Abstract
Background:
Left ventricular ejection fraction (LVEF) is calculated from volumetric change without representing true myocardial properties. Strain echocardiography has been used to objectively measure myocardial deformation. Myocardial strain can give accurate information about intrinsic myocardial function, and it can be used to detect early-stage cardiovascular diseases, monitor myocardial changes with specific therapies, differentiate cardiomyopathies, and predict the prognosis of several cardiovascular diseases. Sacubitril/valsartan has been shown to improve mortality and reduce hospitalizations in patients with heart failure with reduced ejection fraction (HFrEF). The effect of sacubitril/valsartan angiotensin receptor neprilysin inhibitor (ARNI) on left ventricular (LV) ejection fraction (EF) and torsion dynamics in HFrEF patients has not been previously described.
Methods:
The study involved 73 patients with HFrEF, for all patients Full history was taken, full clinical examination was done. Baseline vital signs, ECG, NYHA classification, conventional echocardiography and STE were done at baseline study and after 6 and 11 months.Basal and apical LV short-axis images were acquired for further off-line analysis. Using commercially available two-dimensional strain software, apical, basal rotation, and LV torsion were calculated.
Results:
ARNI group of patients showed improvement of symptoms, LV global longitudinal strain (LVGLS)% and diastolic parameters including, E/A, E/e', TV, untwist onset and rate after 6 months of therapy in comparison to the traditionally treated patients. The improvement continued for 11 months with in additional significant improvement of systolic parameters in the form of LVGLS%, EF%, Twist, Apical and basal rotations, main dependent parameters for improvement of EF% was LVGLS% and Apical rotation.
Conclusion:
To the best of our knowledge, this is the first study to demonstrate that therapy with sacubitril/valsartan in HFrEF patients could create a state of gradual and chronic LV deloading which cause relieving of myocardial wall tensions and decreasing the LV end diastolic pressure this state could cause cardiac reverse remodeling and reestablishment of starling forces proprieties of LV myocardium, which lead to increase of LV EF.
Keywords: Angiotensin receptor neprilysin inhibitor, heart failure with reduced ejection fraction, left ventricle, mics, strain echocardiography, torsion mechanics
BACKGROUND
Left ventricular (LV) systolic function assessment is the cornerstone of an echocardiographic examination. Left ventricular ejection fraction (LVEF) is the most commonly employed. It results from the combined action of basal and apical rotation, longitudinal and circumferential contraction, and radial thickening. However, LVEF has many limitations; some of them related to the definition itself and others to imaging techniques.[1,2]
The characteristic double helical structure of myocardial fibers results in systolic rotation of the base and apex of the LV in opposite directions along its longitudinal axis, and the algebraic subtraction of this rotation causes LV twist. Due to this muscular movement, the base of the LV moves toward the apex, producing a longitudinal LV shortening.[3] Therefore, systolic ventricular contraction results from the combined and simultaneous action of twisting and shortening of LV (Myocardial torsion) which is a fundamental component of cardiac function.[4,5]
Speckle tracking echocardiography[6] (STE) is a useful echocardiographic tool for evaluating myocardial function, due its high spatial and temporal resolution, good intra- and inter-observer reproducibility,[7] being independent of the insonation angle,[8] and it is not affected by translational movements of the heart. It also employed in assessing the torsion mechanics including twist and untwist which has emerged as novel reliable quantitative parameters for the assessment of LV function. Twist and rotational parameters may contribute useful comprehensive information beyond the conventional indices of LV function.[9] Our aim in this study is to assess the effect of sacubitril/valsartan on the myocardial mechanics of the left ventricle in heart failure with reduced ejection fraction (HFrEF) patients.
METHODS
Subjects
The study involved 73 patients with HFrEF where they were randomly assigned into two matched groups. Group (1) including 37 patients with traditional treatment of heart failure and the other Group (2) including 36 patients were treated with sacubitril/valsartan and other evidenced-based medical treatments. Full history was taken, and full clinical examination was done. Baseline vital signs, electrocardiogram (ECG), New York Heart Association (NYHA) classification, conventional echocardiography, and STE were done at baseline study and after 6 and 11 months.
Inclusion criteria:
Written informed consent must be obtained before any assessment was performed
Men and women ≥18 years of age
LVEF ≤40% subjects who were candidates for on-label sacubitril/valsartan treatment per standard of care
NYHA functional Class II-IV
LVEF ≤40% through any local measurement within the past 6 months using echocardiography. If a subject was on a loop diuretic, they must be on a stable dose for 2 weeks prior to baseline.
Exclusion criteria:
Pregnant or nursing women
History of hypersensitivity to any of the study drugs
History of angioedema drug related or otherwise
Subjects with a heart transplant or ventricular assistance device or intent to transplant (on transplant list) or implant a VA
Subjects with a cardioresynchronization therapy device (cardiac resynchronization therapy [CRT]/CRT-Defibrillator) implanted within 6 months of screening visit
Subjects who were currently taking inotropic agents
Bile acid sequestering agents such as cholestyramine or colestipol were prohibited to avoid interference with study drug absorption
Any hospital admission/discharge related to heart failure within 2 weeks prior to baseline
The use of outpatient or inpatient intravenous diuretic therapy within 2 weeks prior to baseline
Potassium >5.2 mEq/L at screening.
Ethical clearance
The local ethics committee of the hosted university approved the study protocol and written consent was obtained from the patients.
Study period
The patients on this study were followed up during the period from February 2019 to December 2019.
Standard echo-Doppler study
Standard echo-Doppler was performed using a Vivid E9 ultrasound system (GE Vingmed Ultrasound AS, Horten, Norway). Cine-loops were recorded on DVDs for offline analysis (EchoPAC PC 6.0.0, GE Medical Systems). All the measurements were analyzed taking the average of three cardiac cycles. LV diameter and wall thickness were measured according to the criteria of the American Society of Echocardiography.[10]
The left atrium volume was determined by the biplane-area-length method.[11] Two-dimensional measurements of LV wall thickness were assessed in four segments (anterior and posterior interventricular septum, inferior, and anterolateral walls) at the mitral valve, papillary muscles, and apical levels by parasternal short-axis views. In addition, LV EF was calculated by the Simpson biplane method.[12] As measures of global LV diastolic function peak velocities at the early (peak E) and late (peak A) diastole, their ratio, deceleration time of the E wave and isovolumic relaxation time were assessed by pulsed-Doppler with the sample volume placed at the mitral valve leaflet tips and at the aortic outflow.[13] Finally, by pulsed tissue Doppler, peak early diastolic velocity on the septal part of the mitral annulus was measured (E′) and E/E′ ratio was calculated.
Speckle tracking imaging study
For the sexually transmitted disease study, the second-harmonic B-mode images of apical (4-chamber, 2-chamber, and 3-chamber) and short-axis (at the mitral valve and apical level) views were obtained. The LV endocardial border was manually traced at the end-systolic frame and a speckle-tracking region of interest was automatically selected. The width of the region of interest was adjusted as necessary to accommodate the total thickness of the LV wall. The computer automatically tracked stable objects in each frame using the sum of absolute differences algorithm. After these steps, the workstation computed and generated strain curves. For assessment of LV rotational mechanics through scanning and recording from the left parasternal short-axis view of both basal and apical short-axis planes to quantify basal and apical LV rotations using the same probe, with a frequency range 1.7–2.0 MHz at a high frame rate (range: 80–115 frame/s).
The basal level was marked as the plane showing the tips of mitral valve leaflets at its center with full-thickness myocardium surrounding the mitral valve. Then, the transducer was positioned one or two intercostal spaces more caudal and slightly lateral from the basal site to be perpendicular to the apical imaging plane.[14] The apical level was defined just proximal to the level of LV apical luminal obliteration at the end-systole. The cross-section must be as circular as possible. We must pay careful attention to ensure that full thickness of myocardium is imaged throughout the cardiac cycle.
To analyze twist and untwist parameters, from the basal and apical short axis, data set with a well-defined endocardial border and the regions of interest were adjusted to include all myocardial thickness without including the pericardium. The endocardial borders of both basal and short axis planes were manually traced and subsequently tracked by the software. If poor tracking quality was observed, the region of interest was readjusted until acceptable tracking was obtained. After processing, curves of basal and apical LV rotation, twist, twist rate, and untwist rate were automatically generated by the software (Excel; Microsoft Corporation, Redmond, Washington, USA). Twist was calculated as apical rotation relative to the basal rotation, with counter clockwise rotation as viewed from LV apex expressed as positive value and clockwise rotation as a negative value. Peak LV twist, peak LV twist rate (as first positive peak after R wave on ECG), and peak LV untwist rate (as the first negative peak after aortic valve closure) were recorded. Cardiac cycle length was measured as R–R interval. Time to peak twist rate was measured as time from R wave to peak twist rate, and time to peak untwist rate was measured as time from R wave to peak untwist rate.
Statistical analysis
Data were analyzed using Statistical Program for Social Science (SPSS) version 22.0 for windows (SPSS Inc., Chicago, IL, USA). Quantitative data of normal distribution were expressed as mean ± standard deviation (SD). Qualitative data were expressed as frequency and percentage. Independent-samples t-test of significance was used when comparing between two means of normally distributed data. Pearson correlation was used to measure the association between two quantitative variables. Multivariable regression analysis is used to predict values of one variable based on two or more other variables. The purpose of the test is to determine whether there is statistical evidence that the mean difference between paired observations on a particular outcome is significantly different from zero. Probability (P value): P < 0.05 was considered significant, P < 0.001 was considered as highly significant and P > 0.05 was considered insignificant.
RESULTS
Our prospective study basically included 73 patients diagnosed as HFrEF based on conventional echocardiography study at the outpatient clinic of Menoufia University Hospital, a total of 12 cases were excluded during the workflow of the follow-ups either because of three cases missed connections, four cases developed severe renal impairment and chronic kidney disease, three cases were uninterested to continue follow-up, and two cases died of sudden cardiac death. Sixty-one patients succeeded to complete the time table of the study, 34 cases were treated by Sacubitril/Valsartan Angiotensin receptor neprilysin Inhibitor (ARNI) group and 27 cases were treated by traditional therapy (Traditional group), data reveled that at baseline there were no significant difference in between the traditionally treated and ARNI treated groups regarding basic characteristics, demographic data, symptoms, conventional, and STE echocardiographic measurements [Table 1].
Table 1.
Comparison between angiotensin receptor neprilysin inhibitor and traditional group at baseline (start of therapy)
| Groups at baseline | n | Mean±SD | t | P |
|---|---|---|---|---|
| Age (years) | ||||
| AR | 34 | 68.03±11.414 | 1.868 | 0.067 |
| TT | 27 | 63.15±8.231 | ||
| LVEDV (mL) | ||||
| AR | 34 | 155.35±29.676 | −0.807 | 0.423 |
| TT | 27 | 161.30±27.091 | ||
| LVESV (mL) | ||||
| AR | 34 | 102.24±19.031 | −1.387 | 0.171 |
| TT | 27 | 109.59±22.385 | ||
| EF (%) | ||||
| AR | 34 | 34.76±3.619 | 1.931 | 0.061 |
| TT | 27 | 32.51±5.649 | ||
| Average LVGLS (%) | ||||
| AR | 34 | −9.765±1.827 | −1.829 | 0.072 |
| TT | 27 | −8.833±2.148 | ||
| TR velocity (m/s) | ||||
| AR | 34 | 2.732±0.300 | −1.437 | 0.156 |
| TT | 27 | 2.848±0.327 | ||
| E/A | ||||
| AR | 34 | 1.144±0.307 | −1.681 | 0.098 |
| TT | 27 | 1.278±0.311 | ||
| E/e’ | ||||
| AR | 34 | 10.62±1.875 | −1.867 | 0.067 |
| TT | 27 | 11.52±1.868 | ||
| DD grade | ||||
| AR | 34 | 2.18±0.521 | −0.656 | 0.514 |
| TT | 27 | 2.26±0.447 | ||
| Untwist rate (°/s) | ||||
| AR | 34 | −61.21±4.728 | −1.234 | 0.222 |
| TT | 27 | −59.74±4.443 | ||
| Untwist onset (ms) | ||||
| AR | 34 | 104.53±16.808 | −1.032 | 0.306 |
| TT | 27 | 109.11±17.723 | ||
| Twist | ||||
| AR | 34 | 8.874±1.468 | −0.521 | 0.604 |
| TT | 27 | 9.044±0.968 | ||
| Basal rotation | ||||
| AR | 34 | −3.5853±0.862 | 0.724 | 0.472 |
| TT | 27 | −3.7444±0.841 | ||
| Apical rotation | ||||
| AR | 34 | 4.515±1.399 | −0.537 | 0.593 |
| TT | 27 | 4.707±1.383 |
AR=ARNI group, TT=Traditional group, ARNI=Angiotensin receptor neprilysin inhibitor, LV=Left ventricular, SD=Standard deviation, LVEDV=LV end-diastolic volume, LVESV=LV end-systolic volume, EF=Ejection fraction, LVGLS=LV global longitudinal strain, TR=Tricuspid regurgitation, DD=Diastolic dysfunction
After 6 months, both the groups recalled for doing the first follow-up and there were remarkable differences in symptoms, conventional and STE echocardiography data [Table 2] there were very high statistically significant improvement in diastolic parameters either conventional E/A, E/e', Tricuspid velocity or STE specifically untwist parameters (Untwist onset, and rate). In addition the ejection fraction and global LV longitudinal strain also showed improvement. For the final recall after 11 months, the data showed exponential improvement for both diastolic and systolic parameters with very high statistically significant improvement of untwist and twist parameters with remarkable improvement of ejection in the ARNI group versus the traditionally treated patients [Table 3].
Table 2.
Comparison between angiotensin receptor neprilysin inhibitor and traditional group at 6 months follow up
| 6 months follow up | n | Mean±SD | t | P |
|---|---|---|---|---|
| LVEDV (mL) | ||||
| AR | 34 | 152.24±30.21 | −0.979 | 0.331 |
| TT | 27 | 159.52±27.03 | ||
| LVESV (mL) | ||||
| AR | 34 | 99.06±22.59 | −1.391 | 0.169 |
| TT | 27 | 107.15±22.51 | ||
| EF (%) | ||||
| AR | 34 | 35.76±3.77 | 2.042 | 0.046 |
| TT | 27 | 33.63±4.45 | ||
| Average LVGLS (%) | ||||
| AR | 34 | −10.118±1.77 | −2.045 | 0.045 |
| TT | 27 | −9.185±1.78 | ||
| TR velocity (m/s) | ||||
| AR | 34 | 2.565±0.31 | −2.901 | 0.005 |
| TT | 27 | 2.804±0.34 | ||
| E/A | ||||
| AR | 34 | 0.947±0.31 | −3.173 | 0.002 |
| TT | 27 | 1.181±0.27 | ||
| E/e’ | ||||
| AR | 34 | 9.21±1.84 | −3.919 | 0.000 |
| TT | 27 | 10.96±1.61 | ||
| Untwist rate (°/s) | ||||
| AR | 34 | −65.00±5.22 | −3.816 | 0.000 |
| TT | 27 | −60.15±4.55 | ||
| Untwist onset (ms) | ||||
| AR | 34 | 98.41±16.87 | −2.152 | 0.036 |
| TT | 27 | 107.48±15.67 | ||
| Twist | ||||
| AR | 34 | 8.979±1.31 | −0.302 | 0.764 |
| TT | 27 | 9.070±0.95 | ||
| Basal rotation | ||||
| AR | 34 | −3.559±0.85 | 1.018 | 0.313 |
| TT | 27 | −3.769±0.73 | ||
| Apical rotation | ||||
| AR | 34 | 4.477±1.29 | −0.718 | 0.475 |
| TT | 27 | 4.715±1.29 |
AR=ARNI group, TT=Traditional group, ARNI=Angiotensin receptor neprilysin inhibitor, SD=Standard deviation, LV=Left ventricular, LVEDV=LV end-diastolic volume, LVESV=LV end-systolic volume, EF=Ejection fraction, LVGLS=LV global longitudinal strain, TR=Tricuspid regurgitation
Table 3.
Comparison between angiotensin receptor neprilysin inhibitor and traditional group at 11 months follow up
| Groups | n | Mean±SD | t | P |
|---|---|---|---|---|
| LVEDV (mL) | ||||
| AR 11 | 34 | 152.24±30.21 | −0.979 | 0.331 |
| TT 11 | 27 | 159.52±27.03 | ||
| LVESV (mL) | ||||
| AR 11 | 34 | 99.06±22.59 | −1.391 | 0.169 |
| TT 11 | 27 | 107.15±22.51 | ||
| EF (%) | ||||
| AR 11 | 34 | 39.35±3.16 | 6.185 | 0.000 |
| TT 11 | 27 | 33.93±3.69 | ||
| Average LVGLS (%) | ||||
| AR 11 | 34 | −11.09±1.34 | −3.994 | 0.000 |
| TT 11 | 27 | −9.63±1.52 | ||
| Untwist rate (°/s) | ||||
| AR 11 | 34 | −65.00±5.22 | −3.816 | 0.000 |
| TT 11 | 27 | −60.15±4.55 | ||
| Untwist onset (ms) | ||||
| AR 11 | 34 | 98.42±16.87 | −2.152 | 0.036 |
| TT 11 | 27 | 107.48±15.67 | ||
| Twist | ||||
| AR 11 | 34 | 10.92±1.46 | 6.279 | 0.000 |
| TT 11 | 27 | 8.87±0.97 | ||
| Basal rotation | ||||
| AR 11 | 34 | −4.59±0.74 | −3.736 | 0.000 |
| TT 11 | 27 | −3.85±0.81 | ||
| Apical rotation | ||||
| AR 11 | 34 | 6.39±1.34 | 4.207 | 0.000 |
| TT 11 | 27 | 4.91±1.30 |
AR=ARNI group follow up after 11 months, TT=Traditional group follow up after 11 months, ARNI=Angiotensin receptor neprilysin inhibitor, SD=Standard deviation, LV=Left ventricular, LVEDV=LV end-diastolic volume, LVESV=LV end-systolic volume, EF=Ejection fraction, LVGLS=LV global longitudinal strain
On the other hand to show the pattern of temporal effect of ARNI therapy among the ARNI group itself, we made comparisons between the ARNI group at different follow-ups and it also showed exponential improvement from baseline, 6 months, and 11 months follow-up with maximal improvement of untwist parameters, twist and EF after 11 months of follow-up [Tables 4 and 5].
Table 4.
Comparison between baseline angiotensin receptor neprilysin inhibitor 0 and angiotensin receptor neprilysin inhibitor 6 after 6 months follow up
| Groups | n | Mean±SD | t | P |
|---|---|---|---|---|
| LVEDV (mL) | ||||
| AR 0 | 34 | 155.35±29.67 | 0.429 | 0.669 |
| AR 6 | 34 | 152.24±30.21 | ||
| LVESV (mL) | ||||
| AR 0 | 34 | 102.23±19.03 | 0.627 | 0.533 |
| AR 6 | 34 | 99.06±22.59 | ||
| EF (%) | ||||
| AR 0 | 34 | 34.77±3.62 | −1.124 | 0.265 |
| AR 6 | 34 | 35.77±3.72 | ||
| Average LVGLS (%) | ||||
| AR 0 | 34 | −9.77±1.83 | 0.811 | 0.420 |
| AR 6 | 34 | −10.12±1.77 | ||
| TR velocity (m/s) | ||||
| AR 0 | 34 | 2.73±0.30 | 2.271 | 0.026 |
| AR 6 | 34 | 2.57±0.31 | ||
| TR grade | ||||
| AR 0 | 34 | 2.50±0.93 | 1.848 | 0.069 |
| AR 6 | 34 | 2.12±0.77 | ||
| MR grade | ||||
| AR 0 | 34 | 2.71±0.84 | 2.951 | 0.004 |
| AR 6 | 34 | 2.12±0.81 | ||
| E/A | ||||
| AR 0 | 34 | 1.14±0.31 | 2.675 | 0.009 |
| AR 6 | 34 | 0.95±0.30 | ||
| E/e’ | ||||
| AR 0 | 34 | 10.62±1.88 | 3.135 | 0.003 |
| AR 6 | 34 | 9.21±1.84 | ||
| DD grade | ||||
| AR 0 | 34 | 2.18±0.52 | 2.651 | 0.010 |
| AR 6 | 34 | 1.82±0.58 | ||
| Untwist rate (°/s) | ||||
| AR 0 | 34 | −61.21±4.73 | 3.142 | 0.003 |
| AR 6 | 34 | −65.00±5.22 | ||
| Untwist onset (ms) | ||||
| AR 0 | 34 | 104.53±16.81 | 1.498 | 0.139 |
| AR 6 | 34 | 98.41±16.87 | ||
| Twist | ||||
| AR 0 | 34 | 8.88±1.49 | −0.313 | 0.755 |
| AR 6 | 34 | 8.98±1.31 | ||
| Basal rotation | ||||
| AR 0 | 34 | −3.56±0.86 | −0.123 | 0.902 |
| AR 6 | 34 | −3.56±0.85 | ||
| Apical rotation | ||||
| AR 0 | 34 | 4.52±1.39 | 0.117 | 0.907 |
| AR 6 | 34 | 4.48±1.29 |
AR 0 baseline, AR 6 6 months follow up. AR=ARNI group, ARNI=Angiotensin receptor neprilysin inhibitor, SD=Standard deviation, LV=Left ventricular, LVEDV=LV end-diastolic volume, LVESV=LV end-systolic volume, EF=Ejection fraction, LVGLS=LV global longitudinal strain, TR=Tricuspid regurgitation, DD=Diastolic dysfunction, MR=Mitral regurgitation
Table 5.
Comparison between angiotensin receptor neprilysin inhibitor 0, angiotensin receptor neprilysin inhibitor 6 and Angiotensin receptor neprilysin inhibitor 11 month’s follow-ups (ANOVA) test
| Descriptives | n | Mean±SD | SE | 95% CI for mean | F | P | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Lower bound | Upper bound | ||||||
| LVEDV (mL) | |||||||
| AR 0 | 34 | 155.35±29.68 | 5.09 | 145.00 | 165.71 | 0.11 | 0.875 |
| AR 6 | 34 | 152.24±31.21 | 5.18 | 141.70 | 162.77 | ||
| AR 11 | 34 | 150.13±30.01 | 5.12 | 140.53 | 161.54 | ||
| LVESV (mL) | |||||||
| AR 0 | 34 | 102.24±19.03 | 3.26 | 95.60 | 108.88 | 0.23 | 0.771 |
| AR 6 | 34 | 99.06±22.59 | 3.87 | 91.18 | 106.94 | ||
| AR 11 | 34 | 98.07±21.48 | 3.75 | 90.14 | 104.23 | ||
| EF (%) | |||||||
| AR 0 | 34 | 34.76±3.62 | 0.62 | 33.50 | 36.03 | 16.09 | 0.000 |
| AR 6 | 34 | 35.76±3.72 | 0.64 | 34.47 | 37.06 | ||
| AR 11 | 34 | 39.35±3.16 | 0.54 | 38.25 | 40.46 | ||
| Average LVGLS (%) | |||||||
| AR 0 | 34 | −9.76±1.83 | 0.31 | −10.40 | −9.13 | 5.84 | 0.004 |
| AR 6 | 34 | −10.12±1.76 | 0.30 | −10.73 | −9.50 | ||
| AR 11 | 34 | −11.09±1.34 | 0.23 | −11.56 | −10.62 | ||
| Untwist rate (°/s) | |||||||
| AR 0 | 34 | −61.21±4.73 | 0.81 | −62.86 | −59.56 | 6.38 | 0.002 |
| AR 6 | 34 | −63.00±5.11 | 0.86 | −63.81 | −61.15 | ||
| AR 11 | 34 | −65.00±5.22 | 0.89 | −66.82 | −63.18 | ||
| Untwist onset (ms) | |||||||
| AR 0 | 34 | 104.53±16.81 | 2.88 | 98.66 | 110.39 | 1.49 | 0.229 |
| AR 6 | 34 | 98.41±16.87 | 2.89 | 92.53 | 104.30 | ||
| AR 11 | 34 | 96.31±17.78 | 2.68 | 91.34 | 103.32 | ||
| Twist | |||||||
| AR 0 | 34 | 8.87±1.47 | 0.25 | 8.36 | 9.39 | 22.49 | 0.000 |
| AR 6 | 34 | 8.98±1.31 | 0.23 | 8.52 | 9.44 | ||
| AR 11 | 34 | 10.91±1.45 | 0.25 | 10.41 | 11.42 | ||
| Basal rotation | |||||||
| AR 0 | 34 | −3.59±0.86 | 0.15 | −3.89 | −3.28 | 17.50 | 0.000 |
| AR 6 | 34 | −3.56±0.85 | 0.15 | −3.86 | −3.26 | ||
| AR 11 | 34 | −4.59±0.73 | 0.13 | −4.84 | −4.33 | ||
| Apical rotation | |||||||
| AR 0 | 34 | 4.51±1.40 | 0.24 | 4.03 | 5.00 | 21.37 | 0.000 |
| AR 6 | 34 | 4.48±1.29 | 0.22 | 4.03 | 4.93 | ||
| AR 11 | 34 | 6.34±1.34 | 0.23 | 5.87 | 6.81 | ||
AR=ARNI group, ARNI=Angiotensin receptor neprilysin inhibitor, SD=Standard deviation, LV=Left ventricular, LVEDV=LV end-diastolic volume, LVESV=LV end-systolic volume, EF=Ejection fraction, LVGLS=LV global longitudinal strain, SE=Standard error, CI=Confidence interval
As regard LVEF of the ARNI group after 11 months, it was positively correlated with LV global longitudinal strain (LVGLS), Twist, Apical rotation, and negatively correlated with untwist onset, and rate and basal rotation [Table 6 and Figures 1-4].
Table 6.
Correlation of ejection fraction percentage and other echocardiographic variables in angiotensin receptor neprilysin inhibitor group after 11 months
| Correlations | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| EF (%) | LVEDV | LVESV | LVGLS (%) | Untwist rate | Untwist onset | Twist | Basal rotation | Apical rotation |
| Pearson correlation | 0.13 | −0.03 | 0.407* | −0.522** | −0.492** | 0.934** | −0.966** | 0.991** |
| Significant (two-tailed) | 0.48 | 0.88 | 0.02 | 0.002 | 0.003 | 0.000 | 0.000 | 0.000 |
| n | 34 | 34 | 34 | 34 | 34 | 34 | 34 | 34 |
*Correlation is significant at the 0.05 level (two-tailed), **Correlation is significant at the 0.01 level (two-tailed). LV=Left ventricular, LVEDV=LV end-diastolic volume, LVESV=LV end-systolic volume, LVGLS=LV global longitudinal strain, EF=Ejection fraction
Figure 1.
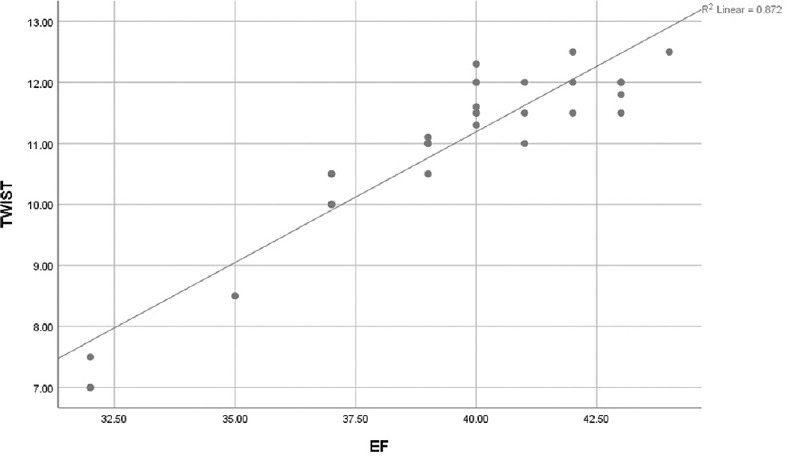
Scattered matrix plot showing positive correlation between ejection fraction % and left ventricular twist
Figure 4.

Scattered matrix plot showing positive correlation between ejection fraction % and average left ventricular global longitudinal strain %
Figure 2.

Scattered matrix plot showing negative correlation between ejection fraction % and left ventricular basal rotation
Figure 3.
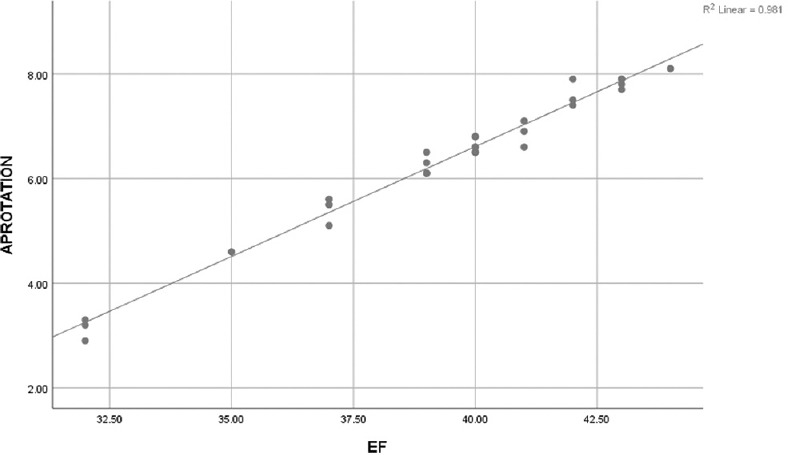
Scattered matrix plot showing positive correlation between ejection fraction % and left ventricular apical rotation
From all of these variables, the only dependent variables for the improvement of EF% were the LVGLS, and Apical rotation [Table 7].
Table 7.
Regression analysis of Echocardiographic parameters in relation to EF % in AR group after 11 months
| Coefficientsa | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Model | Unstandardized coefficients | Standardized coefficients, β | t | Significant | 95% CI for B | ||
|
|
|
||||||
| B | SE | Lower bound | Upper bound | ||||
| EF (%) | |||||||
| Constant | 15.963 | 5.375 | 2.970 | 0.006 | 4.893 | 27.032 | |
| LVEDV | −0.005 | 0.012 | −0.045 | −0.385 | 0.703 | −0.030 | 0.020 |
| LVESV | 0.005 | 0.015 | 0.037 | 0.333 | 0.742 | −0.027 | 0.037 |
| LVGLS (%) | 0.960 | 0.381 | 0.407 | 2.520 | 0.017 | 0.184 | 1.735 |
| Untwist rate | −0.075 | 0.055 | −0.123 | −1.361 | 0.186 | −0.188 | 0.038 |
| Untwist onset | 0.027 | 0.017 | 0.146 | 1.592 | 0.124 | −0.008 | 0.063 |
| Twist | 0.116 | 0.205 | 0.053 | 0.565 | 0.577 | −0.307 | 0.539 |
| Basal rotation | −0.179 | 0.607 | −0.042 | −0.295 | 0.770 | −1.428 | 1.070 |
| Apical rotation | 2.172 | 0.261 | 0.920 | 8.306 | 0.000 | 1.633 | 2.710 |
aDependent variable: EF (%). LV=Left ventricular, LVEDV=LV end-diastolic volume, LVESV=LV end-systolic volume, LVGLS=LV global longitudinal strain, EF=Ejection fraction, SE=Standard error, CI=Confidence interval
DISCUSSION
The sound evaluation of cardiac function plays a crucial role in diagnosing cardiovascular diseases, initiating specific therapeutic interventions, monitoring treatment, and determining the prognosis of a variety of cardiovascular conditions. Echocardiography can provide valuable information about the anatomy and function of the heart.[15,16] LVEF provides objective information about LV systolic function. It has been used to diagnose and classify heart failure,[17,18] determine the suitability of device therapy,[17,19] decide interventions for valvular heart diseases,[20,21] determine the need for specific medications,[19] and predict prognosis.[22] However, LVEF is a volumetric parameter with ventricular load-dependence and had limitations such as significant inter- and intra-observer variability and geometric assumptions.[23] Moreover, LVEF does not represent intrinsic myocardial properties.
Strain is a dimensionless index of a change in length between 2 points before and after movement. Strain echocardiography was introduced to the clinical field about 20 years ago, making it a relatively new echocardiographic modality that can measure myocardial deformation. Unlike LVEF, myocardial strain, as calculated by strain echocardiography, can afford indices of regional and global myocardial systolic function noninvasively and objectively.[24]
Strain echocardiography has been used to diagnose subclinical disease states,[25,26] monitor changes in myocardial function with specific therapies,[27] differentiate cardiomyopathies,[28] and predict the prognosis of several cardiovascular diseases independently of LVEF.[29,30]
Although the physiological mechanisms of action of sacubitril/valsartan are well described, its effects on LV remodeling and LVEF have not been well studied. LV remodeling is a major mechanism underlying disease progression in patients with HFrEF.[31] The degree of improvement in LV end-diastolic volume, LV end-systolic volume, LV dimensions, and LVEF with therapies are strongly correlated with clinical outcomes, including survival.[32]
In the current study, we demonstrated that after 6 months of follow-up on the ARNI-treated group of patients the symptoms of congestion and dyspnea was remarkably improved this is accompanied with the improvement of diastolic indices that might be explained by a chronic state of LV deloading and reduction of LV end diastolic pressure indexed by the reduction of the tricuspid flow velocity, E/A, E/e' also that could help in the initial improvement of LVGLS which indicating that the maximized stretch of LV myocardium and wall tension are reduced with restoration of myocardial starling's forces and this state prepare the LV myocardium recovering the global longitudinal strain. Beta-blockers, Angiotensin converting enzyme inhibitors and receptor blockers, and mineralocorticoids receptor antagonists (MRAs) have demonstrated potent effects on reverse remodeling and improvement in LVEF in multiple studies.[33,34,35,36] Animal studies have shown that treatment with sacubitril/valsartan compared to valsartan alone is associated with a statistically significant increase in LVEF and a trend toward improved reverse remodeling.[37]
Heart failure has recently been classified according to alterations in the mechanical function of the LV.[38] After having observed anomalous specific patterns of ventricular myocardial mechanics in different subsets of patients with heart failure, an alternative approach has been proposed for its characterization.[38,39] Accordingly, heart failure can be classified into three large subgroups: (a) predominant longitudinal dysfunction; (b) transmural dysfunction (longitudinal and circumferential); and (c) predominant circumferential dysfunction. This classification is based on the orientation of the myocardial fibers of the LV, which are arranged obliquely in a double helix shape. Endocardial fibers, which are aligned in a parallel fashion to the LV long axis, are mainly associated with longitudinal mechanics, while transmural fibers are mainly responsible for circumferential mechanics.[40] The action of the latter is predominant due to its greater radius of action.
In the case of systolic dysfunction, twist serves as a compensatory mechanism, and the more its reduction the more advanced stage of the disease. In the dilated left ventricle, the fiber muscles in both layers are stretched and oriented more circumferentially, which leads to additional reduction in chamber contractility and torsion.[41,42] Thus, twist and torsion may be a sensitive marker of remodeling of LV wall architecture, useful in the monitoring of disease progression and response to therapy.
The last follow-up of our patients groups after 11 months, there were a lot of data that strongly supports the idea of the effect of sacubitril/valsartan of LVEF, and reverse myocardial remodeling which might go in agreement with the PROVE-heart failure trial,[43] where the investigators stated that “although improvement in cardiac structure and function was present at 6 months, at 12 months, further improvement in LVEF and volumes was present, with 25% of the study participants experiencing an absolute LVEF increase of more than 13%.” In that trail they depend on changes in LV end systolic, diastolic and left atrial volumes, but in our study, we selected a more precise STE deformational parameters, which had a more sensitive predictive values and accuracy, where we demonstrated that at 11 months most of the torsion mechanics and LVGLS consequently LVEF in addition to the diastolic parameters as untwist rate and onset, that means systolic functional improvement and LV recovery.
CONCLUSION
To the best of our knowledge, this is the first study to demonstrate clear recovery of untwist and twist mechanics, global longitudinal strain, and LVEF in HFrEF patients under 11 months' therapy of sacubitril/valsartan that effect might be explained by a state of chronic deloading with reduction of LV end diastolic pressure which consequently helps in restoration of starling's forces with recovery of LV geometry that lead finally to the improvement of myocardial performance.
Ethical clearance
Human Institutional Ethics Committee (IEC) of Menoufia University, Shebein el koom, Egypt, approved the study protocol. And, the study was performed in accordance with the Declaration of Helsinki and the code of Good Clinical Practice. All patients provided written informed consent to participate after a full explanation of the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Zacà V, Ballo P, Galderisi M, Mondillo S. Echocardiography in the assessment of left ventricular longitudinal systolic function: Current methodology and clinical applications. Heart Fail Rev. 2010;15:23–37. doi: 10.1007/s10741-009-9147-9. [DOI] [PubMed] [Google Scholar]
- 2.Cameli M, Mondillo S, Solari M, Righini FM, Andrei V, Contaldi C, et al. Echocardiographic assessment of left ventricular systolic function: From ejection fraction to torsion. Heart Fail Rev. 2016;21:77–94. doi: 10.1007/s10741-015-9521-8. [DOI] [PubMed] [Google Scholar]
- 3.Torrent-Guasp F, Ballester M, Buckberg GD, Carreras F, Flotats A, Carrió I, et al. Spatial orientation of the ventricular muscle band: Physiologic contribution and surgical implications. J Thorac Cardiovasc Surg. 2001;122:389–92. doi: 10.1067/mtc.2001.113745. [DOI] [PubMed] [Google Scholar]
- 4.Buckberg G, Hoffman JI, Mahajan A, Saleh S, Coghlan C. Cardiac mechanics revisited. Circulation. 2008;118:2571–87. doi: 10.1161/CIRCULATIONAHA.107.754424. [DOI] [PubMed] [Google Scholar]
- 5.Carreras F, García-Barnes J, Gil D, Pujadas S, Li Chi H, Suarez-Arias R, et al. Left ventricular torsion and longitudinal shortening: Two fundamental components of myocardial mechanics assessed by tagged cine-MRI in normal subjects. Int J Cardiovasc Imaging. 2012;28:273–84. doi: 10.1007/s10554-011-9813-6. [DOI] [PubMed] [Google Scholar]
- 6.Mondillo S, Galderisi M, Mele D, Cameli M, Lomoriello VS, Zaca V, et al. Echocardiography study group of the Italian society of C.Speckle tracking echocardiography: A new technique for assessing myocardial function. J Ultrasound Med. 2011;30:7–83. doi: 10.7863/jum.2011.30.1.71. [DOI] [PubMed] [Google Scholar]
- 7.van Dalen BM, Soliman OI, Vletter WB, Kauer F, van der Zwaan HB, ten Cate FJ, et al. Feasibility and reproducibility of left ventricular rotation parameters measured by speckle tracking echocardiography. Eur J Echocardiogr. 2009;10:669–76. doi: 10.1093/ejechocard/jep036. [DOI] [PubMed] [Google Scholar]
- 8.Leitman M, Lysyansky P, Sidenko S, Shir V, Peleg E, Binenbaum M, et al. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr. 2004;17:1021–9. doi: 10.1016/j.echo.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 9.Teske AJ, De Boeck BW, Melman PG, Sieswerda GT, Doevendans PA, Cramer MJ. Echocardiographic quantification of myocardial function using tissue deformation imaging, a guide to image acquisition and analysis using tissue Doppler and speckle tracking. Cardiovasc Ultrasound. 2007;5:27. doi: 10.1186/1476-7120-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantification in M-mode echocardiography: Results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–83. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 11.Lester SJ, Ryan EW, Schiller NB, Foster E. Best method in clinical practice and in research studies to determine left atrial size. Am J Cardiol. 1999;84:829–32. doi: 10.1016/s0002-9149(99)00446-4. [DOI] [PubMed] [Google Scholar]
- 12.Schiller N, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantification of the left ventricle by two-dimensional echocardiography: American Society of Echocardiography Subcommittee on Standards. J Am Soc Echocardiogr. 1989;2:358–68. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 13.Nagueh S, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smisteh OA, et al. Recommandations of the evaluation of diastolic dysfunctions by echocardiography. J Am Soc Echocardiogr. 2009;22:108–28. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Hollekim-Strand SM, Hoydahl SF, Follestad T, Dalen H, Bjørgaas MR, Wisløff U, et al. Exercise training normalizes timing of left ventricular untwist rate, but not peak untwist rate, in individuals with type 2 diabetes and diastolic dysfunction: A pilot study. J Am Soc Echocardiogr. 2016;29:421–30. doi: 10.1016/j.echo.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Kümler T, Gislason GH, Køber L, Torp-Pedersen C. Persistence of the prognostic importance of left ventricular systolic function and heart failure after myocardial infarction: 17-year follow-up of the TRACE register. Eur J Heart Fail. 2010;12:805–11. doi: 10.1093/eurjhf/hfq071. [DOI] [PubMed] [Google Scholar]
- 16.Joyce E, Hoogslag GE, Leong DP, Debonnaire P, Katsanos S, Boden H, et al. Association between left ventricular global longitudinal strain and adverse left ventricular dilatation after ST-segment-elevation myocardial infarction. Circ Cardiovasc Imaging. 2014;7:74–81. doi: 10.1161/CIRCIMAGING.113.000982. [DOI] [PubMed] [Google Scholar]
- 17.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Kim MS, Kim EJ, Park DG, Cho HJ, Yoo BS, et al. KSHF guidelines for the management of acute heart failure: Part I.Definition, epidemiology and diagnosis of acute heart failure. Korean Circ J. 2019;49:1–21. doi: 10.4070/kcj.2018.0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Colvin MM, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: An update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2016;68:1476–88. doi: 10.1016/j.jacc.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–91. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 21.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Fleisher LA, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2017;135:e1159–95. doi: 10.1161/CIR.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 22.Lee JY, Sunwoo JS, Kwon KY, Roh H, Ahn MY, Lee MH, et al. Left ventricular ejection fraction predicts poststroke cardiovascular events and mortality in patients without atrial fibrillation and coronary heart disease. Korean Circ J. 2018;48:1148–56. doi: 10.4070/kcj.2018.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otterstad JE, Froeland G, St John Sutton M, Holme I. Accuracy and reproducibility of biplane two-dimensional echocardiographic measurements of left ventricular dimensions and function. Eur Heart J. 1997;18:507–13. doi: 10.1093/oxfordjournals.eurheartj.a015273. [DOI] [PubMed] [Google Scholar]
- 24.Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S. Myocardial strain imaging: How useful is it in clinical decision making? Eur Heart J. 2016;37:1196–207. doi: 10.1093/eurheartj/ehv529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008;117:1436–48. doi: 10.1161/CIRCULATIONAHA.107.653576. [DOI] [PubMed] [Google Scholar]
- 26.Keramida K, Farmakis D, Bingcang J, Sulemane S, Sutherland S, Bingcang RA, et al. Longitudinal changes of right ventricular deformation mechanics during trastuzumab therapy in breast cancer patients. Eur J Heart Fail. 2019;21:529–35. doi: 10.1002/ejhf.1385. [DOI] [PubMed] [Google Scholar]
- 27.Negishi K, Negishi T, Haluska BA, Hare JL, Plana JC, Marwick TH. Use of speckle strain to assess left ventricular responses to cardiotoxic chemotherapy and cardioprotection. Eur Heart J Cardiovasc Imaging. 2014;15:324–31. doi: 10.1093/ehjci/jet159. [DOI] [PubMed] [Google Scholar]
- 28.Phelan D, Thavendiranathan P, Popovic Z, Collier P, Griffin B, Thomas JD, et al. Application of a parametric display of two-dimensional speckle-tracking longitudinal strain to improve the etiologic diagnosis of mild to moderate left ventricular hypertrophy. J Am Soc Echocardiogr. 2014;27:888–95. doi: 10.1016/j.echo.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 29.Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr. 2013;26:493–8. doi: 10.1016/j.echo.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Levy D. Left ventricular dilatation and the risk of congestive heart failure in people without myocardial infarction. N Engl J Med. 1997;336:1350–5. doi: 10.1056/NEJM199705083361903. [DOI] [PubMed] [Google Scholar]
- 32.Wong M, Johnson G, Shabetai R, Hughes V, Bhat G, Lopez B, et al. Echocardiographic variables as prognostic indicators and therapeutic monitors in chronic congestive heart failure. Veterans Affairs cooperative studies V-HeFT I and II. V-HeFT VA Cooperative Studies Group. Circulation. 1993;87:VI65–70. [PubMed] [Google Scholar]
- 33.Dubach P, Myers J, Bonetti P, Schertler T, Froelicher V, Wagner D, et al. Effects of bisoprolol fumarate on left ventricular size, function, and exercise capacity in patients with heart failure: Analysis with magnetic resonance myocardial tagging. Am Heart J. 2002;143:676–83. doi: 10.1067/mhj.2002.121269. [DOI] [PubMed] [Google Scholar]
- 34.A placebo-controlled trial of captopril in refractory chronic congestive heart failure. Captopril Multicenter Research Group. J Am Coll Cardiol. 1983;2:755–63. doi: 10.1016/s0735-1097(83)80316-7. [DOI] [PubMed] [Google Scholar]
- 35.Matsumori A. Assessment of Response to Candesartan in Heart Failure in Japan (ARCH-J) Study Investigators. Efficacy and safety of oral candesartan cilexetil in patients with congestive heart failure. Eur J Heart Fail. 2003;5:669–77. doi: 10.1016/s1388-9842(03)00162-4. [DOI] [PubMed] [Google Scholar]
- 36.Cicoira M, Zanolla L, Rossi A, Golia G, Franceschini L, Brighetti G, et al. Long-term, dose-dependent effects of spironolactone on left ventricular function and exercise tolerance in patients with chronic heart failure. J Am Coll Cardiol. 2002;40:304–10. doi: 10.1016/s0735-1097(02)01965-4. [DOI] [PubMed] [Google Scholar]
- 37.Suematsu Y, Miura S, Goto M, Matsuo Y, Arimura T, Kuwano T, et al. LCZ696, an angiotensin receptor-neprilysin inhibitor, improves cardiac function with the attenuation of fibrosis in heart failure with reduced ejection fraction in streptozotocin-induced diabetic mice. Eur J Heart Fail. 2016;18:386–93. doi: 10.1002/ejhf.474. [DOI] [PubMed] [Google Scholar]
- 38.Sengupta PP, Narula J. Reclassifying heart failure: Predominantly subendocardial, subepicardial, and transmural. Heart Fail Clin. 2008;4:379–82. doi: 10.1016/j.hfc.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 39.Claus P, Omar AM, Pedrizzetti G, Sengupta PP, Nagel E. Tissue tracking technology for assessing cardiac mechanics: Principles, normal values, and clinical applications. JACC Cardiovasc Imaging. 2015;8:1444–60. doi: 10.1016/j.jcmg.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Omar AM, Bansal M, Sengupta PP. Advances in echocardiographic imaging in heart failure with reduced and preserved ejection fraction. Circ Res. 2016;119:357–74. doi: 10.1161/CIRCRESAHA.116.309128. [DOI] [PubMed] [Google Scholar]
- 41.Sengupta PP, Tajik AJ, Chandrasekaran K, Khandheria BK. Twist mechanics of the left ventricle: Principles and application. JACC Cardiovasc Imaging. 2008;1:366–76. doi: 10.1016/j.jcmg.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Omar AM, Vallabhajosyula S, Sengupta PP. Left ventricular twist and torsion: Research observations and clinical applications. Circ Cardiovasc Imaging. 2015;8:e003029. doi: 10.1161/CIRCIMAGING.115.003029. [DOI] [PubMed] [Google Scholar]
- 43.Januzzi JL, Butler J, Fombu E, Maisel A, McCague K, Piña IL, et al. Rationale and methods of the Prospective Study of Biomarkers, Symptom Improvement, and Ventricular Remodeling During Sacubitril/Valsartan Therapy for Heart Failure (PROVE-HF) Am Heart J. 2018;199:130–6. doi: 10.1016/j.ahj.2017.12.021. [DOI] [PubMed] [Google Scholar]


