1. Introduction
SARS-CoV-2, which causes COVID-19, is a novel virus characterized by its unknown origin and rapid spread of disease. The virus has infected more than 215 million individuals worldwide and its associated disease has caused over 4,493,000 deaths, as of August 28, 2021 [1]. A preliminary study conducted in New York discovered a relationship between blood type and susceptibility to as well as severity of COVID-19 [2]. Blood type A was associated with decreased severity of disease, measured by risk of intubation and death, and Blood type O corresponded with a lower risk of infection.
The hallmark of severe COVID-19 is thromboembolic complications, among other symptoms. The endothelial proliferation of SARS-CoV-2 and its resultant damage to the vascular system can activate the coagulation cascade and result in thrombosis. In addition, the immobility of severely and critically ill patients in ICUs increases their likelihood of developing thrombosis. A study conducted in early 2020 determined that individuals with non-O blood types were at an increased risk for certain thromboembolic events [3]. In addition, blood type O patients typically have lower plasma levels of von Willebrand factor (VWF) [4]. Lower levels of von Willebrand proteins in vascular endothelium have been shown to decrease clotting performance and thus reduce risk for thrombosis. Furthermore, blood type O is thought to protect against vascular complications in malaria [5]. This study was conducted to test whether different blood types in COVID-19 patients are correlated with variable protection against thrombosis.
2. Methods
Throughout this analysis of data, all patients who had at least one positive PCR result indicating the presence of SARS-CoV-2 were considered COVID-19 positive (+). The type and screen (T&S) method was used to determine ABO blood group and Rh type. The present study originally included 1264 COVID-19+ patients hospitalized between March 1, 2020, and June 26, 2020, at our institution; however, 760 of these patients were excluded from study involvement due to lack of blood typing data. 504 patients were thus included in the final analysis. Of these, 102 patients had a thromboembolic event – venous thromboembolism (VTE), pulmonary embolism (PE), arterial embolism (AE), cerebellar infarction (CI) and/or cerebral stroke (CS) – which was verified by CT angiography, MRI, and ultrasound during hospital stay, and these 102 formed the study cohort. Some patients had thrombotic events that fell into more than one category. The other 402 exhibited no signs of thrombosis and composed the control cohort. The distribution of each blood type in the study cohort, both from the ABO blood group and the ABO blood group stratified by Rh factor, was compared to the distribution of blood types in the control cohort.
Pearson's chi-square test of homogeneity and Fisher's exact test were used to test for association between blood type and COVID-19 associated thrombosis. A logistic regression model was conducted to control for the possible confounding effect of race, sex, and age on prevalence of thrombosis in the whole COVID-19+ population, as well as mortality in the study cohort. Chi-square tests were also performed to determine whether there was a difference in clinical outcomes, defined by ICU admission, intubation, blood transfusion, and mortality, between the study cohort and the control cohort. Throughout the analysis, a confidence interval of 95% was selected.
This study was approved by the Institutional Review Board (IRB) at Rush University Medical Center in Chicago, IL and conducted in accordance with the Declaration of Helsinki. Due to the retrospective nature of the study, the requirement of written informed consent was waived.
3. Results
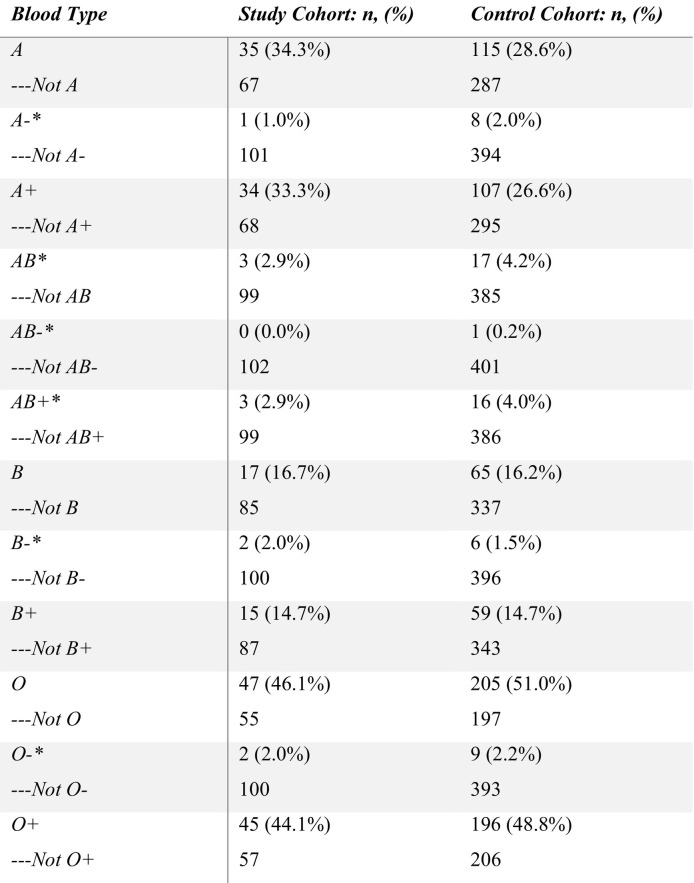
There was no significant difference in prevalence of thrombosis between blood types: the study cohort comprised A (34.3%), AB (2.9%), B (16.7%), and O (46.1%) while the control cohort comprised A (28.6%), AB (4.2%), B (16.2%), and O (51.0%) (p = 0.651). When stratifying by Rh factor, there was still no significant difference in prevalence of thrombosis by blood types: the study cohort was composed of A− (1.0%), A+ (33.3%), AB− (0%), AB+ (2.9%), B− (2.0%), B+ (14.7%), O− (2.0%), O+ (44.1%) while the control cohort was composed of A− (2.0%), A+ (26.6%), AB− (0.2%), AB+ (4.0%), B− (1.5%), B+ (14.7%), O− (2.2%), O+ (48.8%) (p = 0.905). The distribution of blood types in both cohorts is presented in Table 1 . Blood type was not found to be associated with thrombosis after controlling for race, sex, and age. The logistic regression test modeling mortality for patients with both COVID-19 and thrombosis determined that blood type, after controlling for confounders, did not have a significant effect on mortality in the study cohort. Hispanics were found considerably more likely to die than Black or African American individuals after developing COVID-19 associated thrombosis (OR 9.991, 95% CI [3.321–35.085]).
Table 1.
Comparison of the study cohort (COVID-19+ with thrombosis) and control cohort (COVID-19+ without thrombosis) stratified according to the ABO system and Rh factor. * indicates instances where Fisher's exact test was used due to low cell count.
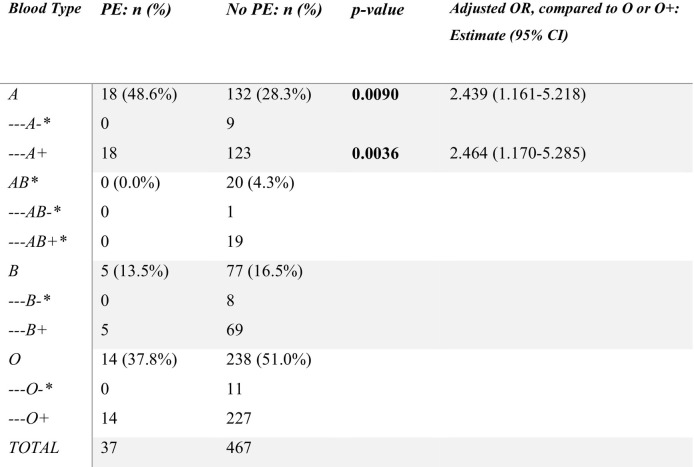
In 102 patients who experienced thrombosis, 65 (63.7%) patients had VTE, 37 (36.3%) patients had PE, 8 (7.8%) had arterial embolism, 23 (22.5%) had cerebral stroke, and 24 (23.5%) had cerebellar infarction. After controlling for race, sex, and age, blood type A+ was associated with higher odds of developing COVID-19 associated PE than blood type O+ (OR 2.464, 95% CI [1.170–5.285]), as shown in Table 2 . Supplementary material #1 contains information about other thrombotic events.
Table 2.
Prevalence of PE in patients with different blood types. * indicates statistical significance.
4. Discussion and conclusion
This study found no increased prevalence of one blood type over another between COVID-19+ patients with thrombosis compared to COVID-19+ patients without thrombosis, implying that patients are not at higher risk for thrombosis in general from COVID-19 infection based on blood type. When further stratifying by Rh factor, there was also no difference in prevalence of thrombosis based on blood types, suggesting that Rh type does not modify the association between blood type and COVID-19 associated thrombosis in general. However, blood type A+ individuals were at an increased risk of developing PE, a specific thrombotic event, as compared to blood type O+, respectively, even after controlling for confounding variables.
While the findings regarding PE suggest that A+ blood type may predispose a patient to certain COVID-19 associated thromboembolic events, this study was limited by its retrospective nature and its setting within a single medical center. Small sample size may have caused a Type II error to occur. Furthermore, severity of disease may have also skewed blood typing data, as patients who were less severely affected by COVID-19 were unlikely to require blood transfusion and hence blood typing. It is also known that individuals with blood type O generally have decreased levels of VWF. Thus, investigating the relationship between VWF level and severity of COVID-19 through prospective studies may bring more insight into COVID-19 associated coagulopathy.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
I would like to offer my special thanks to Perumal Thiagarajan, Robert Taylor, Jan Macfarland, and Ajit Divgi for offering their personal advice on the study and providing editorial assistance. All research was conducted through RUMC. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Presented in abstract form at the virtual 62nd annual meeting of the American Society of Hematology on December 5, 2020.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.thromres.2021.08.024.
Appendix A. Supplementary data
Supplementary tables
References
- 1.Johns Hopkins University. Johns Hopkins Coronavirus Resource Center. Johns Hopkins Coronavirus Resource Center. Published 2021. Accessed August 5, 2021. https://coronavirus.jhu.edu/map.html.
- 2.Zietz M., Zucker J., Tatonetti N.P. Associations between blood type and COVID-19 infection, intubation, and death. Nat. Commun. 2020;11(1):5761. doi: 10.1038/s41467-020-19623-x. Published 2020 Nov 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groot H.E., Villegas Sierra L.E., Said M.A., Lipsic E., Karper J.C., van der Harst P. Genetically determined ABO blood group and its associations with health and disease. Arterioscler. Thromb. Vasc. Biol. 2020;40(3):830–838. doi: 10.1161/ATVBAHA.119.313658. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins P.V., O'Donnell J.S. ABO blood group determines plasma von willebrand factor levels: a biologic function after all? Transfusion. 2006;46(10):1836–1844. doi: 10.1111/j.1537-2995.2006.00975.x. [DOI] [PubMed] [Google Scholar]
- 5.Rowe J.A., Handel I.G., Thera M.A., Deans A.M., Lyke K.E., Koné A., Diallo D.A., Raza A., Kai O., Marsh K., Plowe C.V., Doumbo O.K., Moulds J.M. Blood group O protects against severe Plasmodium falciparum malaria through the mechanism of reduced rosetting. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(44):17471–17476. doi: 10.1073/pnas.0705390104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables