Abstract
Background:
Posttraumatic stress disorder (PTSD) is associated with increased risk for morbidity and mortality, which may be mediated through elevated inflammation. In contrast, social support appears to protect against morbidity and mortality, reduce levels of inflammation, and improve PTSD outcomes.
Methods:
We examined relationships among social isolation, perceived social support, and inflammation in Veterans Affairs (VA) patients with and without PTSD. Our sample included 735 (35% PTSD+) participants from the Mind Your Heart Study (mean age = 58 ± 11; 94% male). Social isolation was assessed with the Berkman Syme Social Network Index; perceived social support with the Multidimensional Scale of Perceived Social Support; and PTSD with the Clinician Administered PTSD Scale. Inflammation was indexed by high sensitivity C-reactive protein, white blood cell count, and fibrinogen. Hierarchical linear regression was used to examine associations between social measures and inflammation. PROCESS was used to examine the interactive effects of social relationships and PTSD on inflammation.
Results:
Social isolation, but not low perceived social support, trended towards an association with elevated inflammation in the full sample. However, considering groups with and without PTSD separately, social isolation was significantly associated with all inflammatory markers among individuals without PTSD, but not among those with PTSD.
Conclusions:
Social integration is associated with reduced inflammation in individuals without, but not with, PTSD. Socially integrated individuals with PTSD did not have lower levels of inflammatory markers than socially isolated individuals with PTSD.
Keywords: Posttraumatic Stress Disorder, PTSD, Social Isolation, Social Network, Perceived Social Support, Social Support, Inflammation, Immune System
1. Introduction
Posttraumatic stress disorder (PTSD) has been repeatedly linked with elevated inflammation, including in a meta-analysis of 20 studies (Passos et al., 2015). In fact, accumulating evidence indicates that inflammation could play a role in the pathophysiology of PTSD (Eraly et al., 2014; O’Donovan et al., 2017), and in the excess physical disease burden associated with PTSD (Boscarino, 2008; Edmondson et al., 2013; O’Donovan et al., 2015). In contrast, social support is associated with lower levels of inflammation (Fagundes et al., 2011; Kiecolt-Glaser et al., 2010) and better physical health (Cacioppo et al., 2015; Holt-Lunstad et al., 2010). Thus, it is plausible that social support could reduce inflammation and improve mental and physical health outcomes in people with PTSD. However, to our knowledge, no studies have examined if social support is associated with inflammation in individuals with PTSD.
Elevated inflammation may play a role in the deleterious health effects of traumatic stress, particularly in individuals who develop PTSD. PTSD has been linked with elevated levels of inflammatory markers, including high sensitivity C-reactive protein (hsCRP), white blood cell (WBC) count, and fibrinogen (O’Donovan et al., 2017). Similarly, a 2015 meta-analysis and meta-regression of the literature concluded that PTSD is associated with increased levels of pro-inflammatory proteins called cytokines, including interleukin-6 (IL-6), interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) (Passos et al., 2015). In turn, high levels of inflammation confer increased risk for numerous chronic diseases, including coronary heart disease, metabolic syndrome, autoimmune disorders, and neurodegenerative diseases (Hansson, 2005; Heppner et al., 2015; Tabas and Glass, 2013). Furthermore, there is emerging evidence that elevated inflammation may elicit psychiatric symptoms, including symptoms of PTSD (Dantzer et al., 2008; Eraly et al., 2014; Raison et al., 2013).
Social support has positive effects on mental and physical health and may protect against adverse health outcomes in PTSD. Previous research has distinguished between two distinct aspects of social support that may have different associations with inflammation and health outcomes. The first is social isolation/integration, reflecting the objective measures of the size of one’s social network, quantified by the number and frequency of social ties (Berkman and Syme, 1979). The second is perceived social support, reflecting subjective relationship quality, as assessed by perceived emotional support available from family, friends, and significant others (Zimet et al., 1988). A recent meta-analysis found that objective social isolation, but not subjective low perceived social support, was significantly associated with increased mortality (Holt-Lunstad et al., 2010). Large-scale studies indicate that socially isolated individuals display elevated levels of systemic inflammatory markers, including hsCRP, WBC count, and fibrinogen (Ford et al., 2006; Kreibig et al., 2014). The data linking low perceived social support with elevated levels of inflammation is far less robust; while there have been modest associations seen in at least one study (Mezuk et al., 2010), other studies have found no association (McDade et al., 2006)or even the opposite relationship (Helminen et al., 1997).
In the present study, we examine whether social isolation and low perceived social support are associated with elevated levels of inflammation in a sample of 735 Veterans Affairs (VA) patients with and without PTSD enrolled in the Mind Your Heart Study. We measured three inflammatory markers: hsCRP, an acute-phase reactant and nonspecific marker of inflammation that is secreted by the liver and adipose tissues (Lowe, 2001); WBC count, a major clinical marker of inflammatory activity (Lowe, 2001); and fibrinogen, an acute phase protein and hemostatic that plays a pro-inflammatory role in the pathogenesis of various diseases (Pearson et al., 2003). We selected these markers given their wide availability in clinical settings and evidence as markers of inflammation. CRP is measured clinically to assess cardiovascular disease risk and monitor infectious and inflammatory conditions (Ridker, 2007). White blood cell count is frequently checked to diagnose infections and as part of routine clinical assessments (Farhangi et al., 2013; Oda and Kawai, 2010). Fibrinogen has been a common marker used particularly in the study of cardiovascular disease but has also been found to play a key role in many other inflammatory/systemic diseases (Davalos and Akassoglou, 2012).Understanding associations between social relationships and inflammation in those with and without PTSD may help tailor interventions to improve health.
2. Methods and Materials
2.1. Participants
The Mind Your Heart Study is a prospective cohort study designed to examine the effects of PTSD on health outcomes in VA patients. Between 2008–2010, data were gathered from adult outpatients at the San Francisco VA Medical Center and the VA Palo Alto Health Care System, California. Patients who planned on leaving the area within three years or who did not have stable contact information were excluded. Because the study involved an exercise-treadmill test, potential participants were also excluded if they had had a myocardial infarction in the prior six months or were unable to walk one block. If participants reported symptoms of acute illness, their appointment was rescheduled. Overall, 1020 patients were recruited; of these, 104 were ineligible and 170 were eligible but did not complete enrollment, leaving 746 enrolled patients. 11 patients were excluded because they did not have complete PTSD assessments; 735 VA patients were included in the present analyses. The study was reviewed by the Committee on Human Research at the University of California, San Francisco, and all participants provided written informed consent.
2.2. Social isolation and perceived social support
Social isolation was assessed with the Berkman Syme Social Network Index (SNI), which assesses marital status; sociability (number and frequency of contacts with extended family and close friends); church group membership; and community organization membership. The index yields continuous scores (range 1–12) and also allows individuals to be classified based on standard cutoff scores established and used in previous research studies: socially isolated, moderately isolated, moderately integrated, and socially integrated (Berkman and Syme, 1979; Berkman and Glass, 2000). SNI data was missing for seven participants.
Perceived social support was measured using the validated 12-item Multidimensional Scale of Perceived Social Support (MSPSS), which assesses perceived support from family, friends, and significant others (Zimet et al., 1988). Respondents answer questions on a 5-point Likert-type scale ranging from 1 (strongly disagree) to 5 (strongly agree). A continuous score is then generated, ranging from 12–60, with a higher score indicating greater perceived social support. The scale has a reported alpha coefficient of 0.88 and test-retest reliability of 0.85 (Kazarian and McCabe, 1991). MSPSS data was missing for five participants.
2.3. Psychiatric diagnoses
PTSD symptoms were assessed with the Clinician-Administered PTSD Scale (CAPS), a structured interview measure based on the DSM-IV (Weathers et al., 2001). PTSD was defined based on the standard CAPS cutoff score of >40 (Weathers et al., 2001; Wingenfeld et al., 2015). All diagnoses were made by trained clinical interviewers who calibrated their assessments under the supervision of a PhD-level clinical psychologist.
2.4. Inflammatory markers
Morning fasting venous blood samples were drawn from participants to measure levels of inflammatory biomarkers. hsCRP was measured using a BNII nephelometer (Siemens Health Care Diagnostics, Tarrytown, New York) with interassay coefficients of variation < 5%. WBC count, measured as the number of WBC per volume of blood, was determined using the Beckman Coulter LH 750 analyzer (Beckman Coulter, Inc., California) with an interassay coefficient of variation < 2%. Serum fibrinogen concentrations were obtained by the Clauss assay with coefficients of variation < 3%. We were missing data for seven participants for hsCRP, four participants for WBC count, and four participants for fibrinogen.
2.5. Covariates
Self-report questionnaires were administered to all patients to assess demographics, including age, sex, race, income, and education. Medications were obtained through self-report, including use of statins and antidepressants (i.e. selective serotonin and norepinephrine reuptake inhibitors, tricyclics, and atypicals). Comorbid depression was assessed using modules of the World Health Organization Composite International Diagnostic Interview (CIDI) Computer Assisted Personal Interview (Version 21.1.1), which generated diagnoses based on Diagnostic and Statistical Manual-IV (DSM-IV) criteria (Wittchen, 1994). History of comorbid health conditions and disease events was obtained through self-report. Participants were asked to report whether a doctor or nurse had diagnosed them with a series of conditions. We calculated a comorbidity severity score by including 1 point for each of the following categories of disorders: cardiovascular disease (heart attack, angina, congestive heart failure, other heart disease, or stroke); chronic lung disease (chronic obstructive lung disease or other chronic lung disease); diabetes; neurologic disease (Parkinson’s, dementia, or other neurologic disease); renal disease; liver disease (hepatitis, cirrhosis, or other liver disease); AIDS/HIV; and cancer excluding skin cancer. Kidney function was also assessed because impaired renal function has been associated with systemic inflammation, such as elevated hsCRP (Ix et al., 2003). This was calculated from serum creatinine levels obtained through fasting morning venous blood samples using the Modification of Diet in Renal Disease (MDRD) equation (Levey et al., 2000).
Questionnaires were used to assess health behaviors; including current tobacco and alcohol use, physical activity, and sleep quality (Cohen et al., 2009). Participants were asked to indicate on a 6-point Likert Scale how often they engaged in vigorous activity for at least 15–20 minutes at a time. As in previous research, the variable was dichotomized with physical inactivity defined as being not at all or a little active (Cohen et al., 2009). Self-report has been shown to be a reliable method of assessing physical activity and has demonstrated excellent construct validity (Jackson et al., 2007). Participants self-rated their overall sleep quality in the prior month, using a 5-point Likert Scale with ratings of “very” or “fairly bad” being coded as poor sleep quality as in previous research (Cohen et al., 2009). The Alcohol Use Disorders Identification Test-consumption questions (AUDIT-C) was used to identify possible problematic alcohol use, using the recommended cut-off scores of 3 for women and 4 for men (Cohen et al., 2009). BMI was assessed by a trained technician. Self-reported use of immunosuppressant or steroid medication was recorded and then verified by chart review. There were nine responses missing for physical activity; 10 for sleep quality; 27 for alcohol use; 10 for smoking; and none for BMI. All covariates had participant response rates of 96% or higher.
2.6. Statistical analysis
Differences in participants’ characteristics by SNI category were assessed using ANOVA for continuous data and Chi-square tests for categorical data. A series of hierarchical linear regression models were used to examine associations of objective and subjective measures of social support (i.e. SNI and MSPSS score) with inflammatory markers. Bootstrapped models were constructed to examine the interaction between social support measures and PTSD using the SPSS macro PROCESS (Hayes and Little, 2018). While the primary inflammation analyses for SNI were completed using the continuous score, illustration of results were done using the standardized categorical cut-offs to better visualize the relationships. All models were adjusted for age, gender, and race. We also analyzed whether results were independent of additional confounders, including income, education, and kidney function. Given their potential effect on inflammatory markers and patient’s symptomatology, the use of statins and antidepressants were also included in this second series of analyses. Furthermore, we examined the potential role of health behaviors by including them as covariates in our models if they were associated with inflammatory markers at p ≤ 0.10 in the full sample. Finally, we ran sensitivity analyses excluding all participants with raw hsCRP values greater than 10 mg/L (n = 24) and individuals taking immunosuppressant or steroid medications (n = 17) to exclude the possibility of acute inflammation due to infection or injury influencing our results. Lastly, given the potential effects of health comorbidities and depression on chronic inflammation as well as patients’ social status, we reran our primary analyses further adjusting for the comorbidity index and depression. We did not adjust for depression and anxiety in our models because their symptoms overlap with PTSD symptoms and therefore can act as a marker of PTSD severity. For all analyses, hsCRP, WBC count, and fibrinogen were log-transformed to produce normal distributions. As PROCESS generates unstandardized coefficients for the conditional effects, standardized betas were subsequently calculated in Stata 15.0 to better compare the relative effects between the three inflammatory markers. All other statistical analyses were performed with SPSS 24.0.
3. Results
3.1. Sample characteristics
Participants had a mean age of 58 ± 11 and were 94% male and 59% white, and 35% had a current PTSD diagnosis. Socially isolated individuals comprised 26% of the sample; moderately isolated individuals comprised 49% of the sample; moderately integrated individuals comprised 16%; and socially integrated individuals comprised 9% (Table 1). Among the potential confounding variables, there were significant differences in age, income, kidney function, and statin use across SNI score. Among health behaviors, there were significant differences in smoking. For perceived social support, as determined by MSPSS score, there were significant differences in sex, income, and antidepressant use among confounders as well as significant differences in smoking, physical inactivity, alcohol, sleep, and BMI among health behaviors (p’s <.05). Additionally, there was also a significant association between perceived social support and social isolation (r = 0.38, p<.001).
Table 1.
Demographic and Clinical Characteristics by Social Network Index (SNI) Categorya
| Characteristic | SNI = 1 (n = 187) | SNI = 2 (n = 360) | SNI = 3 (n = 114) | SNI = 4 (n = 67) | |
|---|---|---|---|---|---|
|
| |||||
| Mean ± SD or N (%) or Min - Max | Mean ± SD or N (%) or Min - Max | Mean ± SD or N (%) or Min - Max | Mean ± SD or N (%) or Min - Max | p valueb | |
| Social network index raw score | 1.0 ± 0.0 | 2.9 ± 1.4 | 6.1 ± 0.3 | 9.2 ± 1.4 | <0.001 |
| MSPSS Score | 35.5 ± 12.7 | 39.9 ± 12.2 | 48.9 ± 7.9 | 45.0 ± 11.3 | <0.001 |
| Age (years) | 57.0 ± 10.0 | 57.7 ± 11.5 | 60.1 ± 11.7 | 64.4 ± 11.6 | <0.001 |
| Male sex, n (%) | 177 (94.7%) | 336 (93.3%) | 109 (95.6%) | 66 (98.5%) | 0.35 |
| White race, n (%) | 107 (57.8%) | 200 (56.7%) | 82 (71.9%) | 34 (51.5%) | 0.02 |
| eGFR (mL/min/1.73 m2) | 84.1 ± 23.0 | 84.2 ± 22.0 | 81.4 ± 21.1 | 77.4 ± 20.4 | 0.09 |
| College graduate, n (%) | 46 (24.7%) | 106 (29.4%) | 47 (41.2%) | 18 (26.9%) | 0.02 |
| Annual income <$20,000, n (%) | 89 (47.6%) | 120 (33.6%) | 12 (10.5%) | 8 (12.1%) | <0.001 |
| Posttraumatic Stress Disorder, n (%) | 72 (38.5%) | 123 (34.2%) | 37 (32.5%) | 21 (31.3%) | 0.61 |
| CAPS Score past month | |||||
| CAPS Score total | 30.3 ± 33.3 | 27.8 ± 31.1 | 27.1 ± 29.5 | 23.9 ± 30.2 | 0.53 |
| CAPS Re-experiencing | 7.5 ± 9.3 | 7.5 ± 9.3 | 7.2 ± 9.6 | 5.9 ± 8.6 | 0.59 |
| CAPS Avoidance | 12.6 ± 14.5 | 10.3 ± 12.7 | 9.5 ± 11.2 | 8.6 ± 11.8 | 0.07 |
| CAPS Hyperarousal | 10.2 ± 11.3 | 9.8 ± 11.0 | 10.4 ± 10.7 | 9.5 ± 11.5 | 0.92 |
| Health related behaviors | |||||
| Current smoker, n (%) | 62 (33.5%) | 90 (25.1%) | 13 (11.5%) | 14 (20.9%) | <0.001 |
| Physical inactivity, n (%) | 61 (32.8%) | 103 (28.8%) | 31 (27.4%) | 15 (22.4%) | 0.41 |
| Problematic alcohol use, n (%) | 77 (42.8%) | 137 (39.7%) | 54 (47.8%) | 27 (40.3%) | 0.49 |
| Poor sleep quality, n (%) | 85 (46.0%) | 129 (36.0%) | 41 (36.3%) | 25 (37.3%) | 0.14 |
| Body mass index (kg/m2) | 28.7 ± 5.7 | 29.0 ± 5.7 | 29.5 ± 5.4 | 29.3 ± 5.1 | 0.66 |
| Inflammatory markers | |||||
| hs-CRP Range (mg/L)c | 0.16 – 44.0 | 0.16 – 88.0 | 0.16 – 24.0 | 0.16 – 21.1 | 0.09 |
| WBC count Range (x109/L)c | 2.4 – 15.4 | 2.8 – 18.2 | 3.2 – 14.0 | 3.4 – 11.3 | 0.07 |
| Fibrinogen Range (mg/dL)c | 154 – 660 | 91 – 761 | 182 – 626 | 196 – 462 | 0.26 |
| Medication use | |||||
| Statins, n (%) | 57 (30.5%) | 121 (33.8%) | 60 (52.6%) | 33 (49.3%) | <.001 |
| Antidepressants, n (%) | 55 (30.7%) | 86 (48.0%) | 23 (12.9%) | 67 (9.2%) | 0.29 |
| Comorbidities | |||||
| Depression, n (%) | 53 (29.1%) | 98 (28.2%) | 23 (20.5%) | 7 (10.8%)` | 0.01 |
| Comorbidity index | 0.95 ± 1.00 | 1.00 ± 1.00 | 1.01 ± 1.00 | 1.20 ± 1.10 | 0.13 |
Notes. SD, standard deviation; MSPSS, Multidimensional Scale of Perceived Social Support; eGFR, estimated glomerular filtration rate; CAPS, Clinically-Administered PTSD Scale; hs-CRP, high-sensitivity C-reactive protein; WBC, white blood cell.
Socially isolated (SNI = 1), moderately isolated (SNI = 2), moderately integrated (SNI = 3), socially integrated (SNI = 4).
p-values are based on chi squared for ratios and ANOVA for continuous variables.
Range refers to the lowest-highest levels of inflammatory markers in each group
3.2. PTSD and social support
Surprisingly, there were no differences between people with and without PTSD in continuous SNI scores (mean 3.28 mg/L ± 2.53 in those with PTSD vs. 3.61 mg/L ± 2.59 in those without PTSD, p =0.10). Moreover, there were no significant differences in distribution of SNI categories between people with and without PTSD (χ = 1.82, p = 0.61). For example, compared to participants without PTSD, those with PTSD were not more likely to be in the socially isolated category (28.5% vs. 24.2%, z = 1.25, p = 0.21) or in the socially integrated category (8.3% vs. 9.7%, z = 0.61, p = 0.54). In contrast, individuals with PTSD did report lower levels of perceived social support by MSPSS (mean 36.6 ± 12.4 vs. 43.5 ± 12.2, t = 7.26, p < 0.01).
3.3. Social isolation and inflammation
We examined whether social isolation was associated with inflammation by comparing levels of inflammatory markers across SNI scores. When adjusting for age, gender and race, social isolation tended to be associated with higher levels of WBC count (β = 0.08, p = 0.03) and fibrinogen (β = 0.08, p = 0.04) but not hsCRP (β = 0.06, p = 0.13). However, after repeating our analyses further controlling for education, income, and kidney function, statin and antidepressant use, social isolation was not significantly associated with any of the inflammatory markers - hsCRP (β = 0.04, p = 0.28), WBC count (β = 0.08, p = 0.09), and fibrinogen (β = 0.07, p = 0.07).
3.4. Perceived social support and inflammation
We also examined whether lack of perceived support was associated with inflammation by examining associations of MSPSS scores with levels of inflammatory markers. Perceived support was not significantly associated with any inflammatory marker in models adjusted for age, gender, and race (β = −0.05, p = 0.16 for hsCRP; β = −0.04, p = 0.33 for WBC count; β = −0.02, p = 0.51 for fibrinogen), or in those additionally adjusted for education, income, kidney function, statins and antidepressant use (β = −0.02, p = 0.58 for hsCRP; β = −0.03, p = 0.51 for WBC count; β = −0.02, p = 0.52 for fibrinogen).
3.5. PTSD as a moderator of the relationship between social support and inflammation
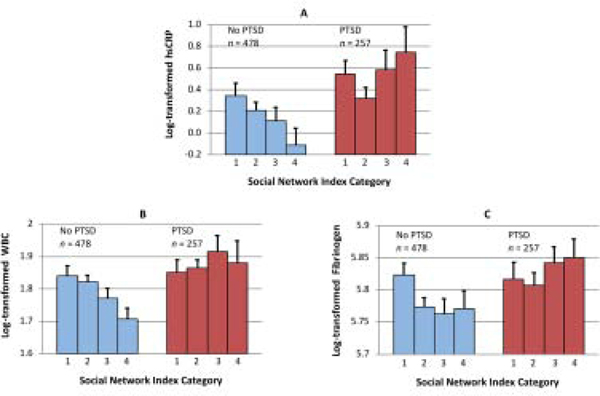
In our moderation analyses, we found that PTSD status moderated the relationship between SNI score and hsCRP (b = 0.09, SE = 0.04, p = 0.02), WBC count (b = 0.02, SE = 0.01, p = 0.02), and fibrinogen (b = 0.02, SE = 0.01, p = 0.04) after adjusting for age, gender and race. In line with our moderating results and a priori goal to examine associations of social support with inflammatory markers separately in individuals with and without PTSD, we examined associations of SNI scores with inflammatory markers in separate models for people with versus without PTSD. Among those without PTSD, greater social isolation was significantly associated with elevated levels of all inflammatory markers (β = 0.12, p = 0.01 for hsCRP; β = 0.14, p = 0.002 for WBC count; β = 0.13, p = 0.01 for fibrinogen). In contrast, social isolation was not associated with any inflammatory marker among those with PTSD (β = 0.08, p = 0.20 for hsCRP; β = 0.04, p = 0.53 for WBC count, β = 0.003, p = 0.69 for fibrinogen) (Figure 1A–C). When additionally adjusting for education, income, kidney function, and statin and antidepressant use, PTSD status remained a significant moderator of the relationship between SNI score and all inflammatory markers [hsCRP (b = 0.09, SE = 0.04, p = 0.01), WBC count (b = 0.02, SE = 0.01, p = 0.01), and fibrinogen (b = 0.02, SE = 0.01, p = 0.02)]. Among those without PTSD, social isolation remained associated with elevated hsCRP (β = 0.09, p = 0.048), WBC count (β = 0.13, p = 0.01) and fibrinogen (β = 0.12, p = 0.01). There were no significant associations between social isolation and inflammation among those with PTSD (p’s >0.10). A similar pattern of results was found after adjusting for health behaviors, with PTSD status continuing to significantly moderate the relationship between SNI score and all inflammatory markers such that social isolation was associated with all inflammatory markers in those without PTSD, but not those with PTSD.
Figure 1A-C.
Relationship between Social Network Index (SNI) Category and Inflammation by Posttraumatic Stress Disorder (PTSD) Status. Figure shows mean levels with standard errors of log-transformed inflammatory markers, including high sensitivity C-reactive protein (hsCRP), white blood cell (WBC) count, and fibrinogen. SNI of 1 is most socially isolated, while SNI of 4 is most socially integrated.
As an exploratory analysis, we then examined if individuals with PTSD, irrespective of their level of social integration, had similar levels of inflammation as socially isolated individuals without PTSD. We compared levels of inflammatory markers between all individuals with PTSD and individuals without PTSD who were in the socially isolated category. Between these two groups, t-tests revealed no significant differences in inflammation (p = 0.39 for hsCRP, p = 0.37 for WBC, p = 0.83 for fibrinogen). Thus, individuals with PTSD on the whole had levels of inflammation comparable to those of socially isolated individuals without PTSD.
In analyses focused on perceived social support, PTSD did not moderate the relationship for any inflammatory marker (b = 0.006, SE = 0.007, p = 0.38 for hsCRP; b = 0.003, SE = 0.002, p = 0.07 for WBC; b = 0.001, SE = 0.001, p = 0.28 for fibrinogen). Moreover, perceived social support was not associated with any inflammatory marker in individuals with and without PTSD considered separately (p’s > 0.10).
3.6. Sensitivity Analyses
We omitted individuals with hsCRP levels > 10 mg/L (n = 24) to exclude the possibility of acute inflammation secondary to infection or injury accounting for our pattern of results. As in our main analyses, relationships between social isolation and elevated inflammatory markers were not significant in models including all participants. Moreover, PTSD remained a significant moderator of the relationship between SNI score for hsCRP, WBC count, and fibrinogen and SNI scores were significantly associated with inflammatory markers in those without but not with PTSD. We also ran our models excluding individuals those who were taking immunosuppressant or steroid medications (n = 17). Again, the pattern of results was similar, although there was now a significant association of social isolation and both WBC count and fibrinogen among all participants (p’s = 0.04, see Supplementary Table 1 for details). However, moderating effects and results stratifying by PTSD status had the same pattern as our main models. Lastly, rerunning our main models adjusted for comorbidities and depression did not substantially change the pattern of results, with social isolation having a significant association with WBC count among all participants in these analyses (p = 0.03), but moderating effects and PTSD-stratified results being similar (see Supplementary Table 2 for details). For these analyses, the comorbidity index was significantly associated with elevated hsCRP (β = 0.11, p = 0.003) and WBC count (β = 0.08, p = 0.03) but not fibrinogen (p = 0.49). Depression was associated with elevated WBC count (β = 0.09, p = 0.02), but not hsCRP or fibrinogen (p’s > 0.10). Additionally, rerunning our main models with our small sample of women excluded also did not change our pattern of results. MSPSS analyses were similar for all sensitivity analyses as well.
4. Discussion
In the present study of 735 Veterans Affairs patients, we found that social isolation was associated with higher levels of inflammatory markers in participants without PTSD, but not in those with PTSD. This pattern of findings was robust to adjustment for race, education, income, kidney function, health behaviors, exclusion of individuals with hsCRP levels > 10 mg/L, and exclusion of individuals taking anti-inflammatory medications. Notably, we found that individuals with PTSD had average levels of inflammation similar to those of socially isolated people without PTSD. We found no associations between perceived social support and inflammation in either group. To our knowledge, we are the first to show that the relationship between social integration and lower levels of inflammation is not present among people with PTSD.
Our findings are consistent with previous studies linking social isolation with increased levels of systemic inflammatory markers in otherwise healthy individuals (Ford et al., 2006; McDade et al., 2006). In our study, socially isolated individuals without PTSD had higher levels of hsCRP, WBC count, and fibrinogen compared to socially integrated individuals. As all of these inflammatory markers are associated with increased risk for physical (Lowe, 2001; Pearson et al., 2003) and mental (Dantzer et al., 2008; Ribeiro-Santos et al., 2014) illness, social isolation may represent a modifiable risk factor for morbidity and mortality in those without PTSD. In contrast, low perceived social support was not associated with elevated inflammation in the current study in patients with or without PTSD. While there have been studies that showed modest associations between low perceived social support and elevated levels of inflammation (Mezuk et al., 2010), our study is in line with other studies demonstrating no relationship between low perceived social support and elevated inflammatory markers (McDade et al., 2006). These contrasting findings for objective and perceived social support may have implications for the design of public health and clinical socially supportive interventions.
In our sample, social isolation was not associated with higher levels of inflammation among individuals with PTSD. However, we also found that mean levels of inflammatory markers in individuals with PTSD were similar to those observed in individuals without PTSD who were socially isolated, with both groups demonstrating elevated inflammation. Individuals with PTSD may be more likely to experience stress as a result of their social relationships; friends and family may cause stress by avoiding discussing the illness, responding critically, acting in an overprotective manner, or provoking experiences of irritability or anger (Graham et al., 2007). Those with PTSD may be especially prone to negative thoughts and emotions following stressful social situations (Hofmann et al., 2003), which may in turn promote inflammation (Yang and Kim, 2015). Inflammation may also be part of the pathophysiology of PTSD itself, and therefore upstream of and not modifiable by, social relationships (Passos et al., 2015). In particular, because inflammatory markers predict PTSD symptomology prospectively (Eraly et al., 2014) and may be essential to the development of PTSD (Rau et al., 2005), PTSD-related inflammation may be a reason that social integration does not appear to be associated with lower inflammatory markers in these individuals.
4.1. Limitations
Our results should be interpreted in the light of several limitations. First, the cross-sectional study design limits our ability to make conclusions about the causal direction of the relationships between social support and inflammation. Moreover, we could not investigate whether there were individuals with PTSD who benefitted from social integration longitudinally, such that they had reductions in levels of inflammation over time. Second, we assessed health behaviors at a single time point using mostly self-report measures, which may be biased towards underestimation, particularly among older males (Newell et al., 1999). Third, we were limited in our ability to adjust for the many types and degrees of severity of chronic health conditions that could influence levels of inflammation, as only self-reported measures of a limited number of conditions were available. Fourth, inflammation is a complex process involving numerous mediators and pathways. Our study included three markers of inflammation that we believe are helpful to evaluate as they are widely available clinically. However, they give an incomplete picture of inflammation and repeating these analyses in studies with inflammatory cytokines and additional biomarkers will be important. Finally, as only 6% of participants were female and all patients were VA patients, findings may not be generalizable to females or to the general population.
5. Conclusions
In the present study, we found that individuals with PTSD had elevated inflammation regardless of level of social integration, whereas social integration was associated with reduced levels of inflammatory markers among individuals without PTSD. Further research could help determine if interventions to improve the nature and quality of social support would be effective in reducing inflammation in those with or without PTSD.
Supplementary Material
Highlights.
PTSD status moderated the relationship between social integration and inflammation
Social integration was associated with lower inflammation in people without PTSD
In PTSD, there was no association between social integration and inflammation
Perceived social support was not associated with levels of inflammation
Acknowledgements
The authors wish to thank Dr. Mary Whooley, the Mind Your Heart Study participants, and Mind Your Heart Study support staff, who made this research possible. BC and the Mind Your Heart Study were supported by the National Heart Lung and Blood Institute, the Northern California Institute for Research and Education, the Brain and Behavior Research Foundation, the Department of Defense, the American Heart Association, and the Irene Perstein Foundation, and the University of California, San Francisco. AOD was supported by a U.S. National Institute of Mental Health K01 Career Development Award (K01MH109871) and a UC Hellman Fellowship. AA and JL were funded by the UCSF Resource Allocation Program for Trainees.
Financial support: The Mind Your Heart Study is funded by the National Heart, Lung, and Blood Institute (to BEC, K23 HL 094765-0); the Irene Perstein Foundation; American Heart Association Clinical Research Program; the Brain and Behavior Research Foundation; NCIRE - The Veterans Health Research Institute; and departmental funds from the University of California, San Francisco. JEL was supported by the Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship. AJA was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (TL1 TR001871), AOD was supported by the National Institute of Mental Health (K01MH109871).
Footnotes
Ethical standards: The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Conflict of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berkman LF, Syme SL, 1979. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol. 109, 186–204. [DOI] [PubMed] [Google Scholar]
- Berkman LF, Glass T, 2000. Social integration, social networks, social support, and health. Social Epidemiology. 1, 137–173. [Google Scholar]
- Boscarino JA, 2008. A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: implications for surveillance and prevention. Psychosom Med. 70, 668–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, et al. , 2015. The neuroendocrinology of social isolation. Annu Rev Psychol. 66, 733–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen BE, et al. , 2009. Posttraumatic stress disorder and health-related quality of life in patients with coronary heart disease: findings from the Heart and Soul Study. Arch Gen Psychiatry. 66, 1214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, et al. , 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 9, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Akassoglou K, 2012. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. 34, 43–62. [DOI] [PubMed] [Google Scholar]
- Edmondson D, et al. , 2013. Posttraumatic stress disorder and risk for coronary heart disease: a meta-analytic review. Am Heart J. 166, 806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraly SA, et al. , 2014. Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry. 71, 423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, et al. , 2011. Relationships and Inflammation across the Lifespan: Social Developmental Pathways to Disease. Soc Personal Psychol Compass. 5, 891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhangi MA, et al. , 2013. White blood cell count in women: relation to inflammatory biomarkers, haematological profiles, visceral adiposity, and other cardiovascular risk factors. J Health Popul Nutr. 31, 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford ES, Loucks EB, Berkman LF, 2006. Social integration and concentrations of C-reactive protein among US adults. Ann Epidemiol. 16, 78–84. [DOI] [PubMed] [Google Scholar]
- Graham JE, Christian LM, Kiecolt-Glaser JK, 2007. Close Relationships and Immunity. In: Psychoneuroimmunology. Vol., Ader R, ed.êds. Elsevier Academic Press, Burlington, pp. 781–798. [Google Scholar]
- Hansson GK, 2005. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 352, 1685–95. [DOI] [PubMed] [Google Scholar]
- Hayes AF, Little TD, 2018. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach, Vol., The Guilford Press, New York, New York. [Google Scholar]
- Helminen A, et al. , 1997. Social network in relation to plasma fibrinogen. J Biosoc Sci. 29, 129–39. [DOI] [PubMed] [Google Scholar]
- Heppner FL, Ransohoff RM, Becher B, 2015. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 16, 358–72. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Litz BT, Weathers FW, 2003. Social anxiety, depression, and PTSD in Vietnam veterans. J Anxiety Disord. 17, 573–82. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB, 2010. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 7, e1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ix JH, et al. , 2003. Association between renal insufficiency and inducible ischemia in patients with coronary artery disease: the heart and soul study. J Am Soc Nephrol. 14, 3233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AW, et al. , 2007. Construct validity evidence for single-response items to estimate physical activity levels in large sample studies. Res Q Exerc Sport. 78, 24–31. [DOI] [PubMed] [Google Scholar]
- Kazarian SS, McCabe SB, 1991. Dimensions of social support in the MSPSS: Factorial structure, reliability, and theoretical implications. Journal of Community Psychology. 19, 150–160. [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Hantsoo L, 2010. Close relationships, inflammation, and health. Neurosci Biobehav Rev. 35, 33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibig SD, Whooley MA, Gross JJ, 2014. Social integration and mortality in patients with coronary heart disease: findings from the Heart and Soul Study. Psychosom Med. 76, 659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AS, et al. , 2000. A simplified equation to predict glomerular filtration rate from serum creatinine. Journal of the American Society of Nephrology. 11, 155A. [Google Scholar]
- Lowe GD, 2001. The relationship between infection, inflammation, and cardiovascular disease: an overview. Ann Periodontol. 6, 1–8. [DOI] [PubMed] [Google Scholar]
- McDade TW, Hawkley LC, Cacioppo JT, 2006. Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: the Chicago health, aging, and social relations study. Psychosom Med. 68, 376–81. [DOI] [PubMed] [Google Scholar]
- Mezuk B, Diez Roux AV, Seeman T, 2010. Evaluating the buffering vs. direct effects hypotheses of emotional social support on inflammatory markers: the multi-ethnic study of atherosclerosis. Brain Behav Immun. 24, 1294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell SA, et al. , 1999. The accuracy of self-reported health behaviors and risk factors relating to cancer and cardiovascular disease in the general population: a critical review. Am J Prev Med. 17, 211–29. [DOI] [PubMed] [Google Scholar]
- O’Donovan A, et al. , 2015. Elevated risk for autoimmune disorders in iraq and afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. 77, 365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, et al. , 2017. Current posttraumatic stress disorder and exaggerated threat sensitivity associated with elevated inflammation in the Mind Your Heart Study. Brain Behav Immun. 60, 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda E, Kawai R, 2010. Comparison between high-sensitivity C-reactive protein (hs-CRP) and white blood cell count (WBC) as an inflammatory component of metabolic syndrome in Japanese. Intern Med. 49, 117–24. [DOI] [PubMed] [Google Scholar]
- Passos IC, et al. , 2015. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry. 2, 1002–12. [DOI] [PubMed] [Google Scholar]
- Pearson TA, et al. , 2003. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 107, 499–511. [DOI] [PubMed] [Google Scholar]
- Raison CL, et al. , 2013. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 70, 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau V, DeCola JP, Fanselow MS, 2005. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev. 29, 1207–23. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Santos A, Lucio Teixeira A, Salgado JV, 2014. Evidence for an immune role on cognition in schizophrenia: a systematic review. Curr Neuropharmacol. 12, 273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, 2007. Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: implications for longevity. Nutr Rev. 65, S253–9. [DOI] [PubMed] [Google Scholar]
- Tabas I, Glass CK, 2013. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 339, 166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR, 2001. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety. 13, 132–56. [DOI] [PubMed] [Google Scholar]
- Wingenfeld K, et al. , 2015. Effect of current and lifetime posttraumatic stress disorder on 24-h urinary catecholamines and cortisol: results from the Mind Your Heart Study. Psychoneuroendocrinology. 52, 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen HU, 1994. Reliability and validity studies of the WHO--Composite International Diagnostic Interview (CIDI): a critical review. J Psychiatr Res. 28, 57–84. [DOI] [PubMed] [Google Scholar]
- Yang SK, Kim E, 2015. The Relationship among the Coping Style, Social Support, and Post-Traumatic Stress Disorder in Breast Cancer Patients Treated with Chemotherapy. Korean J Hosp Palliat Care. 18, 35–41. [Google Scholar]
- Zimet GD, et al. , 1988. The Multidimensional Scale of Perceived Social Support. Journal of Personality Assessment. 52, 30–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.