Abstract
Leishmaniasis, a Neglected Tropical Parasitic Disease (NTPD), is induced by several Leishmania species and is disseminated through sandfly (Lutzomyia longipalpis) bites. The parasite has developed resistance to currently prescribed antileishmanial drugs, and it has become pertinent to the search for new antileishmanial agents. The current study aimed to investigate the in vitro and in silico antileishmanial activity of two newly sourced actinomycins, X2 and D, produced by the novel Streptomyces smyrnaeus strain UKAQ_23. The antileishmanial activity conducted on promastigotes and amastigotes of Leishmania major showed actinomycin X2 having half-maximal effective concentrations (EC50), at 2.10 ± 0.10 μg/mL and 0.10 ± 0.0 μg/mL, and selectivity index (SI) values of 0.048 and 1, respectively, while the actinomycin D exhibited EC50 at 1.90 ± 0.10 μg/mL and 0.10 ± 0.0 μg/mL, and SI values of 0.052 and 1. The molecular docking studies demonstrated squalene synthase as the most favorable antileishmanial target protein for both the actinomycins X2 and D, while the xanthine phosphoribosyltransferase was the least favorable target protein. The molecular dynamics simulations confirmed that both the actinomycins remained stable in the binding pocket during the simulations. Furthermore, the MMPBSA (Molecular Mechanics Poisson-Boltzmann Surface Area) binding energy calculations established that the actinomycin X2 is a better binder than the actinomycin D. In conclusion, both actinomycins X2 and D from Streptomyces smyrnaeus strain UKAQ_23 are promising antileishmanial drug candidates and have strong potential to be used for treating the currently drug-resistant leishmaniasis.
Keywords: actinomycin X2, actinomycin D, in silico molecular modeling, Kala-azar, leishmaniasis, molecular dynamics simulation, Streptomyces smyrnaeus, strain UKAQ_23
1. Introduction
Leishmaniasis, a Neglected Tropical Parasitic Disease (NTPD), is induced by several species of Leishmania and transmitted through the bites of female phlebotomine sandflies [1,2,3,4]. In humans, the parasite lives and reproduces as an intracellular amastigote inside the macrophage phagolysosomes [3]. There have been three major kinds of leishmaniasis reported, i.e., cutaneous leishmaniasis, the most common form of leishmaniasis [5], induced by L. donovani, L. aethiopica, and L. tropica; mucocutaneous leishmaniasis, induced by L. braziliensis; and visceral leishmaniasis or Kala-azar, caused by L. donovani. The most severe form of leishmaniasis is visceral leishmaniasis [3]. Currently, no vaccine against human leishmaniasis is available [1], and chemotherapy is the only treatment option available for the affected population. Amphotericin B, paromomycin, and miltefosine are three commonly prescribed antileishmanial drugs, but their uses are restricted due to their toxicity or high costs. However, the existing drug repertoire is restricted, and increasing resistance to these drugs is a significant concern, especially in Saharan and sub-Saharan regions [1]. Therefore, discovering novel drug targets and evaluating novel drugs are essential for successful leishmaniasis control.
Over the last decade, there has been a paradigm shift in funding for anti-parasitic drug discovery. Certain organizations, including the Institute of One World Health (IOWH), the Drugs for Neglected Diseases Initiative (DNDi), and the Bill and Melinda Gates Foundation, have contributed funds towards the development of tropical disease drugs [1] by encouraging scientific advancements in academia and industry, such as publicly available gene sequencing, which has aided in the drug discovery and development processes. The whole-genome sequencing of several Leishmania species, i.e., L. braziliensis, L. major, and L. infantum, has contributed to significantly advancing drug discovery and development [6].
Streptomyces, a commercially treasured and medically significant actinomycetes genus, produces chemically diversified, biologically active substances, e.g., antibiotics, anticancer, antiviral, herbicidal, and insecticidal agents, which have heightened the interests in the genus and its microbial products [6,7,8]. The actinomycins are well-recognized antibiotics synthesized by various strains of the genus Streptomyces, which have exhibited anticancer and antimicrobial properties [7,8,9]. However, certain reports have suggested that actinomycins, including actinomycin D, also exhibit substantial levels of antileishmanial activity [10,11,12,13]. In this context, the current study aimed to isolate, purify, characterize, and evaluate the antileishmanial activity of two newly-sourced actinomycins, X2 and D, produced by the novel actinomycete strain Streptomyces smyrnaeus UKAQ_23. The study also explored, by the in silico methods, the molecular docking investigations of the isolated actinomycins against various antileishmanial target proteins, reported earlier through detailed analyses of the intermolecular interactions between the target and ligands, followed by molecular dynamics simulation studies and MMPBSA binding energy evaluations of the isolated actinomycins, X2 and D, obtained from the strain, UKAQ_23.
2. Results
2.1. Isolation, Identification, and Characterization of Strain UKAQ_23
The actinomycete strain, UKAQ_23, was isolated from a mangrove sediment sample collected from Jubail, Saudi Arabia, in 2019 (Figure 1), identified as Streptomyces smyrnaeus strain UKAQ_23, and comprehensively characterized. Our earlier published article has further details about this organism [14].
Figure 1.
Growth of isolated strain UKAQ_23 on ISP-4 agar at 28 °C for 7 days.
2.2. Isolation, Purification, and Characterization of Actinomycins X2 and D
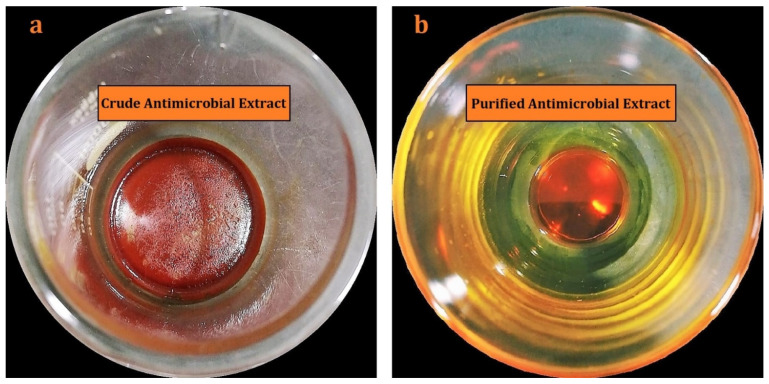
The solid-liquid extraction technique yielded an amorphous, reddish-orange crude antimicrobial extract (Figure 2a). TLC showed two major components, K1, and K2 in the crude antimicrobial extract [14]. As a result, both the compounds were selected for further study. The pure components K1 and K2 were obtained by using various chromatographic techniques (Figure 2b). Additional information on the isolation, purification, and characterization of the actinomycins, X2 and D, are provided in the previously published article by us [14].
Figure 2.
Antimicrobial extracts: (a) crude antimicrobial extract (b) purified antimicrobial extract.
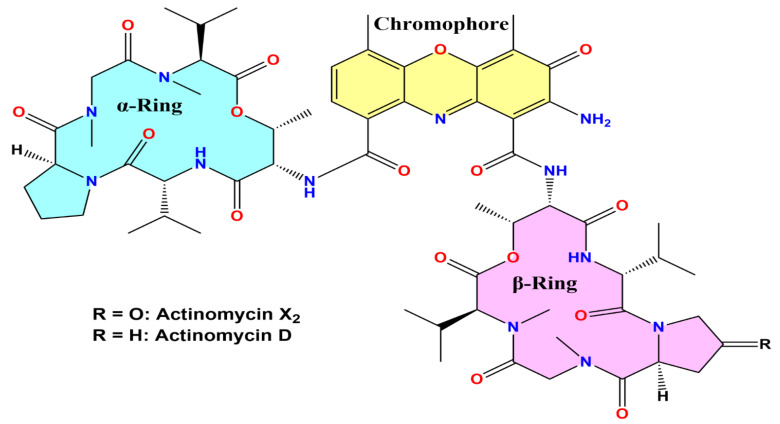
Structural confirmations, and in vitro and in silico antileishmanial activity evaluations of both the components, K1 and K2, were conducted. Thus, based on their physicochemical and spectroscopic analysis results, the isolated components K1 and K2 were identified as actinomycin X2 and actinomycin D, respectively (Figure 3). Additional information on the elucidation of the structures of the actinomycins, X2 and D, was provided in a previously published article by us [14].
Figure 3.
Structure of isolated actinomycin X2 and actinomycin D.
2.3. Antileishmanial Activity Evaluations
2.3.1. Anti-Promastigotes Evaluations
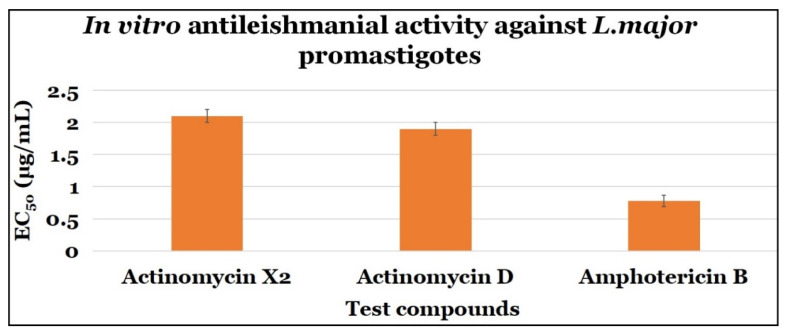
Both the actinomycins X2 and D demonstrated significant anti-parasitic activity against L. major promastigote stages, with EC50 values of 2.10 ± 0.10 μg/mL, and 1.90 ± 0.10 μg/mL with SI values of 0.048 and 0.053, respectively. Amphotericin B, the control drug, had an EC50 value of 0.78 ± 0.09 μg/mL and a SI value of 9.490 (Table 1 and Figure 4).
Table 1.
Anti-promastigotes activity of tested compounds.
| Compounds | Anti-Promastigotes Evaluation | ||
|---|---|---|---|
| EC50 (μg/mL) | CC50 (μg/mL) | SI | |
| Actinomycin X2 | 2.10 ± 0.10 | 0.10 ± 0.0 | 0.048 |
| Actinomycin D | 1.90 ± 0.10 | 0.10 ± 0.0 | 0.053 |
| Amphotericin B | 0.78 ± 0.09 | 7.40 ± 2.64 | 9.490 |
Note: The results are demonstrated in mean ± SD. Each test was performed in triplicate.
Figure 4.
The anti-promastigotes activity of test compounds.
2.3.2. Anti-Amastigotes Evaluations
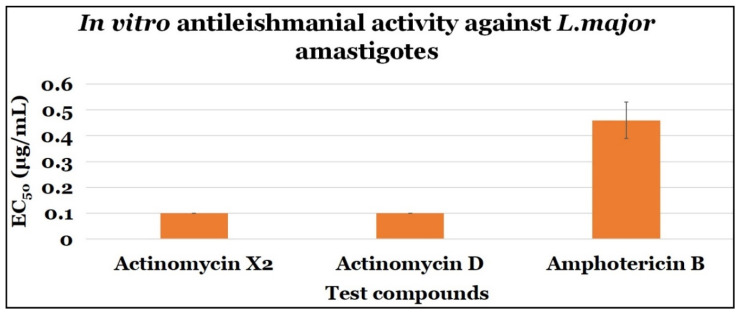
Both actinomycins, X2, and D, demonstrated significant anti-parasitic efficacy against L. major amastigote with EC50 values of 0.10 ± 0.0 μg/mL, and 0.10 ± 0.0 μg/mL, and SI values of 1 and 1, respectively. Amphotericin B, the control drug, had an EC50 value of 0.46 ± 0.07 μg/mL and a SI value of 16.09 (Table 2 and Figure 5).
Table 2.
Anti-amastigotes activity of tested compounds.
| Compounds | Anti-Amastigotes Evaluation | ||
|---|---|---|---|
| EC50 (μg/mL) | CC50 (μg/mL) | SI | |
| Actinomycin X2 | 0.10 ± 0.0 | 0.10 ± 0.0 | 1.0 |
| Actinomycin D | 0.10 ± 0.0 | 0.10 ± 0.0 | 1.0 |
| Amphotericin B | 0.46 ± 0.07 | 7.4 ± 2.64 | 16.09 |
Note: The results are demonstrated in mean ± SD. Each test was performed in triplicate.
Figure 5.
The anti-amastigotes activity of the tested compounds.
The in vitro antileishmanial activity results showed that both the actinomycins, X2, and D, have significant antileishmanial activity, while actinomycin X2 exhibited significantly higher anti-promastigotes activity than the actinomycin D.
2.3.3. Statistical Analysis
There were no statistically significant differences in the mean antileishmanial activity values (in vitro) between the actinomycin X2 (M = 2.1, SD = 0.1) and actinomycin D (M = 1.9, SD = 0.1); t (4) = 2.25, p = 0.070. Additionally, the findings also indicated that the actinomycin X2 was somewhat more effective than the actinomycin D, since the p value (p = 0.070) was near to the statistical significance (p = 0.05).
2.4. In Silico Antileishmanial Activity Evaluations
2.4.1. Molecular Docking Studies
To identify the target proteins for both the actinomycins, X2 and D, 15 previously reported druggable targets involved in several L. major metabolic pathways [1] were screened, except for the trypanothione reductase, which was from L. infantum [3].
The ligand-protein binding energy analysis was carried out, and the results are summarized in Table 3. Both the actinomycins, X2 and D, had the lowest predicted binding energy with squalene synthase, wherein actinomycin X2 predicted a slightly higher calculated affinity with this target protein, squalene synthase, which was consistent with the experimental results. The actinomycin X2 had slightly higher inhibitory activity than the actinomycin D. Several studies suggested that the squalene synthase was a potential target for Leishmania donovani, Leishmania mexicana, Leishmania amazonensis, and Leishmania chagasi [15,16,17,18].
Table 3.
Protein-ligand predicted binding energy obtained from molecular dockings.
| Enzymes | Pathway | Binding Energy (kcal/mol) | |
|---|---|---|---|
| Actinomycin X2 | Actinomycin D | ||
| Trypanothione reductase | Trypanothione pathway | −8.8 | −8.8 |
| Trypanothione synthetase-amidase | Trypanothione pathway | −8.5 | −8.1 |
| Tryparedoxin peroxidase | Trypanothione pathway | −8.0 | −8.1 |
| Squalene synthase | Sterol biogenetic pathway | −10.0 | −9.9 |
| Squalene monooxygenase | Sterol biogenetic pathway | −7.5 | −7.3 |
| Farnesyl pyrophosphate synthase | Sterol biogenetic pathway | −8.3 | −8.0 |
| Glyceraldehyde-3-phosphate dehydrogenase | Glycolytic pathway | −7.7 | −7.5 |
| Triosephosphate isomerase | Glycolytic pathway | −6.8 | −6.7 |
| Phosphoglycerate kinase | Glycolytic pathway | −8.0 | −8.1 |
| Pyruvate kinase | Glycolytic pathway | −8.0 | −7.7 |
| Phosphoglycerate mutase (2,3-diphosphoglycerate-independent) | Glycolytic pathway | −7.9 | −7.8 |
| Fructose-bisphosphate aldolase | Glycolytic pathway | −8.7 | −8.7 |
| Adenine phosphoribosyltransferase | Purine salvage pathway | −7.6 | −7.3 |
| Xanthine phosphoribosyltransferase | Purine salvage pathway | −8.8 | −5.9 |
| Deoxyhypusine hydroxylase | Hypusine biosynthetic pathway |
−8.5 | −8.4 |
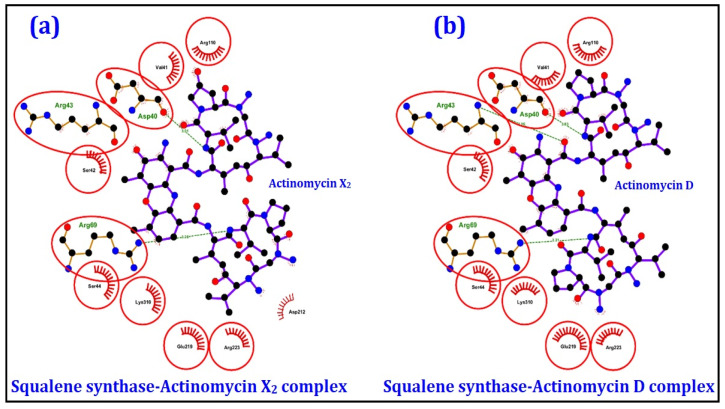
The 2D and 3D ligand-protein interaction analyses for both the actinomycins, X2 and D, are given in Figure 6 and Figure 7, as obtained from Ligplot+ [19] and UCSF Chimera [20] tools, respectively. The results indicated that Val41, Ser42, Ser44, Arg110, Glu219, Arg223, and Lys310 were the common interacting residues in both the complexes, whose side chains predominantly form the hydrophobic interactions with the ligands. Similarly, Asp40, Arg43, and Arg69 residues were common in both complexes and were involved in hydrogen bonding with ligands. The remaining proteins exhibited poor to moderate binding affinities with both the actinomycins, X2 and D, and were not analyzed in detail.
Figure 6.
2D interactions analyses of the squalene synthase complexed with (a) actinomycin X2 and (b) actinomycin D, obtained from the molecular docking exercises. The AAs residues interacting with the ligand are shown in red circles.
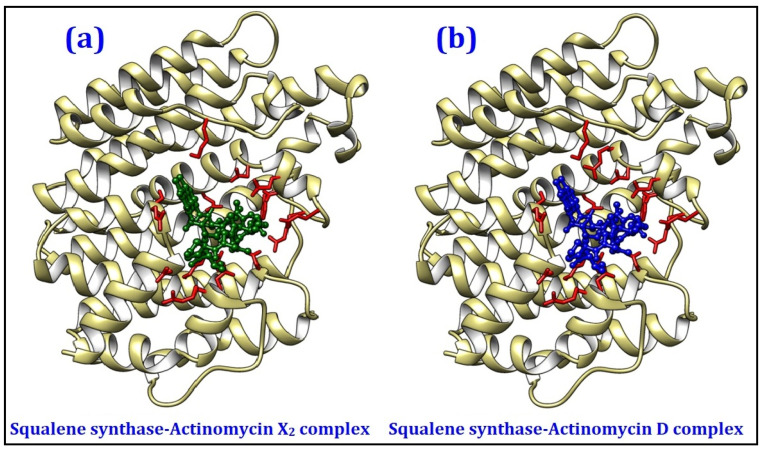
Figure 7.
3D interaction analyses of squalene synthase complexed with (a) actinomycin X2, and (b) actinomycin D as generated by the molecular docking exercises.
2.4.2. Molecular Dynamics (MD) Simulations
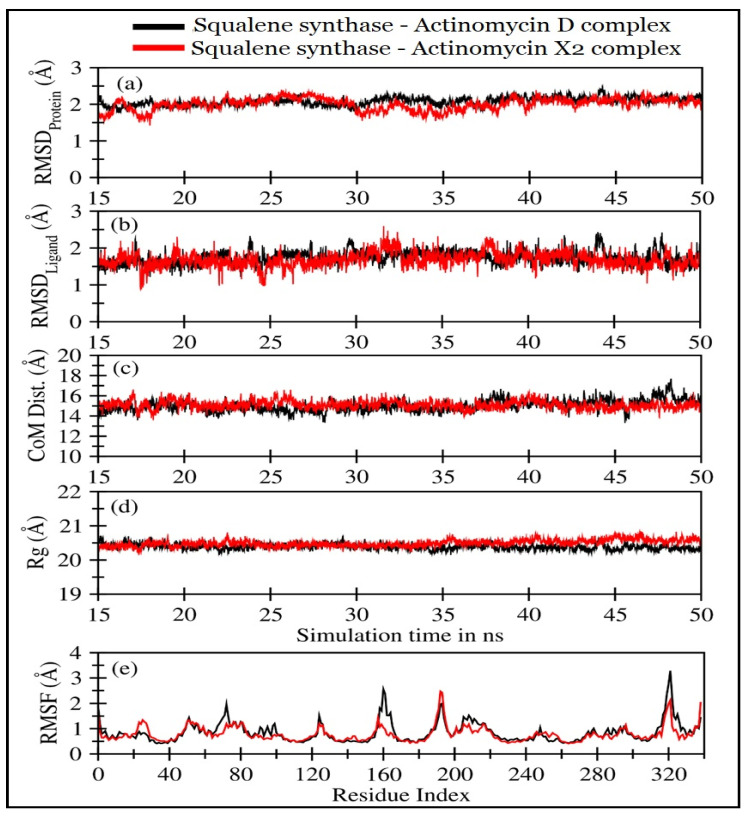
The molecular dynamics simulations of actinomycins, X2 and D, in complexation with squalene synthase, were performed for 50 ns. The first 15 ns were discarded, and the remaining 35 ns trajectory was utilized to ensure that both the systems were completely converged. The trajectory analyses were performed in terms of root mean square deviation (RMSD) of protein and the molecular templates, actinomycins, X2 and D, root mean square fluctuations (RMSF), the radius of gyration (Rg), the center of mass distance (COM) between the protein and the actinomycins, X2 and D (Figure 8), and the number of H-bonds, and MMPBSA [21] ligand-protein binding energy (Figure 8).
Figure 8.
Various trajectory analyses for the squalene synthase complexed with actinomycin D (black lines) and actinomycin X2 (red lines): (a) RMSDprotein, (b) RMSDligand, (c) center-of-mass distance between protein and ligand, (d) protein’s radius of gyration, and (e) RMSF, as calculated from last 35 ns of MD trajectories. RMSDprotein, Rg, and RMSF have been calculated using ‘C-alpha’ atoms using Gromacs or Bio3D modules in R programs.
Figure 8a displayed RMSD for squalene synthase complexed with the actinomycin X2 (1.92 ± 0.25 Å), and actinomycin D (1.96 ± 0.27 Å) (Table 4). The actinomycin X2 showed slightly less deviation in comparison with the actinomycin D. However, both the systems not only displayed RMSD values less than 2 Å but also exhibited highly stable RMSD in the last 35 ns of simulations, indicating that these systems remained stable throughout the simulations. No significant deviations were observed for both the systems involving actinomycins, X2 and D. The RMSD values (Figure 8b) and their mean values for both the ligands are listed in Table 3. It was also observed that the actinomycin X2 showed slightly less RMSDLigand value (1.52 ± 0.30 Å) with a smaller SD in the binding pocket of the squalene synthase than that observed for the actinomycin D (1.58 ± 0.31 Å). Nonetheless, both the ligands remained stable throughout the simulation period with some slight fluctuations within the binding pockets, which did not alter the stability of the ligands systems.
Table 4.
Mean standard deviation values for RMSDProtein, RMSDLigand, center of the mass distance between protein–ligand (CoMProtein-Ligand) and Rg for squalene synthase complexed with actinomycin X2 and actinomycin D ligands.
| Squalene Synthase Complexed with: | RMSDProtein
(Å) |
RMSDLigand
(Å) |
CoMProtein-Ligand
(Å) |
Rg (Å) |
|---|---|---|---|---|
| Actinomycin X2 | 1.92 ± 0.25 | 1.52 ± 0.30 | 14.80 ± 0.69 | 20.48 ± 0.1 |
| Actinomycin D | 1.96 ± 0.27 | 1.58 ± 0.31 | 15.05 ± 0.57 | 20.40 ± 0.09 |
As shown in Figure 8c and Table 4, the center-of-mass distance represented the distance between the center-of-mass of the protein and the center-of-mass of the ligand during MD simulations. It was observed that both the distances remained highly stable throughout the simulation period, representing that both the ligands and the proteins remained in the bound state. However, the actinomycin X2 displayed slightly closer binding with the squalene synthase than the actinomycin D. Figure 8d showed the radii of gyration plots of the squalene synthase in the presence of actinomycins D and X2; the mean values are provided in Table 4. The radius of gyration indicated the compactness of the protein during simulation time, and no significant changes were observed in protein compactness for both the ligands systems involving actinomycin D and actinomycin X2 with values at 20.40Å and 20.48Å, respectively, thereby indicating that both the ligands did not induce any significant changes in the protein folds. The root means square fluctuations (RMSF) for Cα atoms of the squalene synthase, in both the systems, as shown in Figure 8e, indicated that the AAs residues 155–170, and AAs residues 315–330 (representing short helices near the binding pocket) were more flexible in the case of actinomycin D as compared with the actinomycin X2. These observations supported that the actinomycin D ligand complex had a slightly higher RMSD value. Overall, the ligands’ fluctuating patterns were similar, wherein the C-terminal exhibited slightly higher fluctuations than the N-terminal of the binding protein.
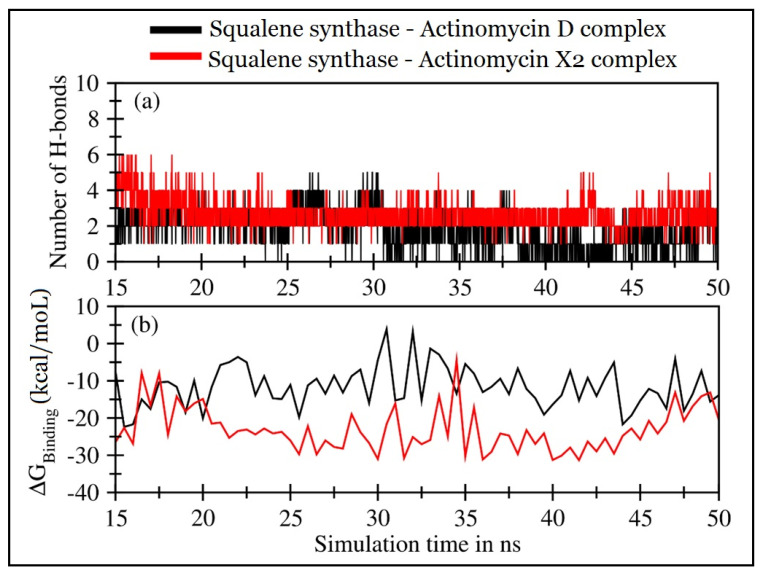
Figure 9a displayed the hydrogen bonds formed between the squalene synthase and the corresponding ligands. Both the actinomycins, X2 and D, systems showed consistent H-bond formations. However, the squalene synthase-actinomycin X2 complex showed consistent H-bonds involving AAs residues Val41, Ser42, and Arg69, as depicted in Figure S1. In contrast, the AAs Arg43 and Arg313 showed non-consistent H-bonds with the ligand as compared with the squalene synthase-actinomycin D complex, wherein the mean number drops to ~2, and only the AA Ser42 residue was consistent, while Arg69, and Arg313, either form H-bond in the beginning or later in the simulation period. Figure S1 also supported that the RMSDLigand and the RMSF were slightly lowered in the case of the actinomycin X2 complex. The MMPBSA protein-ligand binding energy was calculated after every 0.5 ns of simulations for both systems.
Figure 9.
Trajectory analyses for the squalene synthase complexed with actinomycins D (black lines) and X2 (red lines): (a) number of hydrogen-bonds formed between the protein and the actinomycins D (mean: 2.02 ± 1.10) and X2 (mean: 2.99 ± 1.04), and (b) molecular mechanistic Poisson–Boltzmann surface area protein–ligand binding energy calculated after every 0.5 ns.
Figure 9b displayed the binding energies over the simulation’s period, and Table 5 lists the mean values and individual contributions from van der Waal’s electrostatic and polar and non-polar solvation energies.
Table 5.
MMPBSA binding energy in kcal/mol for the protein-ligand complex.
| Squalene Synthase Complexed with: | ∆EVDW (Van der Waal’s Energy) |
∆Eelec (Coulombic Energy) |
∆GPB (Poisson-Boltzmann Polar Solvation Energy) |
∆ESASA (Non-Polar Solvation Energy) |
∆GMMPBSA (Protein-Ligand Binding Energy) |
|---|---|---|---|---|---|
| Actinomycin X2 | −45.14 ± 5.33 | −35.37 ± 10.16 | 62.93 ± 10.62 | −5.49 ± 0.38 | −23.07 ± 5.96 |
| Actinomycin D | −41.78 ± 5.217 | −12.31 ± 11.48 | 47.95 ± 11.05 | −5.42 ± 0.38 | −11.57 ± 5.53 |
Additionally, actinomycin X2 predicted a stronger calculated binding affinity with squalene synthase with a mean binding energy value of −23.07 ± 5.96 kcal/mol than the actinomycin D complex mean energy values at −11.57 ± 5.53 kcal/mol. The significant energy contributions that favored the binding of actinomycin X2 with squalene synthase came from Coulombic interactions and were substantiated by higher H-bond formations between the protein and the actinomycin ligand. Briefly, both the ligands exhibited fair to moderate binding interactions with the squalene synthase. However, actinomycin X2 demonstrated the potential to be a far more active drug than actinomycin D.
3. Discussions
Leishmaniasis, being among the most prevalent diseases in several countries with continuously increasing morbidity and mortality, has posed challenges to health programs and the well-being of populations. The fact that a few drugs are available has made the situation grimmer. The drugs’ toxicity, resistance in treatments, and the availability and cost of the drugs are other concerns. The drug discovery through finding new molecular templates from natural sources has been one of the options. The target identification, molecular docking, binding feasibility, and activity validation through in silico, in vitro, and in vivo approaches have been performed. To establish a successful pipeline for new drug discovery and development, it is necessary to discover new antileishmanial active compounds from the novel sources, novel chemistry, and adaptable appropriate mechanisms of action. The drugs repurposing, structure-, and fragments-based drug designs, chemistry and bioinformatics tools, biological chemistry approaches, structural genomics, and molecular dynamics are some of the newer technological advancements for new drug discovery against several diseases.
Our approach in discovering and developing effective and affordable antileishmanial drugs utilized in silico and in vitro evaluations of the naturally-sourced molecular templates obtained from the Streptomyces smyrnaeus strain UKAQ_23. Supposedly, the low translational outcomes from the in vitro assays were validated by in silico conditions. The thorough and in detail approach to the ligands binding, energy estimations, and geometric preferences outcomes together with the stability of the bound ligand and the target, involvement of amino acids residues facilitating the bindings, and overall energy requirements provided the needed comparison between the predicted efficacy of actinomycins X2 and D bindings led activity projection. The relationship with the in vitro investigations conducted on the isolated products, actinomycin X2, and actinomycin D, indicates the active components between the two targeted compounds isolated during the current study. Hence, the chances of attrition were minimized, and the prospects of the isolated compounds were more relevant to fit the new drug discovery. The molecular dockings against various ligand proteins from two different Leishmania species, L. major and L. infantum, were utilized. A total of 15 enzymes from trypanothione, sterol biogenetic, glycolytic, hypusine biosynthetic, and purine salvage pathways were utilized. The in silico evaluations on squalene synthase from the sterol biogenetic pathway produced the potent interaction towards bindings of the ligands. Molecular dynamics trajectory analyses based on RMSD (root mean square deviation) of the target and the molecular templates, actinomycins, X2 and D, as well as the RMSF (root mean square fluctuations), Rg (radius of gyration), COM (center of mass distance) between the protein and the actinomycins, X2 and D, the number of H-bonds, and MMPBSA ligand-protein binding energy were estimated.
Our findings on actinomycins, X2, and D, produced by Streptomyces smyrnaeus, strain UKAQ_23, are consistent with the previous findings that several Streptomyces strains, including Streptomyces sp. MS449, Streptomyces sp. IMB094, Streptomyces nasri YG62, Streptomyces padanus JAU4234, Streptomyces elizabethii. II, Streptomyces flavogriseus NJ-4, Streptomyces MITKK-103, Streptomyces griseoruber, Streptomyces strain M7, Streptomyces sp. HUST012, Streptomyces heliomycini, Streptomyces hydrogenans IB310 produce actinomycin X2 and actinomycin D, which have provided anti-parasitic and some with anti-leishmanial compounds [14,22,23,24,25,26,27,28,29,30,31,32,33]. Several previously published reports specified that actinomycins D, Z3, Z5 have significant antileishmanial activity [10,11,34]. Jamal reported the antileishmanial activity of actinomycins D, Z3, and Z5 against promastigotes and amastigotes of L. tropica and their cytotoxicity towards human peripheral blood lymphocytes. It was also reported that the IC50 values for the antileishmanial activity of actinomycins D, Z3, and Z5 were at 8.739 μM, 2.135 μM; 5.500 μM, 1.760 μM; 9.529 μM, 1.691 μM concentrations, respectively, against promastigotes and amastigotes of L. tropica, while the cytotoxicity (IC50 values) of the actinomycins D, Z3, and Z5 were at 195.8 μM, 210.1 μM, and 234.9 μM concentrations, respectively, against human peripheral blood lymphocytes [10]. These findings are consistent with our results, which demonstrated substantial antileishmanial activity of actinomycin X2 and D. Another report by Annang et al. found antileishmanial activity (IC50) of actinomycin D against L. donovani to be at 147.9 nM concentration, which is also in agreement with the results obtained in the present study [11], while Kaplum et al. reported the antileishmanial activity (IC50) of actinomycin D against promastigotes of L. amazonensis to be at 50 μM concentration, again consistent with our findings, indicating antileishmanial activity of actinomycin D [34].
4. Materials and Methods
4.1. Isolation, Identification, and Characterization of Strain UKAQ_23
The mangrove sediment sample was collected from Jubail, Saudi Arabia (latitude: 27°00′40″ N; longitude: 49°39′29″ E; altitude: 22 ft; annual rainfall: 97 mm; average temperature: 26.6 °C) by following the standard techniques [35,36]. An actinomycete strain, UKAQ_23, was isolated from the collected mangrove sample by following the standard methods [35,36]. Our previously published article described further details about this organism [14].
4.2. Production, Purification, and Characterization of Actinomycins
The production of actinomycins was conducted on a modified ISP-4 agar medium at pH 6.5 ± 0.1, temperature 35 ± 1 °C, inoculum 5% (v/w), and time duration of 7 days by following the solid-state fermentation [14,37,38].
The antimicrobial extract containing the actinomycins was extracted from the fermented agar using the solid-liquid extraction technique and subsequently purified using various chromatographic techniques [14].
For the physicochemical characterization and structural determination of actinomycins X2 and D, we used the following techniques: color, appearance, solubility, melting point (°C), UV-Visible (λmax, nm) absorbance, FT-IR (υmax, cm−1) absorbance, monoisotopic masses, (+)-HR-ESI-MS, LC-MS, LC-MS-MS, 1D, and 2D NMR spectroscopy. An earlier published article by us described the structure elucidation of the actinomycins X2 and D [14].
4.3. In Vitro Antileishmanial Evaluations
4.3.1. Isolation of L. major and Culture Conditions
The parasite was isolated as promastigotes of L. major from a male patient and cultivated weekly at 26 °C in Schneider’s Drosophila medium (SDM) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS). Promastigotes were cryopreserved at a concentration of 3 × 106 parasites/mL in liquid nitrogen. To maintain virulence, the parasite in the promastigote form (1 × 106) was injected into the hindfoot of female BALB/c mice, and then amastigotes were isolated from mice after 8 weeks. Amastigotes were converted into promastigotes at a temperature of 26 °C using SDM containing 10% (v/v) FBS and antibiotics. Promastigotes (<5 in vitro cycles) were then used for infection and anti-parasitic studies. Mice were kept in a pathogen-free environment [2,3,4].
4.3.2. Anti-Promastigotes Evaluations
The promastigotes with logarithmic-phase were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (phenol red-free and supplemented with 10% (v/v) FBS) and then transferred into 96-welled microtiter plates with a density of 106 cells/mL (200 µL/well). The parasites were counted using a hemocytometer. The anti-promastigotes efficacy of test actinomycins and the control drug was evaluated at different concentrations, including 25, 8.3, 2.7, 0.9, 0.3, and 0.1 µg/mL. Amphotericin B and dimethyl sulphoxide (DMSO) were used as positive and negative controls, respectively. After 72 h of incubation, the parasite inhibition was determined by counting viable promastigotes using a tetrazolium dye (MTT)-based colorimetric technique. The samples were analyzed at 540 nm using an ELISA reader. Each test was performed in triplicate, and the findings were recorded in mean ± SD [2,3,4].
4.3.3. Anti-Amastigotes Evaluations
The anti-amastigotes efficacy of the test compounds was assessed by following a previously published method [2,3,4]. Female BALB/c mice aged 6–8 weeks were used to harvest the peritoneal macrophages. The cells (5 × 104) were then cultured in RPMI-1640 medium containing 10% (v/v) FBS in 96-welled microtiter plates for 4 h at 37 °C with 4% CO2 to promote the cell attachment. After discarding the media, the attached macrophages were washed with PBS and then incubated with 200 µL of RPMI-1640 medium (phenol red-free) supplemented with 10% (v/v) FBS containing promastigotes of L. major in a ratio of 10 promastigotes: 1 macrophage. After that, the plates were incubated at 37 °C with 5% CO2 for 24 h to allow amastigotes to infect and differentiate. The test compounds, including a positive control, were then added to the infected macrophages and 200 µL of RPMI-1640 medium (phenol red-free) containing 10% (v/v) FBS. After 3 washes with PBS to remove free promastigotes, a serial dilution was carried out to obtain final concentrations of 25, 8.3, 2.7, 0.9, 0.3, and 0.1 µg/mL for each of the test compounds and the positive control. The microtiter plates were then incubated for 72 h at 37 °C with 5% CO2. The cultures with DMSO served as the negative control, while cultures with amphotericin B at the same concentrations as test actinomycins served as the positive control. After washing, methanol fixation, and Giemsa staining, the percentage of infected macrophages was assessed microscopically. Each test was conducted in triplicate, and the findings were recorded in mean ± SD [2,3,4].
4.3.4. In Vitro Cytotoxicity Evaluations
The MTT assay was performed to assess the cytotoxicity of actinomycins, X2, and D. The mice macrophages were cultivated for 24 h in RPMI-1640 medium supplemented with 10% (v/v) FBS in microtiter plates (5 × 103 cells/well/200 μL) at 37 °C with 5 % CO2. After washing the cells with PBS, cells were treated with actinomycins, X2, and D for 72 h at differing concentrations, i.e., 25, 8.3, 2.7, 0.9, 0.3, and 0.1 µg/mL in RPMI-1640 medium supplemented with 10% (v/v) FBS. The cells with 10 % (v/v) FBS served as the negative control. After removing the supernatant, a mixture of 50 μL RPMI-1640 medium and 14 μL of MTT (0.5% w/v) was dispensed and incubated for 4 h. After removing the supernatant, 200 μL DMSO was used to solubilize the insoluble formazan produced by living cells from MTT. The colorimetric analysis was performed using the Bio-Rad X-Mark microplate reader at 540 nm. The cytotoxic effects were quantified using CC50 values (the concentration at which 50% of viable cells were killed). Each test was performed in triplicate, and the findings were recorded in mean ± SD [2,3,4].
4.3.5. Statistical Analysis
Microsoft Excel was used to calculate the linear regression equation for EC50 and CC50 values. The SI values were calculated by dividing the CC50 by the EC50 for each parasite. The significance of the variations in means between actinomycin X2 and actinomycin D were calculated using an independent-samples (unpaired) t-test, with a significance level of p = 0.05. SPSS software, version 20.0 (IBM, USA), was used to conduct the statistical analyses [1,2,3,4].
4.4. In Silico Antileishmanial Evaluations
4.4.1. Molecular Docking Evaluations
The UniProt was used to obtain the amino acid sequences of all of the proteins. The 3D structures were either retrieved from PDB and/or model from the Swiss model or I-tasser, depending upon the availability of the template proteins and their percent identity. The FASTA sequences of all the proteins are given in Table S1. After validation of all the structures, the .pdbqt files were generated using AutoDock tools [37] employing Kollman charges. The grid box in x, y, and z dimensional domains were defined to cover the whole protein to perform blind docking. The grid spacing was set to 1 Å, as recommended for the AutoDock vina program [38]. The 3D structure of actinomycin D was retrieved from PDB (PDB entry code: 1A7Y) [39], and it was further modified to generate the structure of actinomycin X2. Before the preparation of .pdbqt files using Gasteiger charges, both the ligands were energy minimized. Finally, the molecular dockings were run utilizing the AutoDock vina program [38] using an exhaustiveness value of 80. A total of 20 docked conformations were generated for each system for analysis.
4.4.2. Molecular Dynamics Simulations
The MD simulations of squalene synthase complexes were performed for 50 ns using the GROningen MAchine for Chemical Simulations (GROMACS) simulation software (GROMACS 2020.4; Department of Biophysical Chemistry, University of Groningen, Groningen, The Netherlands and the Royal Institute of Technology and Uppsala University, Sweden) [40], with the Chemistry at HARvard Macromolecular Mechanics 36m (CHARMM36m) forcefield for proteins, and the CHARMM General Force Field (CGenFF) for actinomycins, X2, and D. The trajectory and energy data were recorded at every 10 ps. The TIP3P water molecules were used to solvate both the systems in a truncated octahedral box. The protein complexes were set in the simulation boxes within 10 Å from the box edge to accurately meet the minimum image convention. Additionally, 12 K+ ions were added to each of the squalene synthase complexes to neutralize the whole system. The systems for actinomycins, X2 and D, contained 46,122 and 46,153 atoms, respectively. The Chemistry at HARvard Macromolecular Mechanics-Graphical User Interface (CHARMM-GUI) webserver was used to generate all input files [41,42].
The system was minimized for 5000 steps using the Steepest Descent technique, and convergence was achieved under the force limit of 1000 (kJ/mol/nm) to exclude any steric disturbances. Finally, the system was equilibrated at NVT (Canonical ensemble: where moles, N; volume, V; and temperature, T were conserved) and NPT (Isothermal-Isobaric ensemble: where moles, N; pressure, P; and temperature, T were conserved) ensembles for 100 ps (50,000 steps) and 1000 ps (1,000,000 steps), respectively, using time steps 0.2 and 0.1 fs, at 300 K to ensure a fully converged system for the production run.
The simulation runs were conducted at a constant temperature of 300 K and a pressure of 1 atm, or 1 bar (using an NPT ensemble), respectively, utilizing weak coupling velocity rescaling (modified Berendsen thermostat) and Parrinello–Rahman algorithms. The relaxation periods were set at τ T = 0.1 ps and τ P = 2.0 ps. Using the LINear Constraint Solver (LINCS) algorithm, all bond lengths involving hydrogen atoms were maintained stiffly at optimal bond lengths, with a time step of 2 fs. The non-bonded interactions were calculated using the Verlet technique. In all x, y, and z directions, Periodic Boundary Conditions (PBC) were applied. Each time, interactions within a short-range cutoff of 12 Å were determined. The electrostatic interactions and forces in a homogeneous medium beyond the long-range cutoff were calculated using the Particle Mesh Ewald (PME) method. For both the complexes, the production ran for 50 ns. The first 15 ns of the trajectories were excluded for comprehensive analysis, and the remaining 35 ns were utilized.
5. Conclusions
The drug-resistant leishmaniasis is becoming more frequent by compromising the therapeutic efficacy of the currently available antileishmanial drugs, thereby emphasizing the urgent need for finding new antileishmanial drugs with enhanced activity to treat drug-resistant leishmaniasis. In this perspective, several pieces of evidence indicated that terrestrial actinomycetes are among the most promising candidates for producing novel bioactive molecules active against a range of pathogenic microorganisms, including the Leishmania parasite. As a result, the isolation of biologically active molecules from terrestrial actinomycetes was undertaken. The current study pursued the antileishmanial activity of actinomycins X2 and D, isolated from Streptomyces smyrnaeus strain UKAQ_23, against promastigotes and amastigotes of Leishmania major. The actinomycin X2 showed higher antileishmanial efficacy than the actinomycin D. However, both the products demonstrated substantial antileishmanial activity against the promastigotes and amastigotes of L. major determined in the in vitro experimentations and validated through the in silico predictions. In conclusion, both the actinomycins X2 and D can be utilized to treat drug-resistant leishmaniasis, and Streptomyces smyrnaeus strain UKAQ_23 has the potential to be a commercially viable source for the production of actinomycins X2 and D, probably implying a paradigm shift in the pharmaceutical industry.
6. Patents
On 22 January 2021, a patent application has been submitted to the Intellectual Property Office of India. The reference number for the patent filing is 202111003185. The final decision is awaited.
Acknowledgments
The authors wish to thank the Faculty of Biosciences and Biotechnology, Invertis University, Bareilly, Uttar Pradesh, India; College of Pharmacy, Qassim University, Buraydah; Unaizah College of Pharmacy, Qassim University, Unaizah; College of Applied Health Sciences, Qassim University, Ar-Rass, Saudi Arabia; and King Abdullah University of Science and Technology (KAUST), Thuwal, Saudi Arabia, for providing the technical and infrastructural support throughout the study.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics10080887/s1, Figure S1: H-bond distances calculated between heavy atoms of protein (i.e., O or N) and ligand (i.e., O or N) for (a) actinomycin D and (b) actinomycin X2 complexes. The hydrogen bonds were calculated using VMD, where the cutoff for Donor-Acceptor distance and angle was set to 3 Å and 20 degrees, respectively, Table S1: The selected proteins for molecular docking studies with actinomycin D and actinomycin X2 ligands targeting multiple pathways in Leishmania major. The table also lists the name of enzymes, their FASTA sequence, amino acid length, their PDB code (if existed) or models (generated from homology modeling approach or threading approach), and the corresponding pathways where the protein is involved.
Author Contributions
Conceptualization, K.A.Q.; methodology, K.A.Q., I.A.N., W.S.K., T.A.K. and R.A.K.; investigation, K.A.Q., I.A.N., W.S.K., T.A.K., M.Q.F., M.I., R.A.K., H.A.M., M.J., A.-H.E., F.A. and G.O.E.; writing—original draft preparation, K.A.Q.; writing—review and editing, K.A.Q., I.A.N., T.A.K., M.Q.F., R.A.K. and F.A.; supervision, D.K.P. and A.D.B.; project administration, K.A.Q.; funding acquisition, K.A.Q. All authors have read and agreed to the published version of the manuscript.
Funding
King Abdullah University of Science and Technology (KAUST), Thuwal, Saudi Arabia, provided financial support for this study.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Committee of Research Ethics, Deanship of Scientific Research, Qassim University, Saudi Arabia (20-03-02/30 September 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are partially available at https://doi.org/10.1038/s41598-021-93285-7, and the complete data can be obtained upon request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chawla B., Madhubala R. Drug targets in Leishmania. J. Parasit. Dis. 2010;34:1–13. doi: 10.1007/s12639-010-0006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al Nasr I., Jentzsch J., Winter I., Schobert R., Ersfeld K., Koko W.S., Mujawah A.A.H., Khan T.A., Biersack B. Antiparasitic activities of new lawsone Mannich bases. Arch. Pharm. 2019;352:1–9. doi: 10.1002/ardp.201900128. [DOI] [PubMed] [Google Scholar]
- 3.Verma R.K., Prajapati V.K., Verma G.K., Chakraborty D., Sundar S., Rai M., Dubey V.K., Singh M.S. Molecular docking and in vitro antileishmanial evaluation of chromene-2-thione analogues. ACS Med. Chem. Lett. 2012;3:243–247. doi: 10.1021/ml200280r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan T.A., Al Nasr I.S., Mujawah A.H., Koko W.S. Assessment of Euphorbia retusa and Pulicaria undulata activity against Leishmania major and Toxoplasma gondii. Trop. Biomed. 2021;38:135–141. doi: 10.47665/tb.38.1.023. [DOI] [PubMed] [Google Scholar]
- 5.Lodi G., Sannino M., Caterino P., Cannarozzo G., Bennardo L., Nisticò S.P. Fractional CO2 laser-assisted topical rifamycin drug delivery in the treatment of pediatric cutaneous leishmaniasis. Pediatr. Dermatol. 2021;38:717–720. doi: 10.1111/pde.14608. [DOI] [PubMed] [Google Scholar]
- 6.Ivens A.C., Peacock C.S., Worthey E.A., Murphy L., Aggarwal G., Berriman M., Sisk E., Rajandream M.A., Adlem E., Aert R., et al. The genome of the kinetoplastid parasite. Leishmania major. Sci. 2005;309:436–442. doi: 10.1126/science.1112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka Y., Omura S. Agroactive compounds of microbial origin. Annu. Rev. Microbiol. 1993;47:57–87. doi: 10.1146/annurev.mi.47.100193.000421. [DOI] [PubMed] [Google Scholar]
- 8.Singh L.S., Baruah I., Bora T.C. Actinomycetes of Loktak habitat: Isolation and screening for antimicrobial activities. Biotechnology. 2006;5:217–221. doi: 10.3923/biotech.2006.217.221. [DOI] [Google Scholar]
- 9.Sanasam S., Ningthoujam D.S. Screening of local actinomycete isolates in Manipur for anticandidal activity. Asian J. Biotechnol. 2010;2:139–145. doi: 10.3923/ajbkr.2010.139.145. [DOI] [Google Scholar]
- 10.Jamal Q. Antileishmanial, Cytotoxic and Genotoxic Effects of Actinomycin D, Z3, Z5 and Hydrazine Derivatives of Isosteviol. University of Peshawar; Peshawar, Pakistan: 2020. [(accessed on 17 June 2021)]. Available online: http://prr.hec.gov.pk/jspui/handle/123456789/12101. [Google Scholar]
- 11.Annang F., Pérez-Moreno G., García-Hernández R., Cordon-Obras C., Martín J., Tormo J.R., Rodríguez L., De Pedro N., Gómez-Pérez V., Valente M., et al. High-throughput screening platform for natural product-based drug discovery against 3 neglected tropical diseases: Human African trypanosomiasis, leishmaniasis, and chagas disease. J. Biomol. Screen. 2015;20:82–91. doi: 10.1177/1087057114555846. [DOI] [PubMed] [Google Scholar]
- 12.Ortiz D., Guiguemde W.A., Hammill J.T., Carrillo A.K., Chen Y., Connlly M., Stalheim K., Elya C., Johnson A., Min J., et al. Discovery of novel, orally bioavailable, antileishmanial compounds using phenotypic screening. PLoS Negl. Trop. Dis. 2017;11:e0006157. doi: 10.1371/journal.pntd.0006157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.David M., Lebrun C., Duguet T., Talmont F., Beech R., Orlowski S., André F., Prichard R.K., Lespine A. Structural model, functional modulation by ivermectin and tissue localization of Haemonchus contortus P-glycoprotein-13. Int. J. Parasitol. Drugs Drug Resist. 2018;8:145–157. doi: 10.1016/j.ijpddr.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qureshi K.A., Bholay A.D., Rai P.K., Mohammed H.A., Khan R.A., Azam F., Jaremko M., Emwas A.-H., Stefanowicz P., Waliczek M., et al. Isolation, characterization, anti-MRSA evaluation, and in-silico multi-target anti-microbial validations of actinomycin X2 and actinomycin D produced by novel Streptomyces smyrnaeus UKAQ_23. Sci. Rep. 2021;11:14539. doi: 10.1038/s41598-021-93285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wadanambi P.M., Mannapperuma U. Computational study to discover potent phytochemical inhibitors against drug target, squalene synthase from Leishmania donovani. Heliyon. 2021;7:e07178. doi: 10.1016/j.heliyon.2021.e07178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urbina J.A., Concepcion J.L., Rangel S., Visbal G., Lira R. Squalene synthase as a chemotherapeutic target in Trypanosoma cruzi and Leishmania mexicana. Mol. Biochem. Parasitol. 2002;125:35–45. doi: 10.1016/S0166-6851(02)00206-2. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues J.C.F., Urbina J.A., De Souza W. Antiproliferative and ultrastructural effects of BPQ-OH, a specific inhibitor of squalene synthase, on Leishmania amazonensis. Exp. Parasitol. 2005;111:230–238. doi: 10.1016/j.exppara.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Granthon A.C., Braga M.V., Rodrigues J.C.F., Cammerer S., Lorente S.O., Gilbert I.H., Urbina J.A., de Souza W. Alterations on the growth and ultrastructure of Leishmania chagasi induced by squalene synthase inhibitors. Vet. Parasitol. 2007;146:25–34. doi: 10.1016/j.vetpar.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 19.Laskowski R.A., Swindells M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011;51:2778–2786. doi: 10.1021/ci200227u. [DOI] [PubMed] [Google Scholar]
- 20.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 21.Miller B.R., McGee T.D., Swails J.M., Homeyer N., Gohlke H., Roitberg A.E. MMPBSA.py: An efficient program for end-state free energy calculations. J. Chem. Theory Comput. 2012;8:3314–3321. doi: 10.1021/ct300418h. [DOI] [PubMed] [Google Scholar]
- 22.Chen C., Song F., Wang Q., Abdel-Mageed W.M., Guo H., Fu C., Hou W., Dai H., Liu X., Yang N., et al. A marine-derived Streptomyces sp. MS449 produces high yield of actinomycin X2 and actinomycin D with potent anti-Tuberculosis activity. Appl. Microbiol. Biotechnol. 2012;95:919–927. doi: 10.1007/s00253-012-4079-z. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q., Zhang Y., Wang M., Tan Y., Hu X., He H., Xiao C., You X., Wang Y., Gan M. Neo-actinomycins A and B, natural actinomycins bearing the 5H-oxazolo[4,5-b]phenoxazine chromophore, from the marine-derived Streptomyces sp. IMB094. Sci. Rep. 2017;7:1–8. doi: 10.1038/s41598-017-03769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Naggar M.Y.M., El-Aassar S.A., Hashem M.A., Stoodley R.J., Raynor C.M., Sigee D.C. Production of actinomycin X2 by immobilized Streptomyces nasri YG62 mycelia. Microbios. 1998;95:165–179. [Google Scholar]
- 25.Xiong Z.Q., Zhang Z.P., Li J.H., Wei S.J., Tu G.Q. Characterization of Streptomyces padanus JAU4234, a producer of actinomycin X2, fungichromin, and a new polyene macrolide antibiotic. Appl. Environ. Microbiol. 2012;78:589–592. doi: 10.1128/AEM.06561-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bird C.W., Latif M. Antibiotics from the newly isolated Streptomyces elizabethii. II. Isolation and characterisation of the antibiotics. J. Chem. Technol. Biotechnol. 2007;31:368–370. doi: 10.1002/jctb.503310151. [DOI] [Google Scholar]
- 27.Wei Z., Xu C., Wang J., Lu F., Bie X., Lu Z. Identification and characterization of Streptomyces flavogriseus NJ-4 as a novel producer of actinomycin D and holomycin. PeerJ. 2017;5:e3601. doi: 10.7717/peerj.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurosawa K., Bui V.P., VanEssendelft J.L., Willis L.B., Lessard P.A., Ghiviriga I., Sambandan T.G., Rha C.K., Sinskey A.J. Characterization of Streptomyces MITKK-103, a newly isolated actinomycin X2-producer. Appl. Microbiol. Biotechnol. 2006;72:145–154. doi: 10.1007/s00253-005-0240-2. [DOI] [PubMed] [Google Scholar]
- 29.Praveen V., Tripathi C.K.M. Studies on the production of actinomycin-D by Streptomyces griseoruber—A novel source. Lett. Appl. Microbiol. 2009;49:450–455. doi: 10.1111/j.1472-765X.2009.02689.x. [DOI] [PubMed] [Google Scholar]
- 30.Sharma M., Manhas R.K. Purification and characterization of actinomycins from Streptomyces strain M7 active against methicillin resistant Staphylococcus aureus and vancomycin resistant Enterococcus. BMC Microbiol. 2019;19:1–14. doi: 10.1186/s12866-019-1405-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khieu T.N., Liu M.J., Nimaichand S., Quach N.T., Chu-Ky S., Phi Q.T., Vu T.T., Nguyen T.D., Xiong Z., Prabhu D.M., et al. Characterization and evaluation of antimicrobial and cytotoxic effects of Streptomyces sp. HUST012 isolated from medicinal plant Dracaena cochinchinensis Lour. Front. Microbiol. 2015;6:574. doi: 10.3389/fmicb.2015.00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang D., Wang C., Gui P., Liu H., Khalaf S.M.H., Elsayed E.A., Wadaan M.A.M., Hozzein W.N., Zhu W. Identification, bioactivity, and productivity of actinomycins from the marine-derived Streptomyces heliomycini. Front. Microbiol. 2017;8:1–12. doi: 10.3389/fmicb.2017.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kulkarni M., Gorthi S., Banerjee G., Chattopadhyay P. Production, characterization and optimization of actinomycin D from Streptomyces hydrogenans IB310, a(n antagonistic bacterium against phytopathogens. Biocatal. Agric. Biotechnol. 2017;10:69–74. doi: 10.1016/j.bcab.2017.02.009. [DOI] [Google Scholar]
- 34.Kaplum V., Cogo J., Sangi D.P., Ueda-Nakamura T., Corrêa A.G., Nakamura C.V. In vitro and in vivo activities of 2,3-diarylsubstituted quinoxaline derivatives against Leishmania amazonensis. Antimicrob. Agents Chemother. 2016;60:3433–3444. doi: 10.1128/AAC.02582-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qureshi K.A., Seroor M., AI-Masabi A., Saykhan M.A., Mutairi Y.A., Elhassan G.O., Khan R.A. Bio-characterizations of some marine bacterial strains isolated from mangrove sediment samples of four major cities of Saudi Arabia. J. Environ. Biol. 2020;41:1003–1012. doi: 10.22438/jeb/41/5/MRN-1317. [DOI] [Google Scholar]
- 36.Maiti P.K., Mandal S. Majority of actinobacterial strains isolated from kashmir himalaya soil are rich source of antimicrobials and industrially important biomolecules. Adv. Microbiol. 2019;09:220–238. doi: 10.4236/aim.2019.93016. [DOI] [Google Scholar]
- 37.Morris G.M., Ruth H., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. Software news and updates AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trott O., Olson A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schäfer M., Sheldrick G.M., Bahner I., Lackner H. Crystal structures of actinomycin D and actinomycin Z3. Angew. Chem. Int. Ed. 1998;37:2381–2384. doi: 10.1002/(SICI)1521-3773(19980918)37:17<2381::AID-ANIE2381>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 40.Abraham M.J., Murtola T., Schulz R., Páll S., Smith J.C., Hess B., Lindah E. Gromacs: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1–2:19–25. doi: 10.1016/j.softx.2015.06.001. [DOI] [Google Scholar]
- 41.Lee J., Cheng X., Swails J.M., Yeom M.S., Eastman P.K., Lemkul J.A., Wei S., Buckner J., Jeong J.C., Qi Y., et al. CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. J. Chem. Theory Comput. 2016;12:405–413. doi: 10.1021/acs.jctc.5b00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jo S., Kim T., Iyer V.G., Im W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008;29:1859–1865. doi: 10.1002/jcc.20945. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are partially available at https://doi.org/10.1038/s41598-021-93285-7, and the complete data can be obtained upon request from the corresponding authors.