Abstract
Aim: We aimed to quantify the effects of immune checkpoint inhibitors (ICIs) on the prognosis of COVID-19. Materials & methods: A meta-analysis was conducted and the hospitalization, severe disease and mortality rates were assessed. Thirteen studies comprising of 4614 cancer patients with COVID-19 were included. Results: When compared with cancer patients without prior ICI exposure, patients with prior ICI treatment exhibited a higher rate of hospitalization (odds ratio [OR] 2.0, 95% CI 1.19–3.38, p = 0.01). However, the OR of severe disease and mortality in ICI exposed cases was similar to non-ICI exposed patients (OR 1.55, 95% CI 0.69–3.51, p = 0.29; OR 1.12, 95% CI 0.85–1.48, p = 0.42, respectively). Conclusion: It is uncertain whether prior exposure to ICIs increases the risk of severe disease and death, however the observed OR suggest a higher rate of hospitalization.
Keywords: : COVID-19, hospitalization, immune checkpoint inhibitor, meta-analysis, mortality, prognosis, severe disease
Lay abstract
COVID-19 is an infectious disease caused by a virus which affected people worldwide in 2020. It mainly attacks the lungs and causes symptoms such as, fever, dry cough and fatigue. However, there is currently are no definite therapies for its treatment. Cancer patients are more vulnerable due to both the tumor itself and the anticancer treatment. At the same time, they are at higher risk of COVID-19 exposure due to the need for regular treatment and testing in hospitals. In this systematic review and meta-analysis we enrolled 13 studies. Firstly, we analyzed the rate of hospitalization, severe disease and death. Additionally, we studied the impact of immune checkpoint inhibitors on the outcome of cancer patients infected with COVID-19. Finally, our discussion focuses on what we can learn from the pandemic to provide guidance for clinical practice.
COVID-19 has already spread quickly on a global scale, evolving into a pandemic and threatening global health [1–3]. The rapid rise of the disease, and the resultant hospitalizations and deaths have strained public health systems [4]. It mainly attacks the lungs and causes related symptoms, including fever, dry cough and fatigue etc., [5]. In addition to lung injury, COVID-19 is associated with hepatitis, gastrointestinal symptoms (such as diarrhea) and damage to other organs [6]. However, there currently are no effective therapies for its treatment. Cancer patients are often at higher risk of COVID-19 exposure due to the need for regular treatment and testing in hospitals [7]. To make matters worse, cancer patients are more vulnerable due to both the tumor itself and the anticancer treatment [8,9]. It remains to be seen whether the application of anticancer drugs results in differential prognoses for patients infected with COVID-19 [10]. It is critically important for clinicians to identify risk factors associated with severity and mortality and take appropriate interventions.
Currently, immunotherapy has raised major concerns amid different therapeutic strategies in cancer treatment, due to its intrinsic and extensive influence on the immune system [11]. Immunotherapy primarily consists of several immune checkpoint inhibitors (ICIs), including inhibitors targeting the CTLA-4, PD-1 and PD-L1. Anti-CTLA-4 and anti-PD-1/-PD-L1 antibodies reactivate cytotoxic CD8+ T cells for antitumor activity through targeting T-cell exhaustion pathways [12]. In patients with malignancy, ICIs sometimes induces adverse events, including liver injury, pneumonia and colitis [13]. Given the convergence of the downstream effects on innate immunity and organ damage caused by both ICIs and COVID-19 infections, we investigated whether patients present worse prognosis due to prior exposure to ICIs.
Aeppli et al. performed an online survey among clinicians involved in the treatment of renal cell carcinoma [14]. The results reflected that over 80% of experts choose pilimumab/nivolumab outside the pandemic, however this figure has fallen by half during the COVID-19 pandemic. Given that ICI therapy represents an important treatment choice for some patients, whether this has an impact on the prognosis of COVID-19 infection in cancer patients should be elucidated. However, whether COVID-19 patients receiving ICI therapy are prone to poorer prognosis remains unknown. In light of this, this systematic review and meta-analysis aimed to assess the safety of ICI application in COVID-19 patients and to make reasonable recommendations by reviewing available publications.
Materials & methods
Search strategy
We searched the PubMed, Embase, and Web of Science databases, limiting our search to papers written in English from the inception of each database until 4 January 2021. The search terms were as follows: ‘severe acute respiratory syndrome coronavirus 2’ or 'SARS-CoV-2’ or '2019-nCoV' or 'COVID-19' and ‘cancer’ or ‘malignancy’ or 'tumor' and ‘immune checkpoint inhibitors’ or 'PD-1/PD-L1' or 'CTLA-4' or 'immunotherapy'. Articles were also retrieved by screening the reference lists of included studies and from related review papers. One reviewer (Y Tian) with experience in database searches designed the search, and two reviewers (W Qian and Y Tian) independently screened the titles, abstracts and full text according to these eligibility criteria, assessing the eligibility of publications.
Inclusion criteria
We included randomized controlled trials, observational studies and case series that reported ICI use in cancer patients and their prognosis in the context of COVID-19. Exclusion criteria were as follows: the same patients enrolled in different studies, studies such as clinical reviews, summaries of meetings, or erratum that did not report original data; and studies containing less than four ICI users. When data was inadequate in some studies, attempts were made to contact the investigators for the missing data.
Data extraction & definitions
Two researchers (W Qian and Y Tian) independently extracted data from the included studies in a double-blind manner. Any disagreements were resolved by a third investigator (L Zuo) or by consensus. The following variables was extracted: name of first author, country, date of COVID-19 diagnosis, study type, age, gender, total number of patients, number of patients receiving ICI, treatment interval before diagnosis of COVID-19 and outcome of infection, such as hospitalization and/or severity and/or mortality (Table 1). Severe disease was defined according to the original studies, primarily based on the symptoms present during treatment – for example, admission to the intensive care unit, development of severe or critical symptoms and utilization of invasive mechanical ventilation [8]. To ensure high-quality evidence, this study was performed in accordance with the Preferred Reporting Items of Systematic Reviews and Meta-analysis statement.
Table 1. Main characteristics of the included studies in meta-analysis.
| Study | Year | Country | Sample (n) | Male | ICI users | Age | Study type | Outcome | Treatment interval before diagnosis | NOS |
|---|---|---|---|---|---|---|---|---|---|---|
| Albiges et al. | 2020 | France | 178 | 76 | 19 | 61 (IQR 52–71) | Retrospective | Severity and mortality | 3 months | 8 |
| Bersanelli et al. | 2020 | Italy | 9 | 8 | 9 | 75 (range 50–82) | Prospective | Mortality | 21 weeks | 6 |
| Dai et al. | 2020 | China | 641 | 302 | 6 | Male median: 64 Female median: 63.5 |
Prospective | Mortality and severity | 40 days | 8 |
| Lara et al. | 2020 | USA | 121 | NR | 8 | 64 (IQR 51–73) | Retrospective | Hospitalization, severity and mortality | NR | 5 |
| Lee et al. | 2020 | UK | 800 | 449 | 44 | 69 (range 59–76) | Prospective | Mortality | 4 weeks | 8 |
| Lievre et al. | 2020 | France | 1289 | 795 | 110 | 67 (range 19–100) | Prospective | Mortality | 3 months | 9 |
| Luo et al. | 2020 | USA | 69 | 33 | 40 | 69 (range 31–91) | Retrospective | Hospitalization, severity and mortality | 6 weeks (n = 20); 6 months (n = 30); ongoing (n = 40). | 6 |
| Moritz et al. | 2020 | Germany | 13 | 7 | 13 | 65 (range 26–88) | Retrospective | Severity and mortality | 51 days | 6 |
| Noguera et al. | 2020 | Spain | 166 | 96 | 58 | 63 (range 33–86) | Retrospective | Hospitalization | 2 months | 7 |
| Pinato et al. | 2020 | UK, Italy, Spain and Germany | 890 | 503 | 56 | 68 (range 21–99) | Retrospective | Mortality | 4 weeks | 6 |
| Robilotti et al. | 2020 | USA | 423 | 212 | 31 | 416 pts >18 (98%) 234 pts >60 (56%) |
Retrospective | Hospitalization and Severity | 90 days | 5 |
| Szabados et al. | 2020 | UK | 4 | 4 | 4 | 67 (range 52–72) | Prospective | Hospitalization, severity and mortality | 90 days | 5 |
| Wu et al. | 2020 | China | 11 | 8 | 11 | 66 (range 29–73) | Retrospective | Severity and mortality | 50 days | 5 |
ICI: Immune checkpoint inhibitor; IQR: Interquartile range; NR: Not reported; NOS: Newcastle–Ottawa Scale; pts: Patients.
Quality assessment
The Newcastle–Ottawa Scale (NOS) was used for observational studies to evaluate the methodological quality of the original study (Table 1) [15]. The NOS consists of three parts: patient selection, study comparability and outcome assessment and produces scores ranging from 0 to 9. Studies with NOS scores of >7 were regarded as high quality. The risk of bias was independently assessed by two authors (W Qian and Y Tian).
Data synthesis & statistical analysis
All statistical analyses in this study were performed using R (version 4.0.2). Odds ratios (OR) were used to describe the ratio of the probability of events occurring in cancer patients treated with different therapies. The q-test was used to calculate heterogeneity among the included studies and I2 test was used to describe the percentage of variation across studies that is due to heterogeneity. p < 0.05 or I2 > 50% indicated substantial heterogeneity across the articles [16], and a random effects model was used [17]. Otherwise, a fixed-effects model was used. Publication bias was assessed using the Begg funnel plot and the Egger test linear regression test (where at least five studies were available). A p < 0.05 was considered statistically significant.
Results
Search results
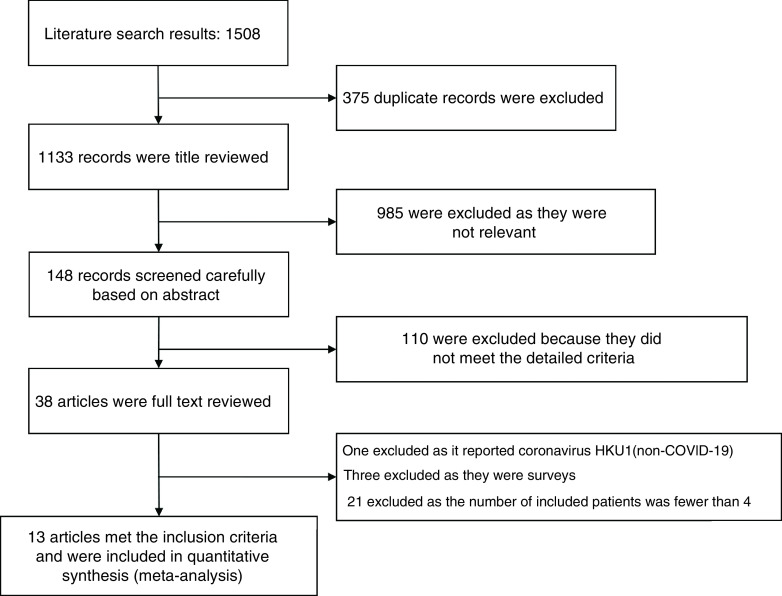
The search strategy identified 1508 articles (Figure 1). Among these studies, 375 were duplicates. After screening the title and abstract, 1085 were excluded, and the full text of the remaining 38 articles was reviewed. Among these, one study about coronavirus, three surveys and 21 researches that included less than four patients were excluded after full text review. Finally, 13 studies reported ICI use in cancer patients and prognosis of COVID-19 infection [8,18–29]. The 13 articles consisted of ten cohort studies and three case series and were included for the meta-analysis.
Figure 1. Flow chart for study selection.
Patient characteristics
Finally, 13 relevant studies were enrolled, including eight retrospective studies and five prospective studies, comprising more than 4600 cancer patients infected by COVID-19. Detailed patient characteristics of the included studies are shown in Table 1. The studies were from eight countries, including China (n = 2), Germany (n = 2), Italy (n = 2), France (n = 2), Spain (n = 2), the UK (n = 3) and the USA (n = 3). These studies included more than four ICI users, and the median age of study participants was 61–67 years old. Of these 13 studies, clinical outcomes were defined as hospitalization in five studies, severity in eight studies and mortality in 11 studies (Table 1). However, there was nonuniformity in the criterion of the time interval from last dose to COVID-19 diagnosis (Table 1) [8,18,19,21,23–25]. Results of the quality assessment of the included studies assessed by NOS scores are presented in Table 1.
ICI use & risk of hospitalization in COVID-19 patients
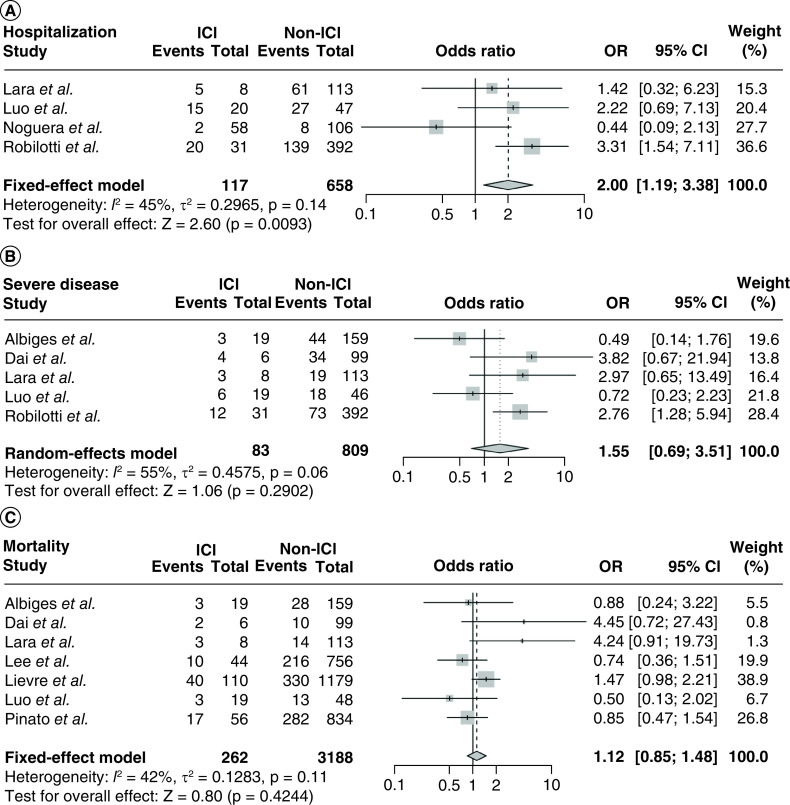
We combined five studies [20,23,25,27,28] reporting the hospitalization of COVID-19 infection in patients on ICI treatment, and a random effects model was used since the heterogeneity test suggested obvious heterogeneity (I2 = 87%, p < 0.01). The pooled estimate of the rate of hospitalization was 0.45 (95% CI 0.15–0.78; Table 2). Four of the five studies [20,23,25,27] contained hospitalization information of patients without ICI exposure. The proportion of hospitalization was markedly increased in patients treated with ICI therapy compared with those without ICI treatment (OR 2.00 [95% CI 1.19–3.38], p = 0.01; I2 = 45%; Figure 2A).
Table 2. The results of the meta-analysis.
| Studies (n) | OR (95% CI) | p-value | Heterogeneity | Model used | Begg’s test | Egger’s test | ||
|---|---|---|---|---|---|---|---|---|
| I2 | p-value | |||||||
| Single proportions | ||||||||
| – Hospitalization | 5 | 0.45 (0.15–0.78) | NA | 87% | <0.01 | Random | 0.33 | 0.45 |
| – Severe disease | 8 | 0.34 (0.26–0.44) | NA | 35% | 0.13 | Fixed | 0.62 | 0.88 |
| – Mortality | 11 | 0.26 (0.17–0.38) | NA | 64% | 0.20 | Random | 0.43 | 0.36 |
| Binary outcome | ||||||||
| Hospitalization | ||||||||
| – ICI vs non-ICI | 4 | 2.00 (1.19–3.38) | <0.01 | 45% | 0.14 | Fixed | NA | NA |
| Severe disease | ||||||||
| – ICI vs non-ICI | 5 | 1.55 (0.69–3.51) | 0.29 | 55% | 0.06 | Random | 1.00 | 0.79 |
| Mortality | ||||||||
| – ICI vs Non-ICI | 7 | 1.12 (0.47–1.54) | 0.42 | 42% | 0.11 | Fixed | 0.65 | 0.85 |
| – ICI vs chemotherapy | 6 | 1.09 (0.54–1.97) | 0.56 | 0% | 0.46 | Fixed | 0.85 | 0.73 |
| – ICI vs hormone therapy | 5 | 1.45 (0.70–2.97) | 0.32 | 53% | 0.08 | Random | 1.00 | 0.80 |
| – ICI vs radiotherapy | 4 | 1.13 (0.74–1.74) | 0.57 | 26% | 0.26 | Fixed | NA | NA |
| – ICI vs surgery | 4 | 1.69 (0.95–2.98) | 0.57 | 0% | 0.64 | Fixed | NA | NA |
| – ICI vs targeted therapy | 6 | 2.13 (1.44–3.14) | <0.01 | 15% | 0.32 | Fixed | 0.85 | 0.65 |
ICI: Immune checkpoint inhibitor; NA: Not available; OR: Odds ratio.
Figure 2. The pooled prognosis of COVID-19 infections compared between patients with prior immune checkpoint inhibitor treatment and those without.
ICI: Immune checkpoint inhibitor; OR: Odds ratio.
ICI use & influence on COVID-19 severity
Eight studies [8,18,20,23,24,27–29] that included 111 COVID-19 cases with ICI exposure reported on COVID-19 severity in relation to ICI exposure. The combined proportion of severe disease was 0.34 (95% CI 0.26–0.44, I2 = 35%; Table 2). Out of these eight studies, five studies [8,18,20,23,27] included 83 COVID-19 cases with ICI exposure and 809 COVID-19 cases unexposed to ICI. A random-effects model was used (I2 = 55%; p = 0.06) and the pooled OR of COVID-19 severity was 1.55 (95% CI, 0.69–3.51, p = 0.29; Figure 2B).
ICI use & risk of mortality in COVID-19 patients
The overall analysis included 11 studies [8,18–24,26,28,29]. Together, 299 COVID-19 cases with ICI exposure and 3188 COVID-19 cases without ICI exposure were included. The pooled proportion of mortality in COVID-19 patients with ICI exposure was 0.26 (95% CI, 0.17–0.38; Table 2). Next, the risk associated with ICI use and mortality was assessed. Overall, the OR of mortality in ICI-exposed cases was similar to non-ICI exposed COVID-19 patients (OR 1.12, 95% CI 0.85–1.48, p = 0.42; Figure 2C). Moderate heterogeneity was observed among the studies (I2 = 42%, p = 0.11).
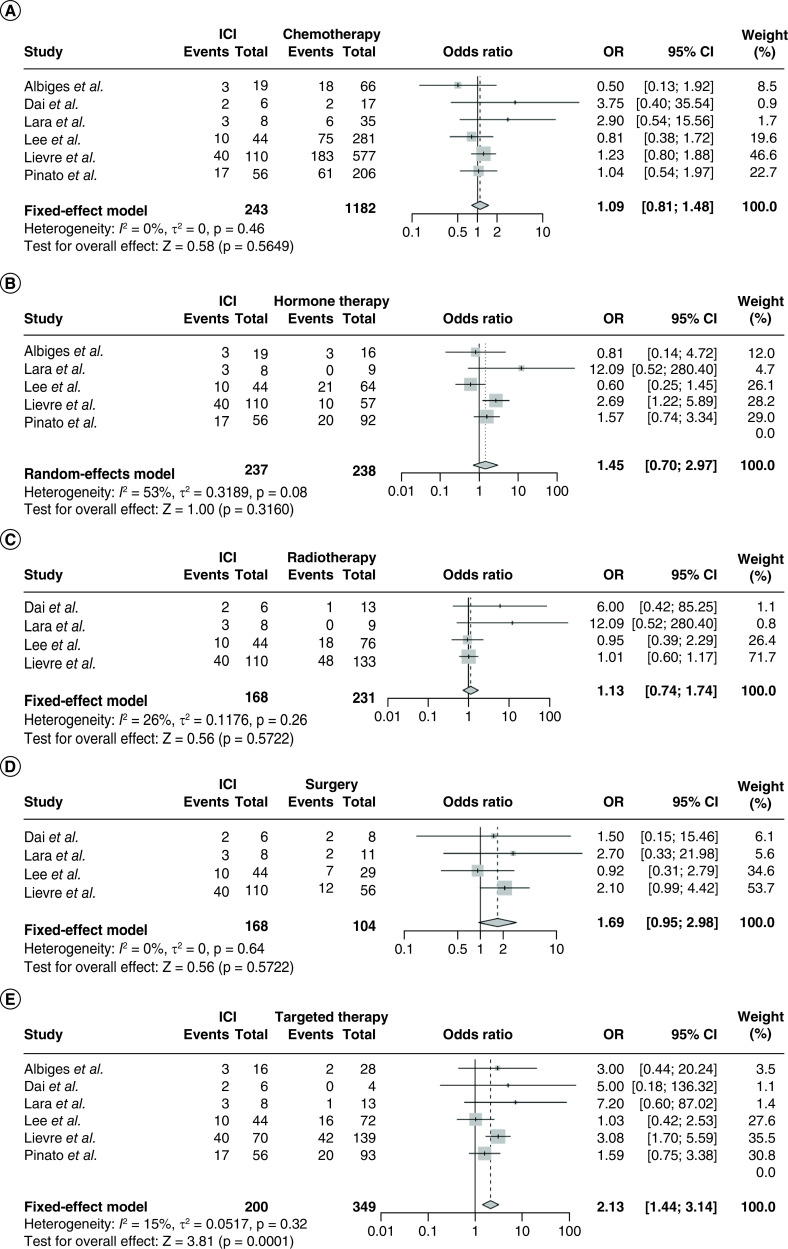
We further examined the mortality between exposure to ICI and other treatments in cancer patients in the context of COVID-19. However, we did not identify significant differences between ICI and chemotherapy (OR 1.09, 95% CI 0.81–1.48, p = 0.56; I2 = 0%; Figure 3A), hormone therapy (OR 1.45, 95% CI 0.70–2.97, p = 0.32; I2 = 53%; Figure 3B), radiotherapy (OR 1.13, 95% CI 0.74–1.74, p = 0.57; I2 = 26%; Figure 3C), surgery (OR 1.69, 95% CI 0.95–2.98, p = 0.57; I2 = 0%; Figure 3D), except for targeted therapy (OR 2.13, 95% CI 1.44–3.14, p < 0.01; I2 = 15%; Figure 3E).
Figure 3. The pooled mortality of COVID-19 infection.
The mortality was compared between patients with prior ICI treatment and those with (A) chemotherapy; p = 0.56, (B) hormone therapy; p = 0.32, (C) radiotherapy; p = 0.57, (D) surgery; p = 0.57 or (E) targeted therapy; p < 0.01.
ICI: Immune checkpoint inhibitor; OR: Odds ratio.
Temporal relationship between prior ICI receipt & diagnosis of COVID-19
Given that the receptor can be occupied for months [30] and the initial start of ICI therapy results in a distinct proliferative burst [31–34], different intervals from the last dose of ICI to the diagnosis of COVID-19 may theoretically influence the prognosis of COVID-19 infection. Luo et al. [23] defined five categories of prior PD-1 blockade, including no prior PD-1, ever received PD-1 blockade, last receipt within 6 months, last receipt within 6 weeks, and first receipt within 3 months, detecting the outcomes of interest. Overall, there was no significant difference in prognosis regardless of PD-1 blockade exposure. We extracted data from this study and regrouped patients according to intervals from last dose of ICI to the diagnosis of COVID-19: no prior PD-1, interval >6 months, interval between 6 months and 6 weeks, interval <6 weeks and initial dose within 3 months (Figure 4). However, we did not capture any statistically significant differences between no prior PD-1 group and the other four groups tested by chi-square test or Fisher’s exact test in terms of prognosis, including hospitalization, severe disease and mortality (Figure 4). Consistent with the above outcomes, Wu et al. [29] observed a similar risk of severity in different intervals from the last ICI administration to COVID-19 diagnosis (interval ≥28 days vs interval <28 days, p = 1.00).
Figure 4. The impact of prior PD-1 exposure on the prognosis of COVID-19 in patients with lung cancer.
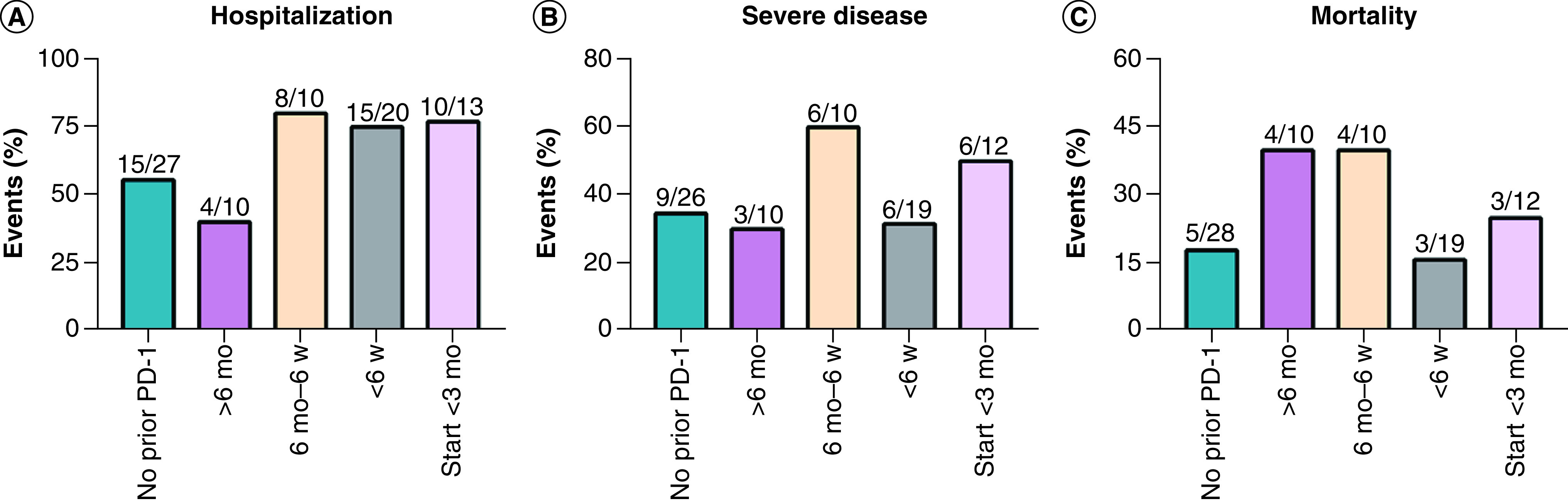
Patients were redistributed into five groups: no prior PD-1, interval >6 months (>6 mo), interval between 6 months and 6 weeks (6 mo–6 w), interval <6 weeks (<6 w) and initial dose within 3 months (start <3 mo). (A) Rate of hospitalization compared between no prior PD-1 and >6 mo (p = 0.48), 6 mo–6 w (p = 026), <6 w (p = 0.17) and start <3 mo (p = 0.30). (B) Rate of severe disease compared between no prior PD-1 and >6 mo (p = 1.00), 6 mo–6 w (p = 0.26), <6 w (p = 0.38) and start <3 mo (p = 0.48). (C) Rate of death compared between no prior PD-1 and >6 mo (p = 0.24), 6 mo–6 w (p = 0.24), <6 w (p = 1.00) and start <3 mo (p = 0.68).
mo: Month; w: Week.
Data taken from [23].
ICI-induced lung injury & COVID-19 infection
ICI-induced pneumonitis presents similar clinical and radiological features to COVID-19, challenging the early diagnosis of COVID-19 [35]. Guerini et al. [36] and Lovly et al. [37] reported two cases where patients experienced misdiagnosis caused by ICI-induced pneumonitis and later died due to an uncontrolled COVID-19 infection. Clinicians should always consider COVID-19 as a differential diagnosis, as few places were spared during the pandemic. In another report [38], two patients were initially highly suspected of COVID-19 infection based on clinical manifestations, imaging findings and epidemiology. Steroids were withheld in one case, and the disease became worse until a third CT scan was obtained, and a second negative RT-PCR test was released after admission. Both patients were eventually diagnosed with ICI-induced pneumonitis, and a mean delay of 3 days in steroid initiation was attributed to the COVID-19 pandemic.
Except for missed window of optimal treatment caused by delayed diagnosis, ICI-induced pneumonitis itself reduces patient resistance and exacerbates COVID-19 infection. Here, we were curious about the influence of ICI in lung cancer patients infected with COVID-19. Data showed that ICI application did not significantly influence the severity of COVID-19 in lung cancer patients (ICI application [7/12] vs no ICI application [8/23], p = 0.181) [27]. Consistently, ICI exposure in lung cancer patients did not exhibit a higher risk for developing severity than in patients with other solid cancers (lung cancer [7/12] vs other solid cancers [5/19], p = 0.13) [27].
Publication bias
The results of publication bias was shown in Table 2, which was assessed using the Begg's funnel plot and Egger's test. There was no significant publication bias in the included studies (all p > 0.5).
Discussion
This review included 13 articles that encompassed 409 ICI users infected with COVID-19. It is uncertain whether prior exposure to ICI increases the risk of severe disease and death but, observed OR suggests a higher rate of hospitalization. In addition, different intervals from the last dose of ICI to diagnosis of COVID-19 might not influence the prognosis of COVID-19 infection. Finally, given the unpredictable duration of the pandemic, we should always keep in mind a differential diagnosis of COVID-19 and rational adjustment of ICI use.
Patients with cancer are theoretically more vulnerable to infection due to poor health status and immunosuppressive conditions provoked by both the cancer and antitumor therapies [39–42]. Poorer prognosis in COVID-19 infection has been associated with several factors, including older age, gender and comorbidities such as pulmonary disease, cardiac disease, hypertension and cancer [26,43]. Liang et al. collected and analyzed 1590 cases from 575 hospitals [7]. In their study, 18 of 1590 (1%; 95% CI 0.61–1.65) COVID-19 cases had a history of cancer, which was higher than the overall incidence of cancer in the population (285.83 [0.29%] per 100,000 people). Importantly, patients with cancer exhibited a higher rate of severe disease than patients without cancer (7/18 [39%] vs 124/1572 [8%], p = 0.0003). Here, we pooled the prognostic data from COVID-19-infected cases with prior exposure to ICI. The rate of hospitalization was 45%, 34% developed severe disease and 26% died.
Physicians worry about the influence of ICI administration on COVID-19 infection for two main reasons [44]. The first is the potential overlap between the two lung injuries: possible pneumological toxicity from ICI use and COVID-19 pneumonia. The incidence of ICI-related pneumonitis was reported to be 2.5–5% with anti-PD-1/PD-L1 monotherapy and 7–10% with anti-CTLA-4/anti-PD-1 combination therapy [45]. These fatal immune-related adverse events accounted for 35% of treatment-related deaths [46]. The second concern is the potential synergy between ICI mechanisms and COVID-19 pathogenesis, both of which are involved in immune hyperactivation [47–49]. Integrating multiple studies into the present study, we found that prior receipt of ICI significantly increased the rate of hospitalization. In contrast, there was no significant difference in severe disease and mortality among patients with or without prior ICI exposure (OR 1.55, 95% CI 0.69–3.51, p = 0.29; OR 1.12, 95% CI 0.85–1.48, p = 0.42; Figure 2B & C). We speculate that prior ICI exposure may lead to gastrointestinal and respiratory symptoms in some patients, which could contribute to more hospitalization.
Most of the included studies focused on the impact of ICI use or not on the prognosis of COVID-19 [8,18–22], but they did not take other important factors into consideration, including courses of ICI use, intervals from the last dose to the diagnosis of COVID-19, and the effect of the first dose. Wu et al. [29] found that patients who received three or more cycles of ICI were more likely to develop severe COVID-19, albeit this difference was not statistically significant (6/7 [85.7%] vs 1/4 [25%], p = 0.09). Another study [23] included 69 patients and defined five groups according to the interval from last ICI receipt to COVID-19 diagnosis. Overall, there was no statistically significant difference in different groups in terms of the rate of hospitalization, severe disease or death.
As the influence of ICI on cancer patients infected with COVID-19 is not clear, there are no authoritative guidelines for ICI modifications in the context of COVID-19. Modifications of drug application are often empirical and based on the mechanism of drug action, taking tumor treatment and epidemic prevention into account [50]. Wang [51] et al. suggested that administration of anticancer drugs should be changed from infusion to oral administration if available. For maintenance therapy, we could appropriately prolong the infusion intervals according to patient condition. Aeppli et al. performed an online survey among physicians involved in the treatment of renal cell carcinoma [14]. Compared with that outside the pandemic, the use of ipilimumab/nivolumab fell by half in intermediate/poor-risk patients during the pandemic (80 vs 41%). In patients responding to established ICI-containing therapies, most participants modified treatment regimen by extending cycle length. Another survey focused on patient perspective on oncological care [52]. In patients with adjusted treatment, immunotherapy (32%) was most frequently adjusted. Consistently, in patients with delay and discontinuation of treatment (39 and 33%, respectively), immunotherapy was the most frequently included modality.
This study has important implications for clinical practice. Given that the pandemic may last for another several months or even years, physicians should balance cancer treatment and COVID-19 infection. Our results indicate that ICI administration increases the rate of hospitalization, though it is uncertain whether prior exposure to ICI increases the risk of severe disease and death. This suggests that we should not easily postpone, suspend or alter our established treatment decisions in clinical practice, especially for patients who are undergoing ICI-containing regimens, because ICI has irreplaceable performance in certain antitumor treatments [12]. Delay or modification of therapy should be considered on a case-by-case basis [53].
This systematic review and meta-analysis has several limitations. The most important limitation is that we could not rule out unknown confounders. Previous studies reported that age, sex, smoking and comorbidities, including pulmonary disease, cardiac disease, and hypertension, significantly affect the prognosis of COVID-19 infection. However, these potential confounders were not considered in most of the included studies. What is more, in the absence of a head-to-head comparison between ICIs, the choice to perform an evaluation as a new pharmacological class is theoretically unsound. However, among all the researches enrolled in the binary outcome, we found that none of the studies could provide prognosis information about the different ICI molecules. Second, due to the relatively small number of studies, we were unable to evaluate the effects of ICI subclasses or line of treatment or their role in individual tumors. Low proportion of patients treated with immunotherapy would unavoidably confound the meta-analysis results to some extent. Additionally, the benefit of longer duration of ICI on the overall survival has been determined, and frequent or early interruption of ICI has been proved to be associated with worse overall survival [12]. It will be worthwhile to comment on the cancer outcome in this population. However, limited by a short follow-up period, we failed to assess the cancer outcome in patients who had delayed or interrupted ICI treatment through published studies. Further, studies on the association between immune-related adverse events and COVID-19 risk, and outcome are needed. At last, included studies defined several intervals from the last dose to the diagnosis of COVID-19 infection, which may have influenced the findings.
Conclusion
The results of this meta-analysis suggest that a higher rate of hospitalization was observed among patients who were undergoing ICI-containing regimens, although it is uncertain whether prior exposure to ICI increases the risk of severe disease and death. Additionally, different intervals from last dose of ICI to the diagnosis of COVID-19 may not influence the prognosis of COVID-19 infection.
Summary points.
The influence of prior exposure to immune checkpoint inhibitors (ICIs) on COVID-19 infection remains largely unknown.
It is necessary to perform a meta-analysis to quantify the effects of ICI on the prognosis of COVID-19.
We systematically searched the PubMed, Embase and Web of Science databases.
We included studies that reported ICI use in cancer patients and their prognosis in the context of COVID-19.
Chi-squared and I2 tests were used to calculate heterogeneity among the included studies, and the choice of random or fixed effects model was made according to the heterogeneity.
Thirteen studies comprising 4614 cancer patients with COVID-19 were included for the systematic review and meta-analysis.
The pooled rate of hospitalization, severe disease, and mortality in patients with prior exposure to ICI was 0.45 (95% CI 0.15–0.78), 0.34 (95% CI 0.26–0.44) and 0.26 (95% CI 0.17–0.38), respectively.
When compared with cancer patients without prior ICI exposure, patients with prior ICI treatment exhibited a higher rate of hospitalization (odds ratio 2.0, 95% CI 1.19–3.38, p = 0.01).
No statistically significant difference in mortality was observed between patients exposed to ICI and other antitumor treatments in the context of COVID-19, except for the targeted therapy.
It is uncertain whether prior exposure to ICI increases the risk of severe disease and death but observed odds ratio suggest a higher rate of hospitalization.
Author contributions
Y Tian, W Qian and J Qian contributed to conception and design. Y Tian, W Qian, Y Ye, L Zuo and T Song contributed to acquisition of data. W Qian, Y Ye, L Zuo and Y Tian contributed to analysis and interpretation of data. W Qian, Y Tian, L Zuo, J Qian and Y Wang contributed to writing, review and revision of the manuscript. All authors contributed to final approval of manuscript.
Footnotes
Financial & competing interests disclosure
This study was supported by Natural Science Foundation of Shanghai (grant 19ZR1447100) and Foundation of Shanghai Dermatology Hospital (grants 17HBDS08 and 2018KYQD02). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Lai CC, Wang CY, Wang YH, Hsueh SC, Ko WC, Hsueh PR. Global epidemiology of coronavirus disease 2019 (COVID-19): disease incidence, daily cumulative index, mortality, and their association with country healthcare resources and economic status. Int. J. Antimicrob. Agents 55(4), 105946 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z, Mcgoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72–314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323(13), 1239–1242 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Rostami A, Sepidarkish M, Leeflang MMGet al. SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis. Clin. Microbiol. Infect. 27(3), 331–340 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jernigan DB. Update: public health response to the coronavirus disease 2019 outbreak – United States, February 24, 2020. MMWR Morb. Mortal. Wkly Rep. 69(8), 216–219 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sudre CH, Lee KA, Lochlainn MN. Symptom clusters in COVID-19: a potential clinical prediction tool from the COVID Symptom Study app. Sci. Adv. 7(12), eabd4177 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta A, Madhavan MV. Extrapulmonary manifestations of COVID-19. Nat. Med. 26(7), 1017–1032 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang W, Guan W, Chen Ret al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 21(3), 335–337 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai M, Liu D, Liu Met al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 10(6), 783–791 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta V, Goel S. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 10(7), 935–941 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sica A, Colombo MP, Trama A, Horn L, Garassino MC, Torri V. Immunometabolic status of COVID-19 cancer patients. Physiol. Rev. 100(4), 1839–1850 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J. Clin. Invest. 125(9), 3335–3337 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp. Mol. Med. 50(12), 1–11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michot JM, Bigenwald C, Champiat Set al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur. J. Cancer 54, 139–148 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Aeppli S, Eboulet EI, Eisen Tet al. Impact of COVID-19 pandemic on treatment patterns in metastatic clear cell renal cell carcinoma. EMSO Open 5(Suppl. 3), e000852 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25(9), 603–605 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Homma S, Thompson JL, Qian Met al. Quality of anticoagulation control in preventing adverse events in patients with heart failure in sinus rhythm: warfarin versus aspirin in reduced cardiac ejection fraction trial substudy. Circ. Heart Fail. 8(3), 504–509 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 327(7414), 557–560 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albiges L, Foulon S, Bayle Aet al. Determinants of the outcomes of patients with cancer infected with SARS-CoV-2: results from the Gustave Roussy cohort. Nat. Cancer 1(10), 965–975 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Bersanelli M, Giannarelli D, De Giorgi U. Symptomatic COVID-19 in advanced-cancer patients treated with immune-checkpoint inhibitors: prospective analysis from a multicentre observational trial by FICOG. Ther. Adv. Med. Oncol. 12, (2020) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lara OD, O'cearbhaill RE, Smith MJ, Sutter ME. COVID-19 outcomes of patients with gynecologic cancer in New York City. Cancer 126(19), 4294–4303 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee LYW, Cazier JB, Starkey T, Turnbull CD, Kerr R, Middleton G. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet 395(10241), 1919–1926 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lièvre A, Turpin A, Ray-Coquard Iet al. Risk factors for Coronavirus disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: a French nationwide cohort study (GCO-002 CACOVID-19). Eur. J. Cancer 141, 62–81 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo J, Rizvi H, Egger JV. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov. 10(8), 1121–1128 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Focuses on the impact of immune checkpoint inhibitor on severity of COVID-19 in patients with cancers and defines five groups according to the interval from last immune checkpoint inhibitor receipt to COVID-19 diagnosis.
- 24.Moritz RKC, Gutzmer R, Zimmer Let al. SARS-CoV-2 infections in melanoma patients treated with PD-1 inhibitors: a survey of the German ADOREG melanoma registry. Eur. J. Cancer 144, 382–385 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garde-Noguera J, Fernández-Murga ML, Giner-Bosch V. Impact of SARS-CoV-2 infection on patients with cancer: retrospective and transversal studies in Spanish population. Cancers (Basel) 12(12), 3513 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinato DJ, Zambelli A, Aguilar-Company J, Bower M. Clinical portrait of the SARS-CoV-2 epidemic in European cancer patients. Cancer Discov. 10(10), 1465–1474 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robilotti EV, Babady NE, Mead PA, Rolling T. Determinants of COVID-19 disease severity in patients with cancer. Nat. Med. 26(8), 1218–1223 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szabados B, Abu-Ghanem Y, Grant M, Choy J, Bex A, Powles T. Clinical characteristics and outcome for four SARS-CoV-2-infected cancer patients treated with immune checkpoint inhibitors. Eur. Urol. 78(2), 276–280 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Q, Chu Q, Zhang Het al. Clinical outcomes of coronavirus disease 2019 (COVID-19) in cancer patients with prior exposure to immune checkpoint inhibitors. Cancer Commun. (London) 40(8), 374–379 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brahmer JR, Drake CG, Wollner Iet al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 28(19), 3167–3175 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Im SJ, Hashimoto M, Gerner MYet al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537(7620), 417–421 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang AC, Postow MA, Orlowski RJet al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545(7652), 60–65 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fairfax BP, Taylor CA, Watson RA, Nassiri I, Danielli S, Fang H. Peripheral CD8(+) T cell characteristics associated with durable responses to immune checkpoint blockade in patients with metastatic melanoma. Nat. Med. 26(2), 193–199 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu TD, Madireddi S, De Almeida PEet al. Peripheral T cell expansion predicts tumour infiltration and clinical response. Nature 579(7798), 274–278 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Artigas C, Lemort M, Mestrez F, Gil T, Flamen P. COVID-19 pneumonia mimicking immunotherapy-induced pneumonitis on 18F-FDG PET/CT in a patient under treatment with nivolumab. Clin. Nucl. Med. 45(8), e381–e382 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guerini AE, Borghetti P, Filippi ARet al. Differential diagnosis and clinical management of a case of COVID-19 in a patient with stage III lung cancer treated with radio-chemotherapy and durvalumab. Clin. Lung Cancer 21(6), e547–e550 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lovly CM, Boyd KL, Gonzalez-Ericsson PIet al. Rapidly fatal pneumonitis from immunotherapy and concurrent SARS-CoV-2 infection in a patient with newly diagnosed lung cancer. medRxiv (2020) (Epub ahead of print). [Google Scholar]
- 38.Souza IL, Fernandes I, Taranto P, Buzaid AC, Schvartsman G. Immune-related pneumonitis with nivolumab and ipilimumab during the coronavirus disease 2019 (COVID-19) pandemic. Eur. J. Cancer 135, 147–149 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamboj M, Sepkowitz KA. Nosocomial infections in patients with cancer. Lancet Oncol. 10(6), 589–597 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Li JY, Duan XF, Wang LP, Xu YJ, Huang L. Selective depletion of regulatory T cell subsets by docetaxel treatment in patients with nonsmall cell lung cancer. J. Immunol. Res. 2014, 286170 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Longbottom ER, Torrance HD, Owen HCet al. Features of postoperative immune suppression are reversible with interferon gamma and independent of interleukin-6 pathways. Ann. Surg. 264(2), 370–377 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Sica A, Massarotti M. Myeloid suppressor cells in cancer and autoimmunity. J. Autoimmun. 85, 117–125 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Wang T, Du Z, Zhu Fet al. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet 395(10228), e52 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bersanelli M. Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy 12(5), 269–273 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi J, Lee SY. Clinical characteristics and treatment of immune-related adverse events of immune checkpoint inhibitors. 20(1), e9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang DY, Salem JE, Cohen JVet al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 4(12), 1721–1728 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rotz SJ, Leino D, Szabo S, Mangino JL, Turpin BK, Pressey JG. Severe cytokine release syndrome in a patient receiving PD-1-directed therapy. Pediatr. Blood Cancer 64(12), e26642 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Chen C, Zhang XR, Ju ZY, He WF. [Advances in the research of mechanism and related immunotherapy on the cytokine storm induced by coronavirus disease 2019]. Zhonghua Shao Shang Za Zhi 36(6), 471–475 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Xu Z, Shi L, Wang Yet al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 8(4), 420–422 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedlaender A, Kim C, Addeo A. Rethinking the optimal duration of immune checkpoint inhibitors in non-small cell lung cancer throughout the COVID-19 pandemic. Front. Oncol. 10, 862 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Z, Wang J, He J. Active and effective measures for the care of patients with cancer during the COVID-19 spread in China. JAMA Oncol. 6(5), 631–632 (2020). [DOI] [PubMed] [Google Scholar]
- 52.De Joode K, Dumoulin DW, Engelen Vet al. Impact of the coronavirus disease 2019 pandemic on cancer treatment: the patients' perspective. Eur. J. Cancer 136, 132–139 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fox TA, Troy-Barnes E, Kirkwood AA, Chan WY, Day JW. Clinical outcomes and risk factors for severe COVID-19 in patients with haematological disorders receiving chemo- or immunotherapy. Br. J. Haematol. 191(2), 194–206 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]