Abstract
Chimeric antigen receptor (CAR) T-cell therapy is a revolutionary treatment modality used to treat haematological malignancies. Lymphocytes are engineered to produce CARs directed towards tumour cell antigens. Clinical trials have demonstrated impressive malignancy-related outcomes. Unfortunately, numerous off-target effects can cause toxicity-related adverse events in this population, the main being cytokine release syndrome and immune effector cell neurotoxicity syndrome. This causes significant patient morbidity and poor outcomes. Patients who receive CAR T-cell therapy are also profoundly immunosuppressed and often cytopenic, which is caused by a multitude of patient- and treatment-related factors. Thus, infection-related complications are also common in this group. Indeed, up to one third of patients will suffer a serious bacterial infection in the first 30 days after therapy. Viral respiratory tract infection appears to be the most common during the late phase and can be severe; one patient has died of influenza A infection. Fungal infection and cytomegalovirus (CMV) reactivation appear to be uncommon. Although institutional guidelines on infection-prevention strategies are available, there is a dearth of evidence to support their approach. Future research needs to target important unanswered questions that remain in this patient population in order to improve their short- and long-term outcomes.
Keywords: chimeric antigen receptor, immunotherapy, infectious complications
Introduction
Chimeric antigen receptor (CAR) T cells are lymphocytes that have been genetically engineered to produce a CAR specifically directed towards tumour cell antigens.1,2 Early clinical trials of CAR T cells targeting CD19 for B-cell malignancies such as acute lymphocytic leukaemia (ALL) and non-Hodgkin’s lymphoma have demonstrated favourable results.3–5 CAR T-cell therapy currently has US Food and Drug Administration approval for use in diffuse large B-cell lymphoma (axicabtagene ciloleucel and lisocabtagene maraleucel), acute lymphoblastic leukaemia (tisagenlecleucel), mantle cell lymphoma (brexucabtagene autoleucel), and multiple myeloma (idecabtagene vicleucel).6–10 CAR T-cell immunotherapies are also being developed for autoimmune diseases and viral infections.11,12 In particular, CAR T-cell therapy has produced impressive outcomes in patients with relapsed or refractory (R/R) B-cell malignancies. The first CAR T-cell therapeutic tisagenlecleucel was given to 93 adult patients with R/R B-cell lymphoma in a phase II trial and demonstrated a 52% response rate overall.10 Although anti-malignancy results of this therapy have been game changing, they do not come without cost. Various off-target effects are frequently observed, which include cytokine-related toxicity that can cause severe morbidity and mortality. One of the most significant complications of this therapy includes infection and its sequelae related to the multifactorial immune suppression affecting this patient group.
Immunosuppression and infection related to CAR T-cell immunotherapy
Despite an excellent anti-malignancy effect, adverse events with CAR T-cell therapy are problematic and include cytokine release syndrome (CRS) (77–93%), neurotoxicity or neurological events (40–64%), neutropaenia (53–87%) and grade 3 or 4 infections (10–31%).6–8 Some of these ‘off-tumour’ effects can be altered by improving the structure and function of CAR T cells.13
CRS
CRS presents with high grade fever, hypotension, hypoxia and reduced cardiac function and usually manifests within 14 days after CAR T infusion.14–16 As the CAR T cells are highly activated and primed to recognise a target, administration can be associated with significant toxicity, including CRS, immune effector cell neurotoxicity syndrome (ICANS) and macrophage activation syndrome.15,17 Risk factors for CRS development include ALL, disease burden and elevated endothelial activation markers at baseline; CRS may also be very difficult to distinguish from sepsis often prompting empirical antibiotic therapy.18 A prediction model of three-cytokines (interleukin 8 [IL-8], IL-1β and interferon gamma [IFN-γ]) was able to predict life-threatening infection in patients with CRS with high sensitivity and specificity (100%, 82.8%).19 Furthermore, treatment of severe CRS often involves administration of IL-6 inhibitors (e.g. tocilizumab) and high-dose corticosteroids. Although there are theoretical concerns regarding worsening of sepsis and increased rates of infection with tocilizumab, a recently published case-control study demonstrated no association of tocilizumab with increased infection risk after CAR T-cell therapy.20 Another study demonstrated an association between increased risk for first infection and tocilizumab use (unadjusted hazard ratio [HR] 3.45, p = 0.19), but failed to demonstrate a risk with corticosteroid use (unadjusted HR 1.50, p = 0.5).21 One cohort did not observe a correlation between CRS and infectious complications overall.22 Further controlled studies in the CAR T-cell therapy population are required to better define postinfusion risk factors for serious infection.
Prior chemotherapy and treatment regimens
The immune suppression experienced in patients receiving CAR T-cell therapy is multifactorial. Prior chemotherapy (including cytotoxic and lymphocyte-depleting chemotherapy), resulting cytopenias and B-cell depletion result in an increased risk of infection.23–25 The specific lymphodepletion regimen may influence duration of marrow suppression,23 but also influences CAR T expansion kinetics; 26,27 38% of patients had received either an autologous or allogeneic haematopoietic stem cell transplant (HSCT) in one post-hoc analysis of infectious complications.21 Another cohort from Seattle reported 49% receiving a prior autologous HSCT and 55% an allogeneic HSCT.28
Cytopenias
Cytokine-mediated cytopenias are not uncommon and also contribute to bleeding and infection risk in this population, and in severe cases stem cell re-infusion may be considered to mediate prolonged cytopenia.24,25 In one analysis of CAR T-cell therapy patients, a breakdown of pre-infusion immunosuppression demonstrated 13% had severe neutropaenia (<0.5 × 109/L) and 80% had lymphopenia (total lymphocytes <0.2 × 109/L).21 Among patients with complete remission and with no myelodysplastic syndrome post-CAR T-cell therapy, 16% experienced prolonged cytopenias requiring transfusions or growth factor support in one study using lisocabtagene maraleucel.29 Neutropaenia within the first week postinfusion is fairly universal; 86.7% developed febrile neutropaenia within the first 30 days in one study using axicabtagene ciloleucel.22 Another small cohort of patients also using axicabtagene ciloleucel, 80% developed febrile neutropaenia.30 Unfortunately, a causative microorganism is only identified in 20–30% of cases of febrile neutropaenia. As a treatment strategy for febrile neutropaenia in this population it has been advocated that cefepime be avoided due to the overlap between central nervous system (CNS) toxicity with ICANS.31
Hypogammaglobulinaemia
Currently registered agents target CD19+ B lymphocytes and B-cell maturation antigen (BCMA) on plasma cells.6–10 Therefore the infusion itself can cause long-standing hypogammaglobulinaemia secondary to depletion of normal CD19+ B cells.29 In addition, there are emerging data on the use of CD22 as a target for B-cell malignancies.32 Agents targeting the B-cell lineage hold the potential for causing long-term immune system dysfunction and hypogammaglobulinaemia. Indeed, in a series of adult patients with diffuse large B-cell lymphoma (DLBCL) treated with tisagenlecleucel, mean time to onset of sustained B-cell recovery was 6.7 months.33 Moreover, in many of the trials investigating CAR T-cell therapy there was a high prevalence of pre-existing humoral immune deficit. The incidence of hypogammaglobulinaemia following CAR T-cell therapy in adults with DLBCL was approximately 15%;6,10 31–64% of patients required intravenous immunoglobulin replacement in these trials.23
Risk of infection
In the registration trials for tisagenlecleucel, axicabtagene ciloleucel and lisocabtagene maraleucel, infection occurred in 12–55% of patients within the first year, with 23–33% of these being severe.6,10,34 Surprisingly, fatality due to infection was low (⩽3%) with most deaths occurring due to relapsed malignancy. In cohort studies, incidence of all infection events within the first 30 days ranged from approximately 27% to 36% of patients (Table 1).22,35–38 Calculated incidence rate of infection overall within the first 28 days was 2.35 infections per 100 days at risk in one study.37 To date, the majority of reported infections complicating CAR T-cell therapy have been from registration clinical trials. Additional risks to this patient group include the requirement of central venous access for cell collection and for pre- and postinfusion care. The incidence of central line-associated infection has been reported as 80–189 episodes per 100,000 patient-years, although may be higher in more vulnerable patient groups.39,40 Strict line care and early removal in the setting of infection is paramount.
Table 1.
Pooled data of infectious complications from CAR-T cell therapy.
| Reference | CAR T-cell therapy | N | Underlying malignancy | Severity grade | Timepoint | Bacterial infection incidence (n, %) | Viral infection incidence (n, %) | Fungal infection incidence (n, %) |
|---|---|---|---|---|---|---|---|---|
| Abramson et al.34 | Lisocabtagene maraleucel | 269 | R/R B-cell lymphoma | ⩾3 | 12 months | 27/269 (10) | 4/269 (1) | 2/269 (1) |
| Locke et al.38 | Axicabtagene ciloleucel | 108 | Refractory B- cell lymphoma | All | 12 months | 44/108 (40) | 11/108 (10) | 7/108 (6) |
| Logue et al.35 | Axicabtagene ciloleucel | 85 | R/R B-cell lymphoma | All | ⩽30 days | 26/85 (31) | 12/85 (14) | 2/85 (2) |
| >30 days | 13/85 (15) | 19/85 (22) | 0/85 (0) | |||||
| Wittmann Dayagi et al.36 | CD28-based CAR T cells | 88 | R/R B-cell lymphoma | All | ⩽30 days | 22/85 (25) | 14/85 (16) | 0/85 (0) |
| 30–60 days | 8/85 (9) | 2/85 (2) | 1/85 (1) | |||||
| Baird et al.37 | Axicabtagene ciloleucel | 41 | R/R B-cell lymphoma | All | ⩽28 days | 7/41 (17.1) | 8/41 (19.5) | 4/41 (9.8) |
| >28 days | 10/41 (24.4) | 10/41 (24.4) | 9/41 (22) | |||||
| Wudhikarn et al.22 | Axicabtagene ciloleucel OR tisagenlecleucel | 60 | R/R DLBCL | All | ⩽30 days | 20/60 (33) | 10/60 (17) | 1/60 (2) |
| >30 days | 14/60 (24) | 17/60 (28) | 3/60 (5) | |||||
| Hill et al.21 | Anti-CD19 CAR autologous T cells | 133 | ALL, CLL, NHL | All | ⩽28 days | 22/133 (16.5) | 11/133 (8.3) | 4/133 (3) |
| >28 days | 7/119 (5.9) | 11/119 (9.2) | 2/119 (1.7) | |||||
| Munshi et al.41 | Idecabtagene vicleucel | 54 | R/R multiple myeloma | All | 12 months | 13/54 (24) | 15/54 (28) | 4/54 (7) |
ALL, acute lymphocytic leukaemia; CAR, chimeric antigen receptor; CLL, chronic lymphocytic leukaemia; DLBCL, diffuse large B-cell lymphoma; NHL, non-Hodgkin’s lymphoma; R/R, relapsed/refractory.
Bacterial infections
Although infection episodes are common post-CAR T-cell therapy, microbiological diagnosis and confirmation are often difficult with pathogenic organisms only identified in 72% of infection episodes (60% bacterial, 92% viral, 50% fungal) in one study.22 Infections due to bacteria in those receiving CAR T-cell therapy are relatively common overall. A good proportion of patients receive antibacterial treatment and multiple cycles of chemotherapy prior to CAR T-cell infusion thereby severely disrupting the composition of their microbiome. This would increase the risk of multidrug-resistant bacterial colonisation and invasive infection during the neutropaenic phase. Approximately 40% of infections occurring in the first 90 days were deemed severe and 6% life threatening; the majority of these were caused by bacteria. In one study, 22/36 (61%) of early infections (within first 30 days post-CAR T-cell infusion) were bacterial in origin.36 This included common sites such as the bloodstream, genitourinary, lung and soft tissue; Clostridioides difficile infection was also seen (Table 2). Indeed, there appears to be a high rate of C. difficile infection among this group with cohort infection rates ranging from 12.5% to 20%.22,30,35–37 Bacterial infection occurred infrequently after 30 days. In another study, bacterial infection occurred in 17% of patients within the first 28 days after infusion: half of these infection episodes were bloodstream infections including a small minority from multidrug-resistant Gram-negative pathogens.21 A similar rate and time of bacterial infection occurred in a cohort of patients with B-cell ALL treated at Memorial Sloan Kettering Cancer Centre,42 as well as that seen in children and young adults from Seattle Children’s Hospital.28,42 Severe CRS has been associated with bacteraemia (HR 19.97, p < 0.001).21,42 In another cohort study of 85 patients with R/R B-cell lymphoma, early and late bacterial infection had an incidence of 31% and 15%, respectively.35 This included one death due to Streptococcus mitis bloodstream infection. In a cohort of 40 patients, a total of 101 infection-events were noted during a 12-month follow-up period, which included 60 bacterial infections.22 Approximately one third of infections occurred within the first 30 days with the median onset of first bacterial infection occurring at 12 days. An even distribution of organ-specific and bloodstream infections were noted including a life-threatening Escherichia coli biliary sepsis.
Table 2.
Microbiological analysis of bacterial, viral and fungal infections in patients receiving CAR T-cell therapy.
| Reference | Chimeric antigen receptor T-cell therapy | N | Syndromes (n) | Bacteria (n) | Viral (n) | Fungal (n) |
|---|---|---|---|---|---|---|
| Abramson et al.34 | Lisocabtagene maraleucel | 269 | Pneumonia (9) Sepsis and septic shock (6) Urinary tract infection (4) Skin infection (2) Appendicitis and peritonitis (2) |
Clostridioides difficile (2) Streptococcus spp. (3) Enterococcus spp. (1) Listeria monocytogenes (1) Salmonella spp. (1) Staphylococcus spp. (2) |
Coronavirus (1) Cytomegalovirus (1) Parainfluenza virus (1) JC virus (1) |
Candida spp. (2) |
| Locke et al.38 | Axicabtagene ciloleucel | 108 | Pneumonia (19) Upper respiratory tract infection (9) Urinary tract infection (10) Sepsis (1) Cellulitis (1) Osteomyelitis (1) Wound infection (1) |
Clostridioides difficile (9) Klebsiella spp. (1) Staphylococcus spp. (1) Salmonella spp. (1) |
Varicella zoster virus (8) Herpes simplex virus (6) Influenza virus (2) Cytomegalovirus (3) Hepatitis B virus (1) Human herpes virus 6 (1) Parainfluenza virus (1) Rhinovirus (1) |
Candida spp. (4) Aspergillus spp. (1) |
| Logue et al.35 | Axicabtagene ciloleucel | 85 | Upper respiratory tract infection (29) Sepsis (7) Urinary tract infection (4) Cellulitis (2) Pneumonia (6) |
Clostridioides difficile (13) Staphylococcus spp. (4) Streptococcus spp. (1) Enterococcus spp. (1) Escherichia coli (1) Bacteroides spp. (1) |
Rhinovirus (11) Influenza (4) Respiratory syncytial virus (2) |
Candida krusei (1) Fusarium spp. (1) |
| Wittmann Dayagi et al.36 | CD28-based CAR T cells | 88 | Bacteraemia (4) Genitourinary tract infection (3) Pneumonia (5) Soft tissue infection (7) Ear infection (1) |
Clostridioides difficile (3) Staphylococcus spp. (2) Streptococcus spp. (1) Enterococcus spp. (1) Bacteroides fragilis (1) Enterococcus spp. (1) Veillonella spp. (1) Klebsiella spp. (1) Pseudomonas aeruginosa (1) |
Cytomegalovirus (2) Epstein-Barr virus (1) Herpes simplex virus (4) BK virus (2) Enterovirus (1) |
Aspergillus niger (1) |
| Baird et al.37 | Axicabtagene ciloleucel | 41 | Lower respiratory tract infection (8) Bacteraemia (1) Urinary tract infection (6) Pneumonia (1) Cellulitis (3) Diverticulitis (1) |
Clostridioides difficile (3) Escherichia coli (6) Mycobacterium chelonae (1) Campylobacter spp. (1) |
Varicella zoster virus (7) Respiratory viruses (11) Cytomegalovirus (4) BK virus (2) Human herpesvirus 6 (1) |
Pneumocystis jirovecii (3) Candida spp. (6) Mould (1) |
| Wudhikarn et al.22 | Axicabtagene ciloleucel OR tisagenlecleucel | 60 | Cellulitis (4) Urinary tract infection (12) Respiratory tract infection (12) Gastrointestinal tract infection (9) |
Clostridioides difficile (9) Escherichia coli (5) Haemophilus influenzae (3) Staphylococcus spp. (2) Pseudomonas aeruginosa (1) Stenotrophomonas maltophilia (1) |
Rhinovirus (27) Parainfluenza virus (10) Coronavirus (2) Human metapneumovirus (4) Adenovirus (2) Influenza virus (2) Cytomegalovirus (3) Norovirus (1) BK virus (4) Varicella zoster virus (3) |
Pneumocystis jirovecii (1) Aspergillus spp. (1) |
| Hill et al.21 | Anti-CD19 CAR autologous T cells | 133 | Upper respiratory tract infection (9) Lower respiratory tract infection (2) Urinary tract infection (2) |
Staphylococcus aureus (1) Enterococcus faecium (1) Stenotrophomonas maltophilia (1) Fusobacterium spp. (1) |
Rhinovirus (4) Parainfluenza virus (2) Influenza 91) Human metapneumovirus (1) Coronavirus (1) Cytomegalovirus (1) BK virus (1) |
Pneumocystis jirovecii (1) Aspergillus spp. (1) |
Predictors of severe bacterial infection are somewhat intuitive in this cohort. There was an association between severe bacterial infection with severe CRS, neurotoxicity, tocilizumab use, steroid use and bridging therapy (p = 0.007, 0.07, 0.03, 0.004 and 0.02, respectively).35 Having no response to CAR T-cell therapy appears to be a strong predictor of severe bacterial infection. This response to treatment correlates both with the quantity of the risk and with time to first severe bacterial infection. Impaired performance status and history of infections within 30 days before CAR T-cell therapy were also risk factors for severe bacterial infection (HR 3.98).22
Viral infections
Unsurprisingly, viral infections appear commonly in this cohort, particularly in the late phase, often due to many of these patients having received lymphocyte-depleting chemotherapy and experiencing profound hypogammaglobulinaemia. Viral infections typically included respiratory syncytial virus, cytomegalovirus, influenza and polyomaviruses. Overall, the most common late infectious aetiology in this cohort appears to be viral respiratory tract infection.37 Indeed, the majority of late infections (>28 days postinfusion) were respiratory viruses in a number of studied cohorts.35,37,42 This was confirmed in another small study where the majority of viral infections occurred after 30 days, and included one death due to influenza A.22 A high incidence of respiratory viral infection during the late phase may be explained by patients who received CAR T-cell therapy swiftly responding to therapy and transitioning back into the community while still at risk. Incidence of viral infection during this time period ranged from 9.2% to 28%. Viral infections occurred in 14% and 22% of patients during the early and late phases after CAR T-cell therapy, respectively, in one cohort.35 Rhinovirus infection was the predominant viral pathogen. Interestingly, this time difference did not occur universally. In one study, respiratory viruses causing disease occurred in equal frequency during early and late periods postinfusion at approximately 8%.21,42 CMV reactivation appears to be uncommon despite the absence of prophylaxis in many guidelines. In one study only 2/88 (2.3%) patients experienced this (viraemia and pneumonitis, respectively).36 Recently there was a case of prolonged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in a patient who had received CAR T-cell therapy targeting the BCMA.43,44 Despite receiving convalescent plasma and the antiviral remdesivir, the patient had persistently elevated viral RNA throughout illness for more than 2 months before succumbing to the infection. CAR T-cell therapy has been used successfully in individuals with HIV and chronic hepatitis B virus (HBV) infection.30 Patients with HIV, HBV or hepatitis C virus were typically excluded from registration trials for CAR T-cell therapy. This centres around concerns for reactivation and uncontrolled viral replication. Patients with chronic HBV infection appear to tolerate therapy well when receiving antiviral therapy. A cohort of 70 patients from China receiving CAR T-cell therapy demonstrated no significant difference in toxicity and response between patients with and without HBV.45 Herpes viruses CMV, Ebstein Barr virus (EBV) and human herpesvirus 6 (HHV-6) appear to be rare. Data regarding the natural history of CMV and EBV infection after CAR T-cell therapy are non-existent. Progressive multifocal leukoencephalopathy has been reported in a patient with relapsed large B-cell lymphoma 1 year after CAR T-cell therapy.46
Risk factors for viral infection in this population have been examined. Interesting, CD4 and CD8 T-cell counts measured at 30 days were not significantly lower in those who would go on to develop viral infection.35 In one study, patients with low immunoglobulin G (IgG) before lymphocyte-depleting chemotherapy had a higher risk of viral infection after CAR T-cell therapy (HR 5.7).22 Intravenous immunoglobulin (IVIG) replacement did not appear to alter the incidence of infection. Baseline depletion of plasma cells and antibodies prior to CAR T-cell therapy may have more of a critical impact on developing viral-specific neutralising antibodies.22 Long-term preservation of antiviral antibodies after CD19 CAR T-cell therapy has been demonstrated in a cohort of 39 adult patients with B-cell malignancies.47 Moreover, anti-measles IgG levels were sustained in 95% of patients.
Fungal infections
Despite the high degree of multifactorial immune suppression, fungal infection has been infrequently reported in recipients of CAR T-cell therapy. Studies providing detailed analysis of fungal infection remain limited. Typically, institutional guidelines have recommended fluconazole prophylaxis in this group. The majority of fungal infection clusters within the first 30 days and typically occurs in the setting of neutropaenia and/or CRS.31 It is infrequent, and perhaps related to the duration of neutropaenia experienced. Overall, invasive fungal infection has a reported incidence of between 1% and 15%, depending on the study; 0–10% and 0–7% of these are yeast and mould infections, respectively.48 Most fungal infections occur as breakthroughs in patients on either fluconazole or an echinocandin. Of those who do have an invasive fungal infection complicating their CAR T-cell therapy, infection-related mortality is low.28,29 Early Candida spp. infection (predominantly bloodstream) commonly occurred in patients receiving fluconazole prophylaxis. Of mould infections, a variety of species have been observed to cause disease (Aspergillus spp., Fusarium spp., Mucorales, Cunninghamella spp.). predominantly pulmonary. One cohort of 85 adult patients with refractory B-cell lymphoma reported only two with fungal infection (both fungal infections due to Candida krusei and Fusarium spp.), both of which died.35 One case of Coccidioides infection has been reported which occurred more than 1 year after CAR T-cell infusion.22 Another cohort of 85 adult and paediatric patients documented only one fungal infection that occurred between 30 days and 60 days post-therapy.36 Late invasive fungal infection typically occurred in patients who had persistent risk factors including neutropaenia and corticosteroid use. Of 40 patients with R/R DLBCL who received CAR T-cell therapy and were followed up for 1 year, only two fungal infections were reported.22 One patient had invasive pulmonary Aspergillosis and the other patient had Pneumocystis jirovecii pneumonia (PJP), both occurring after 30 days. Only three patients have been reported to have had PJP thus far in the literature reflecting the widespread use and effectiveness of prophylaxis strategies.21,29,31 One patient has died from severe Candida glabrata pancolitis and Aspergillus fumigatus pulmonary infection.49 This was likely secondary to profound granulocyte colony stimulating factor (G-CSF) refractory neutropaenia lasting more than 50 days as well as prolonged high-dose corticosteroids for CRS.
Many of the traditional risk factors for invasive fungal infection in haematological malignancy and HSCT patients are common in CAR T-cell recipients. More specifically, prolonged neutropaenia and lymphopaenia are likely to contribute to a durable and high cumulative risk.48 Treatment-experienced patients with a high net state of immunosuppression often have multiple risk factors present and may have a higher overall risk of fungal infection when compared with those who have received CAR T cells early after cancer diagnosis. Tocilizumab, as a treatment for CRS, is unlikely to be a major contributor to risk of fungal disease.
Infection prevention strategies
With the growing use of CAR T-cell therapy globally, institutional guidelines regarding infection prevention and prophylaxis are becoming increasingly available. However, currently there remains no consensus approach to prophylactic strategies in this group and recommendations are largely based on expert opinion and extrapolated evidence from other high-risk groups (e.g. those receiving autologous HSCT). With regards to antimicrobial prophylaxis, some differences and controversies exist. The advantages of antibacterial prophylaxis in CAR T-cell therapy have not been properly evaluated and the use of quinolone prophylaxis remains contentious. The 2018 Infectious Diseases Society of America and American Society of Clinical Oncology guidelines recommend quinolone prophylaxis for patients who are expected to develop profound or protracted neutropaenia and many have included the CAR T-cell therapy population in this group.50 Despite many patients having febrile neutropaenia following therapy, the rate of true infections related to neutropaenia was low in one study.7 Recently developed guidelines from Australia have advocated against the use of antibacterial prophylaxis with a fluoroquinolone.51 Of the guidelines advocating for its use, many recommend cessation once severe neutropaenia has resolved (absolute neutrophil count [ANC] >0.5 × 109/L).52 Universally, herpes simplex virus (HSV) and varicella zoster virus (VZV) antiviral prophylaxis with either acyclovir or valaciclovir is recommended for between 6 months and 12 months postinfusion.51–53 Similarly, it has been strongly advocated that trimethoprim-sulfamethoxazole be used for PJP prophylaxis for a similar time period.36 Some institutions provide PJP and VZV prophylaxis for a minimum of 6 months or until recovery of the CD4 count to over 200 cells/mm3.35 The level of CD4 suppression after CAR T-cell therapy may require prolonged or alternative prophylaxis strategies (e.g. to cover Mycobacterium avium-intracellulare). Patients with latent tuberculosis infection or chronic HBV infection should receive targeted prophylaxis.51 The risk factors for fungal infection in this cohort are not completely defined and therefore there is no consensus about the optimal choice and duration of antifungal prophylaxis after CAR T-cell therapy. Moreover, it is not currently known whether certain subgroups of CAR T-cell recipients may benefit from anti-mould prophylaxis. Guidelines suggest fluconazole or micafungin prophylaxis against Candida spp. during neutropaenia is a good approach.4,5 This is similar to the approach taken for autologous HSCT. A guideline from the European Society for Blood and Marrow Transplantation recommends mould-active azole prophylaxis in patients with prior allogeneic HSCT, prior invasive aspergillosis and those receiving corticosteroids (e.g. prednisolone ⩾20 mg/day for ⩾2 weeks). Similarly, Australian guidelines recommend posaconazole use if there is prolonged neutropaenia, high-dose steroids use or allogeneic HSCT with prior invasive fungal infection.51 Other groups have suggested commencing antifungal prophylaxis only when ANC <0.5 × 109/L.
Indications for prophylactically replacing IgG in the context of CAR T-cell therapies also suffer from the absence of high level data to support decision making. In one study there were no differences in the incidence and spectrum of infections overall despite considerable variability in indications for IgG replacement between treating institutions. In order to manage hypogammaglobulinaemia some experts have suggested screening for serum IgG prior to, and in the first 3 months post-CAR T-cell therapy for B-cell malignancies and to consider prophylactic replacement in patients with both IgG levels ⩽4 g/L and persistent or recurrent bacterial infection.23 Moreover, in patients with B-cell aplasia but normal IgG levels, replacement can be considered if there are repeated episodes of infection or if there is objective evidence of humoral immune dysfunction (e.g. poor response to vaccination).23 Interestingly, one cohort study found that IgG levels at 30 days did not correlate with future infection risk.35
Assessing vaccination strategies in this population has not been performed to date. Some experts have supported the revaccination of patients starting at 3 months post-CAR T-cell therapy (analogous to autologous transplant).51
Future considerations
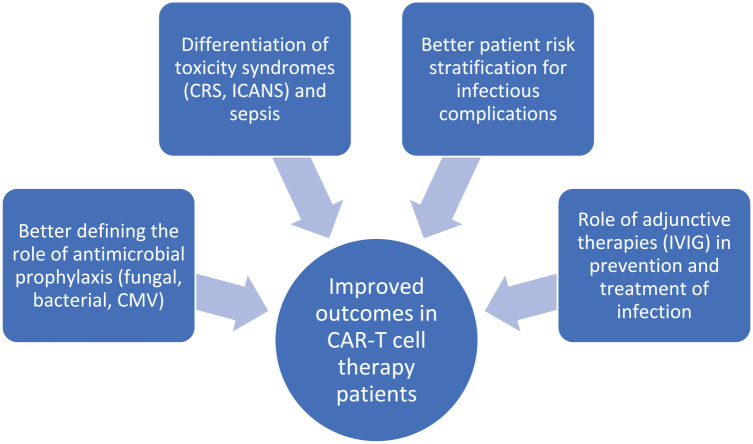
Many unanswered questions remain as to the supportive management of patients receiving CAR T-cell therapy (Figure 1). Moreover, newer generations of CAR T cells are being developed and it remains unclear what the adverse event profile and infection risk will be with each new product and construct. Data on the infection risk with currently available therapies remain limited by low patient numbers, narrow selection of patients and single centre sampling of cohorts. In addition, there are insufficient data on long-term complications and outcomes in this patient population. As this therapy is used more widely, earlier in the course of treatment and for different conditions in the future, additional data on the risk of infection and other toxicities will need to be obtained to better inform prevention strategies in individual groups.
Figure 1.
Current research priorities to improve infectious complications of CAR T-cell therapy.
CAR, chimeric antigen receptor; CMV, cytomegalovirus; CRS, cytokine release syndrome; ICANS, immune effector cell neurotoxicity syndrome; IVIG, intravenous immune globulin.
Prioritising key research questions that will have direct patient impact is critical. Determining the role of antifungal (particularly against moulds) and CMV prophylaxis is important, especially in those with a prior history of invasive infection. Stratification of risk using patient and treatment factors may prove to be useful in a targeted approach to antimicrobial prophylaxis. Improved characterisation of toxicity syndromes including CRS, prolonged neutropaenia and the role of infection in patients suffering from these is crucial in improving management strategies and outcomes (e.g. potential overuse of antimicrobials, growth factors and corticosteroids). Improved rapid non-culture-based diagnostics in microbiology, in addition to the better characterisation of the ‘cytokine signature’ of CRS and the effects on the stem-cell niche, may provide solutions to these problems. Given that the majority of patients suffer from hypogammaglobulinaemia, determining whether administration of IVIG improves infection-related outcomes will also be useful. Standardised approaches to infection prophylaxis are required for this group of patients who have a high burden of infectious risk and a unique immunobiology underpinning this risk.
Conclusion
CAR T-cell therapy provides a revolutionary treatment modality for patients suffering from R/R haematological malignancies. The incidence of therapy-related toxicity is high and contributes to patient morbidity. Infectious complications are similarly high and can potentially be mitigated by prevention strategies including antimicrobial prophylaxis, IVIG and early vaccination. High level data are lacking on short and long infectious complications and how best to prevent them. Institutional guidelines which are largely informed by expert opinion are currently available to support clinical decision making. Targeted research addressing critical questions pertaining to patient outcomes is needed in order to provide optimal care.
Footnotes
Author Contributions: AGS provided conceptualisation, initial manuscript draft and revisions. Both AGS and ASH provided editorial input and contributed to manuscript revision, writing, reviewing and editing, and all authors approved the final version of the manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Adam G. Stewart  https://orcid.org/0000-0002-7262-7529
https://orcid.org/0000-0002-7262-7529
Contributor Information
Adam G. Stewart, Centre for Clinical Research, Faculty of Medicine, The University of Queensland, Royal Brisbane and Women’s Hospital Campus, Brisbane, QLD 4029, Australia.
Andrea S. Henden, Department of Haematology and Bone Marrow Transplantation, Royal Brisbane and Women’s Hospital, Brisbane, AustraliaQIMR Berghofer Institute of Medical Research, Brisbane, QLD, Australia
References
- 1.Kochenderfer JN, Rosenberg SA.Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol 2013; 10: 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson LA, June CH.Driving gene-engineered T cell immunotherapy of cancer. Cell Res 2017; 27: 38–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain T, Bar M, Kansagra AJ, et al. Use of chimeric antigen receptor T cell therapy in clinical practice for relapsed/refractory aggressive B cell non-Hodgkin lymphoma: an expert panel opinion from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant 2019; 25: 2305–2321. [DOI] [PubMed] [Google Scholar]
- 4.Kansagra AJ, Frey NV, Bar M, et al. Clinical utilization of Chimeric Antigen Receptor T-cells (CAR-T) in B-cell Acute Lymphoblastic Leukemia (ALL) – an expert opinion from the European Society for Blood and Marrow Transplantation (EBMT) and the American Society for Blood and Marrow Transplantation (ASBMT). Bone Marrow Transplant 2019; 54: 1868–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kansagra AJ, Frey NV, Bar M, et al. Clinical utilization of chimeric antigen receptor T cells in B cell acute lymphoblastic leukemia: an expert opinion from the European Society for Blood and Marrow Transplantation and the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2019; 25: e76–e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017; 377: 2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018; 378: 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2020; 382: 1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med 2019; 380: 1726–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2019; 380: 45–56. [DOI] [PubMed] [Google Scholar]
- 11.Sadeqi Nezhad M, Seifalian A, Bagheri N, et al. Chimeric antigen receptor based therapy as a potential approach in autoimmune diseases: how close are we to the treatment? Front Immunol 2020; 11: 603237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zmievskaya E, Valiullina A, Ganeeva I, et al. Application of CAR-T cell therapy beyond oncology: autoimmune diseases and viral infections. Biomedicines 2021; 9: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu S, Yi M, Qin S, et al. Next generation chimeric antigen receptor T cells: safety strategies to overcome toxicity. Mol Cancer 2019; 18: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014; 6: 224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brudno JN, Kochenderfer JN.Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 2016; 127: 3321–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 2019; 25: 625–638. [DOI] [PubMed] [Google Scholar]
- 17.Siegler EL, Kenderian SS.Neurotoxicity and cytokine release syndrome after chimeric antigen receptor T cell therapy: insights into mechanisms and novel therapies. Front Immunol 2020; 11: 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zahid A, Siegler EL, Kenderian SS.CART cell toxicities: new insight into mechanisms and management. Clin Hematol Int 2020; 2: 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo H, Wang N, Huang L, et al. Inflammatory signatures for quick diagnosis of life-threatening infection during the CAR T-cell therapy. J Immunother Cancer 2019; 7: 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frigault MJ, Nikiforow S, Mansour MK, et al. Tocilizumab not associated with increased infection risk after CAR T-cell therapy: implications for COVID-19? Blood 2020; 136: 137–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill JA, Li D, Hay KA, et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood 2018; 131: 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wudhikarn K, Palomba ML, Pennisi M, et al. Infection during the first year in patients treated with CD19 CAR T cells for diffuse large B cell lymphoma. Blood Cancer J 2020; 10: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill JA, Giralt S, Torgerson TR, et al. CAR-T – and a side order of IgG, to go? – immunoglobulin replacement in patients receiving CAR-T cell therapy. Blood Rev 2019; 38: 100596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain T, Knezevic A, Pennisi M, et al. Hematopoietic recovery in patients receiving chimeric antigen receptor T-cell therapy for hematologic malignancies. Blood Adv 2020; 4: 3776–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fried S, Avigdor A, Bielorai B, et al. Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transplant 2019; 54: 1643–1650. [DOI] [PubMed] [Google Scholar]
- 26.Ninomiya S, Narala N, Huye L, et al. Tumor indoleamine 2,3-dioxygenase (IDO) inhibits CD19-CAR T cells and is downregulated by lymphodepleting drugs. Blood 2015; 125: 3905–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirayama AV, Gauthier J, Hay KA, et al. The response to lymphodepletion impacts PFS in patients with aggressive non-Hodgkin lymphoma treated with CD19 CAR T cells. Blood 2019; 133: 1876–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vora SB, Waghmare A, Englund JA, et al. Infectious complications following CD19 chimeric antigen receptor T-cell therapy for children, adolescents, and young adults. Open Forum Infect Dis 2020; 7: ofaa121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cordeiro A, Bezerra ED, Hirayama AV, et al. Late events after treatment with CD19-targeted chimeric antigen receptor modified T cells. Biol Blood Marrow Transplant 2020; 26: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abbasi A, Peeke S, Shah N, et al. Axicabtagene ciloleucel CD19 CAR-T cell therapy results in high rates of systemic and neurologic remissions in ten patients with refractory large B cell lymphoma including two with HIV and viral hepatitis. J Hematol Oncol 2020; 13: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haidar G, Garner W, Hill JA.Infections after anti-CD19 chimeric antigen receptor T-cell therapy for hematologic malignancies: timeline, prevention, and uncertainties. Curr Opin Infect Dis 2020; 33: 449–457. [DOI] [PubMed] [Google Scholar]
- 32.Killock D.Anti-CD22 CAR T cells in ALL. Nat Rev Clin Oncol 2020; 17: 391. [DOI] [PubMed] [Google Scholar]
- 33.Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med 2017; 377: 2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 2020; 396: 839–852. [DOI] [PubMed] [Google Scholar]
- 35.Logue JM, Zucchetti E, Bachmeier CA, et al. Immune reconstitution and associated infections following axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma. Haematologica 2021; 106: 978–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wittmann Dayagi T, Sherman G, Bielorai B, et al. Characteristics and risk factors of infections following CD28-based CD19 CAR-T cells. Leuk Lymphoma 2021; 62: 1692–1701. [DOI] [PubMed] [Google Scholar]
- 37.Baird JH, Epstein DJ, Tamaresis JS, et al. Immune reconstitution and infectious complications following axicabtagene ciloleucel therapy for large B-cell lymphoma. Blood Adv 2021; 5: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol 2019; 20: 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 2006; 355: 2725–2732. [DOI] [PubMed] [Google Scholar]
- 40.Patel AR, Patel AR, Singh S, et al. Central line catheters and associated complications: a review. Cureus 2019; 11: e4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munshi NC, Anderson LD, Jr, Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med 2021; 384: 705–716. [DOI] [PubMed] [Google Scholar]
- 42.Park JH, Romero FA, Taur Y, et al. Cytokine release syndrome grade as a predictive marker for infections in patients with relapsed or refractory B-cell acute lymphoblastic leukemia treated with chimeric antigen receptor T cells. Clin Infect Dis 2018; 67: 533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hensley MK, Bain WG, Jacobs J, et al. Intractable COVID-19 and prolonged SARS-CoV-2 replication in a CAR-T-cell therapy recipient: a case study. Clin Infect Dis. Epub ahead of print> 28 January 2021. DOI: 10.1093/cid/ciab072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abbasi J.Prolonged SARS-CoV-2 infection in a CAR T-cell therapy recipient. JAMA 2021; 325: 924. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Liu Y, Tan X, et al. Safety and efficacy of chimeric antigen receptor (CAR)-T-cell therapy in persons with advanced B-cell cancers and hepatitis B virus-infection. Leukemia 2020; 34: 2704–2707. [DOI] [PubMed] [Google Scholar]
- 46.Mian A, Andrapalliyal N, Weathers AL, et al. Late occurrence of progressive multifocal leukoencephalopathy after anti-CD19 chimeric antigen receptor T-cell therapy. Eur J Haematol 2021; 106: 584–588. [DOI] [PubMed] [Google Scholar]
- 47.Hill JA, Krantz EM, Hay KA, et al. Durable preservation of antiviral antibodies after CD19-directed chimeric antigen receptor T-cell immunotherapy. Blood Adv 2019; 3: 3590–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garner W, Samanta P, Haidar G.Invasive fungal infections after anti-CD19 chimeric antigen receptor-modified T-cell therapy: state of the evidence and future directions. J Fungi (Basel) 2021; 7: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rejeski K, Kunz WG, Rudelius M, et al. Severe Candida glabrata pancolitis and fatal Aspergillus fumigatus pulmonary infection in the setting of bone marrow aplasia after CD19-directed CAR T-cell therapy – a case report. BMC Infect Dis 2021; 21: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taplitz RA, Kennedy EB, Flowers CR.Antimicrobial prophylaxis for adult patients with cancer-related immunosuppression: ASCO and IDSA clinical practice guideline update summary. J Oncol Pract 2018; 14: 692–695. [DOI] [PubMed] [Google Scholar]
- 51.Bupha-Intr O, Haeusler G, Chee L, et al. CAR-T cell therapy and infection: a review. Expert Rev Anti Infect Ther 2021; 19: 749–758. [DOI] [PubMed] [Google Scholar]
- 52.Yakoub-Agha I, Chabannon C, Bader P, et al. Management of adults and children undergoing chimeric antigen receptor T-cell therapy: best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE). Haematologica 2020; 105: 297–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gudiol C, Lewis RE, Strati P, et al. Chimeric antigen receptor T-cell therapy for the treatment of lymphoid malignancies: is there an excess risk for infection? Lancet Haematol 2021; 8: e216–e228. [DOI] [PubMed] [Google Scholar]