Abstract
Background:
The great technological advancements in the field of echocardiography have led to applications of stress echocardiography (SE) in almost all diagnostic fields of cardiology, from ischemic heart disease to valvular heart disease and diastolic function. However, the assessment of the right ventricle (RV) in general, and in particular in regard to the contractile reserve of the RV, is an area that has not been previously explored. We, therefore, propose a study to investigate the potential use of SE for the assessment of RV function in adult patients.
Aims and objectives:
The primary aim is to evaluate the feasibility of right ventricular SE. The secondary aim is to assess right ventricular contractile reserve.
Matherials and Methods:
Eighty-one patients undergoing a physical or dobutamine stress echocardiogram for cardiovascular risk stratification or chest pain were the subject of the study. An exercise leg cycle using a standard WHO protocol was used to simultaneously assess the right and left ventricular global and regional function as well as acquiring Doppler data. Whereas the patient had limitations in mobility, a dobutamine SE was be performed. We evaluated the average values of tricuspid annular plane systolic excursion (TAPSE), fractional area change (FAC), S-wave, systolic pulmonary artery pressure (sPAP), and right ventricle global longitudinal (free wall) strain (RVGLS) during baseline and at the peak of the effort. RV contractile reserve was defined as the change in RVGLS from rest to peak exercise. We also assessed the reproducibility of these measurements between two different expert operators (blind analysis).
Results:
At least 3 over 5 RV function parameters were measurable both during baseline and at the peak of the effort in 95% of patients, while all 5 parameters in 65% of our population, demonstrating an excellent feasibility. All RV-studied variables showed a statistically significant increase (P < 0.001) at peak compared to the baseline. The average percentage increases at peak were 31.1% for TAPSE, 24.8% for FAC, 50.6% for S-wave, 55.2% for PAPS, and 39.8% for RV strain. The reproducibility between operators at baseline and peak was excellent. Our study demonstrates that TAPSE, FAC, and S-wave are highly feasible at rest and at peak, while TAPSE, S-wave, and sPAP are the most reliable measurements during RV stress echo.
Conclusion:
RVGLS is useful in the assessment of RV contractile reserve in patients with good acoustic window. Further studies are needed to evaluate the impact of contrast echocardiography in improving RV contractile reserve assessment during SE.
Keywords: Right ventricular function, right ventricular reserve, stress echocardiography
INTRODUCTION
Stress echocardiography (SE) is widely used in the assessment of left ventricular (LV) function[1] for diagnostic and prognostic stratification of patients with coronary artery disease (CAD). The great technological advancements in the field of echocardiography (tissue Doppler imaging, strain technology, three-dimensional (3D) echo, and transpulmonary echo contrast agents) have led to applications of SE in almost all diagnostic fields of cardiology, including valvular heart disease and diastolic function.[2]
However, the assessment of the right ventricle (RV) in general, and in particular in regard to the contractile reserve of the RV, is an area that has been poorly explored.
Resting echocardiographic parameters used so far have shown a limited ability to detect early impairment of right ventricular function. Recently, some studies have suggested that physical or pharmacological stress may unmask RV function abnormalities in patients with repaired tetralogy of Fallot, with normal right ventricular function under resting conditions.[3,4,5]
There has been no similar study performed outside the grown-up congenital heart population.
The use of SE provides the possibility to test both systolic and diastolic functions of the RV in response to increased loading conditions. This can potentially be used to predict which subset of patients may benefit from intervention on the tricuspid valve before the RV displays signs of failure.[6]
We, therefore, propose a study to investigate the potential use of SE for the assessment of RV function in adult patients. The primary aim is to evaluate the feasibility of right ventricular SE in any patient with more than trivial TR. The secondary aim is to assess right ventricular contractile reserve.
METHODS
Eighty-one patients undergoing a physical or dobutamine stress echocardiogram for cardiovascular risk stratification or chest pain were the subject of the study. Inclusion criteria were age >18 years and normal baseline RV function (right ventricular fractional area change [FAC] >35% and tricuspid annular plane systolic excursion [TAPSE] >16 mm). Exclusion criteria were inadequate visualization of the RV, presence of right ventricular dysfunction, systolic pulmonary artery pressure (sPAP) peak >60 mmHg (pulmonary stress hypertension), positive stress test for left myocardial ischemia, presence of more than mild valvular disease, no more than two of the following coronary risk factors: hypertension, dyslipidemia, smoking, and diabetes, Grade III or higher diastolic dysfunction at baseline, and severe respiratory, renal, or hepatic dysfunction.
Echocardiographic examinations were performed on GE Vivid 9.
An exercise leg cycle using a standard WHO protocol[7] was used to simultaneously assess the right and LV global and regional function as well as acquiring Doppler data. Data were acquired at baseline and peak imaging. Exercise leg cycle testing was considered valid if the patient reached a peak systolic blood pressure/heart rate product of 25.000 mmHg beats/min, or at least 85% of predicted maximum heart rate with neither exercise induced angina pectoris neither evidence for reversible ischemia during the study. Whereas the patient had limitations in mobility, a dobutamine SE was performed according to our routine protocol (four stages) with baseline, low dose, intermediate, and peak imaging. Doppler echocardiography was performed with a Vivid 7 ultrasound system (GE Healthcare), using a M3S transducer with a frequency range of 1.5–4.0 Mhz.
Three additional images evaluating the RV will be obtained from the: (i) parasternal short-axis view at the mid-level, (ii) apical four-chamber view of the RV, and (iii) continuous-wave Doppler velocity of tricuspid regurgitation (TR), at baseline and immediately after acquisition of routine peak stress images. Right ventricular function was assessed at baseline and peak stress, by measuring the change of TAPSE and the change in the FAC.
We evaluated the average values of TAPSE, FAC, S-wave, sPAP, and right ventricle global longitudinal (free wall) strain (RVGLS) during baseline and at the peak of the effort. RV contractile reserve was defined as the change in RVGLS from rest to peak exercise. Longitudinal strain is generally reported as a negative number as it is a measure of myocardial fiber longitudinal shortening during systole. Therefore, while more negative strain values reflect greater myocardial deformation when expressed as an absolute value, the higher numbers reflect greater myocardial deformation. We also assessed the reproducibility of these measurements between two different expert operators (blind analysis).
Conventional indices of LV systolic function were also measured at baseline and peak stress.
Statistical analysis
Eleven echocardiographic examinations were randomly selected to assess inter-rater and intra-rater variability. Two distinct operators were asked to perform the echocardiographic assessment in a blinded fashion. In addition, one of the two operators had to analyze the same series of examinations twice without knowing it. Inter- and intra-rater reliability was then assessed using Bland–Altman method.
Continuous variables are represented with median (I and III quartiles) and mean (standard deviation). Categorical data were expressed as percentages. Differences in parameters collected at baseline and at peak were assessed using paired Wilcoxon signed-rank test to deal with repeated measures for each patient. All tests were performed two-sided, and a significance level of P < 0.05 was considered to indicate statistical significance.
Differences in parameters collected at baseline and at peak were assessed using paired Wilcoxon signed-rank test to deal with repeated measures for each patient.
Statistical analyses were performed using SPSS version 22.0 statistical package (SPSS Inc., Chicago, IL, USA).
RESULTS
As shown in Table 1, our study included 81 patients (49 males/32 females) with a mean age of 64.3 ± 11.2 years. The maximum age of participants was 82 years, and the minimum age was 43 years. A submaximal test was achieved by all participants and no tests were terminated because of arrhythmia or other symptoms. During image acquisition at peak exercise, all patients were in normal sinus rhythm.
Table 1.
Clinical and demographic characteristics in patients undergoing exercise and dobutamine stress test
| Variable, n (%) | Exercise 45 (54) | Dobutamine 46 (56) | Total (81) | P |
|---|---|---|---|---|
| Age (years) | 57.4±9.7 | 69.72±9.14 | 64.3±11.2 | <0.001 |
| Sex (male) | 23 (51) | 26 (57) | 49 (60) | 0.408 |
| Hypertension | 10 (22) | 8 (23) | 18 (22) | 0.368 |
| Diabetes | 4 (9) | 7 (20) | 11 (14) | 0.627 |
| Dyslipidemia | 1 (2) | 3 (8) | 4 (5) | 0.457 |
| Family history | 2 (4) | 3 (8) | 5 (6) | 0.883 |
| Smoke | 13 (28) | 15 (42) | 28 (35) | 0.376 |
| VTD (ml/m2) | 59.7±4.6 | 57.4±3.9 | 58.3±4.2 | 0.298 |
| LVEF (%) | 60.1±4.9 | 58.9±4.2 | 59.6±4.6 | 0.248 |
| ATD (cm2/m2) | 10.1±1.5 | 9.4±1.2 | 9.9±1.4 | 0.306 |
| FAC (%) | 41.1±5.5 | 39.4±3.8 | 40.4±4.9 | 0.145 |
*Percentage is referred to the number of patients in the column. FAC=Fractional area change, LVEF=Left ventricular ejection fraction, LVEDV=left ventricle end diastolic volume, RVEDA=right ventricle end diastolic area
Fifteen patients had no risk factors for CAD, while 40 patients had 1 risk factor and 13 patients had 2 risk factors for CAD. Forty-five (54%) patients underwent exercise stress, while 46 (56%) dobutamine stress because of inability to cycle. There was no statistically significant difference in patients undergoing physical or dobutamine test except for age and diabetes [Table 1].
At least 3 over 5 RV function parameterswere measurable both during baseline and at the peak of the effort in 95% of patients, while all 5 parameters in 65% of our population, demonstrating a good feasibility. Table 2 shows the feasibility at rest and peak for the 5 parameters. There was an excellent feasibility at stress for TAPSE, S-wave, and FAC. All RV-studied variables showed a statistically significant increase (P < 0.001) at peak compared to the baseline. The average percentage increases at peak were 31.1% for TAPSE, 24.8% for FAC, 50.6% for S-wave, 55.2% for PAPS, and 39.8% for RVGLS. The mean and median values at rest and peak are reported in Table 3. In particular, the median sPAP was 28 mmHg at rest and 42 mmHg at peak [Table 3].
Table 2.
Feasibility at rest and peak
| Rest (n=81), n (%) | Peak (n=81), n (%) | P | |
|---|---|---|---|
| TAPSE | 79 (98) | 78 (96) | NS |
| FAC | 78 (96) | 77 (95) | NS |
| S-wave | 72 (89) | 70 (86) | NS |
| sPAP | 58 (72) | 47 (58) | 0.07 |
| RVGLS | 71 (87) | 62 (76) | <0.001 |
NS=Not significant, TAPSE=Tricuspid annular plane systolic excursion, FAC=Fractional area change, sPAP=Systolic pulmonary artery pressure, RVGLS=right ventricle global longitudinal strain
Table 3.
Difference of parameters between peak and baseline
| Rest (n=81) | Peak (n=81) | P | |
|---|---|---|---|
| TAPSE | <0.001 | ||
| Median (Q1–Q3) | 21.000 | 29.000 | |
| (18.000–24.000) | (25.000–32.750) | ||
| Mean±SD | 21.861±4.317 | 28.759±5.744 | |
| FAC | <0.001 | ||
| Median (Q1–Q3) | 39.000 | 49.000 | |
| (37.000–42.000) | (45.000–55.000) | ||
| Mean±SD | 40.410±4.940 | 50.000±5.835 | |
| S-wave | <0.001 | ||
| Median (Q1–Q3) | 13.000 | 21.000 | |
| (12.000–17.000) | (18.000–24.000) | ||
| Mean±SD | 14.063±3.015 | 20.842±4.750 | |
| sPAP | <0.001 | ||
| Median (Q1–Q3) | 28.000 | 42.500 | |
| (20.000–29.000) | (38.000–45.000) | ||
| Mean±SD | 25.931±6.316 | 41.370±8.304 | |
| RVGLS | <0.001 | ||
| Median (Q1–Q3) | −28.000 | −37.000 | |
| (24.000–32.000) | (32.000–41.000) | ||
| Mean±SD | −28.932±3.845 | −37.423±4.799 |
TAPSE=Tricuspid annular plane systolic excursion, FAC=Fractional area change, sPAP=Systolic pulmonary artery pressure, RVGLS=right ventricle global longitudinal strain, SD=Standard deviation
Figure 1 shows the reproducibility between operators at baseline and peak with good correlation.
Figure 1.
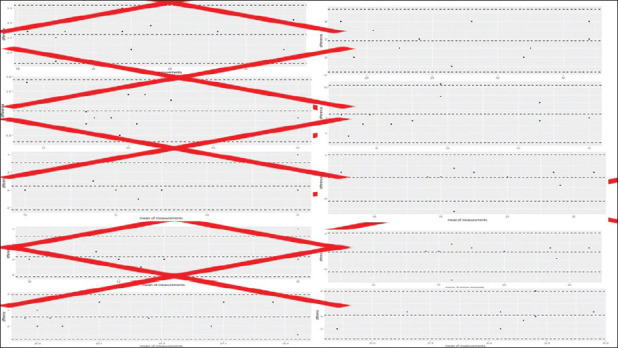
Reproducibility between operators at baseline and peak
Table 4 shows the reproducibility of the data by two separate operators with intraclass correlation coefficients (ICCs).
Table 4.
Differences of means computed as operator 1 minus operator 2
| Parameter | Difference of means (95% CI) |
|---|---|
| TAPSE basal | 0.55 (−4.36–5.45) |
| TAPSE peak | 1.82 (−1.67–5.3) |
| FAC basal | −0.82 (−6.14–4.5) |
| FAC peak | 1.18 (−8.17–10.53) |
| Onda S’ basal | 0.4 (−2.25–3.05) |
| Onda S’ peak | −0.27 (−5.68–5.14) |
| sPAP basal | −1.36 (−6.99–4.27) |
| PAPS peak | −1.2 (−9.71–7.31) |
| RVGLS basal | −0.82 (−3.56–1.93) |
| RVGLS peak | 0.25 (−5.37–5.87) |
TAPSE=Tricuspid annular plane systolic excursion, FAC=Fractional area change, RVGLS=right ventricle global longitudinal strain, CI=Confidence interval, sPAP=systolic pulmonary artery pressure
DISCUSSION
The echocardiogram during physical exertion or during pharmacological stress (dobutamine or dipyridamole) has become a cornerstone of imaging.[8,9,10] Although the prognostic value of the size and function of the RV has been proven by numerous studies,[11,12,13,14,15] guidelines on the echocardiogram during physical exertion or during pharmacological stress have focused almost exclusively on the left ventricle.[8]
Up to now, only a few articles have been published on the RV function during physical exertion or pharmacological stress. Most of these papers have focused on the pediatric population affected by congenital heart disease[16,17,18] or on patients with pulmonary hypertension.[19]
A single study[20] assessed the feasibility of the RV assessment during exercise stress but had the limitation of having analyzed few patients (only 30) and only including patients with a discernible TR signal. Conversely, in our study, we included a large sample of patients (n = 81) and we included also patients with no or trivial TR.
Right ventricular function augments with exercise, and loss of this adaptive ability often determines symptoms. Reported upper normal limits of Doppler-derived sPAP during exercise in normal subjects[21,22,23] are confirmed by our data.
In our cohort of patients, TAPSE, FAC, and S-wave were highly feasible at stress, while sPAP was less feasible because we included in the study also patients with no or trivial TR. RVGLS had a good feasibility at stress of 64%. It has been reported that RVGLS correlates well with RV ejection fraction measured by magnetic resonance and may serve as a predictor of outcome.[24,25,26,27] In our study, there was a median global longitudinal strain of −28% at baseline and of −37% at peak, with a median increase of 39.8%, in agreement with previous studies.[20]
All the RV parameters showed little inter-operator variability when measured at baseline. At the peak of the exercise, M-mode and Doppler-derived parameters (TAPSE, S-wave, and sPAP) showed an excellent reproducibility (ICC >0.86) while it was good for 2D-derived parameters (FAC and RV-free wall strain; ICC = 0.74).
Our results are partially in agreement with Anjak et al.[20] who found a good correlation for TAPSE and S-wave but not for FAC and RV strain. We believe that improvements in the setting of echocardiographic machine and software development may explain the inconsistency.
Our study demonstrates that TAPSE, S-wave, and FAC are the most reliable measurements during RV stress echo. RV strain is less feasible, with a good reproducibility, and it is useful in the assessment of RV contractile reserve in patients with good acoustic window. Further studies are needed to evaluate the impact of contrast echocardiography in improving RV contractile reserve assessment during SE.
Limitations
This is a single-center study. We did not include in our analysis patients with RV disease. Larger multicenter studies including patients with RV diseases are needed.
CONCLUSION
RV SE proved to be feasible and showed little inter-operator variability in patients with at least trivial TR. It provided valuable information about RV contractile reserve that may help stratifying the risk of RV failure in patients undergoing TV surgery.
The ability to couple the left and RVs during stress can provide meaningful information in healthy and diseased individuals as it can support the diagnosis of the etiology of pulmonary hypertension and other conditions and can offer valuable prognostic information, in particular regarding RV contractile reserve. SE appears to be a fascinating and versatile tool for better understanding the pathophysiology of pulmonary circulation and for investigating otherwise unexplained exertional dyspnea.
Ethical clearance
The study was conducted according to the guidelines of the Declaration of Helsinki. Informed consent was obtained from all subjects involved in the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Dr. Chiara Palermo, PhD, for her contribution in the technical help.
REFERENCES
- 1.Sicari R, Nihoyannopoulos P, Evangelista A, Kasprzak J, Lancellotti P, Poldermans D, et al. Stress echocardiography expert consensus statement--executive summary: European Association of Echocardiography (EAE) (a registered branch of the ESC) Eur Heart J. 2009;30:278–89. doi: 10.1093/eurheartj/ehn492. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Fleisher LA, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2017;135:e1159–95. doi: 10.1161/CIR.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 3.Apostolopoulou SC, Laskari CV, Tsoutsinos A, Rammos S. Doppler tissue imaging evaluation of right ventricular function at rest and during dobutamine infusion in patients after repair of tetralogy of Fallot. Int J Cardiovasc Imaging. 2007;23:25–31. doi: 10.1007/s10554-006-9121-8. [DOI] [PubMed] [Google Scholar]
- 4.Brili S, Stamatopoulos I, Barbetseas J, Chrysohoou C, Alexopoulos N, Misailidou M, et al. Usefulness of dobutamine stress echocardiography with Tissue Doppler imaging for the evaluation and follow-up of patients with repaired tetralogy of Fallot. J Am Soc Echocardiogr. 2008;21:1093–8. doi: 10.1016/j.echo.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Ait-Ali L, Siciliano V, Passino C, Molinaro S, Pasanisi E, Sicari R, Pingitore A, Festa P. Role of stress echocardiography in operated fallot: feasibility and detection of right ventricular response. [Epub 2014 Sep 23. PMID: 25260437];J Am Soc Echocardiogr. 2014 (12):1319–28. doi: 10.1016/j.echo.2014.08.006. doi: 10.1016/j.echo.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Sarubbi B, Pacileo G, Pisacane C, Ducceschi V, Iacono C, Russo MG, et al. Exercise capacity in young patients after total repair of tetralogy of fallot. Pediatric Cardiol. 2000;21:211–5. doi: 10.1007/s002460010041. [DOI] [PubMed] [Google Scholar]
- 7.Hecht HS, DeBord L, Sotomayor N, Shaw R, Dunlap R, Ryan C. Supine bicycle stress echocardiography: Peak exercise imaging is superior to postexercise imaging. J Am Soc Echocardiogr. 1993;6:265–71. doi: 10.1016/s0894-7317(14)80062-x. [DOI] [PubMed] [Google Scholar]
- 8.Lancellotti P, Pellikka PA, Budts W, Chaudhry FA, Donal E, Dulgheru R, et al. The clinical use of stress echocardiography in non-ischaemic heart disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2016;17:1191–229. doi: 10.1093/ehjci/jew190. [DOI] [PubMed] [Google Scholar]
- 9.Sicari R, Nihoyannopoulos P, Evangelista A, Kasprzak J, Lancellotti P, Poldermans D, et al. Stress echocardiography expert consensus statement: European Association of Echocardiography (EAE) (a registered branch of the ESC) Eur J Echocardiogr. 2008;9:415–37. doi: 10.1093/ejechocard/jen175. [DOI] [PubMed] [Google Scholar]
- 10.Pellikka PA, Arruda-Olson A, Chaudhry FA, Chen MH, Marshall JE, Porter TR, et al. Guidelines for performance, interpretation, and application of stress echocardiography in ischemic heart disease: From the American Society of Echocardiography. J Am Soc Echocardiogr. 2020;33:1–41.e8. doi: 10.1016/j.echo.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Aymami M, Amsallem M, Adams J, Sallam K, Moneghetti K, Wheeler M, et al. The incremental value of right ventricular size and strain in the risk assessment of right heart failure post-Left ventricular assist device implantation. J Card Fail. 2018;24:823–32. doi: 10.1016/j.cardfail.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Carluccio E, Biagioli P, Lauciello R, Zuchi C, Mengoni A, Bardelli G, et al. Superior prognostic value of right ventricular free wall compared to global longitudinal strain in patients with heart failure. J Am Soc Echocardiogr. 2019;32:836–44.e1. doi: 10.1016/j.echo.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Kusunose K, Tsutsui RS, Bhatt K, Budev MM, Popović ZB, Griffin BP, et al. Prognostic value of RV function before and after lung transplantation. JACC Cardiovasc Imaging. 2014;7:1084–94. doi: 10.1016/j.jcmg.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Mast TP, Taha K, Cramer MJ, Lumens J, van der Heijden JF, Bouma BJ, et al. The prognostic value of right ventricular deformation imaging in early arrhythmogenic right ventricular Cardiomyopathy. JACC Cardiovasc Imaging. 2019;12:446–55. doi: 10.1016/j.jcmg.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Merlo M, Gobbo M, Stolfo D, Losurdo P, Ramani F, Barbati G, et al. The prognostic impact of the evolution of RV function in idiopathic DCM. JACC Cardiovasc Imaging. 2016;9:1034–42. doi: 10.1016/j.jcmg.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 16.Ait-Ali L, Siciliano V, Passino C, Molinaro S, Pasanisi E, Sicari R, et al. Role of stress echocardiography in operated fallot: Feasibility and detection of right ventricular response. J Am Soc Echocardiogr. 2014;27:1319–28. doi: 10.1016/j.echo.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Moller T, Lindberg H, Lund MB, Holmstrom H, Dohlen G, Thaulow E. Right ventricular pressure response to exercise in adults with isolated ventricular septal defect closed in early childhood. Cardiol Young. 2018;28:797–803. doi: 10.1017/S1047951117002979. [DOI] [PubMed] [Google Scholar]
- 18.Dvir-Orgad M, Anand M, De Souza AM, Zadorsky MT, Kiess MC, Potts JE, et al. Stress echocardiographic evaluation for d-transposition of the great arteries after atrial redirection: Unmasking early signs of myocardial dysfunction and baffle stenosis. J Am Soc Echocardiogr. 2017;30:80–9. doi: 10.1016/j.echo.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Almeida AR, Loureiro MJ, Lopes L, Cotrim C, Lopes L, Repolho D, et al. Echocardiographic assessment of right ventricular contractile reserve in patients with pulmonary hypertension. Rev Port Cardiol. 2014;33:155–63. doi: 10.1016/j.repc.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Anjak A, López-Candales A, Lopez FR, Harris D, Elwing J. Objective measures of right ventricular function during exercise: Results of a pilot study. Echocardiography. 2014;31:508–15. doi: 10.1111/echo.12417. [DOI] [PubMed] [Google Scholar]
- 21.Hopkins SR, Schoene RB, Henderson WR, Spragg RG, Martin TR, West JB. Intense exercise impairs the integrity of the pulmonary blood-gas barrier in elite athletes. Am J Respir Crit Care Med. 1997;155:1090–4. doi: 10.1164/ajrccm.155.3.9116992. [DOI] [PubMed] [Google Scholar]
- 22.Naeije R, Chesler N. Pulmonary circulation at exercise. Compr Physiol. 2012;2:711–41. doi: 10.1002/cphy.c100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.La Gerche A, MacIsaac AI, Burns AT, Mooney DJ, Inder WJ, Voigt JU, et al. Pulmonary transit of agitated contrast is associated with enhanced pulmonary vascular reserve and right ventricular function during exercise. J Appl Physiol (1985) 2010;109:1307–17. doi: 10.1152/japplphysiol.00457.2010. [DOI] [PubMed] [Google Scholar]
- 24.Freed BH, Tsang W, Bhave NM, Patel AR, Weinert L, Yamat M, et al. Right ventricular strain in pulmonary arterial hypertension: A 2D echocardiography and cardiac magnetic resonance study. Echocardiography. 2015;32:257–63. doi: 10.1111/echo.12662. [DOI] [PubMed] [Google Scholar]
- 25.Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr. 2011;12:167–205. doi: 10.1093/ejechocard/jer021. [DOI] [PubMed] [Google Scholar]
- 26.Sachdev A, Villarraga HR, Frantz RP, McGoon MD, Hsiao JF, Maalouf JF, et al. Right ventricular strain for prediction of survival in patients with pulmonary arterial hypertension. Chest. 2011;139:1299–309. doi: 10.1378/chest.10-2015. [DOI] [PubMed] [Google Scholar]
- 27.Haeck ML, Scherptong RW, Marsan NA, Holman ER, Schalij MJ, Bax JJ, et al. Prognostic value of right ventricular longitudinal peak systolic strain in patients with pulmonary hypertension. Circ Cardiovasc Imaging. 2012;5:628–36. doi: 10.1161/CIRCIMAGING.111.971465. [DOI] [PubMed] [Google Scholar]


