Abstract
The route used in the transplantation of mesenchymal stem cells (MSCs) can directly affect the treatment success. The transplantation of MSCs via the intrathecal (IT) route can be an important therapeutic strategy for neurological disorders. The objective of this study was to evaluate the safety and feasibility of the IT transplantation of autologous (Auto-MSCs) and allogeneic (Allo-MSCs) bone marrow mesenchymal stem cells (BM-MSCs) in healthy dogs. Based on neurodisability score, cerebrospinal fluid (CSF) and magnetic resonance imaging (MRI), no significant differences from the control group were observed on day 1 or day 5 after IT Auto- or Allo-MSCs transplantation (P > 0.05). In addition, analysis of matrix metalloproteinase (MMP)-2 and MMP-9 expression in the CSF revealed no significant differences (P > 0.05) at 5 days after IT transplantation in the Auto- or Allo-MSCs group when compared to the control. Intrathecal transplantation of BM-MSCs in dogs provides a safe, easy and minimally invasive route for the use of cell-based therapeutics in central nervous system diseases.
Keywords: bone marrow mesenchymal stem cells, cerebrospinal fluid, cell-based therapy, intrathecal route, matrix metalloproteinase
Introduction
The potential use of cell-based therapies in the treatment of neurological disorders is promising in translational medicine. Since companion animal diseases mirror human conditions, naturally occurring diseases offer several advantages as models of human disease1. Dogs represent an important translational model due to their sharing of the human environment and could therefore predict outcomes in human patients1,2. Inflammatory, traumatic and degenerative diseases of the central nervous system (CNS) in dogs are clinically similar to their counterparts in humans3.
Mesenchymal stem cells (MSCs) are easy to obtain, isolate and expand in vitro, express multifactorial therapeutic properties and possess multilineage differentiation capacity4–6 . The application of MSC-based therapies in CNS injuries, including neurodegenerative, genetic, traumatic, vascular and autoimmune diseases, has been widely studied in humans and animals7–12 . The therapeutic effects of MSCs are associated with enhancement of the regenerative microenvironment mediated by their anti-inflammatory/immunomodulatory effects and the promotion of neuroprotective activity through the paracrine secretion of neurotrophic and angiogenic factors13–15 .
The therapeutic effectiveness of MSCs also depends on the migration, engraftment and viability of the cells at the lesion site16. Therefore, concerns exist regarding the selection of an effective route for transplantation17. Intrathecal transplantation (IT) can result in direct cell distribution to the CNS through cerebrospinal fluid (CSF)18, thus avoiding the “first-pass” pulmonary effect, which can result in entrapment of cells and lead to a limited therapeutic effect19,20. The IT route also avoids issues related to dependency on injury-mediated opening of the blood-brain barrier (BBB) to permit access of the cells to the CNS21. Although several studies have demonstrated positive results after IT transplantation of MSCs in animals and humans22–24 , studies evaluating the safety of the IT route of transplantation in companion animals are still lacking.
The host immunological response to MSCs can influence therapeutic effects25. Allogeneic (Allo-MSCs) therapy is useful for transplantation immediately after diagnosis and has been considered safe and tolerable26,27; nevertheless, the production of antibodies against transplanted Allo-MSCs has been previously reported28. On the other hand, the use of autologous (Auto-MSCs) therapy is limited; it can typically only be used at the late stages of disease due to the time needed for expansion and to the limited ex vivo sources of Auto-MSCs29. Research on the in vivo effects of Allo-MSCs and Auto-MSCs transplantation in which clinical and inflammatory parameters are evaluated may help clarify the safety of these cell-based therapies25.
Matrix metalloproteinases (MMPs) are matrix-degrading enzymes that play important roles in many pathophysiological conditions, such as tumor-associated processes, infections and immunomodulation30. The activities of MMPs are regulated by a complex of transcription factors, inflammatory cytokines, chemokines, and growth factors and by the interaction of active MMPs with tissue inhibitors of metalloproteinases (TIMPs)31. Matrix metalloproteinases are secreted as pro-enzyme forms into the extracellular space and require activation by multiple mechanisms to reach their active or mature forms32. In CNS diseases, gelatinases metalloproteinase-2 (MMP-2) and metalloproteinase-9 (MMP-9) contribute to neuroinflammation, disruption of the BBB, oxidative stress and demyelination32,33. Therefore, the measurement of MMPs in the CSF has been considered a biomarker for the neuroinflammatory response34–36 .
We hypothesized that canine bone marrow-derived MSCs (BM-MSCs) transplanted by the intrathecal route are well tolerated and do not induce neurological changes, alterations in CSF (including MMP activity) or produce long-term abnormalities in the magnetic resonance imaging (MRI). In this context, our goal was to evaluate the safety and feasibility of autologous and allogenic BM-MSCs after intrathecal transplantation in healthy dogs.
Materials and Methods
Animal Selection
Fifteen healthy mixed-breed adult dogs (average weight 13 kg) were selected and maintained at the Kennel Veterinary Hospital, São Paulo State University, Brazil. All dogs included in this study had normal clinical and neurological examinations. All experiments involving animals were conducted in accordance with the guidelines of the Institutional Ethics Committee for Experimental Animal Use (CEUA) of São Paulo State University (FMVZ/Unesp, protocol 195/2011).
Experimental Design
The dogs were randomly divided into three groups. The Auto-MSCs group (n = 5) was subjected to the IT transplantation of autologous BM-MSCs. The Allo-MSCs group (n = 5) was subjected to the IT transplantation of allogeneic BM-MSCs. The control group (n = 5) was subjected to the IT injection of phosphate-buffered saline (PBS).
The dogs were clinically evaluated by neurological examination and application of the neurodisability scoring system prior to and at 5 days, 6 months, and 12 months after transplantation. Cerebrospinal fluid and CSF concentrations of the pro- and active forms of MMP-2 and MMP-9 were measured before and at 5 days after transplantation. Magnetic resonance imaging (MRI) was performed 1 year after transplantation (Fig. 1).
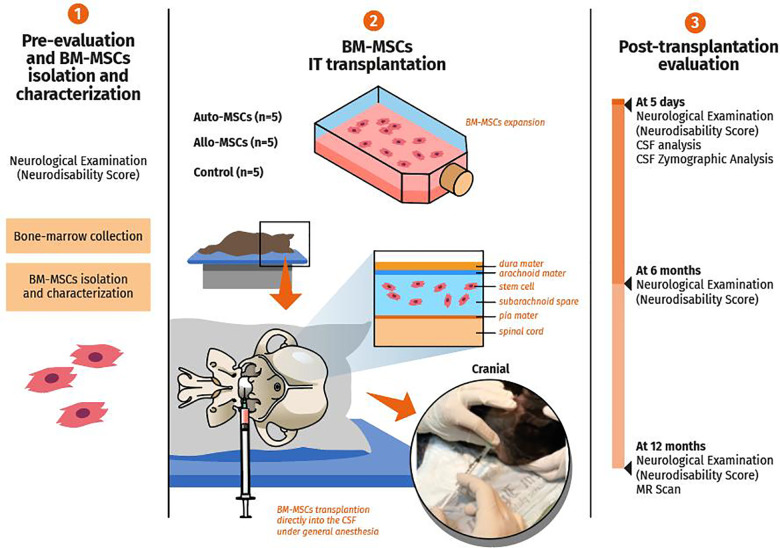
Figure 1.
Evaluation of safety and feasibility of autologous and allogenic BM-MSCs in healthy dogs. (1) The dogs were selected and evaluated with the neurodisability score. The BM-MSCs were harvested, isolated, and characterized with specific positive and negative markers and storage until intrathecal (IT) transplantation. (2) Three groups were selected; Auto-MSCs group (n = 5), Allo-MSCs group (n = 5) and control group (n = 5). After culture and expansion, the Auto-MSCs and All-MSCs groups received by IT route autologous or allogeneic BM-MSC, respectively. The control group received by IT route phosphate-buffered saline (PBS). (3) The neurodisability score, CSF analysis and CSF pro- and active forms of MMP-2 and MMP-9 were performed before IT transplantation and at 5 days. Finally, at long term the dogs were evaluated by neurodisability score and brain MRI. Illustration by Luís Renato do Nascimento.
Isolation and Culture of Canine BM-MSCs
Canine bone marrow was collected from the humerus with the animal under general inhalation anesthesia. The marrow was aspirated into a 20-ml syringe containing 1.000 IU heparin/ml (Cristália, São Paulo, Brazil). The suspension was filtered, centrifuged and mixed with Dulbecco’s modified Eagle’s medium (DMEM/F12, Gibco, Invitrogen, São Paulo, SP, Brazil) at a 1:1 ratio. Subsequently, 5 ml of Ficoll-Paque Premium density 1.077 gradient (GE Healthcare-Sigma Aldrich, São Paulo, SP, Brazil) was slowly added, and the mixture was centrifuged at 1500 rpm for 40 minutes. The mononuclear cell layer was carefully aspirated and centrifuged twice at 1500 rpm for 10 minutes.
The stromal vascular fraction (SVF) was suspended and cultured in DMEM high glucose supplemented with 20% fetal bovine serum (FBS), 1% penicillin/streptomycin (10,000 UI/mL) and 1.2% amphotericin B (250 μg/mL)(all from Gibco, Invitrogen, São Paulo, SP, Brazil). The culture flasks were incubated at 37°C in a humidified incubator with 5% CO2, and the medium was changed twice weekly. When the monolayer reached 80% confluence, the cells were detached with 0.25% trypsin (Gibco, Invitrogen, São Paulo, SP, Brazil) for passage or transplantation. The viability of the cells was measured using the trypan blue method. The cells were stored in liquid nitrogen for further characterization or for transplantation after expansion.
Adipogenic, Osteogenic and Chondrogenic Differentiation of Canine BM-MSCs
To induce trilineage mesodermal differentiation, specific adipogenesis, chondrogenesis, and osteogenesis differentiation kits were used according to the manufacturer’s instructions (Stempro® kit, Gibco, Invitrogen, Grand Island, NE, USA). Adipogenic and osteogenic differentiation was confirmed by histological staining with Oil red O and Alizarin red S, respectively. For chondrogenic differentiation, micromass culture and histological staining with Alcian Blue (pH 2.5) were performed. Cells were cultured in DMEM/20% FBS (all from Gibco, Invitrogen, São Paulo, SP, Brazil) as a negative control.
Flow Cytometry
Canine BM-MSCs were characterized by the presence of the surface markers CD44 and CD105 or the absence of CD3437–39 . Canine BM-MSCs were characterized by flow cytometry on a FACSCalibur cell analyzer (Becton Dickinson Company, San Jose, CA, USA). The cells were incubated with CD44 rat anti-mouse (Sigma-Aldrich, Saint Louis, MO, USA) CD45 rat anti-dog (Abd Serotec, Raileigh, NC, USA), CD90-FITC mouse anti-human (Becton Dickinson Company, San Jose, CA, USA), MHC class II rat anti-dog (Abd Serotec, Raileigh, NC, USA) and and CD34-FITC anti-human antibodies (Becton Dickinson Company, San Jose, CA, USA). A secondary antibody, goat anti-mouse IgG-FITC (Abd Serotec, Raleigh, NC, USA), was used as an unconjugated marker. A standard protocol was used according to the manufacturer’s instructions. The results were analyzed using Cell Quest Pro software (Dickinson Company, San Jose, USA).
Intrathecal Transplantation of Canine BM-MSCs
The dogs were subjected to general inhalation anesthesia, and the atlanto-occipital region was aseptically prepared. A spinal needle (25 G × 3 1/2’’, 0.50 mm × 90) was used according to a previously described protocol40. Briefly, the dogs were positioned in the right lateral recumbency, and cervical ventroflexion was performed. The needle was inserted slowly into the cerebellomedullary cistern, and the CSF was collected. Then, a suspension of 1.5 × 106 cells in 1 mL of PBS or 1 mL of PBS only was injected into the cerebellomedullary cistern over a period of nearly 1 minute.
Neurological Examination
Neurological examination and Neurodisability score were performed in dogs in the Allo-MSCs, Auto-MSCs and control groups prior to and at 5 days, 6 months, and 12 months after BM-MSC transplantation, as previously described. The neurological scoring system was applied to assessment of neurological status (Online Supplemental Material S2). During the neurological examination, behavioral assessment, mental status, posture and gait, cranial nerves, postural reactions, urinary function, sensory deficits, and epaxial palpation were evaluated. The system allocates arbitrary scores to neurological deficits that can be identified in a routine neurological examination, the sum of these providing an overall disability score. Normal scores are close to zero and the dogs with presence of severe neurological deficits show an increase of score close to value of 3041.
Cerebrospinal Fluid Analysis
CSF analysis of the dogs in the Auto-MSCs, Allo-MSCs and control groups was performed prior to and at 5 days after transplantation. During CSF evaluation, cytochemical analysis, including total nucleated cell count (TNCC) and measurement of protein concentration, was performed. The TNCC was considered normal if ≤5 nucleated cells/µL were observed, and protein concentrations ≤30 mg/dL were considered normal. CSF was collected through the cerebellomedullary cistern in all dogs42.
Gelatin Zymography
The CSF concentrations of the pro- and active forms of MMP-2 and MMP-9 of the dogs in the Auto-MSCs, Allo-MSCs, and control groups were determined prior to and at 5 days after transplantation as previously described36. The gelatinolytic activity of the samples was detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gelatin zymography. The results, which are expressed in arbitrary units (A.U.), were calculated by summing the pixel values within the digested area of the band and subtracting the background density using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Magnetic Resonance Imaging
Brain MRIs of the dogs in the Auto-MSCs, Allo-MSCs and control groups were performed 1 year after BM-MSC transplantation to investigate the presence of abnormalities as mass tumors. T1-weighted (T1 W), T1-weighted (T2 W), fluid-attenuation inversion recovery (FLAIR), and postcontrast T1 sequences (Optimark®, Mallinckrodt Inc., Raleigh, NC, USA) in the sagittal, transverse, and dorsal planes were obtained using a 0.25 Tesla scanner (Vet-MR Grande, Esaote, Lígure, GE, Italy).
Statistics Analysis
The quantitative variables CSF protein, CSF TNCC, pro- and active forms of MMP-2 and MMP-9 were evaluated for normality using descriptive statistics, graphical analyses and statistical tests (Shapiro-Wilk). Statistical analysis was performed with the nonparametric Kruskal–Wallis test. Pairwise comparisons were made using the Mann–Whitney test. All boxplots show median values and interquartile ranges and minimum and maximum values. The level of significance between the groups was set at P < 0.05. The differences were denoted by a single asterisk (P < 0.05), two asterisks (P < 0.01), or three asterisks (P < 0.001) (GraphPad Prism version 8 for Mac, San Diego, CA, USA).
Results
Canine Bone Marrow-Derived Mesenchymal Stem Cells Exhibited Mesenchymal Fate and Trilineage Differentiation Potential
The canine BM-MSCs exhibited plastic adherence and fibroblastoid morphology, and the assays of trilineage differentiation revealed their multipotentiality. After 9 days of culture in adipogenic medium, the BM-MSCs differentiated into adipocytes; the core increased in size and formed bright lipid vacuoles or fat droplets that were visible after Oil Red staining (Fig. 2a). Osteogenic differentiation of BM-MSCs, characterized by positive Alizarin Red staining indicating deposits of calcium in the cells, was observed 21 days after the induction of differentiation. Chondrogenic differentiation, indicated by positive Alcian Blue staining showing that the micromass of cells contained sulfated proteoglycans, was observed 21 days after the induction of differentiation (Fig. 2a).
Figure 2.
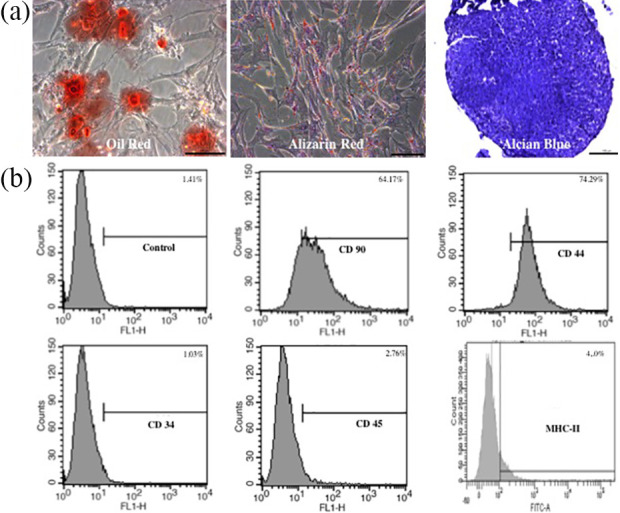
Characterization and immunophenotyping of canine BM-MSCs. (a) Mesodermal differentiation of canine BM-MSCs. The presence of lipid vacuoles was observed after 14 days of adipogenic differentiation (Oil red staining). Calcium phosphate deposits and calcified extracellular matrix were observed after 21 days of osteogenic differentiation (Alizarin red staining). Extracellular matrix deposition with mucopolysaccharides was observed after 21 days of chondrogenic differentiation (Alcian blue staining). Scale bars = 100 µm. (b) Representative immunophenotype of BM-MSCs. BM-MSCs express MSC markers CD90 and CD44 and do not express hematopoietic markers CD34 and CD45 or MHC-II.
To analyze the expression of cell surface markers on canine BM-MSCs, the cells were subjected to immunophenotyping at passage three. The flow cytometry results revealed positive expression of the surface markers CD44 and CD90 and the absence of CD34and MHC-II expression (Fig. 2b). Canine BM-MSC transplantation was performed using cells that exhibited > 90% viability.
Neurological Examination After Intrathecal Transplantation Showed No Alterations
The neurological examination was unremarkable in 100% of the dogs in the Auto-MSCs (5/5), Allo-MSCs (5/5), and control (5/5) groups before transplantation and at 5 days, 6 months, and 12 months after transplantation. Neurodisability score analyses based in cranial nerves, mentation and postural responses, prior to and at 5 days, 6 months, and 12 post-transplantation was unremarkable (score 0) in the dogs of Auto-MSCs, Allo-MSCs and control groups (Online Supplemental Material S3).
Intrathecal Transplantation of Canine BM-MSCs Did not Alter CSF Parameters
Total nucleated cell count analysis did not show significant differences among the Auto-MSCs, Allo-MSCs and control groups prior to and at 5 days after transplantation (P > 0.05) (Fig. 3a). The total nucleated cell count values before transplantation were as follows: Auto-MSCs median 1 cell/µL (range 0-1 cell/µL); Allo-MSCs median 1 cell/µL (range 0–15 cell/µL); and control median 1 cell/µL (range 0–2 cell/µL). The total nucleated cell count values at 5 days after transplantation were as follows: Auto-MSCs median 2 cell/µL (range 1–8 cell/µL); Allo-MSCs median 5 cell/µL (range 1–50 cell/µL); and control median 1 cell/µL (range 1–6 cell/µL).
Figure 3.
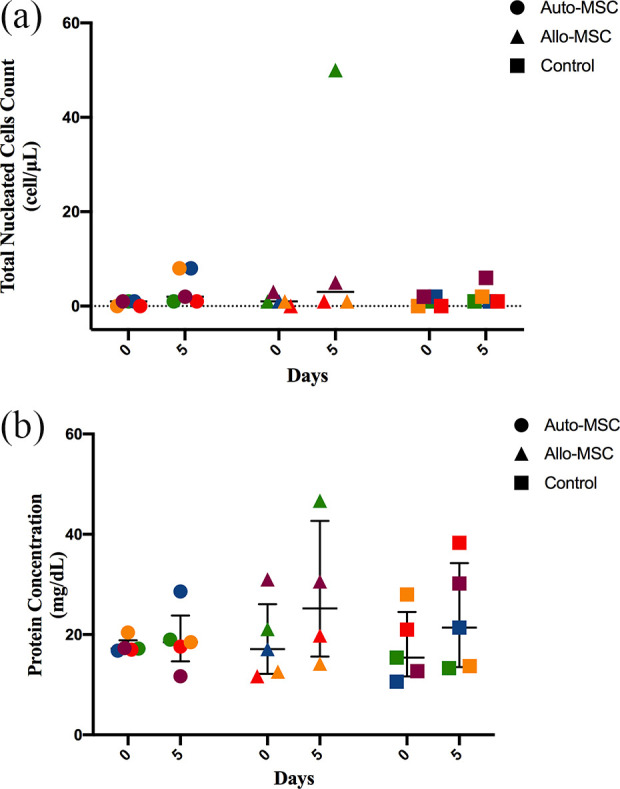
CSF parameters after BM-MSCs transplantation. The analyses were determined prior to and at 5 days after transplantation in all dogs in the Auto-MSCs, Allo-MSCs, and control groups (n = 5 for each group). (a) CSF cytology. No significant differences were found among the groups after BM-MSC transplantation (p > 0.05). (b) CSF protein concentration. No significant differences were found among the groups after BM-MSC transplantation (p > 0.05 .
Five days after transplantation, two dogs in the Auto-MSCs group showed pleocytosis (8 cells/µL, 8 cells/µL), and one dog in the Allo-MSCs group presented pleocytosis (50 cells/µL). For all samples, cell morphology was normal, and no atypical cells were noted in the CSF prior to or after transplantation.
Total protein concentration analysis did not show significant differences among the Auto-MSCs, Allo-MSCs and control groups prior to and at 5 days after transplantation (P > 0.05) (Fig. 3b). The values before transplantation were as follows: median Auto-MSCs 17.2 mg/dL (range 16.8–20.4 mg/dL); median Allo-MSCs 17.1 mg/dL (range 11.7–31.0 mg/dL); and median control 15.4 mg/dL (range 10.6–28.0 mg/dL). The TNCC values at 5 days after transplantation were as follows: Auto-MSCs median 18.5 mg/dL (range 11.7–28.6 mg/dL); Allo-MSCs median 30.0 mg/dL (range 14.2–46.7 mg/dL); and control median 21.4 mg/dL (range 13.3–38.3 mg/dL). Hyperproteinorrachia was observed in one dog in the Allo-MSCs group (46.7 mg/dL) after transplantation (Online Supplemental Material S1).
MMP Expression was not Altered by the Intrathecal Transplantation of Canine BM-MSCs
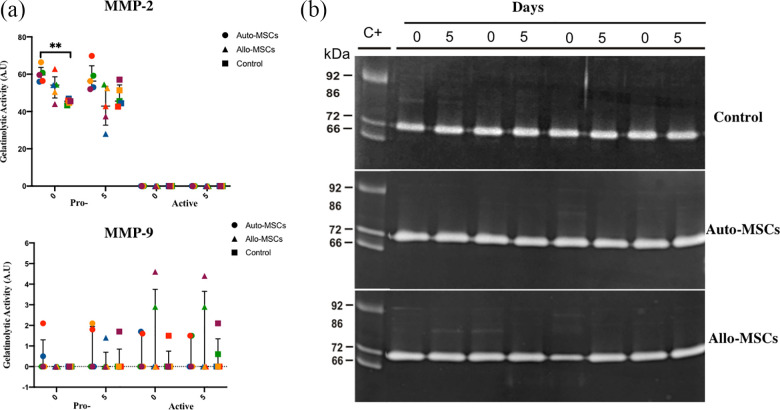
The gelatinolytic activity of pro-MMP-2 and active MMP-2 did not show significant differences among the Auto-MSCs, Allo-MSCs and control groups prior to and at 5 days after transplantation (P > 0.05) (Fig. 4a).
Figure 4.
Gelatin zymographic of matrix metalloproteinases (MMP)-2 and MMP-9 in the CSF. MMP zymographic analysis and zymograms were obtained prior to and after BM-MSC transplantation in all dogs in the Auto-MSCs, Allo-MSCs and control groups. (n = 5 for each group). (a) There were no significant differences in (MMP)-2 and -9 among the groups 5 days after MSCs transplantations (P p > 0.05. (b) Note the presence of the gelatinolytic bands corresponding to pro-MMP-9 (92 kDa), active MMP-9 (86 kDa), pro-MMP-2 (72 kDa), and active MMP-2 (66 kDa).
CSF zymographic analysis revealed the detection of pro-MMP-2 in all of the dogs in the Auto-MSCs (60.8, 56.0, 56.4, 66.4, and 59.6 A.U.), Allo-MSCs (54.4, 54.2, 62.8, 50.5, 44.0 A.U.) and control (43.3, 46.9, 45.9, 44.7, 45.5 A.U.) groups before transplantation. Five days after transplantation, pro-MMP-2 was detected in 5/5 of the dogs in the Auto-MSCs group (59.2, 53.0, 69.8, 56.3, and 52.0 A.U.), Allo-MSCs group (54.5, 28.0, 42.9, 52.5, and 37.4 A.U.) and control group (45.6, 44.4, 42.6, 51.4, and 57.1 A.U.).
Active MMP-2 was not detected by CSF zymography before transplantation or at 5 days after transplantation in any of the dogs in the Auto-MSCs, Allo-MSCs, or control groups.
The gelatinolytic activity of pro-MMP-9 and active MMP-9 did not show significant differences among the Auto-MSCs, Allo-MSCs and control groups before and at 5 days after transplantation (Fig. 4a).
CSF zymographic analysis revealed very low levels of pro-MMP-9 prior to transplantation in two dogs in the Auto-MSCs group (0.5 and 2.1 A.U.). At 5 days after transplantation, pro-MMP-9 displayed very low levels in two dogs in the Auto-MSCs group (1.8 and 2.1 A.U.), in one dog in the Allo-MSCs group (1.5 A.U.), and in one dog in the control group (1.7 A.U.).
Active MMP-9 was detected in very low amounts in two dogs in the Auto-MSCs group (1.7 and 1.6 A.U.), in two dogs in the Allo-MSCs group (2.9 and 4.6 A.U.), and in one dog in the control group (1.5 A.U.) before transplantation. Five days after transplantation, active MMP-9 was observed in one dog in the Auto-MSCs group (1.5 A.U.), in two dogs in the Allo-MSCs group (2.9 and 4.4 A.U.), and in two dogs in the control group (0.6 and 2.1 A.U.), as shown in Fig. 4b (Online Supplemental Material S1).
Long-Term Magnetic Resonance Imaging After Intrathecal Transplantation of Canine BM-MSCs Showed no Alterations
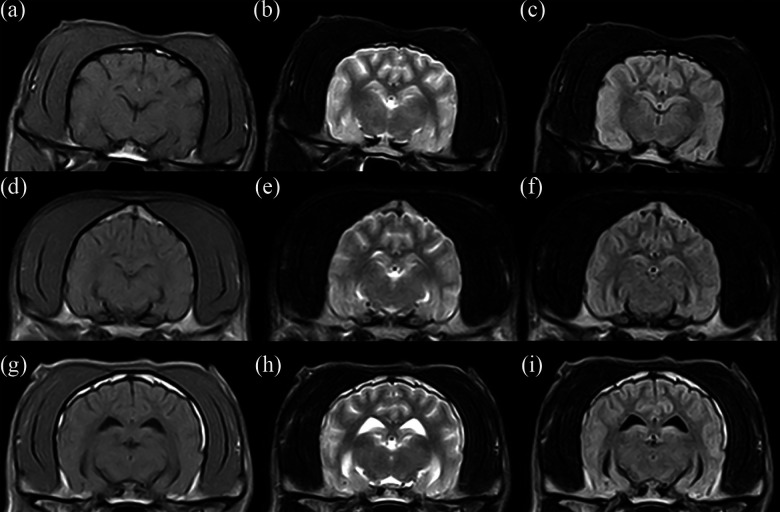
MRI exams performed 1 year after the IT transplantation of BM-MSCs did not reveal alterations or tumor formation in the brains of the dogs in the Auto-MSCs and Allo-MSCs groups (Fig. 5).
Figure 5.
Normal brain magnetic resonance imaging after the IT transplantation of BM-MSCs from the three experimental groups. (a–c) Transverse T1-weighted, T2-weighted, and fluid-attenuated inversion recovery (FLAIR) sequences of the control group (dog 1). (d–f) Transverse T1-weighted, T2-weighted, and FLAIR sequences of the Auto-MSCs group (dog 5). (g–i) Transverse T1-weighted, T2-weighted, and FLAIR sequences of the Allo-MSCs group (dog 1).
Discussion
Central nervous system diseases are damaging to humans and animals, leading to permanent disability and poor quality of life. The promotion of a neuroregenerative and anti-inflammatory microenvironment is the main goal of cell-based therapeutic strategies for CNS diseases. One approach for improving the therapeutic profile of MSCs is the direct transplantation of MSCs into the CSF, thus bypassing the blood-brain barrier obstacle. To date, no studies have evaluated the safety and feasibility of transplantation of canine autologous and allogenic BM-MSCs by the IT route. Herein, canine Auto-MSCs and Allo-MSCs were transplanted by the IT route, and the safety of this method was evaluated by neurological examination, CSF analysis, and long-term brain MRI.
Due to pleiotropic characteristics of the MSCs, the International Society for Cellular Therapy (ISCT) creates guidelines for the definition and characterization of human MSCs. The parameters included plastic adherence, differentiation capacity to lineages adipocytes, chondroblasts, and osteoblasts and expression of surface markers such as CD90, CD73, CD105, associated to absence CD11b, CD14, CD19, CD34, CD45 y MHC- II37. Although the criteria are not fully defined for canine MSCs characterization, the canine BMMSCs showed typical mesenchymal stem cell morphology, adherent property, and multilineage differentiation potential regarding adipogenicity, chondrogenicity, and osteogenicity. Our flow cytometry assay was able to characterize canine BM-MSCs as CD90+, CD44+, CD34−, CD45−, e MHC II−. Herein, canine BM-MSCs exhibited a comparable in vitro profile as described in previous studies39,43–46 .
The self-renewal characteristics and proliferation capacity of MSCs are associated with tumor formation or tumor growth in animal models47. In our study, advanced imaging features related to tumor formation and mass effects in the brain after IT transplantation were not observed in any of the dogs at the on-year long-term evaluation by MRI scanning. These findings indicate that neither allogenic nor autologous BM-MSCs transplanted by the IT route promoted tumor initiation. In this sense, the dogs used in this study showed no neurological deficits at 5 days, 6 months, or 12 months after transplantation. Therefore, clinical aspects also were not affected by allogenic or autologous BM-MSCs delivered intrathecally.
CSF analysis following transplantation revealed the absence of statistical differences in the protein concentration and number of total nucleated cells 5 days after transplantation in both groups Auto-MSCs and Allo-MSCs. One dog in the Allo-MSCs group showed 50 cells/µL and mild proteinorrachia post-transplantation. Two dogs in the Auto-MSCs group showed mild pleocytosis (8 cells/µL) post-transplantation. These findings could be related to some grade of immune response towards the transplanted cells or to the chemical/xenogeneic products used for cell expansion or preservation (i.e., dimethyl sulfoxide and FBS). However, the immune response may not have been sufficient to cause immune rejection since no adverse effects were found upon clinical and neurological examination after the IT transplantation of BM-MSCs. Mesenchymal stem cells secrete various cytokines (i.e., TGF-β, IDO, and PGE2), growth factors and extracellular vesicles that contribute to the modulation of immune responses48,49. Therefore, the balance between the expression of immunogenic and immunosuppressive factors may have contributed to the absence of adverse effects25,29. These features could explain the absence of clinical immune rejection for the allogenic transplantation of BM-MSCs observed in our study. Additionally, pleocytosis could be related to the trauma caused by the puncture itself for CSF collection. Consistent with our findings, Barberini et al. (2018) showed that after IT Auto-MSCs transplantation in six healthy horses, five horses developed mild pleocytosis in the CSF 6 days after transplantation, with no abnormalities on neurological examination50.
Zymographic analysis was used to measure the levels of pro- and active MMP-2 and MMP-9 in the CSFs of healthy dogs prior to and after the transplantation of Auto- and Allo-BM-MSCs as a basis for evaluating the occurrence of neuroinflammation after IT transplantation36,51. In our study, there were no significant differences in pro-MMP-2 and MMP-2 at 5 days after transplantation compared to the control group. Consistent with previous reports about matrix metalloproteinases activity in the CSF in dogs34,36, pro-MMP-2 was detected in the CSF prior to and after BM-MSC transplantation in all experimental groups. This was an expected result since MMP-2 is constitutively expressed in the brain and bodily fluids such as serum and CSF34. Other than this, no expression of active MMP-2 was observed in any of the groups prior to or after transplantation, as evidenced in earlier studies34,36. Higher activity of active MMP-2 has been correlated with immunodeficiency virus infection in human neuronal and glial cells52 and in the CSF of dogs with subacute distemper leukoencephalitis36.
The results of pro-MMP-9 and active MMP-9 were not meaningful at 5 days after transplantation when compared to the control group. Pro- and active forms of MMP-9 were not detected or inconsistently detected in very low amounts in a few samples in the Auto-MSCs, Allo-MSCs, and control groups either before or after BM-MSC transplantation. Importantly, the detected levels were not significant compared to those in subjects with inflammatory/infectious diseases34,42. Similarly, Levine et al. (2006) detected pro-MMP-9 activity in the CSF of one control animal (1/8) in a study that evaluated the activity of metalloproteinases in the CSF and serum of dogs with acute spinal cord trauma due to intervertebral disk disease34. Although the MMP-9 measurements in blood samples may reflect their release by leukocytes during the clotting process in the collection tube53, not all dogs that presented some degree of blood contamination had an increased level of MMP-9. Therefore, the authors believe that this was an unspecific finding associated with sample processing MMP-9 activity.
Herein, BM-MSC transplantation by the IT route in healthy dogs is shown to be a feasible and minimally invasive method for cell-based therapy. The use of the IT route allows the entry of MSCs directly into the subarachnoid space, avoiding the dependency of BBB permeability and resulting in a greater number of cells reaching the site of injury in the CNS16. In comparison, intravenous and intra-arterial routes of transplantation may be less invasive54,55; nevertheless, there is limited evidence showing that a large number of MSCs reach the target in the CNS when these techniques are used56, and most of the cells transplanted by these routes are entrapped in the lung microvasculature and other organs due to the large size of the cells and to the expression of adhesion molecules19,20. Previous studies have suggested that MSC accumulate in the lungs within 24 hours after intravenous administration57,58 and, disappeared from the lungs, but did not reappear in the other tissues, suggesting they did not survive long term in the recipient animals58, as well as, has been suggested that there is no difference in the migration of viable MSC to injured and non-injured organs59. In addition, there is also a significant risk of vascular occlusion by cell aggregates (microembolization) when cells are delivered by the intra-arterial route, which can lead to additional cerebral damage60,61. The intraparenchymal is another alternative route for MSC delivery to treat CNS diseases and may offer benefits regarding local cell biodistribution and migration; nevertheless, it is a very invasive procedure that presents a major risk of CNS injury61.
Since the proposition that the positive effects of MSC-based therapy are mediated via the secretion of trophic and immunoregulatory factors has been proposed, the concept that administered MSC engraft and differentiate in specialized cell types has been abandoned59.
Our study indicates a short- and long-term safety profile of the intrathecal transplantation route for autologous and allogenic BM-MSCs in healthy dogs, revealing potential use of IT route for canine CNS diseases. However, some limitations can be point out related to lack of global MMP analysis including serum samples, since MMP systemic response could to correlate with CSF production35. We provided preliminary data about a new route of MSC transplantation in dogs. Meanwhile, a blinded controlled clinical trial involving more animals with serial transplantations is necessary to improve the protocol.
Conclusions
The IT transplantation of autologous or allogeneic BM-MSCs in healthy dogs did not produce neurological deficits, CSF alterations or induction of MMP activity in the CSF. Upon long-term evaluation by advanced MRI, no changes were detected. MSC transplantation by the IT route in dogs is safe and feasible. However, future research involving multiple IT transplantations, different dosages and MSCs derived from other tissues is necessary to establish a safe therapeutic protocol for canine CNS diseases.
Supplemental Material
Supplemental Material, sj-docx-1-cll-10.1177_09636897211034464 for Intrathecal Transplantation of Autologous and Allogeneic Bone Marrow-Derived Mesenchymal Stem Cells in Dogs by Felipe Pérez Benavides, Giovana Boff Araujo Pinto, Marta Cristina Thomas Heckler, Diana Milena Rodríguez Hurtado, Livia Ramos Teixeira, Marina Mitie de Souza Monobe, Gisele Fabrino Machado, Guilherme Dias de Melo, Diego Noé Rodríguez-Sánchez, Fernanda da Cruz Landim e Alvarenga and Rogério Martins Amorim in Cell Transplantation
Supplemental Material, sj-docx-2-cll-10.1177_09636897211034464 for Intrathecal Transplantation of Autologous and Allogeneic Bone Marrow-Derived Mesenchymal Stem Cells in Dogs by Felipe Pérez Benavides, Giovana Boff Araujo Pinto, Marta Cristina Thomas Heckler, Diana Milena Rodríguez Hurtado, Livia Ramos Teixeira, Marina Mitie de Souza Monobe, Gisele Fabrino Machado, Guilherme Dias de Melo, Diego Noé Rodríguez-Sánchez, Fernanda da Cruz Landim e Alvarenga and Rogério Martins Amorim in Cell Transplantation
Supplemental Material, sj-docx-3-cll-10.1177_09636897211034464 for Intrathecal Transplantation of Autologous and Allogeneic Bone Marrow-Derived Mesenchymal Stem Cells in Dogs by Felipe Pérez Benavides, Giovana Boff Araujo Pinto, Marta Cristina Thomas Heckler, Diana Milena Rodríguez Hurtado, Livia Ramos Teixeira, Marina Mitie de Souza Monobe, Gisele Fabrino Machado, Guilherme Dias de Melo, Diego Noé Rodríguez-Sánchez, Fernanda da Cruz Landim e Alvarenga and Rogério Martins Amorim in Cell Transplantation
Acknowledgement
We would like to acknowledge the School of Veterinary Medicine and Animal Science, São Paulo State University “Júlio de Mesquita Filho” (FMVZ-UNESP, Campus Botucatu).
Footnotes
Author Contributions: FPB and GBAP contributed equally to this manuscript. FPB and RMA made contributions to conception, design and definition of intellectual content. FPB, MTH, DRH, LRM, MSM, GFM, and GDM performed experimental study and data acquisition. FPB, MTH, DRH, and LRM, executed clinical procedures and neurological evaluations. FPB and MTH performed isolation and culture of canine MSC. MSM, GFM, and GDM performed zymography and cerebrospinal fluid analysis. DNRS and GBAP performed MRI analysis, data interpretation, and statistical analysis. DNRS, GBAP, and RMA performed preparation and editing of the manuscript. All authors review the manuscript.
Availability of Data and Materials: All data generated and/or analyzed during this study are included in this published article.
Ethical Approval: Institutional Ethics Committee for Experimental Animal Use (CEUA) of São Paulo State University (FMVZ/UNESP, protocol 195/2011) approved all procedures in this study.
Statement of Human and Animal Rights: All animal experiment protocols in this study were conducted in accordance with Institutional Ethics Committee for Experimental Animal Use (CEUA), School of Veterinary Medicine and Animal Science, São Paulo State University (FMVZ/UNESP).
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was partially funded by the School of Veterinary Medicine and Animal Science, Sao Paulo State University (FMVZ/UNESP), Botucatu, Brazil.
ORCID iDs: Guilherme Dias de Melo  https://orcid.org/0000-0003-0747-7760
https://orcid.org/0000-0003-0747-7760
Diego Noé Rodríguez-Sánchez  https://orcid.org/0000-0001-6524-5939
https://orcid.org/0000-0001-6524-5939
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Hoffman AM, Dow SW. Concise review: stem cell trials using companion animal disease models. Stem Cells. 2016;34(7):1709–1729. [DOI] [PubMed] [Google Scholar]
- 2.Jeffery ND, Barker AK, Alcott CJ, Levine JM, Meren I, Wengert J, Jergens AE, Suchodolski JS. The association of specific constituents of the fecal microbiota with immune-mediated brain disease in dogs Wilson BA, editor. PLoS One. 2017;12(1):e0170589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greer KA, Wong AK, Liu H, Famula TR, Pedersen NC, Ruhe A, Wallace M, Neff MW. Necrotizing meningoencephalitis of Pug dogs associates with dog leukocyte antigen class II and resembles acute variant forms of multiple sclerosis. Tissue Antigens. 2010;76(2):110–118. [DOI] [PubMed] [Google Scholar]
- 4.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110(10):3499–3506. [DOI] [PubMed] [Google Scholar]
- 5.Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cell Dev. 2012;21(14):2724–2752. [DOI] [PubMed] [Google Scholar]
- 6.Uccelli A, Zappia E, Benvenuto F, Frassoni F, Mancardi G. Stem cells in inflammatory demyelinating disorders: a dual role for immunosuppression and neuroprotection. Exp Opin Biolo Therapy. 2006;6(1):17–22. [DOI] [PubMed] [Google Scholar]
- 7.Syková E, Rychmach P, Drahorádová I, Konrádová Š, Růžičková K, Voříšek I, Forostyak S, Homola A, Bojar M.Transplantation of mesenchymal stromal cells in patients with amyotrophic lateral sclerosis: results of phase I/IIA clinical trial. Cell Transplant. 2017;26(4):647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, Pedemonte E, Mantegazza R, Frassoni F, Mancardi G, Pedotti R, Uccelli A.Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Anna Neurol. 2007;61(3):219–227. [DOI] [PubMed] [Google Scholar]
- 9.Stemberger S, Jamnig A, Stefanova N, Lepperdinger G, Reindl M, Wenning GK. Mesenchymal stem cells in a transgenic mouse model of multiple system atrophy: immunomodulation and neuroprotection schuelke m, editor. PLoS One. 2011;6(5):e19808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Detante O, Rome C, Papassin J. How to use stem cells for repair in stroke patients. Rev Neurol. (Paris). 2017;173(9):572–576. [DOI] [PubMed] [Google Scholar]
- 11.Connick P, Kolappan M, Crawley C, Webber DJ, Patani R, Michell AW, Du MQ, Luan SL, Altmann DR, Thompson AJ, et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol. 2012;11(2):150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez AMB.Neurotrauma and mesenchymal stem cells treatment: from experimental studies to clinical trials. World J Stem Cell. 2014;6(2):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paul G, Anisimov SV.The secretome of mesenchymal stem cells: potential implications for neuroregeneration. Biochimie. 2013;95(12):2246–2256. [DOI] [PubMed] [Google Scholar]
- 14.Villatoro AJ, Alcoholado C, Martín-Astorga MC, Fernández V, Cifuentes M, Becerra J.Comparative analysis and characterization of soluble factors and exosomes from cultured adipose tissue and bone marrow mesenchymal stem cells in canine species. Vete Immun Immunopathol. 2019;208(August 2018):6–15. [DOI] [PubMed] [Google Scholar]
- 15.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45(11):e54–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciervo Y, Ning K, Jun X, Shaw PJ, Mead RJ. Advances, challenges and future directions for stem cell therapy in amyotrophic lateral sclerosis. Mol Neurodegenerat. 2017;12(1)85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kabu S, Gao Y, Kwon BK, Labhasetwar V.Drug delivery, cell-based therapies, and tissue engineering approaches for spinal cord injury. J Controlled Release. 2015;219:141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JWM, Petrou P, Ben-Hur T, Abramsky O, Slavin S.Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67(10):1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi Y, Tsuji O, Kumagai G, Hara CM, Okano HJ, Miyawaki A, Toyama Y, Okano H, Nakamura M. Comparative study of methods for administering neural stem/progenitor cells to treat spinal cord injury in mice. Cell Trans. 2011;20(5):727–739. [DOI] [PubMed] [Google Scholar]
- 20.Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cell Dev. 2009;18(5):683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bakshi A, Hunter C, Swanger S, Lepore A, Fischer I. Minimally invasive delivery of stem cells for spinal cord injury: advantages of the lumbar puncture technique. J Neurosurg Spine. 2004;1(3):330–337. [DOI] [PubMed] [Google Scholar]
- 22.Zhang C, Zhou C, Teng J-J, Zhao R-L, Song Y-Q. Multiple administrations of human marrow stromal cells through cerebrospinal fluid prolong survival in a transgenic mouse model of amyotrophic lateral sclerosis. Cytotherapy. 2009;11(3):299–306. [DOI] [PubMed] [Google Scholar]
- 23.Gabr H, El-Kheir WA, Farghali HAMA, Ismail ZMK, Zickri MB, El Maadawi ZM, Kishk NA, Sabaawy HE. Intrathecal transplantation of autologous adherent bone marrow cells induces functional neurological recovery in a canine model of spinal cord injury. Cell Trans. 2015;24(9):1813–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh KW, Noh MY, Kwon MS, Kim HY, Oh SI, Park J, Kim HJ, Ki CS, Kim SH. Repeated intrathecal mesenchymal stem cells for amyotrophic lateral sclerosis. Ann Neurol. 2018;84(3):361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berglund AK, Fortier LA, Antczak DF, Schnabel L V.Immunoprivileged no more: measuring the immunogenicity of allogeneic adult mesenchymal stem cells. Stem Cell Res Therapy. 2017;8(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ. Safety of cell therapy with mesenchymal stromal cells (safecell): a systematic review and meta-analysis of clinical trials beltrami AP, editor. PLoS One. 2012;7(10):e47559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31(10):890–896. [DOI] [PubMed] [Google Scholar]
- 28.Owens SD, Kol A, Walker NJ, Borjesson DL. Allogeneic mesenchymal stem cell treatment induces specific alloantibodies in horses. Stem Cells Int. 2016;2016(5830103):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nature Biotechnol. 2014;32(3):252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158(3):983–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2(7):502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39(3):279–291. [DOI] [PubMed] [Google Scholar]
- 33.Kurzepa J, Kurzepa J, Golab P, Czerska S, Bielewicz J.The significance of matrix metalloproteinase (MMP)-2 and MMP-9 in the ischemic stroke. Int J Neurosci. 2014;124(10):707–716. [DOI] [PubMed] [Google Scholar]
- 34.Levine JM, Ruaux CG, Bergman RL, Coates JR, Steiner JM, Williams DA. Matrix metalloproteinase-9 activity in the cerebrospinal fluid and serum of dogs with acute spinal cord trauma from intervertebral disk disease. Am J Vete Res. 2006;67(2):283–287. [DOI] [PubMed] [Google Scholar]
- 35.Fainardi E, Castellazzi M, Bellini T, Manfrinato MC, Baldi E, Casetta I, Paolino E, Granieri E, Dallocchio F.Cerebrospinal fluid and serum levels and intrathecal production of active matrix metalloproteinase-9 (MMP-9) as markers of disease activity in patients with multiple sclerosis. Multi Sclerosis J. 2006;12(3):294–301. [DOI] [PubMed] [Google Scholar]
- 36.Machado GF, Melo GD, Souza MS, Machado AA, Migliolo DS, Moraes OC, Nunes CM, Ribeiro ÉS.Zymographic patterns of MMP-2 and MMP-9 in the CSF and cerebellum of dogs with subacute distemper leukoencephalitis. Vete Immun Immun opathol. 2013;154(1–2):68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E.Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. [DOI] [PubMed] [Google Scholar]
- 38.Vieira NM, Brandalise V, Zucconi E, Secco M, Strauss BE, Zatz M. Isolation, characterization, and differentiation potential of canine adipose-derived stem cells. Cell Trans. 2010;19(3):279–289. [DOI] [PubMed] [Google Scholar]
- 39.Kisiel a H, McDuffee LA, Masaoud E, Bailey TR, Esparza Gonzalez BP, Nino-Fong R. Isolation, characterization, and in vitro proliferation of canine mesenchymal stem cells derived from bone marrow, adipose tissue, muscle, and periosteum. Am J Vete Res. 2012;73(8):1305–1317. [DOI] [PubMed] [Google Scholar]
- 40.Di Terlizzi R, Platt S.The function, composition and analysis of cerebrospinal fluid in companion animals: Part I – Function and composition. Vete J. 2006;172(3):422–431. [DOI] [PubMed] [Google Scholar]
- 41.Smith PM, Stalin CE, Shaw D, Granger N, Jeffery ND. Comparison of two regimens for the treatment of meningoencephalomyelitis of unknown etiology. J Vete Int Med. 2009;23(3):520–526. [DOI] [PubMed] [Google Scholar]
- 42.Bohn AA, Wills TB, West CL, Tucker RL, Bagley RS. Cerebrospinal fluid analysis and magnetic resonance imaging in the diagnosis of neurologic disease in dogs: a retrospective study. Vete Clini Pathol. 2006;35(3):315–320. [DOI] [PubMed] [Google Scholar]
- 43.de Bakker E, Van Ryssen B, De Schauwer C, Meyer E. Canine mesenchymal stem cells: state of the art, perspectives as therapy for dogs and as a model for man. Vete Quarter. 2013;33(4):225–233. [DOI] [PubMed] [Google Scholar]
- 44.Screven R, Kenyon E, Myers MJ, Yancy HF, Skasko M, Boxer L, Bigley EC, Borjesson DL, Zhu M.Immunophenotype and gene expression profile of mesenchymal stem cells derived from canine adipose tissue and bone marrow. Vete Immun Immunopathol. 2014;161(1–2):21–31. [DOI] [PubMed] [Google Scholar]
- 45.Nantavisai S, Pisitkun T, Osathanon T, Pavasant P, Kalpravidh C, Dhitavat S, Makjaroen J, Sawangmake C.Systems biology analysis of osteogenic differentiation behavior by canine mesenchymal stem cells derived from bone marrow and dental pulp. Scient Rep. 2020;10(1):20703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Humenik F, Cizkova D, Cikos S, Luptakova L, Madari A, Mudronova D, Kuricova M, Farbakova J, Spirkova A, Petrovova E, et al. Canine bone marrow-derived mesenchymal stem cells: genomics, proteomics and functional analyses of paracrine factors. Mol Cell Proteom. 2019;18(9):1824–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F.Concise review: dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.da Silva Meirelles L, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytok Growth Factor Rev. 2009;20(5–6):419–427. [DOI] [PubMed] [Google Scholar]
- 49.McMahill BG, Borjesson DL, Sieber-Blum M, Nolta JA, Sturges BK. Stem cells in canine spinal cord injury – promise for regenerative therapy in a large animal model of human disease. Stem Cell Rev Rep. 2015;11(1):180–193. [DOI] [PubMed] [Google Scholar]
- 50.Barberini DJ, Aleman M, Aristizabal F, Spriet M, Clark KC, Walker NJ, Galuppo LD, Amorim RM, Woolard KD, Borjesson DL. Safety and tracking of intrathecal allogeneic mesenchymal stem cell transplantation in healthy and diseased horses. Stem Cell Res Therapy. 2018;9(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bergman RL, Inzana KD, Inzana TJ. Characterization of matrix metalloproteinase-2 and -9 in cerebrospinal fluid of clinically normal dogs. Am J Vete Res. 2002;63(10):1359–1362. [DOI] [PubMed] [Google Scholar]
- 52.Liuzzi GM, Mastroianni CM, Santacroce MP, Fanelli M, D’agostino C, Vullo V, Riccio P.Increased activity of matrix metalloproteinases in the cerebrospinal fluid of patients with HIV-associated neurological diseases. J NeuroVirol. 2000;6(2):156–163. [DOI] [PubMed] [Google Scholar]
- 53.Zucker S, Hymowitz M, Conner CZarrabi, Hm Hurewitz, An, Matrisian L, Boyd D, Nicolson G, Montana S.Measurement of matrix metalloproteinases and tissue inhibitors of metalloproteinases in blood and tissues: clinical and experimental applications. Anna N Y Acad Sci. 1999;878(1):212–227. [DOI] [PubMed] [Google Scholar]
- 54.Mahmood A, Lu D, Chopp M.Intravenous administration of marrow stromal cells (MSCS) increases the expression of growth factors in rat brain after traumatic brain injury. J Neurot. 2004;21(1):33–39. [DOI] [PubMed] [Google Scholar]
- 55.Chen J, Li YY, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32(4):1005–1011. [DOI] [PubMed] [Google Scholar]
- 56.Leibacher J, Henschler R.Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Therapy. 2016;7(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18(5):683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, Baan CC, Dahlke MH, Hoogduijn MJ. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. 2012;3(SEP):297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eggenhofer E, Luk F, Dahlke MH, Hoogduijn MJ. The life and fate of Mesenchymal stem cells. Front Immunol. 2014;5(MAY):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Argibay B, Trekker J, Himmelreich U, Beiras A, Topete A, Taboada P, Pérez-Mato M, Vieites-Prado A, Iglesias-Rey R, Rivas J, Planas AM, et al. Intraarterial route increases the risk of cerebral lesions after mesenchymal cell administration in animal model of ischemia. Scient Rep. 2017;7(1):40758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang HL, Xie XF, Xiong YQ, Liu SM, Hu GZ, Cao WF, Wu XM. Comparisons of the therapeutic effects of three different routes of bone marrow mesenchymal stem cell transplantation in cerebral ischemic rats. Brain Res. 2018;1680:143–154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-docx-1-cll-10.1177_09636897211034464 for Intrathecal Transplantation of Autologous and Allogeneic Bone Marrow-Derived Mesenchymal Stem Cells in Dogs by Felipe Pérez Benavides, Giovana Boff Araujo Pinto, Marta Cristina Thomas Heckler, Diana Milena Rodríguez Hurtado, Livia Ramos Teixeira, Marina Mitie de Souza Monobe, Gisele Fabrino Machado, Guilherme Dias de Melo, Diego Noé Rodríguez-Sánchez, Fernanda da Cruz Landim e Alvarenga and Rogério Martins Amorim in Cell Transplantation
Supplemental Material, sj-docx-2-cll-10.1177_09636897211034464 for Intrathecal Transplantation of Autologous and Allogeneic Bone Marrow-Derived Mesenchymal Stem Cells in Dogs by Felipe Pérez Benavides, Giovana Boff Araujo Pinto, Marta Cristina Thomas Heckler, Diana Milena Rodríguez Hurtado, Livia Ramos Teixeira, Marina Mitie de Souza Monobe, Gisele Fabrino Machado, Guilherme Dias de Melo, Diego Noé Rodríguez-Sánchez, Fernanda da Cruz Landim e Alvarenga and Rogério Martins Amorim in Cell Transplantation
Supplemental Material, sj-docx-3-cll-10.1177_09636897211034464 for Intrathecal Transplantation of Autologous and Allogeneic Bone Marrow-Derived Mesenchymal Stem Cells in Dogs by Felipe Pérez Benavides, Giovana Boff Araujo Pinto, Marta Cristina Thomas Heckler, Diana Milena Rodríguez Hurtado, Livia Ramos Teixeira, Marina Mitie de Souza Monobe, Gisele Fabrino Machado, Guilherme Dias de Melo, Diego Noé Rodríguez-Sánchez, Fernanda da Cruz Landim e Alvarenga and Rogério Martins Amorim in Cell Transplantation