Abstract
Background
Lung cancer has emerged as a global public health problem and is the most common cause of cancer deaths by absolute cases globally. Besides tobacco, smoke infectious diseases such as human papillomavirus (HPV) might be involved in the pathogenesis of lung cancer. However, data are inconsistent due to differences in study design and HPV detection methods.
Aim
A systematic meta‐analysis was performed to examine the presence of HPV‐infection with lung cancer.
Methods and Results
All studies in all languages were considered for the search concepts “lung cancer” and “HPV” if data specific to HPV prevalence in lung cancer tissue were given. This included Journal articles as well as abstracts and conference reports. As detection method, only HPV PCR results from fresh frozen and paraffin‐embedded tissue were included. Five bibliographic databases and three registers of clinical trials including MEDLINE, Embase, Cochrane Library, and ClinicalTrials.gov were searched through February 2020. A total 4298 publications were identified, and 78 publications were selected, resulting in 9385 included lung cancer patients. A meta‐analysis of 15 case‐control studies with n = 2504 patients showed a weighted overall prevalence difference of 22% (95% CI: 12%‐33%; P < .001) and a weighted overall 4.7‐fold (95% CI: 2.7‐8.4; P < .001) increase of HPV prevalence in lung cancer patients compared to controls. Overall, HPV prevalence amounted to 13.5% being highest in Asia (16.6%), followed by America (12.8%), and Europe (7.0%). A higher HPV prevalence was found in squamous cell carcinoma (17.9%) compared to adenocarcinoma (P < .01) with significant differences in geographic patterns. HPV genotypes 16 and 18 were the most prevalent high‐risk genotypes identified.
Conclusion
In conclusion, our review provides convincing evidence that HPV infection increases the risk of developing lung cancer.
Keywords: carcinogenesis, HPV, lung cancer, meta‐analysis
Abbreviations
- AC
adeno carcinoma
- AhR
aryl hydrocarbon receptor
- ALK
anaplastic lymphoma kinase
- ARI
absolute risk increase
- cIAP‐2
baculoviral IAP repeat‐containing protein3
- E6
E6 oncoprotein of human papillomavirus
- E7
E7 oncoprotein of human papillomavirus
- EGFR
epidermal growth factor receptor
- Embase
biomedical and pharmacological bibliographic database
- EU
European Union
- FHIT
fragile histidine triad protein
- HER‐2
receptor tyrosine‐protein kinase erbB‐2
- HIF‐1α
hypoxia‐inducible factor 1‐alpha
- HPV
human papillomavirus
- hTERT
human telomerase reverse transcriptase
- IL
interleukin
- MCL1
induced myeloid leukemia cell differentiation protein
- MEDLINE
U.S. National Library of Medicine
- NHS
National Health Service
- p53
cellular tumor antigen p53
- PCR
polymerase chain reaction
- PD
prevalence difference
- PR
prevalence ratio
- pRb
retinoblastoma protein
- ROS1
proto‐oncogene tyrosine‐protein kinase ROS
- SCC
squamous cell carcinoma
- VEGF
vascular endothelial growth factor
- WHO
World Health Organization
1. INTRODUCTION
Lung cancer is estimated to be the leading cause of cancer‐related mortality worldwide, with 2.1 million new lung cancer cases and 1.8 million predicted deaths worldwide in 2018.1 Although smoking by far has been identified as the most important risk factor in lung cancer, other interactions with environmental and/or genetic risk factors as well as infectious diseases have been identified to contribute to the pathogenesis of lung cancer as well.
Viral infections, such as human papillomavirus (HPV) infections have been reported to be an important risk factor of cervical cancer if genotypes with a high oncogenic risk are found. Since the first identification of human papillomavirus, more than 200 different subtypes have been identified They are classified into high‐risk HPV types (16, 18, 31, 33, 39, 45, 51, 52, and 58) and low‐risk HPV types (6, 11, 42, 43, and 44).2 In some other publications, a differentiation between high‐, intermediate‐, and low‐risk HPV types can be found.3 Although HPV infection has been identified as a potential contributor to the pathogenesis in lung cancer in certain populations, such as never smokers, its role still remains controversial. Numerous tests, such as nucleic acid amplification, HPV DNA‐based in situ hybridization, immunohistochemistry, and cytology are available for HPV‐testing and screening.4, 5 The current study focused on the prevalence of HPV infections in lung cancer patients in which HPV detection was performed by means of PCR from fresh frozen and/or paraffin‐embedded tissue to first minimize differences in HPV prevalence due to methodological bias and second to rely on the method with the highest sensitivity to detect HPV positivity, which has been proven to have the highest sensitivity in earlier studies.4, 5 We conducted and report here a systematic review on the issue above.
2. METHODS
The methods of the systematic review and meta‐analysis were specified in advance and published in a protocol registered with PROSPERO. Reporting of this meta‐analysis was done according to the recommendation of Stroup et al for reporting observational studies.6
2.1. Evidence search and meta‐analysis
The digital databases Embase (via Ovid, 1974‐present), MEDLINE (via Ovid, 1946‐present), Cochrane Library (Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effect, Cochrane Central Register of Controlled Trials, Health Technology Assessment Database, NHS Economic Evaluation Database; from inception to present), and Science Citation Index Expanded (Web of Science, 1965‐present), as well as the search engine Google Scholar (using Anne‐Wil Harzing's “Publish or Perish” program available from https://harzing.com/resources/publish-or-perish), were searched. From Google Scholar, only the first 200 records (initial search on April 25, 2018; no date limit) and the first 100 records (update search on February 6, 2020; date limit years 2018‐2020) were downloaded (default sort order). In addition, WHO's International Clinical Trials Registry Platform, ClinicalTrials.gov, and the EU Clinical Trials Register were searched for completed studies. All searches were last updated on February 6, 2020. We deviated from the protocol; in that, we did not search the German Clinical Trials Register due to its search interface giving erroneous results. An initial, sensitive search strategy for the concepts “lung cancer” AND “HPV” was developed for Embase by a medical librarian in cooperation with subject matter experts and then adapted to the other databases. Controlled terms from the databases' thesauri and a broad range of synonyms were used. No limits such as for study type, publication type, publication date, or language were applied. Search strategies that allow for reproducing the searches are documented in Appendix 1. Database searches were carried out by a medical librarian. The reference lists of included studies and of relevant systematic reviews were screened for additional studies. Records from the database searches were imported into Endnote software for deduplication. Screening by title and abstract and subsequent full‐text assessment were done in Covidence. Titles and abstracts of the publications were analyzed by three independent reviewers (F.K., J.K., and C.S.) for relevance and matching inclusion criteria. Analysis of the publications was done according to prespecified inclusion and exclusion criteria.
All studies reporting HPV prevalence in primary lung cancer cases in adults were included. Case reports were excluded. As detection method, only PCR from fresh frozen and/or paraffin‐embedded tissue were included. All types of tissue sampling method were included. HPV detection in archival tumor tissue was included as well. Only studies that provide data specific to HPV prevalence in lung cancer tissue were included. No exclusions were made based on language. Journal articles as well as abstracts and conference reports were included if they met the inclusion criteria. Journal articles that reported about not only cases of HPV detection in primary lung cancer but, for example, in head and neck cancer as well, were included but only the data of the primary lung cancer group were extracted.
2.2. Statistical analysis
The total number of cases, as well as the number of positive and negative HPV detections, was collected from the selected records, and HPV prevalences were calculated by means of the extracted patient data. The Chi‐squared‐test of independence was used to analyze whether prevalence rates differ between continents. Furthermore, a meta‐analysis was performed on a small subset of case‐control studies regarding HPV prevalence. Prevalence difference (PD) and prevalence ratio (PR) both accompanied with the corresponding 95% confidence intervals were estimated for each study. To estimate PR in studies with no HPV positive cases, 0.5 was added to each cell of the 2 × 2 table as usually recommended. Random‐effect models were used to determine the weighted averages of PD and PR while allowing for heterogeneity of effects. The Q‐statistic as a measurement for between‐study heterogeneity and I2‐statistic for quantification of the proportion of total variation due to heterogeneity were calculated. Analyses were performed using R version 4.0.3 (The R Foundation for Statistical Computing), the meta‐analysis by using the metafor package. For all comparisons, a P value <.05 was considered as statistically significant.
3. RESULTS
3.1. Evidence Search
The database searches were last updated on February 6, 2020 and yielded a total of 4525 records. Following deduplication, 3135 publications were evaluated on relevance for the research question. A total of 2754 of the titles and abstracts did not relate to the current research and were excluded. In summary, 381 publications were entered into the full text review. Full texts of three possibly relevant publications could not be obtained despite some efforts and therefore were not available7, 8, 9 for further analyses. The remaining 378 full‐texts were assessed for eligibility. After applying the inclusion and exclusion criteria, 78 publications were included in this systematic review. Reasons for exclusion were as follows: No PCR data were reported (n = 80). HPV detection method was not detailed (n = 2). Duplication of the data (n = 22). Case reports (n = 9). Corrections and/or comments on screened publications (n = 15). Systematic reviews and meta‐analysis (n = 29). Overview articles (n = 29). HPV detection was not done in lung biopsies (n = 32). HPV prevalence analyzed in cancers other than lung cancer or on metastasis (n = 6). Missing data on HPV prevalence (n = 40). Same patients in separate publications (n = 7). Same information in different languages (n = 4). Abstract published in a different journal than the full text (n = 12). HPV prevalence in lung cancer in special patient groups, for example, patients after lung transplantation, immunocompromised patients, butchers, and respiratory papillomatosis (n = 7). Unfinished studies (n = 4). No data on sampling method were provided (n = 2). This review process was performed according to the PRISMA statement. Figure 1 depicts the flow of citations reviewed for the meta‐analysis.
FIGURE 1.
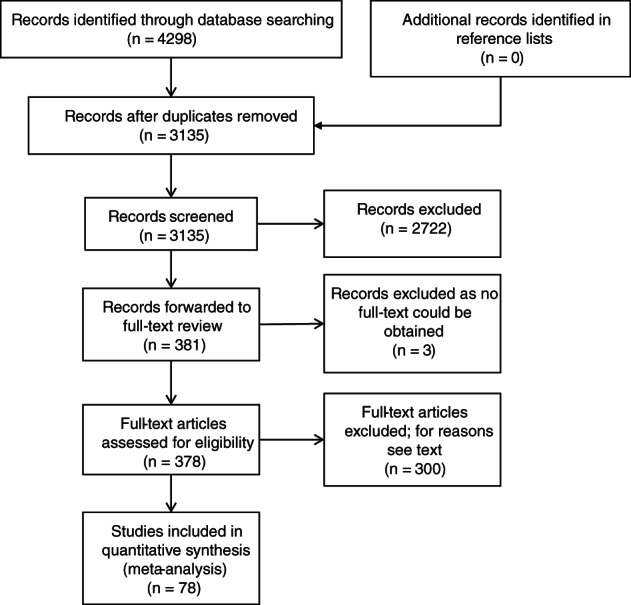
PRISMA flowchart of selected and analyzed studies
A total of 15 publications were case‐control studies, in which normal lung tissue was used as a control (see Table 1).
TABLE 1.
Included case‐control studies
| Author | Year | No. of cases | No. of positive cases | HPV prevalence cases [%] | No. of controls | No. of positive controls | HPV prevalence controls [%] |
|---|---|---|---|---|---|---|---|
| Carpagnano et al10 | 2011 | 89 | 12 | 13.5 | 68 | 0 | 0.0 |
| Cheng et al11 | 2004 | 141 | 54 | 38.3 | 60 | 1 | 1.7 |
| Cheng et al12 | 2001 | 141 | 77 | 54.6 | 60 | 16 | 26.7 |
| Eberlein‐Gonska et al13 | 1992 | 55 | 3 | 5.5 | 15 | 0 | 0.0 |
| Fan et al14 | 2015 | 262 | 22 | 8.4 | 19 | 0 | 0.0 |
| Galvan et al15 | 2012 | 85 | 0 | 0 | 100 | 0 | 0.0 |
| Gatta et al16 | 2012 | 50 | 2 | 4.0 | 23 | 2 | 8.7 |
| Li et al17 | 1995 | 50 | 16 | 32.0 | 22 | 0 | 0.0 |
| Lu et al18 | 2016 | 72 | 33 | 45.8 | 54 | 2 | 3.7 |
| Nadji et al19 | 2007 | 129 | 33 | 25.6 | 89 | 8 | 9.0 |
| Robinson et al20 | 2016 | 70 | 9 | 12.9 | 10 | 1 | 10.0 |
| Wang et al21 | 2008 | 313 | 138 | 44.1 | 96 | 4 | 4.2 |
| Wang et al22 | 2010 | 45 | 19 | 42.2 | 16 | 0 | 0 |
| Yu et al23 | 2015 | 180 | 100 | 55.6 | 110 | 7 | 6.4 |
| Zhang24 | 2009 | 68 | 30 | 44.1 | 12 | 1 | 8.3 |
| Total | 1750 | 548 | 31.3 | 754 | 42 | 5.6 |
The studies were stratified according to the geographical region in which the patients lived. There were 36 studies on patients from Asia, 25 studies on European patients, and 17 studies carried out on the American continent. The countries most represented were Japan (n = 11), China (n = 11), United States (n = 9), and Italy (n = 5). Three studies from Germany met the inclusion criteria. Six studies were done in multiple countries with the information summarized in one publication. Most of the publications were written in English (n = 73). The other publications were published in Chinese (n = 3), French (n = 1), and German (n = 1). In order to get information on as many cases as possible not only journal articles but every type of available study was included. Of the 78 included publications, 67 were journal articles. Of the remaining publications, six were abstracts, three were poster presentations, and two were meeting abstracts.
3.2. Patients characteristics
A total of 9385 lung cancer patients were included into this systematic review. Twenty‐eight studies provided data on the patients' age. The average age of all studies ranged from 51.6 to 70 years. Information on patients' gender was available in 52 out of the 78 studies. Those studies included 6326 patients. Of them, 62.8% were male and 37.2% were female, respectively. The percentage of male patients ranged from 0.0% to 91%. Smoking behavior was detailed in 31 of the studies. There were 3577 current or former smokers, 1958 never smokers, and in 3850 cases, no information on smoking status was available. The rate of smokers was 64.6% and ranged from 0% to 100%.
3.3. Meta‐analysis of 15 case‐control studies
A total of 1750 lung cancer cases and 754 controls were analyzed, which were derived from 15 case‐control studies (Table 1). One of them is from America, 10 are from Asia, and four from Europe. The overall HPV prevalence was detected to be 31.3% (548/1750) in the lung cancer group and 5.5% (42/754) in the control group (P < .001). Figure 2 shows the HPV prevalence derived from case‐control studies as well as divided by different continents. Comparing HPV prevalence of patients with lung cancer and controls in a meta‐analysis, using the 15 case‐control studies with a total of 2504 patients, a higher prevalence could be found for the lung cancer patients for prevalence difference (PD = 0.22; 95%‐CI, 0.12‐0.33; P < .001) as well as prevalence ratio (PR = 4.7; 95% CI, 2.7‐8.4; P < .001). A forest plot summarizing the data and the effect estimates is shown in Figure 3. Due to the large confidence intervals of the PRs, only PDs are presented graphically. According to the Q‐statistic, a significant difference in between‐study heterogeneity could be identified [PD: Q(df = 14) = 344.4, I 2 = 95.94%, P < .001; PR: Q(df = 14) = 33.0, I 2 = 57.6% (PR), P = .003].
FIGURE 2.
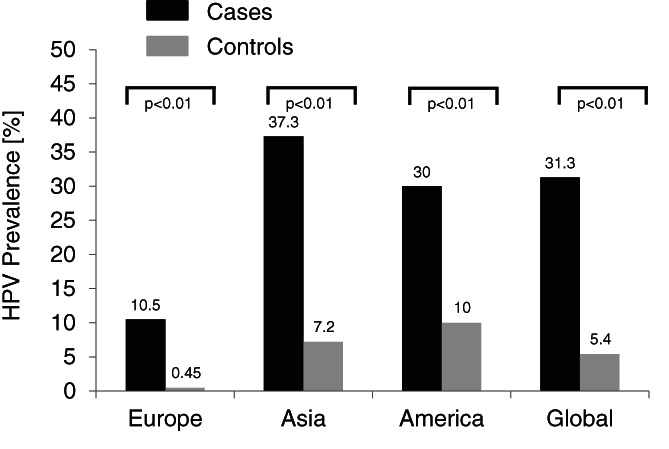
Overall HPV prevalence in case‐control studies as well as divided by different continents. There was a significant difference between the HPV prevalence in cases and controls overall as well as in Europe and Asia (P < .01)
FIGURE 3.
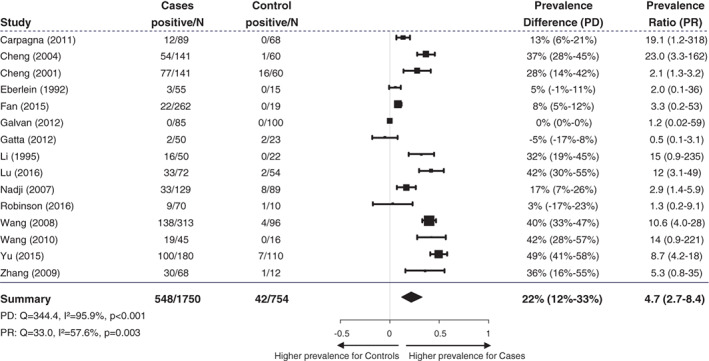
Forest plot demonstrating prevalence difference and prevalence ratio of HPV detection in lung cancer patients compared to control patients without lung cancer. PR of studies with no HPV positive cases in one of the groups was calculated by adding 0.5 to each cell of the 2 × 2 table. Random effect models were used to calculate summary statistics
3.4. HPV prevalence
Of all included patients with lung cancer (n = 9385), HPV was detected to be positive in 1268 cases. The overall HPV prevalence was calculated to be 13.5%. The highest HPV prevalence was detected in Asia with 16.6% (P < .01 vs America and Europe), followed by The Americas (12.8%; P < .01 vs Europe) and Europe (7.0%). The highest HPV 16 prevalence was detected in The Americas (9.4%), followed by Asia (7.5%), and Europe (3.5%). Overall, the HPV 16 prevalence was calculated to be 6.1%. The highest HPV 18 prevalence was found in Asia (4.8%) followed by the Americas (2.3%) and finally Europe (0.7%). Overall, the HPV 18 prevalence was 3.1%. On all three continents, the calculated prevalence of HPV 16 was higher than for HPV 18 (P < .01). Figure 4 depicts the calculated overall HPV prevalence as well as divided by regions and HPV‐genotypes. Tables 2, 3, 4 show the selected studies from Europe, Asia, and America.
FIGURE 4.
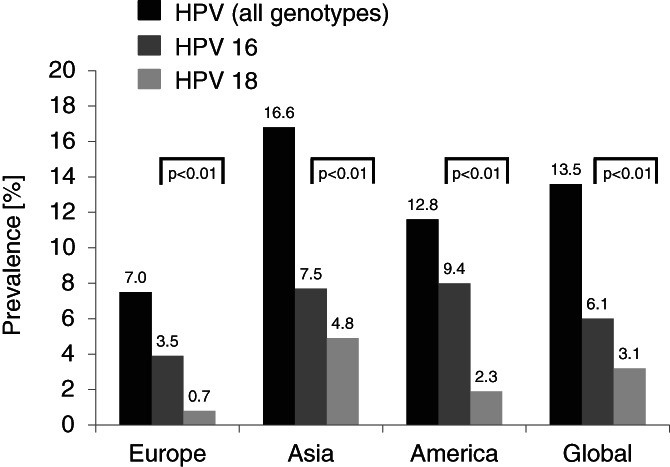
Overall HPV, HPV 16, and HPV 18 prevalence in all analyzed lung cancer cases and between analyzed continents. The highest HPV prevalence was detected in Asia followed by The Americas and Europe. Overall and on all three continents the prevalence of HPV 16 was significantly higher than for HPV 18. The highest HPV 16 prevalence was detected in The Americas followed by Asia and Europe. The highest HPV 18 prevalence was found in Asia followed by The Americas and finally Europe
TABLE 2.
Included studies from Europe
| Reference | Country | No. of cases | Year | HPV prevalence [%] | Specimen type used | Histological subtypes | HPV types detected |
|---|---|---|---|---|---|---|---|
| Anantharaman et al25 | Multiple countries | 290 | 2014 | 9.7 | FFPE, fresh frozen | SCC/AC/others | 11, 16, 51, and 58 |
| Argyri et al26 | Greece | 67 | 2017 | 3.0 | SCC/AC/others | 16 and 53 | |
| Carpagnano et al10 | Italy | 89 | 2011 | 16.4 | FFPE | SCC/AC/others | 16, 30, 31, and 39 |
| Ciotti et al27 | Italy | 38 | 2006 | 8.0 | FFPE, fresh | SCC/AC/others | 16 and 18 |
| Coissard et al28 | France | 218 | 2005 | 1.8 | Fresh frozen | SCC/AC/others | 16 |
| Eberlein‐Gonska et al13 | Germany | 55 | 1992 | 5.5 | Fresh | SCC/AC/others | 16 |
| Galvan et al15 | Italy, United Kingdom | 100 | 2012 | 0 | Fresh frozen | SCC/AC/others | None |
| Gatta et al16 | Italy | 50 | 2012 | 4.0 | FFPE | SCC | |
| Guliani et al29 | Italy | 78 | 2007 | 12.8 | Fresh frozen | SCC/AC/others | 16, 18, 31, and 53 |
| Hennig et al30 | Norway | 22 | 1999 | 13.6 | FFPE | SCC/AC/others | 6 |
| Miasko et al31 | Poland | 94 | 2004 | 12.7 | SCC/AC/others | ||
| Miasko et al32 | Poland | 40 | 2001 | 10.0 | FFPE | SCC/AC/others | |
| Jaworek et al33 | Czech Republic | 80 | 2020 | 0 | FFPE | SCC/AC/others | None |
| Papadopoulou et al34 | Greece | 52 | 1998 | 40.0 | Fresh frozen, FFPE | SCC | 6, 11, 16, and 18 |
| Podsiadlo et al35 | Poland | 33 | 2012 | 3.0 | Fresh | NSCLC/SCLC | 120 |
| Ramqvist, et al36 | Sweden | 87 | 2019 | 0 | FFPE | AC/others | None |
| Sagerup et al37 | Norway | 334 | 2014 | 3.9 | Fresh frozen | SCC/AC/others | 11, 16, 33, and 66 |
| Sarchianaki et al38 | Greece | 100 | 2014 | 19.0 | FFPE | SCC/AC/others | 6, 11, 16, 18, 31, 33, and 59 |
| Shamanin et al39 | Germany | 85 | 1994 | 0 | Fresh frozen | SCC/AC/others | None |
| Spandidos et al40 | Greece | 99 | 1996 | 15.0 | FFPE | SCC/AC/others | 11, 16, 18, and 33 |
| Syrjanen et al41 | Finland | 77 | 2012 | 5.2 | FFPE, archival tissue | SCC/AC/others | 6 and 16 |
| Van Boerdonk et al42 | Netherlands | 211 | 2013 | 0 | FFPE, archival tissue | SCC/AC/others | None |
| Thomas et al43 | France | 31 | 1995 | 16.0 | Fresh frozen | SCC/AC/others | 6, 11 |
| Welt et al44 | Germany | 38 | 1997 | 0 | FFPE | SCC/SCLC | None |
| Zafer et al45 | Turkey | 40 | 2004 | 5.0 | Fresh frozen | SCC/AC/others | 18 |
| Total | 2393 |
TABLE 3.
Included studies from Asia
| Reference | Country | No. of cases | Year | HPV prevalence [%] | Specimen type used | Histologic subtypes | HPV types detected |
|---|---|---|---|---|---|---|---|
| Aguayo et al46 | Pakistan, China | 60 | 2010 | 13.0 | FFPE | SCC/AC/others | 16 |
| Baba et al47 | Japan | 57 | 2010 | 19.3 | FFPE | SCC/AC | 6, 16, 18, and 33 |
| Cheng et al11 | Taiwan | 141 | 2004 | 38.3 | SCC/AC | 6 and 11 | |
| Cheng et al12 | Taiwan | 141 | 2001 | 54.6 | FFPE, fresh frozen | SCC/AC | 16 and 18 |
| Fan et al14 | China | 262 | 2015 | 8.4 | FFPE | SCC/AC | 16, 18, 31, and 58 |
| Goto et al48 | Multiple countries | 304 | 2011 | 7.9 | FFPE | SCC/AC | 6, 11, 16, and 18 |
| Halimi et al49 | Iran | 30 | 2011 | 10.0 | FFPE | SCC | |
| Hartley et al50 | Lebanon | 20 | 2015 | 0 | FFPE | SCLC | none |
| He et al51 | China | 140 | 2019 | 9.3 | Fresh frozen | SCC/AC/others | 16 and 18 |
| Hirayasu et al52 | Japan | 73 | 1996 | 60.3 | FFPE | SCC | 6, 16, and 18 |
| Hiroshima et al53 | Japan | 22 | 1999 | 4.5 | FFPE | AC | 16 |
| Ilahi et al54 | Pakistan | 9 | 2016 | 11.1 | FFPE | SCC/AC/others | 16 |
| Isa et al55 | Japan | 96 | 2015 | 1.0 | FFPE | SCC/AC/others | 6 |
| Ito et al56 | Japan | 901 | 2014 | 0.9 | SCC/AC/others | ||
| Iwakawa et al57 | Japan | 297 | 2010 | 0 | Fresh frozen | AC | none |
| Jafari et al58 | Iran | 50 | 2013 | 18.0 | FFPE | SCC/AC/others | 6 and 18 |
| Jain et al59 | India | 40 | 2005 | 5.0 | Fresh frozen | SCC/AC/others | 18 |
| Kato et al60 | Japan | 42 | 2012 | 16.7 | FFPE | SCC/AC/others | 16 and 58 |
| Kawaguchi et al61 | Japan | 876 | 2016 | 0.3 | FFPE | SCC/AC | 16, 62, and 66 |
| Kinoshita et al62 | Japan | 36 | 1995 | 8.0 | FFPE, fresh frozen | SCC/AC | 18 |
| Lee et al63 | Korea | 233 | 2016 | 0 | FFPE | SCC/AC | none |
| Li et al17 | China | 50 | 1995 | 32.0 | FFPE, fresh frozen | SCC/AC/others | 16 and 18 |
| Lin et al64 | Taiwan | 57 | 2005 | 50.9 | FFPE | SCC/AC | 16 and 18 |
| Lu et al18 | China | 72 | 2016 | 45.8 | FFPE | SCC/AC | 16 and 18 |
| Miyagi et al65 | Japan | 121 | 2001 | 33.9 | FFPE | SCC/AC | 6, 16, and 18 |
| Nadji et al19 | Iran | 129 | 2007 | 25.6 | FFPE | SCC/AC/others | 6, 11, 26, 31, 16, and 18 |
| Ogura et al66 | Japan | 29 | 1993 | 10.3 | Fresh frozen | SCC | 16 and 18 |
| Park et al67 | Korea | 112 | 2007 | 53.6 | AC/NSCLC | 16, 18, and 33 | |
| Wang et al68 | Taiwan | 153 | 2006 | 45.1 | Fresh | SCC/AC | 16 and 18 |
| Wang et al21 | China | 313 | 2008 | 44.1 | Fresh frozen | SCC/AC | 16 and 18 |
| Wang et al22 | China | 45 | 2010 | 42.2 | Fresh frozen | SCC | 16 and 18 |
| Xing et al69 | China | 49 | 1993 | 14.2 | FFPE | SCC | 6, 11, and 16 |
| Yang et al70 | China | 50 | 1998 | 26.0 | FFPE | SCC | 16 |
| Yu et al23 | China | 180 | 2015 | 55.6 | FFPE | SCC/AC/SCLC | 16 and 18 |
| Zhang et al24 | China | 68 | 2009 | 44.1 | Fresh frozen | SCC, AC | 16 and 18 |
| Zhang et al71 | China | 104 | 2010 | 17.3 | FFPE | SCC/AC/others | 16 |
| Total | 5362 |
TABLE 4.
Included studies from The Americas
| Reference | Country | No. of cases | Year | HPV prevalence [%] | Specimen type used | Histologícal subtypes | HPV types detected |
|---|---|---|---|---|---|---|---|
| Aguayo et al72 | Chile | 69 | 2007 | 29.0 | FFPE | SCC/AC/others | 6, 16, 18, 31, and 45 |
| Badillo‐Almaraz et al73 | Mexico | 39 | 2013 | 41.0 | SCC/AC | 16 and 18 | |
| Bohlmeyer et al74 | USA | 34 | 1998 | 5.9 | FFPE | SCC | 18 |
| Cardona et al75 | Multiple South American countries | 132 | 2013 | 39.4 | FFPE | AC | 16 |
| Carlson et al76 | USA | 12 | 2007 | 0 | FFPE | SCLC | None |
| Castillo et al77 | Peru/Colombia/Mexico | 36 | 2006 | 28.0 | FFPE | SCC/AC/others | 16, 18, and 33 |
| de Oliveira et al78 | Brazil | 63 | 2018 | 52,4 | FFPE | SCC/AC/others | 16 and 18 |
| Garcia Falcone et al79 | Argentina | 40 | 2017 | 25.0 | FFPE | SCC | 16 and 18 |
| Joh et al80 | USA | 30 | 2010 | 16.7 | FFPE | SCC/AC/others | 11, 16, and other |
| Koshiol et al81 | USA | 399 | 2011 | 0 | FFPE, ethanol fixed | SCC/AC | none |
| Mehra et al82 | USA | 36 | 2013 | 11.0 | SCC/AC | 16 and 18 | |
| Pillai et al83 | USA | 208 | 2013 | 14.9 | FFPE | NSCLC | 16 and 18 |
| Rezazadeh et al84 | USA | 16 | 2008 | 25.0 | FFPE | NSCLC | 11 and 16 |
| Robinson et al20 | USA | 70 | 2016 | 42.9 | Fresh frozen | SCC/AC | 16, 18, 39, 44, 51, 52, and 68 |
| Silva et al85 | Brazil | 62 | 2019 | 0 | FFPE | SCC/AC/others | None |
| Suh et al86 | USA | 48 | 2010 | 2.0 | FFPE | SCC | No data |
| Yanagawa et al87 | Canada | 336 | 2013 | 1.5 | FFPE | SCC/AC | 16 |
| Total | 1630 |
3.5. Histology and HPV prevalence
Only the information on primary squamous cell carcinoma (SCC) and primary adeno carcinoma (AC) of the lung was collected. In the remaining cases, it was neither one of them or the histological subtype was not detailed. There were 2750 cases of SCC and 2887 cases of AC. In total, 29.3% of the included cases were squamous cell carcinomas and 30.8% were adenocarcinomas.
The overall HPV prevalence in SCC (n = 492) was calculated to be 17.9%. The highest prevalence was calculated in Asia (28.8%), followed by The Americas (10.0%), and Europe (5.1%).
The overall HPV prevalence in adenocarcinomas (n = 265) was calculated to be 9.2%. In contrast, the highest HPV prevalence in AC was calculated in the Americas (11.1%), followed by Asia (10.4%), and Europe (6.0%).
When the HPV prevalences of SCC and AC are compared, the difference is statistically highly significant (P < .01), which is due to a significantly higher HPV prevalence in SCC (P < .01) in Asia, whereas no differences in prevalence were found in The Americas and Europe based on histological subtypes of lung cancer. Figure 5 shows the calculated HPV prevalences.
FIGURE 5.
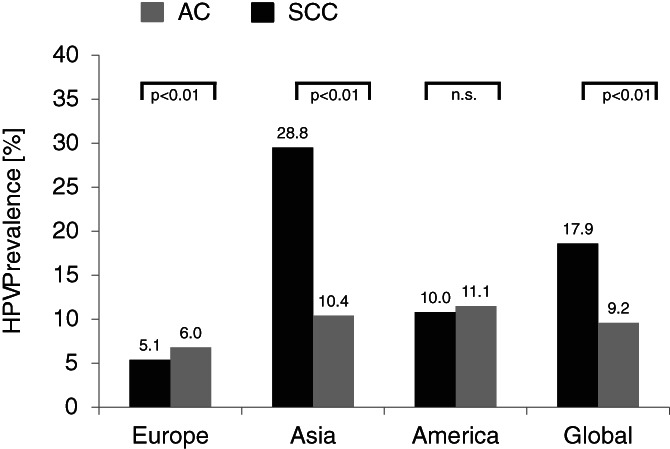
HPV prevalence in SCC vs AC. There was no statistically significant difference between the HPV prevalence in SCC and AC in the studies from America (P = .78). Statistically significant differences were found in studies from Asia (P < .01) and Europe (P < .01). On a global observation HPV prevalence in SCC was significantly higher (P < .01) when compared to AC
4. DISCUSSION
Growing evidence supports the association between HPV‐infection and lung cancer but the relationship is still debatable. The aim of the present study was to conduct a systematic database and literature review by means of a molecular biology based clear definition of HPV positivity and lung cancer. Selection was restricted to studies with lung tissue analysis and PCR‐based confirmation of HPV‐positivity to take advantage of the high specificity and sensitivity of the diagnostic approach. Data of over 9000 lung cancer patients were analyzed, which underlines the robustness of the dataset generated.
The included case‐control studies demonstrated an absolute risk increase of 22% (95% CI: 12%‐33%) in lung cancer patients of being HPV positive, which resulted in a 4.7‐fold (95% CI: 2.7%‐8.4%) increase in the likelihood to detect HPV in patients diagnosed with lung cancer compared to healthy controls regardless of histology or stage of tumor disease.
The meta‐analysis shows that the average HPV infection rate of lung cancer in the world is 13.5% based on PCR‐based assays only. PCR was permitted as the sole method to minimize differences in prevalence related to significant disparities in methodological sensitivity and specificity. Significant regional differences in HPV prevalence in lung cancer patients were found being highest in Asia with 16.6% and lowest in Europe with 7.0%. In addition, the data demonstrate a higher overall HPV prevalence in lung cancer with squamous cell histology, which is mainly due to a significantly higher HPV prevalence in squamous cell carcinoma in Asian regions since this difference was not found in squamous cell carcinoma and adenocarcinoma diagnosed in Europe and America. Most likely, the intriguing different geographic patterns of HPV prevalence in lung cancer are related to the regional differences of the HPV infection itself.
Furthermore, if HPV infection was found, high‐risk genotypes with oncogenic potential were prevalently identified as well. With focus on the most common high‐risk genotypes, overall HPV genotype 16 was the most frequent genotype reported with a twofold higher prevalence compared to HPV genotype 18. With some minor modification, similar findings were reported in all different continents analyzed. These findings additionally support the hypothesis that HPV infections with high‐risk oncogenic potential significantly increase the risk of lung cancer and provide new possibilities in the future in the prevention of lung cancer by means of prophylactic vaccines for the carcinogenic HPV‐16/18 infections.88
The pathogenesis of HPV infection in thoracic visceral lungs is still incompletely understood. Blood based transmission through cervical lesion to the lung, high‐risk sexual behavior, and airborne transmission to the lungs have been discussed.89 HPV oncogenes (eg, HPV E6 and HPV E7) are known to regulate the expression of multiple target genes and proteins such as p53, pRb, HIF‐1α, VEGF, IL‐6, IL‐10, Mcl‐1, Bcl‐2, cIAP‐2, EGFR, FHIT, hTERT, HER‐ 2, ROS1, and AhR, which can facilitate lung cell proliferation, angiogenesis, and cell immortalization by means of various signaling pathways.89
The data of the present study provide evidence for a possible relationship between lung cancer and HPV infection, but the study fails to show a high causal interference since no longitudinal data derived from cohort studies or nested case‐control studies are given. In addition, cofounders of possible importance such as smoking status, gender, age, immunosuppressive co‐medications, oncogenic driver mutations, and estrogenic signaling pathways have not been taken into considerations, which limit the value of the results reported. Furthermore, not all HPV subtypes were assessed due to missing specification in many studies, and no transcriptional activity of the HPV genotypes found was included in the meta‐analysis. Since only PCR was included as HPV detection method but this not being the only way to detect HPV, which can potentially bias the study's results further.
In conclusion, our systematic review provides evidence that HPV infection might increase the risk of developing lung cancer. Whereby relevant regional differences with respect to prevalence and histological subtypes were found with a predominance of squamous cell carcinoma. Consistently, our results support the assumption that the high‐risk genotypes HPV 16 and 18 are risk factors for lung cancer. If the understanding of the process of HPV‐related carcinogenesis in lung cancer could be further elucidated by larger prospective studies, this would facilitate the development of efficient HPV‐targeted prevention strategies.
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
AUTHOR CONTRIBUTIONS
J.K., H.K., M.K., and C.S. provided substantial contributions to the conceptualization of the study. J.K., H.K., W.D., F.Z., and C.S. designed the methodology and were involved in data curation. J.K., W.D., V.F., M.K., F.K., and C.S. wrote the inital draft of the manuscript. All authors critically reviewed the manuscript, and approved the final version for publication.
ETHICAL STATEMENT
Not applicable.
Supporting information
Appendix 1: Search strategies.
Karnosky J, Dietmaier W, Knuettel H, et al. HPV and lung cancer: A systematic review and meta‐analysis. Cancer Reports. 2021;4:e1350. 10.1002/cnr2.1350
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the digital databases Embase (via Ovid, 1974–present), MEDLINE (via Ovid, 1946–present), Cochrane Library (Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effect, Cochrane Central Register of Controlled Trials, Health Technology Assessment Database, NHS Economic Evaluation Database; from inception to present) and Science Citation Index Expanded (Web of Science, 1965–present) as well as the search engine Google Scholar (using Anne‐Wil Harzing's “Publish or Perish” program available from https://harzing.com/resources/publish‐or‐perish).
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 2.Rezazadeh A, Laber DA, Ghim S‐j, Jenson AB, Kloecker G. The role of human papilloma virus in lung cancer: a review of the evidence. Am J Med Sci. 2009;338(1):64‐67. [DOI] [PubMed] [Google Scholar]
- 3.de Cremoux P, La Rochefordière A, de Savignoni A, et al. Different outcome of invasive cervical cancer associated with high‐risk versus intermediate‐risk HPV genotype. Int J Cancer. 2009;124(4):778‐782. [DOI] [PubMed] [Google Scholar]
- 4.Burd EM, Dean CL. Human Papillomavirus. Microbiol Spectr. 2016;4(4):3‐17. [DOI] [PubMed] [Google Scholar]
- 5.Brink AATP, Snijders PJF, Meijer CJLM. HPV detection methods. Dis Markers. 2007;23(4):273‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008‐2012. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Wu CH, Lu Y, Jang CF, Yuan B. The correlation of HPV infection with the expression of epidermal growth factor receptor and vascular endothelial growth factor in non small cell lung cancer. Tumor. 2007;27:821‐824. [Google Scholar]
- 8.Sagawa M, Saito Y, Endo C, et al. Detection of human papillomavirus type 16, 18 and 33 DNA in stage I (pT1N0M0) squamous cell carcinoma of the lung by polymerase chain reaction. Kyobu Geka. 1995;48(5):360‐362. [PubMed] [Google Scholar]
- 9.Singh T. Recent advances in cancer research. J Med Soc. 2007;21:111‐113. [Google Scholar]
- 10.Carpagnano GE, Koutelou A, Natalicchio MI, et al. HPV in exhaled breath condensate of lung cancer patients. Br J Cancer. 2011;105(8):1183‐1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng Y‐W, Chiou H‐L, Chen J‐T, et al. Gender difference in human papillomarvirus infection for non‐small cell lung cancer in Taiwan. Lung Cancer. 2004;46(2):165‐170. [DOI] [PubMed] [Google Scholar]
- 12.Cheng YW, Chiou HL, Sheu GT, et al. The association of human papillomavirus 16/18 infection with lung cancer among nonsmoking Taiwanese women. Cancer Res. 2001;61(7):2799‐2803. [PubMed] [Google Scholar]
- 13.Eberlein‐Gonska M, Gaweco A, Becker H, Otto HF. Polymerase chain reaction demonstration of human papilloma virus type 16 in a lung adenocarcinoma and two squamous cell carcinoma. [German]. Atemwegs‐ und Lungenkrankheiten. 1992;18(9):337. [Google Scholar]
- 14.Fan X, Yu K, Wu J, Shao J, Zhu L, Zhang J. Correlation between squamous cell carcinoma of the lung and human papillomavirus infection and the relationship to expression of p53 and p16. Tumour Biol. 2015;36(4):3043‐3049. [DOI] [PubMed] [Google Scholar]
- 15.Galvan A, Noci S, Taverna F, et al. Testing of human papillomavirus in lung cancer and non‐tumor lung tissue. BMC Cancer. 2012;12:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gatta LB, Balzarini P, Tironi A, et al. Human papillomavirus DNA and p16 gene in squamous cell lung carcinoma. Anticancer Res. 2012;32(8):3085‐3089. [PubMed] [Google Scholar]
- 17.Li Q, Hu K, Pan X, Cao Z, Yang J, Hu S. Detection of human papillomavirus types 16, 18 DNA related sequences in bronchogenic carcinoma by polymerase chain reaction. Chin Med J (Engl). 1995;108(8):610‐614. [PubMed] [Google Scholar]
- 18.Lu Y, Yu L‐Q, Zhu L, Zhao N, Zhou X‐J, Lu X. Expression of HIF‐1α and P‐gp in non‐small cell lung cancer and the relationship with HPV infection. Oncol Lett. 2016;12(2):1455‐1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nadji SA, Mokhtari‐Azad T, Mahmoodi M, et al. Relationship between lung cancer and human papillomavirus in north of Iran. Mazandaran Province Cancer Lett. 2007;248(1):41‐46. [DOI] [PubMed] [Google Scholar]
- 20.Robinson LA, Jaing CJ, Pierce Campbell C, et al. Molecular evidence of viral DNA in non‐small cell lung cancer and non‐neoplastic lung. Br J Cancer. 2016;115(4):497‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Wang A, Jiang R, et al. Human papillomavirus type 16 and 18 infection is associated with lung cancer patients from the central part of China. Oncol Rep. 2008;20(2):333‐339. [PubMed] [Google Scholar]
- 22.Wang Y‐H, D‐j C, Yi T‐N, Liu X‐H. The relationship among human papilloma virus infection, survivin, and p53 gene in lung squamous carcinoma tissue. Saudi Med J. 2010;31(12):1331‐1336. [PubMed] [Google Scholar]
- 23.Yu Y, Liu X, Yang Y, et al. Effect of FHIT loss and p53 mutation on HPV‐infected lung carcinoma development. Oncol Lett. 2015;10(1):392‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang M. The relationship between HPV infection and the expression of insulin‐like growth factor II in lung cancer and its clinical significance. [Chinese]. Tumor. 2009;29:749‐753. [Google Scholar]
- 25.Anantharaman D, Gheit T, Waterboer T, et al. No causal association identified for human papillomavirus infections in lung cancer. Cancer Res. 2014;74(13):3525‐3534. [DOI] [PubMed] [Google Scholar]
- 26.Argyri E, Tsimplaki E, Marketos C, Politis G, Panotopoulou E. Investigating the role of human papillomavirus in lung cancer. Papillomavirus Res. 2017;3:7‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciotti M, Giuliani L, Ambrogi V, et al. Detection and expression of human papillomavirus oncogenes in non‐small cell lung cancer. Oncol Rep. 2006;16(1):183‐189. [PubMed] [Google Scholar]
- 28.Coissard CJ, Besson G, Polette MC, Monteau M, Birembaut PL, Clavel CE. Prevalence of human papillomaviruses in lung carcinomas: a study of 218 cases. Mod Pathol. 2005;18(12):1606‐1609. [DOI] [PubMed] [Google Scholar]
- 29.Giuliani L, Jaxmar T, Casadio C, et al. Detection of oncogenic viruses SV40, BKV, JCV, HCMV, HPV and p53 codon 72 polymorphism in lung carcinoma. Lung Cancer. 2007;57(3):273‐281. [DOI] [PubMed] [Google Scholar]
- 30.Hennig EM, Suo Z, Karlsen F, Holm R, Thoresen S, Nesland JM. HPV positive bronchopulmonary carcinomas in women with previous high‐grade cervical intraepithelial neoplasia (CIN III). Acta Oncol. 1999;38(5):639‐647. [DOI] [PubMed] [Google Scholar]
- 31.Barzał‐Nowosielska M, Miasko A, Chyczewski L. Presences of human papillomavirus DNA (HPV) and immunohistochemical p53 overexpression in papillomas of oral cavity. Rocz Akad Med Bialymst. 2004;49(1):105‐107. [PubMed] [Google Scholar]
- 32.Miasko A, Niklińska W, Nikliński J, Chyczewska E, Naumnik W, Chyczewski L. Detection of human papillomavirus in non‐small cell lung carcinoma by polymerase chain reaction. Folia Histochem Cytobiol. 2001;39(2):127‐128. [PubMed] [Google Scholar]
- 33.Jaworek H, Koudelakova V, Slavkovsky R, Drabek J, Hajduch M. The absence of high‐risk human papillomavirus in Czech non‐small cell lung cancer cases. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2020;164(1):71‐76. [DOI] [PubMed] [Google Scholar]
- 34.Papadopoulou K, Labropoulou V, Davaris P, Mavromara P, Tsimara‐Papastamatiou H. Detection of human papillomaviruses in squamous cell carcinomas of the lung. Virchows Arch. 1998;433(1):49‐54. [DOI] [PubMed] [Google Scholar]
- 35.Podsiadlo L, Mandziuk S, Polz‐Dacewicz M, Stec A, Buczkowski J. Detection, genotyping and phylogenesis of human papillomavirus (HPV) and Epstein‐Barr virus (EBV) in patients with lung cancer. Curr Issue Pharm Med Sci. 2012;25(2):159‐163. [Google Scholar]
- 36.Ramqvist T, Ortiz‐Villalon C, Brandén E, et al. Analysis of human papillomaviruses and human polyomaviruses in lung cancer from Swedish never‐smokers. Acta Oncol. 2020;59(1):28‐32. [DOI] [PubMed] [Google Scholar]
- 37.Sagerup CMT, Nymoen DA, Halvorsen AR, Lund‐Iversen M, Helland A, Brustugun OT. Human papilloma virus detection and typing in 334 lung cancer patients. Acta Oncol. 2014;53(7):952‐957. [DOI] [PubMed] [Google Scholar]
- 38.Sarchianaki E, Derdas SP, Ntaoukakis M, et al. Detection and genotype analysis of human papillomavirus in non‐small cell lung cancer patients. Tumour Biol. 2014;35(4):3203‐3209. [DOI] [PubMed] [Google Scholar]
- 39.Shamanin V, Delius H, de Villiers EM. Development of a broad spectrum PCR assay for papillomaviruses and its application in screening lung cancer biopsies. J Gen Virol. 1994;75(Pt 5):1149‐1156. [DOI] [PubMed] [Google Scholar]
- 40.Noutsou A, Koffa M, Ergazaki M, Siafakas N, Spandidos D. Detection of human papilloma virus (HPV) and K‐ras mutations in human lung carcinomas. Int J Oncol. 1996;8(6):1089‐1093. [DOI] [PubMed] [Google Scholar]
- 41.Syrjänen K, Silvoniemi M, Salminen E, Vasankari T, Syrjänen S. Detection of human papillomavirus genotypes in bronchial cancer using sensitive multimetrix assay. Anticancer Res. 2012;32(2):625‐631. [PubMed] [Google Scholar]
- 42.van Boerdonk RAA, Daniels JMA, Bloemena E, et al. High‐risk human papillomavirus‐positive lung cancer: molecular evidence for a pattern of pulmonary metastasis. J Thorac Oncol. 2013;8(6):711‐718. [DOI] [PubMed] [Google Scholar]
- 43.Thomas P, de Lamballerie X, Garbe L, Castelnau O, Kleisbauer JP. Détection de papillomavirus humain par amplification génique dans les cancers bronchiques primitifs. Bull Cancer. 1996;83(10):842‐846. [PubMed] [Google Scholar]
- 44.Welt A, Hummel M, Niedobitek G, Stein H. Human papillomavirus infection is not associated with bronchial carcinoma: evaluation by in situ hybridization and the polymerase chain reaction. J Pathol. 1997;181(3):276‐280. [DOI] [PubMed] [Google Scholar]
- 45.Zafer E, Ergun MA, Alver G, Sahin FI, Yavuzer S, Ekmekci A. Detection and typing of human papillomavirus in non‐small cell lung cancer. Respiration. 2004;71(1):88‐90. [DOI] [PubMed] [Google Scholar]
- 46.Aguayo F, Anwar M, Koriyama C, et al. Human papillomavirus‐16 presence and physical status in lung carcinomas from Asia. Infect Agents Cancer. 2010;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baba M, Castillo A, Koriyama C, et al. Human papillomavirus is frequently detected in gefitinib‐responsive lung adenocarcinomas. Oncol Rep. 2010;23(4):1085‐1092. [DOI] [PubMed] [Google Scholar]
- 48.Goto A, Li C‐P, Ota S, et al. Human papillomavirus infection in lung and esophageal cancers: analysis of 485 Asian cases. J Med Virol. 2011;83(8):1383‐1390. [DOI] [PubMed] [Google Scholar]
- 49.Halimi M, Morshedi AS. Human papillomavirus infection in lung vs. oral squamous cell carcinomas: a polymerase chain reaction study. PJBS. 2011;14(11):641‐646. [DOI] [PubMed] [Google Scholar]
- 50.Hartley CP, Steinmetz HB, Memoli VA, Tafe LJ. Small cell neuroendocrine carcinomas of the lung do not harbor high‐risk human papillomavirus. Hum Pathol. 2015;46(4):577‐582. [DOI] [PubMed] [Google Scholar]
- 51.He F, Xiong W, Yu F, et al. Human papillomavirus infection maybe not associated with primary lung cancer in the Fujian population of China. Thoracic Cancer. 2020;11(3):561‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hirayasu T, Iwamasa T, Kamada Y, Koyanagi Y, Usuda H, Genka K. Human papillomavirus DNA in squamous cell carcinoma of the lung. J Clin Pathol. 1996;49(10):810‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hiroshima K, Toyozaki T, Iyoda A, et al. Ultrastructural study of intranuclear inclusion bodies of pulmonary adenocarcinoma. Ultrastruct Pathol. 1999;23(6):383‐389. [DOI] [PubMed] [Google Scholar]
- 54.Ilahi NE, Anwar S, Noreen M, Hashmi SN, Murad S. Detection of human papillomavirus‐16 DNA in archived clinical samples of breast and lung cancer patients from North Pakistan. J Cancer Res Clin Oncol. 2016;142(12):2497‐2502. [DOI] [PubMed] [Google Scholar]
- 55.Isa S‐I, Kurahara Y, Yamamoto S, et al. Molecular analysis of human papillomavirus in never‐smokers with non‐small cell lung cancer. Oncol Lett. 2015;9(2):927‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ito N, Kawaguchi T, Koh Y, Isa SI, Shimizu S, Takeo S. Driver mutations associated with smoking and other environmental factors: prospective and integrative genomic analysis from the Japan molecularepidemiology for lung cancer study (JME). J Clin Oncol Conf. 2014;32:7516. [Google Scholar]
- 57.Iwakawa R, Kohno T, Enari M, Kiyono T, Yokota J. Prevalence of human papillomavirus 16/18/33 infection and p53 mutation in lung adenocarcinoma. Cancer Sci. 2010;101(8):1891‐1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jafari H, Gharemohammadlou R, Fakhrjou A, et al. Genotyping of human papillomavirus and TP53 mutations at exons 5 to 7 in lung cancer patients from Iran. Bioimpacts. 2013;3(3):135‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jain N, Singh V, Hedau S, et al. Infection of human papillomavirus type 18 and p53 codon 72 polymorphism in lung cancer patients from India. Chest. 2005;128(6):3999‐4007. [DOI] [PubMed] [Google Scholar]
- 60.Kato T, Koriyama C, Khan N, et al. EGFR mutations and human papillomavirus in lung cancer. Lung Cancer. 2012;78(2):144‐147. [DOI] [PubMed] [Google Scholar]
- 61.Kawaguchi T, Koh Y, Ando M, et al. Prospective analysis of oncogenic driver mutations and environmental factors: Japan molecular epidemiology for lung cancer study. J Clin Oncol. 2016;34(19):2247‐2257. [DOI] [PubMed] [Google Scholar]
- 62.Kinoshita I, Dosaka‐Akita H, Shindoh M, et al. Human papillomavirus type 18 DNA and E6‐E7 mRNA are detected in squamous cell carcinoma and adenocarcinoma of the lung. Br J Cancer. 1995;71(2):344‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee JE, Lee YM, Seong IO, Kang MW, Lee CS, Kim KH. No detection of episomal or integrated high‐risk human papillomavirus in nonsmall cell lung carcinomas among Korean population. Osong Public Health Res Perspect. 2016;7(6):356‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin T‐S, Lee H, Chen R‐A, et al. An association of DNMT3b protein expression with P16INK4a promoter hypermethylation in non‐smoking female lung cancer with human papillomavirus infection. Cancer Lett. 2005;226(1):77‐84. [DOI] [PubMed] [Google Scholar]
- 65.Miyagi J, Kinjo T, Tsuhako K, et al. Extremely high Langerhans cell infiltration contributes to the favourable prognosis of HPV‐infected squamous cell carcinoma and adenocarcinoma of the lung. Histopathology. 2001;38(4):355‐367. [DOI] [PubMed] [Google Scholar]
- 66.Ogura H, Watanabe S, Fukushima K, Masuda Y, Fujiwara T, Yabe Y. Human papillomavirus DNA in squamous cell carcinomas of the respiratory and upper digestive tracts. Jpn J Clin Oncol. 1993;23(4):221‐225. [PubMed] [Google Scholar]
- 67.Park MS, Chang YS, Shin JH, et al. The prevalence of human papillomavirus infection in Korean non‐small cell lung cancer patients. Yonsei Med J. 2007;48(1):69‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang J, Cheng Y‐W, Wu D‐W, et al. Frequent FHIT gene loss of heterozygosity in human papillomavirus‐infected non‐smoking female lung cancer in Taiwan. Cancer Lett. 2006;235(1):18‐25. [DOI] [PubMed] [Google Scholar]
- 69.Xing LQ, Liu HR, Si JY. Detection of human papillomavirus DNA in squamous cell carcinomas of the lung by multiple polymerase chain reaction. Chinese J Tubercul Respirat Dis. 1993;16(5):275‐277.319. [PubMed] [Google Scholar]
- 70.Yang Y, Dong D, Peng L, Ling J, Xiao Y, Zhuang H. A study on the relationship between HPV infection and the oncogenesis of primary squamous carcinoma of the lung. Chin J Lung Cancer. 1998;1(1):35‐36. [DOI] [PubMed] [Google Scholar]
- 71.Zhang J, Wang T, Han M, et al. Variation of human papillomavirus 16 in cervical and lung cancers in Sichuan. China Acta Virol. 2010;54(4):247‐253. [DOI] [PubMed] [Google Scholar]
- 72.Aguayo F, Castillo A, Koriyama C, et al. Human papillomavirus‐16 is integrated in lung carcinomas: a study in Chile. Br J Cancer. 2007;97(1):85‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Badillo‐Almaraz I, Zapata‐Benavides P, Saavedra‐Alonso S, et al. Human papillomavirus 16/18 infections in lung cancer patients in Mexico. Intervirology. 2013;56(5):310‐315. [DOI] [PubMed] [Google Scholar]
- 74.Bohlmeyer T, Le TN, Shroyer AL, Markham N, Shroyer KR. Detection of human papillomavirus in squamous cell carcinomas of the lung by polymerase chain reaction. Am J Respir Cell Mol Biol. 1998;18(2):265‐269. [DOI] [PubMed] [Google Scholar]
- 75.Cardona AF, Rosell R, Vargas C, et al. EGFR and KRAS mutations in patients having lung adenocarcinoma associated with human papilloma virus infection. J Thorac Oncol. 2013;8(2):S428. [Google Scholar]
- 76.Carlson JW, Nucci MR, Brodsky J, Crum CP, Hirsch MS. Biomarker‐assisted diagnosis of ovarian, cervical and pulmonary small cell carcinomas: the role of TTF‐1, WT‐1 and HPV analysis. Histopathology. 2007;51(3):305‐312. [DOI] [PubMed] [Google Scholar]
- 77.Castillo A, Aguayo F, Koriyama C, et al. Human papillomavirus in lung carcinomas among three Latin American countries. Oncol Rep. 2006;15(4):883‐888. [PubMed] [Google Scholar]
- 78.de Oliveira THA, do Amaral CM, de França São Marcos B, et al. Presence and activity of HPV in primary lung cancer. J Cancer Res Clin Oncol. 2018;144(12):2367‐2376. [DOI] [PubMed] [Google Scholar]
- 79.Garcia Falcone MM, Cuello M, Garcia AJ, Avagnina MA, Recondo G, Denninghoff V. Human papillomavirus infection in lung squamous cell carcinoma and correlation to p16 INK4A expression from an argentine population. J Thorac Oncol. 2017;12:S1937‐S1938. [Google Scholar]
- 80.Joh J, Jenson AB, Moore GD, et al. Human papillomavirus (HPV) and Merkel cell polyomavirus (MCPyV) in non small cell lung cancer. Exp Mol Pathol. 2010;89(3):222‐226. [DOI] [PubMed] [Google Scholar]
- 81.Koshiol J, Rotunno M, Gillison ML, et al. Assessment of human papillomavirus in lung tumor tissue. J Natl Cancer Inst. 2011;103(6):501‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mehra R, Egleston B, Yang D, Scott W, Borghaei H, Ragin C. A pilot study of the association and prevalence of the human papillomavirus (HPV) in non‐small cell lung cancer (NSCLC). AACR. 2013;73(8):4785. [Google Scholar]
- 83.Pillai RN, Ragin C, Sica G, Behera M, Chen Z, Kim S. Human papillomavirus (HPV)‐associated early stage non‐small cell lung cancer (NSCLC). J Clin Oncol. 2013;(31):7560. [Google Scholar]
- 84.Rezazadeh A, Desai PC, Laber DA, Ghim S, Schaefer G, Jenson AB. Detection of HPV in different subtypes of non‐small cell lung cancer (NSCLC). J Clin Oncol. 2008;26:22098. [Google Scholar]
- 85.Silva EM, Mariano VS, Pastrez PRA, et al. Human papillomavirus is not associated to non‐small cell lung cancer: data from a prospective cross‐sectional study. Infect Agents Cancer. 2019;14:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Suh JHSKR. A strong inverse correlation between p16INK4a and pRb expression is observed at the level of individual tumor cells in HPV‐negative primary squamous cell lung cancer. FASEB Journal Conference. 2010;24(S1):567.2. [Google Scholar]
- 87.Yanagawa N, Wang A, Kohler D, et al. Human papilloma virus genome is rare in North American non‐small cell lung carcinoma patients. Lung Cancer. 2013;79(3):215‐220. [DOI] [PubMed] [Google Scholar]
- 88.Tota JE, Struyf F, Sampson JN, et al. Efficacy of the AS04‐adjuvanted HPV‐16/18 vaccine: pooled analysis of the Costa Rica vaccine and PATRICIA randomized controlled trials. J Natl Cancer Inst. 2020;112(8):818‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xiong W‐M, Xu Q‐P, Li X, Xiao R‐D, Cai L, He F. The association between human papillomavirus infection and lung cancer: a system review and meta‐analysis. Oncotarget. 2017;8(56):96419‐96432. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: Search strategies.
Data Availability Statement
The data that support the findings of this study are available in the digital databases Embase (via Ovid, 1974–present), MEDLINE (via Ovid, 1946–present), Cochrane Library (Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effect, Cochrane Central Register of Controlled Trials, Health Technology Assessment Database, NHS Economic Evaluation Database; from inception to present) and Science Citation Index Expanded (Web of Science, 1965–present) as well as the search engine Google Scholar (using Anne‐Wil Harzing's “Publish or Perish” program available from https://harzing.com/resources/publish‐or‐perish).


