Abstract
BACKGROUND:
To recruit poorly ventilated lung areas by providing active and adequate oxygenation is a core aspect of treating patients with acute respiratory distress syndrome (ARDS). The airway pressure release ventilation (APRV) mode is increasingly accepted as a means of supporting patients with ARDS. This study aimed to determine whether the APRV mode is effective in improving oxygenation, compared to conventional ventilation, in adult ARDS patients.
METHODS:
We conducted the study according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. We searched for clinical trials in PubMed, Embase, Web of Science, and the Cochrane Library until April 2019. We included all studies comparing APRV and other conventional mechanical ventilation modes for adult ARDS patients. Our primary outcome was oxygenation status (defined as the day 3 PaO2/FiO2 ratio). The secondary outcomes were the length of stay (LOS) in the intensive care unit (ICU) and mortality. Sensitivity analyses were performed including studies with conventional low-tidal volume ventilation as a comparator ventilation strategy.
RESULTS:
We included six clinical trials enrolling a total of 375 patients. The day 3 PaO2/FiO2 was reported in all the studies, and it was significantly higher in patients receiving APRV (mean difference [MD] 51.9 mmHg, 95% confidence intervals (CI) 8.2–95.5, P = 0.02, I2= 92%). There was no significant difference in mortality between APRV and the other conventional ventilator modes (risk difference 0.07, 95% CI: −0.01–0.15, P = 0.08, I20%). The point estimate for the effect of APRV on the LOS in ICU indicated a significant reduction in the ICU LOS for the APRV group compared to the counter group (MD 3.1 days, 95% CI 0.4–5.9, P = 0.02, I2= 53%).
CONCLUSION:
In this study, using the APRV mode may improve oxygenation on day 3 and contribute to reducing the LOS in ICU. However, it is difficult to draw a clinical message about APRV, and well-designed clinical trials are required to investigate this issue.
Keywords: Acute respiratory distress syndrome, airway pressure release ventilation, meta-analysis, oxygenation
Acute respiratory distress syndrome (ARDS) is a serious condition requiring admission to an intensive care unit (ICU), and it is associated with significant hospital mortality.[1] In the clinical progression of ARDS, refractory hypoxemia is the most important pathophysiological feature.[2,3] A core aspect of treating ARDS is to recruit poorly ventilated lung areas by providing active and adequate oxygenation.[4] The management plan of ARDS, therefore, involves invasive mechanical ventilation.[5] Low tidal volume (LTV) ventilation and conventional positive end-expiratory pressure are the standard mechanical ventilation strategies for treating ARDS.[5] However, invasive mechanical ventilatory management of ARDS patients is complicated and associated with ventilator-induced lung injuries due to the heterogeneity in the distribution of alveolar consolidation.[4]
Ideally, invasive mechanical ventilatory modes used for managing ARDS patients should maintain the alveoli open throughout the ventilator cycle to reduce repetitive alveolar collapse and over distention, to minimize lung injuries.[5] Airway pressure release ventilation (APRV) is a ventilation mode proposed as an advantage compared to conventional mechanical ventilation.[6,7,8,9,10] The main feature of APRV is delivering a continuous positive airway pressure (CPAP) from a high pressure (Phigh) to low pressure (Plow), with a brief intermittent release phase.[7,11] This allows using an inversed inspiration to expiration (I: E; Thigh: Tlow) ratio, in which Thigh is the period to use the CPAP to recapture the collapsed alveoli, and Tlow allows both adequate ventilation and complete exhalation. The process advances alveolar recruitment and oxygenation while reducing physical damage to the alveoli.[6,7]
Due to the possible physiologic advantages over other ventilatory modes, many animal and human observational studies explored the immediate hemodynamic and respiratory consequences of using APRV in treating ARDS.[9,12,13,14,15,16] Many studies suggested that applying APRV protocols enhance alveolar recruitment and gas exchange in patients with ARDS.[10] Based on the observational studies, a few clinical trials have been published to assess the efficacy of the early application of APRV in ARDS patients to improve oxygenation and reduce mortality. However, the efficacy of APRV in patients diagnosed with ARDS is still controversial, mainly due to the heterogeneity in APRV application and the initiation time.[17] The aim of this study, therefore, was to assess the efficacy of APRV, compared with other modes of mechanical ventilation, to improve oxygenation and reduce mortality in critically ill adults with ARDS.
Methods
The current study is a meta-analysis conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.[18] The protocol was approved by the King Abdullah International Medical Research Center (protocol number SP19/141/R). The main objective of this study was to assess the efficacy of APRV, compared with other modes of mechanical ventilation, to improve oxygenation in critically ill adults with ARDS.
Eligibility criteria and search strategy
Published clinical trials investigating APRV in the management of adult ARDS patients, admitted to ICU, were retrieved from the following databases: MEDLINE, Embase, Web of Science, and the Cochrane Central Register of Controlled Trials database, from inception to April 2019. All clinical trials in which APRV was compared with any alternative conventional mode have been included. We excluded observational studies, crossover studies, experimental animal studies, and review articles from the search. The following keywords were combined in defining the relevant articles: “Airway Pressure Release Ventilation,” “APRV,” “Continuous Positive Airway Pressure,” “CPAP Ventilation,” “Respiratory Distress Syndrome,” “ARDS,” “Acute Lung Injury,” and “ALI.” We searched for additional articles using the reference list and gray literature. We included only articles published in English.
Study selection and data extraction
Two investigators conducted the primary search independently and screened the titles and abstracts for potentially eligible articles. Subsequently, the full text of potentially relevant articles was assessed for inclusion, according to the documented oxygenation measured as the PaO2/FiO2 on day 3 after the initiation of the mechanical ventilation. The secondary data related to all-cause mortality, i.e., ICU or hospital mortality and ICU length of stay (LOS) were collected.
Data extraction forms were used to extract the data regarding the primary and secondary outcomes, in addition to information on the type of study, year of publication, type of conventional mode, number of patients in APRV group and conventional mode, the proportion of patients who died, and the baseline and day 3 PaO2/FiO2 ratio.
Assessment of the risk of bias in the included studies
The risk of bias was assessed using the Cochrane Collaboration's tool to determine the risk of bias in the clinical trials.[19] The assessment tool has seven domains, assessing random sequence generation, allocation sequence concealment, blinding of participants and personnel, blinding of outcome assessment, completeness of outcome data, selective reporting, and other sources of bias.[19] Based on the assessment of each domain, the quality ranged from low, high risk, or with concern bias. The quality criteria of each article were reviewed by two independent reviewers (TI and FO), and the results compared. The third reviewer resolved any conflicts.
Statistical approach
The meta-analysis was performed using RevMan, version 5.3 (Cochrane Collaboration). For both improving in oxygenation, that measured as the day 3 PaO2/FiO2 ratio, and the LOS in ICU, the outcome variables were continuous variables and expressed as mean differences (MDs). Most of the studies reported this outcome as mean and standard deviation (SD); however, we estimated the mean and SD using the proposed method for the studies that only reported medians.[20] Mortality was managed as a dichotomous variable, expressed as risk differences (RD). The pooled estimate and their 95% confidence interval (CI) were used to summarize the weighted effect size for each study using random effect model, and P < 0.05 was set for statistical significance. Heterogeneity was assessed using the I2 test, with an I2 higher than 70% considered as substantial heterogeneity. We performed a sensitivity analysis by comparing the studies with conventional LVT strategy as the comparator group because it is considered the standard ventilatory strategy to manage ARDS patients.
This study has been registered in King Abdullah International Medical Research Center database reference number SP19.141.R.
Results
Study selection and study characteristics
In the initial search in the electronic databases, we identified 276 citations for review. After the removal of duplicates, we screened the titles and abstracts of 168 records and assessed the full text of six clinical trials, enrolling a total of 375 patients.[21,22,23,24,25,26] The six clinical trials were included in the analysis [Figure 1].
Figure 1.
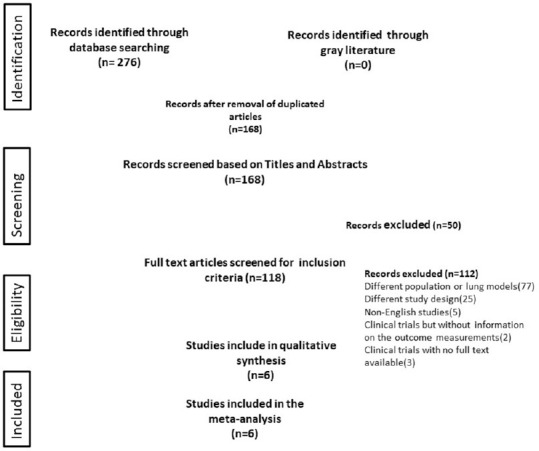
Flowchart of literature screening and study selection of the meta-analysis
Table 1 displays the main characteristics of the selected studies. The mean age of the APRV group was 48 years (range 40–57 years), and for the conventional mode group, 47 years (range 42–53 years). Four studies compared APRV versus conventional modes that use LVT strategy in which tidal volume (Vt) set between 4 and 6 mL/kg,[21,23,25,26] and two studies compared APRV versus synchronized intermittent mandatory ventilation in which they used Vt more than 6 mL/kg. For the main outcome, all studies reported the day 3 PaO2/FiO2, in the tables or figures of the articles. The mortality outcome was reported in all studies, but the LOS in ICU, in only five studies.[21,23,24,25,26] The definition of ARDS varied between the studies, some studies defined ARDS according to the American–European Consensus Conference on ARDS, and others used the Berlin definition of ARDS.[27,28]
Table 1.
Characteristics of the clinical trials that included in the meta-analysis
| Study | Years | Comparator mode and tidal volume strategy | Sample size | Age (years) | PaO2/FiO2 at baseline (mmHg), mean (SD) | PaO2/FiO2 at day 3 (mmHg), mean (SD) |
|---|---|---|---|---|---|---|
| Putensen et al.[21] | 2001 | AC–PC with LVT (Vt=6 ml/kg) | AC–PC: 15 APRV: 15 | AC–PC: 42 (5) APRV: 40 (5) | AC–PC: 250 (14) APRV: 250 (14) | AC–PC: 175 (38) APRV: 320 (58) |
| Varpula et al.[22] | 2004 | SIMV + PS–PC (Vt=8–10 ml/kg) | SIMV–PC: 28 APRV:30 | SIMV–PC: 44 (4) APRV: 49 (5) | SIMV–PC: 164 (6) APRV: 150 (7) | SIMV–PC: 165 (60) APRV: 195 (76) |
| Maxwell et al.[23] | 2010 | SIMV + PS–VC with LVT (Vt=6 ml/kg) | SIMV–VC: 32 APRV:31 | SIMV–VC: 42.4 (16) APRV: 40 (14) | SIMV–VC: 363 (36) APRV: 320 (33) | SIMV-VC: 280 (32) APRV: 300 (45) |
| Li et al.[24] | 2016 | SIMV–VC (Vt=6–8 ml/kg) | SIMV–VC: 26 APRV: 26 | SIMV–VC: 53 (9) APRV: 54 (8) | SIMV–VC: 118 (36) APRV: 119 (35) | SIMV–VC: 212 (55) APRV: 220 (46) |
| Zhou et al.[25] | 2017 | AC–VC with LVT (Vt=6 ml/kg) | AC–VC: 67 APRV: 71 | AC–VC: 52 (15) APRV: 51 (15) | AC–VC: 138 (56) APRV: 121 (46) | AC–VC: 180 (68) APRV: 280 (83) |
| Hirshberg et al.[26] | 2018 | AC–VC with LVT (Vt=6 ml/kg) | AC–VC: 17 APRV: 17 | AC–VC: 51 (14) APRV: 57 (15) | AC–VC: 121 (50) APRV: 109 (67) | AC–VC: 162 (34) APRV: 168 (98) |
A/C=Assist control ventilation, AC-PC=Assisted control with pressure control ventilation, APRV=Airway pressure release ventilation, LTV=Low tidal volume, PC=Pressure control ventilation, SIMV=Synchronized intermittent mandatory ventilation
Risk of bias and quality of evidence
The results of the quality assessment of the studies are provided in Table 2. Two studies had a low bias for the randomization process.[25,26] All studies had a high risk of bias due to deviations from the intended intervention as the investigators were not blinded. The majority of the studies had a moderate risk of bias in terms of the measurement of the outcome, due to the same reason. Based on the direction of the bias for each domain, the overall bias was with concern.
Table 2.
Assessment of the risk of bias in the included studies
| Reference | Comparator | Randomization process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported result | Overall Bias |
|---|---|---|---|---|---|---|---|
| Putensen et al. 2000 | AC–PC versus APRV | Some concerns | High | Some concerns | Some concerns | Low | Some concerns |
| Varpula et al. 2004 | SIMV–PC versus APRV | Some concerns | High | Some concerns | Some concerns | Some concerns | Some concerns |
| Maxwell et al. 2010 | SIMV–VC versus APRV | Some concerns | High | Some concerns | High | Low | Some concerns |
| Li et al. 2016 | SIMV–VC versus APRV | Some concerns | High | Some concerns | Some concerns | Some concerns | Some concerns |
| Zhou et al. 2017 | AC–VC versus APRV | Low | High | Low | Some concerns | Low | Some concerns |
| Hirshberg et al. 2018 | AC–VC versus APRV | Low | High | Low | Low | Low | Low |
A/C=Assist control ventilation, AC-PC=Assisted control with pressure control ventilation, APRV=Airway pressure release ventilation, LTV=Low-tidal volume, PC=Pressure control ventilation, SIMV=Synchronized intermittent mandatory ventilation
Study outcomes
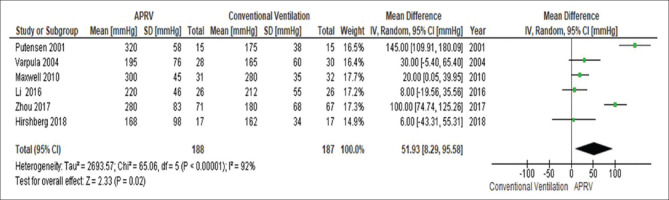
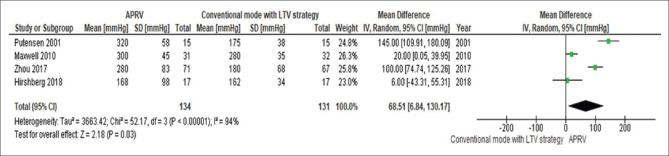
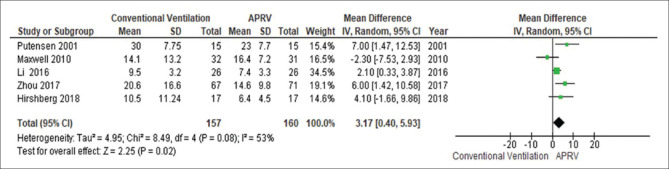
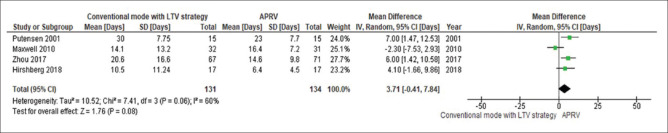
The day 3 PaO2/FiO2 was significantly higher in patients receiving APRV compared to other conventional ventilatory modes (MD 51.9 mmHg, 95% CI 8.2–95.5, P = 0.02, I2= 92%) [Figure 2]. This yield similar results when the analysis includes only conventional mode with LTV strategy as the comparator ventilation approach (MD 68.5 mmHg, 95% CI 6.84–130.1, P = 0.03, I2= 94%) [Figure 3].
Figure 2.
The forest plot comparing the ventilation PaO2/FiO2 at day 3 between airway pressure release ventilation and conventional ventilatory modes
Figure 3.
The forest plot comparing the ventilation PaO2/FiO2 at day 3 between airway pressure release ventilation and conventional mode with low-tidal volume strategy
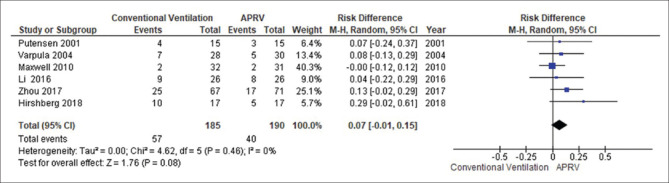
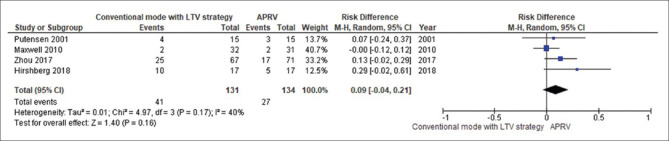
The forest plot comparing the mortality is presented in Figure 4. There was no significant difference in mortality between APRV and the other conventional ventilator modes (RD 0.07, 95% CI: −0.01–0.15, P = 0.08, I2= 0%). With LTV as the comparator ventilation strategy, there was no significant reduction in mortality in the APRV group (RD 0.09, 95% CI: −0.01–0.21, P = 0.16, I2= 40%) [Figure 5]. The point estimate for the effect of APRV on the LOS in ICU indicated a significant reduction in the ICU LOS for the APRV group compared to the counter group (MD 3.1 days, 95% CI 0.4–5.9, P = 0.02, I2 = 53%) [Figure 6]. The point estimate changed with the LTV as the comparator ventilation strategy (MD 3.7, 95% CI: −0.41–7.84, P = 0.08; I2= 60%) [Figure 7].
Figure 4.
The forest plot comparing the mortality between airway pressure release ventilation and conventional ventilatory modes
Figure 5.
The forest plot comparing the mortality between airway pressure release ventilation and conventional mode with low-tidal volume strategy
Figure 6.
The forest plot comparing the length of stay in the intensive care unit between airway pressure release ventilation and conventional ventilatory modes
Figure 7.
The forest plot comparing the length of stay in the intensive care unit between airway pressure release ventilation and conventional mode with low-tidal volume strategy
Discussion
Key finding
This study contributed new evidence to the growing body of literature related to the effectiveness of using APRV in managing ARDS patients. We demonstrated that APRV is associated with an improvement in the day 3 oxygenation and not associated with reduction in mortality. APRV was also associated with a significant reduction in the LOS in ICU. However, the finding was inconsistent when limiting the analysis to the LTV ventilation strategy.
Comparison to the literature
The key concept for managing ARDS patients is to use protective ventilation strategies to avoid over-distension or lung damage from the cyclical opening and closing of the alveoli. The APRV mode is able to optimize gas exchange while reducing the risk of lung injury. In addition, APRV allows for spontaneous breathing, which leads to alveolar recruitment, improve functional residual capacity, and a reduced elastic work of breathing, enhancing gas exchange.[7]
During the study period, three systematic review and meta-analysis studies comparing the efficacy of APRV to other ventilatory modes in managing ARDS patients were published in 1 year (2019–2020).[17,29,30] This indicates the support for using the APRV mode as a concept of open lung ventilation in the management of ARDS. In the first, in April 2019, Carsetti et al. compared the number of ventilator-free days in intubated ARDS patients, using the APRV mode compared with a conventional ventilation strategy.[17] The authors included five clinical trials,[21,22,24,25,26] and they reported a higher number of ventilator-free days at 28 days and lower hospital mortality in the ARDS patients treated with APRV compared to conventional ventilation. Furthermore, they reported no difference in PaO2/FiO2 on day 3 between the APRV group and the conventional ventilatory mode group, which was inconsistent with the result of the current study. This variation can be attributed to different clinical trials that had been included for the pooled estimate for the outcome measured. Thus, Carsetti et al. included only three out of five clinical trials in the analysis of PaO2/ FiO2 at day 3,[17] and only two clinical trials for the mortality (defined as hospital mortality). In the current study, we included six clinical trials in the analysis of PaO2/FiO2 on day 3 and mortality. The mortality outcome was measured as all-cause mortality (hospital or ICU mortality), explaining the difference between the two studies' results.
The second meta-analysis study examined the efficacy of APRV in managing ARDS patients, focused primarily on reviewing the all-cause mortality rate.[29] They reported a reduction in the ARDS adult patients' mortality, managed with APRV compared with conventional ventilation strategies. They included six clinical trials, including a study that was excluded from our meta-analysis because it published an abstract in which we could obtain the full text to assessed it in detail. In this second meta-analysis, the authors used relative risk to estimate the mortality where we expressed the mortality outcome as RDs. Although relative risk and RD provide two different assessments on the same outcome, the RD offers a straightforward interpretation of the absolute difference. Thus, RD describes the difference in the observed risk of mortality between APRV and comparator interventions; therefore, it provides more directly relevant information than relative measures.[31] Although the point estimate for mortality outcome in this current study does not fall across the significant threshold, it still indicates a potential reduction in mortality. The consistency between the literature suggests evidence to support the efficacy of APRV to reduce mortality among ARDS from a clinical perspective.
The last study, in January 2020, including observational studies and clinical trials, demonstrated that using APRV in managing ARDS patients could increase the day 3 PaO2/FiO2.[30] However, a major limitation of the third study was combining the results from observational studies and clinical trials, due to the heterogeneity in the methodology which would affect the validity of the results. In addition, the different characteristics of the pooled population from the observational studies and clinical trials would also affect the results.
Both Lim et al. and Xuri et al. reported an improvement in the day 3 oxygenation PaO2/FiO2 ratio in the APRV group, compared to the conventional ventilatory mode group. In our study, we found a significantly higher day 3 PaO2/FiO2 ratio in the APRV group, and this remained unchanged when we include studies that implement conventional mode with LTV strategy. A possible mechanism that could explain this finding is that APRV increases lung recruitment and oxygenation in addition to the preservation of spontaneous breathing. Those advantages may promote better ventilation/perfusion matching and less need for sedation, and subsequently, higher ventilation free days. Several studies indicated that higher ventilation free days show lower mechanical ventilation duration and decreased ICU LOS.[32,33] This has been demonstrated in the first meta-analysis study that examined the efficacy of APRV in treating ARDS patients. Thus, patients with ARDS who are ventilated with APRV mode have higher number of ventilated free days than the conventional ventilation strategy.
Strength and limitation
Our study has several limitations. The studies included in this meta-analysis have some concerns related to quality as it is difficult to blind the assessor in terms of the intervention arm. Second, the variation of the baseline Pao2/FiO2 ratio due to the severity of the disease, may explain the heterogeneity in the primary outcome. Third, the mortality outcome measure in this study was not standardized in which it was defined either as ICU, hospital, or 28 days mortality. The consequence of this is that it may underestimate the effect of APRV on mortality, which explains the inconsistency of the results in terms of the reduction in mortality. Fourth, the validity of our results could be affected by the publication bias as did not use the funnel plot as a statistical assessment for publication bias due to the low number of included studies.[34] We used a nonstatistical approach. We reviewed the unpublished clinical trials on the clinical trial registries as failure to publish neutral or negative trials could influence the accuracy of reported outcomes.[34] At the time of carrying this study, we found ten registered clinical trials; two were published,[25,26] three have been withdraw due to slow requirment, and the remaining five in the recruiting process. For the selection bias due to restricting our research to the English language literature, we do not anticipate that it will cause deviation of the results as clinical trials tend to register and publish their study in the English language journals.
Since the first description of APRV, the definition of APRV was inconsistent. The terms biphasic and APRV are used interchangeably. In the current analysis, we used different combinations to capture studies with different terminology. The lack of an APRV protocol with standardized settings and parameters, implemented in the different trials, may affect the outcomes that were measured. Not only is there paucity in the number of high-quality trials in humans, but there is a lack of consistency in how APRV is applied. In the literature, there is no standardized protocol for starting APRV as there is a conflict data with regard to the potential risks of using APRV. Many reports demonstrated that barotrauma risk among APRV groups did not differ from the conventional ventilatory mode.[25,26,29] On the other hand, some studies reported a potential safety profile of using APRV including improvement in cardiac function and cardiac index during spontaneous respiration and subsequently improved systematic blood flow.[15,21,35] Other studies reported a potential reduction in the risk of ventilator-associated pneumonia using APRV mode.[36] However, those potential benefits should be carefully reviewed as most of those studies were from lung simulation studies or different study populations.
Conclusion
In this study, using the APRV mode may have improved oxygenation on day 3 and contributed to a reduction of the LOS in ICU. Although the point estimate for mortality outcome does not across the significant threshold, it still indicates a potential reduction in mortality. In light of this study's limitations, it is crucial to consider the effect of the heterogeneity and the quality of the included studies in interpreting those findings. Nevertheless, the data regarding the use of APRV for ARDS appear promising, and further studies are required to validate those results.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Claar DD, Hyzy RC. Refractory hypoxemia and acute respiratory distress syndrome adjunctive therapies: An open question? Ann Am Thorac Soc. 2017;14:1768–9. doi: 10.1513/AnnalsATS.201707-547ED. [DOI] [PubMed] [Google Scholar]
- 2.Blondonnet R, Constantin JM, Sapin V, Jabaudon M. A pathophysiologic approach to biomarkers in acute respiratory distress syndrome. Dis Markers. 2016:350–73. doi: 10.1155/2016/3501373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papazian L, Aubron C, Brochard L, Chiche JD, Combes A, Dreyfuss D, et al. Formal guidelines: Management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9:69. doi: 10.1186/s13613-019-0540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rawal G, Yadav S, Kumar R. Acute respiratory distress syndrome: An update and review. J Transl Intern Med. 2018;6:74–7. doi: 10.1515/jtim-2016-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 6.Maung AA, Luckianow G, Kaplan LJ. Lessons learned from airway pressure release ventilation. J Trauma Acute Care Surg. 2012;72:624–8. doi: 10.1097/TA.0b013e318247668f. [DOI] [PubMed] [Google Scholar]
- 7.Jain SV, Kollisch-Singule M, Sadowitz B, Dombert L, Satalin J, Andrews P, et al. The 30-year evolution of airway pressure release ventilation (APRV) Intensive Care Med Exp. 2016;4:11. doi: 10.1186/s40635-016-0085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Downs JB, Stock MC. Airway pressure release ventilation: A new concept in ventilatory support. Crit Care Med. 1987;15:459–61. [PubMed] [Google Scholar]
- 9.Liu L, Tanigawa K, Ota K, Tamura T, Yamaga S, Kida Y, et al. Practical use of airway pressure release ventilation for severe ARDS A preliminary report in comparison with a conventional ventilatory support. Hiroshima J Med Sci. 2009;58:83–8. [PubMed] [Google Scholar]
- 10.Kollisch-Singule M, Jain S, Andrews P, Smith BJ, Hamlington-Smith KL, Roy S, et al. Effect of airway pressure release ventilation on dynamic alveolar heterogeneity. JAMA Surg. 2016;151:64–72. doi: 10.1001/jamasurg.2015.2683. [DOI] [PubMed] [Google Scholar]
- 11.Frawley PM, Habashi NM. Airway pressure release ventilation: Theory and practice. AACN Clin Issues. 2001;12:234–46. doi: 10.1097/00044067-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Dart IV, Maxwell RA, Richart CM, Brooks DK, Ciraulo DL, Barker DE, et al. Preliminary experience with airway pressure release ventilation in a trauma/surgical intensive care unit. J Trauma Inj Infect Crit Care. 2005;59:71–6. doi: 10.1097/00005373-200507000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida T, Rinka H, Kaji A, Yoshimoto A, Arimoto H, Miyaichi T, et al. The impact of spontaneous ventilation on distribution of lung aeration in patients with acute respiratory distress syndrome: Airway pressure release ventilation versus pressure support ventilation. Anesth Analg. 2009;109:1892–900. doi: 10.1213/ANE.0b013e3181bbd918. [DOI] [PubMed] [Google Scholar]
- 14.Daoud EG, Chatburn RL. Comparing surrogates of oxygenation and ventilation between airway pressure release ventilation and biphasic airway pressure in a mechanical model of adult respiratory distress syndrome. Respir Investig. 2014;52:236–41. doi: 10.1016/j.resinv.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Hering R, Peters D, Zinserling J, Wrigge H, von Spiegel T, Putensen C. Effects of spontaneous breathing during airway pressure release ventilation on renal perfusion and function in patients with acute lung injury. Intensive Care Med. 2002;28:1426–33. doi: 10.1007/s00134-002-1442-z. [DOI] [PubMed] [Google Scholar]
- 16.González M, Arroliga AC, Frutos-Vivar F, Raymondos K, Esteban A, Putensen C, et al. Airway pressure release ventilation versus assist-control ventilation: A comparative propensity score and international cohort study. Intensive Care Med. 2010;36:817–27. doi: 10.1007/s00134-010-1837-1. [DOI] [PubMed] [Google Scholar]
- 17.Carsetti A, Damiani E, Domizi R, Scorcella C, Pantanetti S, Falcetta S, et al. Airway pressure release ventilation during acute hypoxemic respiratory failure: A systematic review and meta-analysis of randomized controlled trials. Ann Intensive Care. 2019;9:44. doi: 10.1186/s13613-019-0518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions. 2nd Ed. Chichester (UK): John Wiley & Sons; 2019. [Google Scholar]
- 20.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Putensen C, Zech S, Wrigge H, Zinserling J, Stüber F, von Spiegel T, et al. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med. 2001;164:43–9. doi: 10.1164/ajrccm.164.1.2001078. [DOI] [PubMed] [Google Scholar]
- 22.Varpula T, Valta P, Niemi R, Takkunen O, Hynynen M, Pettilä V. Airway pressure release ventilation as a primary ventilatory mode in acute respiratory distress syndrome. Acta Anaesthesiol Scand. 2004;48:722–31. doi: 10.1111/j.0001-5172.2004.00411.x. [DOI] [PubMed] [Google Scholar]
- 23.Maxwell RA, Green JM, Waldrop J, Dart BW, Smith PW, Brooks D, et al. A randomized prospective trial of airway pressure release ventilation and low tidal volume ventilation in adult trauma patients with acute respiratory failure. J Trauma Inj Infect Crit Care. 2010;69:501–10. doi: 10.1097/TA.0b013e3181e75961. [DOI] [PubMed] [Google Scholar]
- 24.Li JQ, Li N, Han GJ, Pan CG, Zhang YH, Shi XZ, et al. Clinical research about airway pressure release ventilation for moderate to severe acute respiratory distress syndrome. Eur Rev Med Pharmacol Sci. 2016;20:2634–41. [PubMed] [Google Scholar]
- 25.Zhou Y, Jin X, Lv Y, Wang P, Yang Y, Liang G, et al. Early application of airway pressure release ventilation may reduce the duration of mechanical ventilation in acute respiratory distress syndrome. Intensive Care Med. 2017;43:1648–59. doi: 10.1007/s00134-017-4912-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirshberg EL, Lanspa MJ, Peterson J, Carpenter L, Wilson EL, Brown SM, et al. Randomized feasibility trial of a low tidal volume-airway pressure release ventilation protocol compared with traditional airway pressure release ventilation and volume control ventilation protocols. Crit Care Med. 2018;46:1943–52. doi: 10.1097/CCM.0000000000003437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: The Berlin definition. J Am Med Assoc. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 28.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS: Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 29.Lim J, Litton E. Airway pressure release ventilation in adult patients with acute hypoxemic respiratory failure: A systematic review and meta-analysis. Crit Care Med. 2019;47:1794–9. doi: 10.1097/CCM.0000000000003972. [DOI] [PubMed] [Google Scholar]
- 30.Sun X, Liu Y, Li N, You D, Zhao Y. The safety and efficacy of airway pressure release ventilation in acute respiratory distress syndrome patients: A PRISMA-compliant systematic review and meta-analysis. Med (United States) 2020;99:e18586. doi: 10.1097/MD.0000000000018586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Chichester (UK): John Wiley & Sons; 2019. [Google Scholar]
- 32.Schoenfeld DA, Bernard GR. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30:1772–7. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 33.Yehya N, Harhay MO, Curley MAQ, Schoenfeld DA, Reeder RW. Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med. 2019;200:828–36. doi: 10.1164/rccm.201810-2050CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murad MH, Chu H, Lin L, Wang Z. The effect of publication bias magnitude and direction on the certainty in evidence. BMJ Evidence Based Med. 2018;23:84–6. doi: 10.1136/bmjebm-2018-110891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Putensen C, Mutz NJ, Putensen-Himmer G, Zinserling J. Spontaneous breathing during ventilatory support improves ventilation- perfusion distributions in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;159:1241–8. doi: 10.1164/ajrccm.159.4.9806077. [DOI] [PubMed] [Google Scholar]
- 36.Walkey AJ, Nair S, Papadopoulos S, Agarwal S, Reardon CC. Use of airway pressure release ventilation is associated with a reduced incidence of ventilator-associated pneumonia in patients with pulmonary contusion. J Trauma. 2011;70:E42–7. doi: 10.1097/TA.0b013e3181d9f612. [DOI] [PubMed] [Google Scholar]