Abstract
Escherichia coli represents one of the most common causes of community-onset and nosocomial infections. Strains carrying extended spectrum β-lactamases (ESBL) are a serious public health problem. In Central America we have not found studies reporting the molecular epidemiology of E. coli strains implicated in local infections, so we conducted this study to fill that gap. Materials and Methods: We report on an epidemiological study in two reference hospitals from central Panama, identifying the susceptibility profile, associated risk factors, and molecular typing of E. coli strains isolated between November 2018 and November 2019 using Pasteur’s Multilocus Sequence Typing (MLST) scheme. Results: A total of 30 E. coli isolates with antimicrobial resistance were analyzed, 70% of which came from inpatients and 30% from outpatients (p < 0.001). Two-thirds of the samples came from urine cultures. Forty-three percent of the strains were ESBL producers and 77% were resistant to ciprofloxacin. We identified 10 different sequence types (STs) with 30% of the ESBL strains identified as ST43, which corresponds to ST131 of the Achtman MLST scheme—the E. coli pandemic clone. Thirty-eight percent of the E. coli strains with the ESBL phenotype carried CTX-M-15. Conclusions: To the best of our knowledge, this is the first report confirming the presence of the pandemic E. coli clone ST43/ST131 harboring CTX-M-15 in Central American inpatients and outpatients. This E. coli strain is an important antimicrobial-resistant organism of public health concern, with potential challenges to treat infections in Panama and, perhaps, the rest of Central America.
Keywords: molecular epidemiology, Escherichia coli, Panama, pandemic clone ST43/ST131, CTX-M-15
1. Introduction
Escherichia coli represents one of the most frequent causes of bacterial infections [1] and it accounts for 70% to 95% of community-onset acute urinary tract infections (UTIs) and 50% of nosocomial infections [2]. β-Lactam antibiotics and fluoroquinolones are widely prescribed to treat both community- and hospital-based infections caused by E. coli [3]. However, resistance to these categories of antibiotics has increased worldwide, which represents a major public health problem. For example, it has been reported that third-generation cephalosporins and fluoroquinolones have registered resistance rates greater than 50% in five of the six working regions of the World Health Organization (WHO) [1,4,5].
Among the E. coli strains, the principal mechanism of resistance to β-Lactams is the production of β-lactamase enzymes, all of which differ from each other based on their substrate profile, inhibitor profile, and sequence homology [5]. Extended-spectrum β-lactamases (ESBLs) are a group of enzymes that cause resistance to oxyminocephalosporins (i.e., cefotaxime, ceftazidime, cefuroxime, and cefepime) and monobactams (i.e., aztreonam), but not to cefamycins (i.e., cefoxitin) or carbapenems (i.e., imipenem, meropenem, and ertapenem) [5]. Several risk factors associated with ESBL-producing E. coli infections have been described in the community, including: previous use of antibiotics (especially quinolones and third- or fourth-generation cephalosporins), recurrent E. coli infections, recent hospitalization (within the prior year), artificial nutrition, previous admissions to intensive care units (ICUs), foster home stays, and receiving hemodialysis [6,7,8]. Strains of ESBL-producing E. coli are important antimicrobial-resistant organisms (AROs). In 2017, the WHO defined a list of priority AROs for research purposes, among which ESBL-producing Enterobacteriaceae are in priority group 1 [9].
The E. coli sequence type 43 (ST43) in Pasteur’s multilocus sequence typing (MLST) scheme—which corresponds to ST131 in the Achtman MLST scheme—constitutes a pandemic clonal lineage, responsible for a large number of multidrug resistant infections worldwide. The strains belonging to the ST131 clone differ in serotypes O25b and O16, which correspond, respectively, to STs PST43 and PST506 when using Pasteur’s MLST scheme [10,11]. Global emergence of E. coli ST131 isolates occurred approximately 30 years ago, most likely in a North American context and consistent with strong selection pressure exerted by the widespread introduction and use of extended-spectrum cephalosporins and fluoroquinolones [12,13]. The member strains of ST 131O25b (PST43) are disseminated worldwide and commonly reported in extraintestinal infections as producers of the ESBLs enzymes known as CTX-M-15 or CTX-M-14, which also exhibit resistance to fluoroquinolones [14,15,16,17]. On the basis of their amino acid sequence similarities, the CTX-M family have been classified into five major groups, named CTX-M group-1, -2, -8, -9, and 25/26. CTX-M-15 (of the CTX-M-1 group) and CTX-M-14 (of the CTX-M-9 group) are the most frequent enzymes isolated. The CTX-M-15 β-lactamase is the dominant ESBL in ST131 and is increasingly found in isolates causing both UTI and bacteremia [14]. This dissemination has increased both in the hospital environment and in the community [18]. Some studies from Latin American countries have described the presence of ST131, both in hospitals and the community [19,20,21,22]. In Ecuador, a study of E. coli serotype O25 carrying ESBL belonged to ST 131/PST43 and resulted in carriers of CTX-M-15 [23]. However, to the best of our knowledge, there are still no reports on the molecular epidemiology of infection-causing E. coli strains in Central America [24]. The purpose of this study was to characterize strains of E. coli implicated in infections in hospitalized and outpatients in two reference hospitals from central Panama. By analyzing the E. coli isolates from hospital-based clinical laboratories we aim to identify the susceptibility profile, associated risk factors, characterization of ESBL-producing strains and molecular typing using Pasteur’s MLST scheme.
2. Materials and Methods
2.1. Study Setting
We conducted a prospective epidemiological study between November 2018 and November 2019 in two reference hospitals in central Panama: The Cecilio Castillero General Hospital (CCGH) and the Luis “Chicho” Fábrega Hospital (LCFH), located in the provinces of Herrera and Veraguas, respectively. Both hospitals are public assistance hospitals and represent the main centers that provide medical and laboratory care in the central region of Panama.
2.2. Isolates of E. coli
During the study period, we included E. coli samples that (a) were isolated from diverse samples from outpatients and hospitalized patients, within the first 48 h of admission, (b) were collected as part of routine patient care procedures, and (c) showed resistance to at least one of the antibiotics routinely tested in the hospitals’ clinical microbiology laboratories. The in vitro antimicrobial activity was determined using the Vitek 2 system through Gram-negative susceptibility cards AST-GN69 (Ref. 413 400) and AST-N250 (Ref. 413 573) (BioMérieux; Marcy l’Etoile, France). The test results were interpreted according to breakpoints defined by the Clinical and Laboratory Standards Institute (CLSI) [25].
A technical sheet was completed anonymously for each sample collected, recording the following risk factors: age, sex, hospitalization for 2 or more days in the prior 90 days, antibiotic treatment in the prior 90 days, personal history of immunosuppressive therapy (International Classification of Diseases ICD-10, code Z92.25), wound care at home, hemodialysis within the prior 90 days, and outpatient chemotherapy. The data recorded were captured in MS Excel (The Microsoft Corporation; Redmond, WA, USA). Data analyses were conducted in Stata v. 11.0 (StataCorp, LLC; College Station, TX, USA). We calculated descriptive statistics and estimates with their respective 95% confidence intervals (Cis). We used Fisher’s exact test to compare proportions, setting alpha at 0.05 for statistical significance when comparing the frequencies of ESBL- and non-ESBL-producing isolates.
2.3. Molecular Typing Analyses and β-Lactamase Molecular Identification
Molecular typing analyses were performed using Pasteur’s MLST scheme. We conducted MLST schemes using a standardized protocol specific for E. coli [26]. The sequencing internal fragments of eight housekeeping genes (i.e., dinb, icdA, pabB, polB, putB, trpA, trpB, and uidA) were amplified from chromosomal DNA of the E. coli strain. Sequencing of the polymerase chain reaction (PCR) products was performed using the services of Macrogen (Macrogen Inc.; Seoul, Korea). Sequences of the genes were analyzed by Geneious prime v. 2020.5 (Biomatters, Ltd.; Auckland, New Zealand) and the allelic profile was determined using E. coli MLST datababes (https://bigsdb.pasteur.fr/ecoli/ecoli.html, accessed on 03 May 2021) [27].
All isolates with phenotype ESBL were screened for blaSHV, blaTEM and blaCTX-M gene families by a PCR assay using specific primers as previously described [28]. blaCTX-M groups 1, 2, 8, 9, 25 and group 1 variant (CTX-M-15) were identified by a PCR assay using specific primers as previously described [29,30].
3. Results
A total of 30 isolates of E. coli with antimicrobial resistance were analyzed in this study, 20 (67%) of which came from urine cultures, and 5 each (16%) from blood and wound cultures (p < 0.001). Twenty-one samples came from hospitalized patients and nine came from outpatients (p < 0.001). In general, most (19) patients were female (p < 0.001). The mean age was 57.26 (95% CI [46.82, 68.70]) years.
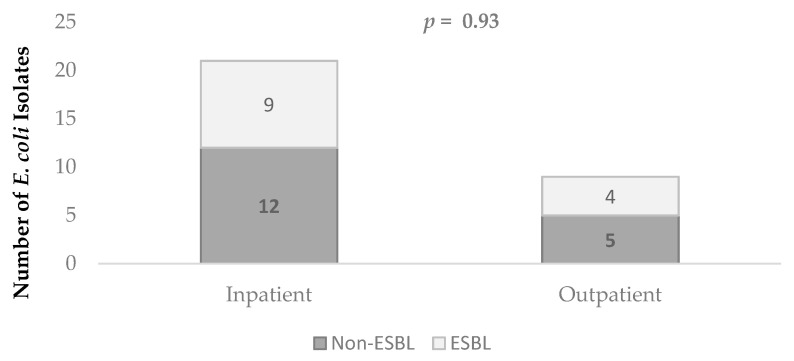
The antimicrobial resistance of the E. coli strains was as follows: 100% were susceptible to carbapenems (i.e., meropenem, imipenem, and ertapenem) and nitrofurantoin; while 13 (43%) were ESBL producers and 17 (57%) were non-ESBL producers. Figure 1 depicts the distribution of ESBL-producing versus non-producing E. coli strains according to patient type (inpatient and outpatient) (p = 0.93). Table 1 compares the percentage of antimicrobial resistance of ESBL-producing versus non-ESBL-producing strains. There were no statistically significant differences between the percentages of resistance to ciprofloxacin (p = 0.376), trimethoprim-sulfamethoxazole (TMP-SMX) (p = 0.184), and gentamicin (p = 0.209) between ESBL-producing and non-ESBL-producing strains. Although comparison of resistances to nalidixic acid did not reach statistical significance (p = 0.083), resistance among non-ESBL-producing E. coli strains tended to be higher than among ESBL-producing E. coli strains.
Figure 1.
Distribution of ESBL-producing versus non-producing E. coli strains according to patient type. ESBL: extended-spectrum β-lactamase.
Table 1.
Antimicrobial resistance of Escherichia coli isolates.
| Antimicrobial Agent | MIC Breakpoint (µg/mL) | Escherichia coli Isolates by Resistance, n (%) | ||
|---|---|---|---|---|
| Total (n = 30) | ESBL (n = 13) | Non-ESBL (n = 17) | ||
| Ampicillin | ≥32 | 27 (90) | 13 (100) | 14 (82) |
| Piperacillin-tazobactam | ≥128/4 | 1 (3) | 0 (0) | 1 (6) |
| Cephalothin | ≥64 | 20 (67) | 13 (100) | 7 (41) |
| Cefuroxime | ≥64 | 16 (53) | 13 (100) | 3 (18) |
| Cefotaxime | ≥64 | 13 (43) | 13 (100) | 0 (0) |
| Ceftazidime | ≥64 | 13 (43) | 13 (100) | 0 (0) |
| Cefepime | ≥64 | 13 (43) | 13 (100) | 0 (0) |
| Amikacin | ≥16 | 2 (7) | 1 (8) | 1 (6) |
| Gentamicin | ≥16 | 8 (27) | 5 (38) | 3 (18) |
| Nalidixic acid | ≥32 | 17 (57) | 5 (38) | 12 (71) |
| Ciprofloxacin | ≥4 | 23 (77) | 11 (85) | 12 (71) |
| Trimethoprim-sulfamethoxazole | ≥320 | 19 (63) | 10 (77) | 9 (53) |
ESBL: extended-spectrum β-lactamase; MIC: minimum inhibitory concentration.
Among the risk factors associated with infections by ESBL-producing E. coli, we observed that 100% of the strains registered at least one of the six risk factors assessed (Table 2). Regarding non-ESBL-producing strains, 35% did not register any of the risk factors analyzed (p = 0.021). The mean age of the patients with E. coli who did not carry ESBL was 49.23 years and of the ESBL carriers was 66.46 years (p = 0.023). The distribution of the strains by age group is observed in Table 2, identifying that the age group most affected by ESBL-producing strains was the one older than 80 years. No significant differences were found in the distribution by sex when comparing both groups.
Table 2.
Identification of risk factors potentially associated with infections by ESBL-producing Escherichia coli.
| Variables, n (%) | ESBL (n = 13) | Non-ESBL (n = 17) | p Value |
|---|---|---|---|
| Age Groups, Years | 0.09 | ||
| 1–19 | 2 (15) | 3 (18) | |
| 20–59 | 1 (8) | 3 (18) | |
| 60–79 | 3 (23) | 9 (53) | |
| ≥80 | 7 (54) | 2 (12) | |
| Sex | 0.42 | ||
| Female | 9 (69) | 10 (59) | |
| Male | 4 (31) | 7 (41) | |
| Risk Factors | |||
| Hospitalized ≥2 d in the prior 90 d | 8 (62) | 7 (41) | 0.28 |
| Antibiotic treatment in the prior 90 d | 7 (54) | 5 (29) | 0.50 |
| Wound care at home | 2 (15) | 0 (0) | 0.10 |
| Outpatient chemotherapy | 1 (8) | 1 (6) | 0.85 |
| Personal history of immunosuppressive therapy | 1 (8) | 0 (0) | 0.25 |
| Hemodialysis in the prior 90 d | 1 (8) | 0 (0) | 0.25 |
| No known risk factors | 0 (0) | 6 (35) | 0.021 |
d: days; ESBL: extended-spectrum β-lactamase.
We found that 92% (12/13) of the ESBL-producing strains carried the CTX-M type. CTX-M group 1 was found in 46% (6/13), (Table 3) whose variant CTX-M-15 was found in 83% (5/6) of them. In addition, CTX-M group 9 genes were found in 46% (6/13) and TEM was identified in 38% (5/13). No SHV genes were identified.
Table 3.
Phenotypic and genotypic characteristics of Escherichia coli isolates.
| Isolate | Sequence Typing | ESBL | β-Lactamases | Originating Site | Source | Phenotypic Profile |
|---|---|---|---|---|---|---|
| 378 | 3 | + | CTX-M-group 9, TEM | H (Surg) | Wound | AMP, CEF, CFZ, CXM, CTX, CAZ, FEP, STX |
| 2724 | 3 | - | Outpatient | Urine | AMP, CEF | |
| 439 | 4 | - | H (Peds) | Urine | AMP, STX | |
| 1232 | 43 | + | CTX-M-group-1 (CTX-M-15) | H (Ort) | Wound | AMP, CEF, CFZ, CXM, CTX, CAZ, FEP, GEN, CIP |
| 2690 | 43 | + | CTX-M-group 9, TEM | Outpatient | Urine | AMP, CEF, CFZ, CXM, CTX, CAZ, FEP, NAL, CIP, STX |
| 0-3630 | 43 | + | CTX-M-group 9, TEM | Outpatient | Urine | AMP, CEF, CFZ, CXM, CTX, CAZ, FEP, NAL, CIP, STX |
| 640 | 53 | + | CTX-M group 9 | H (Surg) | Urine | AMP, CEF, CFZ, CXM, CTX, CAZ, FEP, CIP |
| 370 | 53 | - | H (Ob-Gyn) | Urine | AMP, TZP, CEF, CXM, CAZ, AMK, NAL, CIP | |
| 2685 | 53 | - | Outpatient | Urine | AMP, CEF, NAL, CIP, STX | |
| 2710 | 458 | + | CTX-M-group 9 | Outpatient | Urine | AMP, CEF, CFZ, CXM, CTX, CAZ, FEP, NAL, CIP, STX |
| 19-2410 | 458 | - | H (IM) | Blood | AMP, CIP, STX | |
| 2699 | 458 | - | Outpatient | Urine | AMP, CEF, NAL, CIP | |
| 3627 | 479 | - | H (Surg) | Urine | AMP, GEN, NAL, CIP, STX | |
| 382 | 526 | + | CTX-M-group-1 (CTX-M-15) | H (IM) | Wound | AMP, CEF, CFZ, CXM, CTX, CAZ, FEP, STX |
| HRV-09 | 594 | + | ND | I (ICU) | Wound | AMP, CEF, CFZ, CXM, CTX, CAZ, FEP, GEN, CIP, STX |
| 361 | 621 | + | CTX-M-group-1 (CTX-M-15) | H (IM) | Urine | AMP, CEF, CFZ, CXM, CTX, CAZ, FEP, CIP, STX |
| 2676 | 833 | + | CTX-M-group-1, TEM | Outpatient | Urine | AMP, CEF, CFZ, CXM, CTX, CAZ, FEP, GEN, NAL, CIP, STX |
| 542 | 833 | - | H (IM) | Blood | AMP, NAL, CIP, STX | |
| 375 | N/A | + | CTX-M-group-1 (CTX-M-15), TEM | H (Surg) | Urine | AMP, CEF, CFZ, CXM, CTX, CAZ, FEP, NAL, GEN, CIP |
| 638 | N/A | + | CTX-M-group-1 (CTX-M-15) | H (Surg) | Urine | AMP, CEF, CFZ, CXM, CTX, CAZ, FEP, GEN, CIP, STX |
| CC2 | N/A | + | CTX-M-group 9 | H (IM) | Urine | AMP, CEF, CFZ, CXM, CTX, CAZ, FEP, CIP, STX |
| 435, 655 | N/A | - | H(IM) | Urine | AMP, NAL, CIP, STX | |
| 543, 544 | N/A | - | H (Ob-Gyn) | Blood | AMP, NAL, CIP, STX | |
| 545 | N/A | - | H (IM) | Blood | AMP, CIP, STX | |
| O-2115 | N/A | - | Outpatient | Urine | AMP, CIP, STX | |
| CC1 | N/A | - | H (IM) | Urine | NAL | |
| 519 | N/A | - | H (Ob-Gyn) | Wound | AMP, NAL | |
| 436 | N/A | - | Outpatient | Urine | AMP |
AMP: ampicillin; AMK: amikacin; CAZ: ceftazidime; CEF: cephalothin; CFZ: cefazoline; CIP: ciprofloxacin; CTX: cefotaxime; CXM: cefuroxime; ESBL: extended-spectrum β-lactamase; FEP: cefepime; GEN: gentamicin; ICU: intensive care unit ward; IM: internal medicine ward; NAL: nalidixic acid; SXT: trimethoprim-sulfamethoxazole; TZP: piperacillin-tazobactam; ND: not detected; Ob-Gyn: obstetrics and gynecology ward; Ort: orthopedics ward; Peds: pediatric ward; Surg: surgery ward; +: ESBL; -: non-ESBL; N/A: not applicable.
Table 3 describes the results from the molecular typing using Pasteur’s MLST technique. Of the 10 types of STs in the 30 E. coli samples analyzed, the most frequent were ST43, ST53, ST458 (17% each) and ST833 and ST3 (11% each). In 5.4% of the cases we identified ST4, ST479, ST526, ST621, and ST594. Among the ESBL-producing strains, ST43 of the Pasteur scheme—corresponding to ST131 in Achtman’s MLST scheme—was the most frequently identified ST (30%). The STs identified among outpatients were: ST43, ST53, ST458, and ST3.
4. Discussion
Worldwide, ESBL-producing E. coli strains have been increasingly reported in frequency and severity, making it an important ARO [2] that impacts the duration of hospital stays, delays appropriate antibiotic therapy, and increases healthcare costs [31]. In this study, E. coli strains isolated from clinical samples and showing a phenotype of antimicrobial resistance were analyzed. We observed that of the total isolates, 43% were producers of ESBL and 77% were resistant to ciprofloxacin, urinary tract infections being the main anatomical site of origin (67%) of the samples. In Latin America, the percentage of ESBL-producing E. coli strains is estimated at 24.7%, which has increased in the last decade, exceeded only by the Asia-Pacific region [24,31,32]. Regarding ciprofloxacin, the percentage of resistance in E. coli strains has been reported in 40.2% [31,32]. Panama reported for 2010 a percentage of ESBL-producing strains between 8–16% and 40.2% of strains resistant to fluoroquinolones among isolates of E. coli [24]. This high prevalence of resistance to these antimicrobials represents a great public health problem because both groups of antibiotics are widely used in the treatment of infections associated with E. coli [32].
An ST131 clone detected through the Achtman scheme accounts for nearly two-thirds of ESBL-producing isolates and for approximately 70 to 80% of fluoroquinolone-resistant isolates [33]. Our analysis using Pasteur’s MLST scheme showed the presence of this pandemic clone ST43/ST131 in 30% of the ESBL-producing E. coli strains, both in inpatients and outpatients. The ST43 clone has been described as specific for O25b/ST131, which was identified in 2008 as an important clone linked to CTX-M-15, strongly associated with fluoroquinolone resistance and co-resistance to fluoroquinolones, aminoglycosides, and TMP-SMX [34]. This coincides with our results that all the ST43 (ST131) strains identified in this study were resistant to ciprofloxacin (3/3), two strains (2/3) presented resistance to TMP/SMX and one strain (1/3) presented resistance to gentamicin. Within the three E. coli strains of the pandemic clone ST43/ST131 identified in this study, we found that one strain carried the CTX-M-15 enzyme and two strains carried enzymes corresponding to CTX-M-group 9. The literature describes that CTX-M15 (group-1) and CTX-M-14 (group-9) enzymes are the most commonly identified enzymes in the ST43/ST131 pandemic clone [16,17]. Recent Latin American studies have shown an increase in the prevalence of this group of enzymes in E. coli isolates [34,35,36,37]. In Latin America, clinical isolate studies have been carried out where the dissemination of the ST43/ST131 clone is evidenced in several countries both at the hospital and community level [17,23,24], however, in the Central American region there is little data regarding the identification of this clone. Studies have shown a prevalence of E. coli ST131 among non-ESBL-producing isolates of between 10 and 13% [8], however, in our analyzed strains, all strains belonging to this clone were carriers of ESBL. The frequency of ESBL-producing E. coli strains among inpatients and outpatients was quite similar (Figure 1). Previous studies have reported similar results indicating that ESBL-producing E. coli strains are increasing their dissemination in the community [4,38].
The migration of ESBL-producing Enterobacteriaceae from the hospital to the community setting is on the rise, for which the emergence and spread of CTX-M-type ESBLs throughout the world stands out [4]. Our data reflected that all patients from whom ESBL-bearing E. coli was isolated, were exposed to at least one risk factor associated with ESBL-producing E. coli infections in the community (Table 2), the most prevalent risk factors being a history of previous antibiotic use and previous hospitalization. Studies have shown that in adults with a history of previous antibiotic intake without specifying the classes, they present a risk factor for urinary infection due to ESBL-producing E. coli with an OR (odds ratio) that ranges between 3.1 and 5.6 in adults, while previous hospitalization has been evidenced as a risk factor with an OR that ranges between 1.7 and 3.9, which is influenced by the number of previous hospitalizations and the time elapsed [38]. Similarly, it has been described that people over 55 years of age without risk factors associated with medical care, have a risk two times higher (OR 2.05) of developing a UTI secondary to an ESBL-producing E. coli [39]. In our study, patients older than 80 years presented greater isolation of ESBL-producing E. coli strains.
Our analysis has shown the ST identified in the pediatric population as ST4, which showed resistance to ampicillin, ampicillin-sulbactam, and to TMP-SMX. Logan et al. [40] conducted a study in children and the identified ST of E. coli was ST4, which was described in the phylogroup B2. The authors described two ST4 isolates: in one the ESBL gene CTX-M-1 was identified and in the other, no ESBL genes were detected. This scenario alerts us to the potential difficulties in managing multidrug-resistant E. coli infections in pediatric patients.
As a limitation of the present study, we mention that it has been very difficult to obtain clinical samples due to the low number of requests for cultures by the treating physicians, as well as the low availability of human resources and infrastructure for the processing of cultures in the clinical laboratories of hospitals in Panama. Another limitation of this study is the small sample size, thus the interpretation for its implications, including the risk factor considerations should be prudent.
5. Conclusions
This study makes important contributions to the knowledge of the microbiology, molecular identification of β-lactamases, molecular epidemiology of E. coli strains in Panama, and identifies for the first time the pandemic clone ST43/ST131 harboring CTX-M-15 in the country, which calls our attention to the potential difficulties of treating infections of hospital and community origin, as well as the importance of knowing the composition and distribution of antibiotic resistance genotypes as an important step towards establishing public policies aimed at delimiting the impact of E. coli ARO infections in Panama.
Acknowledgments
The authors would like to acknowledge Humberto López Castillo for his review of the manuscript. The authors also wish to express their gratitude to Lucila Ka, Aracelis Bernal and Aris Jiménez-Domínguez for their contribution in the collection of some isolates of E. coli. Iván Landires is a member of the Sistema Nacional de Investigación (SNI), which is supported by Panama’s Secretaría Nacional de Ciencia, Tecnología e Innovación (SENACYT).
Author Contributions
Conceptualization, V.N.-S. and I.L.; methodology, V.N.-S., M.P., G.P.-P., Y.Q., M.H. and I.L.; software, V.N.-S., M.P., G.P.-P., Y.Q., M.H. and I.L.; validation, V.N.-S., M.P., G.P.-P., Y.Q., M.H. and I.L.; formal analysis, V.N.-S., M.P., G.P.-P., Y.Q., M.H. and I.L.; investigation, V.N.-S., M.P., G.P.-P., Y.Q., M.H. and I.L.; resources, V.N.-S., M.P., G.P.-P., Y.Q., M.H. and I.L.; data curation, V.N.-S. and I.L.; writing—original draft preparation, V.N.-S., M.P. and I.L.; writing—review and editing, V.N.-S., M.P., G.P.-P., Y.Q., M.H. and I.L.; visualization, V.N.-S., M.P., G.P.-P., Y.Q., M.H. and I.L.; supervision, V.N.-S. and I.L.; project administration, V.N.-S. and I.L.; funding acquisition, V.N.-S. and I.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was made possible through support from the Panama’s Secretaría Nacional de Ciencia, Tecnología e Innovación (SENACYT), Project ITE15-010.
Institutional Review Board Statement
The study protocol was reviewed and approved by the Bioethics Committee at the University of Panama (No. CBIUP/070/17, dated 10 February 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization . Antimicrobial Resistance: Global Report on Surveillance. World Health Organization; Geneva, Switzerland: 2014. [Google Scholar]
- 2.Pitout J.D.D. Extraintestinal pathogenic Escherichia coli: A combination of virulence with antibiotic resistance. Front. Microbiol. 2012;3:9. doi: 10.3389/fmicb.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pitout J.D.D. Infections with extended-spectrum β-lactamase-producing enterobacteriaceae: Changing epidemiology and drug treatment choices. Drugs. 2010;70:313–333. doi: 10.2165/11533040-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Chong Y., Shimoda S., Shimono N. Current epidemiology, genetic evolution and clinical impact of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect. Genet. Evol. 2018;61:185–188. doi: 10.1016/j.meegid.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Peirano G., Pitout J.D.D. Extended-spectrum β-lactamase-producing Enterobacteriaceae: Update on molecular epidemiology and treatment options. Drugs. 2019;79:1529–1541. doi: 10.1007/s40265-019-01180-3. [DOI] [PubMed] [Google Scholar]
- 6.Cassier P., Lallechère S., Aho S., Astruc K., Neuwirth C., Piroth L., Chavanet P. Cephalosporin and fluoroquinolone combinations are highly associated with CTX-M β-lactamase-producing Escherichia coli: A case-control study in a French teaching hospital. Clin. Microbiol. Infect. 2011;17:1746–1751. doi: 10.1111/j.1469-0691.2010.03349.x. [DOI] [PubMed] [Google Scholar]
- 7.Calbo E., Romaní V., Xercavins M., Gómez L., Vidal C.G., Quintana S., Vila J., Garau J. Risk factors for community-onset urinary tract infections due to Escherichia coli harbouring extended-spectrum β-lactamases. J. Antimicrob. Chemother. 2006;57:780–783. doi: 10.1093/jac/dkl035. [DOI] [PubMed] [Google Scholar]
- 8.Saely S., Kaye K.S., Fairfax M.R., Chopra T., Pogue J.M. Investigating the impact of the definition of previous antibiotic exposure related to isolation of extended spectrum β-lactamase-producing Klebsiella pneumoniae. Am. J. Infect. Control. 2011;39:390–395. doi: 10.1016/j.ajic.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. World Health Organization; Geneva, Switzerland: 2017. [(accessed on 3 December 2020)]. Available online: http://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/ [Google Scholar]
- 10.Clermont O., Gordon D., Denamur E. Guide to the various phylogenetic classification schemes for Escherichia coli and the correspondence among schemes. Microbiology. 2015;161 Pt 5:980–988. doi: 10.1099/mic.0.000063. [DOI] [PubMed] [Google Scholar]
- 11.Johnson J.R., Clermont O., Johnston B., Clabots C., Tchesnokova V., Sokurenko E., Junka A.F., Maczynska B., Denamur E. Rapid and specific detection, molecular epidemiology, and experimental virulence of the O16 subgroup within Escherichia coli sequence type 131. J. Clin. Microbiol. 2014;52:1358–1365. doi: 10.1128/JCM.03502-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoesser N., Sheppard A.E., Pankhurst L., De Maio N., Moore C.E., Sebra R., Turner P., Anson L.W., Kasarskis A., Batty E.M., et al. Evolutionary History of the Global Emergence of the Escherichia coli Epidemic Clone ST131. MBio. 2016;7:e02162-15. doi: 10.1128/mBio.02162-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben Zakour N.L., Alsheikh-Hussain A.S., Ashcroft M.M., Khanh Nhu N.T., Roberts L.W., Stanton-Cook M., Schembri M.A., Beatson S.A. Sequential Acquisition of Virulence and Fluoroquinolone Resistance Has Shaped the Evolution of Escherichia coli ST131. MBio. 2016;7:e00347-16. doi: 10.1128/mBio.00347-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price L.B., Johnson J.R., Aziz M., Clabots C., Johnston B., Tchesnokova V., Nordstrom L., Billig M., Chattopadhyay S., Stegger M., et al. The epidemic of extended-spectrum-β-lactamase-producing Escherichia coli ST131 is driven by a single highly pathogenic subclone, H30-Rx. MBio. 2013;4:e00377-13. doi: 10.1128/mBio.00377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petty N.K., Ben Zakour N.L., Stanton-Cook M., Skippington E., Totsika M., Forde B.M., Phan M.D., Gomes Moriel D., Peters K.M., Davies M., et al. Global dissemination of a multidrug resistant Escherichia coli clone. Proc. Natl. Acad. Sci. USA. 2014;111:5694–5699. doi: 10.1073/pnas.1322678111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bevan E.R., Jones A.M., Hawkey P.M. Global epidemiology of CTX-M β-lactamases: Temporal and geographical shifts in genotype. J. Antimicrob. Chemother. 2017;72:2145–2155. doi: 10.1093/jac/dkx146. [DOI] [PubMed] [Google Scholar]
- 17.Blanco V.M., Maya J.J., Correa A., Perenguez M., Muñoz J.S., Motoa G., Pallares C.J., Rosso F., Matta L., Celis Y., et al. Prevalence and risk factors for extended-spectrum β-lactamase-producing Escherichia coli causing community-onset urinary tract infections in Colombia. Enferm. Infecc. Microbiol. Clin. 2016;34:559–565. doi: 10.1016/j.eimc.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lahlaoui H., Ben Haj Khalifa A., Ben Moussa M. Epidemiology of Enterobacteriaceae producing CTX-M type extended spectrum β-lactamase (ESBL) Med. Mal. Infect. 2014;44:400–404. doi: 10.1016/j.medmal.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 19.den Reijer P.M., van Burgh S., Burggraaf A., Ossewaarde J.M., van der Zee A. The widespread presence of a multidrug-resistant Escherichia coli ST131 clade among community-associated and hospitalized patients. PLoS ONE. 2016;11:e0150420. doi: 10.1371/journal.pone.0150420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leal A.L., Cortés J.A., Arias G., Ovalle M.V., Saavedra S.Y., Buitrago G., Escobar J.A., Castro B.E., GREBO Emergence of resistance to third generation cephalosporins by Enterobacteriaceae causing community-onset urinary tract infections in hospitals in Colombia. Enferm. Infecc. Microbiol. Clin. 2013;31:298–303. doi: 10.1016/j.eimc.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Peirano G., Asensi M.D., Pitondo-Silva A., Pitout J.D.D. Molecular characteristics of extended-spectrum β-lactamase-producing Escherichia coli from Rio de Janeiro, Brazil. Clin. Microbiol. Infect. 2011;17:1039–1043. doi: 10.1111/j.1469-0691.2010.03440.x. [DOI] [PubMed] [Google Scholar]
- 22.Ruiz S.J., Montealegre M.C., Ruiz-Garbajosa P., Correa A., Briceño D.F., Martinez E., Rosso F., Muñoz M., Quinn J.P., Cantón R., et al. First characterization of CTX-M-15-producing Escherichia coli ST131 and ST405 clones causing community-onset infections in South America. J. Clin. Microbiol. 2011;49:1993–1996. doi: 10.1128/JCM.00045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiluisa-Guacho C., Escobar-Perez J., Dutra-Asensi M. First detection of the CTXM-15 producing Escherichia coli O25-ST131 pandemic clone in Ecuador. Pathogens. 2018;7:42. doi: 10.3390/pathogens7020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guzmán-Blanco M., Labarca J.A., Villegas M.V., Gotuzzo E., Latin American Working Group on Bacterial Resistance Extended spectrum β-lactamase producers among nosocomial Enterobacteriaceae in Latin America. Braz. J. Infect. Dis. 2014;18:421–433. doi: 10.1016/j.bjid.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2020. (CLSI Supplement M100). [Google Scholar]
- 26.Jaureguy F., Landraud L., Passet V., Diancourt L., Frapy E., Guigon G., Carbonnelle E., Lortholary O., Clermont O., Denamur E., et al. Phylogenetic and genomic diversity of human bacteremic Escherichia coli strains. BMC Genom. 2008;9:560. doi: 10.1186/1471-2164-9-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dierikx C.M., van Duijkeren E., Schoormans A.H.W., van Essen Zandbergen A., Veldman K., Kant A., Huijsdens X.W., van der Zwaluw K., Wagenaar J.A., Mevius D.J. Occurrence and characteristics of extended-spectrum-β-lactamase and AmpC-producing clinical isolates derived from companion animals and horses. J. Antimicrob. Chemother. 2012;67:1368–1374. doi: 10.1093/jac/dks049. [DOI] [PubMed] [Google Scholar]
- 28.Dallenne C., Da Costa A., Decré D., Favier C., Arlet G. Development of a set of multiplex PCR assays for the detection of genes encoding important β-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 2010;65:490–495. doi: 10.1093/jac/dkp498. [DOI] [PubMed] [Google Scholar]
- 29.Chen W.-C., Hung C.-H., Chen Y.-S., Cheng J.-S., Lee S.S.-J., Tseng F.-C., Cheng M.-F., Wang J.-L. Bloodstream infections caused by extended-spectrum β-lactamase-producing Escherichia coli in patients with liver cirrhosis. Pathogens. 2021;10:37. doi: 10.3390/pathogens10010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Institut Pasteur Escherichia coli Sequence Typing. [(accessed on 9 December 2020)]; Available online: https://bigsdb.pasteur.fr/ecoli/ecoli.html.
- 31.Boucher H.W., Talbot G.H., Bradley J.S., Edwards J.E., Gilbert D., Rice L.B., Scheld M., Spellberg B., Bartlett J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 32.Barnes A.I., Paraje M.G. Break-down of antibiotic prescription in health centres by infection concerned. Aten Primaria. 2006;37:421–442. doi: 10.1157/13087387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banerjee R., Johnson J.R. A new clone sweeps clean: The enigmatic emergence of Escherichia coli sequence type 131. Antimicrob. Agents Chemother. 2014;58:4997–5004. doi: 10.1128/AAC.02824-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De La Cadena E., Mojica M.F., Castillo N., Correa A., Appel T.M., García-Betancur J.C., Pallares C.J., Villegas M.V. Genomic analysis of CTX-M-group-1-producing extraintestinal pathogenic E. coli (ExPEc) from patients with urinary tract infections (UTI) from Colombia. Antibiotics. 2020;9:899. doi: 10.3390/antibiotics9120899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pavez M., Troncoso C., Osses I., Salazar S., Illescac V., Reydet P., Rodríguezc C., Chahind C., Concha C., Barrientos L. High prevalence of CTX-M-1 group in ESBL-producing enterobacteriaceae infection in intensive care units in southern Chile. Braz. J. Infect. Dis. 2019;23:102–110. doi: 10.1016/j.bjid.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pereira J.L., Volcão L.M., Klafke G.B., Vieira R.S., Gonçalves C.V., Ramis I.V., Almeida da Silva P.E., von Groll A. Antimicrobial resistance and molecular characterization of extended-spectrum β-lactamases of Escherichia coli and Klebsiella spp. Isolates from urinary tract infections in southern Brazil. Microb. Drug Resist. 2019;25:173–181. doi: 10.1089/mdr.2018.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peirano G., van der Bij A.K., Freeman J.L., Poirel L., Nordmann P., Costello M., Tchesnokova V.L., Pitout J.D. Characteristics of Escherichia coli sequence type 131 isolates that produce extended-spectrum β-lactamases: Global distribution of the H30-Rx sublineage. Antimicrob. Agents Chemother. 2014;58:3762–3767. doi: 10.1128/AAC.02428-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larramendy S., Deglaire V., Dusollier P., Fournier J.P., Caillon J., Beaudeau F., Moret L. Risk factors of extended-spectrum β-lactamases-producing Escherichia coli community acquired urinary tract infections: A systematic review. Infect. Drug Resist. 2020;13:3945–3955. doi: 10.2147/IDR.S269033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park S.H., Choi S.M., Lee D.G., Cho S.Y., Lee H.J., Choi J.K., Choi J.H., Yoo J.H. Impact of extended-spectrum β-lactamase production on treatment outcomes of acute pyelonephritis caused by Escherichia coli in patients without health care-associated risk factors. Antimicrob. Agents Chemother. 2015;59:1962–1968. doi: 10.1128/AAC.04821-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Logan L.K., Hujer A.M., Marshall S.H., Domitrovic T.N., Rudin S.D., Zheng X., Qureshi N.K., Hayden M.K., Scaggs F.A., Karadkhele A., et al. Analysis of β-lactamase resistance determinants in Enterobacteriaceae from Chicago children: A multicenter survey. Antimicrob. Agents Chemother. 2016;60:3462–3469. doi: 10.1128/AAC.00098-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available within the article.