Abstract
Objective:
To analyze which parameters, gathered from standard orthodontic diagnostic material, were most relevant for identifying small pharyngeal airway dimensions in preorthodontic children.
Materials and Methods:
The sample was composed of 105 cone beam computed tomography scans of healthy preorthodontic children (44 boys, 61 girls; mean age, 10.7 ± 2.4 years). Airway volume and minimal cross-sectional area were three-dimensionally assessed. Cephalometric features and skeletal maturity were assessed on generated two-dimensional cephalograms. Associations were analyzed and adjusted for age, gender, and skeletal maturity by multiple regression analyses.
Results:
Airway volume and minimal cross-sectional area were significantly smaller in prepubertal children (P < .001, P < .05, respectively) and positively associated with age (P < .001, P < .01, respectively). After adjustment of age, skeletal maturity and gender significant associations were found between pharyngeal airway dimensions and craniofacial morphology. Airway volume was positively associated with maxillary and mandibular width (P < .01; P < .001, respectively) and anterior face height (P < .05; P < .05, respectively). Minimal cross-sectional area was positively associated with maxillary and mandibular width (P < .01; P < .001, respectively) and negatively associated with sagittal jaw relationship (AnPg, P < .05). Mandibular width and age were the most relevant factors for airway volume (r2 = 0.36). Mandibular width and sagittal jaw relationship were the most relevant factors for minimal cross-sectional area (r2 = 0.16).
Conclusion:
Pharyngeal airway dimensions were significantly associated with age, skeletal maturity, and craniofacial morphology in all three planes. Children with a reduced mandibular width and increased sagittal jaw relationship are particularly at risk of having small pharyngeal airway dimensions.
Keywords: Pharyngeal airway, Children
INTRODUCTION
Increased interest in upper airway dimensions and morphology during the past few decades can be attributed to the appreciation that upper airway configuration is associated with sleep disordered breathing (SDB) as well as its general relationship to craniofacial morphology.1,2 Early diagnosis of SDB, or potential associations of SDB, is essential to encourage normal facial development.3,4 Reduced pharyngeal dimensions established early in life could potentially predispose to later development of SDB or even obstructive sleep apnea (OSA) because soft tissue changes related to aging, obesity, or genetic background further reduce oropharyngeal patency.5
Craniofacial morphology has been associated with the upper airway in children. In the sagittal dimension assessed by the sagittal jaw relationship, it was generally found that patients with a Class 3 skeletal pattern have a greater airway volume than skeletal Class 1, which is greater than skeletal Class 2.6–11 However, this difference was not always statistically significant between the groups7 or between skeletal Class 1 and Class 3.6 The minimal cross-sectional area was also found to be greater in skeletal class 1 patients than in Class 2 patients,8,9 and even greater in Class 3 patients.8 Differences in upper airway morphology have been described in individuals with a Class 2 skeletal pattern exhibiting more of a backward orientation of the airway to the Frankfort horizontal (FH) plane when compared with skeletal Class 3 individuals who had a more vertical orientation.7 Class 3 skeletal patients were also found to have a more flat-shaped airway when compared with Class 1 individuals who had a more square oropharyngeal airway.12 However, other studies found no significant differences between skeletal patterns (Classes 1, 2, or 3) and upper airway dimensions.13
Research into associations between the vertical and transverse craniofacial dimensions and the three-dimensional upper airway in children is limited. Correlations have been reported between upper airway dimensions and anterior and posterior face heights.7,11 Di Carlo et al.13 found no associations between upper airway dimensions and craniofacial features in the vertical, transverse, or sagittal dimensions. However, unlike the other studies, the patients were scanned in the supine position and cephalometrically assessed three-dimensionally, and young adults were included in the sample.
Three-dimensional assessments of the upper airway with age14,15 has shown that airway dimensions consistently increase until about 20 years of age. After a slight decrease, airway volume considerably decreases after 50 years of age and minimal cross-sectional area after 30 years of age. Although the walls of the upper airway are constructed of soft tissue structures that influence luminal size, the craniofacial osseous structures determine the general size of the upper airways.16 Therefore, it could be assumed that skeletal maturity is closely associated with upper airway dimensions in children. However, this relationship has not been previously assessed.
Prior to orthodontic treatment, many of the factors associated with pharyngeal airway dimensions are already obtained and analyzed. Study casts and orthopantomograms are obtained for the evaluation of the dentition and occlusion, and lateral and frontal radiographs are obtained for the evaluation of the craniofacial morphology. Explorative studies in which the participants are their own controls have previously been published in the orthodontic literature.17,18
Because the pharyngeal airway is not regularly three-dimensionally assessed in orthodontic clinics, it is relevant to examine which factors are most relevant for pharyngeal airway dimensions based on already existing diagnostic material.
The present study focuses on pharyngeal airway dimensions in relation to craniofacial morphology, morphological occlusion, age, gender, and skeletal maturity in children before orthodontic treatment. The aim of the study was to analyze which parameters from standard orthodontic diagnostic material were most relevant for pharyngeal airway dimensions in preorthodontic children.
MATERIALS AND METHODS
The study was approved by the institutional review board of an established university in Queensland, Australia (H5115). Cone beam computed tomography (CBCT) scans were obtained from an existing database of patients that attended a private practice in Victoria, Australia, for orthodontic treatment from 2011 to 2014. Before they were entered into the database, all CBCT images were anonymized. Sex, age, and morphological occlusion according to Angle's classification were also obtained from the database. These were cross-checked with the CBCT scans and clinical reports. The inclusion criteria were (1) healthy children between 8 to 16 years of age; (2) biting in centric occlusion; and (3) complete imaging of the cranial base, maxilla, mandible, the first four cervical vertebrae (C1–C4), and the associated airway. The exclusion criteria were (1) previous orthodontic treatment and/or orthognathic surgery, (2) previous adeno-tonsillectomy, (3) known syndromal conditions, (4) presence of pathology detectable along the upper airway, (5) history of obstructive sleep apnea, (6) movement artefact, and (7) swallowing during scan acquisition. This resulted in the final sample of 105 scans (Figure 1). The sample consisted of 61 girls (58.1%) and 44 boys (41.9%) with a mean age of 10.7 (±2.4) years.
Figure 1.
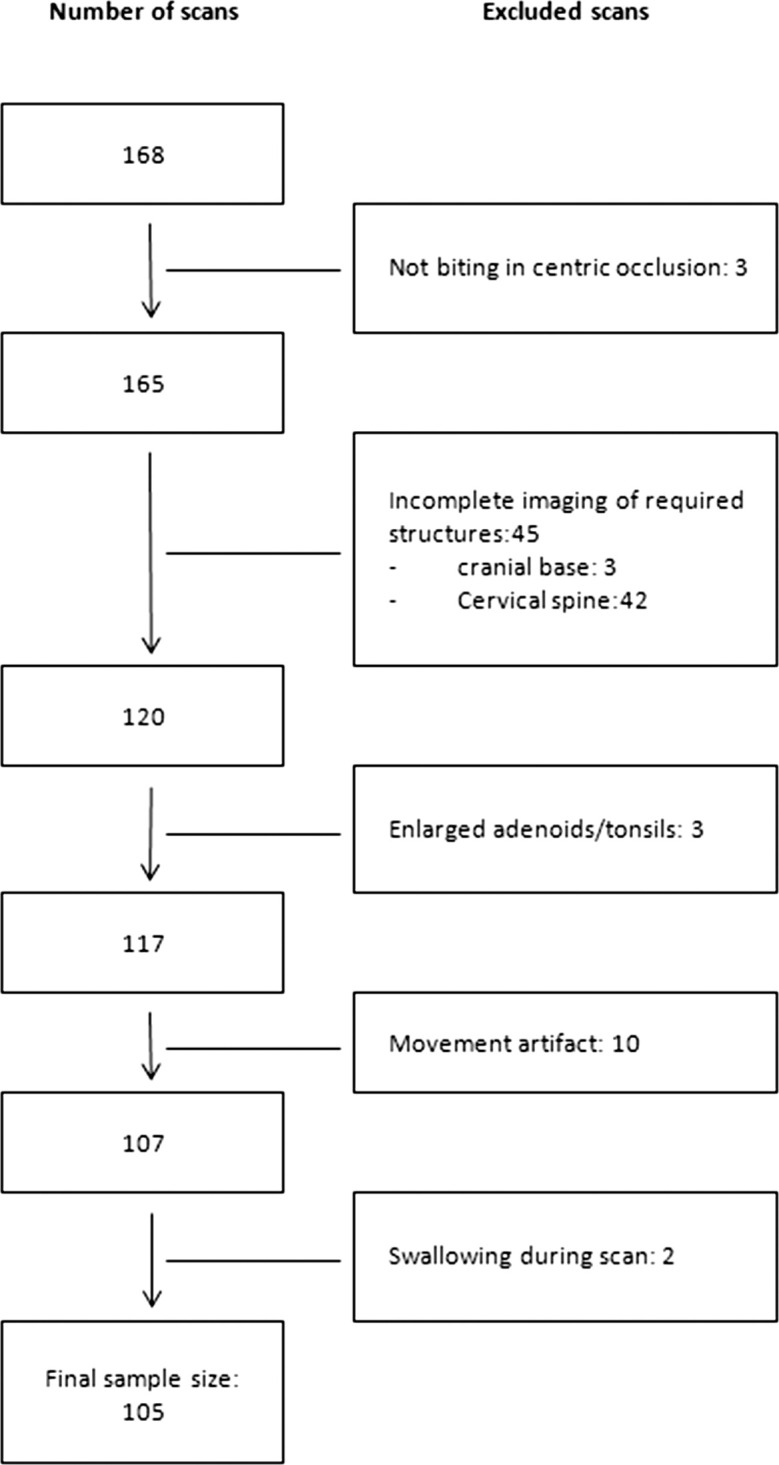
Flowchart of final sample size.
Scan Protocol
All images were taken by the same operator with the same i-CAT Next Generation CBCT machine (Imaging Sciences International, Hatfield, Pa). The following parameters were used: 120 kV, 5 mA, 0.3-mm voxel resolution, 8.9-second scan time, 13 cm (height) × 16 cm (diameter) scan volume. Patients were seated and restrained with a headrest and head strap, but no chin rest to allow Frankfurt horizontal to be positioned parallel to the floor. Patients were instructed to close into centric occlusion, relax their tongue and lips, breathe gently, and not swallow or move during the acquisition. All CBCTs were reviewed by a dento-maxillofacial radiologist (Dr. Dudhia) to ensure that all inclusion and exclusion criteria were met.
Image Preparation and Airway Assessment
The Digital Imaging and Communications in Medicine (DICOM) data were processed using Dolphin Imaging software (version 11.5; Dolphin Imaging and Management Solutions, Chatsworth, Calif). Images were analyzed under the same lighting conditions and by the same investigator as previously described.19 This method was found to be reliable and reproducible. To standardize measurements the skull was reoriented in all three planes using the following guidelines:19
Coronal plane — orbitale of both sides were on the same horizontal plane.
Sagittal plane — Frankfort plane was horizontal.
Axial plane — a line through the crista galli and the basion was vertical.
The upper airway volume and minimal cross-sectional area were assessed three-dimensionally and measured according to Anandarajah et al.19 (Figure 2; Table 1).
Figure 2.
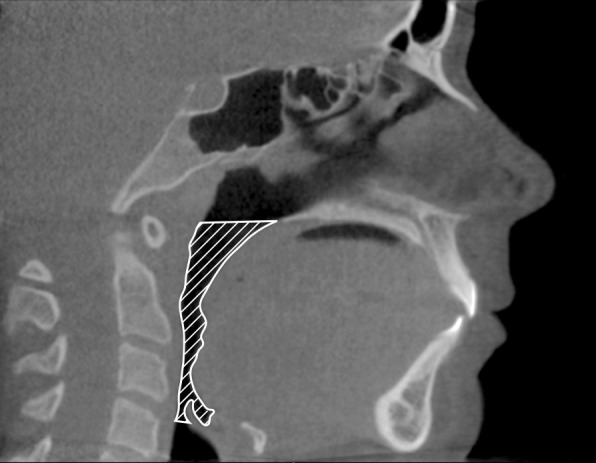
Illustration of margins for delineations of the upper airway (hatched).
Table 1.
Anatomical and Technical Limits of the Upper Airway
| Limit |
Anatomical |
Technical |
| Superior | Hard and soft palate | The line passing from the palatal plane (ANS to PNS) extending to the posterior wall of the pharynxa |
| Inferior | Vallecula (plane of hyoid bone; base of the epiglottis) | Line passing from the antero-superior edge of C4 to menton |
| Anterior | Circumvallate papillae and the oropharyngeal isthmus | Line passing from the soft palate to menton |
| Posterior | Respective pharyngeal walls | Posterior wall of the pharynx |
| Lateral | Respective pharyngeal walls | Respective pharyngeal walls |
ANS indicates anterior nasal spine; PNS, posterior nasal spine.
Craniofacial Morphology Assessment
Craniofacial morphology was digitally assessed on automatically constructed two-dimensional lateral and posteroanterior cephalograms with no magnification. Standard craniofacial measurements were made of the cranial base, maxilla, and mandible according to Björk20,21 and Yoon et al.,22 with reference points, lines, and angles according to Solow and Tallgren23 and Yoon et al.22 (Figure 3, Table 2).
Figure 3.
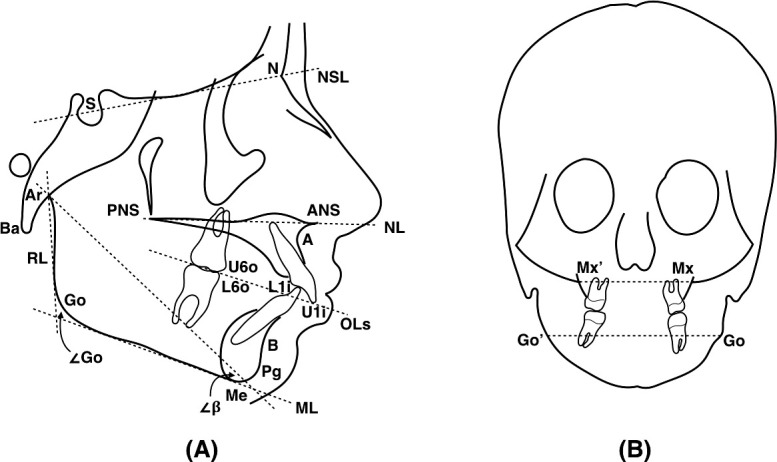
Illustrations of reference points and lines (dotted) describing craniofacial morphology on lateral (A) and postero-anterior (B) cephalograms.22,23
Table 2.
Reference Points, Lines, and Angles According to Solow and Tallgren (1976)23 and Yoon et al. (2004)22
| Landmark |
Abbreviation |
Definition |
| Points | ||
| Sella | S | The centre of sella turcica, the upper limit of which is defined as the line joining the tuberculum and the dorsum sella |
| Nasion | N | The most anterior point of the fronto-nasal suture |
| Basion | Ba | The most postero-inferior point on the clivus |
| A point | A | The most posterior point on the anterior contour of the maxillary alveolar arch |
| B point | B | The most posterior point on the anterior contour of the mandibular alveolar arch |
| Pogonion | Pg | The most anterior point on the mid-sagittal mandibular symphysis |
| Anterior nasal spine | ANS | The apex of the anterior nasal spine |
| Posterior nasal spine | PNS | The tip of the posterior nasal spine |
| Menton | Me | The most inferior point on the mid-sagittal mandibular symphysis |
| Gonion (lat cepha) | Go | The most postero-inferior point on the angle of the mandible, indicated by bisection of the RL to ML |
| Gonion (PA cepha) | Go and Go' | The most lateral point on the convex margin on the angle of the mandible |
| Articulare | Ar | The intersection between the external contour of the cranial base and the dorsal contour of the condylar head or neck |
| Maxillary notch | Mx and Mx' | The intersection of the zygomatic buttress and outline of the tuberosity |
| Upper 6 occlusal | U6o | The mesio-buccal cusp tip of the maxillary molar |
| Lower 6 occlusal | L6o | The mesio-buccal cusp tip of the mandibular molar |
| U1 incisal tip | U1i | The mid-point of the incisal edge of the most prominent upper central incisor |
| L1 incisal tip | L1i | The mid-point of the incisal edge of the most prominent lower central incisor |
| Lines | ||
| Overjet | OJ | The length difference between U1i and L1i as measured along the Mx occlusal line |
| Overbite | OB | The overlap difference between U1i and L1i as measured perpendicular to the Mx occlusal line |
| Maxillary occlusal line | OLs | The line passing through U6o and U1i |
| Nasion-sella line | NSL | The line passing through N and S |
| Nasal line | NL | The line passing through ANS and PNS |
| Mandibular line | ML | The tangent to the lower boarder of the mandible through Me |
| Ramal line | RL | The tangent to the posterior boarder of the mandible through Ar |
| Palatal width | Mx'-Mx | The distance between Mx' and Mx |
| Mandibular width | Go'-Go | The distance between Go' and Go |
| Angles | ||
| Gonial angle | ∠Go | The angle formed between RL and ML |
| Beta angle | ∠β | The angle formed between a ML and a constructed line from Ar to the intersection between ML and a perpendicular line to it through Pg |
lat ceph indicates lateral cephalogram; PA ceph, postero-anterior cephalogram.
Skeletal Maturity Assessment
Skeletal maturity was digitally assessed on constructed two-dimensional lateral cephalograms by the Cervical Vertebral Maturation index according to Baccetti et al.24 and categorized as prepubertal, pubertal, and postpubertal according to Phelan et al.25
Reliability
A total of 25 scans were randomly selected for each variable and remeasured 2 weeks after the initial measurement. The method error for the airway volume and minimal cross-sectional area has been previously reported by the authors (1.90% and 0.49%, respectively).19 For each of the craniofacial measurements, no systematic error was found and the method error ranged from 0.13% to 9.37%. The reliability coefficient ranged from 0.94 to 1.00.
Statistics
The normality of distribution was assessed by parameters of skewness and kurtosis and Shapiro-Wilks W-tests. The airway volume and minimal cross-sectional area differed moderately from the normal distribution and were transformed logarithmically. The sample was screened for outliers by boxplots of each variable. No outliers were found.
Associations between airway dimensions and the continuous variables were assessed by the Spearman correlation analysis. Associations between airway dimensions and categorical variables were analyzed by analysis of variance, followed by post hoc Bonferroni tests. Each of the significant associations was then tested for the effect of gender, age, and skeletal maturity by linear regression analysis. The most relevant variables for airway dimensions were analyzed by linear regression analysis.
All statistical analyses were performed using SPSS for Windows version 22.0 (IBM Corp., Armonk, NY) and considered significant at P < .05.
RESULTS
Of the study population, 54.3% was in the prepubertal stage of skeletal maturity, 29.5% was in the pubertal stage, and 16.2% was in the postpubertal stage. The morphological occlusion according to Angle's classification included 36.2% class 1, 56.2% class 2, and 6.7% class 3. The mean values for the upper airway and craniofacial morphology are presented in Table 3.
Table 3.
Descriptive and Numerical Data
| Dimension or shape |
Minimum |
Maximum |
Mean |
Standard Deviation |
| Upper airway dimensions | ||||
| Volume, mm3 | 2875.1 | 23519.3 | 10077.3 | 4251.7 |
| Minimal cross-sectional area, mm2 | 23.5 | 348.5 | 120.8 | 61.0 |
| Craniofacial morphology | ||||
| Incisal relationships | ||||
| Overjet, mm | −2.5 | 12.7 | 4.3 | 2.8 |
| Overbite, mm | −4.6 | 6.3 | 2.4 | 1.6 |
| Cranial base angle | ||||
| SNBa, degrees | 118.3 | 145.0 | 132.7 | 5.1 |
| Sagittal craniofacial dimension | ||||
| SNA, degrees | 74.7 | 89.1 | 81.2 | 2.9 |
| SNB, degrees | 69.3 | 84.0 | 77.1 | 3.1 |
| SNPg, degrees | 69.3 | 84.9 | 77.2 | 3.2 |
| ANB, degrees | −2.1 | 8.9 | 4.1 | 2.6 |
| ANPg, degrees | −4.4 | 10.5 | 4.0 | 2.9 |
| Vertical craniofacial dimension | ||||
| SN-NL, degrees | 0.6 | 15.9 | 7.7 | 3.2 |
| SN-MP, degrees | 23.0 | 48.0 | 34.3 | 5.4 |
| MMP, degrees | 14.8 | 45.6 | 26.6 | 5.4 |
| ANS-Me, mm | 48.7 | 73.2 | 57.6 | 5.0 |
| N-ANS, mm | 36.7 | 54.1 | 46.6 | 3.5 |
| N-Me, mm | 89.7 | 123.5 | 104.2 | 7.0 |
| LAFH, % | 50.1 | 61.4 | 55.2 | 2.3 |
| S-Ba, mm | 33.7 | 49.5 | 41.2 | 2.6 |
| S-PNS, mm | 37.1 | 50.0 | 42.7 | 2.5 |
| S-Go, mm | 56.5 | 79.8 | 65.7 | 4.6 |
| Transverse craniofacial dimension | ||||
| Mx'-Mx, mm | 46.7 | 68.9 | 60.1 | 3.7 |
| Go'-Go, mm | 71.9 | 96.8 | 86.1 | 5.0 |
| Mandibular shape | ||||
| Gonial angle, degrees | 109.2 | 141.2 | 124.7 | 5.9 |
| β-angle, degrees | 13.3 | 26.0 | 20.0 | 2.4 |
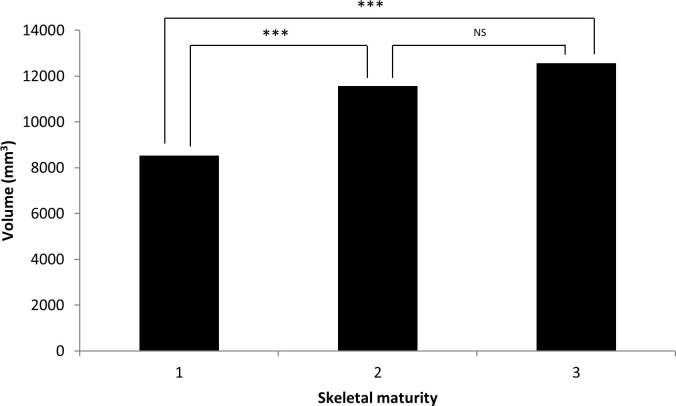
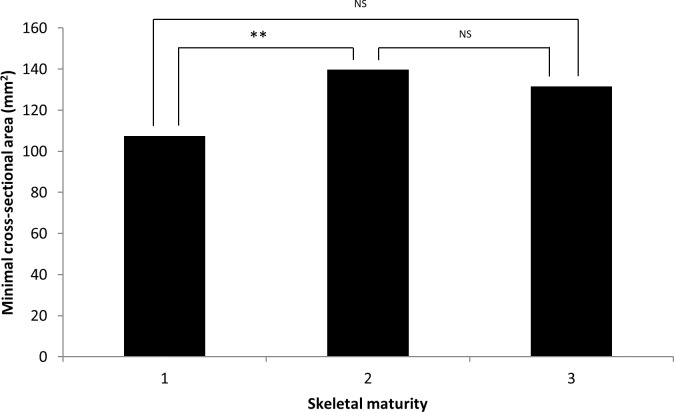
Airway volume and minimal cross-sectional area were significantly smaller in prepubertal children when compared with pubertal children (P < .001, P < .05, respectively; Figures 4 and 5) and positively associated with age (P < .001, P < .01, respectively). Gender and molar occlusion were not significantly associated with airway volume or minimal cross-sectional area.
Figure 4.
Airway volume in relation to skeletal maturity. NS, not significant. 1 = prepubertal, 2 = pubertal, 3 = postpubertal. * P ≤ .05; ** P ≤ .01; *** P ≤ .001.
Figure 5.
Airway minimal cross-sectional area in relation to skeletal maturity. 1 = prepubertal, 2 = pubertal, 3 = postpubertal. NS, not significant. * P ≤ .05; ** P ≤ .01; *** P ≤ .001.
After adjusting for age, skeletal maturity, and gender, significant positive associations were found between airway volume and craniofacial morphology: maxillary and mandibular width (Mx'-Mx, P < .01; Go'-Go, P < .001; Table 4) and anterior face height (N-Me, P < .05; N-ANS, P < .05; Table 4). Minimal cross-sectional area was positively associated with maxillary and mandibular width (Mx'-Mx, P < .01; Go'-Go, P < .001; Table 5) and negatively associated with sagittal jaw relationship (AnPg, P < .05; Table 5). No other significant associations were found.
Table 4.
Significant Associations Between Airway Volume and Gender, Age, Skeletal Maturity, Craniofacial Features, and Occlusion After Adjustment of Gender, Age, and Skeletal Maturity
| Variablea |
Correlation Coefficient, r |
P Value |
| Vertical craniofacial dimension | ||
| N-Me, mmb | 0.51 | .020 |
| N-ANS, mmb | 0.52 | .013 |
| Transverse craniofacial dimension | ||
| Mx'-Mxb | 0.53 | .003 |
| Go'-Gob | 0.60 | .000 |
N-Me indicates the distance between N and Me; N-ANS, the distance between N and ANS; Mx'-Mx, the distance between Mx' and Mx; Go'-Go, the distance between Go' and Go.
Also significant for the effect of age.
Table 5.
Significant Associations Between Airway Minimal Cross-Sectional Area and Gender, Age, Skeletal Maturity, Craniofacial Features, and Occlusion After Adjustment of Gender, Age, and Skeletal Maturity
| Variablea |
Correlation Coefficient, r |
P Value |
| Sagittal craniofacial dimension | ||
| ANPgb | −0.35 | .025 |
| Transverse craniofacial dimension | ||
| Mx'-Mxb | 0.35 | .003 |
| Go'-Go | 0.35 | .000 |
ANPg indicates the angle between A, N and Pg; Mx'-Mx, the distance between Mx' and Mx; Go'-Go, the distance between Go' and Go.
Also significant for the effect of age.
For airway volume, the most relevant variables were mandibular width and age (r2 = 0.36; Table 6). Mandibular width and sagittal jaw relationship were the most relevant factors for minimal cross-sectional area (r2 = 0.16; Table 6).
Table 6.
Most Relevant Factors Associated With Upper Airway Dimensions
| Factora |
Correlation Coefficient, r |
P Value |
| Volume | ||
| Go'-Go | 0.60 | .000 |
| Age | .007 | |
| Minimal cross-sectional airway | ||
| ANPg | 0.40 | .032 |
| Go'-Go | .001 | |
ANPg indicates the angle between A, N and Pg; Go'-Go, the distance between Go' and Go.
DISCUSSION
Dimensions of a healthy pharyngeal airway are influenced by growth,14–16,26,27 anatomical,6–13 postural,28–31 and mechanical factors.32,33 Therefore, it is important to perform multifactorial analyses of the upper airways. Because growth-related and anatomical factors are routinely assessed from standard orthodontic material, it is clinically relevant to investigate which of these parameters are relevant to pharyngeal airway dimensions.
In the present study, no significant differences in airway dimensions were found between molar classes 1, 2, and 3. This is supported by other studies34,35 possibly because any malocclusion can exist on any underlying skeletal pattern as a result of the dentoalveolar compensatory mechanism.36 It is osseous and soft tissue structures that frame the airway that determine its size and morphology.8,16 There was no statistically significant gender difference in airway dimensions, which is also in agreement with previous findings.6–8,11 However, one study found that boys not only had a longer and larger airway than girls but also experienced a quicker increase in dimensions after 11 years of age.15
During active growth, upper airway dimensions increase14–16 as was demonstrated in the present study. Growth and development of the upper airway is influenced by changes in the bony framework until maturity is reached.16 Consequently, it could be assumed that skeletal maturity is closely associated with upper airway dimensions during this period. However, assessment of this relationship is limited. The dimensional airway changes in relation to skeletal maturity observed in the present study could reflect growth-related changes of bony structures surrounding the pharyngeal airways.
Various studies found significant associations with sagittal craniofacial dimensions and upper airway dimensions with regard to volume and minimal cross-sectional area.6,8–11 In general, airway dimensions were negatively correlated to the sagittal jaw relationship, for example, as a result of mandibular retrognathism, and positively correlated to mandibular prognathism. Therefore, it was found that skeletal class 3 patients had greater airway volume than class 1, which was greater than skeletal class 2 patients.6–11 However, this difference was not always statistically significant between the groups7 or between class 1 and class 3.6 Conflicting results have been reported regarding minimal cross-sectional area and sagittal craniofacial dimension; some studies have found an association,8,9 whereas others have not.37 In agreement with most previous studies, the present study found that the sagittal jaw relationship remained significantly negatively associated to minimal cross-sectional area when tested for the effects of age, gender, and skeletal maturity. This suggests that the larger the sagittal jaw relationship, the smaller the airway dimensions.
In relation to vertical craniofacial dimensions, anterior face height was previously found to be significantly associated with upper airway dimensions.7,11 This is in agreement with the present study. However, the association remained statistically significant with only airway volume after the correction for age, gender, and skeletal maturity.
In the present study, associations were found between airway dimensions and the width of the maxilla and mandible. However, Di Carlo et al.13 found no associations between the pharyngeal airway and transverse dimension. This discrepancy could possibly be explained by the population sample being older in the Di Carlo el al. study.14
In the present study, mandibular width and age were the most relevant factors for airway volume. The most relevant factors for the minimal cross-sectional area were mandibular width and sagittal jaw relationship. Previously, age and vertical and sagittal craniofacial morphology have been associated with pharyngeal airway dimensions as single factors,6,7,9–15 but the combinations of age with mandibular width and sagittal jaw relationship with mandibular width have not been previously reported for preorthodontic children. The results may be valuable for orthodontic diagnosis and treatment planning of children, especially those with compromised pharyngeal airway dimensions. Future studies could assess the contribution of other factors that potentially influence airway dimensions, including functional factors.
CONCLUSIONS
Pharyngeal airway dimensions were significantly associated with age, skeletal maturity, and craniofacial morphology in all three planes.
Children with a reduced mandibular width and increased sagittal jaw relationship are particularly at risk of having small pharyngeal airway dimensions.
The results may prove valuable in diagnosis and orthodontics treatment planning, especially in children with compromised pharyngeal airway dimensions.
ACKNOWLEDGMENT
The study was funded by the Australian Society of Orthodontists Foundation for Research and Education as part of a thesis for the degree of Doctor of Clinical Dentistry (Orthodontics).
REFERENCES
- 1.Guijarro-Martínez R, Swennen GRJ. Cone-beam computerized tomography imaging and analysis of the upper airway: a systematic review of the literature. Int J Oral Maxillofac Surg. 2011;40:1227–1237. doi: 10.1016/j.ijom.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 2.Flores-Mir C, Korayem M, Heo G, Witmans M, Major MP, Major PW. Craniofacial morphological characteristics in children with obstructive sleep apnea syndrome a systematic review and meta-analysis. J Am Dent Assoc. 2013;144:269–277. doi: 10.14219/jada.archive.2013.0113. [DOI] [PubMed] [Google Scholar]
- 3.Aboudara C, Nielsen I, Huang JC, Maki K, Miller AJ, Hatcher D. Comparison of airway space with conventional lateral headfilms and 3-dimensional reconstruction from cone-beam computed tomography. Am J Orthod Dentofac Orthop. 2009;135:468–479. doi: 10.1016/j.ajodo.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 4.Peltomäki T. The effect of mode of breathing on craniofacial growth—revisited. Eur J Orthod. 2007;29:426–429. doi: 10.1093/ejo/cjm055. [DOI] [PubMed] [Google Scholar]
- 5.Martin SE, Mathur R, Marshall I, Douglas NJ. The effect of age, sex, obesity and posture on upper airway size. Eur Respir J. 1997;10:2087–2090. doi: 10.1183/09031936.97.10092087. [DOI] [PubMed] [Google Scholar]
- 6.El H, Palomo JM. Airway volume for different dentofacial skeletal patterns. Am J Orthod Dentofac Orthop. 2011;139:e511–e521. doi: 10.1016/j.ajodo.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Oh K-M, Hong J-S, Kim Y-J, Cevidanes LSH, Park Y-H. Three-dimensional analysis of pharyngeal airway form in children with anteroposterior facial patterns. Angle Orthod. 2011;81:1075–1082. doi: 10.2319/010711-8.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng ZH, Yamaguchi T, Kurihara A, Li HF, Maki K. Three-dimensional evaluation of upper airway in patients with different anteroposterior skeletal patterns. Orthod Craniofac Res. 2014;17:38–48. doi: 10.1111/ocr.12029. [DOI] [PubMed] [Google Scholar]
- 9.Alves JM, Franzotti ES, Baratieri C, Nunes LKF, Nojima LI, Ruellas ACO. Evaluation of pharyngeal airway space amongst different skeletal patterns. Int J Oral Maxillofac Surg. 2012;41:814–819. doi: 10.1016/j.ijom.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 10.El H, Palomo JM. An airway study of different maxillary and mandibular sagittal positions. Eur J Orthod. 2013;35:262–270. doi: 10.1093/ejo/cjr114. [DOI] [PubMed] [Google Scholar]
- 11.Kim Y, Hong J, Hwang Y, Park Y. Three-dimensional analysis of pharyngeal airway in preadolescent children with different anteroposterior skeletal patterns. Am J Orthod Dentofac Orthop. 2010;137:306.e301–306.e311. doi: 10.1016/j.ajodo.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaki T, Hayasaki H, Takemoto Y, Kanomi R, Yamasaki Y. Oropharyngeal airway in children with class iii malocclusion evaluated by cone-beam computed tomography. Am J Orthod Dentofac Orthop. 2009;136:318.e311–318.e319. doi: 10.1016/j.ajodo.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Di Carlo G, Polimeni A, Melsen B, Cattaneo P. The relationship between upper airways and craniofacial morphology studied in 3D. A CBCT study. Orthod Craniofac Res. 2015;18:1–11. doi: 10.1111/ocr.12053. [DOI] [PubMed] [Google Scholar]
- 14.Schendel SA, Jacobson R, Khalessi S. Airway growth and development: a computerized 3-dimensional analysis. J Oral Maxillofac Surg. 2012;70:2174–2183. doi: 10.1016/j.joms.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Chiang CC, Jeffres MN, Miller A, Hatcher DC. Three-dimensional airway evaluation in 387 subjects from one university orthodontic clinic using cone beam computed tomography. Angle Orthod. 2012;82:985–992. doi: 10.2319/122811-801.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tourné LP. Growth of the pharynx and its physiologic implications. Am J Orthod Dentofac Orthop. 1991;99:129–139. doi: 10.1016/0889-5406(91)70115-D. [DOI] [PubMed] [Google Scholar]
- 17.Sonnesen L, Bakke M. Molar bite force in relation to occlusion, craniofacial dimensions, and head posture in pre-orthodontic children. Eur J Orthod. 2005;27:58–63. doi: 10.1093/ejo/cjh069. [DOI] [PubMed] [Google Scholar]
- 18.Andersen MK, Sonnesen L. Risk factors for low molar bite force in adult orthodontic patients. Eur J Orthod. 2013;35:421–426. doi: 10.1093/ejo/cjs003. [DOI] [PubMed] [Google Scholar]
- 19.Anandarajah S, Abdalla Y, Dudhia R, Sonnesen L. A proposal of new upper airway margins in children assessed by cone beam CT. Dentomaxillofac Radiol. 2015;44:20140438. doi: 10.1259/dmfr.20140438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Björk A. The face in profile: an anthropological x-ray investigation on Swedish children and conscripts. Sven Tandlak Tidskr. 1947. 40:6.
- 21.Björk A. The relationship of the jaws to the cranium. Introduction to Orthodontics. New York, NY: McGraw-Hill; 1960. pp. 104–140. [Google Scholar]
- 22.Yoon Y-J, Perkiomaki MR, Tallents RH, et al. Transverse craniofacial features and their genetic predisposition in families with nonsyndromic unilateral cleft lip and palate. Cleft Palate Craniofac J. 2004;41:256–261. doi: 10.1597/02-134.1. [DOI] [PubMed] [Google Scholar]
- 23.Solow B, Tallgren A. Head posture and craniofacial morphology. Am J Phys Anthropol. 1976;44:417–435. doi: 10.1002/ajpa.1330440306. [DOI] [PubMed] [Google Scholar]
- 24.Baccetti T, Franchi L, McNamara J. The cervical vertebral maturation (CVM) method for the assessment of optimal treatment timing in dentofacial orthopedics. Semin Orthod. 2005;11:119–129. [Google Scholar]
- 25.Phelan A, Franchi L, Baccetti T, Darendeliler MA, McNamara JA., Jr Longitudinal growth changes in subjects with open-bite tendency: a retrospective study. Am J Orthod Dentofac Orthop. 2014;145:28–35. doi: 10.1016/j.ajodo.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 26.King EW. A roentgenographic study of pharyngeal growth. Angle Orthod. 1952;22:23–37. [Google Scholar]
- 27.Bergland O. The bony nasopharynx. A roentgen-craniometric study. Acta Odont Scand Suppl. 1963;21:1–137. [PubMed] [Google Scholar]
- 28.Muto T, Takeda S, Kanazawa M, Yamazaki A, Fujiwara Y, Mizoguchi I. The effect of head posture on the pharyngeal airway space (pas) Int J Oral Maxillofac Surg. 2002;31:579–583. doi: 10.1054/ijom.2002.0279. [DOI] [PubMed] [Google Scholar]
- 29.Muto T, Yamazaki A, Takeda S, Kawakami J, Tsuji Y, Shibata T, Mizoguchi I. Relationship between the pharyngeal airway space and craniofacial morphology, taking into account head posture. Int J Oral Maxillofac Surg. 2006;35:132–136. doi: 10.1016/j.ijom.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 30.Solow B, Ovesen J, Nielsen PW, Wildschiødtz G, Tallgren A. Head posture in obstructive sleep apnoea. Eur J Orthod. 1993;15:107–114. doi: 10.1093/ejo/15.2.107. [DOI] [PubMed] [Google Scholar]
- 31.Solow B, Skov S, Ovesen J, Norup PW, Wildschiødtz G. Airway dimensions and head posture in obstructive sleep apnoea. Eur J Orthod. 1996;18:571–579. doi: 10.1093/ejo/18.6.571. [DOI] [PubMed] [Google Scholar]
- 32.Strohl KP, Butler JP, Malhotra A. Mechanical properties of the upper airway. Compr Physiol. 2012;2:1853–1872. doi: 10.1002/cphy.c110053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayappa I, Rapoport DM. The upper airway in sleep: physiology of the pharynx. Sleep Med Rev. 2003;7:9–33. doi: 10.1053/smrv.2002.0238. [DOI] [PubMed] [Google Scholar]
- 34.de Freitas MR, Alcazar NMPV, Janson G, de Freitas KMS, Henriques JFC. Upper and lower pharyngeal airways in subjects with class i and class ii malocclusions and different growth patterns. Am J Orthod Dentofac Orthop. 2006;130:742–745. doi: 10.1016/j.ajodo.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 35.Ceylan İ, Oktay H. A study on the pharyngeal size in different skeletal patterns. Am J Orthod Dentofac Orthop. 1995;108:69–75. doi: 10.1016/s0889-5406(95)70068-4. [DOI] [PubMed] [Google Scholar]
- 36.Solow B. The dentoalveolar compensatory mechanism: background and clinical implications. Br J Orthod. 1980;7:145–161. doi: 10.1179/bjo.7.3.145. [DOI] [PubMed] [Google Scholar]
- 37.Kula K, Jeong AE, Stacey H, Kendall D, Ghoneima A. Three dimensional evaluation of upper airway volume in children with different dental and skeletal malocclusions. J Biomed Graph Comput. 2013;3:116–126. [Google Scholar]