Abstract
The current unprecedented COVID-19 pandemic has been another step toward learning about the unique interaction between viral infections and human nervous system. Very few scientific papers explored neuroinvasive and neurotropic potentials of the SARS-CoV-2 virus in children. We report a child with convulsive status epilepticus and confirmed COVID-19 infection. Brief review of current available literature was discussed.
Keywords: SARS-CoV-2, COVID-19, status epilepticus
Background
There has been a rapid increase in the number of confirmed cases of SARS-CoV-2 virus, the cause of COVID-19 infection. More than 11 million cases globally and 2.9 million cases in the United States were infected. Children account for approximately 3% of the patients’ cohort.1 There are some published reports describing neurological complications of SARS-CoV-2 infection supporting both its neuro-invasive and neurotropic potential. However, there are limited data to draw final conclusions in the pediatric age group in particular.2 Although it is believed that various neurological disorders possibly constitute a higher risk for severe COVID-19 disease such as demyelinating and neuromuscular disorders, studies on epilepsy as an independent risk factor had conflicting results.3 Conclusive evidence that COVID-19 infection directly generates cortical excitability is lacking, but the other associated features of COVID-19 such as high fever, hypoxic insult due to severe respiratory compromise, and multi-organ dysfunction are likely to contribute considerably to seizure pathogenesis in rapidly developing, more vulnerable pediatric brains. We report an 8-year-old child with preexisting, well-controlled epilepsy who developed a breakthrough, unusually prolonged seizure (convulsive status epilepticus) in the context of positive COVID-19 infection.
Case Summary
Our patient is an 8-year-old boy with a partial deletion of the short arm of chromosome 4 (4p16.3), motor and expressive language delays, swallowing dysfunction, and epilepsy since the age of 6 months. His initial seizures were in the form of left sided gaze deviation, head turning to the left, body posturing, and clonic rhythmic left lower extremity shaking progressing to bilateral tonic clonic seizures, typically for a total duration of less than 1 minute. Clinical seizures were associated with ictal epileptiform activity in the right hemisphere. Thus, he was diagnosed with focal epilepsy with focal to bilateral tonic-clonic seizures of genetic etiology. Prior to this presentation, his seizures had been completely controlled for more than 3 years with levetiracetam at dose of 30 mg/kg/day, divided over 2 times per day, without any breakthrough activity. He had no prodromal symptoms with no fever, respiratory symptoms, or recent change in overall health. He did not have medication compliance concerns or recent medication changes.
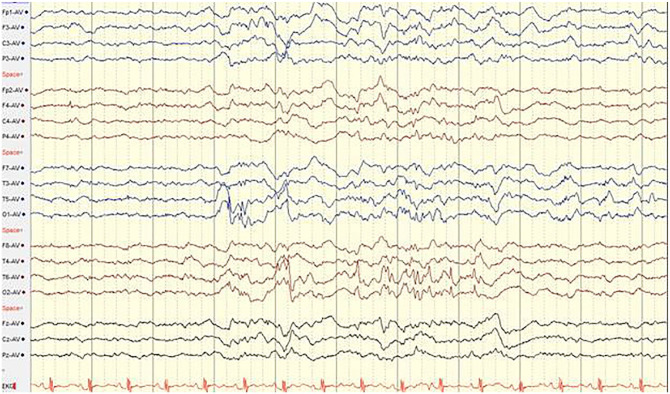
He then presented with a sudden unprovoked seizure comprising eye rolling then left sided eye gazing, drooling, and loss of responsiveness, followed quickly by body tonic posturing and bilateral rhythmic clonic shaking of all extremities. Emergency medical service were called. The episode was estimated to last for 20 to 25 minutes without gaining of consciousness. Thus he was thought to have an episode that was consistent with diagnosis of convulsive status epilepticus. His prolonged seizure was managed with an abortive dose of benzodiazepine (2 mg of Lorazepam which was corresponding to 0.1 mg/kg). He then had a loading dose of levetiracetam of 750 mg, which was corresponding to 40/kg). His seizure resolved while getting the Levetiracetam loading. He was then transferred to be closely observed in the pediatric intensive care unit. Further work up included a complete blood count revealed mild leukocytosis with relative neutrophilia. Serum chemistry profile results were normal, and blood and urine cultures were negative. Electroencephalogram study demonstrated a generalized background slowing and intermittent bilateral multifocal epileptiform discharges without evidence of nonconvulsive status epilepticus (Figure 1). Levetiracetam level drawn prior to loading was found to be sub-therapeutic (10 µg/ml, with reference range of 12-46 µg/ml). COVID-19 screening was positive for SARS-CoV-2 by polymerase chain reaction (PCR) from nasopharyngeal swab specimen. Subsequently, the patient had a largely uncomplicated hospital course. He was discharged next day from the hospital with an adjusted dose of levetiracetam to 40 mg/kg/day divided twice a day and no specific need for respiratory or systemic support measures. No neuroimaging or cerebrospinal fluid analysis studies were obtained. It was concluded the patient trigger of status epilepticus is possibly partially attributed to his concurrent COVID-19 infection.
Figure 1.
Inter-ictal EEG demonstrating: 1. Independent, bi-posterior irregular spike and wave activity in brief bursts. 2. Diffuse background slowing and moderate disorganization for age. 3. No clinical or electrographic seizures identified.
Discussion
Many neurological disorders, including, but not limited to, multiple sclerosis, large-vessel stroke, Parkinson’s disease, dementia, motor neuron disease, and neuromuscular disorders, are considered high risk factors for severe COVID-19 complications. However, substantial evidence that epilepsy by itself constitutes an independent risk factor for COVID-19 infection complications is currently lacking. A knowledge gap persists in our understanding of the exact pathogenic mechanisms of COVID-19 infection and especially potential neurological sequelae in the pediatric age group. Available data suggest that children tend to have a milder phenotype of COVID-19 infections in general.4 However, given the variable presentation of COVID-19, it is plausible that children may not present with typical respiratory symptoms similar to those of adults.4 Among the non-respiratory clinical presentations, many patients have reported both peripheral and central nervous system symptoms, but with seizures being relatively rare.5 A rising number of reports have described acute viral encephalitis, infectious toxic encephalopathy, acute ataxia, worsening headaches, and acute cerebrovascular accidents to be the most prevalent acute neurological complications of COVID-19 infection.6 Possible nervous system mechanisms of dysfunction include direct brain invasion, hematogenous spread, neuronoal migration, secondary hypoxic brain injury, angiotensin converting enzyme dysfunction, autoimmune mediated neurotoxicity, and inflammatory cascade pathways activation.6
Few reports of patients with COVID-19 infection presenting with status epilepticus were recently published, almost exclusively in adult population. Moriguchi et al7 reported a 24 years old young man who presented with acute SARS-CoV-2 viral encephalitis proven by CSF viral PCR and acute prolonged symptomatic seizures. Sohal and Mossammat8 reported a 72 years old patient who developed recurrent tonic seizures on his third day of admission with acute COVID-19 infection. Vollono et al9 reported a 78 years old man with preexisting symptomatic epilepsy secondary to prior HSV encephalitis, who presented with right facial twitching consistent focal status epilepticus needing multiple rounds of anti-seizure medications, who ultimately tested positive for SARS-CoV-2. Somani et al10 has reported 2 adult patients (49 years and 73 years old) who presented with acute COVID-19 infection and concurrent convulsive status epilepticus without prior history of epilepsy. There was another recent report of a 64 years old who developed non convulsive focal status epilepticus along with acute respiratory and psychotic symptoms who was found to have acute COVID-19 infection.11 To date, we are only aware of one 11-year-old child who presented with acute SARS-CoV-2 encephalitis and convulsive status epilepticus.12 However, that patient did have other systemic symptoms including pyrexia, in contrast with our patient.
Pathophysiology of convulsive status epilepticus in the context of acute COVID-19 infection is likely complex and multifactorial. Viral meningoencephalitis infection is one of the most common etiologies for symptomatic acute status epilepticus worldwide with seizure burden being parallel to the degree of brain infection and inflammation.13 SARS-CoV-2 has been isolated in CSF of some patients suggestive a potential causative relationship with concomitant meningoencephalitis and associated seizures in both adults and children.8,11 An obvious limitation to our reported case is unavailability of CSF evaluation during this presentation. Status epilepticus is also a known possible clinical manifestation of autoimmune encephalopathy disorders with several reports of such phenomena among children and adults who were recently infected with COVID-19 infection.14 Zanin et al15 reported a 54 years woman who had status epilepticus and was found to have acute demyelinating encephalomyelitis following an acute COVID-19 infection. Our patient did not have any evolving neurological exam findings to suspect a demyelinating disorder. Nevertheless, and given the lack of an updated neuroimaging, we cannot completely rule out such possibility and constitutes another limitation to our study conclusions. Breakthrough seizures including status epilepticus are observed in some fever sensitive pediatric epilepsy syndromes during acute viral infections such as Dravet syndrome and febrile infection related epilepsy syndrome (FIRES). In a series of 168 children who were hospitalized in Italy for acute COVID-19 infection, 5 children had breakthrough seizures, and out of them 4 children had preexisting epilepsy syndrome.16 Although our reported patient has an established and likely genetic epilepsy diagnosis, he was not diagnosed with a particular epilepsy syndrome. Neurovascular injury is a postulated as a potential neurological implication of COVID-19 infection and is likely contributing to pathogenesis of status epilepticus in some patients, particularly in adults.17 Endothelial cells infection, monocyte activation, secondary lymphohistiocytosis, secondary plagues destabilization, and end organ vasculitis are among the possibly involved mechanisms of injury in this context.18 As stated, our patient did not undergo an updated brain MRI for evaluation of secondary neurovascular injury.
Several metabolic derangements can take place due to both direct and indirect COVID-19 infection. There have been multiple studies of documented multi-organ dysfunction in the setting of acute COVID-19 infection. Hepatic and metabolic dysfunction can alter pharmacokinetics of circulating anti-seizure medications, and thus serve as a potential provoking factor for status epilepticus in susceptible patients. Our reported patient was not noted to have major electrolyte derangements. It is unknown yet if anti-seizure medications do elevate the risk for controlled or uncontrolled epilepsy patients to have a clinically distinct SARS-CoV-2 infection phenotype. However high dose corticosteroids and other immunomodulatory drugs which are occasionally used for their anti-seizure benefits can result in more severe COVID-19 infection phenotype.19 Severe or disseminated COVID-19 might then act as a further trigger for worsening or prolonged seizures. Clinicians must also remain aware that there are potential drug-to-drug interactions between some anti-seizure medications and anti-microbial treatments occasionally used off label in management of COVID-19.20 lastly, it is non debatable that the whole health system has undergone a major shift since the COVID-19 pandemic. Decreased access to primary and specialized care due to patients fears or cancellation of appointments, reduced diagnostic test openings, and delays in dispensing medications have been some of the real life challenges. In fact, possibility of jeopardized epilepsy care quality amid the COVID-19 pandemic and the potential end result of worsening seizure control in some patients is a matter of ongoing discussion.21,22 It is unknown if those logistic barriers may contribute to increased incidences of uncontrolled or prolonged seizures in pediatric epilepsy population.
Conclusion
An association between epilepsy and novel SARS-CoV-2 infection might exist, although it is not currently well understood. Our reported patient’s status epilepticus might have been precipitated by the concurrent SARS-CoV-2 infection. However with limitations of sub-therapeutic anti-seizure medication level and absence of fever or respiratory symptoms present a challenge in arriving confidently at that conclusion. Nevertheless, we believe our reported patient is a useful addition to the library of potential neurological manifestations of the current COVID-19 pandemic. We posit that SARS-CoV-2 testing be considered in pediatric patients with status epilepticus during the pandemic, given its heterogeneous presentation and lack of respiratory symptoms in several patients. Reports such as ours will be central to improving the understanding of the complex relationship between infectious diseases and neurological dysfunction.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author Contributions: AMK: Substantial contributions to the conception and design of the work and the acquisition, analysis, and interpretation of data for the work; drafting the work and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. SH: Substantial contributions to the drafting the work and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. GK: Substantial contributions to the drafting the work and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. SF: Substantial contributions to the drafting the work and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Consent: A parent consented to study publication, provided that name, address, and personal identification info are not publicly published.
ORCID iD: Abdulhafeez M Khair  https://orcid.org/0000-0003-1890-3972
https://orcid.org/0000-0003-1890-3972
References
- 1.Dawood FS, Philip R, Njie GJ, et al. Observations of the global epidemiology of COVID-19 from the prepandemic period using web-based surveillance: a cross-sectional analysis. Lancet Infect Dis. 2020;20:1255-1262. doi: 10.1016/S1473-3099(20)30581-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asadi-Pooya AA, Simani L.Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci. 2020;413:116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhaskar S, Bradley S, Israeli-Korn S, et al. Chronic neurology in COVID-19 era: clinical considerations and recommendations from the REPROGRAM consortium. Front Neurol. 2020;11:664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz AT, Zeichner SL.COVID-19 in children: initial characterization of the pediatric disease. Pediatrics. 2020;145:e20200834. [DOI] [PubMed] [Google Scholar]
- 5.Kuroda N.Epilepsy and COVID-19: associations and important considerations. Epilepsy Behav. 2020;108:107122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sohal S, Mossammat M.COVID-19 presenting with seizures. IDCases. 2020;20:e00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vollono C, Rollo E, Romozzi M, et al. Focal status epilepticus as unique clinical feature of COVID-19: a case report. Seizure. 2020;78:109-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Somani S, Pati S, Gaston T, Chitlangia A, Agnihotri S.De Novo Status Epilepticus in patients with COVID-19. Ann Clin Transl Neurol. 2020;7:1240-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernard-Valnet R, Pizzarotti B, Anichini A, et al. Two patients with acute meningoencephalitis concomitant with SARS-CoV-2 infection. Eur J Neurol. 2020;27:e43-e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAbee GN, Brosgol Y, Pavlakis S, Agha R, Gaffoor M.Encephalitis associated with COVID-19 infection in an 11-year-old child. Pediatr Neurol. 2020;109:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowenstein DH, Walker M, Waterhouse E.Status epilepticus in the setting of acute encephalitis. Epilepsy Curr. 2014;14(suppl 1):43-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Correia AO, Feitosa P, Moreira J, et al. Neurological manifestations of COVID-19 and other coronaviruses: a systematic review. Neurol Psychiatry Brain Res. 2020;37:27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zanin L, Saraceno G, Panciani PP, et al. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. 2020;162:1491-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garazzino S, Montagnani C, Donà D, et al. Multicentre Italian study of SARS-CoV-2 infection in children and adolescents, preliminary data as at 10 April 2020. Euro Surveill. 2020;25:2000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.TunÇ A, ÜnlÜbaŞ Y, Alemdar M, AkyÜz E.Coexistence of COVID-19 and acute ischemic stroke report of four cases. J Clin Neurosci. 2020;77:227-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Z, Liu J, Zhou Y, Zhao X, Zhao Q, Liu J.The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect. 2020;81:e13-e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fırat O, Yalçın N, Demirkan K.COVID-19 & antiepileptic drugs: Should we pay attention? Seizure. 2020;80:240-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Assenza G, Lanzone J, Ricci L, et al. Electroencephalography at the time of Covid-19 pandemic in Italy. Neurol Sci. 2020;41:1999-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adan GH, Mitchell JW, Marson T.Epilepsy care in the COVID-19 era. Clin Med (Lond). 2020;20:e104-e106. [DOI] [PMC free article] [PubMed] [Google Scholar]