Abstract
Objective:
To compare the histological responses in corticotomy- and corticision-assisted tooth movement.
Materials and Methods:
Ninety Wistar rats were divided into three groups: C (control—tooth movement only), CT (tooth movement + corticotomy), and CI (tooth movement + corticision). Surgeries were performed on the vestibular and lingual cortical bone of the maxillary first molar. Tooth movement was carried out with nickel-titanium closed coil springs having a force of 30 g. The rats were sacrificed at 3, 14, and 28 days. To evaluate the number of osteoclasts and amount of root resorption, a tartrate-resistant acid phosphatase stain was used. Hematoxylin and eosin staining was performed for areas of hyalinization, and the organic bone matrix was stained with picrosirius.
Results:
The CT group showed a greater number of osteoclasts than did the C group on day 3 (P < .05). At the same time point, the CT and CI groups showed a delayed onset of organic bone matrix remodeling and a lower incidence of root resorption than did the C group (P < .05). There were also fewer hyalinization areas in the CI group than in the C group on day 3 (P < .05).
Conclusions:
Corticotomy effectively increased bone resorption during the early stages of tooth movement, but this increase was not observed for corticision. The surgical procedures did not accelerate organic bone matrix remodeling. Corticotomies and corticisions decreased the risk of root resorption only during the early stages of movement. Corticision reduced the level of hyalinization, while corticotomy did not.
Keywords: Corticotomy, Corticision, Tooth movement
INTRODUCTION
Selective alveolar corticotomy, a surgical procedure used to increase the speed of tooth movement,1 is defined as any intentional surgical injury in which only the cortical bone is cut, drilled, or altered, while the bone marrow remains intact.2,3 The goal of this strategy is to create bone blocks containing one or more teeth that could be moved more quickly than individual teeth.1
Studies involving clinical3–7 and histological1,6–8 evaluation have demonstrated that corticotomy is effective. However, due to the postoperative discomfort and risk of complications,7–9 Kim et al.9 in 2009 introduced corticision as a surgery complementary to orthodontic treatment to accelerate tooth movement via regional acceleration9,10 with minimal surgical intervention.
Although the effectiveness of corticotomies1,3,6,7 and corticisions9,10 have been documented, no studies have compared these techniques. Therefore, the present study was conducted to comparatively evaluate the tooth movement rate and histological responses arising from induced tooth movement assisted by corticotomy and cortical incision with regard to bone resorption, remodeling of the organic bone matrix, and amount of root resorption and hyalinization occurring.
MATERIALS AND METHODS
The sample consisted of 90 male Wistar rats 9 weeks of age, weighing 300 to 350 g each. The animals were housed in vivarium under photoperiod and temperature control. Ground dry feed was used to avoid damage to the orthodontic device, and filtered water was available ad libitum.7,11,12 Body weight was recorded throughout the experimental period.13 The rights of the animal subjects were protected, and the study was approved by the Ethics Committee on Animal Use (No. 629).
The animals were divided into three groups of 30 rats each: tooth movement only (C, control), tooth movement surgically assisted by corticotomy (CT), and tooth movement surgically assisted by corticision (CI).
For the surgical procedures and installation of the orthodontic device, the animals were anesthetized with Tiletamine/Zolazepan (Zoletil 50, Virbac, Jurubatuba, Brazil) at 50 mg/kg and an average volume of 0.25 mL/animal.
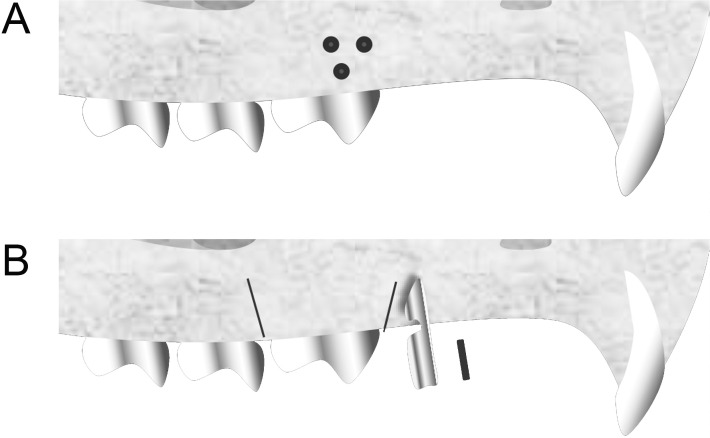
The corticotomy procedure (Figure 1A) involved an intrasulcular incision and folding of the flap in the vestibular and palatal faces of the maxillary right first molar region. With the aid of a low-speed, ¼-mm spherical carbide bur1 (KG Sorensen Ltd, Cotia, Brazil), three perforations were created in the vestibular bone plate and two in the palatal bone plate of the first molar to perforate the cortical bone and reach the medullary bone. The perforations were made under irrigation, with a size corresponding to the bur diameter (0.25 mm width and 0.25 mm depth).1 The tissues were subsequently sutured with absorbable thread (Vicryl-Ethicon 5-0, Johnson & Johnson, São José dos Campos, Brazil).
Figure 1.
Schematic drawing showing corticotomy (A) and corticision (B) procedures adjacent to the maxillary right first molar.
The corticision (Figure 1B) was made on the mesial and distal faces of the maxillary right first molar in both the vestibular and palatal bone plates. A scalpel blade was positioned between the roots at an angle of 45° to 60° to the long axis of the tooth and was gradually inserted into the alveolar bone with the aid of a surgical hammer (16 CM.QD 992-16 Quinelato, Schobell, Rio Claro, Brazil) to perforate the gum, cortical bone, and cancellous bone. The blade was later removed with oscillatory movements to prevent fracturing. The methodology used was described by Kim et al.9; however, in the present study, a 15C blade was used (Swann-Morton, Sheffield, England), due to the small size of the experimental model.
After the surgical procedures, a periodontal probe was used to confirm that the medullary bone had been reached.
The orthodontic device, consisting of a nickel-titanium (G&H Wire, Franklin, Ind) closed-spring coil, was installed,12 fixed to the maxillary right first molar and central incisors with a 0.010-inch stainless steel ligature wire (Morelli, São Paulo, Brazil), tied around the teeth,14 promoting mesial molar displacement with 30 g of force.15 Activation of the spring was achieved and standardized with a dynamometer (Correx Tension Gauge, Haag-Sreit International, Koeniz, Switzerland) only once during the experiment. To ensure greater spring stability, the mandibular incisors were worn down and the maxillary incisors bonded together with the composite resin Charisma (Heraeus, Hanau, Germany).
Tooth Movement Rate
The tooth movement rate was ascertained using a digital caliper (Absolute-Mitutoyo, Kawasaki-Shi, Japan) directly in the dental arch of the animals.14 The distance between the most mesial point of the maxillary right first molar and the center of the palatal face of the ipsilateral maxillary central incisor, at the gingival level, was measured before placing the orthodontic device (initial measurement) and after euthanasia (final measurement). The tooth movement rate was calculated by subtracting the final measurement from the initial measurement.
Histological Analysis
The animals were sacrificed by intraperitoneal injection of sodium pentobarbital 100 mg/kg (Thiopentax, Cristal Pharma Ltd, Contagem, Brazil) on days 3, 14, and 28 postsurgery and the onset of tooth movement. Each of those days represented a subgroup and contained 10 rats.
The right hemimaxilla was sectioned and the laboratory procedures were performed. Fifteen sections of 4-μm thickness were collected starting from the cervical third in the apical direction in the distal vestibular (DV) root of the maxillary first molar of each specimen. Initially, three adjacent cross-sections of 4-μm thickness were collected, and then 60 μm skipped, and this procedure was repeated five times. The sections were stained with H&E, Picrosirius, and the TRAP technique (totaling five sections each). The DV root was chosen based on a previous study.16
TRAP staining (TRAP 387 Kit, Sigma-Aldrich Chemicals, St Louis, Mo), a marker of osteoclasts, was used to evaluate alveolar bone resorption.7,12,13,17–19 The osteoclasts were multinucleated, TRAP-positive cells in contact with or near bone surfaces12,13 and were evaluated in the periodontal ligament (PL) of the mesial face of the DV root of the maxillary right first molar.18,20,21 From each of the five sections, images were captured from five consecutive microscopic fields18 using an Olympus BX40 light microscope (Olympus, Tokyo, Japan) coupled to a Dinolite AM 423X microcamera (AmMo Electronics Corp, New Taipei City, Taiwan) at 400× magnification.12,17,18 The osteoclasts were counted by the same operator with the aid of Image Pro Plus 4.5 (Media Cybernetics, Rockville, Md), with a grid mask. The total number of osteoclasts for each animal was calculated by averaging the values obtained for each of the five sections.18
TRAP was used to identify the presence of root resorption.17 Evaluation was performed on the entire circumference of the DV root of the first molar with the same microscope and magnification, registering the presence or absence of root craters (cementum or dentin), in which, most of the time, there were cementoclasts (TRAP-positive multinucleated cells) in contact with the root.
The presence of hyalinized areas was evaluated throughout the periodontal ligament (PL) of the same root using H&E staining in the previously described microscope at 100× magnification. The hyalinized areas were identified by PL degenerative changes and appeared both homogeneous and cell free.13,22
Neoformation of organic bone matrix was verified with picrosirius (Aldrich Chemical Co, Milwaukee, Wisc). One area on the distal side of the referred root from each section was selected, and an image was captured with an Olympus BX50 light microscope (Olympus) coupled to the same microcamera and a polarizer lens (Olympus U–P110) at 100× magnification.15,23,24 The images were edited in Adobe Photoshop CS6 software (Adobe Systems Inc, San Jose, Calif), in which the PL and teeth were excluded, leaving only the bone tissue to be analyzed. Image Pro Plus 4.5 was also used to measure the percentages of the mature and immature collagen areas.12,15 The percentage of each collagen type was obtained by averaging the values acquired for the five sections per animal.
Mature collagen is known as “type I collagen,” and the immature collagen is known as “type III.”25
Statistical Analysis
Statistical analysis was made with SPSS software 22.0 (IBM SPSS, Armonk, NY), with a significance level of 0.05. Comparison of the mean was performed with the two-way ANOVA parametric test followed by either the Games-Howell test or Tukey's HSD, when indicated. In addition, the power test was calculated. A chi-square test was performed for root resorption and hyalinization, followed by a test for the difference between the two proportions. The Dahlberg error was less than 6.2%, indicating reliable reproducibility of the osteoclast values. The Kappa coefficient of concordance was 0.659 for the variable of root resorption and 0.861 for that of the hyalinization areas. The results show that the evaluator reproduced the measurements in a reliable way.
RESULTS
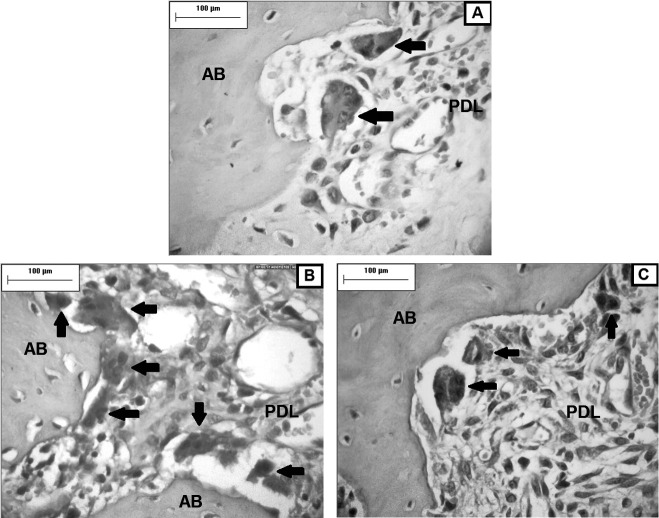
At day 3, a greater number of osteoclasts were found in the CT group than in the C group (P < .05), but the number was similar between the CI and C groups and between the CI and CT groups (P > 0.05) (Table 1; Figure 2). Based on the two-way ANOVA parametric test, (power test >99%), there was a difference between the groups, independent of time.
Table 1.
Means and Standard Deviations of Variablesa
| Day |
Games-Howell Test |
|||||
| Group C (Mean ± SDb) |
Group CT (Mean ± SD) |
Group CI (Mean ± SD) |
C × CT (P Value) |
C × CI (P Value) |
CT × CI (P Value) |
|
| Number of osteoclasts* | ||||||
| 3 | 3.759 ± 1.611 | 8.172 ± 1.8413 | 6.899 ± 2.793 | .000* | .125 | .944 |
| 14 | 2.729 ± 1.054 | 7.233 ± 4.416 | 7.763 ± 4.472 | .141 | .088* | 1.00 |
| 28 | 3.969 ± 2.403 | 4.718 ± 1.388 | 8.35 ± 4.116 | .992 | .165 | .268 |
| % Mature collagen** | ||||||
| 3 | 82.681 ± 8.124 | 96.122 ± 0.728 | 94.998 ± 1.181 | .009* | .016* | .278 |
| 14 | 77.263 ± 12.223 | 88.621 ± 5.112 | 81.79 ± 11.756 | .238 | .993 | .745 |
| 28 | 86.294 ± 7.090 | 92.214 ± 3.148 | 85.101 ± 6.693 | .353 | 1.000 | .141 |
| % Immature collagen** | ||||||
| 3 | 17.319 ± 8.12 | 3.878 ± 0.728 | 5.002 ± 1.181 | .009* | .016* | .278 |
| 14 | 22.737 ± 12.223 | 11.379 ± 5.112 | 18.21 ± 11.756 | .238 | .993 | .745 |
| 28 | 13.706 ± 7.090 | 7.786 ± 3.148 | 14.899 ± 6.693 | .353 | 1.000 | .141 |
Two-way ANOVA, full factorial model; * P = .0747; ** P = .0725; b SD indicates standard deviation.
Figure 2.
Photomicrographs of the mesial face of the periodontal ligament (PDL) and alveolar bone (AB) of the distovestibular root of the maxillary right first molar. Greater numbers of osteoclasts (arrows) were observed in the CT group (B) compared with the C (A) and CI (C) groups on the third day. (TRAP, original magnification ×400).
There was a lower percentage of immature collagen fibers in the bone tissue in the experimental groups than in the C group on day 3 (P < .05). For this time point, the CT and CI groups showed similar percentages of these fibers (P > .05) (Table 1). The power test for the between-group difference was >99% and for time was >98%.
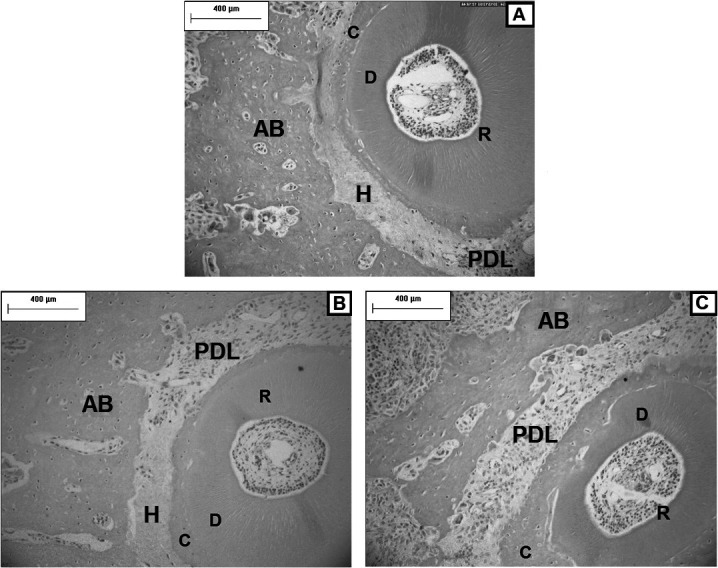
A lower amount of root resorption was observed in the CT and CI groups than in the C group on day 3 (P < .05). For this time point, no difference in the presence of resorption was observed between the CI and CT groups (P > .05) (Table 2; Figure 3).
Table 2.
Presence (PRES) of Root Resorption and Hyalinization in Groups.
| Day |
Unilateral Test of the Difference Between Two Proportions |
|||||
| Group C PRES % |
Group CT PRES % |
Group CI PRES % |
C × CT (P Value) |
C × CI (P Value) |
CT × CI (P Value) |
|
| Root resorptiona | ||||||
| 3 | 70 | 30 | 10 | .042* | .006* | .139 |
| 14 | 70 | 60 | 40 | .324 | .097 | .191 |
| 28 | 90 | 80 | 90 | .269 | .5 | .269 |
| Hyalinized areasb | ||||||
| 3 | 100 | 100 | 70 | .5 | .038* | .038* |
| 14 | 10 | 20 | 30 | .269 | .139 | .305 |
| 28 | 0 | 20 | 0 | .076 | .5 | .076 |
Chi-square test; a P = .001; b P = .000; SD indicates standard deviation.
Figure 3.

Photomicrographs of the PDL and distovestibular root of the maxillary right first molar. A lower presence of resorption (arrow) was observed in the CT group (B) and CI (C) compared with C (A) on day 3; C, cementum; D, dentin. (TRAP, original magnification ×400).
Fewer hyalinization areas were observed in the CI group than in the C and CT groups on day 3 (P < .05). For the same time point, no differences were found between the C and CT groups (P > .05). In the last stage of movement, an absence or substantial reduction in the number of hyaline areas was observed in the three study groups (Table 2; Figure 4).
Figure 4.
Photomicrographs of the mesial face of the periodontal ligament (PDL) and AB of the distovestibular root of the maxillary right first molar. Fewer hyalinization areas (H) were observed in the CI group (C) compared with the C (A) and CT (B) groups on the third day; C, cementum; D, dentin. (H&E, original magnification ×100).
No significant differences were found in the tooth movement rate among the studied groups at time intervals (P > .05; Table 3) (power test = 53.08%).
Table 3.
Means and Standard Deviations of Tooth Movement Rate in Groups (mm)a
| Day |
C (Mean ± SD) |
CT (Mean ± SD) |
CI (Mean ± SD) |
Tukey's HSD |
||
| C × CT (P Value) |
C × CI (P Value) |
CT × CI (P Value) |
||||
| 3 | 0.843 ± 0.2233 | 1.026 ± 0.2349 | 0.874 ± 0.2641 | .9773 | 1.000 | .9931 |
| 14 | 1.427 ± 0.5154 | 1.794 ± 0.940 | 1.31 ± 0.3341 | .4544 | .9989 | .1250 |
| 28 | 1.854 ± 0.4905 | 2.246 ± 0.3443 | 1.956 ± 0.5709 | .3630 | .9996 | .7502 |
Two-way ANOVA, full factorial model; P = .6606; SD indicates standard deviation.
DISCUSSION
The present study is the first revealing that corticotomy and corticision exert the same effect in inducing tooth movement. Only the CT group showed a higher number of osteoclasts on the third day than did the C group, observed also by Wang et al.20 Despite this fact, the tooth movement rate was similar among the three studied groups.
It has been reported that short time periods between activations of orthodontic devices in patients subjected to corticotomy is indicated.5 It is possible that these shorter activation periods could hypothetically leverage the increase in the number of osteoclasts induced by corticotomy, as noted at the beginning of tooth movement in this research. Studies should be conducted to verify whether additional activations of the device or surgical procedure should be performed to increase the number of osteoclasts.
In the present study, there was no significant increase in the number of osteoclasts or in the tooth movement rate in the corticision group than in the control group. These results disagree with those of Kim et al.,9 who found a higher number of osteoclasts at 7 and 14 days in cats subjected to corticision, suggesting improved tooth movement due to an increase of bone resorption during these time periods.
With regard to corticision, organic bone matrix neoformation in the CI group was initially lower than in the control group and similar to the control group after 14 days. These results differ from those of previous studies showing greater and faster bone apposition in cats9 and dogs10 subjected to corticision.
Orthodontic movement assisted by corticotomy26 and corticision9 has been reported to decrease the rate of root resorption. In our study, the CT and CI groups showed a lower degree of root resorption compared with the C group only at day 3. However, the total absence of root resorption in dogs and cats subjected to corticotomy6,7 and corticision9 has been verified, suggesting a greater resistance to resorption in these animals than with rats.
The presence of hyalinization is not favorable for recruiting osteoclasts within the periodontal tissue, and it leads to delayed alveolar bone resorption.13 Although the present study found fewer hyalinization areas in the CI group, this reduction did not result in an increased number of osteoclasts, as found by Kim et al.9 in cats. Lino et al.7 observed a lower incidence of hyalinization in the groups subjected to corticotomy compared with the control group in the early stages of treatment.
CONCLUSIONS
Alveolar corticotomy increased bone resorption in the early stages of tooth movement. This did not occur when corticision was performed.
The surgical procedures employed did not accelerate the remodeling of organic bone matrix.
Both surgeries were effective in decreasing the incidence of root resorption only during the early stages of tooth movement.
Corticision reduced the incidence of hyalinization during the early stages of tooth movement. The same reduction was not observed for corticotomies.
The surgical procedures studied did not modify the tooth movement rate in the studied period.
REFERENCES
- 1.Baloul SS, Gerstenfeld LC, Morgan EF, Carvalho RS, Van Dyke TE, Kantarci A. Mechanism of action and morphologic changes in the alveolar bone in response to selective alveolar decortication-facilitated tooth movement. Am J Orthod Dentofacial Orthop. 2011;139:S83–S101. doi: 10.1016/j.ajodo.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 2.Murphy KG, Wilcko MT, Wilcko WM, Ferguson DJ. Periodontal accelerated osteogenic orthodontics: a description of the surgical technique. J Oral Maxillofac Surg. 2009;67:2160–2166. doi: 10.1016/j.joms.2009.04.124. [DOI] [PubMed] [Google Scholar]
- 3.Aboul-Ela S-D, El-Sayed K, Selim E, El-Mangoury N, Mostafa Y. Miniscrew implant-supported maxillary canine retraction with and without corticotomy-facilitated orthodontics. Am J Orthod Dentofacial Orthop. 2011;139:252–259. doi: 10.1016/j.ajodo.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 4.Oliveira DD, de Oliveira BF, de Araujo Brito HH, de Souza MM, Medeiros PJ. Selective alveolar corticotomy to intrude overerupted molars. Am J Orthod Dentofacial Orthop. 2008;133:902–908. doi: 10.1016/j.ajodo.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 5.Fischer TJ. Orthodontic treatment acceleration with corticotomy-assisted exposure of palatally impacted canines. Angle Orthod. 2007;77:417–420. doi: 10.2319/0003-3219(2007)077[0417:OTAWCE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Mostafa Y, Fayed M, Mechanni S, ElBokle N, Heider A. Comparison of corticotomy-facilitated vs standard tooth-movement techniques in dogs with miniscrews as anchor units. Am J Orthod Dentofacial Orthop. 2009;136:570–577. doi: 10.1016/j.ajodo.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 7.Lino S, Sakoda S, Ito G, Nishimori T, Ikeda T, Miyawaki S. Acceleration of orthodontic tooth movement by alveolar corticotomy in the dog. Am J Orthod Dentofacial Orthop. 2007;131:448.e.441–448.e.448. doi: 10.1016/j.ajodo.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Iglesias-Linares A, Moreno-Fernandez AM, Yanez-Vico R, Mendoza-Mendoza A, Gonzalez-Moles M, Solano-Reina E. The use of gene therapy vs. corticotomy surgery in accelerating orthodontic tooth movement. Orthod Craniofac Res. 2011;14:138–148. doi: 10.1111/j.1601-6343.2011.01519.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim SJ, Park YG, Kang SG. Effects of corticision on paradental remodeling in orthodontic tooth movement. Angle Orthod. 2009;79:284–291. doi: 10.2319/020308-60.1. [DOI] [PubMed] [Google Scholar]
- 10.Kim SJ, Moon SU, Kang SG, Park YG. Effects of low-level laser therapy after corticision on tooth movement and paradental remodeling. Lasers Surg Med. 2009;41:524–533. doi: 10.1002/lsm.20792. [DOI] [PubMed] [Google Scholar]
- 11.Kawarizadeh A, Bourauel C, Zhang D, Götz W, Jäger A. Correlation of stress and strain profiles and the distribution of osteoclastic cells induced by orthodontic loading in rat. Eur J Oral Sci. 2004;112:140–147. doi: 10.1111/j.1600-0722.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- 12.Marquezan M, Bolognese AM, Araujo MT. Effects of two low-intensity laser therapy protocols on experimental tooth movement. Photomed Laser Surg. 2010;28:757–762. doi: 10.1089/pho.2009.2694. [DOI] [PubMed] [Google Scholar]
- 13.Tomizuka R, Shimizu Y, Kanetaka H, Suzuki A, Urayama S, Kikuchi M, et al. Histological evaluation of the effects of initially light and gradually increasing force on orthodontic tooth movement. Angle Orthod. 2007;77:410–416. doi: 10.2319/0003-3219(2007)077[0410:HEOTEO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Frigotto GCF, de Araujo CM, Guariza Filho O, Tanaka OM, Johann ACBR, Camargo ES. Effect of fluoxetine on induced tooth movement in rats. Am J Orthod Dentofacial Orthop. 2015;148:450–456. doi: 10.1016/j.ajodo.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 15.Retamoso LB, da Cunha Tde M, Knop LA, Shintcovsk RL, Tanaka OM. Organization and quantification of the collagen fibers in bone formation during orthodontic tooth movement. Micron. 2009;40:827–830. doi: 10.1016/j.micron.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Fracalossi ACC, Santamaria M, Jr, Consolaro M, Consolaro A. Movimentação dentária experimental em murinos: período de observação e plano dos cortes microscópicos. Rev Dent Press Ortodon Ortoped Facial. 2009;14:143–157. [Google Scholar]
- 17.Liu Z, Xu J, Lingling E, Wang D. Ultrasound enhances the healing of orthodontically induced root resorption in rats. Angle Orthod. 2012;82:48–55. doi: 10.2319/030711-164.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braga S.de Albuquerque Tadei S, Andrade IJ, Queiroz-Junior C. GarletRepeke C, Teixeira M, et al., editors. Effect of diabetes on orthodontic tooth movement in a mouse model. Eur J Oral Sci. 2011;119:7–14. doi: 10.1111/j.1600-0722.2010.00793.x. [DOI] [PubMed] [Google Scholar]
- 19.Hayman AR. Tartrate-resistant acid phosphatase (TRAP) and the osteoclast/immune cell dichotomy. Autoimmunity. 2008;41:218–223. doi: 10.1080/08916930701694667. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Lee W, Lei DL, Liu YP, Yamashita DD, Yen SL. Tisssue responses in corticotomy- and osteotomy-assisted tooth movements in rats: histology and immunostaining. Am J Orthod Dentofacial Orthop. 2009. 136:770 e771–711; discussion 770–771. [DOI] [PubMed]
- 21.Noxon SJ, King GJ, Gu G, Huang G. Osteoclast clearance from periodontal tissues during orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2001;120:466–476. doi: 10.1067/mod.2001.117912. [DOI] [PubMed] [Google Scholar]
- 22.Miyoshi K, Igarashi K, Saeki S, Shinoda H, Mitani H. Tooth movement and changes in periodontal tissue in response to orthodontic force in rats vary depending on the time of day the force is applied. Eur J Orthod. 2001;23:329–338. doi: 10.1093/ejo/23.4.329. [DOI] [PubMed] [Google Scholar]
- 23.Retamoso LB, Montagner F, Camargo ES, Vitral RW, Tanaka OM. Polarized light microscopic analysis of bone formation after inhibition of cyclooxygenase 1 and 2. Anat Rec (Hoboken) 2010;293:195–199. doi: 10.1002/ar.21035. [DOI] [PubMed] [Google Scholar]
- 24.Retamoso L, Knop L, Shintcovsk R, Maciel JV, Machado MA, Tanaka O. Influence of anti-inflammatory administration in collagen maturation process during orthodontic tooth movement. Microsc Res Tech. 2011;74:709–713. doi: 10.1002/jemt.20947. [DOI] [PubMed] [Google Scholar]
- 25.Montes GS. Structural biology of the fibres of the collagenous and elastic systems. Cell Biol Int. 1996;20:15–27. doi: 10.1006/cbir.1996.0004. [DOI] [PubMed] [Google Scholar]
- 26.Wilcko WM, Wilcko T, Bouquot JE, Ferguson DJ. Rapid orthodontics with alveolar reshaping: two case reports of decrowding. Int J Periodontics Restorative Dent. 2001;21:9–19. [PubMed] [Google Scholar]