Abstract
Attention-Deficit/Hyperactivity Disorder (ADHD) is associated with impaired cognitive functioning and increased delay discounting (i.e., a stronger preference for immediate reward). At the group level, stimulant medication improves cognition and delay discounting; yet, not all children exhibit problems in these domains, and previous work has not examined whether stimulant-induced improvements are moderated by baseline performance. To address this question in the current study, 82 children with ADHD (9–12 years old) attended a week-long research camp. On the baseline day (Monday), participants completed tasks of inhibitory control, visuo-spatial working memory, reaction time variability, and delay discounting. Children then completed a 3-day, randomized, double-blind, placebo-controlled trial of ~1 mg/kg and 2 mg/kg long-acting methylphenidate (mean doses = 39.1 and 74.3 mg, respectively), during which they were re-administered the battery of tasks. Cognitive composites (mean of inhibitory control, working memory, and reaction time variability performance) were created for the baseline and medication evaluation phases. As predicted, the extent to which cognition was improved with medication compared to placebo, and with 2 mg/kg compared to 1 mg/kg, was greatest among children with poorer baseline cognitive function. Children with stronger baseline cognition exhibited less improvement with methylphenidate compared to placebo and did not benefit from the 2 compared to 1 mg/kg dose. In contrast, medication-related improvement in delay discounting was unrelated to baseline discounting. Given that improving cognitive function is one potential mechanisms by which stimulants exert their therapeutic effects, this study has significant implications for understanding how and for whom stimulant medication works.
Keywords: ADHD, methylphenidate, cognition, moderation, delay discounting
Attention-Deficit/Hyperactivity Disorder (ADHD) is the most commonly diagnosed mental health condition in childhood (Polanczyk et al., 2014). As a group, children with ADHD perform more poorly on tasks assessing a variety of cognitive processes theorized to be involved in the etiology of ADHD, including working memory (Kasper et al., 2012), inhibitory control (Lipszyc & Schachar, 2010), and reaction time variability (Kofler et al., 2013). Numerous studies have attempted to remediate cognitive difficulties via stimulant medication, and meta-analytic work suggests that stimulants result in small-to-moderate improvements across a range of cognitive functions (Coghill et al., 2014). Yet, effect size estimates are variable across individual studies, and effect sizes are notably smaller than those observed for clinical outcomes (e.g., Faraone & Buitelaar, 2010).
As discussed by Coghill and colleagues, these two patterns may be driven, at least in part, by heterogeneity of cognitive functioning in ADHD. There is increasing recognition that the pattern of cognitive functioning in ADHD is heterogeneous; only a subset of children will have cognitive problems in any particular domain, and few will have deficits across multiple domains (Kofler et al., 2018; Nigg et al., 2005). Thus, evaluations of stimulant effects on cognition in ADHD will invariably attempt to remediate “deficits” that do not exist for some children.
Though it seems intuitive that baseline performance would moderate the impact of medication on cognition, empirical evidence is lacking. There is some evidence from studies of healthy adults that baseline working memory is correlated with greater methylphenidate-induced working memory improvement (Mehta et al., 2000), and that amphetamine improves inhibitory control only for those with poor baseline inhibition (De Wit, Crean, & Richards, 2000). In the ADHD literature, we are aware of only one study that has evaluated this question. Hale and colleagues (2011) administered a battery of neuropsychological tasks to children and adolescents with ADHD under conditions of baseline, placebo, and two doses of methylphenidate (MPH). Based on factor scores derived from the task battery, participants were classified as having no, low, moderate, and high executive impairment. The authors found that individuals with the greatest cognitive impairment at baseline showed the best response to MPH, whereas individuals with intact cognition showed only small improvements with MPH.
While this study was an important first step in understanding heterogeneity in MPH effects on cognition, analytic concerns limit the interpretability of results. Most notably, although an independent baseline cognitive assessment was collected, baseline performance was used in the evaluation of medication effects (the medication evaluation included four levels: baseline, placebo, low, and high MPH doses), which artificially inflates the association between “baseline” performance and the medication effect (Baschnagel & Hawk, 2008). To illustrate this point, consider a scenario in which performance under placebo is used as either a predictor or moderator of a difference score between placebo and active medication (i.e., the medication effect). In this case, placebo performance contributes approximately half of the variance to the difference score, so placebo performance and the medication effect will be related because of statistical artifact, regardless of the true association.
The current study builds upon this initial work and is the first to utilize an independent baseline cognitive assessment as a moderator of medication response on cognition among youth with ADHD (but see Hale et al., 2011). We focused on inhibitory control as measured by the stop signal task, reaction time variability (RTV) from a simple 2-choice discrimination task, and visuo-spatial working memory (VSWM) because all three domains are central in cognitive theories of ADHD etiology, and multiple studies have evaluated their response to MPH (see Coghill et al., 2014; Pietrzak, Mollica, Maruff, & Snyder, 2006). We have previously reported robust medication effects on each of these cognitive variables (Rosch et al., 2016; Shiels et al., 2009; Spencer et al., 2009). The novelty of the current analysis lies in the evaluation of whether these medication effects on cognitive task performance are moderated by baseline cognition. Baseline cognition was predicted to moderate cognitive response to methylphenidate (MPH), such that children with good baseline cognitive function would demonstrate minimal improvements with MPH, whereas children with poor baseline cognitive functioning would demonstrate large improvements with MPH.
We also expand upon previous work to examine whether the moderation effects predicted for cognition are observed in the motivation domain, given the emphasis on atypical response to reward in multifactorial causal models of ADHD (Luman et al., 2010; Sonuga-Barke, Bitsakou, & Thompson, 2010) and the impact of stimulant medication on dopaminergic systems central in neural circuitry governing response to reward (Volkow et al., 2001). In particular, individuals with ADHD tend to show a stronger preference for smaller, immediate rewards over larger, delayed rewards, referred to as delay discounting (Jackson & MacKillop, 2016; Patros et al., 2016). Using an experiential discounting task (EDT), we anticipated that baseline delay discounting may relate to the extent to which stimulant medication reduces discounting; yet, this hypothesis is tentative because little research has evaluated stimulant effects on discounting (cf. Shiels et al., 2009), and heterogeneity of delay discounting has not been investigated in ADHD.
Method
All study procedures were approved by the Institutional Review Board at the University at Buffalo, SUNY. Participants included 821 children (9–12 years old) with a DSM-IV (American Psychiatric Association, 2000) diagnosis of ADHD (see Table 1). Diagnoses were made via structured diagnostic interviews (Diagnostic Interview Schedule for Children – 4th Edition; Shaffer et al., 2000) and parent/teacher rating scales of symptoms (Disruptive Behavior Disorders Rating Scale; Pelham et al., 1992) and impairment (Impairment Rating Scale; Fabiano et al., 2006; see Rosch et al., 2016 for details).
Table 1.
Participant characteristics.
| Age, mean (SD) | 10.8 (1.1) |
| Sex (% male:female) | 74:26 |
| % stimulant naïve | 21 |
| WISC Full-Scale IQ, mean (SD) | 103 (14) |
| Race (% white:black:other) | 81:12:7 |
| Hyp/Imp symptoms, mean (SD) | |
| Parent | 5.2 (2.6) |
| Teacher | 3.7 (2.9) |
| Inattentive symptoms, mean (SD) | |
| Parent | 6.7 (2.4) |
| Teacher | 4.9 (2.7) |
| ODD symptoms, mean (SD) | |
| Parent | 3.3 (2.6) |
| Teacher | 1.7 (2.2) |
Note. Symptoms represent the total number endorsed in each domain on the DBD-rating scale. ODD = oppositional defiant disorder.
Children participated in a week-long Summer Research Camp, consisting of academic periods, recreational activities, and computerized cognitive tasks. Task order was counterbalanced across participants. Participants currently taking stimulant medication discontinued use ≥24 hours prior to baseline testing. Baseline testing occurred Monday of camp; children then completed a 3-day, randomized, double-blind, placebo-controlled medication assessment (T-Th). Medication was extended-release OROS MPH. Mean standard and high doses were 1.06 mg/kg (SD=0.12) and 2.02 mg/kg (SD=0.23), respectively, and equates to the nearest commercially-available equivalents of 0.3 and 0.6 mg/kg immediate-release MPH dosed TID. Twenty-four children who were either stimulant-naïve or were previously taking MPH at doses <40% of the high dose (~ 2mg/kg) had their medication order restricted to ensure they received the standard dose (~1 mg/kg) prior to the high dose. Otherwise, dosing order was counterbalanced across participants.
Inhibitory control was assessed with the stop signal task (SST; Logan, Cowan, & Davis, 1984; Rosch et al., 2016). The SST required children to press a button to indicate the directionality of an arrow on the screen (the “go” stimulus). After a brief “go” practice, a “stop” practice introduced the auditory stop signal (1000 Hz tone; 25% of trials). Children then completed four continuous blocks of 64 trials. The initial stop delay (SD) was set at 350 ms and adjusted dynamically in 50-ms increments. Stop signal reaction time (SSRT=mean RT – mean SD) was the primary outcome; smaller SSRTs reflect better inhibitory control. Baseline SST data have been reported elsewhere to evaluate diagnostic group differences (Rosch et al., 2016); no other baseline data have been previously reported.
To assess RTV, children completed a simple discrimination task (“X” vs. “O”) by pressing buttons as quickly as possible while maintaining accuracy (Spencer et al., 2009). The task included 10 practice trials and 100 test trials (2.8-s stimulus duration; 1-s ITI). RTV was quantified with the ex-Gaussian parameter tau, which estimates the skewed tail of an RT distribution due to infrequent, slow RTs (Leth-Steensen et al., 2000).
The computerized visuo-spatial working memory task (Shiels et al., 2008) presented an array of 10 white boxes on a black background. On each trial, a sequence of smiley faces ( ) appeared within these squares. In the backward span condition examined here, children were instructed to click on the squares in the reverse order that they were presented. After a practice trial, children completed trials that advanced in difficulty from a two-location sequence to a maximum of an eight-location sequence, with two trials in each difficulty level. The task terminated when the child missed both trials in the same level. The total number of stimuli correct was the primary outcome variable (see Conway et al., 2005).
) appeared within these squares. In the backward span condition examined here, children were instructed to click on the squares in the reverse order that they were presented. After a practice trial, children completed trials that advanced in difficulty from a two-location sequence to a maximum of an eight-location sequence, with two trials in each difficulty level. The task terminated when the child missed both trials in the same level. The total number of stimuli correct was the primary outcome variable (see Conway et al., 2005).
The experiential discounting task (EDT) is a computerized, real-time delay discounting task during which participants actually receive chosen rewards at specified time delays (Reynolds et al., 2004; Shiels et al., 2009). During the task, participants made choices between a smaller, adjusting immediate reward (initially $0.15) and a larger, fixed ($0.30) delayed reward (7s, 14s, or 28s) delivered in real-time (either immediately or at the specified delay depending on their choice) from a coin dispenser attached to the computer. Participants responded until an indifference point was reached, which was defined by a participant choosing both immediate and delayed options three times within six consecutive choice trials. Indifference values for each delay session were used to calculate area under the curve (AUC; Myerson, Green, & Warusawitharana, 2001); higher AUC values indicate less discounting by delay/less impulsivity.
Rather than presenting analyses for each separate cognitive domain, we created composites of the three cognitive domains for several reasons. First, no task is “process pure,” measuring only its intended construct, and the underlying constructs do not function independently of one another (Karr et al., 2018; Snyder, Miyake, & Hankin, 2015). Second, we had no reason to expect that a pattern of moderation by baseline cognition would vary across cognitive domains and are interested in cognition generally, rather than a specific process. Nevertheless, we present analyses for each domain separately in supplemental online material.
For the baseline cognition composite, SSRT, tau, and stimuli correct from the WM task were each standardized to z-scores. SSRT and tau z-scores were multiplied by −1 so that higher scores reflect better performance, and the average of the three z-scores was computed2. Composites during the medication trial were computed in a similar manner, except that the distribution used for computing z-scores included all three medication conditions: placebo, 1 mg/kg, and 2 mg/kg MPH. Delay discounting scores were standardized in a similar manner for consistency, such that higher scores reflect less delay discounting/less impulsive responses.
Analyses were hierarchical linear models with a random subject intercept. Contrasts were computed for placebo vs. medication (average of 1 and 2 mg/kg) and standard dose vs. high dose. The intercept represents performance under placebo for the placebo-medication contrast and performance under the standard dose for the dose-response contrast. Models for both the cognitive composite and delay discounting include main effects of medication condition as a level 1 predictor, baseline performance as a time-invariant level 2 predictor, and a Medication x Baseline interaction to test whether the strength of medication effects vary as a function of baseline performance. Though many studies examining cognitive heterogeneity in ADHD dichotomize children into impaired and non-impaired groups for each domain (Kofler et al., 2018; Nigg et al., 2005), we did not take this approach because dichotomizing dimensional distributions reduces power and would omit important information about variation within the impaired and non-impaired subgroups (e.g., Cohen, 1983; Decoster et al., 2009).
Results
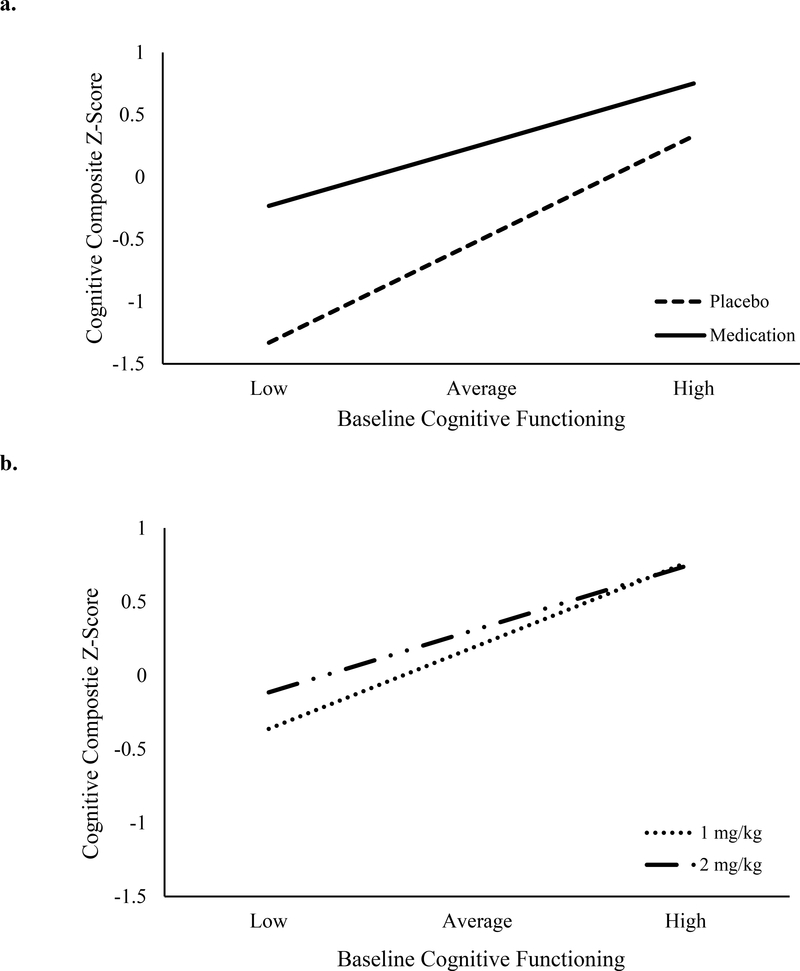
Medication improved the cognitive composite score, β=0.76, p<.001, and baseline cognitive function significantly predicted the cognitive composite (the average of the cognitive composite for placebo and active MPH), β=0.83, p<.001. As predicted, these main effects were qualified by a Medication (placebo vs. average of standard and high doses) x Baseline interaction, β= −0.34, p<.001 (see Figure 1a)3. Specifically, children 1 SD below the mean on the baseline composite showed greater improvement in cognition with medication, β=1.10, p<.001, than those 1 SD above the mean, β=0.42, p<.001. As shown in Supplemental Materials, the pattern of results is similar when cognitive processes are examined separately.
Figure 1. Medication effects on cognition moderated by baseline cognition.
Values for baseline cognitive functioning represent 1 SD below the mean (low) to 1 SD above the mean (high). Cognitive composites are the average z-score of Stop Signal Reaction Time from the stop signal task, tau from a choice discrimination task, and stimuli correct from a visuospatial backwards span task. The top graph represents the contrast between placebo and active methylphenidate (average of the 1 mg/kg and 2 mg/kg dose). The bottom graph represented the dose-response contrast.
The high dose of MPH resulted in better cognitive performance than the standard dose, β=0.11, p=.03, and baseline cognition significantly predicted the cognitive composite (average performance at standard and high doses), β=0.55, p<.001. Main effects were again qualified by a Dose x Baseline interaction, β= −0.13, p=.05 (see Figure 1b). Individuals with relatively poor baseline cognition showed additional improvement from the increase in MPH dose, β=0.23, p=.004, but children with relatively high baseline cognition did not, β= −0.02, p=.82.
For delay discounting, medication reduced AUC, β=0.21, p=.04, and baseline AUC significantly predicted the average AUC across placebo and active medication, β=0.30, p=.004. However, baseline AUC and medication condition did not interact, β=0.04, p=.68. There was no dose effect on AUC, β=0.03, p=.79. Although baseline discounting predicted the average AUC across standard and high doses, β=0.37, p<.001, the effect of dose did not interact with baseline discounting, β= −0.08, p=.45.
Discussion
There is significant interest in using cognitive heterogeneity to better understand ADHD symptoms (Karalunas et al., 2017) and functional impairment (Kofler et al., 2018). Somewhat surprisingly, very little attention has been paid to how cognitive heterogeneity informs medication response. The present study demonstrated that the degree to which MPH improves cognition among children with ADHD depends on children’s initial performance. Although children across the range of baseline cognitive functioning exhibited improvement with MPH relative to placebo, the medication effect was stronger for those with poorer baseline cognition4.
Furthermore, only children with relatively poor baseline cognition benefitted from increasing the MPH dose from ~1 mg/kg to 2 mg/kg. This pattern highlights that, for studies in which medication effects (or dose effects) are small or non-significant, it is especially important to take into account individual difference variables that may moderate medication response. It also suggests that individuals with low versus high baseline cognitive functioning may require different dosages for maximizing cognitive performance. That is, in the current study children with low baseline cognition demonstrated their best performance under the high dose, whereas children with high baseline cognition reached their peak performance at the standard dose.
The robust baseline moderation observed among the cognitive variables was not observed for delay discounting. That is, medication did improve delay discounting, but baseline performance did not significantly impact the strength of the medication effect. These null findings suggest that the cognitive results were not simply an artifact of extreme initial scores showing the greatest change with medication (i.e., those who start further from the ceiling have more room for improvement). One potential explanation for these null findings is the poor testretest reliability of experiential discounting paradigms (Smits et al., 2013). In the present study, the correlation between cognition during baseline and during placebo was high (r= .77) whereas delay discounting showed poor reliability from baseline to placebo (r= .29). Beyond psychometric issues, we assessed a single motivational domain, which may only be impaired in a subset of children with ADHD, as has been shown for cognitive deficits. A thorough examination of heterogeneity of motivational deficits in ADHD and the impact of stimulant medication on motivation is needed to more clearly interpret the present results.
The finding that baseline cognition moderates cognitive response to stimulant medication has significant implications for understanding individual differences in medication response and, relatedly, may inform our understanding of how stimulant medication works. Recent evidence suggests that stimulant-induced improvements in inhibitory control and working memory partially mediate therapeutic effects of MPH on classroom behavior (Hawk et al., 2018). If the magnitude of medication effects on cognition (“a” pathway in a mediation framework) depends on the extent to which those processes are impaired, then improved cognitive function may only be a significant mediator of stimulant response for children with impaired cognitive function; children with intact cognitive function may either benefit clinically via other mechanisms or simply be less likely to exhibit clinical improvement with stimulants. It will be important for future studies to consider heterogeneity in cognition, motivation, and their interaction as potential moderators and mediators of treatment response in order to better integrate evaluations of treatment outcome with theories of ADHD etiology.
Supplementary Material
Public significance:
Among children with Attention-Deficit/Hyperactivity Disorder, this study suggests that stimulant medication markedly improves cognitive functioning for children with cognitive difficulties, and that high doses of medication result in further improvement beyond standard doses. For children with relatively good cognition, medication still improved performance, but to a smaller degree, and there was no additional benefit from the high dose of medication.
Acknowledgments
This work was sponsored by NIMH grant R01 MH069434 to LWH. The funding source had no role in the project other than financial support.
We thank Jerry Richards and Rosemary Tannock, for invaluable contributions to the overall design of the project and input on numerous clinical and methodological details; Beth Gnagy and Greg Fabiano for assistance with making a summer research camp work; Dominica Vito and Brian Gangloff for project coordination; Mark Kugotowski for e-prime programming (programs available from the authors); Louise Cooper and the Research Pharmacy at UB, the many trainees who worked long hours on the project; and the children and families who allowed us to work and play with them in the name of science.
Footnotes
In the past three years, Dr. Waxmonsky has received research funding from Supernus and Pfizer and has served on the advisory board for NLS Pharma and Purdue Pharma. All other authors declare that they have no conflicts of interest.
All authors significantly contributed to this manuscript and all have read and approved the manuscript in its final form.
Prior dissemination of data in the current manuscript:
The medication data (placebo, and two active doses of MPH) have been previously reported to examine stimulant effects on these processes (Rosch et al., 2016; Shiels et al., 2008; Spencer et al., 2009) and the extent to which stimulant effects on various cognitive functions mediates stimulant effects on classroom behavior (Hawk et al., 2018). However, baseline data for most of the tasks have not been previously reported. Only baseline stop signal task performance has been previously reported to evaluate diagnostic-group differences (Rosch et al., 2016).
Seventy-nine children had data for delay discounting analyses, and 80 children had data to evaluate dose-response effects on cognitive function.
We also computed composites based on Bartlett weighted factor scores (DiStefano, Zhu, & Mindrila); the factor scores were correlated rs > .95 with the simple unweighted composites reported here, so the unweighted composites are reported for simplicity.
To ensure that results were driven by baseline cognition, we evaluated the extent to which correlates of baseline cognition moderated medication response. Only age and IQ significantly correlated with baseline cognition, and neither moderated cognitive response to medication, ps > .5.
It is important to keep in mind that “above” and “below” average in the current study is defined relative to other children with ADHD and does not reflect “above” or “below” average in a normative sense.
References
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders, 4th ed. Washington, D.C.: American Psychiatric Association. [Google Scholar]
- Baschnagel JS, & Hawk LW (2008). The effects of nicotine on the attentional modification of the acoustic startle response in nonsmokers. Psychopharmacology, 198, 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill D, Seth S, Pedrosos S, Usala T, Currie J, & Gagliano A (2014). Effects of methylphenidate on cognitive functions in children and adolescents with attention-deficit/hyperactivity disorder: Evidence from a systematic review and a meta-analysis. Biological Psychiatry, 76, 603–615. [DOI] [PubMed] [Google Scholar]
- Cohen JB (1983). The cost of dichotomization. Applied Psychological Measurement, 7, 240–253. [Google Scholar]
- Conway AR, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, & Engle RW (2005). Working memory span tasks: A methodological review and user’s guide. Psychonomic Bulletin & Review, 12, 769–786. [DOI] [PubMed] [Google Scholar]
- de Wit H, Crean J, & Richards JB (2000). Effects of d-amphetamine and ethanol on a measure of behavioral inhibition in humans. Behavioral neuroscience, 114, 830–837. [DOI] [PubMed] [Google Scholar]
- DeCoster J, Iselin AMR, & Gallucci M (2009). A conceptual and empirical examination of justifications for dichotomization. Psychological Methods, 14, 349. [DOI] [PubMed] [Google Scholar]
- DiStefano C, Zhu M, & Mindrila D (2009). Understanding and using factor scores: Considerations for the applied researcher. Practical Assessment, Research & Evaluation, 14, 1–11. [Google Scholar]
- Fabiano GA, Pelham WE Jr, Waschbusch DA, Gnagy EM, Lahey BB, Chronis AM, … & Burrows-MacLean L (2006). A practical measure of impairment: Psychometric properties of the impairment rating scale in samples of children with attention deficit hyperactivity disorder and two school-based samples. Journal of Clinical Child and Adolescent Psychology, 35, 369–385. [DOI] [PubMed] [Google Scholar]
- Faraone SV, & Buitelaar J (2010). Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. European Child and Adolescent Psychiatry, 19, 353–364. [DOI] [PubMed] [Google Scholar]
- Hale JB, Reddy LA, Semrud-Clikeman M, Hain LA, Whitaker J, Morley J, … & Jones N (2011). Executive impairment determines ADHD medication response: implications for academic achievement. Journal of Learning Disabilities, 44, 196–212. [DOI] [PubMed] [Google Scholar]
- Hawk LW Jr., Fosco WD, Colder CR, Waxmonsky JG, Pelham WE Jr., & Rosch KS (2018). How do stimulant treatments for ADHD work? Evidence for mediation by improved cognitive function. Journal of Child Psychology and Psychiatry, 59, 1271–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JN, & MacKillop J (2016). Attention-deficit/hyperactivity disorder and monetary delay discounting: a meta-analysis of case-control studies. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 1, 316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas SL, Gustafsson HC, Dieckmann NF, Tipsord J, Mitchell SH, & Nigg JT (2017). Heterogeneity in development of aspects of working memory predicts longitudinal attention deficit hyperactivity disorder symptom change. Journal of Abnormal Psychology, 126, 774–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr JE, Areshenkoff CN, Rast P, Hofer SM, Iverson GL, & Garcia-Barrera MA (2018). The unity and diversity of executive functions: A systematic review and re-analysis of latent variable studies. Psychological Bulletin, 144, 1147–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper LJ, Alderson RM, & Hudec KL (2012). Moderators of working memory deficits in children with attention-deficit/hyperactivity disorder (ADHD): a meta-analytic review. Clinical Psychology Review, 32, 605–617. [DOI] [PubMed] [Google Scholar]
- Kofler MJ, Irwin LN, Soto EF, Groves NB, Harmon SL, & Sarver DE (2018). Executive functioning heterogeneity in pediatric ADHD. Journal of Abnormal Child Psychology, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler MJ, Rapport MD, Sarver DE, Raiker JS, Orban SA, Friedman LM, & Kolomeyer EG (2013). Reaction time variability in ADHD: A meta-analytic review of 319 studies. Clinical Psychology Review, 33, 795–811. [DOI] [PubMed] [Google Scholar]
- Leth-Steensen C, Elbaz ZK, Douglas VI (2000). Mean response times, variability and skew in the responding of ADHD children: A response time distributional approach. Acta Psychologica, 104, 167–190. [DOI] [PubMed] [Google Scholar]
- Lipszyc J, & Schachar R (2010). Inhibitory control and psychopathology: A meta-analysis of the stop signal task. Journal of the International Neuropsychological Society, 16, 1064–1076. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, & Davis KA (1984). On the ability to inhibit simple and choice reaction time responses: A model and a method. Journal of Experimental Psychology: Human Perception and Performance, 10, 276–291. [DOI] [PubMed] [Google Scholar]
- Luman M, Tripp G, & Scheres A (2010). Identifying the neurobiology of altered reinforcement sensitivity in ADHD: A review and research agenda. Neuroscience and Biobehavioral Reviews, 34, 744–754. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, & Robbins TW (2000). Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. Journal of Neuroscience, 20, RC65–RC65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerson J, Green L, & Warusawitharana M (2001). Area under the curve as a measure of discounting. Journal of the Experimental Analysis of Behavior, 76, 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, & Sonuga-Barke ES (2005). Causal heterogeneity in Attention-Deficit/ Hyperactivity Disorder: Do we need neuropsychologically impaired subtypes?. Biological Psychiatry, 57, 1224–1230. [DOI] [PubMed] [Google Scholar]
- Patros CH, Alderson RM, Kasper LJ, Tarle SJ, Lea SE, & Hudec KL (2016). Choice-impulsivity in children and adolescents with attention-deficit/hyperactivity disorder (ADHD): A meta-analytic review. Clinical Psychology Review, 43, 162–174. [DOI] [PubMed] [Google Scholar]
- Pelham WE Gnagy EM, Greenslade KE, & Milich R (1992). Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 31, 210–218. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Mollica CM, Maruff P, & Snyder PJ (2006). Cognitive effects of immediate-release methylphenidate in children with attention-deficit/hyperactivity disorder. Neuroscience & Biobehavioral Reviews, 30, 1225–1245. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, De Lima MS, Horta BL, Biederman J, & Rohde LA (2007). The worldwide prevalence of ADHD: A systematic review and metaregression analysis. American Journal of Psychiatry, 164, 942–948. [DOI] [PubMed] [Google Scholar]
- Reynolds B, & Schiffbauer R (2004). Measuring state changes in human delay discounting: an experiential discounting task. Behavioral Processes, 67, 343–356. [DOI] [PubMed] [Google Scholar]
- Rosch KS, Fosco WD, Pelham WE, Waxmonsky JG, Bubnik MG, & Hawk LW, (2016). Reinforcement and stimulant medication ameliorate deficient response inhibition in children with Attention-Deficit/Hyperactivity Disorder. Journal of Abnormal Child Psychology, 44, 309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, & Schwab-Stone ME (2000). NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry, 39, 28–38. [DOI] [PubMed] [Google Scholar]
- Shiels K, Hawk LW, Lysczek CL, Tannock R, Pelham WE, Spencer SV, Gangloff BP, & Waschbusch DA (2008). The effects of incentives on visual-spatial working memory in children with Attention-Deficit/Hyperactivity Disorder. Journal of Abnormal Child Psycholgoy, 36, 903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels K, Hawk LW Jr, Reynolds B, Mazzullo RJ, Rhodes JD, Pelham WE Jr, … & Gangloff BP (2009). Effects of methylphenidate on discounting of delayed rewards in attention deficit/hyperactivity disorder. Experimental and Clinical Psychopharmacology, 17, 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits RR, Stein JS, Johnson PS, Odum AL, & Madden GJ (2013). Test–retest reliability and construct validity of the Experiential Discounting Task. Experimental and Clinical Psychopharmacology, 21, 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Miyake A, & Hankin BL (2015). Advancing understanding of executive function impairments and psychopathology: Bridging the gap between clinical and cognitive approaches. Frontiers in Psychology, 6, 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke E, Bitsakou P, & Thompson M (2010). Beyond the dual pathway model: evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 49, 345–355. [DOI] [PubMed] [Google Scholar]
- Spencer SV, Hawk LW, Richards JB, Shiels K, Pelham WE, & Waxmonsky JG (2009). Stimulant treatment reduces lapses in attention among children with ADHD: The effects of methylphenidate on intra-individual response time distributions. Journal of Abnormal Child Psychology, 37, 805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gerasimov M, Maynard L, … & Franceschi D (2001). Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. Journal of Neuroscience, 21, RC121–RC121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.