElvery HL, Lawson ML, et al. An integration of vibration and cold relieves venipuncture pain in a pediatric emergency department. Pediatr Emerg Care 2011;27:1151–6. [DOI] [PubMed] [Google Scholar]
- 53.Baxter AL, Leong T, Mathew B. External thermomechanical stimulation versus vapocoolant for adult venipuncture pain: pilot data on a novel device. Clin J Pain 2009;25:705–10. [DOI] [PubMed] [Google Scholar]
- 54.Sharma P, Czyz CN, Wulc AE. Investigating the efficacy of vibration anesthesia to reduce pain from cosmetic botulinum toxin injections. Aesthet Surg J 2011;31:966–71. [DOI] [PubMed] [Google Scholar]
- 55.Shilpapriya M, Jayanthi M, Reddy VN, Sakthivel R, et al. Effectiveness of new vibration delivery system on pain associated with injection of local anesthesia in children. J Indian Soc Pedod Prev Dent 2015;33:173–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


