Abstract
CONTEXT:
Exploring clinical characteristics of coronavirus disease-19 (COVID-19) in children may help in prevention and treatment guidelines.
AIMS:
The aim of the to describe the spectrum of pediatric COVID-19 in Saudi Arabia.
SETTINGS And DESIGN:
A multicenter, retrospective, cross-sectional study involving pediatric COVID-19 patients across all Saudi regions.
METHODS:
All patients aged between 2 months and 18 years with a confirmed diagnosis of COVID-19 were included. The primary end point was the hospitalization.
STATISTICAL ANALYSIS USED:
Descriptive statistics were used to describe the baseline demographic data and clinical characteristics. Numerical data were explored using Kolmogorov–Smirnov test and Shapiro–Wilk test, while Chi-square or Fisher's exact test were used for categorical data.
RESULTS:
Among the 654 pediatric COVID-19 patients, 4.7% (n = 31) were hospitalized, with one patient only needing pediatric intensive care admission. Sex, breastfeeding, birth status, and the patients' living environment showed no significant association with hospitalization. Most children (80.3%, n = 525) were symptomatic, with two symptoms that were significantly associated with admission, namely, vomiting (P = 0.007) and nausea (P = 0.026). History of admission within the last year was identified in 10.4% (n = 68) children but had no association with worse outcome. The median duration of hospitalization for the entire group was 5.5 days, with longest hospital stay for age group 7–12 years (median 6 days).
CONCLUSIONS:
COVID-19 is usually a milder disease in children. Although having preexisting medical conditions was linked to a longer hospitalization, it was not associated with worse outcome. Continuous surveillance will allow additional characterization of the burden and outcomes of pediatric COVID-19-associated hospitalizations.
Keywords: Children, coronavirus, coronavirus disease 19, infection, risk factors
In December 2019, a novel coronavirus disease-19 (COVID-19) due to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) emerged in Wuhan, China.[1] This coronavirus rapidly spread worldwide over the next months, causing a Public Health Emergency of International Concern as declared on the January 30, 2020, by the World Health Organization (WHO). Soon after, COVID-19 was characterized as a pandemic on March 11, 2020.[2] Interestingly, poor outcomes of COVID-19 varied tremendously with higher morbidity and mortality rates reported among older adults and in patients with preexisting comorbidities including diabetes, hypertension, and obesity.[3,4] As of October 1, 2020, the WHO declared more than 35 million confirmed COVID-19 cases and more than 1 million deaths reported worldwide because of this pandemic.[5] Although the incidence of COVID-19 is less common among children, the data on the pediatric clinical presentation spectrum and pathogenesis have not yet been fully established.[6] Data from several countries worldwide show pediatric cases comprise 1.2%–2% of COVID-19 cases. The vast majority of reported pediatric cases were either asymptomatic or mild diseases, with death rarely reported. A recent systematic review revealed that severe and critical pediatric COVID-19 cases constituted only 5% and 0.6% of pediatric cases, respectively. Children with previous chronic conditions represent the most vulnerable pediatric population with more significant/higher disease severity.[7,8,9,10] Defining the epidemiological and clinical characteristics of COVID-19 in the pediatric population is an urgent need. It will help in the identification of suspected cases, determination of the restrictive measures needed to control the disease spread, and the development of prevention and treatment guidelines for the community's COVID-19 cases. Therefore, we conducted a retrospective review of the reported pediatric COVID-19 cases in the Saudi pediatric population to describe the spectrum of epidemiological and clinical characteristics, associated risk factors, medications intake, and definitive outcomes of the cases.
Methods
This was a multicenter, retrospective, cross-sectional study involving 654 consecutive pediatric COVID-19 patients presenting to health-care facilities across all Saudi Arabia regions between March 2, 2020, and April 19, 2020. All patients aged between 2 months and 18 years with a confirmed diagnosis of COVID-19 were included in this study. Data were extracted from the Health Electronic Surveillance Network (HESN) Database. This system contains demographic, comorbidities, medication intake, and raw outcomes data of COVID-19-positive patients from all regions of Saudi Arabia. Demographic, comorbidities, medications intake, and outcomes data of COVID-19-positive pediatric patients based on the Ministry of Health Guidelines were retrieved from HESN. Phone call interviews were also conducted with the parents to obtain further details. Two expert data collectors entered all the data using a designated data collection form. An independent reviewer resolved any discrepancies based on the final medical reports on the records.[11] COVID-19 was diagnosed based on quantitative reverse transcription polymerase chain reaction testing of nasopharyngeal samples according to the WHO protocols.[12] We divided patients into the following age groups: 2 months to <1 year (infant), 1–3 years (toddler), 4–6 years (preschool), 7–12 years (school), and 13–18 years (adolescent) according to the Centers for Disease Control and Prevention (CDC) classifications.[13] The birth status was divided into full term (>37 weeks pregnancy) and preterm (<37 weeks pregnancy) based on the WHO preterm key facts.[14] Comorbidities were classified based on the International Classification of Diseases, Revision 10 diagnostic codes.[15] Alternative medicine data were classified based on the WHO global report on traditional and complementary medicine 2019.[16]
The primary end point of this study was the hospitalization of the pediatric COVID-19 cases. Data concealment was maintained throughout the study by generating strong passwords for the electronic system and limiting access to designated investigators who had signed nondisclosure agreement forms. The study was approved by the Central Ministry of Health Institutional Review Board Committee (NO: 20-198M). Descriptive statistics were used to describe the baseline demographic data and clinical characteristics. Counts and percentages represented categorical variables, whereas numerical data were explored for normality using the Kolmogorov–Smirnov test and Shapiro–Wilks test and were represented by the median and interquartile range. Categorical variables underwent a test of association using a Chi-square test or Fisher's exact test. Graphical presentations of some crucial variables were done using Microsoft Office Excel. All mean and median values and their measures of variability were formatted to one decimal place. All percentages were rounded to one decimal place. The significance level was two sided, with a Type 1 error of 5%. The analysis was done using the Statistical Package for the Social Sciences version 24 (SPSS-24) IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp. A P < 0.05 was considered statistically significant.
Results
From March 2 to April 19, 2020, we recruited 654 pediatric COVID-19 patients across all Saudi Arabia regions through the HESN database; 4.7% (n = 31) of the patients were hospitalized, whereas 95.3% (n = 623) were not hospitalized. Out of the hospitalized patient, 97% (n = 30) were admitted to the wards, and 3% (n = 1) were admitted to the intensive care unit (ICU). Sociodemographic characteristics of the pediatric COVID-19 patients were summarized based on their hospitalization status. The patients' ages varied from infants to adolescents. The stratification of age by hospitalization status (hospitalized/nonhospitalized) gave a significant P value (P = 0.024), demonstrating a difference in hospitalization among age groups. In the 2 months to <1 year age group, three patients (11.1%) were hospitalized (9.7% of all hospitalized patients) and 24 patients (88.9%) were not hospitalized (3.9% of all nonhospitalized patients). Moreover, in the 1–3-year age group, 9.1% (n = 11) were hospitalized (35.5% of all hospitalized cases) versus 90.9% (n = 110) were not (17.7% of all nonhospitalized patients). All groups had more patients who were not hospitalized than were hospitalized [Table 1]. Females constituted 51.4% (n = 336) of the study sample. Sex, breastfeeding for at least 6 months, birth status, and the patients' living environment showed no significant association with the hospitalization status [Table 1]. Most children (80.3%, n = 525) were symptomatic, but 19.7% (n = 129) did not report any symptoms. Comorbidities were reported for 15.6% (n = 102) of the population and included different conditions such as allergic diseases in 6.6% (n = 43), bronchial asthma in 4.3% (n = 28), chronic lung diseases in 1.2% (n = 8), and other conditions that were less frequent such as congenital heart diseases in 0.8% (n = 5), hematological diseases in 0.6% (n = 4), and others [Table 1 and Figure 1].
Table 1.
Sociodemographic characteristics, comorbidities, and history of hospitalization of pediatric coronavirus disease-19 cases based on their hospitalization status
| Characteristic | Total, n (%) | Hospitalized, n (%) | Nonhospitalized, n (%) | P |
|---|---|---|---|---|
| Total | 654 (100) | 31 (4.7) | 623 (95.3) | |
| Age | ||||
| 2 months to <1 year (infant) | 27 (4.1) | 3 (9.7) | 24 (3.9) | 0.024 |
| 1-3 years (toddler) | 121 (18.5) | 11 (35.5) | 110 (17.7) | |
| 4-6 years (preschool) | 120 (18.3) | 4 (12.9) | 116 (18.6) | |
| 7-12 years (school) | 289 (44.2) | 12 (38.7) | 277 (44.5) | |
| 13-18 years (adolescent) | 97 (14.8) | 1 (3.2) | 96 (15.4) | |
| Sex | ||||
| Male | 318 (48.6) | 15 (48.4) | 303 (48.6) | 0.978 |
| Female | 336 (51.4) | 16 (51.6) | 320 (51.4) | |
| Breastfeeding for at least 6 months | ||||
| Yes | 372 (56.9) | 18 (58.1) | 354 (56.8) | 0.437 |
| No | 251 (38.4) | 13 (41.9) | 238 (38.2) | |
| Don’t know | 31 (4.7) | - | 31 (5.0) | |
| Birth status | ||||
| Full-term | 629 (96.2) | 30 (96.8) | 599 (96.1) | 0.859 |
| Preterm | 25 (3.8) | 1 (3.2) | 24 (3.9) | |
| Health care worker co-living in the house | ||||
| Yes | 132 (20.2) | 4 (12.9) | 128 (20.5) | 0.301 |
| No | 522 (79.8) | 27 (87.1) | 495 (79.5) | |
| Smoker in the house (passive smoking) | ||||
| Yes | 214 (32.7) | 6 (19.4) | 208 (33.4) | 0.104 |
| No | 440 (67.3) | 25 (80.6) | 415 (66.6) | |
| Presence of symptoms | ||||
| Asymptomatic | 129 (19.7) | 2 (6.5) | 127 (20.4) | 0.057 |
| Symptomatic | 525 (80.3) | 29 (93.5) | 496 (79.6) | |
| Comorbidities | ||||
| All | 102 (15.6) | 6 (19.4) | 96 (15.4) | 0.555 |
| Diabetes mellitus | 3 (0.5) | - | 3 (0.5) | >0.999 |
| Endocrine diseases | 1 (0.2) | - | 1 (0.2) | >0.999 |
| Psychiatric disorders | 4 (0.6) | - | 4 (0.6) | >0.999 |
| Allergic diseasesa | 43 (6.6) | 2 (6.5) | 41 (6.6) | 0.977 |
| Bronchial asthma | 28 (4.3) | 3 (9.7) | 25 (4.0) | 0.128 |
| Chronic lung diseases | 8 (1.2) | - | 8 (1.3) | 0.526 |
| Congenital heart diseases | 5 (0.8) | 1 (3.2) | 4 (0.6) | 0.216 |
| Chronic liver diseases | 1 (0.2) | - | 1 (0.2) | >0.999 |
| Chronic renal diseases | 1 (0.2) | - | 1 (0.2) | >0.999 |
| Hematological diseases | 4 (0.6) | 1 (3.2) | 3 (0.5) | 0.177 |
| Neurological diseases | 2 (0.3) | - | 2 (0.3) | >0.999 |
| Cancer | 2 (0.3) | - | 2 (0.3) | >0.999 |
| Genetic disorder | 69 (10.6) | 2 (6.5) | 67 (10.8) | 0.784 |
| History of previous hospitalization (within the last 12 months) | ||||
| Yes | 68 (10.4) | 6 (19.4) | 62 (10.0) | 0.094 |
| No | 586 (89.6) | 25 (80.6) | 561 (90.0) |
aIncludes atopic dermatitis, allergic rhinitis, food allergy, and anaphylaxis
Figure 1.
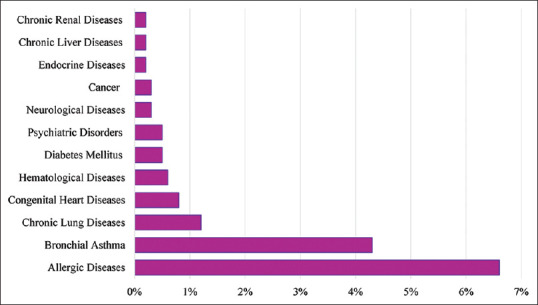
Percentage of pediatric coronavirus disease-19 cases with comorbidities
The clinical characteristics were studied in the 654 pediatric cases based on the hospitalization status (hospitalized/nonhospitalized). The frequently occurring symptoms were fever in 61% (n = 399), followed by fatigue in 40.2% (n = 263), sore throat in 26.3% (n = 172), cough in 25.5% (n = 167), diarrhea in 23.1% (n = 151), loss of smell or taste in 22.2% (n = 145), runny nose in 21.3% (n = 139), and headache in 20.3% (n = 133). Other less frequent symptoms included loss of appetite in 15% (n = 98), shortness of breath in 10.7% (n = 70), vomiting in 10.4% (n = 68), and other symptoms [Table 2 and Figure 2]. Moreover, only two symptoms were significantly associated with admission, which were vomiting (P = 0.007) and nausea (P = 0.026) [Table 2 and Figure 2]. Genetic disorders were present in 10.6% (n = 69) of the patient population with 2.9% (n = 2) being hospitalized (6.5% of all hospitalized patients) versus 97.1% (n = 67) not hospitalized (10.8% of all nonhospitalized patients). However, this was not statistically significant. Furthermore, history of admission within the last year was identified in 10.4% (n = 68) of the cases and had no significant association with the outcome as well [Table 2].
Table 2.
Clinical Characteristics of the pediatric coronavirus disease-19 cases based on their hospitalization status
| Characteristics | Total (n=654; 100%), n (%) | Hospitalized (n=31; 4.7%), n (%) | Nonhospitalized (n=623; 95.3%), n (%) | P |
|---|---|---|---|---|
| Symptoms | ||||
| Fever | 399 (61.0) | 24 (77.4) | 375 (60.2) | 0.155 |
| Cough | 167 (25.5) | 11 (35.5) | 156 (25.0) | 0.421 |
| Sore throat | 172 (26.3) | 10 (32.3) | 162 (26.0) | 0.630 |
| Runny nose | 139 (21.3) | 9 (29.0) | 130 (20.9) | 0.471 |
| Shortness of breath | 70 (10.7) | 6 (19.4) | 64 (10.3) | 0.171 |
| Vomiting | 68 (10.4) | 9 (29.0) | 59 (9.5) | 0.007* |
| Nausea | 50 (7.6) | 6 (19.4) | 44 (7.1) | 0.026* |
| Dizziness | 57 (8.7) | 1 (3.2) | 56 (9.0) | 0.440 |
| Fatigue | 263 (40.2) | 16 (51.6) | 247 (39.6) | 0.315 |
| Loss of smell or taste | 145 (22.2) | 5 (16.1) | 140 (22.5) | 0.530 |
| Diarrhea | 151 (23.1) | 12 (38.7) | 139 (22.3) | 0.089 |
| Headache | 133 (20.3) | 3 (9.7) | 130 (20.9) | 0.131 |
*Pvalue of less than 0.05 is considered significant
Figure 2.
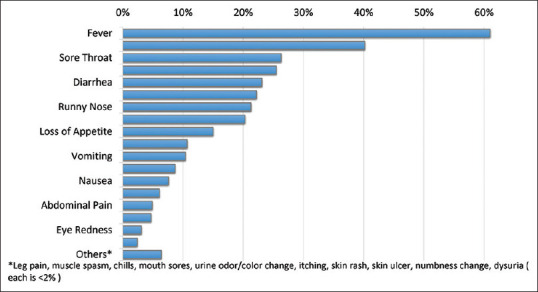
Percentage of pediatric coronavirus disease-19 cases showing symptoms
The median duration of hospitalization for the entire group was 5.5 days. Among the age groups, the longest duration was for age group of 7–12 years (median 6 days), followed by age group 1–3 (median 5.5 days), then age group 4–6 years (median 4 days) and the shortest was for those between 2 months and <1 year with (median 1 day). The median hospitalizations for females and males were very close at 6.5 and 5 days, respectively. Moreover, those with any comorbidity had a median hospitalization of 8 days, whereas those with symptoms had medians ranging from 5 to 7.5 days [Figure 3].
Figure 3.
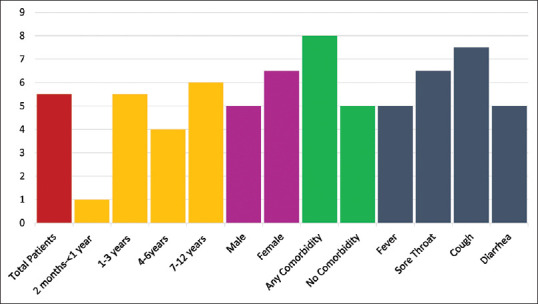
Median length of hospitalization (days) for pediatric coronavirus disease-19 casesdisease-19 cases
The most common medications given during hospitalization were paracetamol (51.1%, n = 334), followed by nonsteroidal anti-inflammatory drugs (8.7%, n = 57), and antibiotics (8.1%, n = 53). Other medications included inhaled steroids, bronchodilators, and cough suppressant and are shown in Table 3. Alternative medicine was taken by 85.2% (n = 557) of the pediatric COVID-19-positive patients. The most common alternative medicines taken were honey (60.9%, n = 398), lemon (56.6%, n = 370), vitamin C (39.6%, n = 259), ginger (32.9%, n = 215), and many others as shown in Table 3.
Table 3.
Medication/alternative medicine intake among pediatric coronavirus disease-19 patients
| Count (%) | |
|---|---|
| Medications name | |
| Paracetamol | 334 (51.1) |
| NSAID | 57 (8.7) |
| Antibiotics | 53 (8.1) |
| Bronchodilators | 9 (1.4) |
| Cough suppressants | 8 (1.2) |
| Inhaled steroids | 8 (1.2) |
| Alternative medicine | |
| Yes | 557 (85.2) |
| No | 97 (14.8) |
| Alternative medicine/product | |
| Honey | 398 (60.9) |
| Lemon | 370 (56.6) |
| Vitamin C | 259 (39.6) |
| Ginger | 215 (32.9) |
| Vitamin D | 75 (11.5) |
| Garlic | 44 (6.7) |
| Multivitamins | 44 (6.7) |
| Anise | 25 (3.8) |
| Zinc | 16 (2.4) |
| Olive oil | 10 (1.5) |
| Calcium | 9 (1.4) |
| Omega 3 | 8 (1.2) |
| Magnesium | 6 (0.9) |
| Iron | 6 (0.9) |
NSAID=Nonsteroidal anti-inflammatory drug
Discussion
To the best of our knowledge, this is one of the first national reports on the pediatric COVID-19 population in the Middle East. The inclusion of such a considerable number of cases was accomplished by including many centers across Saudi Arabia through a well-established electronic surveillance system at the Saudi Ministry of Health. This study has primarily compiled data from children and adolescents managed in different health-care facilities both at primary care centers and hospital settings. Therefore, the study population represents a variable spectrum of disease presentation. In our report, approximately 5% of the patients required hospitalization, with children aged 1–3 years and 7–12 years accounting for the greatest proportions of all hospitalized patients. This is somewhere near the international figures, with a 5.7%–20% hospitalization rate for pediatric patients reported by the CDC.[10] Thus far, our data indicated that children and adolescents, particularly adolescents, were overall less severely affected by COVID-19 than adults. Contrary to some previous reports, our data show that severe COVID-19 is uncommon for infants, despite their incomplete immune maturation.[10,17] Overall, 15.6% of the children in this analysis had an underlying medical condition. This suggests that the presence of underlying conditions may not place children at a higher risk for COVID-19-associated hospitalizations. Underlying conditions in the United States were more common among school-aged children with severe COVID-19; 16% were hospitalized, 27% were admitted to the ICU, and 28% died.[18] In Italy, a recent multicenter study reported that preexisting medical conditions were not different in severe and critical patients compared with asymptomatic, mild, and moderate cases. Furthermore, the ICU admission rate was not different in patients with and without comorbidities.[19] Among our cases, allergic diseases and bronchial asthma were the most frequent underlying illnesses; however, allergy was not common in those who required hospitalization. Reports from a pediatric series showed that neither condition caused exacerbation during hospitalization in COVID-19-infected patients.[20] However, several studies in the United States showed that asthma was a common comorbidity in children infected with SARS-CoV-2, with a prevalence of 20%–24%.[21,22] This finding could be affected by the overall prevalence of asthma in different countries and the possibility that many asthmatic children infected with COVID-19 could be asymptomatic and thus underreported.
Of note, obesity was not a common underlying comorbidity in our series, although childhood obesity is a major public health issue in the region. The prevalence of childhood obesity in Saudi Arabia is 34.3% and 17.3% in girls and boys, respectively.[23] Other studies of hospitalized children with COVID-19 in the United States found that obesity was the most prevalent underlying medical condition.[22,24,25] There is growing evidence of other clinical manifestations, apart from acute respiratory illness in pediatrics, suggesting that children's COVID-19 spectrum and pathogenesis need to be elucidated. Asymptomatic infection was reported in 20.4% of our cases. A similar rate was reported from Wuhan among 171 confirmed cases.[26] Although fever was the most commonly reported symptom, cough, sore throat, and runny nose were less frequent in our patients compared to previous reports.[26,27] In contrast to adults, children are more likely to present with extra-respiratory symptoms.[28] In our cases, almost a quarter had gastrointestinal (GI) symptoms at presentation, including diarrhea, vomiting, nausea, and abdominal pain. Interestingly, those who presented with vomiting and nausea were more likely to require hospitalization. Increasing evidence showed that the GI tract might represent a target for SARS-CoV-2 because of the expression of the angiotensin-converting enzyme 2, a major virus receptor.[29]
A positive correlation between a history of GI symptoms with severe/critical status and a higher ICU admission rate was documented. Moreover, GI symptoms were more frequently reported in patients who developed cardiac impairment due to SARS-CoV-2 infection complications.[19] The development of hyperinflammatory syndromes and Kawasaki-like disease in children exposed to SARS-CoV-2 infection has been recently reported and subsequently named pediatric inflammatory, multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS; sometimes known as MIC-S). The high proportion of patients aged 7–12 years in the hospitalized group may include some of those cases; however, because data on MIS-C was not systematically surveyed, this would require further research. In a recent report of 186 cases of hyperinflammatory syndrome with cardiac involvement, 40% were in the age group of 5–12 years and presented with fever and significant GI symptoms (diarrhea, vomit, and abdominal pain).[30]
The use of alternative medicine and herbal products was reported in most of our patients and was more common in nonhospitalized children. In pediatric patients, this would reflect the actual belief and practice of the parents and caregivers. Use of herbal medicine and supplements during the current COVID-19 pandemic were commonly reported in various countries including Saudi Arabia.[31,32] In the present study, all patients had a good outcome. This is in agreement with previous reports of the outcome in pediatric COVID-19 patients.[33,34,35] In these reports, patients with and without pneumonia recovered without sequelae. The hospitalization duration in our study was shorter than that reported for adult patients, possibly because of the higher proportion of adult patients with COVID-19 who have pneumonia and other related complications.[27] Only one of our patients required ICU admission and mechanical ventilation with no reported mortality. Other studies have found that one-third of children hospitalized with COVID-19 required ICU care and that the case-fatality rate was low, even among children who have more severe COVID-19-related complications such as MIS-C.[22,25,30] Early recognition of these hospitalized children with COVID-19 is advisable before further deterioration in their clinical status occurs.
Limitation
Because of the study's retrospective nature, we may not have captured all comorbidities or signs and symptoms. In the situation of widespread community transmission, SARS-CoV-2 detection by polymerase chain reaction (PCR) test could also be coincidental with other diagnoses. It could represent previous illness with prolonged viral shedding, asymptomatic infection, or mild symptoms, leading to misclassification of the status. Information on the children's exposure history was not retrieved so that further analysis of such data could not be performed. Finally, the current national surveillance program did not systematically collect information on MIS-C. Furthermore, given that PCR tests can miss approximately 50% of patients with MIS-C despite positive serology or an epidemiologic exposure link to SARS-CoV-2 infection, this surveillance will likely underestimate the incidence of MIS-C cases among children with SARS-CoV-2 infections in Saudi Arabia. Despite these limitations, this study provides a major focus on the epidemiological characteristics and hospitalization of children and adolescents with COVID-19 in Saudi Arabia. Future longitudinal studies are needed to confirm our findings and better explore which patients' populations are at increased risk for developing severe disease.
Conclusions
Our data from multiple centers across Saudi Arabia confirm that COVID-19 is usually a milder disease in children. Although having preexisting medical conditions was linked to a longer hospitalization, it was not associated with worse outcome. As community transmission of SARS-CoV-2 continues, clinicians must be vigilant to the variable presentations of COVID-19 in children. Continuous surveillance will allow additional characterization of the burden and outcomes of COVID-19-associated hospitalizations among children and adolescents.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors thank the Deanship of Scientific Research and RSSU at King Saud University for their technical support.
References
- 1.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Statement on the Second Meeting of the International Health Regulations. Emergency Committee Regarding the Outbreak of Novel Coronavirus (2019-nCoV) 2005. [Last accessed on 2020 Nov 01]. Available from: https://www.who.int/news/item/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-nove-l-coronavirus-
- 3.Cao Y, Liu X, Xiong L, Cai K. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2: A systematic review and meta-analysis. J Med Virol. 2020;92:1449–59. doi: 10.1002/jmv.25822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan AA, Althunayyan SM, Alsofayan YM, Alotaibi R, Mubarak A, Arafat M, et al. Risk factors associated with worse outcomes in COVID-19: A retrospective study in Saudi Arabia. East Mediterr Health J. 2020;26:1371–80. doi: 10.26719/emhj.20.130. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Coronavirus Disease 2019 (COVID-19): Situation Report, 110. World Health Organization. 2020. [Last accessed on Sep 2021]. Available from: https://apps.who.int/iris/handle/10665/332065 .
- 6.Alsofayan YM, Althunayyan SM, Khan AA, Hakawi AM, Assiri AM. Clinical characteristics of COVID-19 in Saudi Arabia: A national retrospective study. J Infect Public Health. 2020;13:920–5. doi: 10.1016/j.jiph.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–95. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–51. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Livingston E, Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA. 2020;323:1335. doi: 10.1001/jama.2020.4344. [DOI] [PubMed] [Google Scholar]
- 10.Bialek S, Gierke R, Hughes M, McNamara L, Pilishvili T, Skoff T. Coronavirus disease 2019 in children — United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:422–6. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kollmann TR, Kampmann B, Mazmanian SK, Marchant A, Levy O. Protecting the newborn and young infant from infectious diseases: Lessons from immune ontogeny. Immunity. 2017;46:350–63. doi: 10.1016/j.immuni.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Coronavirus-Disease-2019-Guidelines-v1.2.pdf. [Last accessed on 2020 Nov 01]. Available from: https://www.moh.gov.sa/Ministry/MediaCenter/Publications/Documents/Coronavirus-Disease-2019-Guidelines-v1.2.pdf .
- 13.Centers for Disease Control and Prevention (CDC). Preschooler (3–5 Years Old) [Last accessed on 2020 Nov 01]. Available from: https://www.cdc.gov/ncbddd/childdevelopment/positiveparenting/preschoolers.html .
- 14.World Health Organization. Preterm Birth. 2018. Feb 19, [Last accessed on 2020 Nov 01]. Available from: https://www.who.int/news-room/fact-sheets/detail/preterm-birth .
- 15.International Statistical Classification of Diseases and Related Health Problems 10th Revision. 2019. [Last accessed on 2020 Nov 01]. Available from: https://icd.who.int/browse10/2019/en .
- 16.World Health Organization. WHO Global Report on Traditional and Complementary Medicine. Geneva, Switzerland: 2019. [Last accessedon 2020 Nov 26]. Available from: https://www.who.int/traditional-complementary-integrative-medicine/WhoGlobalReportOnTraditionalAndComplementaryMedicine2019.pdf . [Google Scholar]
- 17.Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183:7150–60. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leeb RT, Price S, Sliwa S, Kimball A, Szucs L, Caruso E, et al. COVID-19 trends among school-aged children — United States, March 1–September 19, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1410–5. doi: 10.15585/mmwr.mm6939e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giacomet V, Barcellini L, Stracuzzi M, Longoni E, Folgori L, Leone A, et al. Gastrointestinal symptoms in severe COVID-19 children. Pediatr Infect Dis J. 2020;39:e317–20. doi: 10.1097/INF.0000000000002843. [DOI] [PubMed] [Google Scholar]
- 20.Du H, Dong X, Zhang JJ, Cao YY, Akdis M, Huang PQ, et al. Clinical characteristics of 182 pediatric COVID-19 patients with different severities and allergic status. Allergy. 2021;76:510–32. doi: 10.1111/all.14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeBiasi RL, Song X, Delaney M, Bell M, Smith K, Pershad J, et al. Severe coronavirus disease-2019 in children and young adults in the Washington, DC, Metropolitan Region. J Pediatr. 2020;223:199–2030. doi: 10.1016/j.jpeds.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chao JY, Derespina KR, Herold BC, Goldman DL, Aldrich M, Weingarten J, et al. Clinical characteristics and outcomes of hospitalized and critically ill children and adolescents with coronavirus disease 2019 at a Tertiary Care Medical Center in New York City. J Pediatr. 2020;223:14–9.e2. doi: 10.1016/j.jpeds.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alqarni SS. A review of prevalence of obesity in Saudi Arabia. J Obes Eat Disord. 2016;2:2. [Google Scholar]
- 24.Kim L, Whitaker M, O'Halloran A, Kambhampati A, Chai SJ, Reingold A, et al. Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19 - COVID-NET, 14 states, March 1-July 25, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1081–8. doi: 10.15585/mmwr.mm6932e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zachariah P, Johnson CL, Halabi KC, Ahn D, Sen AI, Fischer A, et al. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a children's hospital in New York City, New York. JAMA Pediatr. 2020;174:e202430. doi: 10.1001/jamapediatrics.2020.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. SARS-CoV-2 infection in children. N Engl J Med. 2020;382:1663–5. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: Different points from adults. Pediatr Pulmonol. 2020;55:1169–74. doi: 10.1002/ppul.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castagnoli R, Votto M, Licari A, Brambilla I, Bruno R, Perlini S, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: A systematic review. JAMA Pediatr. 2020;174:882–9. doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- 29.Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: A systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667–78. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MB, et al. Multisystem inflammatory syndrome in U.S. Children and adolescents. N Engl J Med. 2020;383:334–46. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alyami HS, Orabi MAA, Aldhabbah FM, Alturki HN, Aburas WI, Alfayez AI, et al. Knowledge about COVID-19 and beliefs about and use of herbal products during the COVID-19 pandemic: A cross-sectional study in Saudi Arabia. Saudi Pharm J. 2020 Nov;28(11):1326–1332. doi: 10.1016/j.jsps.2020.08.023. doi: 10.1016/j.jsps.2020.08.023. Epub 2020 Sep 1. PMID: 32904846; PMCID: PMC7462475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmed I, Hasan M, Akter R, Sarkar BK, Rahman M, Sarker MS, et al. Behavioral preventive measures and the use of medicines and herbal products among the public in response to Covid-19 in Bangladesh: A cross-sectional study. PLoS One. 2020 Dec 11;15(12):e0243706. doi: 10.1371/journal.pone.0243706. doi: 10.1371/.journal.pone.0243706. PMID: 33306725; PMCID: PMC7732085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiehao C, Jin X, Daojiong L, Zhi Y, Lei X, Zhenghai Q, et al. A case series of children with 2019 novel coronavirus infection: Clinical and epidemiological features. Clin Infect Dis. 2020;71:1547–51. doi: 10.1093/cid/ciaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y, et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–5. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: An observational cohort study. Lancet Infect Dis. 2020;20:689–96. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


