Abstract
Objective
The main aim of the study was to evaluate the association between non-alcoholic fatty liver disease (NAFLD), estimated by fatty liver index (FLI), and the development of type 2 diabetes (T2D) in a large cohort of adult workers with pre-diabetes.
Design
Prospective cohort study.
Setting
Occupational health services from Spain.
Participants
16 648 adult workers (aged 20–65 years) with pre-diabetes (fasting plasma glucose (FPG) of 100–125 mg/dL).
Outcome and measures
FLI was calculated based on measurements of triglycerides, body mass index, waist circumference and γ-glutamyltransferase. The population was classified into three categories: FLI<30 (no hepatic steatosis), FLI 30–60 (intermediate status) and FLI>60 (hepatic steatosis). Sociodemographic, anthropometric, dietary habits, physical activity and clinical data were collected from all subjects. The incidence rate of T2D was determined after 5 years of follow-up.
Results
After 5 years of follow-up, 3706 of the 16 648 participants (22.2%) were diagnosed with T2D, corresponding to an annual rate of progression of 4.5%. FLI was strongly associated with T2D conversion. The incidence rates of T2D in the FLI<30, FLI 30–60 and FLI>60 groups were significantly different after 5 years of follow-up were 19/6,421 (0.3%), 338/4,318 (7.8%) and 3,349/5,909 (56.7%), respectively. This association remained significant for FLI>60 after adjustment for, age, diet, physical activity, FPG, blood pressure, social class and smoking habits (adjusted HR=6.879; 95% CI 5.873 to 8.057 for men, and HR=5.806; 95% CI 4.863 to 6.932 for women).
Conclusion
NAFLD assessed by FLI independently predicted the risk of conversion to T2D among people with pre-diabetes. FLI may be an easily determined and valuable early predictor for T2D in people with pre-diabetes. FLI-based assessment of NAFLD in subjects with pre-diabetes in routine clinical practice could allow the adoption of effective measures to prevent and reduce their progression to T2D.
Keywords: diabetes & endocrinology, nutrition & dietetics, primary care, occupational & industrial medicine
Strengths and limitations of this study.
This is a prospective study, with large sample size and 5-year follow-up.
Study participants had multiple occupations and were from several geographical locations.
The study sample included only adult workers; therefore, the results cannot be generalised to the general population.
Introduction
Type 2 diabetes (T2D) is closely associated with a constellation of metabolic comorbidities, including obesity, hypertension, hypercholesterolaemia, dyslipidaemia and non-alcoholic fatty liver disease (NAFLD).1 The main characteristic of NAFLD is the infiltration of hepatocytes by free fatty acids and triglycerides (TGs) not related to significant alcohol intake. NAFLD is an entity that encompasses a wide spectrum of lesions ranging from indolent liver fat storage followed by lipotoxicity,2 to hepatic inflammation, also known as non-alcoholic steatohepatitis (NASH). NAFLD is the most common chronic liver disease worldwide that is associated with excess health-related expenditures, making it a community health problem.3
Mounting evidence indicates a close association between the pathogenesis of T2D and NAFLD4–8; evidence suggests a complex bidirectional relationship, whereby presence of one leads to the progression of the other.9 The presence of NAFLD increases the incidence of T2D, while diabetes might contribute to the worsening of NAFLD to more advanced stages such as steatohepatitis and even hepatocellular carcinoma.10
NAFLD is strongly associated with insulin resistance such that prevalence of NAFLD is fivefold higher in patients with T2D compared with those without.8 Recent data showed that there is a solid genetic basis that support their association, since gene variants in numerous proteins related to lipid and glucose metabolism, appear to significantly raise the risk of NAFLD and T2D.10 11 These genetic abnormalities are directly linked to hepatic and peripheral insulin resistance, resulting in a deficient inhibition of hepatic gluconeogenesis, diminished glycogen synthesis and increased extrahepatic lipid accumulation. Other mechanisms underlying these NAFLD-T2D pathogenic duo involve excessive hepatic fat accumulation, diverse alterations in energy metabolism, altered microbiome, comorbidities, increased reactive oxygen species production and inflammatory signals derived from different cell types including immune cells, such as proinflammatory cytokines.12
The estimated overall worldwide prevalence of NAFLD in the general adult population is about 25%–30%,3 13 but ranges from 40% to 70% in subjects with established T2D.14 15 In fact, NAFLD and T2D are conditions that frequently coexist and can act synergistically to drive adverse outcomes.16 NAFLD is considered the hepatic manifestation of metabolic syndrome (MetS)17 because epidemiological studies have consistently shown that NAFLD is strongly linked to obesity, dyslipidaemia, and insulin resistance.18 19 Therefore, NAFLD is thought to be an independent risk factor for incident T2D16 and cardiovascular disease.20
Liver biopsy is currently the gold standard for diagnosing progressive NAFLD.21 Biopsies are invasive procedures with several drawbacks, including sampling error, interobserver variability, high cost, patient discomfort and risk of complications.14 Moreover, obtaining liver biopsies from all patients with NAFLD is unrealistic. Abdominal ultrasonography is a simple, inexpensive, widely available and minimally invasive technique that is used to diagnose fatty liver in most subjects. However, its sensitivity is low in subjects with fatty retention less than 20%–30% and it does not provide information on the degree of fibrosis.22 Consequently, attempts have been made to diagnose NAFLD/NASH using clinical and laboratory-based biomarkers and scoring systems that can predict fatty changes in the liver. These indices for the diagnosis of NAFLD/NASH include the fatty liver index (FLI),23 NAFLD liver fat score,24 the hepatic steatosis index,25 the ALD/NAFLD index,26 the lipid accumulation product27 and the SteatoTest.28 These indices require the measurement of patient characteristics, including concentrations of TG, γ-glutamyl transpeptidase (GGT), aspartate aminotransferase, alanine transaminase, insulin, body mass index (BMI), waist circumference (WC), gender, mean corpuscular value and presence or absence of T2D or MetS.29 The FLI is a simple and accurate algorithm that combines routine measurements of TG and GGT concentrations, WC and BMI, showing an excellent discriminative ability to predict ultrasonographic NAFLD and hepatic steatosis in the general population.23 30
The FLI has been reported to correlate with: (1) insulin resistance; (2) risk of coronary heart disease; (3) MetS; (4) early atherosclerosis; and (5) rates of non-hepatic-related morbidity and mortality in nondiabetic subjects.31 Thus, FLI-diagnosed NAFLD may be an indicator of incident T2D.19 Nonetheless, the risk of progression to T2D determined by FLI in patients with pre-diabetes remains poorly understood.
Few studies have evaluated the influence of NAFLD as a risk factor for T2D development in a cohort of workers with pre-diabetes. Determining FLI in subjects with pre-diabetes may be highly relevant, as both epidemiological and clinical evidence have shown that primary healthcare prevention programmes should target people at greater risk of developing T2D. The present study was therefore designed to evaluate the association between NAFLD, as estimated by FLI, and the development of T2D in a large cohort of South-European Mediterranean workers with pre-diabetes.
Methods
Study population and design
This cohort study included 16 648 Spanish working adults with pre-diabetes who worked in public administration, construction, health departments or post offices. The study methods have been described in detail previously.32 Briefly, participants were carefully chosen from 234 995 potentially eligible individuals who underwent periodic occupational health assessments between 2012 and 2013. Participants were included if they were aged 20–65 years and had an fasting plasma glucose (FPG) of 100–125 mg/dL.33 Subjects were excluded if they had a history of physician-diagnosed diabetes, had been treated with an oral antidiabetic agent or a systemic glucocorticoid, had an FPG≥126 mg/dL or an HbA1c≥6.5% at baseline, had received cancer treatment during the preceding 5 years, had anaemia (haematocrit <36% in men and <33% in women) or were pregnant. All subjects underwent standard health examinations, anthropometric measurements, and metabolic tests at baseline and were followed up 5 years later, in 2017 and 2018.
Patient and public involvement
People were not involved in setting the research question nor in the study design. Participants were interviewed face to face by trained researchers for a detailed explanation of the purpose of this research and informed consent at the beginning. Results of the research will be disseminated to the participants.
Data collection
At baseline, anthropometric measurements and fasting blood sample were taken from all subjects during occupational health examinations. A questionnaire was administered to collect data on sociodemographic characteristics, dietary habits, physical activity (PA) and clinical data. Participants were asked to report if they performed moderate and/or vigorous exercise (at least 150 min/week, according to WHO recommendations) and if they consumed fruits and vegetables daily. Each participant was also categorised as a current smoker (habitual or casual), former smoker, or never smoker, according to WHO criteria. Social class was defined using the Spanish Epidemiology Society classification, which is based on occupation, and it has shown high correlation with level of education.34 Class I (upper class) includes executives, managers and university professionals; class II (middle class) includes intermediate occupations and employees; and class III (lower class) includes manual workers.
All anthropometric measurements were made in the morning, after an overnight fast, at the same time and according to the guidelines and recommendations in the International Standards for Anthropometric Assessment manual.35 All measurements were performed by well trained technicians or researchers to minimise coefficients of variation. Body weight was measured to the nearest 0.1 kg using an electronic scale (Seca 700 scale, Hamburg); height was measured to the nearest 0.5 cm using a stadiometer (Seca 220) Telescopic Height Rod for Column Scales, Hamburg); and BMI was calculated as weight (kg) divided by height (m) squared (kg/m2). Obesity was defined as BMI ≥30.0 kg/m2, in agreement with WHO guidelines. Blood pressure was measured after a resting period of 10 min, with the subject in the supine position, using an electric and calibrated sphygmomanometer (OMRON M3, Healthcare Europe, Spain). Blood pressure in each subject was measured three times with a 1 min gap between measurements and their average was calculated.
Venous blood samples were taken from the antecubital vein of each subject in a sitting position, in the morning after a 12 hours overnight fast. Blood samples were collected in suitable vacutainers without anticoagulant to obtain serum. Serum concentrations of glucose, TG and cholesterol were measured by standard procedures using a Beckman Coulter SYNCHRON CX 9 PRO clinical system (La Brea, California, USA).
The main outcome variable of the study was the time elapsed until T2D onset, defined as FPG≥126 mg/dL,36 or the time until initiation of antihyperglycaemic medications for diabetes control in people with pre-diabetes during the follow-up period.
FLI as a surrogate measure of fatty liver
The FLI was calculated based on measurements of TG, GGT, BMI and WC, using the formula23:
Fatty liver index (FLI) = ey / (1 + ey) × 100
Where y=0.953 × ln(TG)+0.139 × BMI+0.718 × ln(GGT)+0.053 × WC – 15.745
Here, TG indicates triglyceride concentration, measured as mg/dL; BMI indicates body mass index, measured as kg/m2; GGT indicates γ-glutamyl transpeptidase, measured as U/L; and WC indicates waist circumference, measured as cm.
FLI, which ranges from 0 to 100, has shown good diagnostic accuracy in detecting fatty liver, with an area under the curve of 0.85 and a 95% CI of 0.81 to 0.88.19 23 FLI<30 was found to rule out steatosis with a sensitivity of 87% and a specificity of 64%, whereas FLI>60 was indicative of the presence of steatosis with a sensitivity of 61% and specificity of 86%.23 FLI scores have been validated by comparison with the results of liver ultrasound and nuclear magnetic resonance spectroscopy. An FLI of 30–60 indicated indeterminate risk, in which fatty liver could not be ruled in or out.
Statistical analyses
Continuous variables were expressed in means (±SDs) and compared by Student’s t-test and one-way analysis of variance, with post-hoc Bonferroni contrast method. Categorical variables were expressed as n (%) and compared by χ2 tests with Bonferroni post-hoc method. Crude and multivariable Cox regression analyses were performed to calculate FLI, diet and PA HRs for the development of diabetes, adjusting for potential confounders (age, social class, BMI, smoking, systolic blood pressure (SBP), FPG) that showed significant association in univariate analysis. Schoenfeld residuals were used to check the proportional hazard assumption. For this analysis, participants were classified into two categories: those with FLI>60 and FLI<60.
All analyses were performed using the Statistical Package for the Social Sciences (SPSS) V.25.0 (IBM Company) for Windows. All statistical tests were two sided, and p values<0.05 were considered statistically significant.
Results
Baseline demographic and anthropometric characteristics of the study subjects by sex are shown in table 1. The sample included 16 648 individuals with pre-diabetes, comprising 12 080 (72.6%) men and 4568 (27.4%) women, of mean age 44.81±9.91 years. The prevalence of obesity in the entire sample was 26.9%. The percentage of men was significantly higher among subjects with than without NAFLD. There were also significant differences in all anthropometrical and biochemical parameters analysed, with BMI, WC, TG, FPG, cholesterol, GGT and SBP and diastolic blood pressure (DBP) being significantly higher in subjects with than without NAFLD. The percentages of subjects who performed at least 150 min per week of PA (4.3% vs 61.8%; p<0.001) and who did not consume fruits and vegetables every day (12.0% vs 56.4%; p<0.001) were significantly lower in subjects with than without NAFLD.
Table 1.
Basal anthropometric characteristics and biochemical parameters of subjects by sex
| Characteristics | All (n=16 648) |
Men (n=12 080) |
Women (n=4568) |
P value |
| Age (years) | 44.51±9.89 | 44.38±9.87 | 44.84±9.94 | <0.01 |
| Social class | <0.001 | |||
| I | 741 (4.5%) | 558 (4.6%) | 183 (4.0%) | |
| II | 2779 (16.7%) | 1902 (15.7%) | 877 (19.2%) | |
| III | 13 128 (78.9%) | 9620 (79.6%) | 3508 (76.8%) | |
| BMI (kg/m2) | 27.66±4.81 | 27.76±4.47 | 27.42±5.61 | <0.001 |
| BMI categories | <0.001 | |||
| Normal weight | 5049 (30.3%) | 3300 (27.3%) | 1749 (38.3%) | |
| Overweight | 7120 (42.8%) | 5596 (46.3%) | 1524 (33.4%) | |
| Obese | 4479 (26.9%) | 3184 (26.4%) | 1295 (28.3%) | |
| WC (cm) | 87.00±9.95 | 90.28±8.62 | 78.32±7.78 | <0.001 |
| Triglycerides (mg/dL) | 137.66±106.39 | 150.08±117.11 | 104.81±59.14 | <0.001 |
| Glucose (mg/dL) | 106.22±5.82 | 106.43±5.90 | 105.68±5.56 | <0.001 |
| Cholesterol (mg/dL) | 202.40±38.09 | 202.49±38.59 | 202.18±36.74 | 0.642 |
| GGT (UI/L) | 44.20±55.68 | 48.03±59.07 | 34.08±33.69 | <0.001 |
| SBP (mm Hg) | 127.86±16.74 | 130.16±16.10 | 121.79±16.88 | <0.001 |
| DBP (mm Hg) | 78.32±11.01 | 79.51±10.94 | 75.18±10.58 | <0.001 |
| PA (≥150 min/week) | 6892 (41.4%) | 4787 (39.6%) | 2105 (46.1%) | <0.001 |
| Diet (daily fruits and vegetables) | 6771 (40.7%) | 4654 (38.5%) | 2117 (46.3%) | <0.001 |
| Smoking habit | <0.001 | |||
| Never | 7645 (45.9%) | 5124 (42.4%) | 2521 (55.2%) | |
| Former | 3549 (21.3%) | 2750 (22.8%) | 799 (17.5%) | |
| Current | 5454 (32.8%) | 4206 (34.8%) | 1248 (27.3%) |
Results are reported as mean ± SD or n (%).
Continuous variables were compared by Student’s t-test, whereas categorical variables were compared by χ2 tests.
BMI, body mass index; DBP, diastolic blood pressure; GGT, γ-glutamyl transpeptidase; PA, physical activity; SBP, systolic blood pressure; WC, waist circumference.
General characteristics of the study population, such as anthropometric and biochemical data, are shown in table 2, according to FLI categories. Data stratified by gender and FLI categories are shown in table 3 for men and table 4 for women. In both men and women, those with FLI>60 presented a significantly worse anthropometric and biochemical profile, as compared with the other two groups.
Table 2.
Basal anthropometric characteristics and biochemical parameters of men and women according to fatty liver index (FLI) categories (n=16 648)
| Characteristics | FLI<30 n=6421 (29.8%) (a) |
FLI 30–60 n=4318 (29.5%) (b) |
FLI>60 n=5909 (40.7%) (c) |
P value | Post-hoc |
| Sex (ref: male) | 3605 (56.1%) | 3558 (82.4) | 4927 (83.2%) | 0.008 | |
| Age (years) | 42.35±10.57 | 45.34±9.53 | 46.32±8.89 | <0.001 | a<b< c |
| Social class | 0.107 | NS | |||
| I | 248 (4.4%) | 218 (5.0%) | 239 (4.0%) | ||
| II | 1129 (17.6%) | 689 (16.0%) | 961 (16.3%) | ||
| III | 5008 (78.0%) | 3411 (79.0%) | 4709 (79.6%) | ||
| BMI (kg/m2) | 23.91±2.61 | 27.27±2.39 | 32.04±4.39 | <0.001 | c<b<a |
| BMI categories | <0.001 | ||||
| Normal weight | 4340 (67.6%) | 626 (14.5%) | 83 (1.4%) | a>b, c; b>c | |
| Overweight | 2014 (31.4%) | 3.218 (74.5%) | 1888 (32.0%) | b>a, c; c>a | |
| Obese | 67 (1%) | 474 (11.0%) | 4.479 (26.9%) | b>a; c>a, b | |
| WC (cm) | 78.87±7.06 | 87.92±6.55 | 95.16±7.34 | <0.001 | c>b>a |
| Triglycerides (mg/dL) | 87.52±36.34 | 129.90±60.74 | 19 197.81±146.19 |
<0.001 | c>b>a |
| Glucose (mg/dL) | 105.52±5.28 | 106.01±5.65 | 107.30±6.32 | <0.001 | c>b>a |
| Cholesterol (mg/dL) | 192.04±35.64 | 204.66±36.98 | 212.01±38.69 | <0.0 | c>b>a |
| GGT (UI/L) | 21.35±13.19 | 39.22±31.37 | 72.68±76.26 | <0.001 | c>b>a |
| SBP (mm Hg) | 121.54±15.31 | 128.56±15.01 | 134.24±16.91 | <0.001 | c>b>a |
| DBP (mm Hg) | 74.22±10.09 | 78.72±10.13 | 82.50±10.96 | <0.001 | c>b>a |
| PA (≥150 min/week) | 5156 (80.3%) | 1480 (34.3%) | 256 (4.3%) | <0.001 | a>b, c; b>c |
| Diet (daily fruits and vegetables) | 4502 (66.5%) | 1560 (23.0%) | 709 (12.0%) | <0.001 | a>b, c; b>c |
| Smoking habit | 0.930 | NS | |||
| Never | 3065 (42.7%) | 1981 (45.9%) | 2599 (44.0%) | ||
| Former | 1077 (16.8%) | 953 (22.1%) | 1519 5.7%) | ||
| Current | 2279 (35.5%) | 1384 (32.1%) | 1791 (30.3%) |
BMI, body mass index; GGT, γ-glutamyl transpeptidase; PA, physical activity; SBP, systolic blood pressure; WC, waist circumference.
Table 3.
Basal anthropometric characteristics and biochemical parameters of men according to fatty liver index (FLI) categories (n=12 080)
| Men characteristics | FLI<30 n=3605 (29.8%) (a) |
FLI 30–60 n=3558 (29.5%) (b) |
FLI>60 n=4917 (40.7%) (c) |
P value | Post-hoc |
| Age (years) | 41.02±10.66 | 45.08±9.55 | 46.34±8.81 | <0.001 | |
| Social class | 0.137 | NS | |||
| I | 153 (4.2%) | 191 (5.4%) | 214 (4.4%) | ||
| II | 559 (15.5%) | 554 (15.6%) | 789 (16.0%) | ||
| III | 2893 (80.2%) | 2813 (79.1%) | 3914 (79.6%) | ||
| BMI (kg/m2) | 23.74±2.24 | 26.78±2.02 | 31.41±4.09 | <0.001 | c<b<a |
| BMI categories | <0.001 | ||||
| Normal weight | 2616 (72.6%) | 603 (16.9%) | 81 (1.6%) | a>b, c; b>c | |
| Overweight | 980 (27.2%) | 2787 (78.3%) | 1829 (37.2%) | b>a, c; c>a | |
| Obese | 9 (0.2%) | 168 (4.7%) | 3007 (61.2%) | b>a; c>a, b | |
| WC (cm) | 82.67±5.85 | 89.13±6.06 | 96.68±6.83 | <0.001 | c>b>a |
| Triglycerides (mg/dL) | 88.46±37.22 | 130.95±60.70 | 209.1–0±153.25 | <0.001 | c>b>a |
| Glucose (mg/dL) | 105.56±5.36 | 106.05±5.63 | 107.34±6.34 | <0.001 | c>b>a |
| Cholesterol (mg/dL) | 187.32±34.39 | 203.72±37.20 | 212.71±38.95 | <0.001 | c>b>a |
| GGT (UI/L) | 23.02±13.22 | 38.89±31.70 | 72.98±81.09 | <0.001 | c>b>a |
| SBP (mm Hg) | 124.08±14.42 | 129.23±14.63 | 135.30±16.60 | <0.001 | c>b>a |
| DBP (mm Hg) | 74.98±10.08 | 79.03±10.02 | 83.18±10.86 | <0.001 | c>b>a |
| PA (≥150 min/week) | 3108 (86.2%) | 1425 (40.1%) | 254 (5.2%) | <0.001 | a>b, c; b>c |
| Diet (daily fruits and vegetables) | 2693 (74.7%) | 1372 (38.6%) | 589 (12.0%) | <0.001 | a>b, c; b>c |
| Smoking habit | <0.001 | ||||
| Never | 1539 (42.7%) | 1571 (44.2%) | 2014 (41.0%) | b>c | |
| Former | 620 (17.2%) | 800 (22.5%) | 1330 (27.0%) | b>a; c>a, b | |
| Current | 1446 (40.1%) | 1187 (33.4%) | 1573 (32.0%) | a>b, c |
Results are reported as mean ± SD or n (%).
Continuous variables were compared by analysis of variance, whereas categorical variables were compared by χ2 tests.
BMI, body mass index; DBP, diastolic blood pressure; GGT, γ-glutamyl transpeptidase; PA, physical activity; SBP, systolic blood pressure; WC, waist circumference.
Table 4.
Anthropometric characteristics and biochemical parameters of women according to fatty liver index (FLI) categories (n=4568)
| Women characteristics | FLI<30 n=2816 (61.7%) (a) |
FLI 30–60 n=760 (16.6%) (b) |
FLI>60 n=992 (21.7%) (c) |
P value | Post-hoc |
| Age (years) | 43.91±10.22 | 46.55±9.31 | 46.20±9.28 | <0.001 | a<b, c |
| Social class | <0.01 | ||||
| I | 131 (4.7%) | 27 (3.6%) | 25 (2.5%) | a>c | |
| II | 570 (20.2%) | 135 (17.8%) | 172 (17.3%) | ||
| III | 2115 (75.1%) | 598 (78.7%) | 795 (80.1%) | c>a | |
| BMI (kg/m2) | 24.13±3.02 | 29.56±2.62 | 35.12±4.49 | <0.001 | c>b>a |
| BMI categories | <0.001 | ||||
| Normal weight | 1724 (61.2%) | 23 (3.0%) | 2 (0.2%) | a>b, c; b>c | |
| Overweight | 1034 (36.7%) | 431 (56.7%) | 59 (5.9%) | a>c; b>a, c | |
| Obese | 58 (2.1%) | 306 (40.3%) | 931 (93.9%) | b>a; c>a, b | |
| WC (cm) | 74.00±5.35 | 82.22±5.70 | 87.63±4.60 | <0.001 | c>b>a |
| Triglycerides (mg/dL) | 86.33±35.15 | 125.00±60.71 | 141.83±84.45 | <0.001 | c>b>a |
| Glucose (mg/dL) | 105.13±5.17 | 105.84±5.73 | 107.12±6.18 | <0.001 | c>b>a |
| Cholesterol (mg/dL) | 198.09±36.29 | 209.10±35.61 | 208.52±37.20 | <0.001 | a<b, c |
| GGT (UI/L) | 19.21±12.83 | 40.74±29.70 | 71.16±45.26 | <0.001 | c>b>a |
| SBP (mm Hg) | 118.28±15.80 | 125.42±16.34 | 128.98±17.42 | <0.001 | c>b>a |
| DBP (mm Hg) | 73.25±10.02 | 77.25±10.50 | 79.08±10.82 | <0.001 | c>b>a |
| PA (≥150 min/week) | 2048 (72.7%) | 55 (7.2%) | 2 (0.2%) | <0.001 | a>b, c; b>c |
| Diet (daily fruits and vegetables) | 1809 (64.2%) | 188 (24.7%) | 120 (12.1%) | <0.001 | a>b, c; b>c |
| Smoking habit | <0.001 | ||||
| Never | 1526 (54.2%) | 410 (53.9%) | 585 (59.0%) | c>a | |
| Former | 457 (16.2%) | 153 (20.1%) | 189 (19.1%) | b>a | |
| Current | 833 (29.6%) | 197 (25.9%) | 218 (22.0%) | a>c |
Results are reported as mean ± SD or n (%).
Continuous variables were compared by analysis of variance, whereas categorical variables were compared by χ2 tests.
BMI, body mass index; DBP, diastolic blood pressure; GGT, γ-glutamyl transpeptidase; PA, physical activity; SBP, systolic blood pressure; WC, waist circumference.
Among men, 40.7% presented a FLI>60, 29.5% a FLI 30–60, and 29.8% a FLI<30. As compared with men in the other two categories, those with FLI>60 were older, more obese, and presented higher values of WC, TGs, glucose, cholesterol, GGT, SBP, and DBP (all p<0.001). Men with FLI<30 consumed more fruits and vegetables daily, and dedicated more time to PA, than men with FLI>60 (all p<0.001).
Among women, 21.7% had a FLI>60, 16.6% a FLI 30–60, and 61.7% a FLI<30. As compared with women in the other two categories, those with FLI>60 were more obese, and had worse anthropometric and biochemical values (WC, TGs, glucose, cholesterol, GGT, SBP and DBP) (all p<0.001). Women with FLI<30 also consumed more fruits and vegetables daily, and dedicated more time to PA, than women with FLI>60 (all p<0.001).
Baseline FLI showed a significant correlation with FPG concentration at 5-year follow-up with a Pearson’s correlation coefficient of 0.528 (p<0.001; figure 1).
Figure 1.
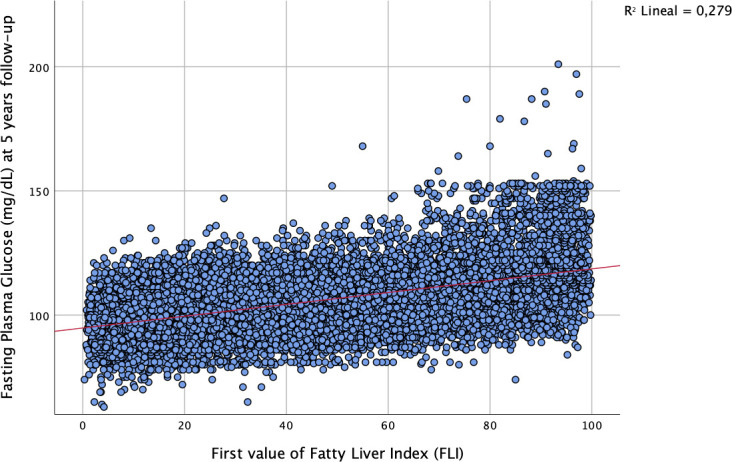
Correlation of baseline FLI and FPG after 5 years of follow-up.
Of the 16 648 subjects with pre-diabetes, 3706 (22.2%) progressed to T2D at 5 years, corresponding to an annual rate of 4.5%. The incidence of T2D after 5 years was similar between men (22.1%) and women (22.6%). When specifically looking at FLI categories, 0.2% (6/3605) of men and 0.5% (13/2816) of women in the low-risk group (FLI<30), progressed to T2D, corresponding to an annual rate of 0.04% for men and 0.1% for women. In the intermediate risk group (FLI 30–60), progression to T2D occurred in 4.3% (152/3558) of men and 24.5% (186/760) in women, corresponding to an annual rate of 0.86% and 4.9%, respectively. Finally, in the high-risk group (FLI>60), incidence of T2D was 51.2% (2516/4917) in men and 84.0% (833/992) in women, corresponding to an annual rate of 11.34% and 16.8% respectively. Rates of progression to T2D in men and women according baseline FLI categories are shown in figure 2.
Figure 2.
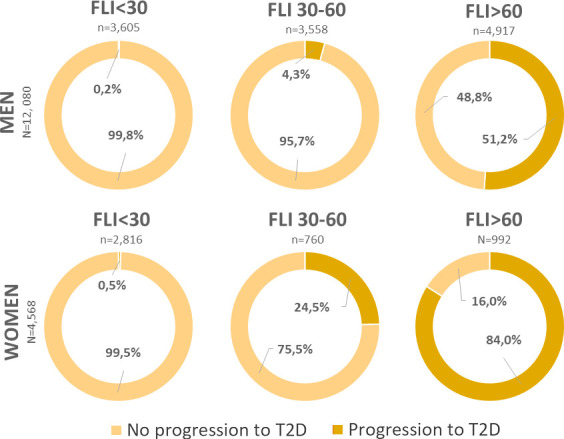
Incidence of type 2 diabetes (T2D) after 5-year follow-up according to baseline fatty liver index (FLI) classification.
In bivariate analysis (table 5), high FLI (>60) was strongly associated with progression to T2D in both genders (HR=24.361; 95% CI 21.020 to 28.233 for men, and HR=17.816; 95% CI 15.400 to 20.611 for women), as were age, social class, BMI, smoking habits, FPG and SBP. An adjusted cox regression model showed that high FLI scores (>60) remained independently associated with progression to T2D (adjusted HR=6.879; 95% CI 5.873 to 8.057 for men, and HR=5.806; 95% CI 4.863 to 6.932 for women). BMI was also associated to progression to T2D in both genders after adjustment (HR=1.041; 95% CI 1.036 to 1.045 for men, and HR=1.104; 95% CI 1.036 to 1.045 for women). Some of the evaluated factors also remained significant after adjustment. Performing at least 150 min/week of PA (adjusted HR=0.215; 95% CI 0.173 to 0.268 for men, and HR=0.070; 95% CI 0.043 to 0.112 for women) was significantly protective against progression to T2D in both genders. Current male smokers were also less likely to progress to T2D (adjusted HR=0.909; 95% CI 0.834 to 0.991).
Table 5.
HRs for progression from pre-diabetes to type 2 diabetes (T2D)
| Variables | HR crude (95% CI) | HR adjusted (95% CI) | ||||
| Men | Women | All subjects | Men | Women | All subjects | |
| Age | 1.054 (1.051 to 1.058) | 1.035 (1.030 to 1.050) | 1.042 (1.040 to 1.054) | 1.041 (1.036 to 1.045) | 1.018 (1.011 to 1.026) | 1.029 (1.024 to 1.036) |
| Social class (Ref: I) | ||||||
| II | 1.147 (0.984 to 1.338) | 1.475 (1.102 to 1.974) | 1.331 (1.043 to 1.656) | 1.009 (0.839 to 1.212) | 1.216 (0.818 to 1.809) | 1.113 (0.826 to 1.511) |
| III | 1.087 (0.994 to 1.251) | 1.845 (1.402 to 2.428) | 1446 (1.198 to 1.840) | 1.005 (0.851 to 1.186) | 1.170 (0.793 to 1.140) | 1.088 (0.822 to 1.163) |
| PA (≥150 min/week) | 0.037 (0.031 to 0.046) | 0.027 (0.019 to 0.038) | 0.032 (0.025 to 0.042) | 0.215 (0.173 to 0.268) | 0.070 (0.043 to 0.112) | 0.143 (0.108 to 0.190) |
| Diet (daily fruits and vegetables) | 0.126 (0.112 to 0.142) | 0.141 (0.120 to 0.166) | 0.134 (0.116 to 0.154) | 0.959 (0.843 to 1.091) | 0.951 (0.793 to 1.140) | 0.955 (0.818 to 1.116) |
| Smoking habits (Ref: never smoker) | ||||||
| Former | 1.244 (1.153 to 1.343) | 1.010 (0.875 to 1.165) | 1.749 (1.014 to 1.254) | 0.985 (0.904 to 1.072) | 1.017 (0.873 to 1.184) | 1.046 (1.340 to 1.128) |
| Current | 0.770 (0.719 to 0.824) | 0.714 (0.633 to 0.804) | 0.742 (0.676 to 0.814) | 0.909 (0.834 to 0.991) | 0.959 (0.830 to 1.107) | 0.934 (0.832 to 1.049) |
| BMI | 1.174 (1.170 to 1.178) | 1.161 (1.154 to 1.167) | 1.168 (1.162 to 1.172) | 1.041 (1.036 to 1.045) | 1.104 (1.101 to 1.107) | 1.073 (1.069 to 1.076) |
| SBP | 1.023 (1.021 to 1.024) | 1.023 (1.021 to 1.025) | 1.023 (1.021 to 1.025) | 0.999 (0.996 to 1.001) | 1.001 (0.997 to 1.004) | 1.000 (0.997 to 1.003) |
| FPG | 1.037 (1.034 to 1.039) | 1.027 (1.023 to 1.030) | 1.032 (1.029 to 1.035) | 1.021 (1.018 to 1.024) | 1.018 (1.013 to 1.023) | 1.020 (1.016 to 1.024) |
| FLI (Ref: FLI<60) | ||||||
| FLI>60 | 24.361 (21.020 to 28.233) | 17.816 (15.400 to 20.611) | 21.089 (18.210 to 24.422) | 6.879 (5.873 to 8.057) | 5.806 (4.863 to 6.932) | 6.343 (5.368 to 7.495) |
BMI, body mass index; FLI, fatty liver index; FPG, fasting plasma glucose; PA, physical activity; SBP, systolic blood pressure.
Discussion
The present study aimed to evaluate the possible association between hepatic steatosis, as estimated by FLI, and T2D progression in a large and representative sample of Mediterranean workers with pre-diabetes. The main finding of the study was that FLI was a strong independent risk factor for the progression of T2D, in both men and women with baseline pre-diabetes, after a 5-year follow-up. Moreover, FLI could preventively identify subjects at high risk of progression to T2D. Other risk factor associated with progression T2D were older age, male sex, higher BMI, higher FPG, low consumption of fruits and vegetables, and performing less than 150 min/week of PA.
The results of the present study are in accordance with previous evidence reporting that NAFLD is a strong predictor of T2D in subjects with pre-diabetes.14 37 The baseline prevalence of hepatic steatosis (ie, FLI>60) in our study population was 35.4%, higher than the 19.3% reported in a Japanese study,37 lower than the 55.7% observed in the Primary Health Care Study on the Evolution of Patients with Prediabetes (PREDAPS study),14 and closer to the 22%–40% reported by studies using ultrasonography-diagnosed NAFLD.38 39
Patients with a higher FLI score, independently of gender, presented a higher BMI, a worse cardiometabolic profile and less healthy lifestyle habits. Previous studies40 similarly observed that patients with FLI >60 were more metabolically impaired compared with patients with lower FLI, they also presented a higher risk for MetS, as well as worse lipid profile.41 Accordingly, the degree of liver fat content correlates with MetS components,42 and that this correlation may be due to NAFLD and T2D sharing a series of common physiopathological pathways.43 44
At 5-year follow-up, nearly 1 in 4 individuals (22.2%) with pre-diabetes progressed to T2D resulting in an annual rate of progression of 4.5%. In comparison, the French IT-DIAB study,45 a 5-year, prospective observational study reported an annual progression rate of 7.1%. The study also reported that FLI could predict the risk of progressing to T2D as well as the possibility of reverting to normoglycaemia in clinical practice, independently of classical glucose parameters.46 Moreover, normalisation of glycaemia was higher in subjects with FLI<30 than in those with higher FLI scores. The incidence of T2D observed in our study was higher than in previous ones47–49 probably due to differences in sociodemographic characteristics between study populations. The ARIC (Atherosclerosis Risk in Communities) study,47 which reported an annual progression rate to T2D of 2.3%, included a higher percentage of women than in our cohort, whereas the ELSA-Brasil study,49 which found that the annual progression rate to T2D was 3.5%, included a higher percentage of subjects with high educational level. On the other hand, the PREDAPS study50 showed a similar annual conversion rate (4.2%). The incidence rate of T2D in our sample was lower than that shown (5.8%) in a previous Korean study51 of 7680 subjects who had undergone general routine health evaluations. Nevertheless, similar to what was observed in our study, 65.5% of the Korean subjects were men, and male sex was a risk factor for development of T2D in patients with pre-diabetes.
When stratifying for gender, the proportion of women in the FLI>60 category who progressed to T2D was significantly higher (80%) than the proportion of men in the same category (50%), at 5 year follow-up. Although women are generally less likely to suffer from hepatic steatosis,52 once they do, they might present a higher risk of developing T2D than males.53 Genetic predisposition and epigenetic mechanisms, nutritional components and lifestyle exert effects differently in both sexes. Furthermore, sexual hormones directly impact on energy metabolism, body composition, inflammatory cascades and vascular functioning. Particularly, low levels of 17β-estradiol are associated with increased risk of T2D, independently of established risk factors, including BMI and insulin resistance.54 Thus, endocrine imbalances might relate to unfavourable cardiometabolic traits observable in female sex.55
Of note, results from our study show an apparently protective effect of smoking on progression to diabetes. However this could be due to the anorexigenic effect of tobacco, more than tobacco consumption itself. Smokers are generally leaner than average as nicotine may affect energy homeostasis and food consumption at brain level.56 Accordingly, the proportion of smokers with a lower FLI was higher than that of smokers in the other two categories.
The FLI could be utilised in primary care as a practical tool for early detection of NAFLD in subjects with pre-diabetes, while predicting their risk of developing T2D.57 This would benefit patients at greater risk, allowing more careful monitoring and providing an opportunity for early interventions to prevent and reduce both the progression of hepatic disease and T2D. The present study also highlights the importance of weight control, promotion of PA and of fruits and vegetables consumption in the prevention of T2D progression. Determining lifestyle-related factors, particularly PA, together with repeated anthropometrical measurements in subjects with pre-diabetes may be crucial in properly assessing the risks of progression to T2D and of cardiovascular events.58
Strengths and limitations
This study had some limitations. First, this work incorporated data from periodic health assessments performed in the workplace. None of these subjects underwent oral glucose tolerance tests, which is considered more sensitive but slightly less specific than FPG for identifying people at risk of developing T2D.59 However, the low reproducibility, high cost and prolonged time required for this test have limited its use in clinical practice.60 Second, possible misclassification bias could have occurred as subjects were categorised as having pre-diabetes based on a single FPG sample, thus limiting the possibility to account for intraindividual variability and increasing the possibility of a regression-toward-the-mean effect, possibly affecting the progression rate. Third, diet and PA were only evaluated at baseline, thus lifestyles changes were not recorded during follow-up, possibly resulting in misclassification bias. Moreover, specific separate information on fruits and vegetable consumption could not be assessed, thus limiting the possibility of studying the confounding effect of excessive fruit consumption on NAFLD risk. Finally, we cannot discard the effect of job-related confounders such as job stress or the healthy worker effect. The main strengths of this study were the large sample size (16 648 subjects) and the relatively long follow-up period. Study participants had multiple occupations and were from several geographical locations, suggesting that the study population was representative of the Spanish workforce, although, our results are not applicable to the general population.
Clinical implications
This study highlights the importance of FLI as an easily calculated and valuable early indicator for high risk of T2D in subjects with pre-diabetes. FLI-based screening could allow the adoption of effective measures to prevent and reduce the progression of NAFLD. The workplace could be a feasible setting for implementing diabetes prevention programmes based on early detection and lifestyle changes.
Conclusion
Because of the progressive nature of NAFLD and the risk of serious consequences, healthcare providers should be strongly advised to screen routinely for NAFLD in all subjects with pre-diabetes or at risk of T2D. Fatty liver indices are simple clinical tools for evaluating the extent of liver fat and are predictive of incident diabetes. Concretely, the FLI is a simple, effective and practical method of stratifying the risk of conversion to T2D based on the degree of hepatic steatosis. FLI may be useful in routine clinical practice as an additional screening tool to identify subjects with pre-diabetes who are at high risk of progression and could benefit from early interventions. Identification of subjects who could benefit from preventive strategies represents an opportunity to assist vulnerable individuals to understand their health risks and encourage them to adopt preventive behaviours.
The workplace may be a feasible setting for the assessment of risk factors, allowing early detection of NAFLD in younger subjects with pre-diabetes who are likely to progress to T2D and the implementation of T2D prevention programmes.
Supplementary Material
Acknowledgments
The authors are grateful to the field staff and participants of this study.
Footnotes
Twitter: @miquelbennasar
Contributors: CB-C, MB-V, A-AL-G, AA and AY were responsible for the conception and design of the study. A-AL-G, SF and AA acquired the data, supervised the study and had full access to all study data. CB-C, MB-V and AY analysed and interpreted the data and drafted the manuscript. SF, A-AL-G and AA participated in critical revision of the manuscript for important intellectual content. All authors read and approved the final version of the manuscript.
Funding: This project was funded by the Carlos III Health Institute (Ministry of Economy and Competitiveness, Spain) through the Network for Prevention and Health Promotion in Primary Care (redIAPP, RD16/0007/008), and by European Union ERDF funds.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. Data are available upon reasonable request. Readers may contact Dr. Arturo Lopez (angarturo@gmail.com) regarding the data.
Ethics statements
Patient consent for publication
Obtained.
Ethics approval
All the procedures in the study protocol were in accordance with the Declaration of Helsinki for research on human participants and were approved by the Balearic Islands Ethical Committee of Clinical Research (Ref. No: CEI-IB-1887). All participants were carefully informed of the purpose and demands of the study. Informed consent was obtained from all participants included in the study.
References
- 1.Younossi ZM, Marchesini G, Pinto-Cortez H, et al. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: implications for liver transplantation. Transplantation 2019;103:22–7. 10.1097/TP.0000000000002484 [DOI] [PubMed] [Google Scholar]
- 2.Cobbina E, Akhlaghi F. Non-alcoholic fatty liver disease (NAFLD) - pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab Rev 2017;49:197–211. 10.1080/03602532.2017.1293683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellentani S. The epidemiology of non-alcoholic fatty liver disease. Liver Int 2017;37:81–4. 10.1111/liv.13299 [DOI] [PubMed] [Google Scholar]
- 4.Alisi A, Manco M, Panera N, et al. Association between type two diabetes and non-alcoholic fatty liver disease in youth. Ann Hepatol 2009;8:44–50. [PubMed] [Google Scholar]
- 5.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013;10:330–44. [DOI] [PubMed] [Google Scholar]
- 6.Williams KH, Shackel NA, Gorrell MD, et al. Diabetes and nonalcoholic fatty liver disease: a pathogenic Duo. Endocr Rev 2013;34:84–129. 10.1210/er.2012-1009 [DOI] [PubMed] [Google Scholar]
- 7.Tilg H. NAFLD and diabetes mellitus Herbert. Nat Rev 2017. [DOI] [PubMed] [Google Scholar]
- 8.Fujii H, Kawada N, Japan Study Group of NAFLD (JSG-NAFLD) Japan Study Group of NAFLD (JSG-NAFLD) . The role of insulin resistance and diabetes in nonalcoholic fatty liver disease. Int J Mol Sci 2020;21:3863. 10.3390/ijms21113863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parry SA, Hodson L. Managing NAFLD in type 2 diabetes: the effect of lifestyle interventions, a narrative review. Adv Ther 2020;37:1381–406. 10.1007/s12325-020-01281-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia M-F, Bian H, Gao X. Nafld and diabetes: two sides of the same coin? rationale for gene-based personalized NAFLD treatment. Front Pharmacol 2019;10:1–11. 10.3389/fphar.2019.00877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashanian S, Fuchs M. Non-alcoholic fatty liver disease in patients with diabetes mellitus: a clinician’s perspective. Int J Dig Dis 2015;01:1–9. 10.4172/2472-1891.100010 [DOI] [Google Scholar]
- 12.Friedman SL, Neuschwander-Tetri BA, Rinella M, et al. Mechanisms of NAFLD development and therapeutic strategies. Nat Med 2018;24:908–22. 10.1038/s41591-018-0104-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tana C, Ballestri S, Ricci F, et al. Cardiovascular risk in non-alcoholic fatty liver disease: mechanisms and therapeutic implications. Int J Environ Res Public Health 2019;16:3104. 10.3390/ijerph16173104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franch-Nadal J, Caballeria L, Mata-Cases M, et al. Fatty liver index is a predictor of incident diabetes in patients with prediabetes: the PREDAPS study. PLoS One 2018;13:1–17. 10.1371/journal.pone.0198327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caballería L, Auladell MA, Torán P, et al. Prevalence and factors associated with the presence of non alcoholic fatty liver disease in an apparently healthy adult population in primary care units. BMC Gastroenterol 2007;7:1–6. 10.1186/1471-230X-7-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hazlehurst JM, Woods C, Marjot T, et al. Non-alcoholic fatty liver disease and diabetes. Metabolism 2016;65:1096–108. 10.1016/j.metabol.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falguera M, Vilanova MB, Alcubierre N. Prevalence of prediabetes and undiagnosed diabetes in the Mollerussa prospective observational cohort study rural area of Catalonia in a semi- rural area of Catalonia. BMJ Open 2019;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanyal AJ. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 2019;16:377–86. 10.1038/s41575-019-0144-8 [DOI] [PubMed] [Google Scholar]
- 19.Jäger S, Jacobs S, Kröger J, et al. Association between the fatty liver index and risk of type 2 diabetes in the EPIC-Potsdam study. PLoS One 2015;10:1–14. 10.1371/journal.pone.0124749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013;10:330–44. 10.1038/nrgastro.2013.41 [DOI] [PubMed] [Google Scholar]
- 21.Wieckowska A, Feldstein AE. Diagnosis of nonalcoholic fatty liver disease: invasive versus noninvasive. Semin Liver Dis 2008;28:386–95. 10.1055/s-0028-1091983 [DOI] [PubMed] [Google Scholar]
- 22.Papagianni M, Sofogianni A, Tziomalos K. Non-invasive methods for the diagnosis of nonalcoholic fatty liver disease. World J Hepatol 2015;7:638–48. 10.4254/wjh.v7.i4.638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bedogni G, Bellentani S, Miglioli L. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 2006;6:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dyson JK, Anstee QM, McPherson S. Non-alcoholic fatty liver disease: a practical approach to diagnosis and staging. Frontline Gastroenterol 2014;5:211–8. 10.1136/flgastro-2013-100403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J-H, Kim D, Kim HJ, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis 2010;42:503–8. 10.1016/j.dld.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Li P, Jiang Z, et al. Diagnostic value of alcoholic liver disease (ALD)/nonalcoholic fatty liver disease (NAFLD) index combined with γ-glutamyl transferase in differentiating ALD and NAFLD. Korean J Intern Med 2016;31:479–87. 10.3904/kjim.2015.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rotter I, Rył A, Szylińska A, et al. Lipid accumulation product (LAP) as an index of metabolic and hormonal disorders in aging men. Exp Clin Endocrinol Diabetes 2017;125:176–82. 10.1055/s-0042-116071 [DOI] [PubMed] [Google Scholar]
- 28.Poynard T, Lassailly G, Diaz E, et al. Performance of biomarkers FibroTest, ActiTest, SteatoTest, and NashTest in patients with severe obesity: meta analysis of individual patient data. PLoS One 2012;7:e30325–8. 10.1371/journal.pone.0030325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayensa-Vazquez JA, Leiva A, Tauler P, et al. Agreement between type 2 diabetes risk scales in a Caucasian population: a systematic review and report. J Clin Med 2020;9:1546–19. 10.3390/jcm9051546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calori G, Lattuada G, Ragogna F, et al. Fatty liver index and mortality: the Cremona study in the 15th year of follow-up. Hepatology 2011;54:145–52. 10.1002/hep.24356 [DOI] [PubMed] [Google Scholar]
- 31.Zhou K, Cen J. The fatty liver index (FLI) and incident hypertension: a longitudinal study among Chinese population. Lipids Health Dis 2018;17:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Bennasar-Veny M, Fresneda S, López-González A. Lifestyle and progression to type 2 diabetes in a cohort of workers with prediabetes. Nutrients 2020;12:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2012;35:S64–71http://www.ncbi.nlm.nih.gov/pubmed/22187472DiagnosisandClassification 10.2337/dc12-s064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Domingo-Salvany A, Bacigalupe A, Carrasco JM. Propuestas de clase social neoweberiana Y neomarxista a partir de la Clasificación Nacional de Ocupaciones 2011. Gac Sanit 2013;27:263–72. [DOI] [PubMed] [Google Scholar]
- 35.Stewart A, Marfell-Jones M, Olds T de RH. International standards for anthropometric assessment. ISAK. 3rd edn. New Zealand: Lower Hutt, 2011. [Google Scholar]
- 36.American Diabetes Association, Care D, Suppl SS. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care 2021;44:S15–33. 10.2337/dc21-S002 [DOI] [PubMed] [Google Scholar]
- 37.Nishi T, Babazono A, Maeda T, et al. Evaluation of the fatty liver index as a predictor for the development of diabetes among insurance beneficiaries with prediabetes. J Diabetes Investig 2015;6:309–16. 10.1111/jdi.12290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bae JC, Rhee EJ, Lee WY, et al. Combined effect of nonalcoholic fatty liver disease and impaired fasting glucose on the development of type 2 diabetes: a 4-year retrospective longitudinal study. Diabetes Care 2011;34:727–9. 10.2337/dc10-1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong VWS, Hui AY, Tsang SWC, et al. Prevalence of undiagnosed diabetes and postchallenge hyperglycaemia in Chinese patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2006;24:1215–22. 10.1111/j.1365-2036.2006.03112.x [DOI] [PubMed] [Google Scholar]
- 40.Leutner M, Göbl C, Schlager O, et al. The fatty liver index (FLI) relates to diabetes-specific parameters and an adverse lipid profile in a cohort of nondiabetic, dyslipidemic patients. J Am Coll Nutr 2017;36:287–94. 10.1080/07315724.2016.1262802 [DOI] [PubMed] [Google Scholar]
- 41.Rogulj D, Konjevoda P, Milić M, et al. Fatty liver index as an indicator of metabolic syndrome. Clin Biochem 2012;45:68–71. 10.1016/j.clinbiochem.2011.10.014 [DOI] [PubMed] [Google Scholar]
- 42.Lonardo A, Ballestri S, Marchesini G, et al. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis 2015;47:181–90. 10.1016/j.dld.2014.09.020 [DOI] [PubMed] [Google Scholar]
- 43.Saponaro C, Gaggini M, Gastaldelli A. Nonalcoholic fatty liver disease and type 2 diabetes: common pathophysiologic mechanisms. Curr Diab Rep 2015;15:1–13. 10.1007/s11892-015-0607-4 [DOI] [PubMed] [Google Scholar]
- 44.Forlani G, Giorda C, Manti R. The burden of NAFLD and its characteristics in a nationwide population with type 2 diabetes. J Diabetes Res 2016:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wargny M, Smati S, Pichelin M, et al. Fatty liver index is a strong predictor of changes in glycemic status in people with prediabetes: the IT-DIAB study. PLoS One 2019;14:1–14. 10.1371/journal.pone.0221524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Busquets-Cortés C, Bennasar-Veny M, López-González Ángel Arturo, et al. Utility of fatty liver index to predict reversion to normoglycemia in people with prediabetes. PLoS One 2021;16:e0249221. 10.1371/journal.pone.0249221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selvin E, Steffes MW, Gregg E, et al. Performance of A1C for the classification and prediction of diabetes. Diabetes Care 2011;34:84–9. 10.2337/dc10-1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giráldez-García C, María Hernández A, Gamarra J. Evolución de pacientes con prediabetes en Atención Primaria de Salud (PREDAPS): resultados del quinto año de seguimiento. Diabetes Práctica 2018;09:37–80. [Google Scholar]
- 49.Schmidt MI, Bracco PA, Yudkin JS, et al. Intermediate hyperglycaemia to predict progression to type 2 diabetes (ELSA-Brasil): an occupational cohort study in Brazil. Lancet Diabetes Endocrinol 2019;7:267–77. 10.1016/S2213-8587(19)30058-0 [DOI] [PubMed] [Google Scholar]
- 50.Giráldez-García C, García-Soidán FJ, Serrano Martín R. Evolución de pacientes con prediabetes en Atención Primaria de Salud (PREDAPS): resultados del primer año de seguimiento. Diabetes Práctica 2014;05:1–48. [Google Scholar]
- 51.Jung CH, Lee WJ, Hwang JY, et al. Assessment of the fatty liver index as an indicator of hepatic steatosis for predicting incident diabetes independently of insulin resistance in a Korean population. Diabet Med 2013;30:428–35. 10.1111/dme.12104 [DOI] [PubMed] [Google Scholar]
- 52.Ballestri S, Nascimbeni F, Baldelli E, et al. NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv Ther 2017;34:1291–326. 10.1007/s12325-017-0556-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tokita Y, Maejima Y, Shimomura K, et al. Non-alcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men and women. Intern Med 2017;56:763–71. 10.2169/internalmedicine.56.7115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mauvais-Jarvis F. Is estradiol a biomarker of type 2 diabetes risk in postmenopausal women? Diabetes 2017;66:568–70. 10.2337/dbi16-0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev 2016;37:278–316. 10.1210/er.2015-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stojakovic A, Espinosa EP, Farhad OT, et al. Effects of nicotine on homeostatic and hedonic components of food intake. J Endocrinol 2017;235:R13–31. 10.1530/JOE-17-0166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ciardullo S, Muraca E, Perra S, et al. Screening for non-alcoholic fatty liver disease in type 2 diabetes using non-invasive scores and association with diabetic complications. BMJ Open Diabetes Res Care 2020;8:1–9. 10.1136/bmjdrc-2019-000904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vistisen D, Witte DR, Brunner EJ, et al. Risk of cardiovascular disease and death in individuals with prediabetes defined by different criteria: the Whitehall II study. Diabetes Care 2018;41:899–906. 10.2337/dc17-2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Unwin N, Shaw J, Zimmet P, et al. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med 2002;19:708–23. 10.1046/j.1464-5491.2002.00835.x [DOI] [PubMed] [Google Scholar]
- 60.Perry RC, Shankar RR, Fineberg N, et al. Hba1C measurement improves the detection of type 2 diabetes in high-risk individuals with nondiagnostic levels of fasting plasma glucose: the early diabetes intervention program (EDIP). Diabetes Care 2001;24:465–71. 10.2337/diacare.24.3.465 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. Data are available upon reasonable request. Readers may contact Dr. Arturo Lopez (angarturo@gmail.com) regarding the data.


