Abstract
Objective:
Long-term safety of pembrolizumab in melanoma was analyzed in KEYNOTE-001, KEYNOTE-002, and KEYNOTE-006.
Patients and methods:
Analysis involved patients who received ≥1 pembrolizumab dose. Lead-time bias was addressed via landmark analyses in patients who were progression-free before day 147.
Results:
Adverse events (AEs) were analyzed for 1567 patients (median follow-up, 42.4 months). Most AEs were mild/moderate; grade 3/4 treatment-related AEs occurred in 17.7% of patients. Two pembrolizumab-related deaths occurred. Any-grade immune-mediated AEs (imAEs) occurred in 23.0%, most commonly hypothyroidism (9.1%), pneumonitis (3.3%), and hyperthyroidism (3.0%); grade 3/4 imAEs occurred in 6.9% of patients. Most imAEs occurred within 16 weeks of treatment. In landmark analysis, patients who did (n = 79) versus did not (n = 384) develop imAEs had similar objective response rates (ORRs) (64.6% versus 63.0%); median time to response (TTR), 5.6 months for both; median duration of response (DOR), 20.0 versus 25.3 months; median progression-free survival (PFS), 17.0 versus 17.7 months; median overall survival (OS), not reached (NR) versus 43 months (p = 0.1104). Patients who did (n = 17) versus did not (n = 62) receive systemic corticosteroids had similar ORRs (70.6% vs. 62.9%) and median TTR(6.4 vs. 5.6 months) but numerically shorter median PFS(9.9 vs. 17.0 months); median DOR, 14.2 months versus NR; median OS, NR for both.
Conclusions:
These results enhance the knowledge base for pembrolizumab in advanced melanoma, with no new toxicity signals after lengthy follow-up of a large population. In landmark analyses, pembrolizumab efficacy was similar regardless of imAEs or systemic corticosteroid use.
Clinical trial registry:
Keywords: Pembrolizumab, Advanced melanoma, Immune-related adverse events, Immune-checkpoint inhibitors, PD-1 inhibitors, Immunomodulating drugs, Corticosteroid use
1. Introduction
The immune-checkpoint inhibitors pembrolizumab, nivolumab, and ipilimumab have significantly improved overall survival (OS) in patients with metastatic melanoma [1]. Pembrolizumab is approved for the treatment of various malignancies, including advanced melanoma [2]. The safety and efficacy of pembrolizumab monotherapy in melanoma was established through three studies, KEYNOTE-001, KEYNOTE-002, and KEYNOTE-006 [3–6]. The phase Ib KEYNOTE-001 study included patients with melanoma irrespective of prior ipilimumab therapy [3,7]. The phase II KEYNOTE-002 study compared pembrolizumab with standard-of-care chemotherapy in ipilimumab-refractory melanoma [8]. The phase III KEYNOTE-006 study compared pembrolizumab with ipilimumab in ipilimumab-naive advanced melanoma [5,9]. Across these trials, pembrolizumab induced robust and durable antitumor responses and had a favorable safety profile.
Immune-mediated adverse events (imAEs) have occurred with programmed death 1 (PD-1) inhibitors, including pembrolizumab [10]. Pembrolizumab is generally associated with low-grade and manageable AEs, including dermatologic, gastrointestinal, endocrine, hepatic, renal, and pulmonary toxicities [4,7–9]. This analysis examined the long-term safety of pembrolizumab monotherapy across KEYNOTE-001, KEYNOTE-002, and KEYNOTE-006. In addition, the relationship between imAE occurrence and efficacy was examined. Potential correlations between imAEs and clinical outcomes after checkpoint inhibition are unclear, and many studies have not considered lead-time bias that might give rise to misinterpretation of data [11].
2. Material and methods
2.1. Patients
Pembrolizumab data from 3 trials were pooled (details in Appendix). Follow-up was defined as time from randomization to database cutoff. As the safety profile of pembrolizumab has been shown to be similar across dosing regimens, the data from all pembrolizumab dose groups in KEYNOTE-001, KEYNOTE-002, and KEYNOTE-006 were pooled [5,8,9].
2.2. Safety and efficacy assessments
Safety evaluations included treatment-related AEs (TRAEs), time to onset and resolution of AEs, and use of systemic corticosteroids to manage TRAEs or imAEs. Adverse events were considered an imAE based on mechanism of action and a prespecified list of terms provided by the sponsor (details in Appendix). AEs were graded per the Common Terminology Criteria for Adverse Events (version 4.0) [12]. Time to AE resolution was defined as the longest time from onset to complete resolution or improvement to baseline grade. Resolved AEs were defined as events in which the outcome was recorded as ‘recovered/resolved’ or ‘recovered/resolved with sequelae’ based on the investigator’s discretion, and an end date was specified. Because there were no specific guidelines for resolution of AEs, endocrine AEs that were managed with hormone supplements might have been categorized as resolved or unresolved by different investigators. An exploratory analysis evaluated the relationship between imAE development and efficacy endpoints, objective response rate (ORR), time to response (TTR), duration of response (DOR), and progression-free survival(PFS). AEs occurring before the date of PFS event were included. Tumor assessment was conducted at baseline, followed by imaging every 12 weeks. By week 21, 997 (63.6%) patients experienced disease progression and 107 (6.82%) patients died. To reduce lead-time bias, a landmark analysis [13] was conducted of ORR, TTR, DOR, and PFS by occurrence of imAEs (none vs. any) in patients (n = 463) still on therapy per protocol and without progression before day 147 (week 21). The best overall responses (BORs) before week 21 were not considered, and BOR was re-evaluated at week 21. To manage imAEs, systemic corticosteroids were allowed (Appendix Table A1). To evaluate the potential impact of AE management on pembrolizumab efficacy, ORR, TTR, DOR, PFS, and OS were assessed in patients who did and did not receive systemic corticosteroids. Tumor responses and PFS were assessed by investigator review per Response Evaluation Criteria in Solid Tumors, version 1.1 [14].
2.3. Statistical analysis
Safety and efficacy were assessed in patients who received ≥1 dose of pembrolizumab. ORR and 95% confidence intervals (CIs) were estimated using the Clopper-Pearson method; the difference in ORR among subgroups and 95% CIs were estimated using the Miettinen and Nurminen method; p values were one-sided. TTR and DOR were assessed in patients with confirmed complete or partial response. PFS and DOR were estimated using the Kaplan-Meier method; associated 95% CIs for median PFS were estimated using the Greenwood formula. The hazard ratio (HR) for PFS comparison was based on the Cox regression model, with treatment as a covariate; p values were one-sided and based on the log-rank test. SAS software, version 9.4 (Cary, NC), was used for all analyses.
3. Results
3.1. Patients
Overall, 1567 (616 women, 951 men) pembrolizumab-treated patients with melanoma were included from KEYNOTE-001 (n = 655), KEYNOTE-002 (n = 357), and KEYNOTE-006 (n = 555). Median age was 62.0 years (range, 15‒94); 577 (36.8%) patients had elevated lactate dehydrogenase (LDH) levels; 157 (10.0%) had stable, previously treated brain metastases; and 44.6% previously received ipilimumab therapy (Appendix Table A2). Median treatment duration was 5.1 months (range, 0.0‒46.3); patients received a median of nine doses of pembrolizumab (range, 1‒91). At data cutoff (KEYNOTE-001: September 18, 2015; KEYNOTE-002: February 3, 2017; KEYNOTE-006: December 4, 2017), median follow-up was 42.4 months (range, 24.6‒50.6). Among patients who did (n = 1265) versus did not (n = 302) experience a TRAE, baseline characteristics were generally balanced; however, a greater proportion of patients who had TRAEs had an Eastern Cooperative Oncology Group performance score of 0 (69.0% vs. 49.7%), had normal LDH levels (65.8% vs. 44.7%), had longer median treatment duration (7.7 vs. 2.1 months), and received more pembrolizumab doses (median, 13 vs. 4) (Appendix Table A3).
3.2. Pooled safety analysis
3.2.1. Adverse events
3.2.1.1. Any-cause AEs and TRAEs.
At data cutoff, 1546 (98.7%) patients had any-cause AEs of any grade and 787 (50.2%) had grade 3/4 AEs (Appendix Table A4). A total of 165 (10.5%) patients experienced ≥1 AEs, most commonly malignant neoplasm progression (n = 111; 7.1%) and died subsequently. Any-grade TRAEs occurred in 80.7% of patients (Appendix Table A5), most commonly (≥15%) fatigue (32.5%), pruritus (24.4%), rash (18.6%), and diarrhea (17.8%) (Appendix Table A6). A total of 278 (17.7%) patients experienced grade 3/4 TRAEs, most commonly colitis (1.5%), diarrhea (1.4%), and fatigue (1.3%). Two deaths were considered pembrolizumab-related (sepsis in one patient in KEYNOTE-006 [9]; general physical health deterioration in the setting of grade 3 diarrhea and pneumonia in one patient [85 years] in KEYNOTE-002 [4]). A total of 300 (19.1%) patients received systemic corticosteroids for a TRAE, 1267 (80.9%) did not. TRAEs led to discontinuation in 138 (8.8%) patients; the most frequently reported (>1%) were colitis and pneumonitis, each occurring in 18 (1.1%) patients. Additionally, autoimmune hepatitis led to discontinuation in 7 (0.4%) patients; fatigue and arthralgia in 6 (0.4%) patients; and diarrhea in 4 (0.3%) patients.
3.2.1.2. imAEs: Incidence, onset, and resolution.
Any-grade imAEs occurred in 361 (23.0%) patients, most commonly (≥1.0%) hypothyroidism (9.1%), pneumonitis (3.3%), hyperthyroidism (3.0%), colitis (2.7%), skin and subcutaneous disorders (1.8%), infusion reactions (1.8%), hypophysitis (1.4%), hepatitis (1.2%), uveitis (1.1%), and thyroiditis (1.0%) (Figure 1A). Grade 3/4 imAEs occurred in 108 (6.9%) patients. Median time (in weeks) to onset and resolution of the most common imAEs were hypothyroidism, 15.9 and 8.6; pneumonitis, 36.0 and 8.1; hyperthyroidism, 7.3 and 6.1; colitis, 34.7 and 6.0; skin and subcutaneous disorders, 41.1 and 3.1 (Figures 1B and C). Overall, 75% of imAEs were resolved at data cutoff. Most imAEs (251 new imAEs in 196 [12.5%] patients) occurred within 16 weeks of the first pembrolizumab administration (Figure 2A). Within 160 weeks of treatment initiation, 3 (0.7%) patients of 429 still receiving study treatment developed three new imAE events (Figure 2A). Median time (in weeks) to resolution of the first imAEs treated with systemic corticosteroids: pneumonitis, 7.5; colitis, 6.3; hypophysitis, 9.0; hepatitis, 8.6; adrenal insufficiency, 2.9 (Figure 2B).
Fig. 1.
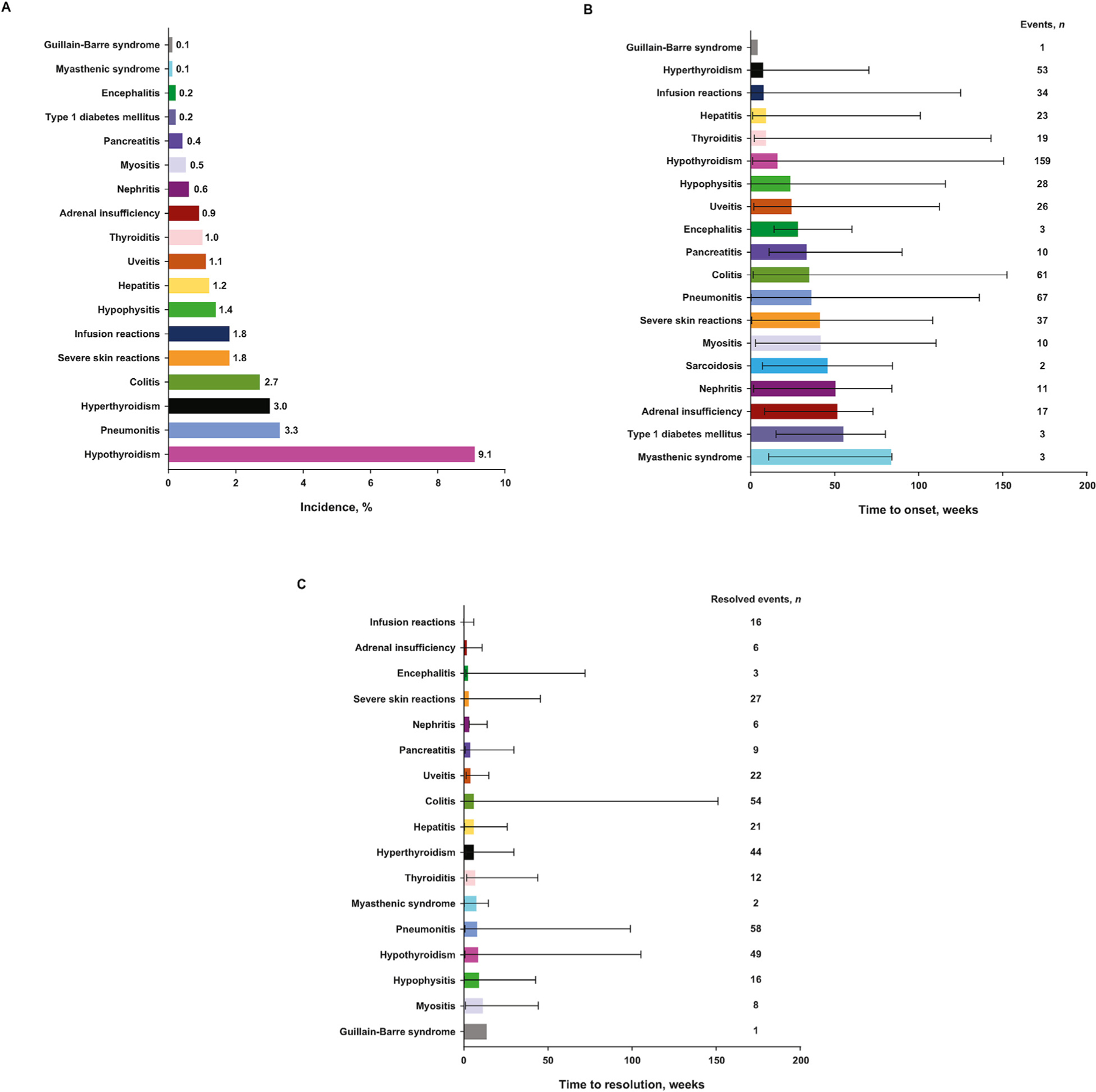
Incidence of any-grade imAEsa occurring in at least one patient (N = 1567) (A); median (range) time to onset (B) and resolution (C) of any-grade imAEs (incidence >0%). imAEs, immune-mediated adverse events. aEndocrine AEs that were managed with hormone supplements might have been categorized as resolved or unresolved by different investigators.
Fig. 2.
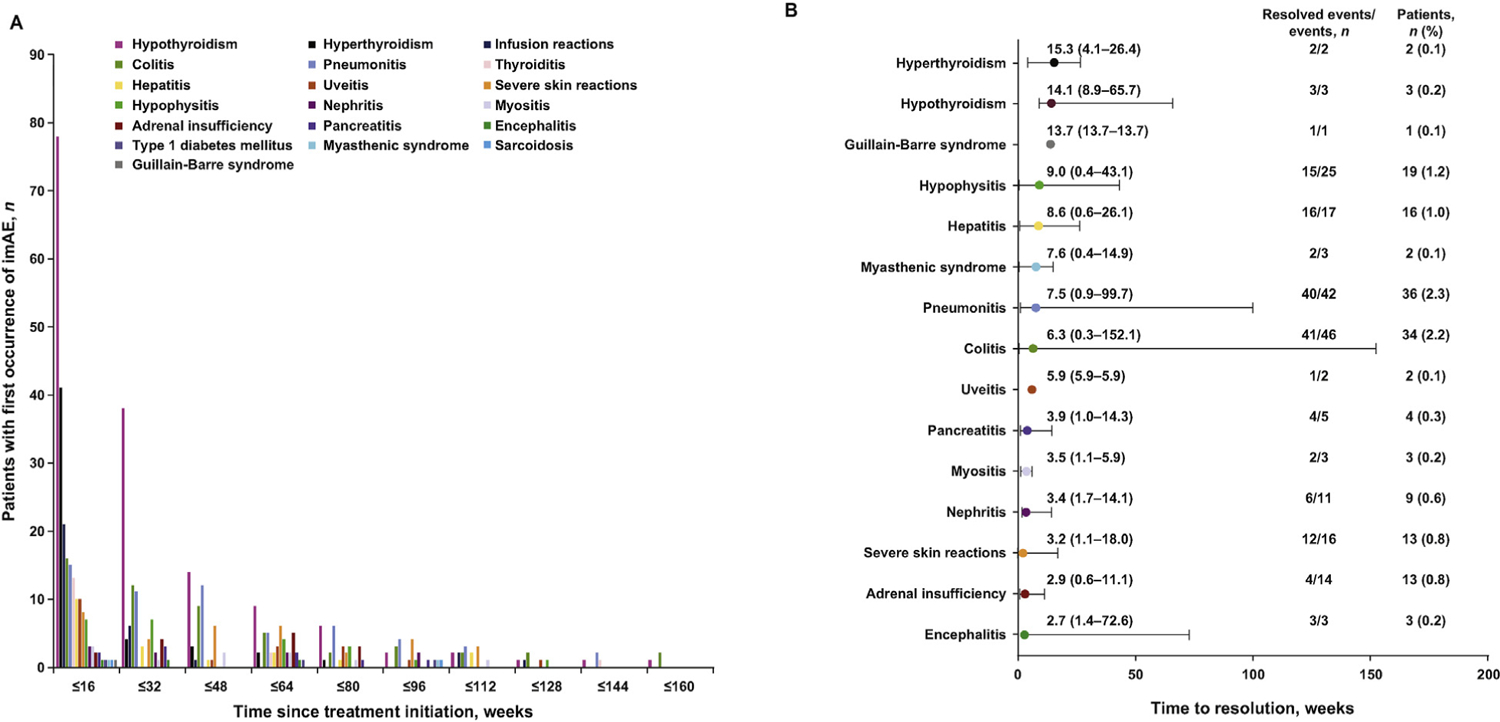
First occurrence of imAEsa of any grade over time (A) and median (range) time to resolution of imAEs of any grade with corticosteroids (B). AEs, adverse events; imAEs, immune-mediated adverse events. aEndocrine AEs that were managed with hormone supplements might have been categorized as resolved or unresolved by different investigators.
3.2.1.3. imAEs managed with systemic corticosteroids: Time to resolution.
Of 361 patients with imAEs, 156 (43.2%) received systemic corticosteroids for treatment of an imAE. Of the 202 any-grade imAEs that were managed using systemic corticosteroids, 152 (75.2%) resolved, with a median time to resolution of 6.4 weeks (range, 0.3‒152.1). A total of 36 (23.1%) patients required a second course of the same systemic corticosteroid or a first dose of a new systemic corticosteroid. Of the 93 grade 3/4 imAEs that occurred in 87 (5.6%) patients and managed using systemic corticosteroids, 75 (86%) resolved with a median time to resolution of 5.1 weeks (range, 0.4‒152.1).
3.2.2. Impact of imAEs on clinical response, PFS, and OS
Of the 463 patients who were progression-free before day 147 (week 21) with available data (291 were still on study), 79 (17.1%) experienced imAEs before week 21 and 384 (82.9%) did not. ORR, median TTR, and median DOR were similar among those who had and had not experienced any-grade imAEs (ORR, 64.6% vs 63.0%, p = 0.3983; median TTR, 5.6 months each; median DOR, 20.0 vs. 25.3 months, respectively) (Table 1).
Table 1.
Impact of imAEs and systemic corticosteroid use for imAEs on overall response, time to response, and duration of response to pembrolizumab therapy in progression-free patients on pembrolizumab at week 21.
| Any-grade imAEs |
Systemic corticosteroid for imAEs |
|||
|---|---|---|---|---|
| Yes (n = 79) | No (n = 384) | Yes (n = 17) | No (n = 62) | |
| Overall response | ||||
| No. of overall responses | 51 | 242 | 12 | 39 |
| ORR, % (95% CI) | 64.6 (53.0‒75.0) | 63.0 (58.0‒67.9) | 70.6 (44.0‒89.7) | 62.9 (49.7‒74.8) |
| Comparison of ORR | Yes vs. no | Yes vs. no | ||
| Estimated difference, % (95% CI)a | 1.5 (−10.5 to 12.5) | 7.7 (−18.9 to 29.0) | ||
| p valueb | 0.3983 | 0.2799 | ||
| Time to response | ||||
| No. of responders | 51 | 242 | 12 | 39 |
| Median (range), months | 5.6 (4.8‒34.7) | 5.6 (4.9‒27.8) | 6.4 (5.‒25.3) | 5.6 (4.8‒34.7) |
| DOR | ||||
| Median (range), months | 20.0 (0.0+ to 26.4+) | 25.3 (0.0+ to 34.3+) | 14.2 (0.‒14.2) | NR (0.0+ to 26.4+) |
A total of 156 (10.0%) patients who received systemic corticosteroids, and 1411 (90.0%) patients who did not receive systemic corticosteroids to manage imAEs of any grade were evaluated for tumor response.
AE, adverse event; CI, confidence interval; DOR, duration of response; imAE, immune-mediated adverse event; NR, not reached; ORR, objective response rate.
Based on Miettinen & Nurminen method.
One-sided p value for testing. H0: difference in % = 0 versus H1: difference in % > 0.
indicates the response duration is censored.
Median PFS was similar among patients who had and had not experienced any-grade imAEs (17.0 vs. 17.7 months; p = 0.4522) (Table 2; Figure 3). Median OS was not reached (NR) for patients who experienced any-grade imAEs and was 43 months for those who did not (p = 0.1104). Three-year OS rates were 66.2% and 59.4%, respectively. Grade 3/4 imAEs occurred in 10 of 463 (2.2%) patients included in the landmark analysis; median OS was not significantly different between these patients (NR) and those who did not (n = 453) experience grade 3/4 imAEs (43.0 months; p = 0.6472).
Table 2.
Impact of imAEs and systemic corticosteroid use for imAEs on PFS and OS in patients on pembrolizumab therapy at week 21.
| Any-grade imAEs |
Systemic corticosteroid for imAEs |
|||
|---|---|---|---|---|
| Yes (n = 79) | No (n = 384) | Yes (n = 17) | No (n = 62) | |
| PFSa | ||||
| No. of events | 51 | 227 | 12 | 39 |
| Median, months (95% CI) | 17.0 (12.1‒23.6) | 17.7 (15.4‒20.1) | 9.9 (6.8‒23.6) | 17.0 (13.6‒25.8) |
| Comparison of PFS | Yes vs. no | Yes vs. no | ||
| HR (95% CI)b | 0.98 (0.72‒1.33) | 1.45 (0.76‒2.79) | ||
| p valuec | 0.4522 | 0.8692 | ||
| OSa | ||||
| No. of events | 26 | 154 | 5 | 21 |
| Median, months (95% CI) | NR (38.2‒NR) | 43.0 (37.9‒NR) | NR (13.8‒NR) | NR (34.3‒NR) |
| Comparison of OS | Yes vs no | Yes vs no | ||
| HR (95% CI)b | 0.77 (0.51‒1.17) | 0.86 (0.32‒2.28) | ||
| p valuec | 0.1104 | 0.3799 | ||
AE, adverse event; CI, confidence interval; HR, hazard ratio; imAEs, immune-mediated adverse events; NR, not reached; ORR, objective response rate; OS, overall survival; PFS, progression-free survival.
From product-limit (Kaplan-Meier) method for censored data.
Based on Cox regression model with treatment as a covariate.
One-sided p value based on log-rank test.
Fig. 3.
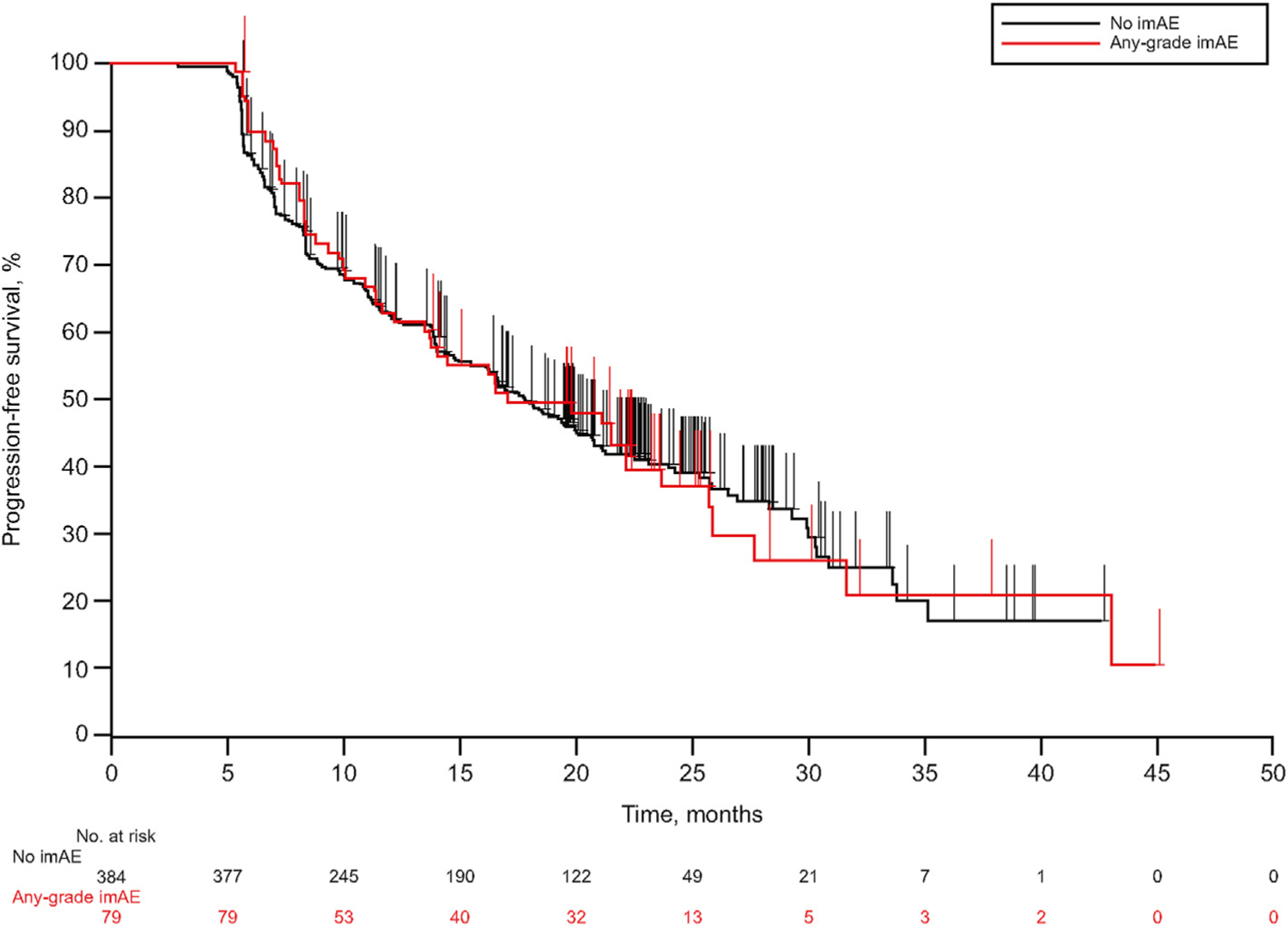
Kaplan-Meier plot of PFS in patients with or without imAEs who were receiving pembrolizumab before week 21. imAEs, immune-mediated adverse events; PFS, progression-free survival.
3.2.3. Impact of systemic corticosteroid use on clinical response, PFS, and OS
In landmark analysis, 17 of 79 (21.5%) patients who experienced imAEs received systemic corticosteroids for the management of imAEs and 62 (78.5%) did not. Among patients with imAEs, ORR and median TTR were similar for patients who did and did not receive systemic corticosteroids (ORR, 70.6% vs. 62.9%, p = 0.2799; median TTR, 6.4 vs. 5.6 months, respectively) (Table 1). Median DOR was 14.2 months (range, 0.0‒14.2) for patients who did and NR for patients who did not receive systemic corticosteroids. The percentage of patients with responses lasting ≥12 months was 80.8% and 92.8%, respectively. Median PFS was numerically shorter among patients who did versus did not (9.9 vs. 17.0 months) receive systemic corticosteroids for imAEs; however, the 95% CI for the HR was wide (0.76‒2.79) and associated with a large p value (p = 0.8692). Median OS was NR in both groups (p = 0.3799). Three-year OS rates were 70.1% and 64.7%, respectively (Table 2; Appendix Figure A1).
4. Discussion
This pooled analysis of KEYNOTE-001, KEYNOTE-002, and KEYNOTE-006 is the most comprehensive analysis of long-term pembrolizumab safety in advanced melanoma. No new toxicity signals (AE type or incidence) were identified. Notably, classification of AEs as immune-mediated was based on a prespecified AE list, regardless of their suspected underlying immune mechanism. AEs that are classically not considered immune mediated—such as fatigue, arthralgia, or pruritus—but might result from an immune reaction were therefore excluded. Historically, definition of imAEs has varied significantly, resulting in disparate data on the incidence and severity of immune-related toxicities [15]. As more data become available from patients treated with checkpoint inhibitors, understanding of etiology of imAEs will improve and allow better comparability between studies.
Results show that most TRAEs, including imAEs associated with pembrolizumab, were low grade. The incidence of any-grade TRAEs, grade 3/4 TRAEs, and TRAEs leading to discontinuation were similar to those reported previously for pembrolizumab, as was the imAE profile [6,8,9,16]. Most imAEs seen with pembrolizumab resolved without sequelae, except for endocrine AEs such as adrenal insufficiency and hypophysitis, which often require long-term endocrine therapy.
The association of tumor response and improved relapse-free survival with the occurrence of imAEs has been reported previously in melanoma treated with immune-checkpoint inhibitors [17–20]. However, performing such analyses without recognizing potential guarantee-time bias, the time period a patient has to remain free of the AEs to be included in the analysis, might lead to misinterpretation because the occurrence of the AE is linked to the duration of follow-up of each patient [11]. In this analysis, we removed guarantee-time bias by conducting a conditioned landmark analysis at week 21 and considering the occurrence of imAEs in patients without progression before day 147. This revealed similar response characteristics and similar PFS and OS among patients who did or did not experience imAEs.
Successful management of imAEs is critical to reducing sequelae and ensuring continued treatment benefit [10,18]. Data on time to onset and resolution of imAEs can inform clinicians of the similarities and differences between therapies and assist in early recognition and management of these events. Mostly, the first occurrence of imAEs associated with pembrolizumab happened within the first 16 weeks, similar to what is reported with nivolumab [17,18]. However, three (0.7%) patients did experience the first occurrence of imAEs later (at week 160 of treatment initiation). Thus, careful monitoring of imAEs throughout treatment is warranted.
Systemic corticosteroids may potentially mitigate antitumor immune response [17,21,22]. In this analysis, no significant differences were observed in efficacy among patients who received and did not receive systemic corticosteroids to manage imAEs. A finding similar to that of other studies of checkpoint inhibitors which have reported that systemic corticosteroids did not negatively affect antitumor responses [17,21,22]. While these results suggest that systemic corticosteroids do not impair the antitumor activity of checkpoint inhibitors, a caveat could be the masking of the effect of corticosteroids on PD-1 efficacy because patients requiring systemic corticosteroids may also be responsive to anti-PD-1 therapy. Another parameter that may influence the effect of corticosteroids on PD-1 efficacy in the current analysis is the dose and duration of corticosteroids. The immunosuppressive effects of corticosteroids are dose-dependent, with higher doses leading to greater immunosuppressive effects [23]. In the current analysis, systemic corticosteroid use varied in dose and duration among the 156 patients who received corticosteroids. Therefore, current results should be interpreted with caution.
Limitations of this analysis include lack of multiplicity adjustments for hypothesis testing of association between imAE occurrence or systemic corticosteroid use and efficacy, and the choice of an appropriate landmark. Ideally, one chooses a landmark at a time when all of the classification events (imAEs) and none of the outcome events (progression) have occurred [24]. For this analysis, setting the landmark at week 12 was considered too soon because those having an imAE after week 12 would be classified as having “no imAE” at week 12. Setting the landmark at week 26 (or later) was considered too late because patients who already had experienced progression would have been excluded. Week 21 was thought to balance the goal of maximizing the number of patients having imAE before the landmark, while minimizing the number of patients having progression before the landmark. Additional limitations include the small subgroup size of patients in the landmark analysis whereby of 79 patients who experienced imAEs, only 17 received systemic corticosteroids to manage imAEs. The sample size for this subgroup may be considered insufficient to accurately assess the impact of corticosteroid treatment on pembrolizumab efficacy.
Potential sources of bias include investigator discretion in assessment of AE as treatment-related and differences in baseline characteristics between patients who had versus had not experienced imAEs. Data pooling from three studies could also be considered a limitation due to differences in patient populations. However, KEYNOTE-001, KEYNOTE-002, and KEYNOTE-006 had similar eligibility criteria. This population is therefore likely to be more homogeneous and have more complete data collection than that of a real-world analysis, the only other study type that would include such large patient numbers.
Results of this large pooled analysis have demonstrated the long-term safety profile of pembrolizumab monotherapy in advanced melanoma. TRAEs and imAEs were primarily mild to moderate, and the efficacy of pembrolizumab was similar regardless of the occurrence of imAEs or the use of systemic corticosteroids for management of imAEs in the landmark analysis. These results further enhance understanding of pembrolizumab safety for the treatment of advanced/metastatic melanoma.
Supplementary Material
Acknowledgements
The authors thank Hesham Aboshady for technical help, James R. Anderson for input on statistical approach, and Scot W. Ebbinghaus for research supervision and critical review of this manuscript. Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Additionally, this research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Funding
This work was supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. No grant number is applicable.
Role of the funding source
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, sponsored this study. The sponsor collaborated with academic advisors to design the study and gather, analyze, and interpret the results. All authors had full access to all study data and approved the decision to submit the manuscript for publication.
Footnotes
Conflict of interest statement
C.R. has participated in advisory boards for Roche; Pierre Fabre; Merck; Novartis; Amgen; Bristol-Myers Squibb; Novartis; Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, U.S.A. (MSD); and Sanofi. O.H. reports personal fees from Merck for consulting during the conduct of the study; as well as contracted research for his institution from Arcus, Aduro, Akeso, Amgen, Array, Bristol-Myers Squibb, CytomX, Exelixis, Genentech, GSK, Immunocore, Incyte, Iovance, Merck, Moderna, Merck Serono, NextCure, Novartis, Regeneron, Roche, Seattle Genetics, Torque, and Zelluna outside the submitted work. A.R. has received personal fees as honoraria for consulting from Amgen, Chugai, Genentech-Roche, Novartis, and Merck; and personal fees, as a scientific advisory member and stockholder from Arcus, Bioncotech, Compugen, CytomX, Five Prime, FLX-Bio, Merus, Rgenix, PACT Pharma, and Tango Therapeutics, outside the submitted work. J.S.W. reports personal fees for honoraria for advisory boards and transportation from Merck, Bristol-Myers Squibb, Genentech, Celldex, Pfizer, and AstraZeneca, outside the submitted work. In addition. J.S.W. has a patent named on a PD-1 biomarker patent by Biodesix issued. I.D. reports grants from Merck, Bristol-Myers Squibb, Incyte, OncoSec, and Regeneron, and personal fees from Regeneron, during the conduct of the study. F.S.H. reports other from Merck to their institution for clinical trial support during the conduct of the study; grants, personal fees, and consulting from Bristol-Myers Squibb; personal fees from Merck, EMD Serono, Genentech/Roche, Bayer, Partners Therapeutics, Sanofi, Pfizer, and Kairos for consulting; grants and personal fees from Novartis for consulting; personal fees from Takeda, Surface, Compass Therapeutics, Verastem, and Rheos for advisory boards; personal fees from Apricity and Bicara for scientific advisory board and equity; personal fees from Aduro for advisor consulting; personal fees from Pionyr for advisory board and equity; personal fees from 7 Hills Pharma for advisor; other from Torque for scientific advisory board and equity; outside the submitted work. In addition, F.S.H. has a patent for Methods for Treating MICA-Related Disorders (#20100111973) with royalties paid, a patent for Tumor antigens and uses thereof issued (#7250291), a patent for Angiopoieten-2 Biomarkers Predictive of Anti-immune checkpoint response pending (#20170248603), a patent for Compositions and Methods for Identification, Assessment, Prevention, and Treatment of Melanoma using PD-L1 Isoforms pending (#20160340407), five patents for Therapeutic peptides pending (#20160046716, #20140004112, #20170022275, #20170008962, #9402905), and a patent for “methods of using pembrolizumab and trebananib.” J.D.W. reports personal fees for consulting from Adaptive Biotech, Amgen, Apricity, Ascentage Pharma, Astellas, AstraZeneca, Bayer, BeiGene, Bristol-Myers Squibb, Celgene, Chugai, Eli Lilly, F-star, Imvaq, Kyowa Hakko Kirin, Linnaeus, MedImmune, Merck, Neon Therapeutics, Ono, Polaris Pharma, Polynoma, PsiOxus, Puretech, Recepta, Takara Bio, Trieza, Truvax, Serametrix, Surface Oncology, Syndax, and Syntalogic; research support from Bristol-Myers Squibb and AstraZeneca; and equity in Potenza Therapeutics, Tizona Pharmaceuticals, Adaptive Biotechnologies, Imvaq, BeiGene, Trieza, and Linnaeus. T.C.M. reports personal fees for honorarium from Bristol-Myers Squibb, Aduro, Merck, and Incyte outside the submitted work. R.W.J. reports other from Bristol-Myers Squibb, and Gilead for consulting, outside the submitted work. C.B. reports personal fees from Bristol-Myers Squibb (board advisor), speaker fees from Merck, and travel fees from Amgen and Sandoz outside the submitted work. GVL is consultant advisor for Aduro Biotech Inc, Amgen Inc, Array Biopharma inc, Boehringer Ingelheim International GmbH, Bristol-Myers Squibb, Highlight Therapeutics S.L., MSD, Novartis Pharma AG, Pierre Fabre, QBiotics Group Limited, Regeneron Pharmaceuticals Inc, SkylineDX B.V. I.P. is a consultant for Amgen, Merck, and Iovance, outside the submitted work. R.Dummer reports intermittent, project focused consulting and/or advisory relationships with Novartis, MSD, Bristol-Myers Squibb, Roche, Amgen, Takeda, Pierre Fabre, Sun Pharma, Sanofi, Catalym, Second Genome, Regeneron, Alligator, MaxiVAX SA and touchIME outside the submitted work. J.L. reports employment at MSD, and GSK. S.J.D. reports personal fees as an employee of MSD, and shareholder inMerck & Co., Inc., Kenilworth, NJ, USA. M.S.C. has served on advisory boards for Bristol-Myers Squibb, MSD, Amgen, Novartis, Pierre Fabre, Roche, Sanofi, Merck, Ideaya, Regeneron, Nektar, Eisai, Oncosec and Qbiotics., outside the submitted work. A.J. reports consulting and/or advisory role for Neolukin, Janssen Oncology, Ipsen, AstraZeneca, Sanofi, Noxopharm, IQvia, Pfizer, Novartis, Bristol-Myers Squibb, and Merck Serono, outside the submitted work. A.J. also reports research funding from Bristol-myers Squibb, Janssen Oncology, MSD, Mayne Pharma, Roche/Genentech, Bayer, Macrogenics, Lilly, Pfizer, AstraZeneca, and Corvus Pharmaceuticals. All remaining authors have declared no conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2020.11.010.
Writing assistance
Medical writing and/or editorial assistance was provided by Jemimah Walker, PhD, and Doyel Mitra, PhD, CMPP, of ApotheCom (Yardley, PA, USA). This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
References
- 1.Karlsson AK, Saleh SN. Checkpoint inhibitors for malignant melanoma: a systematic review and meta-analysis. Clin Cosmet Invest Dermatol 2017;10:325–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.KEYTRUDA® (pembrolizumab) for injection, for intravenous use. 11/2020. Merck Sharp & Dohme Corp. Whitehouse Station, NJ, USA; 2020. [Google Scholar]
- 3.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol 2019;30(4):582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamid O, Puzanov I, Dummer R, Schachter A, Daud A, Schadendorf D, et al. Final analysis of a randomized trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Canc 2017;86:37–45. [DOI] [PubMed] [Google Scholar]
- 5.Robert C, Ribas A, Schachter J, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol 2019;20(9):1239–51. [DOI] [PubMed] [Google Scholar]
- 6.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372(26):2521–32. [DOI] [PubMed] [Google Scholar]
- 7.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369(2):134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16(8):908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017;390(10105): 1853–62. [DOI] [PubMed] [Google Scholar]
- 10.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36(17):1714–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol 2013;31(23):2963–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) version 4. In: National Cancer Institute website; 2009. https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. [Accessed27 July 2020]. [Google Scholar]
- 13.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol 1983;1(11):710–9. [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Canc 2009;45(2): 228–47. [DOI] [PubMed] [Google Scholar]
- 15.Puzanov I, Diab A, Abdallah K, Bingham CO III, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) toxicity management working group. J Immunother Canc 2017;5(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 2016; 315(15):1600–9. [DOI] [PubMed] [Google Scholar]
- 17.Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol 2017;35(7):785–92. [DOI] [PubMed] [Google Scholar]
- 18.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 2012;30(21):2691–7. [DOI] [PubMed] [Google Scholar]
- 19.Sarnaik AA, Yu B, Yu D, Morelli D, Hall M, Bogle D, et al. Extended dose ipilimumab with a peptide vaccine: immune correlates associated with clinical benefit in patients with resected high-risk stage IIIc/IV melanoma. Clin Canc Res 2011;17(4): 896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Canc Res 2016;22(4):886–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Canc Res 2007;13(22 Pt 1):6681–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harmankaya K, Erasim C, Koelblinger C, Ibrahim R, Hoos A, Pehamberger H, et al. Continuous systemic corticosteroids do not affect the ongoing regression of metastatic melanoma for more than two years following ipilimumab therapy. Med Oncol 2011; 28(4):1140–4. [DOI] [PubMed] [Google Scholar]
- 23.Yasir M, Goyal YM, Bansal A, Sonthalia S. Corticosteroid adverse effects. In: Statspearls (internet). Treasure Island, FL: Statpearls Publishing; 2020. [PubMed] [Google Scholar]
- 24.Dafni U Landmark analysis at the 25-year landmark point. Circ Cardiovasc Qual Outcomes 2011;4(3):363–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


