Abstract
Objective:
In the context of the opioid epidemic, there is value in examining the use of opioids in specific cancer patient cohorts. We analyzed opioid use in patients undergoing adjuvant therapy for oral cavity cancer to define the incidence of new persistent use beyond 3 months.
Study Design:
Retrospective.
Setting:
Comprehensive academic cancer center.
Subjects and Methods:
We performed a retrospective IRB-approved analysis of opioid use in patients who received adjuvant radiotherapy with or with concurrent systemic therapy for surgically resected oral cavity cancer between 2003 and 2016. Factors associated with opioid use were evaluated by Chi-square test and one-way ANOVA. The Kaplan-Meier method was used to estimate overall survival.
Results:
Of 77 identified patients, 10 (13%) patients received opioid prescriptions at 3 months or greater following completion of radiotherapy. Patients who were opioid naive prior to surgery required significantly fewer opioid prescriptions than intermittent or chronic opioid users. No specific factors were associated with new persistent opioid use.
Conclusions:
Patients undergoing surgery and adjuvant radiotherapy for oral cavity cancer who required opioids for cancer treatment related pain are at minimal risk for new dependency. Judicious pain management should be applied for patients with a history of prior opioid use. Larger patient cohorts will be needed to identify patient, disease, and treatment characteristics associated with new persistent use given its limited incidence.
Keywords: Oral cavity cancer, opioids, pain, radiotherapy, surgery
Introduction
The prevalence of pain at time of diagnosis for patients with cancers of the head and neck has been estimated at 40–84% (1–5) with the highest pain intensity reported by those with advanced disease with primary tumors of the oral cavity and oropharynx (5,6). Heterogeneity of tumor burden, proximity and access to cranial nerves, variation in the extent of surgical resection, and differences in pain evaluation have revealed a range of acute post-operative pain assessments ranging from 30–76% (7, 8). In the chronic setting, 25% of patients will report pain for up to at least 2 years after surgery (1).
Opioids are the cornerstone of management of severe pain in patients with cancer (9). A comprehensive study of opioid use for pain control in patients undergoing surgery for non-head and neck malignancies demonstrated new persistent opioid use in 7–11% and 15–21% in patients not receiving or receiving adjuvant chemotherapy, respectively (10). Similarly, evaluation of chronic opioid use in patients with various head and neck cancers undergoing curative intent therapy was 13% (11). The diversity of patient and disease characteristics as well as differences in treatment in these studies limits their applicability to specific disease sites, which maintain distinct patient characteristics, tumor-related pain syndromes, and treatment-induced toxicities.
Given the severity of pain associated with head and neck cancers, especially advanced tumors of the oral cavity, this patient population has a high likelihood of being prescribed opioids for long periods throughout their management. This raises the potential risk of creating opioid dependence. Indeed, a recent retrospective study reported chronic opioid use in 41% of patients undergoing curative surgery for oral cavity squamous cell carcinoma (OCSCC) (12). Furthermore, the addition of adjuvant radiotherapy with or without chemotherapy following surgery for OCSCC may intensify and prolong the pain related to cancer treatment and increases the number of potential prescribers. Thus, this population of patients is a selected subset of individuals at higher risk of becoming new persistent opioid users.
Time from surgery to completion of RT is associated with overall survival in patients with OCSCC (13–16). Therefore, the use of narcotics to optimize pain control in order to prevent treatment delays may play a role in patient outcomes. In light of the conflicting priorities of minimizing the potential for new persistent opioid use and controlling pain to enable timely completion of cancer therapy, we evaluated the use of opioids in patients undergoing adjuvant therapy following curative-intent surgery for OCSCC.
Methods
We performed an IRB-approved retrospective analysis of opioid prescribing patterns and clinical outcomes of patients identified in our institutional head and neck cancer database who received adjuvant radiotherapy with or without concurrent systemic therapy for resected OCSCC between 2003 and 2016. Patient and disease characteristics, including smoking and alcohol use, treatments rendered, and clinical outcomes were obtained from the database. Patients’ electronic medical records were reviewed to collect information regarding type of opioid prescribed, the number and dates of prescriptions, and the number of providers. Patients whose narcotic use was documented in the physician note but not recorded in their electronic medical record with dates of initiation and/or completion were excluded from analysis.
Opioid use
Patients were categorized as naïve, intermittent, or chronic users of opioids, using previously published criteria (10). Opioid naïve patients were defined as those who had no opioid prescriptions between 12 months and 31 days prior to surgery. Patients who were chronic users were those that filled at least 120 days’ worth of supply between 12 months and 31 days prior to surgery or documented at least 3 prescriptions in the 90 days prior to surgery. Intermittent users were those whose narcotic prescription pattern fell between naïve and chronic users. Narcotic use was presumed to be associated with cancer treatment and management was designated as prescriptions written within 30 days pre-operatively, as well as those during and 30 days immediately after radiotherapy. Persistent opioid use was defined as consecutive prescription dates no longer than 2 months apart. New persistent opioid use was defined as consecutive prescriptions written 90 days after completion of radiotherapy in patients who were previously opioid naïve.
Statistical analysis
Chi-square test and one-way ANOVA were used to test independence of association between patient factors and opioid use. The Kaplan-Meier method was used to estimate overall survival.
Results
We identified 77 patients with OCSCC managed with curative intent surgery (see table 1). Fifty-one (66.2%) patients received adjuvant RT and 27 patients (34.6%) were managed with adjuvant chemoradiotherapy. The median follow-up was 36.9 months (range 5.5–117.5 months). Oral tongue was the most common oral cavity subsite (41%). The majority of patients had pT4 disease (57%) and were node positive (62%). Tobacco smoking and alcohol use was reported by 70% and 83% of patients, respectively. Nearly 90% of patients were opioid naïve.
Table 1.
Baseline patient and treatment characteristics
| Patient characteristics | number (%) |
|---|---|
| Age (median) | 61 years |
| Sex | |
| Male | 45 (58) |
| Female | 32 (42) |
| Oral cavity subsite | |
| Alveolar ridge | 12 (15.6) |
| Buccal mucosa | 7 (9.1) |
| Floor of mouth | 15 (19.5) |
| Hard palate | 1 (1.3) |
| Lip | 1 (1.3) |
| Oral tongue | 32 (41.6) |
| Retromolar trigone | 9 (11.7) |
| T-stage (AJCC 7th edition) | |
| T1 | 11 (14.3) |
| T2 | 19 (24.7) |
| T3 | 4 (5.2) |
| T4 | 43 (55.8) |
| N-stage (AJCC 7th edition) | |
| N0 | 29 (37.7) |
| N1 | 7 (9.1) |
| N2a | 0 (0) |
| N2b | 32 (41.6) |
| N2c | 9 (11.7) |
| N3 | 0 (0) |
| Type of narcotic user | |
| aNaïve | 69 (89.6) |
| bIntermittent | 6 (7.8) |
| cChronic | 2 (2.6) |
| Tobacco (smoking) history | |
| Non-smoker | 21 (27.3) |
| Current smoker or quit < 1 month prior | 33 (42.9) |
| Quit > 1 month prior | 1 (1.3) |
| Quit > 1 year prior | 4 (5.2) |
| Quit > 5 years prior | 4 (5.2) |
| Quit > 15 years prior | 13 (16.9) |
| No data on smoking history | 1 (1.3) |
| Pack-years, mean (s.d.) | 31.1 (32.8) |
| Alcohol history | |
| None | 2 (2.6) |
| dSocial | 24 (30.8) |
| eConsistent | 16 (20.5) |
| fHigh volume | 19 (24.4) |
| Previously high but now none | 6 (7.7) |
| No data on alcohol use | 11 (14.1) |
| Radiation dose (median) | 63 Gy |
| Type of Adjuvant Therapy | |
| Adjuvant radiotherapy | 51 (66.2) |
| Adjuvant chemoradiotherapy | 26 (33.8) |
no opioid prescription between 12 and 1 months prior to surgery,
between naïve and chronic,
opioid prescription filled at least 12 and 1 month prior to surgery or filled at least 3 prescriptions in the 3 months prior to surgery,
0–6 drinks per week,
7–20 drinks per week,
21 or more drinks per week
Among patients that were naïve, intermittent, or chronic opioid users prior to surgery, there was a positive correlative relationship with the number of prescriptions provided during treatment, as well as the duration of opioid use. There was also a difference in the time from surgery to initiation of adjuvant therapy between these three groups of patients. We did not detect a relationship between the groups with respect to the number of prescription providers, nor in the duration of adjuvant therapy (see table 2).
Table 2.
Impact of prior opioid use on future opioid prescriptions and radiotherapy timing
| All patients median (range) | Naïve median (range) | Intermittent median (range) | Chronic median (range) | p-value | |
|---|---|---|---|---|---|
| Number of scripts | 4 (1–50) | 3 (1–31) | 7 (1–50) | 15 (13–16) | 0.03 |
| Duration of opioid use (days) | 54 (1–532) | 37 (1–532) | 110 (1–474) | 289 (265–313) | 0.03 |
| Number of providers | 2 (1–15) | 2 (1–15) | 5 (1–120 | 5 (5–5) | 0.07 |
| Time from surgery to start of RT | 49 (21–107) | 49 (21–107) | 41 (35–58) | 81 (78–84) | 0.03 |
| Duration of RT (days) | 42 (32–65) | 42 (32–65) | 42.5 (41–63) | 41.5 (39–44) | 0.41 |
RT=radiotherapy
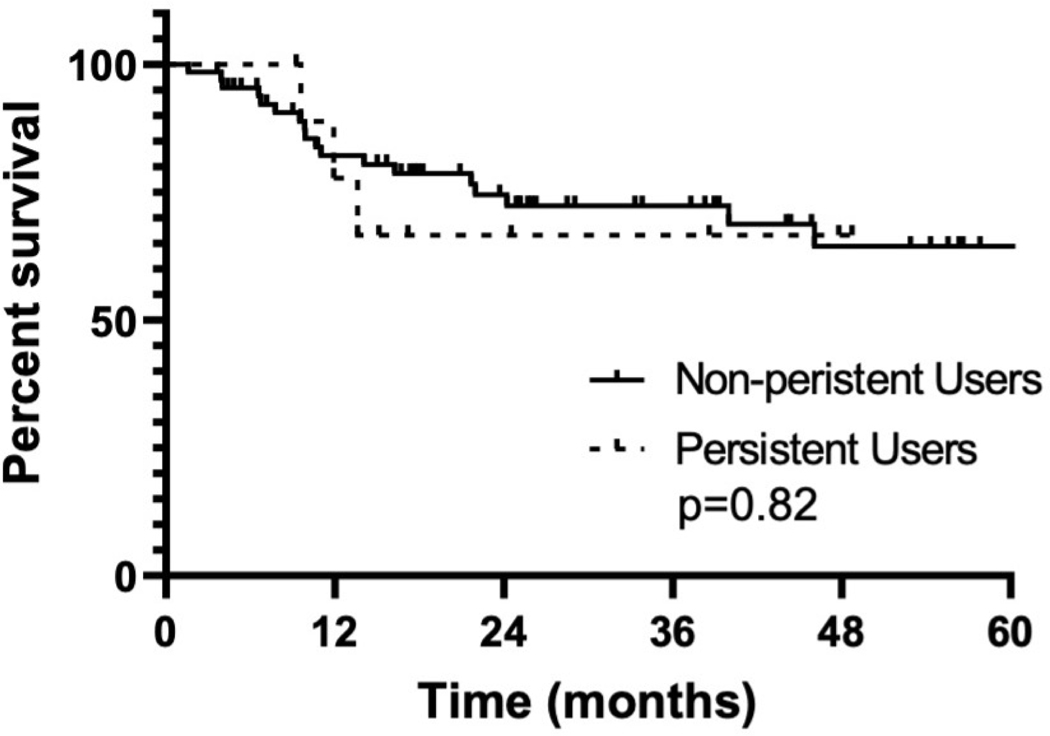
Of the entire 77 patient cohort, 13% (n=10) of patients were new persistent users, all of which were opioid naïve, none being prior intermittent users. There were no significant associations between new persistent opioid use and primary tumor site, smoking history, alcohol consumption, T-stage, N-stage, type of adjuvant therapy (radiotherapy versus chemoradiotherapy) or type of neck dissection (bilateral versus unilateral) (seevtable 3). Estimated 3-year overall survival for non-persistent users was 64.5% (95% CI: 47.8%–75.5%) and new persistent opioids users was 66.7% (95% CI: 28.2%–87.8%) (p = 0.82) (see figure 1).
Table 3.
Association of non-opioid related factors and new persistent opioid use
| Not persistent number, (%) | New Persistent Number, (n) | p-value | |
|---|---|---|---|
| Oral cavity subsite | 0.73 | ||
| Alveolar ridge | 12 (17.9) | 0 (0) | |
| Buccal mucosa | 6 (8.9) | 1 (10) | |
| Floor of mouth | 12 (17.9) | 3 (30) | |
| Hard palate | 1 (1.5) | 0 (0) | |
| Lip | 1 (1.5) | 0 (0) | |
| Oral tongue | 27 (40.3) | 5 (50) | |
| Retromolar trigone | 8 (11.9) | 1 (10) | |
| T-stage (AJCC 7th edition) | 0.73 | ||
| T1 | 9 (13.4) | 2 (20) | |
| T2 | 17 (25.4) | 2 (20) | |
| T3 | 3 (4.5) | 1 (10) | |
| T4 | 38 (56.7) | 5 (50) | |
| N-stage (AJCC 7th edition) | 0.73 | ||
| N0 | 25 (37.3) | 4 (40) | |
| N1 | 6 (8.9) | 1 (10) | |
| N2a | 0 (0) | 0 (0) | |
| N2b | 28 (41.8) | 4 (40) | |
| N2c | 8 (11.9) | 1 (10) | |
| N3 | 0 (0) | 0 (0) | |
| Tobacco (smoking) history | 0.184 | ||
| Non-smoker | 21 (31.8) | 0 (0) | |
| Current smoker or quit < 1 month prior | 26 (39.4) | 7 (70) | |
| Quit > 1 month prior | 1 (1.5) | 0 (0) | |
| Quit > 1 year prior | 4 (6.1) | 0 (0) | |
| Quit > 5 years prior | 4 (6.1) | 0 (0) | |
| Quit > 15 years prior | 10 (15.2) | 3 (30) | |
| Alcohol history | 0.41 | ||
| None | 2 (3.4) | 1 (10) | |
| Social | 22 (37.3) | 2 (20) | |
| Consistent | 15 (25.4) | 1 (10) | |
| High volume | 16 (27.1) | 3 (30) | |
| Previously high but now none | 4 (6.8) | 2 (20) | |
| Adjuvant treatment | 0.73 | ||
| Radiotherapy | 44 (65.7) | 6 (60) | |
| Chemoradiotherapy | 23 (34.3) | 4 (40) | |
| Type of neck dissection | 0.68 | ||
| None | 3 (4.4) | 0 (0) | |
| Unilateral | 54 (79.4) | (90) | |
| Bilateral | 11 (16.2) | 1 (10) |
Figure 1.
Kaplan-Meier estimated 3-year overall survival for non-persistent users (64.5% (95% CI: 47.8%–75.5%)) and new persistent opioid users (66.7% (95% CI: 28.2%–87.8%)), p = 0.82
Discussion
The time from initiation of adjuvant RT following surgery (14) and the duration of adjuvant RT (13) impacts the clinical outcomes of patients with OCSCC. Moreover, time from surgery to completion of adjuvant therapy of greater than 14 weeks has been associated with worse overall survival (17). A significant challenge to achieving these goals is adequate pain management. Beyond topical and over-the-counter oral analgesics, opioids form the foundation for severe pain management (9). Retrospective reviews have reported that the most severe pain for patients with head and neck cancers is associated with advanced disease especially arising from primary tumors of the oral cavity (5,6). Given the potential addicting properties of opioids combined with pain management needs, patients with OCSCC undergoing surgery and adjuvant therapy are at risk for developing long term opioid dependency.
In the present study, we demonstrate that the percentage of patients continuing opioids at three months or greater following completion of radiotherapy was 13%. This is similar to data found in cancer patients undergoing treatment for head and neck cancers from various primary tumor sites (11) as well as therapy for malignancies arising in other anatomic sites (10). Patients that were opioid naive prior to treatment were prescribed significantly less opioids than those with a prior history of opioid prescriptions. There was a difference in time from surgery to initiation of adjuvant therapy amongst patients that were either naïve, intermittent, and chronic opioid users. This was driven by pre-surgery chronic pain patients who represented < 3% of the entire cohort making it difficult to draw conclusions from this finding. There was no difference in the time to receive adjuvant therapy between the cohorts. We did not identify patient, disease, or treatment factors that were associated with new persistent use. The median time from surgery to completion of radiotherapy for the entire cohort was 13 weeks. These data suggest that judicious use of opioids results in a minimal risk of new persistent use and may aid in the delivery of adjuvant therapy within a favorable timeframe for patients with locally advanced OCSCC.
The data presented here contrast with outcomes reported in a recent publication of patients undergoing surgery with or without adjuvant therapy for OCSCC (12) in which 41% of the entire cohort required opioids more than 90 days after surgery. Of those patients, nearly 25% were new persistent users. Here we report only 13% of patients became new persistent users. These differences persisted despite more advanced primary tumors and regional disease and all patients receiving at least bimodality (surgery and adjuvant radiotherapy) and approximately 25% receiving trimodality therapy (surgery and adjuvant chemoradiotherapy). Smoking (18) and alcohol use (11), which have been demonstrated in prior studies to be associated with opioid use, showed minimal if no impact on persistent opioid use in either study. Given the small number of patients evaluated and found to be new persistent users, this may reflect a lack of power to identify a difference. The primary difference between the patient cohorts was the number of chronic users prior to surgery, which was associated with increased opioid prescription use in both studies. Hyperalgesia is a well described phenomenon for chronic opioid use (19), which is not accounted for in this study, but undoubtedly contributes to the clinical picture. It is unclear whether the increased opioid prescriptions for patients with previous use reflects this phenomenon, or use of opioids for other chronic pain conditions (19). These findings support cautious pain management approaches in patients with a history of chronic opioid use.
Previous data suggests that patients with OCSCC who are chronic users of opioids exhibit worse clinical outcomes (12). A possible rationale is that dysregulation of the immune system by opioids may enable tumors to escape immune-dependent tumoricidal activity (20). Other hypotheses suggest that opioids may result in tumor growth by activating the mu opioid receptors located on tumors, which can increase angiogenesis (21). There also is data to support worse outcomes with increasing doses of intra-operative opioid use (22,23). Here we did not identify a difference in overall survival in patients that were non-persistent users compared to new persistent users. The differences in reports likely represent a lack of power and would require larger datasets to answer this question definitively.
Despite similar new persistent use of opioids identified in this study compared to others of approximately 10–15% (10,11), strategies to reduce this risk should be pursued given their potential negative side-effects. As there is a neuropathic component to toxicities arising from treating OCSCC with surgery and radiotherapy, medications that block these signals may reduce pain and mitigate the need for opiates. Indeed, a recent randomized trial in patients with head and neck cancer receiving chemoradiotherapy demonstrated a reduction in opioid use with high-dose gabapentin compared to low dose gabapentin (24). However, pain continued to worsen over time in both treatment arms suggesting the need for further research in managing pain for patient with head and neck cancer receiving definitive treatment.
This study is limited by its sample size. Our ability to identify factors associated with new persistent use was limited given the small number of patients who required opioid prescriptions 3 months from the end of radiotherapy. Finally, we used opioid prescriptions as a surrogate use of opioid use. Without available records documenting a positive opioid drug test, the prescription surrogate was the best data available.
We demonstrate that persistent opioid use in patients with oral cavity squamous cell carcinoma undergoing curative intent surgery and adjuvant therapy is 13% which is similar to that reported by patients undergoing definitive treatment for various head and neck cancers (11). Caution should be taken when deciding about pain management approaches for patients with a recent history of opioid use given their increased propensity for use of these medications beyond cancer therapy. Future attempts at identifying novel agents beyond opioids should be pursued. However, given the strong association between the time of treatment and overall survival and the minimal risk of new persistent use and uncertain impact on overall survival, opioids should be used when clinically necessary.
Acknowledgements
This work was supported in part by the NIH/NCI P30 CA014520-UW Comprehensive Cancer Center Grant and NIH P50 DE026787-UW Head and Neck SPORE Grant. Heather Geye for maintaining the Head and Neck cancer database. The authors declare that they have no conflict of interest. Informed consent was obtained from each individual participant involved in this study. This study was conducted in accordance with the 1964 Declaration of Helsinki and its subsequent amendments. There were no animals involved in this study.
References
- 1.Chaplin JM, Morton RP. A prospective, longitudinal study of pain in head and neck cancer patients. Head Neck 1999;21(6):531–7. [DOI] [PubMed] [Google Scholar]
- 2.Epstein JB, Stewart KH. Radiation therapy and pain in patients with head and neck cancer. Eur J Cancer B Oral Oncol 1993;29B(3):191–9. [DOI] [PubMed] [Google Scholar]
- 3.Keefe FJ, Manuel G, Brantley A, Crisson J. Pain in the head and neck cancer patient: Changes over treatment. Head Neck Surg 1986;8(3):169–76. [DOI] [PubMed] [Google Scholar]
- 4.Portenoy RK, Lesage P. Management of cancer pain. Lancet 1999;353(9165):1695–700. [DOI] [PubMed] [Google Scholar]
- 5.Saxena A, Gnanasekaran N, Andley M. An epidemiological study of prevalence of pain in head and neck cancers. Indian J Med Res.1995;102:28–33. [PubMed] [Google Scholar]
- 6.Cuffari L, Tesseroli de Siqueira JT, Nemr K, Rapaport A. Pain complaint as the first symptom of oral cancer: a descriptive study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;102(1):56–61. [DOI] [PubMed] [Google Scholar]
- 7.Frenette L. The acute pain service. Crit Care Clin 1999;15(1):143–50. [DOI] [PubMed] [Google Scholar]
- 8.Svensson I, Sjostrom B, Haljamae H. Influence of expectations and actual pain experiences on satisfaction with postoperative pain management. Eur J Pain 2001;5(2):125–33. [DOI] [PubMed] [Google Scholar]
- 9.Mandala M, Moro C, Labianca R, Cremonesi M, Barni S. Optimizing use of opiates in the management of cancer pain. Ther Clin Risk Manag 2006;2(4):447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JS, Hu HM, Edelman AL, Brummett CM, Englesbe MJ, Waljee JF, et al. New persistent opioid use among patients with cancer after curative-intent surgery. J Clin Oncol 2017;35(36):4042–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith WH, Luskin I, Resende Salgado L, Scarborough BM, Lin JY, Ozbek U, et al. Risk of prolonged opioid use among cancer patients undergoing curative intent radiation therapy for head and neck malignancies. Oral Onco. 2019;92:1–5. [DOI] [PubMed] [Google Scholar]
- 12.Pang J, Tringale KR, Tapia VJ, Moss WJ, May ME, Furnish T, et al. Chronic opioid use following surgery for oral Cavity cancer. JAMA Otolaryngol Head Neck Surg 2017;143(12):1187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujiwara RJ, Judson BL, Yarbrough WG, Husain Z, Mehra S. Treatment delays in oral cavity squamous cell carcinoma and association with survival. Head Neck 2017;39(4):639–46. [DOI] [PubMed] [Google Scholar]
- 14.Langendijk JA, de Jong MA, Leemans CR, de Bree R, Smeele LE, Doornaert P, et al. Postoperative radiotherapy in squamous cell carcinoma of the oral cavity: the importance of the overall treatment time. Int J Radiat Oncol Biol Phys 2003;57(3):693–700. [DOI] [PubMed] [Google Scholar]
- 15.Suwinski R, Sowa A, Rutkowski T, Wydmanski J, Tarnawski R, Maciejewski B. Time factor in postoperative radiotherapy: a multivariate locoregional control analysis in 868 patients. Int J Radiat Oncol Biol Phys 2003;56(2):399–412. [DOI] [PubMed] [Google Scholar]
- 16.Vikram B. Importance of the time interval between surgery and postoperative radiation therapy in the combined management of head & neck cancer. Int J Radiat Oncol Biol Phys 1979;5(10):1837–40. [DOI] [PubMed] [Google Scholar]
- 17.Goel AN, Frangos MI, Raghavan G, Lazaro SL, Tang B, Chhetri DK, et al. The impact of treatment package time on survival in surgically managed head and neck cancer in the United States. Oral Oncol 2019;88:39–48. [DOI] [PubMed] [Google Scholar]
- 18.Bastian LA, Driscoll MA, Heapy AA, Becker WC, Goulet JL, Kerns RD, et al. Cigarette smoking status and receipt of an opioid prescription among veterans of recent wars. Pain Med 2017;18(6):1089–97. [DOI] [PubMed] [Google Scholar]
- 19.Boudreau D, Von Korff M, Rutter CM, Saunders K, Ray GT, Sullivan MD, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf 2009;18(12):1166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boland JW, McWilliams K, Ahmedzai SH, Pockley AG. Effects of opioids on immunologic parameters that are relevant to anti-tumour immune potential in patients with cancer: a systematic literature review. Br J Cancer 2014;111(5):866–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singleton PA, Moss J, Karp DD, Atkins JT, Janku F. The mu opioid receptor: A new target for cancer therapy? Cancer 2015;121(16):2681–8. [DOI] [PubMed] [Google Scholar]
- 22.Cata JP, Keerty V, Keerty D, Feng L, Norman PH, Gottumukkala V, et al. A retrospective analysis of the effect of intraoperative opioid dose on cancer recurrence after non-small cell lung cancer resection. Cancer Med 2014;3(4):900–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cata JP, Zafereo M, Villarreal J, Unruh BD, Truong A, Truong DT, et al. Intraoperative opioids use for laryngeal squamous cell carcinoma surgery and recurrence: a retrospective study. J Clin Anesth 2015;27(8):672–9. [DOI] [PubMed] [Google Scholar]
- 24.Hermann GM, Iovoli AJ, Platek AJ, Wang C, Miller A, Attwood K, et al. A single-institution, randomized, pilot study evaluating the efficacy of gabapentin and methadone for patients undergoing chemoradiation for head and neck squamous cell cancer. Cancer 2020;126(7):1480–91. [DOI] [PMC free article] [PubMed] [Google Scholar]