Abstract
Background:
A randomized, placebo-controlled phase III study (AB10015) previously demonstrated that orally administered masitinib (4.5 mg/kg/day) slowed rate of functional decline, with acceptable safety, in amyotrophic lateral sclerosis (ALS) patients having an ALS Functional Rating Scale-revised (ALSFRS-R) progression rate from disease onset to baseline of <1.1 points/month. Here we assess long-term overall survival (OS) data of all participants from study AB10015 and test whether a signal in OS is evident in an enriched patient population similar to that prospectively defined for confirmatory study AB19001.
Methods:
Survival status of all patients originally randomized in AB10015 was collected from participating investigational sites. Survival analysis (using the multivariate log-rank test and Cox proportional hazards model, with stratification factors as covariates) was performed on the intention-to-treat population and enriched subgroups, which were defined according to initial randomization, baseline ALSFRS-R progression rate and baseline disease severity.
Results:
A significant survival benefit of 25 months (p = 0.037) and 47% reduced risk of death (p = 0.025) was observed for patients receiving 4.5 mg/kg/day masitinib (n = 45) versus placebo (n = 62) in an enriched cohort with ⩾2 on each baseline ALSFRS-R individual component score (i.e. prior to any complete loss or severe impairment of functionality) and post-onset ALSFRS-R progression rate <1.1 (i.e. exclusion of very fast progressors) [median OS of 69 versus 44 months, respectively; hazard ratio, 0.53 [95% CI (0.31–0.92)]]. This corresponds to the population enrolled in confirmatory phase III study, AB19001.
Conclusions:
Analysis of long-term OS (75 months average follow-up from diagnosis) indicates that oral masitinib (4.5 mg/kg/day) could prolong survival by over 2 years as compared with placebo, provided that treatment starts prior to severe impairment of functionality.
This trial was registered at www.ClinicalTrials.gov under identifier NCT02588677 (28 October 2015).
Keywords: clinical trials, masitinib, therapy, tyrosine kinase inhibitor
Introduction
Masitinib, a selective oral tyrosine kinase inhibitor, exerts experimental neuroprotection in both central and peripheral nervous systems.1–5 We report long-term survival data from the previously published masitinib phase IIb/III study (AB10015, ClinicalTrials.gov identifier: NCT02588677) in amyotrophic lateral sclerosis (ALS) (see Supplementary Appendix for summary).6,7 Briefly, the primary endpoint of study AB10015, decline in ALS Functional Rating Scale-revised (ALSFRS-R) from baseline to week 48 (∆ALSFRS-R), showed benefit for masitinib administered at 4.5 mg/kg/day as an add-on to riluzole over placebo in ALS patients having an ALSFRS-R progression rate from disease onset to baseline (ΔFS) of <1.1 points/month. The between-group difference was 3.39 [95% confidence interval (CI) 0.65–6.13; p = 0.016], corresponding to a 27% slowing of ALSFRS-R deterioration over the 48-week treatment period. This significant outcome was corroborated by numerous sensitivity analyses, including the conservative multiple imputation jump-to-reference technique with a between-group difference of 2.80 [95% CI (0.15–5.46); p = 0.039].6,7
At the time of final readout for study AB10015, overall survival (OS) data were too immature for interpretation. The rate of decline in ALSFRS-R is considered a sensitive and independent clinical prognostic parameter in ALS that correlates with OS8–11; nevertheless, median OS analysis remains the gold standard for demonstration of a drug’s therapeutic benefit in ALS. Hence, post-study long-term OS assessment represents a valuable complement to the main study results.
Included in the published results from study AB10015 were subgroup analyses based on revised ALS trial guidelines that recommend patients should be selected as early as possible in the course of their disease.12–14 This showed that initiation of masitinib at a less severe stage of disease produced greater treatment effect.6,15 The benefit of patient enrichment strategies based on ALSFRS-R has also been recognized by other studies; for example, the edaravone, rasagiline and high-fat intervention clinical trials.16–18 Hence, findings from this analysis, in conjunction with scientific advice from regulatory authorities, have been incorporated into the confirmatory masitinib study design (AB19001, ClinicalTrials.gov identifier: NCT03127267). Such a design is consistent with masitinib’s therapeutic objective for conservation of neuromuscular functions as opposed to repair of existing neurological damage.1,19
Importantly, identification of this enriched population suggested that a long-term OS signal could be detectable within the available AB10015 dataset given sufficient follow-up. The main objective of the current analysis was therefore to assess whether a signal in OS is evident in an enriched patient population, similar to that prospectively defined for the confirmatory phase III study (AB19001). It also allowed us to explore reports of greatly improved survival from individual sites participating in an international early access Named Patient Program (NPP) using a larger, multicenter patient cohort.20
Method
Details of the method and patient selection criteria of study AB10015 (ClinicalTrials.gov identifier: NCT02588677) have been published6 (see Supplementary Appendix for additional information). The protocol for study AB10015 was approved by the institutional review board or ethics committee at each participating clinical site and was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent, which included provision to review medical records for completion of undetermined endpoints, such as OS.
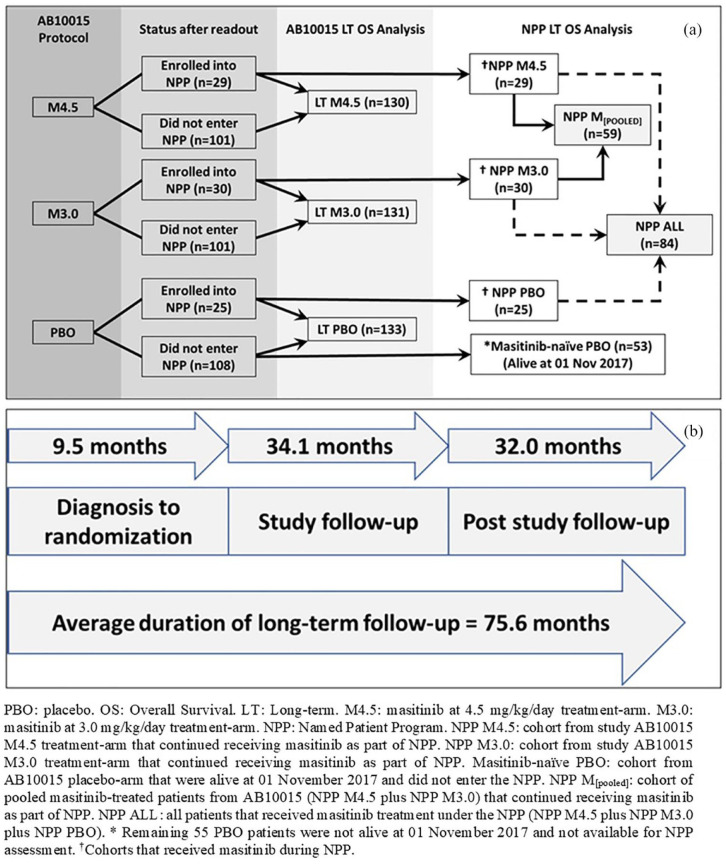
Figure 1 shows the relation between randomized treatment arms of study AB10015, cohorts used for the AB10015 long-term OS analysis, and subpopulations of interest for the NPP long-term OS analysis. OS was defined as the time elapsed between randomization and death from any cause. OS p-values were calculated via the multivariate log-rank test using the covariates of age and ALSFRS-R score at randomization, site of onset (spinal versus bulbar), geographical region, and ΔFS. All covariates were prespecified in the AB10015 protocol as randomization stratification factors.6 Hazard ratios (HR) and 95% CIs were calculated via the Cox proportional hazards model using the abovementioned covariates.
Figure 1.
(a) Relationship between randomized treatment arms of study AB10015, cohorts used for the AB10015 long-term OS analysis, and subpopulations of interest for the NPP long-term OS analysis. (b) Time frame for study AB10015 long-term OS analysis showing a 75-month average duration of observation (time of diagnosis until cut-off of long-term OS analysis).
LT, long term; NPP, Named Patient Program; OS, overall survival; PBO, placebo.
Procedure for long-term OS analysis of the AB10015 study population
In the current analysis, vital status (i.e. survival status of alive or dead, including date of death) of all patients originally randomized to study AB10015 was collected from each participating investigational site. As such, three patient groups were defined [see Figure 1(a)]: long-term high-dose (4.5 mg/kg/day) masitinib (referred to hereafter as ‘LT-M4.5’), long-term low-dose (3.0 mg/kg/day) masitinib (referred to hereafter as ‘LT-M3.0’), and long-term placebo (referred to hereafter as ‘LT-PBO’). Long-term assessment for study AB10015 encompassed the prospectively declared 48-week treatment period with associated double-blind extension (commencing in April 2013 until data readout), and a post-study (unblinded) follow-up period (from November 2017 until June 2020) [see Figure 1(b)]. Patients still alive at the time of analysis were censored at the date of last contact.
Long-term OS analysis was performed on the intention-to-treat (ITT) population (i.e. all those patients randomized to study AB10015) as well as on enriched subgroups, the latter of which were defined after the prospectively declared study period had ended, according to initial randomization, ΔFS, and baseline disease severity. For example, exclusion of patients with high baseline disease severity was achieved by identifying those patients with any zero-point ALSFRS-R items or any one-point ALSFRS-R items at baseline, corresponding to disease that has advanced to the point of a permanent loss of function or a severe impairment of functionality, respectively. In this manner, three enriched subgroups were defined, namely: patients with ΔFS < 1.1 regardless of baseline ALSFRS-R score; patients with ⩾2 on each baseline ALSFRS-R item, regardless of baseline ΔFS; and patients with ⩾2 on each baseline ALSFRS-R item and ΔFS < 1.1 (the latter cohort corresponding most closely to the confirmatory AB19001 study population). Potential between-group baseline bias in the enriched patient subgroups was assessed by comparison of baseline parameters for each cohort. Supportive analyses using the same patient cohorts were also performed according to the endpoints of ∆ALSFRS at week 48, and a time-to-event analysis referred to as progression-free survival (PFS) (an endpoint driven by both death and a fixed disease progression on the ALSFRS-R scale, the latter being defined in study AB10015 as a nine-point deterioration of ALSFRS-R from baseline).
Procedure for long-term OS analysis of the Named Patient Program subpopulations
Following data readout for the main protocol period of study AB10015, treatment assignment was unblinded and an optional, open-label, early access NPP was initiated. This allowed those patients still receiving masitinib to continue treatment, while also allowing patients from the AB10015 placebo arm to begin masitinib treatment. NPPs typically concern individual patients, designated by name, and are issued at the request of the prescribing physician. Such programs are not clinical trials or compassionate use programs as per the European Union regulations but are intended to allow patients access to medicinal products that have not yet received a marketing authorization. Conditions required to run such a program are that the product must be intended for treatment of a serious or orphan disease, there must be an absence of a suitable therapeutic alternative available, and the efficacy/safety ratio can be presumed favorable based on available evidence.21 Another feature distinct to NPPs is that dosing decisions are made solely by the prescribing physician, albeit based on best available evidence, such as results from study AB10015 and recommendations on use of a masitinib dose-escalation scheme.6,22 It is also important to note that only participants of study AB10015 were included in this analysis of long-term OS.
The NPP permits comparison between patients randomized to either one of the AB10015 masitinib treatment arms and who continued to receive masitinib treatment under the NPP, versus patients from the AB10015 placebo arm that did not participate in the NPP and were therefore never treated with masitinib. This latter subpopulation is referred to hereafter as the ‘masitinib-naïve placebo (PBO) cohort’ [Figure 1(a)]. Because only patients alive at the time of study data readout were available to enroll into the NPP, it is necessary to apply a similar condition to the comparator placebo subpopulation, i.e. exclusion of patients who had died prior to 1 November 2017; thereby, avoiding bias arising from the implicit condition that patients had survived at least the randomized study period prior to entering the NPP.
To define NPP subpopulations for long-term OS analysis, all patients enrolled into the NPP retained their initial treatment-arm designations. For example, patients from the AB10015 masitinib 4.5 and 3.0 mg/kg/day treatment arms who continued to receive masitinib on the NPP, were designated as the ‘NPP-M4.5’ and ‘NPP-M3.0’ subpopulations, respectively [Figure 1(a)]. Likewise, patients from the AB10015 placebo arm who started masitinib treatment as part of the NPP were designated as the ‘NPP-PBO’ subpopulation. Further cohorts of interest were patients pooled from both masitinib treatment-arms (i.e. ‘NPP-M4.5’ plus ‘NPP-M3.0’, referred to hereafter as the ‘NPP-M(pooled)’ subpopulation), and also patients pooled from all NPP groups (i.e. ‘NPP-M4.5’ plus ‘NPP-M3.0’ plus ‘NPP-PBO’, referred to hereafter as the ‘NPP-ALL’ subpopulation). NPP long-term OS analyses were performed regardless of baseline ΔFS or individual ALSFRS-R component scores. Potential sources of baseline bias in the NPP subpopulations of interest were assessed by comparison of baseline parameters for each cohort. Because any treatment effect during the randomized study period is expected to have impacted on ALSFRS-R, respiratory function and duration-related variables, comparison of NPP baseline characteristics is made at the time of randomization rather than at NPP enrolment.
Results
Patients and follow-up analysis
This long-term survival analysis encompassed all participants randomized to study AB10015. A total of 394 patients from 34 sites in nine countries were available for the main study efficacy analysis (133, 131, and 130 patients in the placebo, masitinib 3.0 mg/kg/day, and masitinib 4.5 mg/kg/day treatment arms, respectively). All investigational sites from study AB10015 were contacted with a request for an update on each patient’s vital (survival) status. As of June 2020, 96% of patients (378/394) had their vital status verified (Table 1). The remaining 16 patients (4%) had a status that dated back further than 7 months from this cut-off, with a patient distribution that was evenly spread across treatment arms (five, five, and six patients in the placebo, masitinib 3.0 mg/kg/day, and masitinib 4.5 mg/kg/day treatment arms, respectively).
Table 1.
Disposition of populations for long-term OS analysis of study AB10015 and its associated NPP.
| Cohort | PBO (n) | M4.5 (n) | M3.0 (n) | Total (n) |
|---|---|---|---|---|
| Overall (mITT) population | 133 | 130 | 131 | 394 |
| Survival status verified less than 7 months prior to cut-offa | 128 (96.2%) | 124 (95.4%) | 126 (96.2%) | 378 (95.9%) |
| Survival status older than 7 months | 5 (3.8%) | 6 (4.6%) | 5 (3.8%) | 16 (4.1%) |
| Patients available for NPP OS analysisb | 25 (18.8%) | 29 (22.3%) | 30 (22.9%) | 84 (21.3%) |
| Patients not available for NPP OS analysis | 108 (81.2%) | 101 (77.7%) | 101 (77.1%) | 310 (78.7%) |
Survival status (also referred to as vital status) verified by investigational site within 7 months prior to cut-off (June 2020) for long-term OS analysis.
Patients from study AB10015 that entered the optional NPP.
NPP, Named Patient Program; OS, overall survival; PBO, placebo.
Concerning the NPP, a total of 84 patients from 19 sites in eight countries chose to enter the program following unblinding of study AB10015 and were therefore available for NPP long-term OS analysis. This included 19% (25/133) of patients from the placebo arm switching to receive masitinib, 23% (30/131) of patients from the masitinib 3.0 mg/kg/day treatment arm, and 22% (29/130) of patients from the masitinib 4.5 mg/kg/day treatment arm. A total of 53/133 (40%) patients from the placebo arm were alive at November 2017 but did not participate on the masitinib NPP, and were thereby defined as the ‘masitinib-naïve PBO’ cohort.
Patient disposition from study AB10015 and the masitinib NPP, including breakdown of updated survival status, is shown in Table 1. A summary of the overall population’s average follow-up time and disease duration at various stages of study AB10015 and its post-study follow-up period is presented in Figure 1(b) and Table 2. Based on the inclusion criterion of patients having less than 36 months disease duration from baseline, the overall average time from diagnosis until randomization was 9.5 ± 7.5 months. The average follow-up of patients on study AB10015 and its associated double-blind extension period, i.e. from randomization of the first patient (April 2013) until date of study data readout (November 2017), was 34.1 ± 10.1 months. The duration of observation with respect to diagnosis (i.e. time elapsed from diagnosis until date of study data readout, regardless of patient censoring) was 43.4 ± 24.4 months. The duration of the unblinded post-study follow-up period, including optional NPP, was an additional 32 months, i.e. November 2017 until June 2020. Hence, from the date of diagnosis, patients have been under long-term OS observation for an average duration of approximately 75 months.
Table 2.
Summary of study AB10015 time intervals showing a 75-month average duration of long-term OS observation (time of diagnosis until cut-off of long-term OS analysis).
| PBO (n = 133) | M4.5 (n = 130) | M3.0 (n = 131) | |
|---|---|---|---|
| Average duration from diagnosis until randomization (months) (mean ± SD) | 9.0 ± 6.8 | 9.7 ± 8.4 | 10.0 ± 7.4 |
| Average duration from disease onset until randomization (months) (mean ± SD) | 18.1 ± 8.6 | 19.2 ± 9.6 | 19.2 ± 8.2 |
| Overall study population (n = 394) | |||
| Overall average duration from diagnosis until randomization (months) (mean ± SD) | 9.5 months ± 7.5 | ||
| Overall average duration of study follow-up (April 2013 – end of study) (mean ± SD) | 34.1 months ± 10.1 | ||
| Duration of post-study follow-up (November 2017 – long-term OS cut-off June 2020) | 32 months | ||
| Average duration of long-term OS observation from date of diagnosis (estimate) | 75.6 months | ||
M3.0, masitinib at 3.0 mg/kg/day treatment arm; M4.5, masitinib at 4.5 mg/kg/day treatment arm; OS, overall survival; PBO, placebo; SD, standard deviation.
Baseline characteristics
Treatment arms for the primary efficacy analysis population of study AB10015 were well balanced, with patients having been randomized using a computerized central randomization system and minimization method according to the covariates of ALSFRS-R score, age, geographical region, site of onset (spinal versus bulbar), and post-onset ΔFS.6
Representative data for the long-term M4.5 cohorts showing the smallest and greatest levels of enrichment are presented in Table 3. As expected, the overall population (i.e. LT-M4.5, regardless of baseline ΔFS or individual ALSFRS-R component scores), maintained balanced baseline characteristics between treatment arms. Balance was also observed for the enriched patient subgroup of LT-M4.5 with ⩾2 on each baseline ALSFRS-R item and ΔFS < 1.1, for which the only notable difference (>10%) was that the masitinib arm had a longer average disease duration from time of diagnosis compared with placebo (8.2 versus 7.6 months, respectively). Baseline characteristics for additional long-term M4.5 enriched patient subgroups and the long-term M3.0 cohorts are presented in Tables S1 and S2, respectively.
Table 3.
Baseline characteristics for the overall (ITT) masitinib 4.5 mg/kg/day cohort versus associated placebo group (regardless of baseline ΔFS or individual ALSFRS-R component scores) and for the enriched cohort most closely matched to the AB19001 study design.
| M4.5, regardless of baseline ALSFRS-R or ΔFS | M4.5 (⩾2 each baseline ALSFRS-R item, ΔFS < 1.1) | |||||
|---|---|---|---|---|---|---|
| PBO (N = 133) | M4.5 (N = 130) | Deltab (%) | PBO (N = 62) | M4.5 (N = 45) | Deltab (%) | |
| Sex; n (%) | ||||||
| Male | 80 (60.2) | 83 (63.8) | +3.6 | 39 (62.9) | 31 (68.9) | −6.0 |
| ΔFS < 1.1; n (%) | ||||||
| Yes | 114 (85.7) | 106 (81.5) | −4.2 | 62 (100.0) | 45 (100.0) | 0 |
| Average ΔFS (points/month) | ||||||
| Mean ± SD | 0.71 ± 0.69 | 0.73 ± 0.63 | +2.8 | 0.41 ± 0.23 | 0.39 ± 0.25 | −4.9 |
| Range | 0.05; 5.00 | 0.03; 3.69 | 0.05; 1.07 | 0.03; 1.01 | ||
| Average ALSFRS-R score | ||||||
| Mean ± SD | 38.1 ± 5.5 | 37.5 ± 5.5 | −1.6 | 42.0 ± 3.2 | 42.2 ± 3.1 | +0.5 |
| Range | 21.0; 47.0 | 23.0; 47.0 | 34.0; 47.0 | 36.0; 47.0 | ||
| Age (years) | ||||||
| Mean ± SD | 55.2 ± 10.6 | 55.5 ± 10.6 | +0.5 | 55.0 ± 10.1 | 55.6 ± 11.5 | +1.1 |
| Range | 27.0; 75.0 | 24.0; 79.0 | 28.0; 73.0 | 24.0; 78.0 | ||
| ALS diagnosis; n (%) | ||||||
| Definite | 79 (59.4) | 76 (58.5) | +0.9 | 39 (62.9) | 25 (55.6) | −7.3 |
| Probable | 43 (32.3) | 44 (33.8) | −1.5 | 19 (30.6) | 16 (35.6) | +5.0 |
| Probable, lab | 11 (8.3) | 10 (7.7) | +0.6 | 4 (6.5) | 4 (8.9) | +2.4 |
| Disease durationa (months) | ||||||
| Mean ± SD | 9.0 ± 6.8 | 9.7 ± 8.4 | +7.8 | 7.3 ± 5.9 | 8.2 ± 7.6 | +12.3 |
| FVC (predicted) | ||||||
| Mean ± SD | 89.2 ± 18.7 | 87.5 ± 16.9 | +1.9 | 92.9 ± 17.9 | 93.8 ± 17.0 | +1.0 |
| Site of onset; n (%) | ||||||
| Spinal | 109 (82.0) | 107 (82.3) | +0.3 | 46 (74.2) | 35 (77.8) | +3.6 |
| Bulbar | 24 (18.0) | 23 (17.7) | −0.3 | 16 (25.8) | 10 (22.2) | −3.6 |
| Region; n (%) | ||||||
| North America and Western Europe | 86 (64.7) | 81 (62.3) | −2.4 | 35 (56.5) | 24 (53.3) | −3.2 |
| Eastern Europe | 8 (6.0) | 8 (6.2) | +0.2 | 4 (6.5) | 4 (8.9) | +2.4 |
| Other countries | 39 (29.3) | 41 (31.5) | +2.2 | 23 (37.1) | 17 (37.8) | +0.7 |
Average disease duration from time of diagnosis.
Delta, relative difference between treatment arms (%) with respect to placebo.
ALSFRS-R, Amyotrophic Lateral Sclerosis Functional Rating Scale-revised; FVC, forced vital capacity; M4.5, masitinib 4.5 mg/kg/day; PBO, placebo; SD, standard deviation; ΔFS, ALSFRS-R progression rate from disease onset to baseline.
Considering baseline parameters from representative NPP subpopulations, i.e. NPP-M4.5 and masitinib-naïve PBO, it is seen that the majority of baseline characteristics (e.g. ALSFRS-R score, age, respiratory function, and disease duration from diagnosis) showed no indication of self-selection bias (see Table 4). Some imbalance between treatment arms had been introduced regarding the distribution of ALS diagnosis (El Escorial diagnostic criteria), site of onset (bulbar), and post-onset ΔFS. More specifically, patients opting into the NPP-M4.5 cohort were progressing at a slower rate than patients in the masitinib-naïve PBO cohort (average ΔFS at randomization of 0.5 versus 0.62, respectively, a relative difference of 19%). This situation could potentially favor survival in the masitinib cohort. Conversely, a higher proportion of bulbar onset patients opted into the NPP-M4.5 cohort relative to the masitinib-naïve PBO cohort (20.7% versus 11.3%, respectively, a relative difference of 83%), which could potentially favor survival in the masitinib-naïve PBO cohort because bulbar onset is an independent risk factor for poor survival.23
Table 4.
Baseline patient characteristics for the NPP subgroup analysis.
| Masitinib-naïve PBO (N = 53) | NPP-M4.5 (N = 29) | Deltab (%) | NPP-M(pooled) (N = 59) | Deltab (%) | NPP-ALL (N = 84) | Deltab (%) | |
|---|---|---|---|---|---|---|---|
| Sex; n (%) | |||||||
| Male | 33 (62.3) | 20 (69.0) | +6.7 | 39 (66.1) | +3.8 | 55 (65.5) | +3.2 |
| ΔFS < 1.1; n (%) | |||||||
| Yes | 48 (90.6) | 28 (96.6) | +6.0 | 57 (96.6) | +6.0 | 80 (95.2) | +4.6 |
| Average ΔFS (points/month) | |||||||
| Mean ± SD | 0.62 ± 0.7 | 0.5 ± 0.5 | −19.4 | 0.46 ± 0.39 | −25.8 | 0.46 ± 0.38 | −25.8 |
| ALSFRS-R score | |||||||
| Mean ± SD | 38.5 ± 5.1 | 40.4 ± 5.6 | +4.9 | 40.1 ± 4.8 | +4.2 | 40.2 ± 4.8 | +4.4 |
| Age (years) | |||||||
| Mean ± SD | 54.0 ± 10.8 | 50.0 ± 9.7 | −7.4 | 50.7 ± 9 .7 | −6.1 | 50.9 ± 10.0 | −5.7 |
| ALS diagnosis; n (%) | |||||||
| Definite | 34 (64.2) | 13 (44.8) | −19.4 | 29 (49.2) | −15.0 | 36 (42.9) | −21.3 |
| Probable | 12 (22.6) | 14 (48.3) | +25.7 | 22 (37.3) | +14.7 | 38 (45.2) | +22.6 |
| Probable, lab | 7 (13.2) | 2 (6.9) | −6.3 | 8 (13.6) | +0.4 | 10 (11.9) | −1.3 |
| Disease durationa | |||||||
| Mean ± SD | 9.5 ± 7.1 | 10.0 ± 10.0 | +5.3 | 11.2 ± 9.3 | +17.9 | 10.7 ± 8.7 | +12.6 |
| FVC (% predicted) | |||||||
| Mean ± SD | 91.7 ± 20.6 | 93.9 ± 15.9 | +2.4 | 91.4 ± 15.7 | −0.3 | 92.7 ± 15.4 | +1.1 |
| Site of onset; n (%) | |||||||
| Bulbar | 6 (11.3) | 6 (20.7) | +9.4 | 10 (16.9) | +5.6 | 14 (16.7) | +5.4 |
| Region; n (%) | |||||||
| Other countries | 21 (39.6) | 10 (34.5) | −5.1 | 23 (39.0) | −0.6 | 25 (29.8) | −9.8 |
| Eastern Europe | 6 (11.3) | 2 (6.9) | −4.4 | 2 (3.4) | −7.9 | 2 (2.4) | −8.9 |
| North America/Western Europe | 26 (49.1) | 17 (58.6) | +9.5 | 34 (57.6) | +8.5 | 57 (67.9) | +18.8 |
Average disease duration from time of diagnosis.
Delta, relative difference between treatment arms (%) with respect to masitinib-naïve PBO.
ALSFRS-R, Amyotrophic Lateral Sclerosis Functional Rating Scale-revised; FVC, forced vital capacity; masitinib-naïve PBO, cohort from AB10015 placebo arm (regardless of baseline ΔFS or baseline ALSFRS-R scores) who were alive at 1 November 2017 and did not enter the NPP; NPP M4.5, cohort from study AB10015 M4.5 treatment arm (regardless of baseline ΔFS or baseline ALSFRS-R scores) who continued receiving masitinib as part of NPP; NPP, Named Patient Program; SD, standard deviation; ΔFS, ALSFRS-R progression rate calculated from disease onset to baseline.
Hence, overall baseline characteristics were similar across treatment arms with no indication of bias due to self-selection.
Results from long-term OS analysis of the AB10015 study population
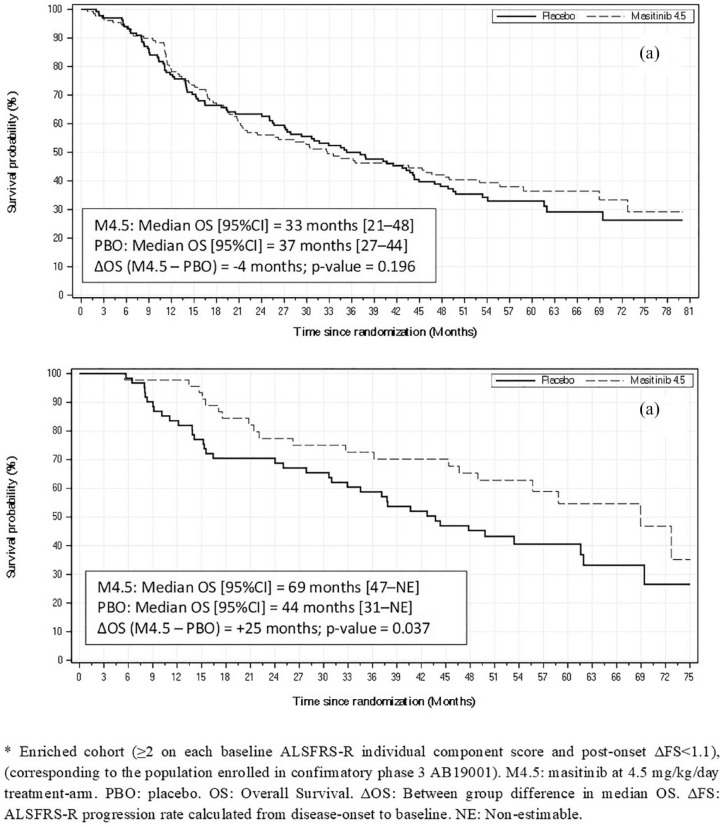
A summary of long-term median OS results for various patient cohorts of the AB10015 study population is presented in Table 5. No long-term survival advantage was observed for the overall masitinib 4.5 mg/kg/day cohort of study AB10015 (i.e. LT-M4.5, regardless of baseline ALSFRS-R scores or ΔFS) over placebo. Median OS for masitinib (n = 130) and placebo (n = 133) was 33 months [95% CI (21–48)] versus 37 months [95% CI (27–44)], respectively; p = 0.196 [Figure 2(a)]. However, as enrichment of patient selection was applied, an increasing improvement in median OS for masitinib with respect to placebo was observed. Statistical significance was reached for the cohort ‘LT-M4.5 with ⩾2 on each baseline ALSFRS-R item, regardless of baseline ΔFS’, with a median OS for masitinib (n = 50) of 69 months [95% CI (44–NE)] versus 44 months [95% CI (31–62)] for placebo (n = 63). This corresponds to a median OS difference of 25 months in favor of masitinib, p = 0.037. HR analysis was also significant for this cohort with a 44% reduced risk of death for masitinib-treated patients compared with those originally randomized to placebo [HR, 0.56 (95% CI (0.33–0.97)); p = 0.037] (Table 6). A similar survival advantage in favor of masitinib was seen for the cohort corresponding most closely to the confirmatory AB19001 study population, i.e. ‘LT-M4.5 with ⩾2 on each baseline ALSFRS-R item and ΔFS < 1.1’, with a significant median OS difference of 25 months in favor of masitinib, p = 0.037 [Figure 2(b)] and a significant 47% reduced risk of death [HR, 0.53 (95% CI (31–92)); p = 0.025] (Table 6).
Table 5.
Long-term OS from study AB10015 according to Kaplan–Meier survival analysis, including assessment of enriched subgroups and subpopulations of the associated NPP.
| Cohort | MAS (n) | PBO (n) | Deaths | Median OS (95% CI) | ΔOS (months) | p-value (log-rank) | ||
|---|---|---|---|---|---|---|---|---|
| MAS | PBO | MAS (months) | PBO (months) | |||||
| Study AB10015 high-dose masitinib arm versus placebo | ||||||||
| LT-M4.5 (regardless of baseline ALSFRS-R or ΔFS) | 130 | 133 | 80 (62%) | 88 (66%) | 33 (21; 48) | 37 (27; 44) | −4 | 0.196 |
| LT-M4.5 (ΔFS < 1.1, any baseline ALSFRS-R score) | 106 | 114 | 59 (56%) | 72 (63%) | 47 (30; 69) | 41 (28; 49) | +6 | 0.102 |
| LT-M4.5 (⩾2 each baseline ALSFRS-R item, any ΔFS) | 50 | 63 | 24 (48%) | 38 (60%) | 69 (44; NE) | 44 (31; 62) | +25 | 0.037 |
| LT-M4.5 (⩾2 each baseline ALSFRS-R item, ΔFS < 1.1) | 45 | 62 | 20 (44%) | 38 (61%) | 69 (47; NE) | 44 (31; 62) | +25 | 0.037 |
| Study AB10015 low-dose masitinib arm versus placebo | ||||||||
| LT-M3.0 (regardless of baseline ALSFRS-R or ΔFS) | 131 | 133 | 91 (69%) | 88 (66%) | 30 (25; 40) | 37 (27; 44) | −7 | 0.70 |
| LT-M3.0 (⩾2 each baseline ALSFRS-R item, ΔFS < 1.1) | 46 | 62 | 27 (59%) | 38 (61%) | 48 (26; 62) | 44 (31; 62) | +4 | 0.70 |
| NPP subpopulations (regardless of baseline ALSFRS-R or ΔFS) | ||||||||
| NPP-M4.5 versus masitinib-naïve PBO | 29 | 53 | 6 (21%) | 22 (42%) | 73 (69; NE) | 62 (49; NE) | +11 | 0.008 |
| NPP-M(pooled) versus masitinib-naïve PBO | 59 | 53 | 16 (27%) | 22 (42%) | 73 (69; NE) | 62 (49; NE) | +11 | 0.008 |
| NPP-ALL versus masitinib-naïve PBO | 84 | 53 | 27 (32%) | 22 (42%) | NR (69; NE) | 62 (49; NE) | NE | 0.016 |
p-value calculated using the log-rank test.
LT, long-term; M3.0, masitinib at 3.0 mg/kg/day treatment arm; M4.5, masitinib at 4.5 mg/kg/day treatment arm; MAS, masitinib; masitinib-naïve PBO, cohort from AB10015 placebo arm (regardless of baseline ΔFS or baseline ALSFRS-R scores) that were alive at 1 November 2017 and did not enter the NPP; NE, non-estimable; NPP, Named Patient Program; NPP-ALL, all patients that entered the NPP (NPP-M4.5 plus NPP-M3.0 plus NPP-PBO); NPP-M(pooled), cohort of pooled masitinib-treated patients from AB10015 (NPP-M4.5 plus NPP-M3.0) who continued receiving masitinib as part of NPP; NPP-M4.5, cohort from study AB10015 M4.5 treatment arm (regardless of baseline ΔFS or baseline ALSFRS-R scores) who continued receiving masitinib as part of NPP; NR, not reached; OS, overall survival; PBO, placebo; ΔFS, ALSFRS-R progression rate calculated from disease onset to baseline; ΔOS, between-group difference in median OS (MAS minus PBO).
Figure 2.
Kaplan–Meier survival curves from masitinib study AB10015 long-term survival analysis. (a) Overall masitinib 4.5 mg/kg/day cohort versus placebo (regardless of baseline ΔFS or individual component scores). (b) Enriched* masitinib 4.5 mg/kg/day cohort versus placebo (⩾2 on each baseline ALSFRS-R individual component score and post-onset ΔFS < 1.1).
Table 6.
Multivariate Cox proportional hazards ratio analysis, including assessment of enriched subgroups and subpopulations of the associated NPP.
| Cohort | MAS (n) | PBO (n) | Hazard ratio | 95% CI | Reduced risk of death (%) | p-value |
|---|---|---|---|---|---|---|
| Study AB10015 high-dose masitinib arm versus placebo | ||||||
| LT-M4.5 (regardless of baseline ALSFRS-R or ΔFS) | 130 | 133 | 0.82 | 0.60–1.13 | 18 | 0.226 |
| LT-M4.5 (ΔFS < 1.1, any baseline ALSFRS-R score) | 106 | 114 | 0.78 | 0.54–1.11 | 22 | 0.161 |
| LT-M4.5 (⩾2 each baseline ALSFRS-R item, any ΔFS) | 50 | 63 | 0.56 | 0.33–0.97 | 44 | 0.037 |
| LT-M4.5 (⩾2 each baseline ALSFRS-R item, ΔFS < 1.1) | 45 | 62 | 0.53 | 0.31–0.92 | 47 | 0.025 |
| Study AB10015 low-dose masitinib arm versus placebo | ||||||
| LT-M3.0 (regardless of baseline ALSFRS-R or ΔFS) | 131 | 133 | 1.02 | 0.75–1.38 | 0 | 0.903 |
| LT-M3.0 (⩾2 each baseline ALSFRS-R item, ΔFS < 1.1) | 46 | 62 | 1.00 | 0.60–1.66 | 0 | 0.996 |
| NPP subpopulations (regardless of baseline ALSFRS-R or ΔFS) | ||||||
| NPP-M4.5 versus masitinib-naïve PBO | 29 | 53 | 0.33 | 0.12–0.88 | 67 | 0.027 |
| NPP-M(pooled) versus masitinib-naïve PBO | 59 | 53 | 0.58 | 0.28–1.19 | 42 | 0.134 |
| NPP-ALL versus masitinib-naïve PBO | 84 | 53 | 0.71 | 0.38–1.31 | 29 | 0.267 |
LT, long-term; M3.0, masitinib at 3.0 mg/kg/day treatment arm; M4.5, masitinib at 4.5 mg/kg/day treatment arm; MAS, masitinib; masitinib-naïve PBO, cohort from AB10015 placebo arm (regardless of baseline ΔFS or baseline ALSFRS-R scores) who were alive at 1 November 2017 and did not enter the NPP; NPP, Named Patient Program; NPP-ALL, all patients who entered the NPP (NPP-M4.5 plus NPP-M3.0 plus NPP-PBO); NPP-M(pooled), cohort of pooled masitinib-treated patients from AB10015 (NPP-M4.5 plus NPP-M3.0) who continued receiving masitinib as part of NPP; NPP-M4.5, cohort from study AB10015 M4.5 treatment arm (regardless of baseline ΔFS or baseline ALSFRS-R scores) who continued receiving masitinib as part of NPP; PBO, placebo; ΔFS, ALSFRS-R progression rate calculated from disease onset to baseline.
In contrast, results from the low-dose masitinib arm of study AB10015 (LT-M3.0) showed that none of the enriched patient subgroups tested produced a significant improvement in median OS with respect to placebo (Tables 5 and 6).
Results from long-term OS analysis of the Named Patient Program subpopulations
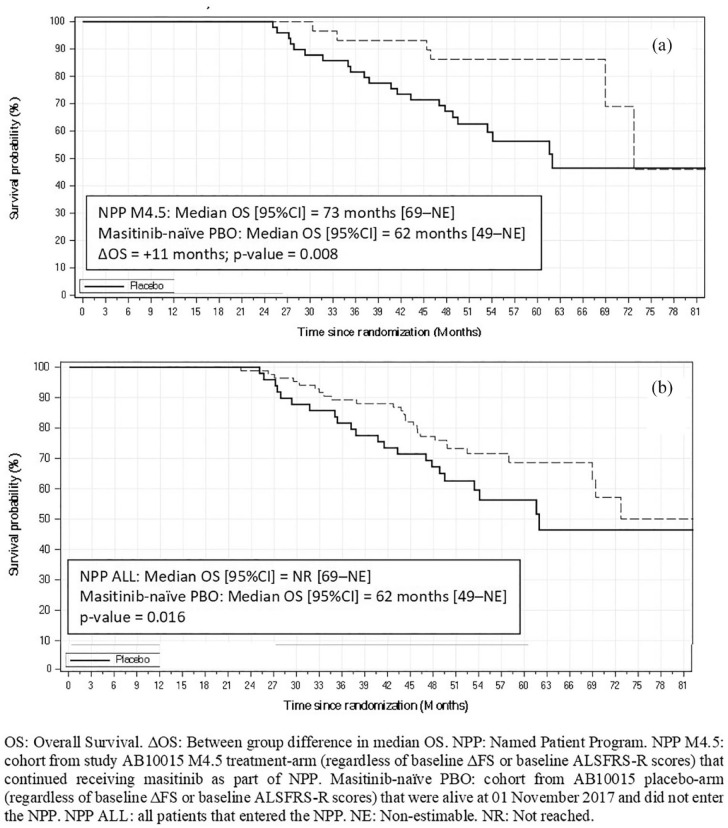
A summary of long-term median OS results for subpopulations of the AB10015 NPP is presented in Table 5. Kaplan–Meier survival curves visually showed divergence between NPP subpopulations and the placebo comparator arm, indicating a consistent survival advantage in favor of masitinib (Figure 3). For example, the subpopulations of ‘NPP-M4.5’ (n = 29) versus ‘masitinib-naïve PBO’ (n = 53) showed a significant difference in median OS of 11 months (p = 0.008) [Figure 3(a); Table 5]. The corresponding hazard ratio analysis showed a significant 67% reduced risk of death in favor of masitinib [HR, 0.33 [95% CI (0.12–0.88)]; p = 0.027] (Table 6). Of note, this analysis effectively compares patients that have remained in their assigned treatment arms following study AB10015 data readout, i.e. those originally randomized to active treatment compared with those originally randomized to placebo, and therefore avoids possible confounding effects related to treatment switch-over (i.e. placebo patients switching to masitinib).
Figure 3.
Kaplan–Meier survival curves from masitinib NPP subgroup long-term OS analysis. (a) NPP-M4.5 versus masitinib-naïve PBO. (b) NPP-ALL versus masitinib-naïve PBO.
Likewise, the analysis of ‘NPP-M(pooled)’ (n = 59) versus ‘masitinib-naïve PBO’ (n = 53) also showed a significant difference in median OS (p = 0.008) with a between-group difference of 11 months and a 42% reduced risk of death [HR, 0.58 [95% CI (0.28–1.19)]; p = 0.134] (Tables 5 and 6). Considering, the subpopulation of ‘NPP-ALL’ (n = 84) versus ‘masitinib-naïve PBO’ (an analysis that estimates the NPPs overall impact on OS), a significant difference between survival curves (p = 0.016) and 29% reduced risk of death (p = 0.267) was seen in favor of masitinib, despite inevitable dilution from placebo patients that had switched [Figure 3(b); Tables 5 and 6].
Supportive analyses for masitinib patient enrichment
The enriched patient cohorts used for long-term OS analysis of study AB10015 were also tested in the context of that study’s main endpoints, i.e. ∆ALSFRS-R at week-48 and PFS (respectively, Tables S3 and S4). The between-group difference in both ∆ALSFRS-R and PFS improved with increasing degrees of enrichment. Considering the cohort most closely matched to the target population of study AB19001 (i.e. ‘M4.5 with ⩾2 on each baseline ALSFRS-R item and ΔFS < 1.1’), ∆ALSFRS-R for masitinib was −6.36 versus −11.03 for placebo, corresponding to a significant 42% slowing in rate of decline (p = 0.018), while PFS for this cohort showed a trend improvement of 13 months in favor of masitinib (p = 0.060).
One concern when performing subgroup analysis is whether an observed treatment effect (i.e. a significant difference in favor for masitinib relative to placebo) in a given subgroup, is simply reflected in an opposite sense for its complement subgroup (i.e. a significant difference in favor for placebo relative to masitinib). This was tested in a cohort with suitably large enriched subgroup and complement subgroup sample sizes (see Supplemental Appendix and Table S5). Results showed that there was no discernable (non significant) difference for the complement subgroup, therefore, supporting plausibility of the significant treatment effect observed in the enriched subgroup.
Discussion
Findings from this long-term OS analysis are consistent with the favorable results from study AB10015.6 Notably, a statistically significant survival benefit of over 2 years and 47% reduced risk of death for patients receiving masitinib (4.5 mg/kg/day) as compared with placebo was observed for the enriched cohort of ‘⩾2 on each baseline ALSFRS-R item and ΔFS < 1.1’ (Tables 5 and 6). This subgroup is comprised of ALS patients that have not suffered a complete loss or severe impairment of ALSFRS-related functionality at the time of masitinib treatment initiation and have not experienced a rapid (ΔFS ⩾ 1.1) ALSFRS-R progression rate from disease onset to baseline. Likewise, a substantial effect was observed in the endpoints of ∆ALSFRS-R at week 48 and PFS for this enriched cohort relative to the overall population of study AB10015 (Tables S3 and S4), supporting the premise of greater treatment effect when masitinib is initiated at an earlier stage of disease. Additionally, long-term OS results from the low-dose (3.0 mg/kg/day) masitinib treatment arm are consistent with an observed dose-dependency in the 48-week endpoint analysis, confirming that a target dose of at least 4.5 mg/kg/day is necessary to produce clinically meaningful improvement.6
It should be noted that the current OS analysis represents new data, which were unavailable at the time when masitinib was considered for marketing authorization by the European Medicine Agency Committee for Medicinal Products for Human Use; an application that was based on interim results from study AB10015.24 An advantage of the current analysis is that OS is considered a robust and reliable endpoint; highly accurate for the event and time, relatively simple to collect and assess, and of unquestionable clinical relevance. Nevertheless, the nature of long-term survival data does also present a number of challenges. For example, these data were not generated under conditions of a randomized clinical trial but rather as part of an open-label follow-up period that also incorporated an optional NPP. Furthermore, long-term observational studies following clinical trials are susceptible to selection bias from missing data because of patient discontinuation or loss to follow-up. This risk has been mitigated in the current analysis via confirmation of vital status in all patients from the overall study population (Table 1); information for 96% of patients being verified less than 7 months prior to cut-off, while 4% had a status older than 7 months. A threshold of 5% for missing data (which would be a worst-case scenario for these censored patients) is commonly reported to represent a low risk of bias25–27; moreover, these patients were distributed evenly across treatment arms (see Table 1) and represents a small proportion (at least 10-fold fewer) relative to the outcome event rate (i.e. number of deaths). There is also a risk of introducing baseline bias when comparing subgroups taken from a larger randomized population; however, assessment of baseline parameters showed that enriched patient subgroups maintained a good balance between treatment-arm baseline variables. Likewise, there was no indication that patient self-selection had introduced confounding bias to the NPP analysis (Table 4). For those parameters that did show some baseline imbalance between treatment arms, distribution of bulbar onset patients could have potentially favored survival in masitinib-naïve PBO cohorts, whereas a slower post-onset ΔFS could have potentially favored masitinib cohorts. Distribution of baseline El Escorial diagnostic classification is unlikely to have generated bias as this is not a predictive indicator of survival.23 Hence, despite the risk of bias associated with data generated outside of a prospectively declared randomized protocol, it would appear that long-term OS analysis of the AB10015 study population is robust.
A significant masitinib effect on OS and HR was also apparent for the NPP-M4.5 subpopulation as compared with patients that received no form of masitinib treatment (i.e. the masitinib-naïve placebo subpopulation) (Tables 5 and 6). This provides valuable insight into the potential long-term treatment benefits of masitinib in a broader ALS population (i.e. regardless of baseline ΔFS or individual ALSFRS-R component scores). Results from the NPP dataset also showed a significant treatment effect for the NPP-M(pooled) and NPP-ALL subpopulations, indicating that therapeutic benefit was possible for those patients switching to masitinib (4.5 mg/kg/day) from either the placebo or low-dose masitinib arms, despite there being a delay in receiving this treatment.
One limitation of this analysis is that no information on the post-study use of tracheostomy or invasive ventilation was collected, nor was information regarding which drugs were taken following withdrawal from study AB10015 or its associated NPP. Regarding the latter point, no other drug has been convincingly shown to improve OS, with the exception of riluzole, to which masitinib was administered as an add-on. Edaravone is the only other drug to have received marketing authorization for ALS in certain countries, but this drug was not available to the vast majority of the AB10015 population.16,28 Hence, the risk that these currently reported OS results have been confounded by subsequent successful treatment with other drugs is small. It is of interest that 25 placebo patients switched to masitinib as part of the NPP; however, any impact from this cross-over would manifest as a bias in favor of the study’s overall placebo arm and does not therefore weaken the AB10015 long-term OS results.
The magnitude of the observed OS signal for masitinib (4.5 mg/kg/day) as compared with placebo is encouraging and also provides evidence that the main efficacy outcomes of study AB10015, i.e. ∆ALSFRS-R at week 48 (according to the primary endpoint and sensitivity analyses based on multiple imputation jump-to-reference techniques) and PFS, are legitimate surrogate endpoints for long-term OS. It is equally encouraging in terms of having an optimized design for the current confirmatory phase III study (AB19001), the primary efficacy population of which will be ALS patients with mild or moderate impairment of functionality at baseline (i.e. a score of at least two on each ALSFRS-R individual component scores). Results were also in agreement with our previous observation of dose-dependent efficacy between target doses of 3.0 and 4.5 mg/kg/day, which in turn supports the higher target dose of the AB19001 study design (i.e. 6.0 mg/kg/day administered using a titrated dosing scheme for enhanced safety) on the premise that this could yield an optimized benefit–risk balance.
In conclusion, findings from this long-term OS analysis, with an average follow-up of 75 months since diagnosis, indicate that oral masitinib (4.5 mg/kg/day) could prolong survival by over 2 years and reduces risk of death by at least 44% as compared with placebo, provided that treatment starts early in disease (i.e. prior to severe impairment of functionality). Confirmatory study AB19001 is ongoing with a primary endpoint of decline in ALSFRS-R from baseline to week 48 in the enriched patient population described herein. Considering the consistency of significant treatment effect in terms of median OS, HR of death, ∆ALSFRS-R and PFS, a benefit to patients in the current NPP can be strongly presumed.
Acknowledgments
We thank the study participants, their families and caregivers. We also thank all investigators and collaborators from study AB10015.
Footnotes
Authors’ contributions: JSM, DC, MHB, JM, JG, GMGM and AM contributed to data collection and conception of the work. JSM, ACL, WGB, OH, AM and CDM did the initial data interpretation. CDM wrote the manuscript with contributions from JSM, ACL, WGB, AM and OH. The sponsor collected and analyzed the data in conjunction with the authors, who contributed to manuscript draft revisions, provided critical comment, and approved submission for publication.
Conflict of interest statement: Masitinib is under clinical development by the study funder, AB Science. AM, OH and CDM are employees and shareholders of AB Science. JSM has received research funding from AB Science. All remaining authors have no competing interests. AL, an Associate Editor of Therapeutic Advances in Neurological Disorders, is an author of this paper, therefore, the peer review process was managed by alternative members of the Board and the submitting Editor was not involved in the decision-making process.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: AB Science, Paris, France.
ORCID iDs: Josep Gamez  https://orcid.org/0000-0003-3127-7486
https://orcid.org/0000-0003-3127-7486
Colin D. Mansfield  https://orcid.org/0000-0001-9175-5051
https://orcid.org/0000-0001-9175-5051
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jesus S. Mora, ALS Unit, Hospital San Rafael, Madrid, Spain
Walter G. Bradley, Department of Neurology, University of Miami School of Medicine, Miami, FL, USA
Delia Chaverri, ALS Unit, Department of Neurology, University Hospital La Paz-Carlos III, Madrid, Spain.
María Hernández-Barral, ALS Unit, Department of Neurology, University Hospital La Paz-Carlos III, Madrid, Spain.
Javier Mascias, ALS Unit, Department of Neurology, University Hospital La Paz-Carlos III, Madrid, Spain.
Josep Gamez, Neurology Department, GMA Clinic, Autonomous University of Barcelona, European Reference Network on Rare Neuromuscular Diseases (ERN EURO-NMD), Barcelona, Spain.
Gisella M. Gargiulo-Monachelli, Hospital Universitario CEMIC-CONICET Buenos Aires, Argentina
Alain Moussy, AB Science, Paris, France.
Colin D. Mansfield, AB Science, Paris, France
Olivier Hermine, Department of Hematology, Necker Hospital, University of Paris, 149 Rue de Sèvres, Paris 75015, France; AB Science, Paris, France; Laboratory of Cellular and Molecular Mechanisms of Hematological Disorders and Therapeutic Implication, Imagine Institute, INSERM UMR 1163 and CNRS ERL 8254, Hôpital Necker, Paris, France.
Albert C. Ludolph, Department of Neurology, University of Ulm, Oberer Eselsberg 45, Ulm 89081, Germany; German Center for Neurodegenerative Diseases, Ulm, Germany.
References
- 1.Harrison JM, Rafuse VF.Muscle fiber-type specific terminal Schwann cell pathology leads to sprouting deficits following partial denervation in SOD1G93A mice. Neurobiol Dis 2020; 145: 105052. [DOI] [PubMed] [Google Scholar]
- 2.Trias E, Kovacs M, King PH, et al. Schwann cells orchestrate peripheral nerve inflammation through the expression of CSF1, IL-34, and SCF in amyotrophic lateral sclerosis. Glia 2020; 68: 1165–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trias E, King PH, Si Y, et al. Mast cells and neutrophils mediate peripheral motor pathway degeneration in ALS. JCI Insight 2018; 3: e123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trias E, Ibarburu S, Barreto-Núñez R, et al. Evidence for mast cells contributing to neuromuscular pathology in an inherited model of ALS. JCI Insight 2017; 2: e95934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trias E, Ibarburu S, Barreto-Núñez R, et al. Post-paralysis tyrosine kinase inhibition with masitinib abrogates neuroinflammation and slows disease progression in inherited amyotrophic lateral sclerosis. J Neuroinflammation 2016; 13: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mora JS, Genge A, Chio A, et al. Masitinib as an add-on therapy to riluzole in patients with amyotrophic lateral sclerosis: a randomised clinical trial. Amyotroph Lateral Scler Frontotemporal Degener 2020; 21: 5–14. [DOI] [PubMed] [Google Scholar]
- 7.Hermine O, Arnold V, Mansfield CD, et al. Sensitivity analyses from the first phase 3 clinical study of masitinib (AB10015) in ALS demonstrate robustness of the positive primary analysis. Paper presented at the ENCALS Annual Meeting, 20–22June2018, Oxford, England: Abstract C35, https://www.encals.eu/meetings/encals-meeting-2018-oxford-england/ (2018, accessed 7 July 2020). [Google Scholar]
- 8.Kimura F, Fujimura C, Ishida S, et al. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology 2006; 66: 265–267. [DOI] [PubMed] [Google Scholar]
- 9.Labra J, Menon P, Byth K, et al. Rate of disease progression: a prognostic biomarker in ALS. J Neurol Neurosurg Psychiatry 2016; 87: 628–632. [DOI] [PubMed] [Google Scholar]
- 10.Kollewe K, Mauss U, Krampfl K, et al. ALSFRS-R score and its ratio: a useful predictor for ALS-progression. J Neurol Sci 2008; 275: 69–73. [DOI] [PubMed] [Google Scholar]
- 11.Gordon PH, Cheung YK.Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology 2006; 67: 1314–1315. [DOI] [PubMed] [Google Scholar]
- 12.Van Den Berg LH, Sorensen E, Gronseth G, et al. Revised Airlie House consensus guidelines for design and implementation of ALS clinical trials. Neurology 2019; 92: e1610–e1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ludolph A, Drory V, Hardiman O, et al. A revision of the El Escorial criteria. Amyotroph Lateral Scler Frontotemporal Degener 2015; 16: 291–292. [DOI] [PubMed] [Google Scholar]
- 14.Mitsumoto H, Brooks BR, Silani V.Clinical trials in amyotrophic lateral sclerosis: why so many negative trials and how can trials be improved? Lancet Neurol 2014; 13: 1127–1138. [DOI] [PubMed] [Google Scholar]
- 15.Mora JS, Genge A, Mansfield CD, et al. Initiation of masitinib at a less severe stage of disease produces greater treatment effect: subgroup analyses from masitinib study AB10015. Presented at the ENCALS Annual Meeting, 20–22June2018, Oxford, England: Abstract C35, https://www.encals.eu/meetings/encals-meeting-2018-oxford-england/ (2018, accessed 7 July 2020). [Google Scholar]
- 16.Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomized double-blind, placebo-controlled trial. Lancet Neurol 2017; 16: 505–512. [DOI] [PubMed] [Google Scholar]
- 17.Ludolph AC, Schuster J, Dorst J, et al. Safety and efficacy of rasagiline as an add-on therapy to riluzole in patients with amyotrophic lateral sclerosis: a randomised, double-blind, parallel-group, placebo-controlled, phase 2 trial. Lancet Neurol 2018; 17: 681–688. [DOI] [PubMed] [Google Scholar]
- 18.Ludolph AC, Dorst J, Dreyhaupt J, et al. Effect of high-caloric nutrition on survival in amyotrophic lateral sclerosis. Ann Neurol 2020; 87: 206–216. [DOI] [PubMed] [Google Scholar]
- 19.Kwon HS, Koh SH.Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener 2020; 9: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gamez J.Vall d’Hebron participates in an international study to validate masitinib for amyotrophic lateral sclerosis treatment. VHIR.org News, 20March, http://en.vhir.org/portal1/news-detail.asp?t=vall-dhebron-participates-in-an-international-study-to-validate-masitinib-for-amyotrophic-lateral-sclerosis-treatment&contentid=214927&s=actualitat (2020, accessed 7 July 2020).
- 21.Whitfield K, Huemer KH, Winter D, et al. Compassionate use of interventions: results of a European Clinical Research Infrastructures Network (ECRIN) survey of ten European countries. Trials 2010; 11: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lortholary O, Chandesris MO, Livideanu CB, et al. Masitinib for treatment of severely symptomatic indolent systemic mastocytosis: a randomised, placebo-controlled, phase 3 study. Lancet 2017; 389: 612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zoccolella S, Beghi E, Palagano G, et al. Analysis of survival and prognostic factors in amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry 2008; 79: 33–37. [DOI] [PubMed] [Google Scholar]
- 24.AB Science. AB science announces that the CHMP has adopted a negative opinion for marketing authorization of masitinib in ALS, https://www.ab-science.com/ab-science-announces-that-the-chmp-has-adopted-a-negative-opinion-for-the-marketing-authorization-of-masitinib-in-amyotrophic-lateral-sclerosis/ (2018, accessed 10 May 2021).
- 25.Schulz KF, Grimes DA.Sample size slippages in randomised trials: exclusions and the lost and wayward. Lancet 2002; 359: 781–785. [DOI] [PubMed] [Google Scholar]
- 26.Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol 2011; 64: 407–415. [DOI] [PubMed] [Google Scholar]
- 27.Dettori JR.Loss to follow-up. Evid Based Spine Care J 2011; 2: 7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardiman O, Van Den Berg LH.Edaravone: a new treatment for ALS on the horizon? Lancet Neurol 2017; 16: 490–491. [DOI] [PubMed] [Google Scholar]