Abstract
Leiomyosarcoma is a rare aggressive malignant mesenchymal tumour, accounting for 1% of all uterine malignancies. It spreads rapidly through the intraperitoneal and haematogenous pathways. It is often diagnosed postoperatively following myomectomy, hysterectomy or supracervical hysterectomy for presumed benign disease. It has a predilection for perimenopausal women with a median age of 50 years. Individuals may describe symptoms of vaginal or abdominal pressure. Physical examination may reveal a large palpable pelvic mass, which may haemorrhage. Surgery remains the mainstay of treatment. Hysterectomy and a bilateral salpingo-oophorectomy may be considered, depending on the individual’s menopausal status. Ovarian preservation can be considered in young patients. Adjuvant systemic treatment and radiotherapy are of no benefit. Gemcitabine/docetaxel and doxorubicin have shown benefit in the treatment of advanced or recurrent disease. The authors present the case of a 44-year-old woman with lower abdominal pain, vaginal bleeding and a uterine fibroid. Laboratory investigations confirmed a leucocytosis, neutrophilia and a thrombocythaemia. Further investigation with an MRI pelvis showed a very large, heterogeneous, malignant appearing pelvic mass compressing the right ureter and it appeared uterine in nature. Her staging CT showed multiple lung metastases. The diagnosis of uterine leiomyosarcoma was subsequently established. Due to the aggressive behaviour of this sarcoma subtype, novel early detection strategies and targeted therapies are required.
Keywords: cancer - see oncology, gynecological cancer
Background
The most common histological subtype of uterine sarcoma includes leiomyosarcomas, smooth muscle tumours expressing desmin, h-caldesmon and smooth muscle actin.1 They are highly aggressive with a poor prognosis. They spread by the haematogenous route and metastasise primarily to the lung. Uterine leiomyosarcoma is challenging to diagnose preoperatively. It can mimic the appearance of benign uterine leiomyomas, otherwise known as a fibroid, and can present with heavy menstrual bleeding, pelvic pain and a palpable mass.
It is often an incidental histological finding following removal of the uterus or a single leiomyoma without morcellation. It is relatively resistant to chemotherapy and radiotherapy; however, targeted therapies with trabectedin, pazopanib or olaratumab offer future promise.
Due to its rarity and histopathological diversity, efficacious therapies to achieve prolonged survival or cure in those with both early and advanced-stage uterine leiomyosarcoma have remained elusive.
Case presentation
A 44-year-old woman, UK resident, was referred to the gynaecology department from the medical ward with lower abdominal pain, a history of intermittent vaginal bleeding for 12 months and a 1 stone weight loss over 6 weeks. She was gravida 12 and para 3 (three normal vaginal deliveries, nine early miscarriages). One year earlier, she attended the gynaecology department as she was unable to palpate the threads of her Mirena intrauterine device (IUD). Physical examination followed by an ultrasound scan (USS) showed no evidence of her IUD. Further investigation with an abdominal radiograph showed no evidence of an extrauterine IUD and it was thought likely that she had unwittingly expelled it. Her cervical smear was unremarkable. Her general practitioner (GP) confirmed the diagnosis of microcytic anaemia thought likely due to her menorrhagia. She commenced treatment with norethisterone 5 mg/day, tranexamic acid 1 g four times a day and ferrous sulphate 200 mg. She was referred to the ‘One Stop Menstrual Disorders Clinic’ for further investigation. She was a smoker with a 20-year pack history. A transvaginal ultrasound scan revealed a posterior myometrial heterogeneous area 58×51×56 mm, likely a subserosal fibroid impinging on the endometrium (figure 1). She was admitted to hospital with a severe vaginal haemorrhage and her haemoglobin (Hb) dropped to 29 g/dL. She required 4 units of packed red blood cells (PBCs). The haematologist advised vitamin K 10 mg intravenously and to repeat her full blood count as well as her clotting, including fibrinogen. Fresh frozen plasma was considered after the 4th unit of PBCs. In addition, she received an iron infusion (ferinject/ferric carboxymaltose). She was investigated for a suspected malignancy. Hysteroscopy was performed using a cervical block and histology was normal. She commenced treatment with a (gonadotropin-releasing hormone) GnRh analogue, in this case, prostap and was placed on the waiting list for a hysterectomy. She was recently admitted under the medical team with a urinary tract infection and sepsis. She was treated with nitrofurantoin and trimethoprim in the community. She received intravenous tazobactam 4.5 g three times a day. Her inflammatory markers improved and her antimicrobial therapy was changed to intravenous ceftriaxone and intravenous metronidazole. Her observations were stable: temperature 36.5°C, heart rate 70 bpm, blood pressure (BP) 120/90 mm Hg, Spo2 98% on air and respiratory rate 14. Physical examination revealed a large, palpable abdominal pelvic mass, and the uterine fundus extended beyond the umbilicus. Speculum and bimanual examination showed a normal cervix with blood collecting in the vagina. Respiratory examination confirmed decreased air entry bilaterally.
Figure 1.
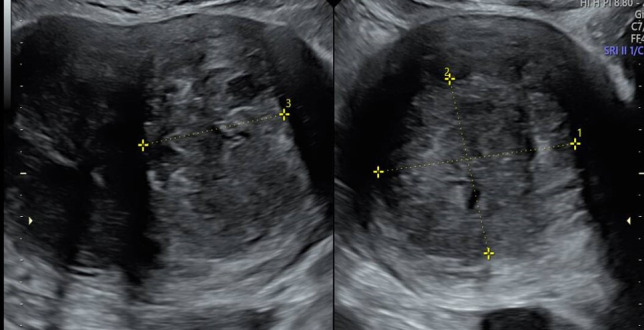
There appeared to be a heterogeneous area within the posterior myometrium measuring 58×51×56 mm suggestive of a subserosal fibroid that appeared to impinge on the endometrium.
Investigations
Laboratory investigations showed a microcytic anaemia (Hb 82 g/dL, mean cell volume (MCV) 72 fl). She had an elevated white cell count (38.9×109/L), a neutrophilia 35.8×109/L and a thrombocythaemia (1031×109/L) (table 1). As she had unprotected sexual intercourse since starting her prostap, a urine pregnancy test was requested. Her bHCG was elevated at
Table 1.
Laboratory investigations showed a leucocytosis and a thrombocythaemia
| Haematology | Biochemistry |
| Haemoglobin 82 g/L (118–148) | Sodium 137 mmol/L (133–146) |
| White cell count 38.9×109/L (3.5–11) | Potassium 4.2 mmol/L (3.5–5.3) |
| Platelet count 1031×109/L (150–400) | Urea 4 mmol/L (1.0–7.8) |
| Mean cell volume 72 fl (80–100) | Creatinine 60 umol/L (50-130) |
| Neutrophil count 35.8×109/L (2–7.5) | eGFR>90 mL/min/1.73m2>60 |
| Monocyte count 0.9×109/L (0.2–0.8) | Alkaline phosphatase 185 U/L (30–300) |
| Eosinophil count 0.6×109/L (0.0–0.4) | Alanine transaminase 7 U/L (<21) |
| Basophil count 0.2×109/L (0.0–0.2) | Bilirubin<3 umol/L (<35) |
| Clotting | Albumin 37 g/L (30-50) |
| APTT 28.3 s (30–40 s) | C reactive protein 56 mg/L (<5) |
| Prothrombin time 13.2 s (10–14 s) | Gamma GT 83 U/L (<40) |
APTT, activated partial thromboplastin time; eGFR, estimated glomerular filtration rate; GGT, gamma-glutamyl transferase.
53 (range 1–10) raising the suspicion of a paraneoplastic condition or an ectopic pregnancy. However, it was difficult to establish her last menstrual period due to her ongoing bleeding.
A repeated transvaginal (TV) USS showed her posterior myometrium fibroid, which had increased in size and now measured 71×58×68 mm and her fundal myometrium fibroid measured 64×76×52 mm. The echotexture was heterogeneous and the appearances were suggestive of a degenerating fibroid (figure 2). The endometrium was completely obscured by the fibroids and could not be well assessed, however, no evidence of an intrauterine gestation sac or an ectopic was seen. Neither ovary was definitively identified. Neither adnexae was well visualised due to the fibroids, however, no obvious adnexal mass or free fluid was seen. Due to her abnormal blood test results and decreased air entry on auscultation, a chest radiograph was performed. It showed a normal heart and mediastinal outline. Multiple small nodular densities were seen in both of her lungs, not present on her chest radiograph in May 2020.
Figure 2.
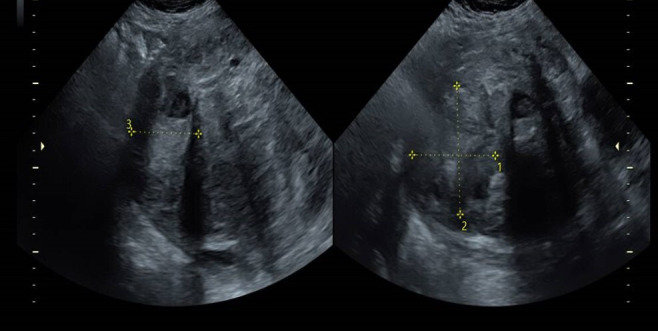
The posterior myometrium fibroid measured 71×58×68 mm. The fundal myometrium fibroid measured 64×76×52 mm. The appearances were suggestive of a degenerating fibroid.
The differential diagnosis included multiple pulmonary nodules including tumour deposits, small abscesses and connective tissue diseases (figure 3). An MRI pelvis was requested to further delineate her pelvic mass. Unfortunately, the patient was unable to tolerate the study very well, therefore, only a T2 sagittal and a coronal section were performed. A huge heterogeneous mass measuring 21×15×19 cm, centred on and replacing the uterus and cervix, was observed (figure 4). It was difficult to delineate the endometrial cavity. It exhibited an intermediate heterogeneous signal on T2 and had a well-defined border. Extensive metastatic lymphadenopathy was present with multiple enlarged pelvic and para-aortic lymph nodes, for example, a common iliac node (67×41 mm) and a para-aortic node (48×33 mm). A confluent right pelvic sidewall mass measuring 12.8×8.5 cm was present. Cranially this mass filled the space between the right paravertebral region and the psoas muscle. Right hydronephrosis was present, likely due to compression from the mass. The visualised bowel appeared unremarkable. The ovaries were not clearly seen. A trace of ascites was noted. The differential diagnosis included a uterine or cervical malignancy or lymphoma with nodal metastasis. A staging CT of the neck, thorax, abdomen and pelvis was performed. Small neck, axillary and mediastinal lymph nodes not enlarged by CT criteria were identified.
Figure 3.
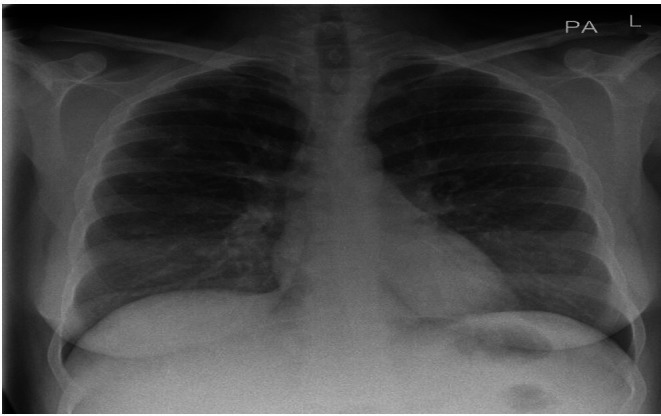
Her chest radiograph showed multiple small nodular densities in both of her lungs. The differential diagnosis included multiple pulmonary nodules including tumour deposits, small abscesses and connective tissue diseases. L, left side; PA, posteroanterior.
Figure 4.
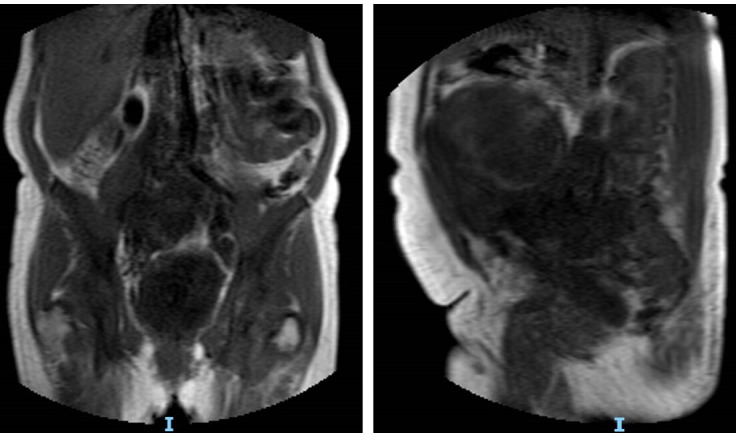
Further investigation with a MRI pelvis demonstrated a huge heterogeneous mass measuring 21×15×19 cm, centred on and replacing the uterus and cervix was observed.
Within the abdomen, several para-aortic and iliac lymph nodes with a large malignant appearing nodal mass in the right side of the pelvis (120×65 mm) surrounding the internal and external iliac arteries were noted. Multiple pulmonary nodules scattered throughout the lungs in keeping with metastases were observed. A mixed solid cystic mass lesion within the uterus and extending to the cervical regions was present (figure 5). No focal liver lesions were demonstrated. In addition, minor nodularity of the omentum was noted raising the suspicion of omental deposits. The bowel appeared normal.
Figure 5.
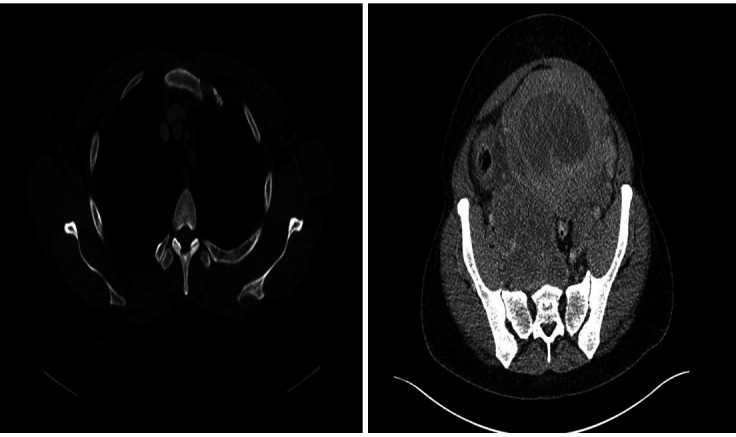
A staging CT chest, abdomen and pelvis showed a large mediastinal lymph node in the anterior mediastinum (8 mm) and a right pelvic nodal mass lesion (120×65 mm). A mixed solid cystic mass lesion within the uterus was observed.
Following discussion with interventional radiology, it was decided that the retroperitoneal nodes were amenable to percutaneous image-guided biopsy. The lung metastasis also represented an appropriate target for a biopsy. Given the diagnostic uncertainty, the multidisciplinary team (MDT) meeting agreed to pursue a biopsy from the lymph nodes of the lung metastasis.
Differential diagnosis
Histopathological assessment confirmed the diagnosis of stage 4 leiomyosarcoma with pulmonary metastasis and extensive metastatic lymphadenopathy.
Treatment
She continued treatment with ferrous sulphate 200 mg three times a day to correct her microcytic anaemia. She received tranexamic acid 1 g to help with her vaginal bleeding. She continued treatment with prostap/provera 400 mg/day and low-molecular weight heparin, 5000 units/day.
Outcome and follow-up
Following discussion at the MDT, it was concurred that she had inoperable disease, and due to her poor performance status, she was not suitable to undergo chemotherapy. Therefore, she commenced palliative care. She died 8 weeks following her diagnosis.
Discussion
Uterine sarcomas account for 3%–7% of all uterine cancers, and leiomyosarcoma is the most common subtype, occurring in 1%–2% of cases. It has a predilection for perimenopausal women aged >40 years. It presents with a plethora of symptoms including abnormal vaginal bleeding (56%), a palpable pelvic mass (54%) and pelvic pain or pressure (22%).2 A further cohort of individuals are asymptomatic. Other recognised risk factors for its development include tamoxifen, a uterine oestrogen receptor agonist and radiation therapy.
Leiomyosarcomas are typically voluminous tumours with a mean diameter of 10 cm. The cut surface is typically soft, bulging, fleshy, necrotic, haemorrhagic and lacks the prominent whorled appearance of leiomyomas. The year 2009 heralded the advent of a new FIGO staging system for uterine sarcomas and has two divisions, one for leiomyosarcoma and endometrial stromal sarcoma and one for adenosarcoma.3 Histologically, they exhibit hypercellularity, severe nuclear atypia and high mitotic rate (>15 mitotic figures per 10 high-power-fields).4 Smooth muscle markers such as desmin, h-caldesmon, smooth muscle actin and histone deacetylase 8 are usually expressed. They are often immunoreactive for CD10 and epithelial markers including keratin and epithelial membrane antigen. Conventional leiomyosarcomas express oestrogen, progesterone and androgen receptors in 30%–40% of cases. Overexpression of p16 and high levels of Ki67 have been described.5 The vast majority of uterine leiomyosarcomas are sporadic and individuals with germline mutations in fumarate hydratase are believed to be at an increased risk.6 In addition, leiomyoma variants may mimic malignancy and include the following: mitotically active leiomyoma, cellular leiomyoma, haemorrhagic leiomyoma and hormone-induced changes, atypical leiomyoma, myxoid leiomyoma, epithelioid leiomyoma and leiomyoma with massive lymphoid infiltration.
A risk score for uterine leiomyosarcoma (uLMS) has been developed.7 Moreover, one or more supportive clinicopathologic features such as peri or postmenopausal age, extrauterine extension, large size (>10 cm), infiltrating border, necrosis and atypical mitotic figures are frequently present. Neither preoperative imaging with ultrasonography nor PET scans are capable of differentiating between benign or malignant smooth muscle masses. Patients with suspected or confirmed leiomyosarcoma (LMS) should have their uterus removed en bloc with a total abdominal hysterectomy or tumour debulking if present outside the uterus, with maximal effort to avoid intraoperative rupture, morcellation (intraperitoneal dissemination of disease) or spillage of tumour into the peritoneal cavity. Ovarian preservation may be considered in premenopausal patients with early stage disease.
There is no valid data on the use of endocrine therapies, for example, letrozole or anastrozole in the management of metastatic LMS. Oestrogen and progesterone receptor expression have been identified in 40%–80% of cases. Trabectedin is now approved in advanced and recurrent LMS.8 It has been suggested that radiation therapy may be of benefit in cases of unresectable locoregional relapse, however, a trial performed by the European Organisation for Research and Treatment for Cancer concluded that it did not improve local or distant progression rates and had no impact on survival.9 10
Considering palliative single chemotherapy, there are only few effective substances, (eg, ifosfamide, gemcitabine, doxorubicin) with moderate remission rates of 15%–25%.11 The multitargeted tyrosine kinase inhibitor pazopanib may induce disease stabilisation in metastatic disease.12 The platelet derived growth factor (PDGFR) antibody olaratumab has shown significant prolonged disease-free survival, prompting an accelerated FDA approval of its use in soft tissue sarcoma.13
Leiomyosarcomas are associated with a poor prognosis even when confined to the uterus at the time of diagnosis.4 14 Recurrence rates range from 53% to 71% and common sites include the lung, liver, pelvic lymph nodes, vertebral and long bones.7 First recurrences occur in the lungs in 40% of patients and in the pelvis in only 13%.8 Overall, 5-year survival rate ranges from 15% to 25%.9 Due to the rarity and heterogeneity of these tumours, there are no suitable biomarkers or treatment methods and the past few decades have not seen marked improvements in survival. Further large prospective studies are required using risk stratification models and multivariate analysis. Future efforts should focus on better defining the molecular aetiology of uterine leiomyosarcoma in order to make advances in the care of patients with this disease.
Learning points.
Uterine leiomyosarcomas are rare aggressive tumours, with high recurrence rates. They typically spread haematogenously and are most commonly diagnosed in women in their perimenopausal years. Presenting symptoms may be vague and mimic other benign uterine conditions, such as a leiomyoma. However, fibroids rarely develop into cancer and the risk is <0.1%.
Surgery remains the mainstay of treatment. Ovarian preservation may be considered in premenopausal patients with early stage disease.
Due to aggressive tumour biology and relative chemotherapy and radiotherapy resistance, efficacious therapies have been elusive.
Footnotes
Contributors: LD: wrote the case report. GS: edited the case report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Prat J, Mbatani N. Uterine sarcomas. Int J Gynaecol Obstet 2015;131 Suppl 2:S105–10. 10.1016/j.ijgo.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 2.Giuntoli RL, Metzinger DS, DiMarco CS, et al. Retrospective review of 208 patients with leiomyosarcoma of the uterus: prognostic indicators, surgical management, and adjuvant therapy. Gynecol Oncol 2003;89:40–69. 10.1016/s0090-8258(03)00137-9 [DOI] [PubMed] [Google Scholar]
- 3.Prat J. Figo staging for uterine sarcomas. Int J Gynaecol Obstet 2009;104:177–8. 10.1016/j.ijgo.2008.12.008 [DOI] [PubMed] [Google Scholar]
- 4.Oliva E, Carcangiu ML, Carinelli SG, et al. Mesenchymal tumours [chapter 5: tumours of the uterine corpus]. In: WHO classification of tumours of female reproductive organs. 4th edn. Lyon: IARC, 2014: 135–47. [Google Scholar]
- 5.Silverberg SG, Major FJ, Blessing JA, et al. Carcinosarcoma (malignant mixed mesodermal tumor) of the uterus. a gynecologic Oncology group pathologic study of 203 cases. Int J Gynecol Pathol 1990;9:1–19. 10.1097/00004347-199001000-00001 [DOI] [PubMed] [Google Scholar]
- 6.Ylisaukko-oja SK, Kiuru M, Lehtonen HJ, et al. Analysis of fumarate hydratase mutations in a population-based series of early onset uterine leiomyosarcoma patients. Int J Cancer 2006;119:283–7. 10.1002/ijc.21798 [DOI] [PubMed] [Google Scholar]
- 7.Köhler G, Belau A, Krichbaum J. Deutsches klinisches Kompetenzzentrum für genitale sarkome und mischtumoren an der universitätsmedizin greifswald [DKMS] und kooperationspartner VAAO deutschland und FK Frankfurt/Sachsenhausen. In: Datenbank und Promotionsgruppe genitale Sarkome Greifswald, 2015. [Google Scholar]
- 8.Ricci S, Stone RL, Fader AN. Uterine leiomyosarcoma: epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol Oncol 2017;145:208–16. 10.1016/j.ygyno.2017.02.019 [DOI] [PubMed] [Google Scholar]
- 9.Reed NS, Mangioni C, Malmström H, et al. Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: an European organisation for research and treatment of cancer gynaecological cancer Group study (protocol 55874). Eur J Cancer 2008;44:808–18. 10.1016/j.ejca.2008.01.019 [DOI] [PubMed] [Google Scholar]
- 10.Wright JD, Seshan VE, Shah M, et al. The role of radiation in improving survival for early-stage carcinosarcoma and leiomyosarcoma. Am J Obstet Gynecol 2008;199:536.e1–536.e8. 10.1016/j.ajog.2008.04.019 [DOI] [PubMed] [Google Scholar]
- 11.Look KY, Sandler A, Blessing JA, et al. Phase II trial of gemcitabine as second-line chemotherapy of uterine leiomyosarcoma: a gynecologic oncology group (GOG) study. Gynecol Oncol 2004;92:644–7. 10.1016/j.ygyno.2003.11.023 [DOI] [PubMed] [Google Scholar]
- 12.van der Graaf WTA, Blay J-Y, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879–86. 10.1016/S0140-6736(12)60651-5 [DOI] [PubMed] [Google Scholar]
- 13.Tobias A, O'brien MP, Agulnik M. Olaratumab for advanced soft tissue sarcoma. Expert Rev Clin Pharmacol 2017;10:699–705. 10.1080/17512433.2017.1324295 [DOI] [PubMed] [Google Scholar]
- 14.D'Angelo E, Spagnoli LG, Prat J. Comparative clinicopathologic and immunohistochemical analysis of uterine sarcomas diagnosed using the world Health organization classification system. Hum Pathol 2009;40:1571–85. 10.1016/j.humpath.2009.03.018 [DOI] [PubMed] [Google Scholar]


