Abstract
Metabolic reprogramming is one of the most common characteristics of cancer cells. The metabolic alterations of glucose, amino acids and lipids can support the aggressive phenotype of cancer cells. Exosomes, a kind of extracellular vesicles, participate in the intercellular communication through transferring bioactive molecules. Increasing evidence has demonstrated that enzymes, metabolites and non-coding RNAs in exosomes are responsible for the metabolic alteration of cancer cells. In this review, we summarize the past and recent findings of exosomes in altering cancer metabolism and elaborate on the role of the specific enzymes, metabolites and non-coding RNAs transferred by exosomes. Moreover, we give evidence of the role of exosomes in cancer diagnosis and treatment. Finally, we discuss the existing problems in the study and application of exosomes in cancer diagnosis and treatment.
Keywords: exosomes, metabolic reprogramming, cancer cells, glucose, amino acids, lipids, biomarkers
Introduction
Metabolic reprogramming is one of the common features of cancer cells. Compared with normal cells, cancer cells need more glucose, amino acids and lipids to support their growth and survival in a nutrient-deprived environment.1 Instead of using oxidative phosphorylation (OXPHOS), cancer cells up-regulate the rate of glycolysis to produce more energy, which is called the Warburg effect.2 Besides glucose, cancer cells also need more amino acids and lipids to build biomass and provide more energy. Therefore, the major steps of metabolic reprogramming have the potential to become therapeutic targets for cancer treatment.
In recent years, the function of extracellular vesicles (EVs) in mediating long-distance intercellular communication is emerging.3 The bioactive molecules encapsulated by EVs are protected by the bilayer lipid structure from degradation during transportation.
Exosomes are a kind of bilayer membrane EVs with bioactive molecules released from various cells in the tumor microenvironment (Figure 1). The bioactive molecules in exosomes could influence the physiological and pathological functions of recipient cells. The internalization of exosomes to recipient cells includes fusion with the cell membrane, interaction with receptors on the cell membrane, and phagocytosis.4 It has been found that exosomes participate in the whole process of tumor metastasis, including epithelial-mesenchymal transition (EMT), organ-specific metastasis and the formation of pre-metastatic niches.5 Nevertheless, few studies have investigated the role of exosomes in regulating intercellular communication and altering cancer metabolism.6,7 The enrichment of glucose transporter-1 (GLUT-1) has been detected in exosomes from breast cancer cells.8 Exosomes released by cancer cells can be found in blood, urine, saliva and milk.9 A study used metabolomics to analyze EVs from body fluids, and the results suggested that the selected metabolites in EVs could be used as diagnostic biomarkers.10 In addition, microRNAs transported by exosomes can modulate the metabolic features of recipient cells in the tumor microenvironment, and can also be utilized as biomarkers for cancer diagnosis.11
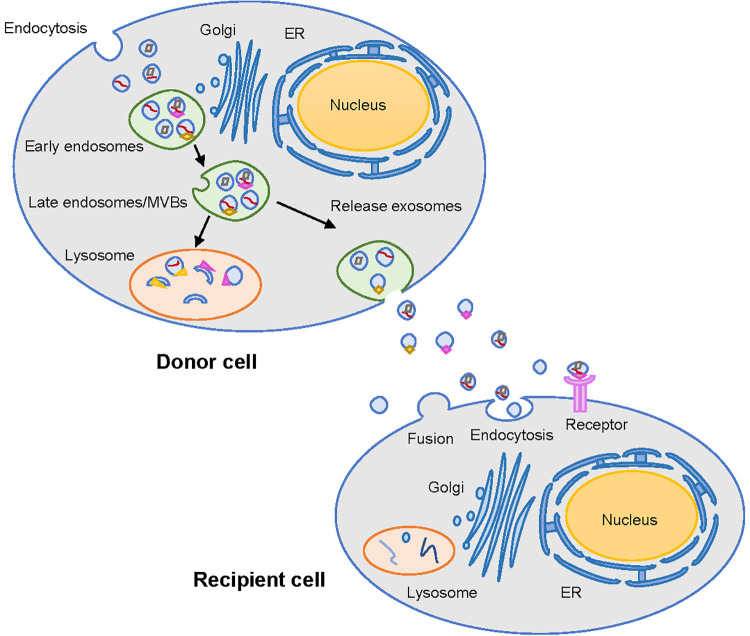
Figure 1.
Biogenesis and secretion of exosomes. Exosomes are originated from early endosomes and then form multivesicular bodies (MVBs). The fusion of MVBs and lysosomes results in the degradation of the contents of exosomes. Exosomes could be secreted to the extracellular space when MVBs fuse with the cell membrane. The exosomes from donor cells can transfer bioactive molecules through the main 3 ways: fusion, endocytosis and receptor-ligand mediated interaction.
In this review, we will first describe the characteristics of exosomes and then summarize the major alterations of cancer metabolism, as well as the regulatory functions of exosomes during this process. Furthermore, we will discuss the role of exosomes in cancer diagnosis and treatment.
The Composition, Characteristics and Functions of EVs
In 1983, Pan and Johnstone showed that peptides containing vesicles are released from sheep reticulocytes to the extracellular space. Since then, a term “exosome” was used to describe EVs.12-14 Generally, the classification of EVs contains 3 types of vesicles: exosomes (30-120 nm in diameter), microvesicles (0.1-1.0 µm in diameter) and apoptotic bodies (0.8-5.0 µm in diameter).15 Exosomes originate from the early endosomes and then form multivesicular bodies (MVBs) that are secreted into the extracellular space through fusing with the plasma membrane.16 Microvesicles are EVs that budding from the plasma membrane directly.17 During the process of apoptosis, dying cells are divided into several apoptotic bodies with fluctuated sizes, which are released through membrane budding.18,19 Many research works have focused on exosomes and microvesicles, but the formation of apoptotic bodies and their functions in cancer progression has been largely ignored.20
The identification of subtypes of EVs often uses the specific proteins enriched in EVs as markers. However, due to the lack of isolation standard, the features among exosomes, microvesicles and apoptotic bodies have not been fully illustrated. In previous studies, it has been found that the protein contents of exosomes are associated with the biogenesis of MVBs.21 Specifically, the proteomic analysis of exosomes from dendritic cells has proved the presence of Alix and TSG101.22 By using immunoelectron microscopy, researchers discovered the presence of tetraspanin family members in exosomes, such as CD37, CD53, CD63, CD81 and CD82.23 Among them, CD9, CD63, CD81, as the typical markers of exosomes, are used to identify the pellets containing exosomes. Some studies have used CD9 and CD63 to isolate exosomes.24,25 However, this sorting strategy is open for discussion, because CD9 and CD63 are also present in other types of EVs.26 Therefore, some studies use TSG101 and Alix, which are involved in the biogenesis of MVBs, as the markers for isolation.27,28 During the formation of microvesicles, the inhibition of small GTP-binding protein ARF6 can reduce the shedding of microvesicles from the plasma membrane.29 Thus, some studies have now used ARF6 and VAMP3 as markers for the detection of microvesicles.30 In general, programmed apoptosis is accompanied by the exposure of phosphatidylserine (PS) on the cell membrane. Annexin V has been used as a typical marker of apoptosis because it can interact with PS.31 Thus, the identification of apoptotic bodies relies on the presence of Annexin V on EVs.30 There is no criterion for the classification of EVs, so the discovery of more precise markers of different subtypes of EVs is urgent in the future.
As key players of intercellular communication, EVs transfer multiple biological molecules from donor cells to recipient cells, changing their physiological and pathological characteristics.32 The most common cargoes of EVs include proteins, lipids and nucleic acids.33,34 The bilayer structure of EVs can protect the contents from degradation during transportation.35 It is worth noting that EVs promote the aggressiveness of cancer cells,36,37 so that the metabolites in exosomes are getting more attention. Researchers have found that glycolytic enzymes are frequently detected in exosomes.38 Moreover, the application of metabolomic analysis of EVs is emerging, especially in the discovery of diagnostic biomarkers.39,40 The transcriptome analysis of non-coding RNAs from colorectal cancer revealed that microRNAs carried by EVs are significant in patient prognosis.41 The RNA sequencing analysis of exosomal circular RNAs has identified circ-PNN as a biomarker for colorectal cancer diagnosis.42 It has been found that the quantity and contents of EVs varied when cells respond to different stimuli.43 Thus, the regulatory functions of EVs in cancer progression and their application in cancer diagnosis are worthy to be fully studied.
The Metabolic Reprogramming of Cancer Cells
In the past few years, a lot of studies have focused on cancer metabolic alteration. The main changes were found in the metabolic processes of glucose, amino acids and lipids. Cancer cells change the metabolism of these substances and provide metabolic intermediates and energy to promote their malignant phenotype.
Glucose
In the 1920s, Warburg found that tumors need more glucose than normal tissues.44 In the following experiments, he discovered that the respiration of cancer cells was injured irreversibly, and cancer cells preferred to use energy from fermentation.2 However, Weinhouse performed isotope-tracing experiments and concluded that the respiratory function of cancer cells was not impaired in comparison to normal cells.45 This result indicated that cancer cells preferred to use glycolysis to produce energy even in the presence of oxygen. Thus, aerobic glycolysis has been considered as a trait to distinguish cancer cells from normal cells.46,47 Cancer cells can proliferate rapidly in the tumor microenvironment and use glucose as main energy resource. The metabolism of glucose in cancer cells includes glycolysis and the pentose phosphate pathway (PPP).48 Evidence showed that the increased glycolysis is associated with drug resistance in colon carcinoma.49 Correspondingly, there are accumulating studies targeting the key enzymes of glycolysis to inhibit ATP production in cancer treatment. Apart from this, the increased glycolysis also provides intermediates for PPP, such as ribose-5-phosphate (R5P) and NADPH, which result in the biosynthesis of nucleotides and redox balance in cancer cells.50 Thus, drugs targeting PPP are of great potential to be developed, and the emerged inhibitors of PPP, including 6-AN, DHEA and OT, have made progress.51 However, the systemic cytotoxicity and low specificity hampered the application of these inhibitors in cancer treatment.
Amino Acids
The amino acids can be used as alternative fuels and biosynthetic materials to support the growth of cancer cells or provide antioxidants to maintain reactive oxygen species (ROS). Glutamine is a multifunctional nutrient that participates in several metabolic processes, such as the synthesis of biological molecules, and the generation of energy and redox balance in cancer cells.52 A study on branched-chain amino acids (BCAAs) catabolism found that the carbon atoms were incorporated into the intermediates of the tricarboxylic acid (TCA).53 The biosynthesis of nucleotides is dependent on the amino acids that are involved in the synthesis of purine and pyrimidine.54 However, the abundance of amino acids and the flexibility of cancer cells using them make them difficult to be targeted. Another study has shown that L-asparaginases can block the supply of asparagine in lymphoblastic leukemia by depleting it in the serum.55 Several cancer cell lines are sensitive to the deprivation of glutamine, and this trait makes suppressing glutamine addiction a promising strategy for cancer treatment.56
Lipids
Lipids are significant molecules that support the growth of cancer cells and they also comprise several molecules, including phospholipids, sphingolipids, fatty acids and cholesterol. Cancer cells can increase the uptake of lipids from outer space to enhance the synthesis of fatty acids, which act as the source of energy and participate in the formation of cell membranes.57 Studies have investigated the effects of blocking fatty acids synthesis and increasing fatty acid degradation, that is, inhibiting the proliferation of cancer cells. For instance, the apoptosis of glioblastoma cells was increased by inhibiting sterol regulatory element binding proteins (SREBPs), the main regulators of fatty acid synthesis help to prevent ER stress in cells.58 Traditionally, numerous studies have demonstrated the function of glycolysis in generating ATP to support the growth of cancer cells. However, the energy-providing function of fatty acid oxidation (FAO) has been largely ignored. A study found that the inhibition of FAO in human leukemia cells can sensitize these cells to apoptosis, which may be a promising therapeutic strategy for the treatment of leukemia.59
Exosomes Are Involved in the Metabolic Alteration of Cancer Cells
Exosomes can perform similar behaviors to the cells from which they are derived. The contents transported via exosomes also reflect the characteristics of their parental cells. Thus, exosomes are involved in the metabolism alterations of cancer cells. The transportation of enzymes, metabolites and non-coding RNAs by exosomes has significant role in promoting cancer progression (Table 1). In this part, we will summarize the main findings related to exosomes-mediated tumor metabolic reprogramming, including glycolysis, PPP and metabolism of amino acids and lipids (Figure 2).
Table 1.
EV Cargoes and Functions in Cancer.
| Metabolism process | EV cargoes | Functions | Ref. |
|---|---|---|---|
| Glycolysis | miR-105 | Promote the growth of cancer cells in nutrient-deprived conditions | 60 |
| HISLA | Induce aerobic glycolysis and chemoresistance of breast cancer cells | 61 | |
| GLUT1 | Increase the glucose consumption of recipient cells | 62 | |
| HK2 | ATP generation | 63 | |
| PKM2 | Support the growth of prostate cancer cells | 64 | |
| PKM2 | Promote HCC tumor growth | 65 | |
| ciRS-122 | Promote oxaliplatin resistance of colorectal cancer cells | 66 | |
| miR-122 | Promote breast cancer metastasis | 67 | |
| miR-155, miR-210 | Promote the pre-metastatic niche formation of melanoma | 68 | |
| PPP | G6PDH, TKT, TALDO1 | Promote progression of osteosarcoma | 69 |
| Amino acids | lactate, glutamate | Support breast cancer development | 70 |
| ARG1 | Inhibit the proliferation of T cells through deleting L-arginine | 71 | |
| Lipids | miRNA-144, miRNA-126 | Promote the aggressiveness of breast cancer progression | 72 |
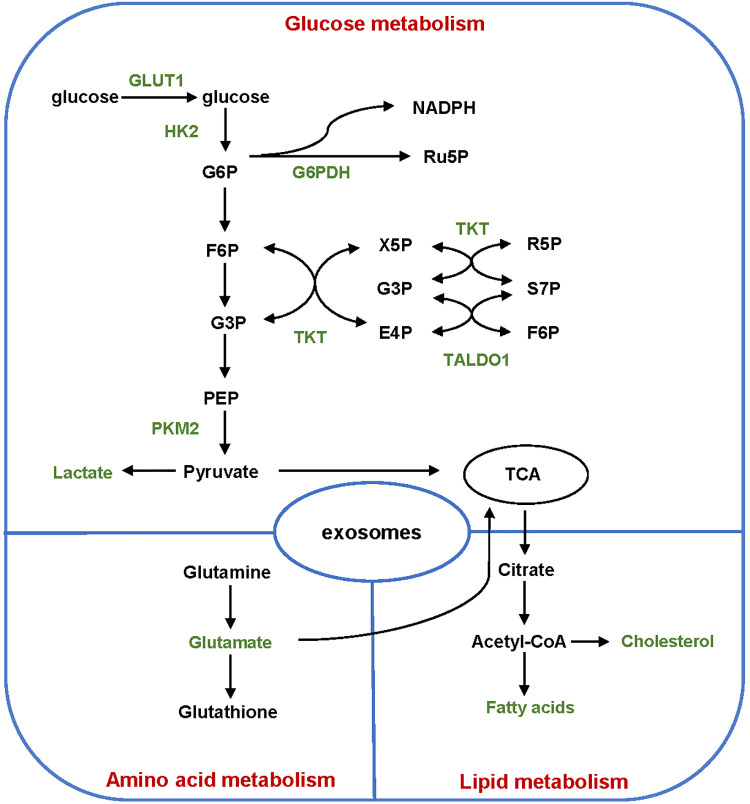
Figure 2.
Regulation of exosomes on the metabolism of cancer cells. Exosomes participate in the metabolism of glucose, amino acids and lipids during cancer progression. Major enzymes and metabolites transported by exosomes are shown in green words. GLUT1 indicates glucose transporter 1; HK2, hexokinase 2; PKM2, pyruvate kinase M2; G6PDH, glucose-6-phosphate dehydrogenase; TKT, transketolase; TALDO1, transaldolase 1; G6P, glucose-6-phosphate; F6P, fructose-6-phosphate; G3P, glyceraldehyde-3-phosphate; PEP, phosphoenolpyruvate; Ru5P, Ribulose-5-phosphate; X5P, Xylulose-5-phosphate; R5P, Ribose-5-phosphate; S7P, sedoheptulose-7-phosphate; E4P, erythrose-4-phosphate; TCA, tricarboxylic acid.
Glycolysis
Glucose is the main energy resource for cancer cell proliferation. The high rate of aerobic glycolysis is one of the hallmarks of cancer metabolism. Compared with normal cells, cancer cells live in an environment that lacks nutrients and oxygen. Exosomal miR-105 from breast cancer cells can alter the glucose metabolism of stromal cells and thus promote the growth of cancer cells under nutrient-deprived conditions.60 To increase the energy supply for the growth of cancer cells, the flux of glucose keeps changing during cancer development. The products of glycolysis, including pyruvate, NADH and ATP, provide energy to fuel metabolic reactions and transportation of substances. The metabolism of 1 mole of glucose consumes 2 moles of ATP and produces 4 moles of ATP, which is much less than OXPHOS that generates 36 moles of ATP from 1 mole of glucose.73 Cancer cells overcome this disadvantage by increasing the uptake of glucose, which result in the high rate of glycolysis and production of lactate. The lactate in the tumor microenvironment can up-regulate HIF-1α-stabilizing long non-coding RNA (HISLA) in tumor associated macrophages (TAMs). HISLA transported by EVs from TAMs can induce aerobic glycolysis and chemoresistance of breast cancer cells.61
The transport of glucose by glucose transporters (GLUTs) on the cell membrane is a vital step of glucose metabolism, which brings glucose into cells continuously. It has been found that GLUTs are up-regulated in several kinds of cancer cells. The overexpression of oncoprotein c-Myc up-regulates the transcription of GLUT1 and increased glycolysis.74 Caveolin-1 (CAV1) can increase the glucose consumption and ATP production of colorectal cancer cells through regulating the transcription of GLUT3, and this promotes the growth and survival of cells under a nutrient-deprived environment.75 The down-regulation of GLUT4 can decrease the glycolytic rate of breast cancer cells and convert the metabolic flux into mitochondrial oxidation, which also impairs cell viability and reduces cell proliferation.76 KRAS mutation is associated with aerobic glycolysis in colorectal cancer. Exosomes from KRAS mutant colorectal cancer cells can increase the glucose consumption of recipient cells via the transportation of GLUT1.62 When glucose enters the cell, it is converted to glucose-6-phosphate (G6P) by hexokinases (HK), which is the first-rate limiting step of glycolysis. The high efficiency of this step decreases the concentration of G6P in cells and thus keeps the import of glucose by GLUTs continuously. G6P is also the convergent point of glycolysis and PPP. In an early study, investigators detected the expression of HK isozymes in various cells and found that HK2 is highly expressed in rat hepatoma cell lines, while HK1 is only activated in normal cells when they need more energy.77 This finding showed that the expression of HK between tumor cells and normal cells is different. In malignant cells, the increased expression of HK2 up-regulates the first-rate limiting step of glycolysis, which produces more G6P than normal cells. The HK2 was activated in hepatoma cells under hypoxia conditions.78 The presence of HK2 in exosomes from prostate cancer was identified by proteomics and HK2 was highly expressed in the plasma of prostate cancer patients with poor prognosis.63 Pyruvate kinase (PK) catalyzes phosphoenolpyruvate (PEP) to pyruvate and produces ATP. PKM2 is one of the isoenzymes of PK and it is correlated with cell proliferation.79 GLUT1 and PKM2 were found in exosomes from activated hepatic stellate cells (HSCs), and the transportation of these molecules to liver nonparenchymal cells changed their metabolism and induced liver fibrosis, which was correlated with most hepatocellular carcinoma (HCC).80,81 The metastatic prostate cancer cells prefer to localize at bone due to the effect of exosomal transfer of PKM2 from prostate cancer cells to bone marrow stromal cells (BMSCs), which up-regulated the CXCL12 of BMSC and supported the growth of cancer cells.64 PKM2 encapsulated by microvesicles from HCC can induce the differentiation of macrophages. The secretion of CCL1 from macrophages supported HCC tumor growth, and PKM2 in microvesicles has the potential to be used as a diagnostic marker.65 Circular RNA-122 (ciRS-122) transported by exosomes from oxaliplatin-resistant colorectal cancer cells can promote drug resistance through upregulating the expression of PKM2 in sensitive cells.66 The exosomal miR-122 secreted by breast cancer cells is associated with cancer metastasis and can suppress the glucose consumption of fibroblasts by downregulating PKM2.67 In addition, the secretion of exosomes can be influenced by PKM2. A study found that the phosphorylated PKM2 can promote the secretion of exosomes through phosphorylating synaptosome-associated protein 23 (SNAP-23), indicating that aerobic glycolysis played an essential role in the secretion of exosomes.82 More importantly, PKM2 is not only correlated with remodeling of tumor microenvironment but also participates in the release of exosomes, which further promotes the alteration of metabolism in tumor stromal cells.82
Pentose Phosphate Pathway
PPP is another branch of glycolysis that starts from G6P, the first metabolite of glucose. PPP is made up of 2 branches: the oxidative branch and the non-oxidative branch. The conversion of these 2 branches is mediated by the different metabolic situations of cells.83 The main enzymes of PPP include glucose-6-phosphate dehydrogenase (G6PDH), the gatekeeper of oxidative PPP, and 2 enzymes in non-oxidative PPP, transketolase (TKT) and transaldolase 1 (TALDO1). The identification of proteins in exosomes from ovarian cancer cell lines confirmed the presence of G6PDH, TKT and TALDO1.84 A study has compared the expression of G6PDH in the serum of osteosarcoma patients and healthy donors, and the proteomic results showed that exosomes from osteosarcoma patients increased the proliferation, adhesion and migration of osteosarcoma cells.69 When glucose enters the cell and is catalyzed by HK, it might be converted to ATP, pyruvate and lactate through glycolysis.73 G6P could also be metabolized by G6PDH through oxidative PPP and 2 other important metabolites, NADPH and ribulose-5-phosphate (Ru-5-P), are produced. These products participate in maintaining cellular redox homeostasis, fatty acid synthesis, RNA or DNA synthesis in cells. The non-oxidative branch of PPP could be activated by stressed conditions. The metabolites in non-oxidative PPP, such as fructose-6-phosphate (F-6-P) and glyceraldehyde-3-phosphate (G-3-P), can also enter the glycolytic pathway and produce ATP.
Amino Acids
Amino acids are also an indispensable source of metabolic intermediates for cancer cells. The dysregulated uptake of amino acids is one of the hallmarks of cancer metabolism. To survive in a nutrient-deprived microenvironment, cancer cells require more amino acids to support their rapid proliferation, such as arginine, serine and glycine.85-87 Glutamine can provide nitrogen and carbon to cancer cells. Moreover, it is also an important source of α-ketoglutarate, which can support the production of ATP through TCA.52 BCAAs, such as leucine, isoleucine and valine, can be converted into α-ketoglutarate and produce energy through TCA.53 Moreover, a lot of cancer cells rely on the exogenous supply of amino acids. SLC6A14, a typical transporter of several amino acids, is up-regulated in cervical cancer and colorectal cancer, which can promote the transportation of arginine.88,89 LAT1 and ASCT1 are associated with the transportation of glutamine and they can work coordinately in many tumors to support their growth and survival.90 Transaminases are a kind of enzyme that can promote the interconversion of different amino acids. Phosphoserine aminotransferase 1 (PSAT1) is associated with serine synthesis. It has been found that the high level of PSAT1 is associated with tamoxifen resistance and poor prognosis in breast cancer patients.91 In pancreatic cancer, researchers found that the overexpression of aspartate transaminase can promote the growth of pancreatic ductal adenocarcinoma cells (PDAC) and maintain the intercellular redox balance by providing NADPH.92
In the tumor microenvironment, the supply of amino acids relies not only on the transporters or enzymes but the transportation of amino acids from donor cells to recipient cells via EVs. Mesenchymal Stem Cells (MSCs) are able to promote the growth or angiogenesis of tumor.93 Researchers have used metabolomics to analyze the metabolites of EVs from human Mesenchymal Stem Cells (hMSCs) and detected the presence of lactate and glutamate, which may play an important role in breast cancer development.70 One study used intra-exosomal metabolomics to analyze the metabolites in exosomes derived from cancer-associated fibroblasts (CAFs), and the results indicated that they can supply amino acids to promote the progression of cancer cells in a nutrient-deprived condition.94 The poor efficacy of immunotherapy in ovarian carcinoma patients is caused by exosomal arginase 1 (ARG1), which can inhibit the proliferation of T cells through deleting L-arginine.71
Lipids
Besides glucose and amino acids, the metabolism of lipids is also changing in many cancers. Lipids are important components of various molecules, including phosphoglycerides, sterols, sphingolipids and fatty acids.95 Fatty acids are not only essential constituents of membrane lipids but substrates for energy storage.96,97 The metabolic pathway of lipids includes synthesis, transportation and degradation. Cancer cells rely on the uptake of lipids from the cultural medium. These cancer cells can grow in a lipid-limited condition through increasing lipogenic activity.98
Lipid metabolic reprograming has played a critical role in the development of human cancer. Lipogenesis is up-regulated in several cancers to satisfy their demand for cell membrane synthesis.99 Researchers have analyzed the lipid profiles of breast cancer tissue and found that the increased fatty acid synthesis is associated with patients’ survival.100 Recently, the functions of EVs in cancer progression are getting more attention. It has been found that exosomes can transfer cholesterol and fatty acids between different cells.101 Exosomes from lung cancer cells can be internalized by human adipose tissue-derived mesenchymal stem cells (hAD-MSCs) and have a negative effect on hAD-MSC adipogenic differentiation.102 Similarly, exosomes from pancreatic cancer (PC) can induce lipolysis in subcutaneous adipocytes, and it is associated with the weight loss of patients.103 The transportation of miRNA-144 and miRNA-126 by exosomes between breast cancer cells and adipocytes could promote breast cancer aggressiveness.72
The Potential Role of EVs in Cancer Diagnosis and Treatment
EVs released by cancer cells contain proteins, RNAs, lipids and metabolites that reflect the characteristics of their donor cells and can alter the metabolism of recipient cells. The contents of EVs are protected by the lipid bilayer membrane from degradation during transportation. Exosomes can be identified in various types of body fluids, such as blood, urine, saliva and milk. Studies have demonstrated the role of exosomes in cancer diagnosis. One study analyzed the circulating exosomes from patients’ blood, and the results stressed the value of exosomal microRNAs as diagnostic markers.104
The molecules in exosomes could be used as drug targets for cancer treatment. For instance, exosomal miR-155 and miR-210 can promote glycolysis of human adult dermal fibroblasts (HADF) and are involved in pre-metastatic niche formation. However, this effect is reversed by miRNA inhibitors transfected into exosomes from melanoma cells.68 Exosomes from activated T cells take Fas ligand (FasL) on their surface, and the interaction between cancer cells and FasL positive exosomes can induce lung metastasis of melanoma cells.105 Due to the metabolic reprogramming function of exosomes, several studies have focused on inhibiting the secretion of exosomes as a therapeutic strategy to treat cancer. For instance, GW4869 is a widely used inhibitor that can block the release of exosomes from cells, and it might be a promising drug to inhibit the communication between different cells.106
EVs are considered as a native way to deliver bioactive molecules with low cytotoxicity, and researchers are trying to use exosomes as nanoparticle delivery systems in cancer treatment. It has been found that EVs can transport proteins and nucleic acids through the plasma membrane barriers with low cytotoxicity. In one study, the researchers compared the biological effect of the liposomal delivery systems and EVs, and they found that EVs are more effective.107 Various molecules, such as miRNAs, siRNAs and therapeutic molecules, can be incorporated into exosomes and they can pass through the blood-brain barrier to treat brain tumors more efficiently.108 Moreover, exosomes can be used as the delivery system, which can increase the concentration of drugs in specific cells or organs.109 Red blood cell extracellular vesicles (RBC-EVs) have shown liver accumulation through a macrophage-dependent manner, which can be used to deliver drugs in liver cancer treatment.110 Exosomes from marrow stromal cells have been used to deliver miR-146b to inhibit the growth of malignant glioma in rat models.111 Researchers have used exosomes as vehicles to transfer interfering RNA to target KrasG12D , and this is more effective than the liposome delivery system in suppressing pancreatic cancer in mouse models.112
According to the molecules encapsulated by exosomes, the treatment strategy should focus on specific molecules transported by exosomes from cancer cells or other cells in the tumor microenvironment, such as miRNAs, proteins or enzymes associated with the metabolic alteration. If tumor-promoting effect is induced by metabolites in exosomes, we ought to explore the process of cargo selection in the formation of exosomes and the secretion pathway of exosomes in the future study.
Conclusions
In summary, EVs play significant roles in cancer progression through transporting bioactive molecules among different cells. As mentioned in this review, EVs can regulate the metabolism of cancer cells, and we mainly focused on the metabolism of glucose, amino acids and lipids. The past studies have focused on RNAs and proteins transported by EVs, and the reported findings partially illustrate the effects of EVs on cancer metabolic reprogramming. With the exploitation of targeting cancer metabolism as a treatment strategy, the regulatory role of EVs is emerging. The function of EVs in glucose metabolism has been studied but there are few researches explore the contents of EVs in altering amino acids and lipids metabolism. The way that EVs interact with recipient cells and change their metabolism is still unclear. EVs are also recognized as biomarkers for cancer diagnosis and prognosis in clinical studies. Thus, developing EVs as therapeutic targets and drug delivery system is a promising strategy in the future.
Although the research of EVs in cancer have made advances in the past few years, questions about their roles in cancer metabolic reprogramming are still unsolved. First of all, the differences between metabolic alteration induced by genes or EVs from other cells remain unclear. Second, the most effective content in EVs in regulating metabolic reprogramming has not been determined. And the question how cells in the tumor microenvironment select intercellular molecules and encapsulate them specifically, such as enzymes or metabolites that can promote cancer progression. Thirdly, it is not yet known whether the contents of EVs are the unwanted components that cells choose to export under stimulation or selected to promote the malignant phenotype of other cells in tumor progression. Lastly, EVs have the potential to be used in clinical diagnosis and prognosis. Nevertheless, there are still technological obstacles for the isolation and purification of EVs from multiple body fluids. Thus, further research should focus on developing standard methods for the isolation of EVs, exploring the possibility of targeting the bioactive molecules in EVs and using EVs as vesicles to carry therapeutic drugs for cancer treatment.
Acknowledgments
We would like to acknowledge Ka-Wo Chan and Yiran Duan for the linguistic assistance of our manuscript.
Abbreviations: OXPHOS, oxidative phosphorylation; EVs, extracellular vesicles; EMT, epithelial-mesenchymal transition; MVBs, multivesicular bodies; PS, phosphatidylserine; PPP, pentose phosphate pathway; R5P, ribose-5-phosphate; ROS, reactive oxygen species; SREBPs, sterol regulatory element binding proteins; FAO, fatty acid oxidation; GLUTs, glucose transporters; CAV1, Caveolin-1; G6P, glucose-6-phosphate; HK, hexokinases; PK, Pyruvate kinase; PEP, phosphoenolpyruvate; HSCs, hepatic stellate cells; HCC, hepatocellular carcinoma; BMSCs, bone marrow stromal cells; SNAP-23, synaptosome-associated protein 23; G6PDH, glucose-6-phosphate dehydrogenase; TAMs, tumor associated macrophages; TKT, transketolase; TALDO1, transaldolase 1; Ru-5-P, ribulose-5-phosphate; F-6-P, fructose-6-phosphate; G-3-P, glyceraldehyde-3-phosphate; BCAAs, branched-chain amino acids; PSAT1, phosphoserine aminotransferase 1; PDAC, pancreatic ductal adenocarcinoma; MSCs, mesenchymal Stem Cells; HMSCs, human Mesenchymal Stem Cells; CAFs, cancer-associated fibroblasts; ARG1, arginase 1; hAD-MSCs, human adipose tissue-derived mesenchymal stem cells; PC, pancreatic cancer; HADF, human adult dermal fibroblasts; FasL, Fas ligand.
Authors’ Note: HL, XY and QZ conceived the work. ZQ wrote the manuscript and made the figures. XY and HL revised the manuscript. All authors read the paper and approved the final version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Scientific Foundation of China (81672413). Project to Attract High Level Foreign Experts (G20200019016).
ORCID iD: Huanliang Liu  https://orcid.org/0000-0002-1006-6666
https://orcid.org/0000-0002-1006-6666
References
- 1.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23(1):27-47. doi:10.1016/j.cmet.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309-314. doi:10.1126/science.123.3191.309 [DOI] [PubMed] [Google Scholar]
- 3.Desrochers LM, Antonyak MA, Cerione RA. Extracellular vesicles: satellites of information transfer in cancer and stem cell biology. Dev Cell. 2016;37(4):301-309. doi:10.1016/j.devcel.2016.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73(10):1907-1920. doi:10.1016/j.jprot.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 5.Li K, Chen Y, Li A, Tan C, Liu X. Exosomes play roles in sequential processes of tumor metastasis. Int J Cancer. 2019;144(7):1486-1495. doi:10.1002/ijc.31774 [DOI] [PubMed] [Google Scholar]
- 6.Wendler F, Favicchio R, Simon T, Alifrangis C, Stebbing J, Giamas G. Extracellular vesicles swarm the cancer microenvironment: from tumor-stroma communication to drug intervention. Oncogene. 2017;36(7):877-884. doi:10.1038/onc.2016.253 [DOI] [PubMed] [Google Scholar]
- 7.Yang E, Wang X, Gong Z, Yu M, Wu H, Zhang D. Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal Transduct Target Ther. 2020;5(1):242. doi:10.1038/s41392-020-00359-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Risha Y, Minic Z, Ghobadloo SM, Berezovski MV. The proteomic analysis of breast cell line exosomes reveals disease patterns and potential biomarkers. Sci Rep. 2020;10(1):13572. doi:10.1038/s41598-020-70393-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu S, Cao H, Shen B, Feng J. Tumor-derived exosomes in cancer progression and treatment failure. Oncotarget. 2015;6(35):37151-37168. doi:10.18632/oncotarget.6022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puhka M, Takatalo M, Nordberg ME, et al. Metabolomic profiling of extracellular vesicles and alternative normalization methods reveal enriched metabolites and strategies to study prostate cancer-related changes. Theranostics. 2017;7(16):3824-3841. doi:10.7150/thno.19890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomasetti M, Lee W, Santarelli L, Neuzil J. Exosome-derived microRNAs in cancer metabolism: possible implications in cancer diagnostics and therapy. Exp Mol Med. 2017;49(1):e285. doi:10.1038/emm.2016.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967-978. doi:10.1016/0092-8674(83)90040-5 [DOI] [PubMed] [Google Scholar]
- 13.Harding CV, Heuser JE, Stahl PD. Exosomes: looking back three decades and into the future. J Cell Biol. 2013;200(4):367-371. doi:10.1083/jcb.201212113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C.Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412-9420. [PubMed] [Google Scholar]
- 15.Han L, Lam EW, Sun Y. Extracellular vesicles in the tumor microenvironment: old stories, but new tales. Mol Cancer. 2019;18(1):59. doi:10.1186/s12943-019-0980-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255-289. doi:10.1146/annurev-cellbio-101512-122326 [DOI] [PubMed] [Google Scholar]
- 17.Muralidharan-Chari V, Clancy JW, Sedgwick A, D’Souza-Schorey C. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci. 2010;123(Pt 10):1603-1611. doi:10.1242/jcs.064386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauser P, Wang S, Didenko VV. Apoptotic bodies: selective detection in extracellular vesicles. Methods Mol Biol. 2017;1554:193-200. doi:10.1007/978-1-4939-6759-9_12 [DOI] [PubMed] [Google Scholar]
- 19.Battistelli M, Falcieri E. Apoptotic bodies: particular extracellular vesicles involved in intercellular communication. Biology (Basel). 2020;9(1):21. doi:10.3390/biology9010021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caruso S, Poon IKH. Apoptotic cell-derived extracellular vesicles: more than just debris. Front Immunol. 2018;9:1486. doi:10.3389/fimmu.2018.01486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol. 2002;3(12):893-905. doi:10.1038/nrm973 [DOI] [PubMed] [Google Scholar]
- 22.Thery C, Boussac M, Veron P, et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166(12):7309-7318. doi:10.4049/jimmunol.166.12.7309 [DOI] [PubMed] [Google Scholar]
- 23.Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273(32):20121-20127. doi:10.1074/jbc.273.32.20121 [DOI] [PubMed] [Google Scholar]
- 24.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285(23):17442-17452. doi:10.1074/jbc.M110.107821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jansen FH, Krijgsveld J, van Rijswijk A, et al. Exosomal secretion of cytoplasmic prostate cancer xenograft-derived proteins. Molecu Cellu Proteom. 2009;8(6):1192-1205. doi:10.1074/mcp.M800443-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113(8):E968-E977. doi:10.1073/pnas.1521230113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lasser C, Eldh M, Lotvall J. Isolation and characterization of RNA-containing exosomes. J Vis Exp. 2012(59):e3037. doi:10.3791/3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez-Llama P, Khositseth S, Gonzales PA, Star RA, Pisitkun T, Knepper MA. Tamm-Horsfall protein and urinary exosome isolation. Kidney Int. 2010;77(8):736-742. doi:10.1038/ki.2009.550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muralidharan-Chari V, Clancy J, Plou C, et al. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol. 2009;19(22):1875-1885. doi:10.1016/j.cub.2009.09.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of Extracellular Vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113(1):1-11. doi:10.1007/s11060-013-1084-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31(1):1-9. doi:10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r [DOI] [PubMed] [Google Scholar]
- 32.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654-659. doi:10.1038/ncb1596 [DOI] [PubMed] [Google Scholar]
- 33.van Balkom BWM, Eisele AS, Pegtel DM, Bervoets S, Verhaar MC. Quantitative and qualitative analysis of small RNAs in human endothelial cells and exosomes provides insights into localized RNA processing, degradation and sorting. J Extracell Vesicles. 2015;4:26760. doi:UNSP 26760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tkach M, Thery C.Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164(6):1226-1232. doi:10.1016/j.cell.2016.01.043 [DOI] [PubMed] [Google Scholar]
- 35.Koga Y, Yasunaga M, Moriya Y, et al. Exosome can prevent RNase from degrading microRNA in feces. J Gastrointest Oncol. 2011;2(4):215-222. doi:10.3978/j.issn.2078-6891.2011.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das A, Mohan V, Krishnaswamy VR, Solomonov I, Sagi I. Exosomes as a storehouse of tissue remodeling proteases and mediators of cancer progression. Cancer Metast Rev. 2019;38(3):455-468. doi:10.1007/s10555-019-09813-5 [DOI] [PubMed] [Google Scholar]
- 37.Xiang X, Poliakov A, Liu C, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124(11):2621-2633. doi:10.1002/ijc.24249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goran Ronquist K. Extracellular vesicles and energy metabolism. Clin Chim Acta. 2019;488:116-121. doi:10.1016/j.cca.2018.10.044 [DOI] [PubMed] [Google Scholar]
- 39.Williams C, Palviainen M, Reichardt NC, Siljander PR, Falcon-Perez JM. Metabolomics applied to the study of extracellular vesicles. Metabolites. 2019;9(11):276. doi:10.3390/metabo9110276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davison J, O’Gorman A, Brennan L, Cotter DR. A systematic review of metabolite biomarkers of schizophrenia. Schizophr Res. 2018;195:32-50. doi:10.1016/j.schres.2017.09.021 [DOI] [PubMed] [Google Scholar]
- 41.Francavilla A, Turoczi S, Tarallo S, Vodicka P, Pardini B, Naccarati A. Exosomal microRNAs and other non-coding RNAs as colorectal cancer biomarkers: a review. Mutagenesis. 2020;35(3):243-260. doi:10.1093/mutage/gez038 [DOI] [PubMed] [Google Scholar]
- 42.Xie Y, Li J, Li P, et al. RNA-Seq profiling of serum exosomal circular RNAs reveals Circ-PNN as a potential biomarker for human colorectal cancer. Front Oncol. 2020;10:982. doi:10.3389/fonc.2020.00982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aatonen MT, Ohman T, Nyman TA, Laitinen S, Gronholm M, Siljander PR. Isolation and characterization of platelet-derived extracellular vesicles. J Extracell Vesicles. 2014;3. doi:10.3402/jev.v3.24692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8(6):519-530. doi:10.1085/jgp.8.6.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinhouse S. Studies on the fate of isotopically labeled metabolites in the oxidative metabolism of tumors. Cancer Res. 1951;11(8):585-591. [PubMed] [Google Scholar]
- 46.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269-270. [PubMed] [Google Scholar]
- 47.Weinhouse S. On respiratory impairment in cancer cells. Science. 1956;124(3215):267-269. doi:10.1126/science.124.3215.267 [DOI] [PubMed] [Google Scholar]
- 48.Hay N. Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat Rev Cancer. 2016;16(10):635-649. doi:10.1038/nrc.2016.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fanciulli M, Bruno T, Giovannelli A, et al. Energy metabolism of human LoVo colon carcinoma cells: correlation to drug resistance and influence of lonidamine. Clin Cancer Res. 2000;6(4):1590-1597. [PubMed] [Google Scholar]
- 50.Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18(1):54-61. doi:10.1016/j.gde.2008.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riganti C, Gazzano E, Polimeni M, Aldieri E, Ghigo D. The pentose phosphate pathway: an antioxidant defense and a crossroad in tumor cell fate. Free Radic Biol Med. 2012;53(3):421-436. doi:10.1016/j.freeradbiomed.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 52.Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest. 2013;123(9):3678-3684. doi:10.1172/JCI69600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Green CR, Wallace M, Divakaruni AS, et al. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat Chem Biol. 2016;12(1):15-21. doi:10.1038/nchembio.1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moffatt BA, Ashihara H. Purine and pyrimidine nucleotide synthesis and metabolism. Arabidopsis Book. 2002;1:e0018. doi:10.1199/tab.0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Covini D, Tardito S, Bussolati O, et al. Expanding targets for a metabolic therapy of cancer: L-asparaginase. Recent Pat Anticancer Drug Discov. 2012;7(1):4-13. doi:10.2174/157489212798358001 [DOI] [PubMed] [Google Scholar]
- 56.Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010;35(8):427-433. doi:10.1016/j.tibs.2010.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Currie E, Schulze A, Zechner R, Walther TC, Farese RV, Jr. Cellular fatty acid metabolism and cancer. Cell Metab. 2013;18(2):153-161. doi:10.1016/j.cmet.2013.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Griffiths B, Lewis CA, Bensaad K, et al. Sterol regulatory element binding protein-dependent regulation of lipid synthesis supports cell survival and tumor growth. Cancer Metab. 2013;1(1):3. doi:10.1186/2049-3002-1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Samudio I, Harmancey R, Fiegl M, et al. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J Clin Invest. 2010;120(1):142-156. doi:10.1172/JCI38942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yan W, Wu XW, Zhou WY, et al. Cancer-cell-secreted exosomal miR-105 promotes tumour growth through the MYC-dependent metabolic reprogramming of stromal cells. Nat Cell Biol. 2018;20(5):597-609. doi:10.1038/s41556-018-0083-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen F, Chen J, Yang L, et al. Extracellular vesicle-packaged HIF-1alpha-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat Cell Biol. 2019;21(4):498-510. doi:10.1038/s41556-019-0299-0 [DOI] [PubMed] [Google Scholar]
- 62.Preet R, Dixon DA. Mutant KRAS exosomes influence the metabolic state of the colon microenvironment. Cell Mol Gastroenter. 2018;5(4):647-648. doi:10.1016/j.jcmgh.2018.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ronquist KG, Sanchez C, Dubois L, et al. Energy-requiring uptake of prostasomes and PC3 cell-derived exosomes into non-malignant and malignant cells. J Extracell Vesicles. 2016;5:29877. doi:UNSP 2987710.3402/jev.v5.29877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dai JL, Escara-Wilke J, Keller JM, et al. Primary prostate cancer educates bone stroma through exosomal pyruvate kinase M2 to promote bone metastasis. J Exp Med. 2019;216(12):2883-2899. doi:10.1084/jem.20190158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hou PP, Luo LJ, Chen HZ, et al. Ectosomal PKM2 promotes HCC by inducing macrophage differentiation and remodeling the tumor microenvironment. Mol Cell. 2020;78(6):1192-1206. doi:10.1016/j.molcel.2020.05.004 [DOI] [PubMed] [Google Scholar]
- 66.Wang X, Zhang H, Yang H, et al. Exosome-delivered circRNA promotes glycolysis to induce chemoresistance through the miR-122-PKM2 axis in colorectal cancer. Mol Oncol. 2020;14(3):539-555. doi:10.1002/1878-0261.12629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fong MY, Zhou W, Liu L, et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol. 2015;17(2):183-194. doi:10.1038/ncb3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.La Shu S, Yang YC, Allen CL, et al. Metabolic reprogramming of stromal fibroblasts by melanoma exosome microRNA favours a pre-metastatic microenvironment. Sci Rep. 2018;8(1):12905. doi:ARTN 1290510.1038/s41598-018-31323-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shen RK, Zhu X, Yi H, et al. Proteomic identification of osteosarcoma-derived exosomes and their activation of pentose phosphate pathway. Int J Clin Exp Patho. 2016;9(3):4140-4148. [Google Scholar]
- 70.Vallabhaneni KC, Penfornis P, Dhule S, et al. Extracellular vesicles from bone marrow mesenchymal stem/stromal cells transport tumor regulatory microRNA, proteins, and metabolites. Oncotarget. 2015;6(7):4953-4967. doi:10.18632/oncotarget.3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sosnowska A, Czystowska-Kuzmicz M, Golab J. Extracellular vesicles released by ovarian carcinoma contain arginase 1 that mitigates antitumor immune response. Oncoimmunology. 2019;8(11):e1655370. doi:10.1080/2162402x.2019.1655370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu Q, Li J, Li Z, et al. Exosomes from the tumour-adipocyte interplay stimulate beige/brown differentiation and reprogram metabolism in stromal adipocytes to promote tumour progression. J Exp Clin Cancer Res. 2019;38(1):223. doi:10.1186/s13046-019-1210-3 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 73.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029-1033. doi:10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Osthus RC, Shim H, Kim S, et al. Accelerated publication—deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J Biol Chem. 2000;275(29):21797-21800. doi:10.1074/jbc.C000023200 [DOI] [PubMed] [Google Scholar]
- 75.Ha TK, Chi SG. CAV1/caveolin 1 enhances aerobic glycolysis in colon cancer cells via activation of SLC2A3/GLUT3 transcription. Autophagy. 2012;8(11):1684-1685. doi:10.4161/auto.21487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garrido P, Osorio FG, Moran J, et al. Loss of GLUT4 induces metabolic reprogramming and impairs viability of breast cancer cells. J Cell Physiol. 2015;230(1):191-198. doi:10.1002/jcp.24698 [DOI] [PubMed] [Google Scholar]
- 77.Shinohara Y, Ichihara J, Terada H. Remarkably enhanced expression of the type II hexokinase in rat hepatoma cell line AH130. FEBS Lett. 1991;291(1):55-57. doi:10.1016/0014-5793(91)81102-e [DOI] [PubMed] [Google Scholar]
- 78.Mathupala SP, Rempel A, Pedersen PL. Glucose catabolism in cancer cells: identification and characterization of a marked activation response of the type II hexokinase gene to hypoxic conditions. J Biol Chem. 2001;276(46):43407-43412. doi:10.1074/jbc.M108181200 [DOI] [PubMed] [Google Scholar]
- 79.Li YH, Li XF, Liu JT, et al. PKM2, a potential target for regulating cancer. Gene. 2018;668:48-53. doi:10.1016/j.gene.2018.05.038 [DOI] [PubMed] [Google Scholar]
- 80.Wan L, Xia T, Du Y, et al. Exosomes from activated hepatic stellate cells contain GLUT1 and PKM2: a role for exosomes in metabolic switch of liver nonparenchymal cells. FASEB J. 2019;33(7):8530-8542. doi:10.1096/fj.201802675R [DOI] [PubMed] [Google Scholar]
- 81.Matsuda M, Seki E. Hepatic stellate cell-macrophage crosstalk in liver fibrosis and carcinogenesis. Semin Liver Dis. 2020;40(3):307-320. doi:10.1055/s-0040-1708876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wei Y, Wang D, Jin F, et al. Pyruvate kinase type M2 promotes tumour cell exosome release via phosphorylating synaptosome-associated protein 23. Nat Commun. 2017;8:14041. doi:10.1038/ncomms14041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cho ES, Cha YH, Kim HS, Kim NH, Yook JI. The pentose phosphate pathway as a potential target for cancer therapy. Biomol Ther. 2018;26(1):29-38. doi:10.4062/biomolther.2017.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yi H, Zheng X, Song J, Shen R, Su Y, Lin D. Exosomes mediated pentose phosphate pathway in ovarian cancer metastasis: a proteomics analysis. Int J Clin Exp Pathol. 2015;8(12):15719-15728. [PMC free article] [PubMed] [Google Scholar]
- 85.Maddocks OD, Berkers CR, Mason SM, et al. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature. 2013;493(7433):542-546. doi:10.1038/nature11743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jain M, Nilsson R, Sharma S, et al. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. 2012;336(6084):1040-1044. doi:10.1126/science.1218595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Feun LG, Kuo MT, Savaraj N. Arginine deprivation in cancer therapy. Curr Opin Clin Nutr Metab Care. 2015;18(1):78-82. doi:10.1097/MCO.0000000000000122 [DOI] [PubMed] [Google Scholar]
- 88.Gupta N, Miyauchi S, Martindale RG, et al. Upregulation of the amino acid transporter ATB0,+ (SLC6A14) in colorectal cancer and metastasis in humans. Biochim Biophys Acta. 2005;1741(1-2):215-223. doi:10.1016/j.bbadis.2005.04.002 [DOI] [PubMed] [Google Scholar]
- 89.Gupta N, Prasad PD, Ghamande S, et al. Up-regulation of the amino acid transporter ATB(0,+) (SLC6A14) in carcinoma of the cervix. Gynecol Oncol. 2006;100(1):8-13. doi:10.1016/j.ygyno.2005.08.016 [DOI] [PubMed] [Google Scholar]
- 90.Fuchs BC, Bode BP. Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin Cancer Biol. 2005;15(4):254-266. doi:10.1016/j.semcancer.2005.04.005 [DOI] [PubMed] [Google Scholar]
- 91.De Marchi T, Timmermans MA, Sieuwerts AM, et al. Phosphoserine aminotransferase 1 is associated to poor outcome on tamoxifen therapy in recurrent breast cancer. Sci Rep. 2017;7(1):2099. doi:ARTN 209910.1038/s41598-017-02296-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Son J, Lyssiotis CA, Ying H, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496(7443):101-105. doi:10.1038/nature12040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang WH, Chang MC, Tsai KS, Hung MC, Chen HL, Hung SC. Mesenchymal stem cells promote growth and angiogenesis of tumors in mice. Oncogene. 2013;32(37):4343-4354. doi:10.1038/onc.2012.458 [DOI] [PubMed] [Google Scholar]
- 94.Zhao H, Yang L, Baddour J, et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife. 2016;5:e10250. doi:10.7554/eLife.10250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J. 2012;279(15):2610-2623. doi:10.1111/j.1742-4658.2012.08644.x [DOI] [PubMed] [Google Scholar]
- 96.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Bio. 2008;9(2):112-124. doi:10.1038/nrm2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7(10):763-777. doi:10.1038/nrc2222 [DOI] [PubMed] [Google Scholar]
- 98.Daniels VW, Smans K, Royaux I, Chypre M, Swinnen JV, Zaidi N. Cancer cells differentially activate and thrive on de novo lipid synthesis pathways in a low-lipid environment. PLoS One. 2014;9(9):e106913. doi:10.1371/journal.pone.0106913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schulze A, Harris AL. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature. 2012;491(7424):364-3673. doi:10.1038/nature11706 [DOI] [PubMed] [Google Scholar]
- 100.Hilvo M, Denkert C, Lehtinen L, et al. Novel theranostic opportunities offered by characterization of altered membrane lipid metabolism in breast cancer progression. Cancer Res. 2011;71(9):3236-3245. doi:10.1158/0008-5472.CAN-10-3894 [DOI] [PubMed] [Google Scholar]
- 101.Wang W, Zhu N, Yan T, et al. The crosstalk: exosomes and lipid metabolism. Cell Commun Signal. 2020;18(1):119. doi:10.1186/s12964-020-00581-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang SH, Li XX, Xu MQ, Wang J, Zhao RC. Reduced adipogenesis after lung tumor exosomes priming in human mesenchymal stem cells via TGF beta signaling pathway. Molecul Cellu Biochem. 2017;435(1-2):59-66. doi:10.1007/s11010-017-3056-3 [DOI] [PubMed] [Google Scholar]
- 103.Sagar G, Sah RP, Javeed N, et al. Pathogenesis of pancreatic cancer exosome-induced lipolysis in adipose tissue. Gut. 2016;65(7):1165-1174. doi:10.1136/gutjnl-2014-308350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteom. 2009;6(3):267-283. doi:10.1586/epr.09.17 [DOI] [PubMed] [Google Scholar]
- 105.Cai Z, Yang F, Yu L, et al. Activated T cell exosomes promote tumor invasion via Fas signaling pathway. J Immunol. 2012;188(12):5954-5961. doi:10.4049/jimmunol.1103466 [DOI] [PubMed] [Google Scholar]
- 106.Menck K, Sonmezer C, Worst TS, et al. Neutral sphingomyelinases control extracellular vesicles budding from the plasma membrane. J Extracell Vesicles. 2017;6(1):1378056. doi:10.1080/20013078.2017.1378056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang X, Xu Q, Zi Z, et al. Programmable extracellular vesicles for macromolecule delivery and genome modifications. Dev Cell. 2020;55(6):784-801.e9. doi:10.1016/j.devcel.2020.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Katakowski M, Chopp M. Exosomes as tools to suppress primary brain tumor. Cell Mol Neurobiol. 2016;36(3):343-352. doi:10.1007/s10571-015-0280-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11(7):3183-3195. doi:10.7150/thno.52570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang G, Huang X, Xiu H, et al. Extracellular vesicles: natural liver-accumulating drug delivery vehicles for the treatment of liver diseases. J Extracell Vesicles. 2020;10(2):e12030. doi:10.1002/jev2.12030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Katakowski M, Buller B, Zheng XG, et al. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335(1):201-204. doi:10.1016/j.canlet.2013.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kamerkar S, LeBleu VS, Sugimoto H, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498-503. doi:10.1038/nature22341 [DOI] [PMC free article] [PubMed] [Google Scholar]