Abstract
Clostridioides (also known as Clostridium) difficile is a Gram-positive anaerobic, spore producing bacterial pathogen that causes severe gastrointestinal infection in humans. The current chemotherapeutic options are inadequate, expensive, and limited, and thus inexpensive drug treatments for C. difficile infection (CDI) with improved efficacy and specificity are urgently needed. To improve the solubility of our cationic amphiphilic 1,1′-binaphthylpeptidomimetics developed earlier that showed promise in an in vivo murine CDI model we have synthesized related compounds with an N-arytriazole or N-naphthyltriazole moiety instead of the 1,1′-biphenyl or 1,1′-binaphthyl moiety. This modification was made to increase the polarity and thus water solubility of the overall peptidomimetics, while maintaining the aromatic character. The dicationic N-naphthyltriazole derivative 40 was identified as a C. difficile-selective antibacterial with MIC values of 8 µg/mL against C. difficile strains ATCC 700057 and 132 (both ribotype 027). This compound displayed increased water solubility and reduced hemolytic activity (32 µg/mL) in an in vitro hemolysis assay and reduced cytotoxicity (CC50 32 µg/mL against HEK293 cells) relative to lead compound 2. Compound 40 exhibited mild efficacy (with 80% survival observed after 24 h compared to the DMSO control of 40%) in an in vivo murine model of C. difficile infection by reducing the severity and slowing the onset of disease.
Keywords: antibacterial, Clostridioides (Clostridium) difficile, peptidomimetic, triazole
1. Introduction
Clostridioides (also known as Clostridium) difficile is a Gram-positive, anaerobic spore-forming bacterium that causes mild to serious infections in the gastrointestinal tract (GIT) due to the production of potent exotoxins (TcdA, TcdB, and CDT) that cause severe gastrointestinal damage [1,2,3]. The resilient endospores contaminate healthcare environments and facilitate disease initiation, dissemination, and re-infection. In the GIT, spores require glycine and cholate derivatives for germination. In a healthy GIT, the microbiota metabolizes cholate derivatives preventing germination of C. difficile spores. CDI occurs when the normal GIT microbiota is disrupted or killed by conventional broad-spectrum antimicrobials [1]. Under these conditions the metabolism of cholate is significantly compromised, facilitating the germination of spores into C. difficile vegetative cells [4,5].
CDI has a mortality rate of up to 8% [2] with the reoccurrence of infections occurring in up to 20% of cases treated with vancomycin or metronidazole [6]. A 2019 Antibiotic Resistance Threat Report from the US Centers for Disease Control and Prevention indicated that in the USA in 2017 an estimated 223,900 cases of CDI in hospitalized patients resulted in 12,800 deaths and $1 billion in attributed healthcare costs [7]. Thus, there is a significant and important incentive to develop novel therapeutics that show selectivity for C. difficile over other gut bacteria to effectively combat CDI. While fecal microbiota transplantation can be effective for recurrent CDI, there can be adverse effects and the long-term impacts are unknown [1,2,8].
Fidaxomicin was specifically approved by the FDA in 2011 for treating CDI [9]; resulting in approximately 50% less CDI recurrence compared to vancomycin [10] most likely due to its greater selectivity for C. difficile, less impact on commensal enteric microflora (i.e., Bacteroides spp.), and its ability to reduce C. difficile sporulation [11]. There are many potential chemotherapeutics undergoing clinical trials for the treatment of CDI [12]. Other small molecule chemotherapeutics currently under investigation for use against C. difficile, include antimicrobial peptidomimetics [13,14,15], glycopeptides [16], bis-indoles [17], purine derivatives [18], tetramic acids [19], nitroheterocycles [20], macrolides [21], and nylon-3 polymers [22]. Two vaccines are being investigated in clinical trials (Pfizer and Intercell [23]), whereas bezlotoxumab (a monoclonal antibody targeting C. difficile TcdB) was given FDA approval in 2016 as adjunctive therapy for patients undergoing antimicrobial treatment who were at high risk of recurrent infection [24].
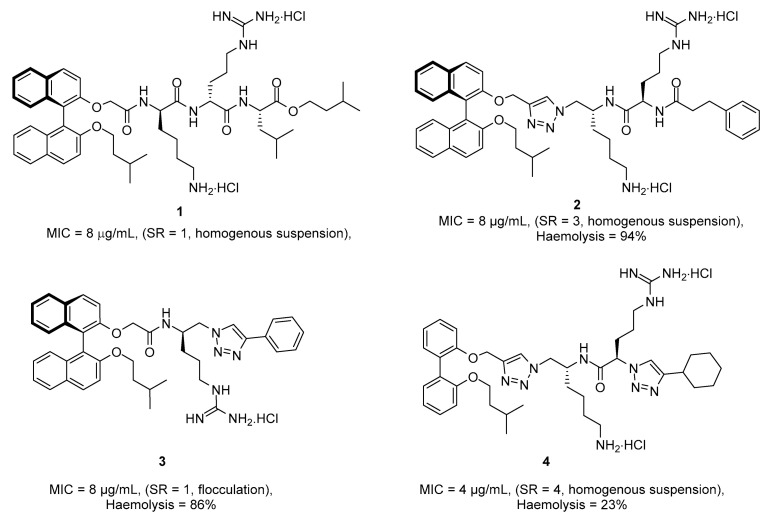
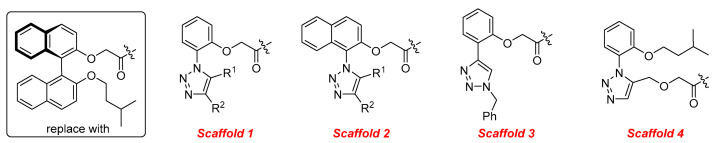
In our earlier work on the development of the cationic amphiphilic 1,1′-binaphthylpeptidomimetics, we established the pharmacophoric importance of a hydrophobic head group (e.g., a binaphthyl moiety) connected to a dicationic peptide in the development of broad-spectrum antibacterial agents. This led to the identification of compound 1 with potent antibacterial activity against drug resistant Gram-positive bacteria with potential for topical applications (Figure 1) [25]. More recent work in our laboratory has identified compounds 2–4 from a class of small molecule cationic amphiphilic 1,1′-biarylpeptidomimetics that exert antibacterial activity through cytoplasmic membrane disruption [13,14]. These compounds have IC50 values of 4–8 μg/mL against C. difficile (Figure 1). The efficacy of these compounds at treating CDI in an in vivo murine CDI model was assessed against vancomycin as a positive control with 10% DMSO as the negative control. Compound 2 appeared to protect the mice from disease at the 24 h point with a 50% survival rate (2/4 mice) vs. 0% survival in the 10% DMSO group; this was not statistically significant due to the small sample size. These results clearly showed that compound 2 exhibited a notable positive effect in the treatment of CDI. Unfortunately compound 3 showed poor solubility with precipitation during preparation in a 10% DMSO solution, and high in vitro hemolytic activity against HEK293 cells. While compound 4 showed promising in vitro properties, it performed poorly in the C. difficile murine model with a survival rate of 60% after 24 h, but a 0% rate after 48 h [13], despite its low hemolytic activity. Despite some positive results, more water-soluble derivatives with lower hemolytic activity for further in vivo murine CDI model studies needed to be developed. To achieve this aim, we replaced the hydrophobic binaphthyl group found in 2 and 3 with an N-arytriazole or N-naphthyltriazole moiety as shown in Figure 2. These modifications should retain the aromatic character of these molecules while inducing a better polarity profile and thereby increasing the water solubility of the overall peptidomimetics. It was not clear at the start what effect these modifications would have on the antibacterial activities of these newly proposed compounds or their specificity for C. difficile over other pathogenic bacteria. Herein, we disclose the results of this investigation.
Figure 1.
Previously published cationic amphiphilic hydrophobic anchored peptidomimetic antimicrobial agents. MIC values against C. difficile in µg/mL. SR = solubility ratio relative to that of compound 1–see Ref [13].
Figure 2.
Hydrophobic scaffold replacements of the binaphthyl moiety for the target peptidomimetics.
2. Results and Discussion
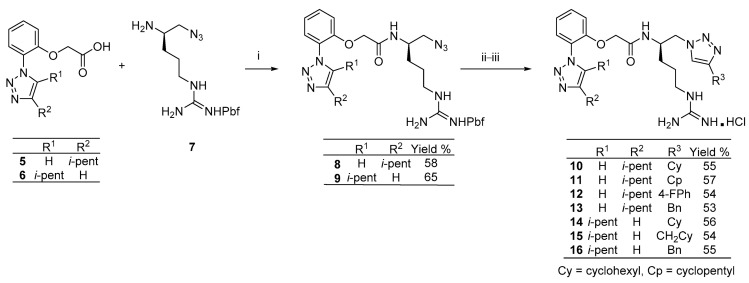
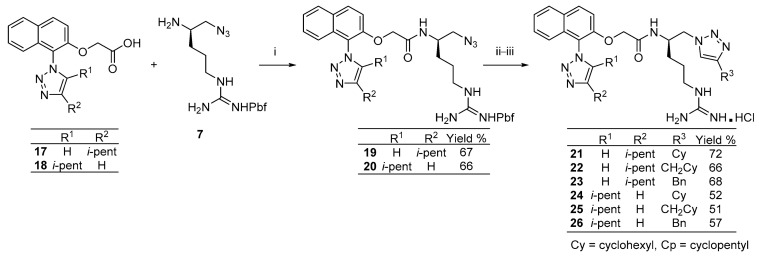
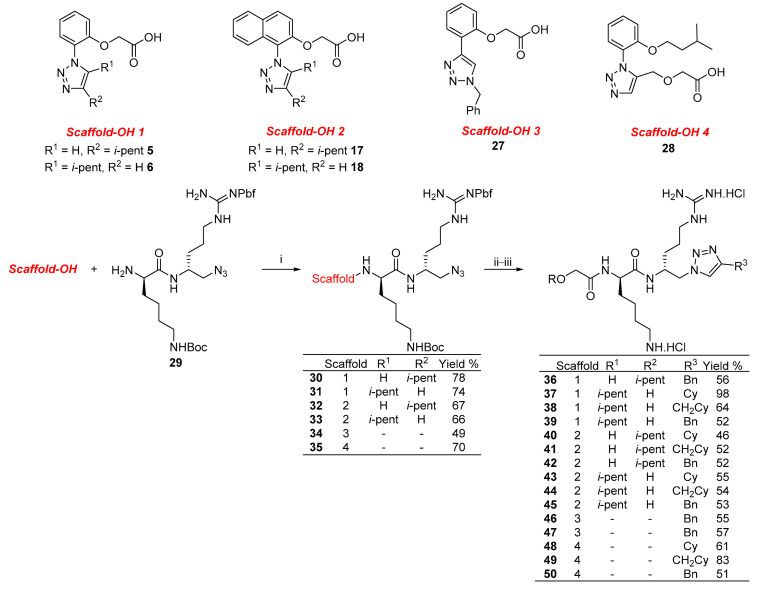
Preparation of the target N-arytriazole or N-naphthyltriazole peptidomimetics required the synthesis of the carboxylic acid derivatives 5, 6, 17, 18, 27, and 28 based on scaffolds 1–4 (Figure 2); the syntheses of acid 17 is described in the experimental section with the other acid syntheses described in the Supporting Information.
The synthesis of the new peptidomimetic derivatives is described in Scheme 1, Scheme 2 and Scheme 3. In a typical example, derivative 40 (Scheme 3) was generated starting from acid 17 coupling with the protected azidodipetide 29 under standard peptide coupling conditions (EDCI/HOBt) [26,27] to give amide 32 in 67% yield. This was followed by a standard copper-catalyzed azide-alkyne cycloaddition reactions [28] with ethenylcyclohexane to give the corresponding 1,4-disubstituted 1,2,3-triazole product which was deprotected using TFA/CH2Cl2/H2O followed by treatment with ethereal HCl to yield the dicationic amphiphile 40 in 46% yield over two steps. The synthesis of the additional mono- and dicationic peptidomimetic amphiphiles 10–16, 21–26, and 36–50 followed an analogous strategy and is summarized in Scheme 1, Scheme 2 and Scheme 3 with experimental and characterization details provided in the Supporting Information.
Scheme 1.
Synthesis of N-aryltriazole monocationic peptidomimetics 10–16. i. HOBt (1.1 eq), EDCI.HCl (1.1 eq), Et3N (1.0 eq), CH2Cl2, rt, 16 h. ii. R3C≡CH, CuSO4.5H2O (0.2 eq), Na.ascorbate (0.4 eq), t-BuOH:H2O (4:1), rt, 16 h. iii. TFA/H2O/DCM, rt, 16 h; then HCl in Et2O.
Scheme 2.
Synthesis of N-naphthyltriazole monocationic peptidomimetics 21–26. i. HOBt (1.1 eq), EDC.HCl (1.1 eq), Et3N (1.0 eq), CH2Cl2, rt, 16 h. ii. R3C≡CH, CuSO4.5H2O (0.2 eq), Na.ascorbate (0.4 eq), t-BuOH:H2O (4:1), rt, 16 h. iii. TFA/H2O/DCM, rt, 16 h; then HCl in Et2O.
Scheme 3.
Synthesis of dicationic peptidomimetics 36–50-i. HOBt (1.1 eq), EDC.HCl (1.1 eq), Et3N (1.0 eq), CH2Cl2, rt, 16 h. ii. R′C≡CH, CuSO4.5H2O (0.2 eq), Na.ascorbate (0.4 eq), t-BuOH:H2O (4:1), rt, 16 h. iii. TFA/H2O/DCM, rt, 16 h; then HCl in Et2O.
The N-arytriazole and N-naphthyltriazole peptidomimetics were subjected to antimicrobial screening. In the first instance, minimum inhibitory concentrations (MICs) were determined against a panel of Gram-positive (including two strains of C. difficile) and Gram-negative pathogenic bacteria with vancomycin and the commercially available peptide colistin as positive controls, respectively; the MICs are displayed in Table 1. The compounds were then tested against a second panel of Gram-positive and Gram-negative pathogenic bacteria and two fungi strains at the Community for Open Antimicrobial Drug Discovery (CO-ADD)-these results are reported in the Supporting Information (Table S1) [29]. A cytotoxicity concentration (CC50) assay was also performed by CO-ADD; the synthesized compounds were tested at concentrations ≤32 µg/mL on human embryonic kidney cells (HEK293 cells; ATCC CRL-1573) while hemolysis assays for lysis of human erythrocytes were also performed. Vancomycin, colistin, fluconazole, and tamoxifen were used as positive controls (see Table 1 for details). The CC50 and HC50 values are also shown in Table 1.
Table 1.
Preliminary antibacterial screening a.
| Compound |
C. difficile ATCC 700057 |
C. difficile 132 b (RT027) |
S. aureus ATCC 29213 |
S. aureus NCTC 10442 c |
E. faecalis ATCC 29212 |
S. pneumoniae ATCC 49619 |
E. coli ATCC 25922 |
CC50 d | HC50 e | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 32 | 64 | 32 | 32 | 32 | 16 | 128 | >32 | >32 |
| 2 | 11 | 32 | 64 | 32 | 32 | 32 | 16 | 128 | >32 | >32 |
| 3 | 12 | 32 | 64 | 32 | 32 | 32 | 16 | 128 | >32 | >32 |
| 4 | 13 | 32 | 64 | 32 | 32 | 32 | 16 | 128 | >32 | >32 |
| 5 | 14 | 128 | 128 | 32 | 32 | 32 | 32 | >128 | >32 | >32 |
| 6 | 15 | 64 | 64 | 16 | 16 | 16 | 16 | 128 | >32 | >32 |
| 7 | 16 | 32 | 32 | 32 | 32 | 32 | 32 | >128 | >32 | >32 |
| 8 | 21 | 128 | >128 | 8 | 8 | 16 | 16 | 128 | >32 | >32 |
| 8 | 22 | 32 | 32 | 4 | 4 | 8 | 8 | 32 | >32 | >32 |
| 9 | 23 | 32 | 32 | 4 | 4 | 4 | 4 | 64 | >32 | >32 |
| 10 | 24 | 32 | 32 | 8 | 8 | 8 | 8 | 32 | 21.9 | >32 |
| 11 | 25 | 32 | 64 | 4 | 4 | 8 | 8 | 16 | >32 | 10.6 |
| 12 | 26 | 64 | >128 | 8 | 8 | 16 | 4 | 64 | 23.5 | >32 |
| 13 | 36 | 128 | 128 | 32 | 32 | 64 | 16 | 128 | >32 | >32 |
| 14 | 37 | 64 | 64 | 16 | 32 | 16 | 8 | 64 | >32 | >32 |
| 15 | 38 | 128 | >128 | 128 | 128 | >128 | 128 | >128 | 32 | 32 |
| 16 | 39 | 64 | 32 | 32 | 64 | 32 | 16 | 128 | >32 | >32 |
| 17 | 40 | 8 | 8 | 16 | 16 | 32 | 16 | 64 | 32 | 32 |
| 18 | 41 | 16 | 16 | 8 | 8 | 8 | 16 | 128 | 16 | 32 |
| 19 | 42 | 8 | 8 | 8 | 8 | 8 | 8 | 32 | 32 | 32 |
| 20 | 43 | 128 | 128 | 16 | 16 | 64 | 4 | 64 | >32 | >32 |
| 21 | 44 | 32 | 32 | 8 | 4 | 16 | 4 | 64 | >32 | >32 |
| 22 | 45 | 128 | 128 | 16 | 16 | 64 | 4 | 128 | >32 | >32 |
| 23 | 46 | 128 | 128 | 32 | 32 | 64 | 16 | 128 | >32 | >32 |
| 24 | 47 | 128 | 128 | 32 | 32 | 64 | 16 | 128 | >32 | >32 |
| 25 | 48 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | 24.5 |
| 26 | 49 | >32 | >32 | 32 | >32 | >32 | >32 | >32 | >32 | 17.1 |
| 27 | 50 | >32 | >32 | 32 | >32 | >32 | >32 | >32 | >32 | 19.8 |
| vanc | 0.5 | 0.5 | 1 | 1 | 4 | 1 | >16 | - | - | |
| colistin | 0.25 | 0.25 | 0.25 | 0.125 | ||||||
| tamoxifen | 13.1 |
a Values are reported as MIC values in μg/mL. b C. difficile PCR Ribotype (RT027). c Methicillin resistant S. aureus (MRSA). d Cytotoxicity; determined on HEK293 cells. e Hemolysis; HC50 values determined by lysis of human erythrocytes and % hemolysis was determined by lysis of sheep erythrocytes. Vanc = vancomycin. Coloured cells refer to the same activities.
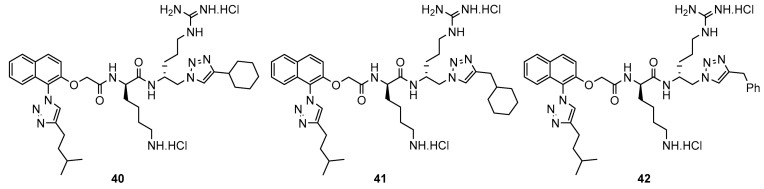
Preliminary screening revealed that compared to the previously synthesized compounds 1–4, the new N-naphthyltriazole dicationic derivatives 40 and 42 showed the best activities against the two C. difficile RT 027 strains, ATCC 700,057 and 132 with a similar activity of 8 µg/mL compared to compounds 1, 3, and 4. However, they were generally less active against the other Gram-positive and Gram-negative bacteria (Table 1). The relative solubility ratios (relative to compound 1) [13] for 40 and 42 were 5 and 4 with CLogP values of 4.46 and 4.39, respectively, when compared to 1 with a ClogP of 7.47. Therefore, despite the better solubility profiles of these compounds, they failed to show better activity against C. difficile. However, the increased solubility (enhanced polarity) of derivatives 40–42 could be a factor in the reduced activities against the other bacteria, when compared to compounds 1–4 (see Table 2). None of the other derivatives synthesized in this study showed appreciable activity against C. difficile with MIC values ranging from 32 to 128 µg/mL (Table 1). Importantly, the remaining anti-bacterial results were generally poor, however for these specific derivatives, these reduced activities could indicate reduced capacity to interfere with normal GIT microbiota (Table 2). Compounds 40 and 42 showed a slight reduction in cytotoxicity against HEK293 cells compared to compounds 2 and 4. The hemolytic activity of these compounds was 32 µg/mL against human erythrocytes, 2-fold more than their IC50 values against C. difficile.
Table 2.
Antimicrobial, cytotoxicity, and hemolytic activities of the three most active derivatives synthesized in this study a.
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound |
C. difficile ATCC 700057 |
C. difficile 132 b (RT027) |
S. aureus ATCC 29213 |
S. aureus ATCC 43300 c |
S. aureus NCTC 104422 c,d |
E. faecalis ATCC 29212 |
S. pneumoniae ATCC 49619 |
E. coli ATCC 25922 |
CC50 e | HC50 f |
| 40 | 8 | 8 | 16 | 8 | 16 | 32 | 16 | 64 | 32 | 32 |
| 41 | 16 | 16 | 8 | 4 | 8 | 8 | 16 | 128 | 16 | 32 |
| 42 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 32 | 32 | 32 |
| 1 [30] | 8 g | 8 h | 2 | - | - | 2 | 2 | 16 | - | - |
| 2 [31] | - | 32 | 4 | 2 | 4 | 4 | 4 | 8 | 27.4 | 94% i |
| 3 [19] | 8 g | 8 h | 2 | - | 2 | 4 | 8 | >128 | - | - |
| 4 [31] | - | 8 | 8 | 4 | 4 | 8 | 4 | 8 | 14.2 | 23% i |
| vanc | 0.5 | 0.5 | 1 | - | 1 | 4 | 1 | >16 | - | - |
a Values are reported as MIC values in μg/mL. b C. difficile PCR Ribotype (RT027). c Methicillin resistant S. aureus (MRSA). d Testing performed by the Community for Open Antimicrobial Drug Discovery (CO-ADD). e Cytotoxicity; determined on HEK293 cells. f Hemolysis; determined by lysis of sheep erythrocytes g C. difficile strain tested M7404 (RT027). h C. difficile strain tested R20291 (RT207). i % hemolysis at 50 µg/mL. vanc = vancomycin. Coloured cells refer to the same activities.
Analysis of the anti-bacterial activities against other bacterial species indicated that the monocationic naphthyltriazole derivatives 21–26 showed appreciable activity against Staphylococcus aureus (including an MRSA strain) with MIC values between 4 and 8 µg/mL (Table 1). Additionally, compound 21 had notable MIC values of 4 µg/mL against Enterococcus faecalis and Streptococcus pneumoniae. An overview of activity shown in Table 1 showed “pockets” of activities focused on the naphthyl-based derivatives (21–26 and 40–45, columns 1–4), with the monocationic examples (21–26) producing better outcomes against the Gram positive strains. The second screening results (Table S1, Supporting Information) were consistent with these results with analogous trends in activity against an additional S. aureus strain.
The secondary testing (Table S1, Supporting Information) also identified compounds 21, 25, and 40–46 as having activity against the fungal strain Cryptococcus neoformans var. grubii (ATCC208821) (MIC 4-8 µg/mL).
3. In Vivo Assay: Murine Model of CDI
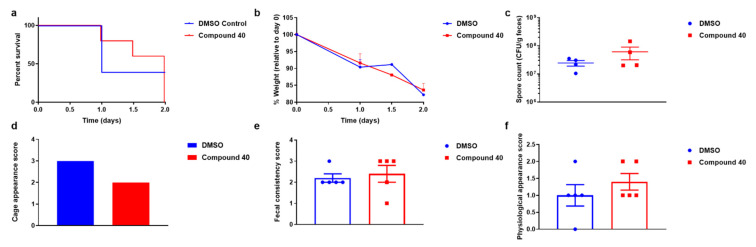
Compound 40 was selected for further evaluation as an effective treatment for C. difficile using a murine model of CDI study because of its sustained antimicrobial potency against C. difficile and its better water solubility profile. The results from these studies are summarized in Figure 3.
Figure 3.
C57BL/6J mice (n = 5 per group) were infected with 105 spores of C. difficile strain M7404 (RT027). Six hours post-infection and then every 12 h thereafter, mice were administered either 10% DMSO (blue) or 2.5 mg (100 mg/kg in 10% DMSO) of compound 40 (red) by oral gavage. Mice were monitored daily for survival (a) and weight loss relative to day 0, which was the day of infection (b). Fecal spore load at 1-day post-infection was determined by plating (c). Data are presented as CFU/gram feces, with each point representing a single mouse. Mouse cages were scored on day 1 post-infection for appearance (d) and mice were individually assessed for fecal consistency (e) and physiological appearance (f). Data represent the mean ±S.E.M. and statistical significance was assessed using a log-rank (Mantel–Cox) test or one-way ANOVA with a post hoc Tukey’s multiple comparison test.
The mice treated with compound 40 (red) showed delayed disease onset compared to mice treated with DMSO (blue; Figure 3), although they still succumbed to infection by day 2. Notably, at day 1 post-infection, mice treated with compound 40 showed 40% greater survival compared with mice treated with DMSO (Figure 3a), although there was no effect on mouse weight (Figure 3b), or spore numbers shed in the feces of these animals (Figure 3c), suggesting that compound 40 was not impacting C. difficile colonization. Furthermore, on day 1 post-infection, treatment with compound 40 resulted in a lower overall cage appearance score when compared to DMSO (Figure 3d), which suggested that this compound was delaying diarrheal onset although there was no significant difference in individual fecal score (Figure 3e) or physiological appearance score (Figure 3f) detected between the two groups of mice (Figure 3e). Thus, collectively these data suggest that compound 40 may reduce the severity of disease caused by C. difficile.
4. Materials and Methods
Synthetic methods and general characterization and analysis were as described previously [13].
Notes and other considerations. Known reagents that were not available commercially were prepared as reported using known methods and is detailed in the Supporting Information, [14,32,33,34,35].
4.1. General Synthesis Procedures
4.1.1. General Procedure I: Alkylation of Phenols (with Ethyl Bromoacetate)
A solution of the phenol (1 eq) in dry DMF (5 mL/mmol substrate) was stirred during the addition of K2CO3 (3 eq). Ethyl bromoacetate (1.3 eq) was added at room temperature and stirring was continued at rt for 12 h, before being diluted with EtOAc (2 × 50 mL). The resulting mixture was washed with water (2 × 50 mL), brine (2 × 50 mL), dried (MgSO4), filtered, and concentrated under vacuum. The residue was subjected to silica gel flash column chromatography to afford the desired ester product.
4.1.2. General Procedure II: Ester Hydrolysis
A solution of the ester (1 eq) in ethanol (10 mL/mmol substrate) was stirred followed by the addition of 7% KOH solution (5 mL/mmol) at rt. The mixture was stirred at rt for 2 h, then acidified with 1 M HCl (25 mL). The resulting mixture was extracted with EtOAc (2 × 25 mL) and the combined extracts washed with brine (50 mL), dried (MgSO4), filtered, and concentrated under vacuum to afford the acid product.
4.1.3. General Procedure III: Amide Coupling
A mixture of the amine (1.0 eq), carboxylic acid (1.0 eq), EDC.HCl (1.2 eq), HOBt (1.1 eq), and TEA (1 eq) in dichloromethane/acetonitrile solution (10 mL/mmol amine) was stirred at rt for the specified time. The mixture was concentrated (if >5.0 mL dichloromethane/acetonitrile), and then the resulting residue dissolved in EtOAc (25 mL for reactions that contained ≤1.0 mmol amine or 25 mL/mmol amine for larger scale reactions) and washed with aqueous HCl (1.0 M–2 × 25 mL), saturated aqueous NaHCO3 (3 × 25 mL), and brine (1 × 25 mL). The organic solution was dried (MgSO4), filtered, concentrated and subjected to further purification via flash chromatography (if required) to furnish the targeted amide product.
4.1.4. General Procedure IV: Copper-Catalyzed Azide-Alkyne Cycloaddition
To a stirred solution of the azide (1.0 eq) and alkyne (2.0–3.0 eq) in tert-butanol/water (4:1) at rt was added CuSO4∙5H2O (0.2 eq), followed by sodium ascorbate (0.4 eq). The reaction was stirred at rt (unless noted otherwise) for the specified time. To the mixture was added aqueous saturated NH4Cl solution (1 mL), and water (20 mL) with the mixture then extracted with EtOAc (20 mL for reactions that contained ≤1.0 mmol azide or 20 mL/mmol azide for larger scale reactions). The organic layers were back-washed with water (2 × 25 mL), brine (2 × 25 mL), then dried (MgSO4), filtered, concentrated under vacuum and subjected to flash chromatography to afford the desired 1,4-disubstituted 1,2,3-triazole product.
4.1.5. General Procedure VII: Amine Deprotection (N-Boc and/or N-Pbf Removal)
To a solution of the N-protected amine (1.0 eq) in CH2Cl2 (30 mL/mmol substrate) (if the substrate contained an N-Pbf moiety, H2O (20.0 eq) was added to the solution) was added TFA (30.0 mL/mmol substrate) and then stirred at rt overnight (>16 h). The solvent was removed and the resulting residue dissolved in CH2Cl2 (30 mL/mmol substrate). Excess anhydrous HCl (2.0 M in Et2O, 15 mL/mmol substrate, 30.0 eq) was added and the solvent was then removed. The residue was then dissolved in a minimal volume of CH2Cl2 (or MeOH) and excess Et2O (25 mL for ≤0.1 mmol substrate) was added, resulting in a precipitate of the hydrochloride salt of the amine. The reaction mixture was filtered; the resulting filtrate collected, concentrated, triturated with Et2O (3 × 20 mL); and the solids then dissolved in MeOH. The solution was concentrated and dried in vacuo to yield the mono or di-hydrochloride salt as a thin, translucent film that usually required scratching with a spatula, producing a fine hygroscopic powder or amorphous gum.
4.2. Representative Synthesis of Compound 40
4.2.1. Ethyl 2-((1-iodonaphthalen-2-yl)oxy)acetate
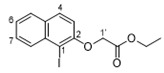
Following General Procedure I, 1-iodonaphthol (1.00 g, 3.70 mmol), K2CO3 (1.53 g, 11.11 mmol), and ethyl bromoacetate (0.80 g, 4.81 mmol) were stirred in DMF (8 mL) at rt for 16 h to give the titled ester (0.68 g, 52%) as a pale yellow waxy solid after flash chromatography over silica gel (EtOAc/n-hexane-10:90). TLC (EtOAc/n-hexane-20:80): Rf = 0.6; 1H NMR (400 MHz, CDCl3) δ 8.16 (d, J = 7.2 Hz, 1H, H8), 7.78 (d, J = 7.2 Hz, 1H, H5), 7.72 (d, J = 8.0 Hz, 1H, H4), 7.54 (t, J = 7.2 Hz, 1H, H7), 7.39 (t, J = 7.2 Hz, 1H, H6), 7.08 (d, J = 8.0 Hz, 1H, H3), 4.80 (s, 2H, H1′), 4.27 (q, J = 5.6 Hz, 2H, OCH2CH3), 1.29 (t, J = 5.6 Hz, 3H, OCH2CH3); 13C NMR (101 MHz, CDCl3) δ 168.7 (C = O), 155.6 (C2), 135.8 (C8a), 131.7 (C4a), 130.6 (C4), 130.5 (C8), 128.4 (C7), 128.3 (C5), 121.1 (C6), 114.4 (C3), 89.47 (C1), 67.6 (C1′), 61.7 (OCH2CH3) 14.3 (OCH2CH3); IR (neat) max 2981, 1756, 1622, 1593, 1502, 1462, 1349, 1291, 1200, 1151, 1134, 1096, 1028, 801, 764, 747 cm−1; MS (ESI +ve) m/z 379 ([M + Na]+, 100%); HRMS (ESI + ve TOF) calcd for C14H13O3NaI 378.9807, found 378.9801 ([M + Na]+).
4.2.2. Ethyl 2-((1-(4-isopentyl-1H-1,2,3-triazol-1-yl)naphthalen-2-yl)oxy)acetate
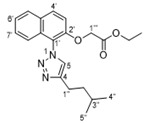
To a stirred solution of ethyl 2-(2-iodophenoxy)acetate (0.20 g, 0.54 mmol), 5-methyl-1-hexyne (0.16 g, 1.64 mmol), CuI (0.02 g, 0.11 mmol), NaN3 (0.04 g, 0.60 mmol), and sodium ascorbate (0.04 g, 0.22 mmol) in DMSO (2.5 mL) in H2O (0.5 mL) was added racemic trans-N,N′-dimethyl cyclohexane-1,2-diamine (0.016 g, 0.11 mmol) at rt under a nitrogen atmosphere. The reaction mixture was stirred and heated at 75 °C for 16 h. The reaction was cooled to rt and aqueous saturated NH4Cl solution (3 mL) was added, and the mixture was extracted with EtOAc (2 × 25 mL). The combined extracts were washed with water (25 mL), brine (25 mL) and dried (MgSO4). The solution was filtered, concentrated under vacuum and the residue was subjected to silica gel flash column chromatography (EtOAc/n-hexane-10:90 → 100:0) to afford the titled compound (0.05 g, 25%) as a yellow waxy solid. TLC (EtOAc/n-hexane-33:67); Rf = 0.4; 1H NMR (400 MHz, CDCl3) δ 7.97 (d, J = 7.2 Hz, 1H, H8′), 7.84 (d, J = 6.4 Hz, 1H, H5′), 7.67 (s, 1H, H5), 7.49–7.41 (m, 2H, H6′/H7′), 7.27–7.25 (m, 2H, H3′/H4′), 4.67 (s, 2H, H1′′′), 4.22 (q, J = 5.6 Hz, 2H, OCH2CH3), 2.89 (t, J = 5.6 Hz, 2H, H1′′), 1.73–1.67 (m, 3H, H2′′/H3′′), 1.26 (t, J = 5.6 Hz, 3H, OCH2OCH3), 0.99 (d, J = 4.0 Hz, 6H, H4′′/H5′′); 13C NMR (101 MHz, CDCl3) δ 168.5 (C = O), 150.5 (C2′), 148.1 (C8a′), 131.6 (C4), 131.3 (C4a′), 129.5 (C4′), 128.5 (C5′), 127.9 (C7′), 125.3 (C8′), 124.7 (C6′), 122.1 (C5), 121.3 (C3′), 114.3 (C1′), 66.7 (C1′′′), 61.6 (OCH2CH3), 38.6 (C2′′), 27.9 (C1′′), 23.8 (C3′′), 22.5 (C4′′/C5′′; Observed by gHMBC), 14.2 (OCH2CH3); IR (neat) max 2954, 2928, 2868, 1748, 1632, 1600, 1513, 1483, 1454, 1430, 1366, 1288, 1206, 1150, 1117, 1087, 1042, 806, 749 cm−1; MS (ESI +ve) m/z 390 ([M +Na]+, 100%); HRMS (ESI +ve TOF) calcd for C21H26N3O3 368.1974, found 368.1985 ([M + H]+).
4.2.3. 2-((1-(4-Isopentyl-1H-1,2,3-triazol-1-yl)naphthalen-2-yl)oxy)acetic acid (17)
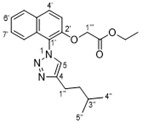
Following General Procedure II, ethyl 2-((1-(4-isopentyl-1H-1,2,3-triazol-1-yl)naphthalen-2-yl)oxy)acetate (0.07 g, 0.19 mmol) and 7% KOH solution (0.5 mL) were stirred in ethanol (2 mL) at rt for 2 h to give after acidification the acid 17 (0.04 g, 62%) as a white solid. M.P: 152–154 °C. TLC (EtOAc/n-hexane-100:0): Rf = 0.2; 1H NMR (500 MHz, CDCl3) δ 8.00 (d, J = 9.0 Hz, 1H, H8′), 7.88 (d, J = 7.5 Hz, 1H, H5′), 7.69 (s, 1H, H5), 7.54–7.46 (m, 2H, H6′/H7′), 7.47–7.29 (m, 2H, H3′/H4′), 4.78 (s, 2H, H1′′′), 2.91–2.87 (m, 2H, H1′′), 1.71–1.68 (m, 3H, H2′′/H3′′), 0.98 (d, J = 6.0 Hz, 6H, H4′′/H5′′), COOH resonance was not observed; 13C NMR (126 MHz, CDCl3) δ 170.6 (C = O), 150.4 (C2′), 148.4 (C8a′), 132.2 (C4), 130.7 (C4a′), 129.6 (C4′), 128.8 (C5′), 128.2 (C7′), 125.6 (C8′), 124.9 (C6′), 121.7 (C5), 120.9 (C3′), 114.4 (C1′), 66.8 (C1′′′), 38.5 (C2′′), 28.0 (C1′′), 23.7 (C3′′), 22.6 (C4′′/C5′′; Observed by gHMBC); IR (neat) max 3147, 2954, 2929, 2868, 1731, 1631, 1600, 1514, 1483, 1429, 1366, 1284, 1213, 1151, 1118, 1087, 1062, 923, 806, 748 cm−1; MS (ESI +ve) m/z 362 ([M + Na]+, 40%), 340 ([M + H]+, 100%); HRMS (ESI + ve TOF) calcd for C19H22N3O3 340.1661, found 340.1667 ([M + H]+).
4.2.4. (9H-Fluoren-9-yl)methyl tert-butyl ((R)-6-(((R)-1-azido-5-(2-((2,2-dimethyl-2,3-dihydro benzofuran-5-yl)sulfonyl)guanidino)pentan-2-yl)amino)-6-oxohexane-1,5-diyl) dicarbamate
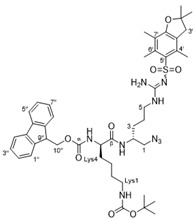
To a reaction vessel charged with azide 7 [30] (1.38 g, 3.16 mmol), Fmoc-L-Lys(Boc)-OH (1.62 g, 3.50 mmol), EDCI (0.67 g, 3.50 mmol) and HOBt (0.53 g, 3.50 mmol) was added CH2Cl2 (10 mL) and the mixture was stirred at rt for 12 h. The reaction mixture was concentrated and diluted with water (100 mL) and extracted with EtOAc (3 × 100 mL). The organic extracts were combined and washed with HCl (1 M–100 mL), aqueous NaHCO3 (100 mL), brine (25 mL), dried (MgSO4) and concentrated to give a pale-yellow residue. This residue was purified via flash chromatography over SiO2 (MeOH/CH2Cl2 = 4:96) to afford the titled compound as an off-white foam (1.50 g, 54%). TLC (MeOH/CH2Cl2–10:90) Rf = 0.52; 1H-NMR (400 MHz, CDCl3) δ 7.77–7.70 (m, 2H, H4′′/H5′′), 7.55 (d, J = 7.5 Hz, 2H, H1′′/H8′′), 7.55 (brs, 1H, βCONH), 7.41–7.32 (m, 2H, H2′′/H7′′), 7.29–7.21 (m, 2H, H3′′/H6′′), 7.17 (brs, 1H, αCONH), 6.31–6.24 (m, 2H, NH2 (guanidine)), 6.19–6.09 (brs, 1H, N5-H), 4.82–4.72 (brs, 1H, LysN1-H), 4.33 (d, J = 7.4 Hz, 2H, H10′′), 4.25–4.07 (m, 2H, Lys5/H9′′), 4.07–3.97 (m, 1H, H2), 3.41–3.23 (m, 2H, H1), 3.23–2.98 (m, 4H, H5/Lys1), 2.89 (s, 2H, H3′), 2.55 (s, 3H, C6′-CH3), 2.48 (s, 3H, C4′-CH3), 2.06 (s, 3H, C7′-CH3), 1.67 (s, 6H, C2′-CH3), 1.55–1.35 (m, 19H, H3/H4/Lys2/Lys3/Lys4/C(CH3)3); 13C NMR (101 MHz, CDCl3) δ 172.7 (Cβ), 158.8 (C7a′), 156.7 (Cα), 156.4 (C = N), 156.2 (COOC(CH3)3), 143.85 (C1a′′or C8a′′), 143.83 (C8a′′ or C1a′′), 143.82 (C4a′′ or C5a′′), 143.6 (C5a′′ or C4a′′), 138.3 (C3a′), 132.8 (C6′), 132.2 (C4′), 127.8 (C3′′/C6′′), 127.1 (C4′′/C5′′), 125.0 (C2′′/C7′′), 124.7 (C5′), 120.0 (C1′′/C8′′), 117.6 (C7′), 86.4 (C2′), 79.3 (C(CH3)3), 67.3 (C10′′), 55.1 (Lys5), 54.8 (C1), 48.8 (C2), 47.0 (C9′′), 43.2 (C3′), 40.9 (C5), 39.9 (Lys1), 31.9 (Lys2), 29.5 (Lys4), 29.3 (C3), 28.6 (C2′-(CH3)2), 28.4 (C(CH3)3), 25.5 (C4), 22.5 (Lys3), 19.3 (C6′-CH3), 17.9 (C4′-CH3), 12.5 C7′-CH3); IR (neat) max 3322, 2101, 1634, 1548, 1450, 1248, 1165, 1092, 739, 567 cm−1; MS (ESI +ve) m/z 888 ([M + H]+), 910 ([M + Na]+); HRMS (ESI +ve TOF) calcd for C45H61N9O8SNa 910.4262, found 910.4218 ([M + Na]+).
4.2.5. Tert-butyl ((R)-5-amino-6-(((R)-1-azido-5-(2-((2,2-dimethyl-2,3-dihydrobenzofuran-5-yl)sulfonyl)guanidino)pentan-2-yl)amino)-6-oxohexyl)carbamate (31)
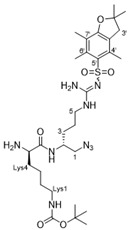
To a solution of the above Fmoc-protected amine (1.50 g, 1.69 mmol) in acetonitrile (15 mL) was added piperidine (0.25 mL, 1.5 eq.) and the reaction was stirred vigorously at rt for 12 h. The reaction mixture was diluted with MeOH (50 mL) and extracted with hexane (50 mL) multiple times until TLC analysis showed no byproduct (dibenzofulvene piperidine adduct) present in the MeOH layer. The MeOH extract was concentrated under reduced pressure to give 31 as an off-white foam (0.80 g, 71%). TLC (MeOH/CH2Cl2–10:90) Rf = 0.2; 1H-NMR (500 MHz, CDCl3) δ 7.61 (brs, 1H, N2-H), 6.42–6.20 (m, 3H, N5-H/NH2 (guanidine)), 4.82–4.72 (m, 1H, LysN1-H), 4.12–3.99 (m, 1H, Lys5), 3.46–3.29 (m, 3H, H1/H2), 3.29–3.14 (m, 2H, H5), 3.14–3.04 (m, 2H, Lys1), 2.96 (s, 2H, C3′), 2.58 (s, 3H, C6′-CH3), 2.52 (s, 3H, C4′-CH3), 2.10 (s, 3H, C7′-CH3), 1.62–1.31 (m, 25H, H3/H4/Lys2/Lys3/Lys4/C(CH3)3/C2′-(CH3)2), N5H2 resonance was not observed; 13C-NMR (126 MHz, CDCl3) δ 158.8 (C7a′), 156.6 (C = O), 156.4 (C = N), 138.5 (C3a′), 133.2 (C4′), 132.4 (C6′), 124.7 (C5′), 117.6 (C7′), 86.5 (C2′), 79.4 ((C(CH3)3), 55.1 (Lys5), 55.0 (C1), 46.9 (C2), 43.4 (C3′), 40.9 (C5), 40.4 (Lys1), 34.7 (Lys4), 30.1 (Lys2), 29.8 (C3), 28.8 (C2′-(CH3)2), 28.6 (C(CH3)3), 25.8 (C4), 22.7 (Lys3), 19.4 (C6′-CH3), 18.1 (C4′-CH3), 12.6 (C7′-CH3), COO(C(CH3)3) resonance was not observed; IR (neat) max 3327, 2101, 1685, 1620, 1551, 1454, 1366, 1278, 1250, 1168, 1094, 665, 569 cm−1; MS (ESI +ve) m/z 666 ([M + H]+); HRMS (ESI +ve TOF) calcd for C30H52N9O6S 666.3761, found 666.3741 ([M + H]+).
4.2.6. Tert-butyl ((R)-6-(((R)-1-azido-5-(2-((2,2,4,6,7-pentamethyl-2,3-dihydrobenzofuran-5-yl)sulfonyl)guanidino)pentan-2-yl)amino)-5-(2-((1-(4-isopentyl-1H-1,2,3-triazol-1-yl)naphthalen-2-yl)oxy)acetamido)-6-oxohexyl)carbamate (32)
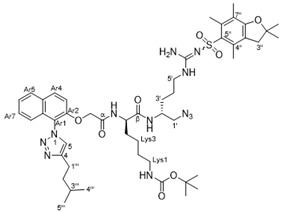
Following General Procedure III, 2-((1-(4-isopentyl-1H-1,2,3-triazol-1-yl)naphthalen-2-yl)oxy)acetic acid 17 (0.12 g, 0.35 mmol), tert-butyl ((R)-5-amino-6-(((R)-1-azido-5-(2-((2,2-dimethyl-2,3-dihydrobenzofuran-5-yl)sulfonyl)guanidino)pentan-2-yl)amino)-6-oxohexyl) carbamate 57 (0.24 g, 0.35 mmol), EDCI.HCl (0.08 g, 0.39 mmol), HOBt (0.06 g, 0.39 mmol), and TEA (0.03 g, 0.35 mmol) were stirred in CH2Cl2 (5 mL) at rt for 12 h to give the acetamide 65 (0.22 g, 64%) as an off-white solid. M.P: 236–238 °C. TLC (MeOH/CH2Cl2-10:90): Rf = 0.5; 1H NMR (400 MHz, CDCl3) δ 8.05 (d, J = 8.5 Hz, 1H, Ar8), 7.89 (d, J = 8.5 Hz, 1H, Ar5), 7.65 (s, 1H, H5), 7.54–7.47 (m, 2H, Ar4/βCONH), 7.37 (d, J = 8.5 Hz, 1H, Ar7), 7.26–7.19 (m, 2H, Ar6/Ar3), 6.85 (brs, 1H, αCONH), 6.36–6.08 (m, 3H, N5′-H/NH2 (guanidine)), 5.00 (brs, 1H, LysN1-H), 4.69 (ABq, J = 16.5 Hz, 2H, OCHAHB), 4.42–4.36 (m, 1H, Lys5), 4.02–3.96 (m, 1H, H2′), 3.44–2.94 (m, 6H, H1′/H5′/Lys1), 2.88 (s, 2H, H3′′), 2.88–2.84 (m, 2H, H1′′′), 2.55 (s, 3H, C4′′-CH3), 2.48 (s, 3H, C6′′-CH3), 2.06 (s, 3H, C7′′-CH3), 2.00–1.86 (m, 4H, H4′/Lys4), 1.84–1.60 (m, 7H, H3′/Lys3/H2′′′/H3′′′), 1.44 (s, 6H, C2′′(CH3)2), 1.39 (s, 9H, C(CH3)3), 1.32–1.22 (m, 2H, Lys2), 0.9 (d, J = 5.0 Hz, 6H, H4′′′/H5′′′); 13C NMR (101 MHz, CDCl3) δ 171.9 (βC = O), 168.1 (αC = O), 158.7 (C7a′′), 156.4 (C = N), 150.1 (Ar2), 149.1 (COOC(CH3)3), 138.4 (Ar8a), 133.4 (C4), 132.6 (C4′′), 132.46 (C6′′), 132.44 (C3a′′), 130.6 (C5′′), 129.4 (Ar4), 129.1 (Ar4a), 128.48 (C7′′), 128.47 (Ar5), 125.7 (Ar7), 124.7 (Ar8), 121.1 (C5), 120.3 (Ar6), 117.5 (Ar3), 113.8 (Ar1), 86.5 (C2′′), 79.2 (C(CH3)3), 68.0 (OCHAHB), 54.8 (Lys5), 53.6 (C1′), 43.4 (C2′), 40.8 (C2′′′), 40.2 (C5′), 38.6 (C3′′), 38.5 (Lys1), 31.79 (Lys4), 31.74 (C3′), 29.4 (Lys2), 28.7 (C2′′-(CH3)2), 28.6 ((CH3)3), 28.0 (C1′′′), 25.5 (C4′), 23.8 (C3′′′), 22.8 (C4′′′/C5′′′), 22.6 (Lys3), 19.4 (C4′′-CH3), 18.1 (C6′′-CH3), 12.6 (C7′′-CH3); IR (neat) max 3405, 3317, 3415, 3057, 2953, 2868, 2100, 1664, 1631, 1600, 1546, 1514, 1484, 1452, 1406, 1390, 1366, 1265, 1247, 1165, 1106, 1090, 1044, 994, 970, 852, 781, 733, 661, 641 cm−1; MS (ESI +ve) m/z 987 ([M + H]+, 100%); HRMS (ESI +ve TOF) calcd for C49H71N12O8S 987.5239, found 987.5272 ([M + H]+).
4.2.7. (R)-6-Amino-N-((R)-1-(4-cyclohexyl-1H-1,2,3-triazol-1-yl)-5-guanidinopentan-2-yl)-2-(2-((1-(4-isopentyl-1H-1,2,3-triazol-1-yl)naphthalen-2-yl)oxy)acetamido)hexanamide dihydrochloride (40)
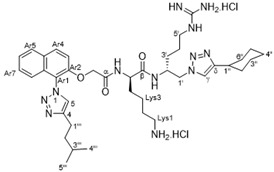
Following General Procedure IV, azide 32 (0.08 g, 0.08mmol), cyclohexylacetylene (0.03 g, 0.24 mmol), CuSO4∙5H2O (0.004 g, 0.01 mmol) and sodium ascorbate (0.006 g, 0.03 mmol) were stirred in t-BuOH (2.0 mL) and H2O (0.5 mL) for 16 h to give the triazole product as an off-white gum after flash chromatography over SiO2 gel (MeOH/CH2Cl2–0:100 → 8:92). Following General Procedure VII, the intermediate (0.06 g, 0.05 mmol) was dissolved in CH2Cl2 (2 mL), treated with H2O (0.02 g, 1.00 mmol) and CF3COOH (1 mL) followed by work-up with ethereal HCl (3 mL) to give the amine salt 40 (0.03 g, 46% over two steps) as an off-white solid that rapidly transitioned to a sticky gum. + 59.1 (c 0.0052, MeOH); 1H NMR (400 MHz, CD3OD) δ 8.30 (s, 1H, H5), 8.29 (s, 1H, Hγ), 8.18 (d, J = 9.2 Hz, 1H, Ar8), 7.98 (d, J = 7.5 Hz, 1H, Ar5), 7.61 (ddd, J = 9.2, 9.2, 1.7 Hz, 1H, Ar7), 7.57–7.49 (m, 2H, Ar6/Ar4), 7.14 (d, J = 8.3 Hz, 1H, Ar3), 4.93–4.89 (m, 2H, OCHAHB), 4.77–4.72 (m, 1H, H1′), 4.59–4.53 (m, 1H, H1′), 4.37–4.32 (m, 1H, Lys5), 4.12–4.09 (m, 1H, H2′), 3.18–3.14 (m, 2H, H5′), 2.95–2.91 (m, 2H, Lys1), 2.84–2.78 (m, 3H, H1′′′/H1′′), 2.00–1.96 (m, 2H, Lys4), 1.74–1.60 (m, 14H, H2′′′/H3′′′/Lys2/H3′/H4′/H2′′/H3′′/H4′′/H5′′/H6′′), 1.48–1.21 (m, 7H, Lys3/H2′′/H3′′/H4′′/H5′′/H6′′), 1.01 (d, J = 6.2 Hz, 6H, H4′′′/H5′′′); 13C NMR (101 MHz, CD3OD) δ 173.0 (βC = O), 169.1 (αC = O), 157.1 (C = N), 150.8 (Ar2), 148.7 (C4), 147.6 (Cδ), 132.5 (Ar8a), 130.3 (Ar4), 129.1 (Ar4a), 128.6 (Ar5), 128.0 (Ar7), 126.9 (Ar8), 125.5 (C5), 125.1 (Cγ), 120.2 (Ar6), 119.1 (Ar3), 113.9 (Ar1), 67.4 (OCHAHB), 55.7 (C1′), 53.5 (Lys5), 49.3 (C2′), 40.4 (C5′), 39.0 (Lys1), 37.9 (C2′′′), 33.4 (C1′′), 31.6 (C2′′), 31.5 (C6′′), 30.9 (Lys4), 28.1 (Lys2), 27.5 (C1′′′), 26.5 (C3′), 25.2 (C4′′), 25.0 (C3′′/C5′′), 24.8 (C3′′′), 22.6 (C4′), 22.5 (C4′′′/C5′′′), 21.3 (Lys3); IR (neat) max 3348, 3265, 3202, 3066, 2932, 2860, 1662, 1544, 1514, 1483, 1451, 1384, 1366, 1349, 1279, 1220, 1168, 1117, 1081, 1049, 816, 749, 668, 585 cm−1; MS (ESI + ve) m/z 743 ([M–2HCl + H]+, 60%), 372 ([M–2HCl + H]2+, 100%); HRMS (ESI + ve TOF) calcd for C39H59N12O3 743.4833, found 743.4866 ([M–2HCl + H]+).
4.3. Microbiological Assays
Primary screening (Gram-positive bacteria). Primary MIC assays were performed as described by the Clinical and Laboratory Standards Institute for aerobic [36] and anaerobic [37] bacteria. MIC values for vancomycin were within acceptable QC ranges [38].
Secondary screening (MRSA and Gram-negative bacteria) and cytotoxicity assay–performed by the Community for Open Antimicrobial Drug Discovery (CO-ADD). Samples were provided to CO-ADD [29] for antimicrobial screening by whole cell growth inhibition assays.
Bacterial Inhibition–MIC Assay. These were performed as described previously [13,29].
Cytotoxicity Assay. These were performed as described previously [13,29].
Haemolysis assay (sheep erythrocytes). These were performed as described previously [13].
Hemolysis assay (human erythrocytes)–HC50 determination. These were performed as described previously [13,29].
4.4. In Vivo Murine Model of CDI Treatment
Disease Treatment Model. These experiments were performed as previously described [39,40,41,42]. Mice were humanely killed at the onset of severe disease or at the end of the experiment (day 4), as previously described [43].
Statistical Analysis. Statistical analysis was performed using Prism 7 (GraphPad Software). The Kaplan–Meier survival curves were assessed using a log-rank (Mantel–Cox) test. Weight loss, spore shedding, fecal consistency, and physiological appearance data were analyzed by one-way ANOVA with a post hoc Tukey’s multiple comparison test. Differences in data values were considered significant at a p value of <0.05.
5. Conclusions
This study reported the next generation of hydrophobic anchored cationic peptidomimetics as antibacterial agents, with a focus on targeting CDI. A major aim was to improve the solubility profile of these compounds to allow for sufficient solubility for efficient administration of the drug while maintaining gut availability and antibacterial activity. The naphthyltriazole derivates containing either a monocationic or dicationic amino acid side chain were generally the most effective, with compounds 40 and 42, possessing terminal cyclohexyl and benzyl moieties, respectively, exhibiting MIC values of 8 µg/mL.
Naphthyltriazole 40 was selected for an in vivo murine model trials of CDI but exhibited only mild evidence of in vivo efficacy indicating that further investigation into the structural and biological parameters affecting the in vivo efficacy of these antibacterial peptidomimetics is required, as the observed in vitro efficacy did not translate directly into in vivo efficacy. We have already reported that a correlation exists between increased hemolytic activity and an increase in hydrophobic/cationic ratio [15]; unfortunately, compound 40 exhibited a slight increase in hemolytic activity relative to the majority of tested compounds in this class with an HC50 value of 32 µg/mL. While the selectivity ratio could be more substantial, this is acceptable for the future development of these gastrointestinal focused compounds. We have previously reported a comparative solubility assay for this class of antimicrobial agents with increasing numerical values corresponding to better aqueous solubility relative to compound 1 (which possesses a value of 1) [13]. Compound 40 showed a better solubility ratio with an assay value of 5, relative to our lead compound 2 with a value 3—this is also reflected in the CLogP values of 4.46 and 5.76 for 41 vs. 2, respectively. These outcomes were confirmed with no issues during the mouse model trials with sufficient solubility in the dosage regimen. Variations on the triazole and O-naphthyl substituents could be made in future studies with the view of enhancing antibacterial activity against C. difficile.
Acknowledgments
The authors thank the National Health and Medical Research Council (NHMRC) Australia for financial support (Grant #APP1145760). The authors also thank Meagan James and Chris Evans for assistance with mouse infection experiments.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics10080913/s1, Figures S1–S85: Details of synthesis and characterization data for compounds; Table S1: Secondary antimicrobial screening a–(bacteria and fungi), Murine model studies experimental procedures.
Author Contributions
Conceptualization, P.A.K. and S.G.P.; methodology, P.A.K., S.G.P., D.L., and T.V.R.; validation, S.G.P., P.A.K., D.L., and T.V.R.; formal analysis, M.K.M., A.J.T., S.J., P.P., M.L.H., K.A.H., D.R.K., P.A.K., S.G.P., D.L., and T.V.R.; resources, P.A.K., S.G.P., D.L., and T.V.R.; writing—original draft preparation, M.K.M., P.P., M.L.H., P.A.K., and S.G.P.; writing—review and editing, S.G.P., P.A.K., A.J.T., D.L., T.V.R., M.L.H., and D.R.K.; supervision, P.A.K., S.G.P., D.L., and T.V.R.; project administration, P.A.K., S.G.P., D.L., and T.V.R.; funding acquisition, P.A.K., S.G.P., D.L., and T.V.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Health and Medical Research Council of Australia, grant number #APP1124032.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and Victorian State Government regulations, and was approved by the Monash University Animal Ethics Committee (Monash University AEC no. MARP/2014/142).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in supplementary material.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Leffler D.A., Lamont J.T. Treatment of Clostridium difficile-Associated Disease. Gastroenterology. 2009;136:1899–1912. doi: 10.1053/j.gastro.2008.12.070. [DOI] [PubMed] [Google Scholar]
- 2.Knight D.R., Elliott B., Chang B.J., Perkins T.T., Riley T.V. Diversity and Evolution in the Genome of Clostridium difficile. Clin. Microbiol. Rev. 2015;28:721–741. doi: 10.1128/CMR.00127-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eaton S.R., Mazuski J.E. Overview of Severe Clostridium difficile Infection. Crit. Care Clin. 2013;29:827–839. doi: 10.1016/j.ccc.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Di Bella S., Ascenzi P., Siarakas S., Petrosillo N., Di Masi A. Clostridium difficile Toxins A and B: Insights into Pathogenic Properties and Extraintestinal Effects. Toxins. 2016;8:134. doi: 10.3390/toxins8050134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandrasekaran R., Lacy D.B. The role of toxins in Clostridium difficile infection. FEMS Microbiol. Rev. 2017;41:723–750. doi: 10.1093/femsre/fux048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson A.P. New antibiotics for selective treatment of gastrointestinal infection caused by Clostridium difficile. Expert Opin. Ther. Pat. 2010;20:1389–1399. doi: 10.1517/13543776.2010.511177. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Clostridium Difficile Update. [(accessed on 26 June 2021)];2019 Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/CRE-508.pdf.
- 8.Stanley J.D., Bartlett J.G., Dart B.W., Ashcraft J. Clostridium difficile infection. Curr. Probl. Surg. 2013;50:302–337. doi: 10.1067/j.cpsurg.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Ritter A.S., Petri W.A. New developments in chemotherapeutic options for Clostridium difficile colitis. Curr. Opin. Infect. Dis. 2013;26:461–470. doi: 10.1097/QCO.0b013e328363456e. [DOI] [PubMed] [Google Scholar]
- 10.Cornely O.A., Miller M.A., Louie T.J., Crook D.W., Gorbach S.L. Treatment of First Recurrence of Clostridium difficile Infection: Fidaxomicin Versus Vancomycin. Clin. Infect. Dis. 2012;55:S154–S161. doi: 10.1093/cid/cis462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hostler C.J., Chen L.F. Fidaxomicin for treatment of Clostridium difficile-associated diarrhea and its potential role for prophylaxis. Expert Opin. Pharmacother. 2013;14:1529–1536. doi: 10.1517/14656566.2013.802307. [DOI] [PubMed] [Google Scholar]
- 12.Cho J.M., Pardi D.S., Khanna S. Update on Treatment of Clostridioides difficile Infection. Mayo Clin Proc. 2020;95:758–769. doi: 10.1016/j.mayocp.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Tague A.J., Putsathit P., Hammer K.A., Wales S.M., Knight D.R., Riley T.V., Keller P.A., Pyne S.G. Cationic biaryl 1,2,3-triazolyl peptidomimetic amphiphiles targeting Clostridioides (Clostridium) difficile: Synthesis, antibacterial evaluation and an in vivo C. difficile infection model. Eur. J. Med. Chem. 2019;170:203–224. doi: 10.1016/j.ejmech.2019.02.068. [DOI] [PubMed] [Google Scholar]
- 14.Wales S.M., Hammer K.A., King A.M., Tague A.J., Lyras D., Riley T.V., Keller P.A., Pyne S.G. Binaphthyl-1,2,3-triazole peptidomimetics with activity against Clostridium difficile and other pathogenic bacteria. Org. Biomol. Chem. 2015;13:5743–5756. doi: 10.1039/C5OB00576K. [DOI] [PubMed] [Google Scholar]
- 15.Tague A.J., Putsathit P., Riley T.V., Keller P.A., Pyne S.G. Positional Isomers of Biphenyl Antimicrobial Peptidomimetic Amphiphiles. ACS Med. Chem. Lett. 2021;12:413–419. doi: 10.1021/acsmedchemlett.0c00611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S.J., Yang Q., Xu L., Chang J., Sun X. Synthesis and antibacterial activity against Clostridium difficile of novel demethylvancomycin derivatives. Bioorg. Med. Chem. Lett. 2012;22:4942–4945. doi: 10.1016/j.bmcl.2012.06.039. [DOI] [PubMed] [Google Scholar]
- 17.Butler M.M., Williams J.D., Peet N.P., Moir D.T., Panchal R.G., Bavari S., Shinabarger D.L., Bowlin T.L. Comparative In Vitro Activity Profiles of Novel Bis-Indole Antibacterials against Gram-Positive and Gram-Negative Clinical Isolates. Antimicrob. Agents Chemother. 2010;54:3974–3977. doi: 10.1128/AAC.00484-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dvoskin S., Xu W.-C., Brown N.C., Yanachkov I.B., Yanachkova M., Wright G.E. A Novel Agent Effective against Clostridium difficile Infection. Antimicrob. Agents Chemother. 2012;56:1624–1626. doi: 10.1128/AAC.06097-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ueda C., Tateda K., Horikawa M., Kimura S., Ishii Y., Nomura K., Yamada K., Suematsu T., Inoue Y., Ishiguro M., et al. Anti-Clostridium difficile Potential of Tetramic Acid Derivatives from Pseudomonas aeruginosa Quorum-Sensing Autoinducers. Antimicrob. Agents Chemother. 2010;54:683–688. doi: 10.1128/AAC.00702-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballard T.E., Wang X., Olekhnovich I., Koerner T., Seymour C., Hoffman P.S., Macdonald T.L. Biological Activity of Modified and Exchanged 2-Amino-5-Nitrothiazole Amide Analogues of Nitazoxanide. Bioorg. Med. Chem. Lett. 2010;20:3537–3539. doi: 10.1016/j.bmcl.2010.04.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirst H.A., Toth J.E., Debono M., Willard K.E., Truedell B.A., Ott J.L., Counter F.T., Felty-Duckworth A.M., Pekarek R.S. Synthesis and evaluation of tylosin-related macrolides modified at the aldehyde function: A new series of orally effective antibiotics. J. Med. Chem. 1988;31:1631–1641. doi: 10.1021/jm00403a025. [DOI] [PubMed] [Google Scholar]
- 22.Liu R., Suárez J.M., Weisblum B., Gellman S.H., McBride S.M. Synthetic Polymers Active against Clostridium difficile Vegetative Cell Growth and Spore Outgrowth. J. Am. Chem. Soc. 2014;136:14498–14504. doi: 10.1021/ja506798e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarrad A.M., Karoli T., Blaskovich M.A.T., Lyras D., Cooper M.A. Clostridium difficile Drug Pipeline: Challenges in Discovery and Development of New Agents. J. Med. Chem. 2015;58:5164–5185. doi: 10.1021/jm5016846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowes R. FDA Approves Zinplava for Preventing Return of C. difficile. [(accessed on 26 June 2021)]; Available online: https://www.medscape.com/viewarticle/870887.
- 25.Bremner J.B., Keller P.A., Pyne S.G., Boyle T.P., Brkic Z., David D.M., Garas A., Morgan J., Robertson M., Somphol K., et al. Binaphthyl-Based Dicationic Peptoids with Therapeutic Potential. Angew. Chem. Int. Ed. 2010;49:537–540. doi: 10.1002/anie.200904392. [DOI] [PubMed] [Google Scholar]
- 26.Bremner J.B., Keller P.A., Pyne S.G., Boyle T.P., Brkic Z., David D.M., Robertson M., Somphol K., Baylis D., Coates J.A., et al. Synthesis and antibacterial studies of binaphthyl-based tripeptoids. Part 1. Bioorg. Med. Chem. 2010;18:2611–2620. doi: 10.1016/j.bmc.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 27.Bremner J.B., Keller P.A., Pyne S.G., Boyle T.P., Brkic Z., Morgan J., Somphol K., Coates J.A., Deadman J., Rhodes D.I. Synthesis and antibacterial studies of binaphthyl-based tripeptoids. Part 2. Bioorg. Med. Chem. 2010;18:4793–4800. doi: 10.1016/j.bmc.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Mahadari M.K., Tague A.J., Keller P.A., Pyne S.G. Synthesis of sterically congested 1,5-disubstituted-1,2,3-Triazoles using chloromagnesium acetylides and hindered 1-naphthyl azides. Tetrahedron. 2021;81:131916. doi: 10.1016/j.tet.2020.131916. [DOI] [Google Scholar]
- 29.Blaskovich M.A.T., Zuegg J., Elliott A.G., Cooper M.A. Helping chemists discover new antibiotics. ACS Infect. Dis. 2015;1:285–287. doi: 10.1021/acsinfecdis.5b00044. [DOI] [PubMed] [Google Scholar]
- 30.Wales S.M., Hammer K.A., Somphol K., Kemker I., Schröder D.C., Tague A.J., Brkic Z., King A.M., Lyras D., Riley T.V., et al. Synthesis and antimicrobial activity of binaphthylbased, functionalized oxazole and thiazole peptidomimetics. Org. Biomol. Chem. 2019;13:10813–10824. doi: 10.1039/C5OB01638J. [DOI] [PubMed] [Google Scholar]
- 31.Tague A.J., Putsathit P., Hammer K.A., Wales S.M., Knight D.R., Riley T.V., Keller P.A., Pyne S.G. Cationic biaryl 1,2,3-triazolyl peptidomimetic amphiphiles: Synthesis, antibacterial evaluation and preliminary mechanism of action studies. Eur. J. Med. Chem. 2019;168:386–404. doi: 10.1016/j.ejmech.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Zhu D., Ma J., Luo K., Fu H., Zhang L., Zhu S. Enantioselective Intramolecular C-H Insertion of Donor and Donor/Donor Carbenes by a Nondiazo Approach. Angew. Chem. Int. Ed. 2016;55:8452–8456. doi: 10.1002/anie.201604211. [DOI] [PubMed] [Google Scholar]
- 33.Maehr H., Smallheer J. Total syntheses of rivularins D1 and D3. J. Am. Chem. Soc. 1985;107:2943–2945. doi: 10.1021/ja00296a018. [DOI] [Google Scholar]
- 34.Gamble A.B., Garner J., Gordon C.P., O’Conner S.M.J., Keller P.A. Aryl Nitro Reduction with Iron Powder or Stannous Chloride under Ultrasonic Irradiation. Synth. Commun. 2007;37:2777–2786. doi: 10.1080/00397910701481195. [DOI] [Google Scholar]
- 35.Zilla M.K., Nayak D., Vishwakarma R.A., Sharma P.R., Goswami A., Ali A. A convergent synthesis of alkyne-azide cycloaddition derivatives of 4-α,β-2-propyne podophyllotoxin depicting potent cytotoxic activity. Eur. J. Med. Chem. 2014;77:47–55. doi: 10.1016/j.ejmech.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 36.Clinical and Laboratory Standards Institute . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. 9th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2015. CLSI Document M07-A10. [Google Scholar]
- 37.Clinical and Laboratory Standards Institute . Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria. 8th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2012. CLSI Document M11-A8. [Google Scholar]
- 38.Clinical Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2018. 28th Informational Supplement; CLSI Document M100-S28. [Google Scholar]
- 39.Carter G.P., Lyras D., Allen D.L., Mackin K.E., Howarth P.M., O’Connor J.R., Rood J.I. Binary toxin production in Clostridium difficile is regulated by CdtR, a LytTR family response regulator. J. Bacteriol. 2007;189:7290–7301. doi: 10.1128/JB.00731-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hutton M.L., Cunningham B.A., Mackin K.E., Lyon S.A., James M.L., Rood J.I., Lyras D. Bovine antibodies targeting primary and recurrent Clostridium difficile disease are a potent antibiotic alternative. Sci. Rep. 2017;7:3665. doi: 10.1038/s41598-017-03982-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyon S.A., Hutton M.L., Rood J.I., Cheung J.K., Lyras D. CdtR regulates TcdA and TcdB production in Clostridium difficile. PLoS Pathog. 2016;12:e1005758. doi: 10.1371/journal.ppat.1005758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Awad M.M., Hutton M.L., Quek A.J., Klare W.P., Mileto S.J., Mackin K., Ly D., Oorschot V., Bosnjak M., Jenkin G., et al. Human Plasminogen Exacerbates Clostridioides difficile Enteric Disease and Alters the Spore Surface. Gastroenterology. 2020;159:1431–1443. doi: 10.1053/j.gastro.2020.06.032. [DOI] [PubMed] [Google Scholar]
- 43.Carter G.P., Chakravorty A., Pham Nguyen T.A., Mileto S., Schreiber F., Li L., Howarth P., Clare S., Cunningham B., Sambol S.P., et al. Defining the roles of TcdA and TcdB in localized gastrointestinal disease, systemic organ damage, and the host response during Clostridium difficile infections. mBio. 2015;6:e00551. doi: 10.1128/mBio.00551-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in supplementary material.