Abstract
The v-Rel oncoprotein belongs to the Rel/NF-κB family of transcription factors and induces aggressive lymphomas in chickens and transgenic mice. Current models for cell transformation by v-Rel invoke the combined activation of gene expression and the dominant inhibition of transcription mediated by its cellular homologs. Here, we mapped a serine-rich transactivation domain in the C terminus of v-Rel that is necessary for its biological activity. Specific serine-to-alanine substitutions within this region impaired the transcriptional activity of v-Rel, whereas a double mutant abolished its function. In contrast, substitutions with phosphomimetic aspartate residues led to a complete recovery of the transcriptional potential. The transforming activity of v-Rel mutants correlated with their ability to inhibit programmed cell death. The transforming and antiapoptotic activities of v-Rel were abolished by defined Ser-to-Ala mutations and restored by most Ser-to-Asp substitutions. However, one Ser-to-Asp mutant showed wild-type transactivation ability but failed to block apoptosis and to transform cells. These results show that the transactivation function of v-Rel is necessary but not sufficient for cell transformation, adding an important dimension to the transformation model. It is possible that defined protein-protein interactions are also required to block apoptosis and transform cells. Since v-Rel is an acutely oncogenic member of the Rel/NF-κB family, our data raise the possibility that phosphorylation of its serine-rich transactivation domain may regulate its unique biological activity.
The v-rel oncogene of reticuloendotheliosis virus strain T (Rev-T) was the first member of the Rel/NF-κB gene family to be discovered (65, 69, 77, 78). v-rel induces aggressive lymphoma or leukemia in chickens and transgenic mice and immortalizes and transforms spleen and bone marrow cells in vitro (5, 7, 13, 20, 41, 61). v-Rel is structurally related to its cellular homologs in the Rel/NF-κB family of transcription factors: c-Rel, RelA, RelB, p105/NF-κB1, p100/NF-κB2, Dorsal, Dif, and Relish (reviewed in references 3 and 72). These proteins play fundamental roles in immune, inflammatory, and acute-phase responses and in the control of cell proliferation and limb development (1–3, 10, 35, 46, 68, 72). As with v-rel, the rearrangement, overexpression, or amplification of the human c-rel, rela, nf-κb1, and nf-κb2 genes has been associated with leukemia, lymphoma, and other lymphoproliferative diseases (reviewed in references 24 and 43).
v-rel arose from the transduction of the turkey c-rel proto-oncogene by nontransforming reticuloendotheliosis virus strain A (Rev-A) (reviewed in reference 8). Consequently, v-Rel contains viral envelope-derived amino acids at its N and C termini together with internal mutations that act in concert to increase its oncogenicity (6, 31, 53). However, the alteration that is most significant for its transforming activity is the deletion of 118 C-terminal amino acids that function as a strong transactivation domain in c-Rel (12, 34, 58). The removal of these sequences from c-Rel greatly enhanced its transforming potential (17, 31, 34, 38, 39). The N-terminal Rel homology domain (RHD) of v-Rel is necessary for its nuclear localization, its association with other Rel factors, and its binding to κB DNA sites (reviewed in references 24 and 43). The sequences located C terminal to the RHD preferentially activate κB site-dependent transcription in undifferentiated cells and interact with the general transcription factors TBP and TFIIB (33, 34, 59, 64, 73, 82). Importantly, this region is also essential for cell transformation (59). The deletion of 100 amino acids from the C terminus of v-Rel was shown to dramatically reduce its oncogenic potential (21).
Early studies showed that v-Rel altered gene expression in a promoter- and cell-specific manner, suggesting a possible mechanism for its transforming activity (22, 33, 73). It is now clear that v-Rel must bind to DNA and activate gene expression to transform cells. Mutations inactivating its DNA-binding, dimerization, or transactivation functions invariably abolish its transforming potential (reviewed in references 24 and 43). The transactivating sequences found in the C-terminal half of v-Rel do not show any significant homology with acidic, glutamine-rich, or proline-rich activation motifs. However, this region contains multiple serine residues and is highly phosphorylated in transformed lymphoid cells (81). This fact raises the possibility that phosphorylation could confer upon this region the charge and/or the conformation of an activation domain.
With few exceptions, a common consequence of rel gene alterations in oncogenesis is the inappropriate activation of cellular gene expression (43). It is therefore important to elucidate the mechanisms that control the transcriptional activity of Rel proteins to evaluate their contribution to the oncogenic process. In this study, we used deletion and site-directed mutagenesis to delineate the transcriptional activation domain of v-Rel and to identify serine residues important for its function. The results demonstrated that specific serines in the C-terminal half of v-Rel are essential for its transcriptional, antiapoptotic, and transforming activities.
MATERIALS AND METHODS
Plasmids.
v-Rel deletion mutants Δ139, Δ102, Δ73, Δ63, and Δ53 lacked 139, 102, 73, 63, or 53 amino acids, respectively, from the C terminus of v-Rel. They were generated by introducing stop codons at defined positions in v-Rel by use of the Altered Sites mutagenesis system (Promega Corp., Madison, Wis.). Mutant B10 contained a stop codon after the initiating ATG of v-rel (45). The alanine (A1 to A11) and aspartate (D6, D7, and D10) point mutants of v-Rel and the double and triple alanine mutants (A6.7 and A6.7.10) were generated by site-directed mutagenesis (Promega).
rel genes were expressed in vitro from the SP6 promoter of pGEM-2 (wild-type v-rel; pCG129) (40) or the T7 promoter of pAlter-1 (v-rel mutants). Wild-type and mutant Rel proteins were expressed in vivo under the control of the cytomegalovirus (CMV) immediate-early promoter of pJDCMV19SV (19) for chloramphenicol acetyltransferase (CAT) assays, under the control of the spleen necrosis virus long terminal repeat promoter of pJD214 (18) for cell transformation assays, or in pUHD-10-3-hygro for conditional expression in stable cell lines. pUHD10-3-v-rel plasmids expressed the wild-type or mutant v-rel genes under the control of the minimal CMV promoter and seven tetracycline operator sites of pUHD10-3 (27). pUHD10-3-hygro-v-rel plasmids were constructed by inserting the thymidine kinase promoter-hygromycin resistance gene cassette from pUHD10-3 ENV (a gift from J. Sodroski, Dana-Farber Cancer Institute, Boston, Mass.) into the XhoI site of pUHD10-3-v-rel plasmids. pIL6κBCAT expressed the CAT reporter gene under the control of the interleukin 6 promoter with three NF-κB DNA-binding motifs (51). SW253 carried replication-competent Rev-A and was used to generate helper virus (74). pCG147 carried residues 1 to 147 from the yeast GAL4 DNA-binding domain (66). GAL4-cc fusion constructs expressed residues 1 to 147 of GAL4 fused in frame to C-terminal v-Rel sequences derived by PCR amplification. GAL4-cc1 contained v-Rel amino acids 332 to 503; GAL4-cc2 contained amino acids 345 to 450; GAL4-cc3 contained amino acids 364 to 396; GAL4-cc4 contained amino acids 397 to 440; GAL4-cc5 contained amino acids 364 to 440; GAL4-cc6 contained amino acids 345 to 503; GAL4-cc7 contained amino acids 345 to 485; and GAL4-cc8 contained amino acids 345 to 467. pG5BCAT contained five copies of the yeast GAL4 DNA-binding motif inserted upstream of the adenovirus E1B gene TATA box (42). In all cases, mutations were confirmed by DNA sequence analysis with Sequenase (U.S. Biochemical Corp.) or by automated DNA sequencing (Molecular Resource Facility, University of Medicine and Dentistry of New Jersey-New Jersey Medical School, Newark, N.J.).
Transient cell transfection and CAT assays.
Human Tera-2 cells (human embryonal carcinoma; HTB-106; American Type Culture Collection) were maintained in McCoy’s 5a medium supplemented with 10% fetal bovine serum (FBS; Gibco), 100 U of penicillin per ml, and 100 μg of streptomycin per ml and grown at 37°C in an atmosphere of 5% CO2. Six-well plates were seeded with 1.5 × 105 cells in 2 ml of complete medium per well. The cells were cotransfected on the following day with 1.2 μg of CMV v-rel plasmid DNA and 0.8 μg of pIL6κBCAT by use of Lipofectin (Gibco Life-Technology). COS-7 simian virus 40-transformed African green monkey kidney cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS, penicillin, and streptomycin. Transfections were carried out with a modified calcium phosphate procedure (15). Cells (5 × 105) in 60-mm dishes were transfected with 2 μg of CMV c-rel or CMV vector plasmid DNA together with 10 μg of wild-type or mutant CMV v-rel plasmid DNA and 3 μg of pIL6κBCAT DNA. The total amount of plasmid DNA transfected (15 μg) was kept constant by the addition of the CMV vector. In both cases, cell extracts were prepared 36 to 48 h after transfection, and the total protein concentration was measured by the method of Bradford (9). CAT activities were determined within the linear range of the assay (22). The products and substrates were quantitated with the Image-Quant program on a phosphorimager. Normalized CAT activity from a minimum of three independent experiments is shown.
Transformation of chicken spleen cells.
Spleens from 3-week-old chickens were gently dissociated in EF20 medium (DMEM supplemented with 20% FBS and 1% chicken serum [Gibco]) at room temperature. The cell suspension was decanted, and the upper phase was centrifuged for 3 min at 940 × g and room temperature. The pellet was resuspended in EF20 medium. Cells (3 × 107) were resuspended in 100 μl of EF20 medium together with 20 μg of pJD214v-rel DNA or pJD214b10 control DNA and 10 μg of SW253 helper virus DNA and were incubated on ice for 10 min. Cells were electroporated with a Bio-Rad Gene Pulser at 250 V and 960 μF and incubated on ice for 10 min as described previously (50). Cells were cultured for 3 days in EF20 medium at 40.5°C in an atmosphere of 5% CO2 to allow virus spread and were plated in soft agar. Transformed colonies were scored 10 to 14 days later. The colonies were picked and maintained in EF20 medium.
DNA-binding assays.
DNA-binding assays were performed with v-Rel proteins produced by in vitro translation. Wild-type or mutant v-rel genes expressed from the SP6 or T7 promoter of pGEM-2 or pAlter-1 were translated with a TNT-coupled rabbit reticulocyte lysate system (Promega). Protein expression was verified by Western blotting and quantitated by densitometry. Equal amounts of proteins were incubated with a 32P-labeled IL6-κB oligonucleotide probe (3 × 104 cpm) (80) in 12.5 mM HEPES (pH 7.9)–12% glycerol–5 mM MgCl2–60 mM KCl–0.2 mM EDTA–1 mM dithiothreitol–1 μg of bovine serum albumin per μl–1 μg of poly(dI-dC) per μl and analyzed on 5% native polyacrylamide gels.
Establishment of tetracycline-regulated cell lines.
HeLa cell-derived HtTA-1 cells, which stably expressed a fusion protein comprised of the Escherichia coli tetracycline repressor and the activation domain of the herpes simplex virus VP16 protein (tTA), were a gift from H. Bujard, Heidelberg, Germany (27). Cells were grown in DMEM supplemented with 10% FBS, 2 mM l-glutamine, 1× vitamin solution, 1× nonessential amino acids, antibiotics (100 U of penicillin per ml and 100 μg of streptomycin per ml), and 125 μg of microbiological potency units of G418 per ml. Cells were maintained at 37°C in an atmosphere of 5% CO2. HtTA-1 cells were conditioned to tetracycline HCl (2 μg per ml; Sigma) for 4 days prior to transfection. The cells were transfected with pUHD10-3-hygro-v-rel vectors encoding wild-type or mutant v-Rel proteins with a modified calcium phosphate procedure (15). Cell clones were selected in the presence of hygromycin B (225 U per ml; Calbiochem). Drug-resistant colonies were picked and screened for the inducible expression of v-Rel proteins by immunoblotting. Cell clones were maintained in the presence of tetracycline (2 μg per ml) and refed every 3 days. The cell clones used in this study were v-Rel#4, A6#1-12, D6#9-4, A7#2-4, D7#3-1, A10#5-1, D10#6-16, A6.7#7-10, and A6.7.10#8-1.
Immunoblotting.
Cells maintained in the presence of tetracycline were induced to express wild-type or mutant v-Rel proteins in medium lacking tetracycline for 48 h. Cell extracts were prepared in lysis buffer (50 mM Tris HCl [pH 7.5], 150 mM sodium chloride, 1% sodium deoxycholate, 1% Triton X-100, 10 μg of leupeptin per ml, 10 μg of pepstatin per ml, 20 μg of aprotinin per ml, 10 mM sodium pyrophosphate, 50 mM sodium fluoride, 0.5 mM sodium orthovanadate) (57) and quantitated for total protein concentration by the method of Bradford (9). Proteins (20 μg) were resolved by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes (Schleicher & Schuell). Immunoblotting was performed by enhanced chemiluminescence (Amersham). Wild-type and mutant v-Rel proteins were detected with rabbit polyclonal antibody 1967 specific for the unique N terminus of v-Rel (82). An antiactin antibody (Sigma) was used as a control.
TNF-α-induced apoptosis.
Tumor necrosis factor alpha (TNF-α; Sigma)-induced apoptosis was analyzed as previously described (76, 83). Briefly, cell clones containing wild-type or mutant v-rel genes (105 cells) were seeded into six-well plates in the presence of tetracycline (2 μg per ml). Rel protein expression was induced by removal of the drug for 48 h, and cells were treated with cycloheximide (CHX; 30 μg per ml) alone or together with TNF-α (1,000 U per ml) for 14 h. Cell survival was quantitated by crystal violet staining with a modified protocol (86). The optical density of the eluate was determined at 595 nm. Results are expressed as the ratio of the optical density of cells treated with TNF-α plus CHX to that of cells treated with CHX alone.
RESULTS
Deletion mapping defines v-Rel sequences essential for v-Rel transcriptional and transforming activities.
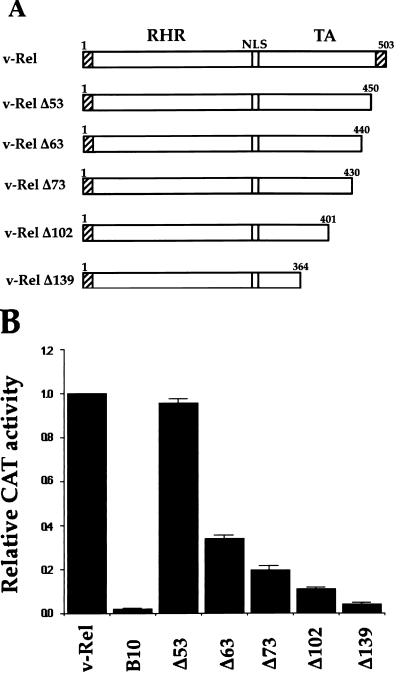
Previous studies by us and others showed that the deletion of all sequences mapping 3′ to the RHD of v-Rel abolished its transcriptional activity (v-HincII) (59, 82). Progressive deletions were introduced in the C-terminal half of v-Rel to map the sequences necessary for this function (Fig. 1A). Wild-type and mutant v-rel genes expressed from a CMV immediate-early promoter were cotransfected into human Tera-2 cells along with the pIL6κBCAT reporter plasmid. As shown in Fig. 1B, pCMV-v-rel activated κB site-dependent transcription approximately 20-fold more efficiently than pCMV-b10 (control), which contained a stop codon after the initiating ATG of v-rel (45). A mutant with a deletion of 53 amino acids from the C terminus of v-Rel retained wild-type activity (Δ53). However, further deletion of C-terminal sequences sharply reduced the transactivating potential of v-Rel. Whereas mutant Δ63 lost more than 60% of wild-type v-Rel activity, mutant Δ73 showed a nearly 80% loss of function. Further deletions virtually abolished the transcriptional potential of v-Rel (Δ102 and Δ139).
FIG. 1.
Effect of C-terminal deletions on the transcriptional and transforming activities of v-Rel. (A) Structures of v-Rel deletion mutants. RHR, Rel homology region; NLS, nuclear localization sequence; TA, transactivation domain. v-Rel sequences derived from the envelope gene of Rev-A are shown as hatched boxes. (B) Effect of deletion mutants on the transcriptional activity of v-Rel. Undifferentiated Tera-2 cells were cotransfected with 1.2 μg of CMV expression vectors for wild-type v-rel or mutant v-rel genes and 0.8 μg of reporter plasmid pIL6κBCAT. pCMV-b10 was used as a negative control. Assays were performed with 50 μg of protein for 2.5 h. The average fold activation normalized to that for pCMV-v-rel from three independent experiments is plotted. Error bars show standard deviations.
The transforming potential of v-Rel deletion mutants was examined with primary chicken spleen cells to assess their biological activity. As expected, v-Rel was strongly transforming, yielding an average of 45 to 50 transformed spleen cell colonies (Table 1). In contrast, mutant B10, which did not synthesize any v-Rel protein, failed to transform cells. While mutant Δ53 transformed cells almost as efficiently as v-Rel, mutant Δ63 transformed cells at about 50% the efficiency of v-Rel. This finding agreed with its reduced transcriptional activity (Fig. 1B). Deletion of 73 amino acids from the C terminus of v-Rel decreased its transforming activity fourfold (Δ73), whereas no transformation was observed with mutants Δ102 and Δ139. Combined, these results indicated a strong correlation between the transactivating potential and transforming activity of v-Rel. This deletion analysis also defined the region between amino acids 401 and 450 in v-Rel as being essential for its transcriptional and biological functions (between mutants Δ102 and Δ53).
TABLE 1.
Transactivation and transforming properties of wild-type v-Rel and deletion mutantsa
| v-Rel mutant | Transactivation | Transformation |
|---|---|---|
| None (wild type) | ++++ | ++++b |
| B10 | − | − |
| Δ53 | ++++ | ++++ |
| Δ63 | ++ | ++ |
| Δ73 | + | + |
| Δ102 | − | − |
| Δ139 | − | − |
Relative efficiency: ++++, 100; ++, 35 to 60; +, 20 to 35; −, <15.
v-Rel yielded an average of 45 to 50 transformed spleen cell colonies.
Sequences between amino acids 345 and 450 in v-Rel function as an autonomous transactivation domain.
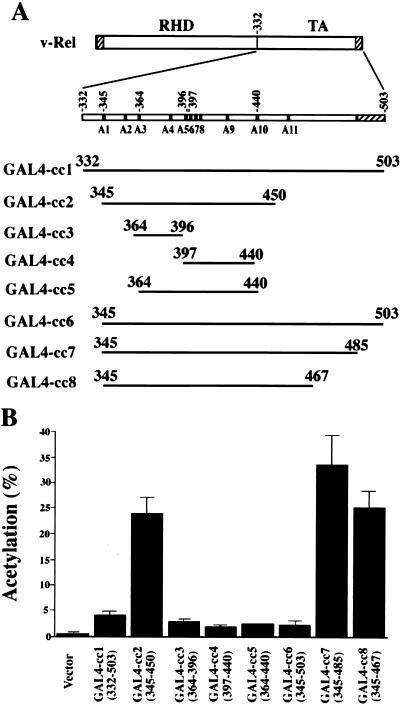
GAL4-Rel fusion proteins containing various subfragments derived from the C-terminal half of v-Rel were used to further delineate its transactivation domain (Fig. 2A, GAL4-cc1 to GAL4-cc8). As shown in Fig. 2B, a GAL4-Rel protein containing the complete C-terminal half of v-Rel activated the expression of the pG5BCAT reporter sevenfold over that of the pCG147 control in Tera-2 cells (GAL4-cc1; amino acids 332 to 503). Importantly, the GAL4-cc2 fusion (amino acids 345 to 450), extending from the first serine residue in the C terminus of v-Rel to the deletion endpoint of mutant Δ53, activated transcription 42-fold over that of the pCG147 control. In sharp contrast, GAL4 fusions to v-Rel amino acids 364 to 396, 397 to 440, or 364 to 440 failed to significantly activate transcription above that of the control (Fig. 2B, GAL4-cc3, GAL4-cc4, and GAL4-cc5). These findings demonstrated that the region comprising amino acids 345 to 450 of v-Rel can function as an autonomous transactivation domain when fused to a heterologous DNA-binding motif. The results also suggested that sequences present in the GAL4-cc1 fusion and absent in the GAL4-cc2 fusion may act as a transcriptional repression domain. To address this issue, v-Rel sequences unique to GAL4-cc1 were deleted. The GAL4-cc6 fusion, with a deletion of v-Rel amino acids 332 to 345, activated transcription within the same range as GAL4-cc1 (Fig. 2B). In contrast, deletion of the extreme C terminus of v-Rel greatly increased its transcriptional activity. Both GAL4-cc7 and GAL4-cc8 efficiently activated the expression of the pG5BCAT reporter, similar to GAL4-cc2 (Fig. 2B). Together, these assays defined the region comprising amino acids 345 to 450 of v-Rel as an autonomous transactivation domain and showed that sequences found between amino acids 485 and 503 of v-Rel decrease its activity.
FIG. 2.
Transcriptional activity of GAL4-Rel fusion proteins. (A) v-Rel subfragments fused to the yeast GAL4 DNA-binding domain. v-Rel sequences derived from the envelope gene of Rev-A are shown as hatched boxes. The positions of Ser-to-Ala mutations are indicated as black boxes (see Fig. 3A). TA, transactivation domain. (B) Transcriptional activity of GAL4-Rel fusion proteins. Undifferentiated Tera-2 cells were cotransfected with 1.2 μg of GAL4-Rel fusion genes and 0.8 μg of pG5BCAT. The pCG147 vector was used as a negative control. Assays were performed with 10 μg of protein for 2 h. Relative CAT activity from the average of three independent experiments is plotted. Error bars show standard deviations.
Serines 398, 399, 402, 438, and 439 are important for the transcriptional activity of v-Rel.
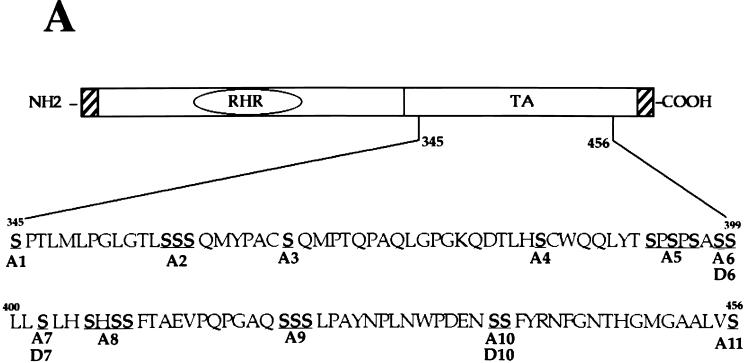
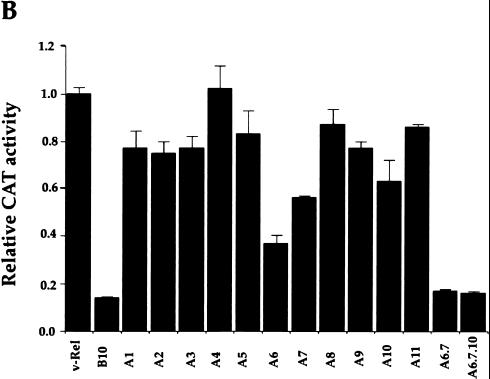
Although the C-terminal half of v-Rel does not show any significant homology with conventional acidic, glutamine-rich, or proline-rich activation domains, it contains 27% serine residues. This fact suggested that serines may confer upon this region the properties of an activation domain. To address this issue, serine-to-alanine substitutions were introduced in the activation region defined by our deletion mutagenesis (Fig. 3A, mutants A1 to A11). The transactivation potential of v-Rel point mutants was assayed by transient transfection of Tera-2 cells with a pIL6κBCAT reporter plasmid. Mutant B10, which does not express any v-Rel protein, was used as a negative control. As shown in Fig. 3B, the transcriptional activity of v-Rel was marginally affected by the majority of alanine substitutions (mutants A1, A2, A3, A4, A5, A8, A9, and A11). In contrast, the activity of v-Rel was decreased to 35, 55, and 60% by mutants A6, A7, and A10, respectively. The ability of v-Rel to activate κB site-dependent gene expression was abolished in double mutant A6.7. Similar results were obtained with triple mutant A6.7.10. The complete loss of transactivation observed with double mutant A6.7 indicated that serines 398, 399, and 402 in v-Rel are essential for its transcriptional activity.
FIG. 3.
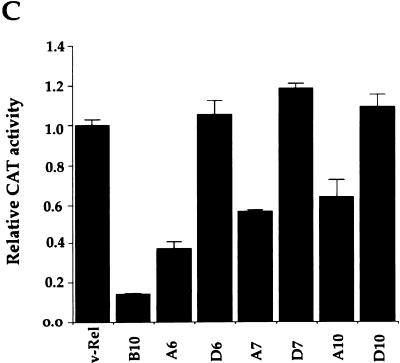
Transcriptional activity of serine mutants of v-Rel. (A) Serine mutations in the C-terminal half of v-Rel. “A” mutants contained substitutions of alanine for serine residues. “D” mutants contained substitutions of aspartate for serine residues. RHR, Rel homology region; TA, transactivation domain. (B) Effect of alanine substitutions on the transcriptional activity of v-Rel. Undifferentiated Tera-2 cells were cotransfected with 1.2 μg of CMV expression vectors for wild-type v-rel or mutant v-rel genes and 0.8 μg of pIL6κBCAT. pCMV-b10 was used as a negative control. Assays were performed with 50 μg of protein for 2.5 h. The average fold activation normalized to that for pCMV-v-rel from three independent experiments is plotted. Error bars show standard deviations. (C) Effect of alanine and aspartate substitutions on the transcriptional activity of v-Rel. Undifferentiated Tera-2 cells were cotransfected and analyzed for CAT activity as described above. The average fold activation normalized to that for pCMV-v-rel from three independent experiments is plotted. Error bars show standard deviations.
Importantly, the substitution of aspartate residues for the serines mutated in mutants A6, A7, and A10 led to a full recovery of transcriptional activity (Fig. 3C, mutants D6, D7, and D10). These data are consistent with a model in which the transcriptional activity of v-Rel may depend upon the charge or the conformation brought about by the phosphorylation of one or more serines at these positions.
Mutation of serines 398, 399, 402, 438, and 439 does not adversely affect v-Rel DNA binding.
Since v-Rel-mediated gene activation is strictly dependent on the binding of v-Rel to κB DNA sites (47, 59), we verified the ability of v-Rel mutants to interact with a consensus κB DNA motif in gel retardation assays. Wild-type and mutant v-Rel proteins produced by in vitro translation were tested for binding to a 32P-labeled oligonucleotide containing an IL6-κB DNA site. Wild-type v-Rel bound efficiently to the probe, in comparison to the endogenous NF-κB DNA-binding activity observed in a mock translation reaction (Fig. 4, compare lanes 2 and 3). Similarly, all of the v-Rel alanine and aspartate mutants bound efficiently to the probe (Fig. 4, lanes 4 to 11). This finding is in sharp contrast to that for mutants with mutations mapping in the Rel homology domain of v-Rel, which completely abrogated its DNA-binding activity (40, 47). The results suggested that the amino acid substitutions that were introduced in the C-terminal region of v-Rel did not impair its transcriptional potential by antagonizing its interaction with DNA.
FIG. 4.
Effect of C-terminal serine mutations on the DNA-binding activity of wild-type or mutant v-Rel. 35S-labeled v-Rel proteins were incubated with a 32P-labeled oligonucleotide containing an IL6-κB DNA-binding motif. DNA-protein complexes were resolved from the unbound probe on a 5% native polyacrylamide gel.
The transforming activity of v-Rel mutants A6 and A7 is restored by Ser-to-Asp substitutions, whereas that of mutant A10 is not.
Next, we investigated the role of serines 398, 399, 402, 438, and 439 in the biological function of v-Rel by testing the transforming activity of alanine and aspartate mutants. The mutants were subcloned into the pJD214 retroviral vector and assayed for the transformation of primary chicken spleen cells. Mutant B10 was used as a negative control. Whereas most of the alanine mutants depicted in Fig. 3A exhibited wild-type transforming activity, mutants A6, A7, and A10, the double mutant A6.7, and the triple mutant A6.7.10 failed to transform cells (Table 2 and data not shown). Importantly, the aspartate substitutions in mutants D6 and D7, which fully restored the transcriptional activity of v-Rel, also rescued its transforming potential (Fig. 3C and Table 2). Surprisingly, aspartate mutant D10 failed to transform spleen cells despite its strong transcriptional activity and the fact that it was expressed in vivo at a level comparable to that of v-Rel (data not shown; see Fig. 6A).
TABLE 2.
Transcriptional and transforming activities of wild-type v-Rel and point mutantsa
| v-Rel mutant | Transactivation | Transformation |
|---|---|---|
| None (wild type) | ++++ | ++++b |
| B10 | − | − |
| A1 | +++ | +++ |
| A2 | +++ | +++ |
| A5 | +++ | +++ |
| A6 | ++ | ±c |
| D6 | ++++ | ++++ |
| A7 | ++ | ±c |
| D7 | ++++ | ++++ |
| A8 | +++ | +++ |
| A9 | +++ | +++ |
| A10 | ++ | − |
| D10 | ++++ | − |
| A6.7 | − | − |
| A6.7.10 | − | − |
See Table 1, footnote a, for explanations of symbols. Relative efficiency: +++, 60 to 85.
v-Rel yielded an average of 45 to 50 transformed spleen cell colonies.
±, abortive colonies that could not be propagated in liquid cultures.
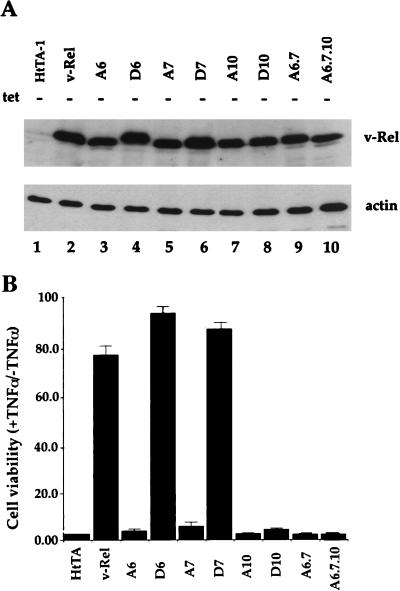
FIG. 6.
Antiapoptotic activity of wild-type or mutant v-Rel proteins. (A) Immunoblot analysis of wild-type or mutant v-Rel protein expression in tetracycline-regulated HtTA-1-derived cell clones. Cells were maintained in the presence of tetracycline, and v-Rel expression was induced by the removal of the drug for 48 h. Extracts (20 μg) were resolved by SDS-PAGE and analyzed by enhanced chemiluminescence-immunoblotting with an antibody specific for the N terminus of v-Rel (1967). An antiactin antibody was used as a control. (B) Analysis of cell survival of TNF-α-induced apoptosis. The parental HtTA-1 cell clone (control) and HtTA-derived cells expressing wild-type or mutant v-Rel proteins were induced for protein production following the removal of tetracycline for 48 h. Cells were treated with CHX alone or together with TNF-α for 14 h. Cell survival was quantitated by crystal violet staining. Relative cell viability represents the ratio of the optical density of cells treated with TNF-α together with CHX to that of cells treated with CHX alone. The average viability observed in three independent experiments is plotted. Error bars show standard deviations.
Thus, while mutation of most serines in the C-terminal half of v-Rel had no detrimental effect on its transforming activity, serines 398, 399, 402, 438, and 439 were essential (compare mutants A1, A2, A5, A8, and A9 to mutants A6, A7, and A10). Furthermore, whereas the negative charge brought about by aspartate substitutions at positions 398, 399, and 402 was sufficient to restore the transforming potential of v-Rel, the substitution of negatively charged residues at positions 438 and 439 was not (compare mutants D6 and D7 to mutant D10).
Wild-type and mutant v-Rel proteins can competitively inhibit c-Rel-mediated transcription.
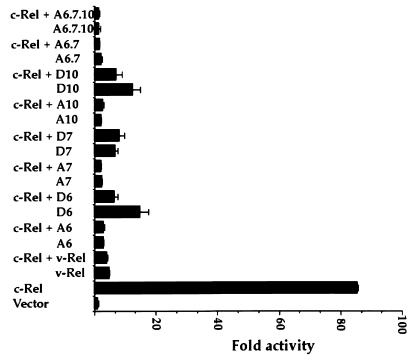
c-Rel was previously shown to efficiently activate κB site-dependent gene expression, whereas v-Rel competitively inhibited this activity and that of endogenous NF-κB factors (4, 32, 45, 58, 73). The abilities of v-Rel to activate transcription and to interfere with transactivation by its cellular homologs were both proposed to be important for its transforming function (11, 16, 37, 48, 62, 63, 79). To determine which of these two activities of v-Rel was responsible for the transformation-defective phenotype of Ser-to-Ala mutants, mutants were evaluated for competitive inhibition of c-Rel-mediated transcription in cotransfection assays. As expected, c-Rel alone strongly activated the expression of pIL6κBCAT in COS-7 cells in comparison to that of the pJDCMV19SV control vector (Fig. 5). As anticipated, v-Rel weakly activated transcription in these cells and strongly inhibited c-Rel-mediated transcription (4, 32, 45, 58, 73). This finding agreed with previous reports (22, 33, 73). Importantly, all of the alanine and aspartate mutants of v-Rel efficiently inhibited activation by c-Rel, regardless of their different transforming potentials. This result showed that the loss of transforming activity in mutants A6 and A7 did not result from an inability to function as competitive inhibitors of c-Rel but rather correlated with their inability to efficiently activate transcription. This result indicated that the ability of v-Rel to act as a dominant inhibitor of cellular Rel/NF-κB factors is not sufficient for cell transformation. This conclusion is consistent with studies showing that v-Rel must activate transcription in order to transform cells (reviewed in references 24 and 43).
FIG. 5.
Competitive inhibition of c-Rel-mediated transcription by wild-type or mutant v-Rel proteins. COS-7 cells were cotransfected with 3 μg of pIL6κBCAT and 2 μg of CMV c-rel plasmid alone or together with 10 μg of CMV v-rel vectors. The total amount of transfected DNA (15 μg) was kept constant by the addition of pJDCMV19SV vector DNA. CAT assays were performed with 10 μg of total cellular protein for 1 h. Error bars show standard deviations.
Absolute correlation between the antiapoptotic activity of v-Rel mutants and their transforming potential.
Recent studies demonstrated that v-Rel protects cells from programmed cell death and raised the possibility that this antiapoptotic activity may be critical for the transforming function of v-Rel (54, 75, 86). We investigated whether serine point mutants with mutations in the C terminus of v-Rel were affected in this activity by characterizing their ability to protect cells from TNF-α-induced apoptosis. Alanine and aspartate mutants of v-Rel were conditionally expressed under tetracycline-regulated control in the HtTA-1 cell line (27). In this system, mutant v-rel genes were expressed under the control of a tTA transactivator comprised of the E. coli tetracycline repressor fused to the activation domain of the VP16 protein of herpes simplex virus. The addition of tetracycline to the culture medium prevented the association of the tTA transactivator with the operator sites, thereby arresting rel gene transcription. The inducible expression of mutant v-Rel proteins was analyzed by immunoblotting. As shown in Fig. 6A, lanes 2 to 10, the removal of tetracycline led to the significant accumulation of wild-type and mutant proteins, which were expressed at approximately equivalent levels. As expected, no v-Rel expression was observed in parental HtTA-1 cells grown in the absence of tetracycline (Fig. 6A, lane 1) or in individual mutant v-Rel cell clones cultured in the presence of the drug (data not shown).
Cells were assayed for resistance to TNF-α-induced killing, and cell survival was quantitated by crystal violet staining as described previously (86). As expected, parental HtTA-1 cells underwent massive cell death following treatment with TNF-α together with CHX (Fig. 6B). The expression of wild-type v-Rel in cell clone HtTA-v-Rel#4 protected approximately 78% of the cells from cytolysis. Whereas the Ser-to-Ala mutants invariably failed to protect cells from TNF-α-induced cytolysis, transformation-competent Ser-to-Asp mutants D6 and D7 allowed 94 and 89% of the cells, respectively, to survive this treatment. Importantly, Ser-to-Asp mutant D10 failed to show any antiapoptotic activity, despite its strong transcriptional potential (Fig. 6B). This finding agreed with its transformation-defective phenotype (Table 2). Together, these experiments demonstrated an absolute correlation between the antiapoptotic and transforming activities of v-Rel.
DISCUSSION
Current models for cell transformation by v-Rel invoke the combined activation of gene expression and the dominant inhibition of transcription mediated by its cellular homologs (reviewed in references 24 and 43). The experiments described here demonstrate that the competitive inhibition of NF-κB-mediated transcription by v-Rel is not sufficient to transform cells. Moreover, our results obtained with the D10 mutant add an important dimension to the transformation model by showing that the transactivation function of v-Rel is necessary but not sufficient for cell transformation. This study establishes an absolute correlation between the ability of v-Rel to block programmed cell death and the oncogenic transformation of lymphoid cells. The results emphasize the need for v-Rel to activate gene expression and promote cell survival for the manifestation of its malignant phenotype and raise the possibility that defined protein-protein interactions also may be involved.
Serine-rich transactivation domain in the C terminus of v-Rel.
The transactivation domain of v-Rel was previously reported to map 3′ to the RHD and downstream of amino acid 388 (33, 34, 59). Our deletion analysis further delineated the sequences necessary for transactivation. While the extreme C-terminal 53 amino acids of v-Rel were dispensable for κB site-dependent gene activation, further deletion of 49 amino acids virtually abolished its activity (mutants Δ53 and Δ102, respectively). When fused to a yeast GAL4 DNA-binding region, v-Rel amino acids 345 to 450 functioned as an autonomous transactivation domain that could not be further divided without a significant loss of function. The strong activity of this region is consistent with that seen for other transactivation domains isolated as fusions to heterologous DNA-binding motifs (reviewed in reference 56). This analysis also revealed that the deletion of retroviral envelope-derived sequences that encode the C-terminal 18 amino acids of v-Rel significantly increased its transcriptional activity. Deletion of this repressive region may allow the v-Rel transactivation region to efficiently interact with the transcriptional machinery or with coactivators. According to this model, phosphorylation of the serine transactivation domain defined in this study may help to unmask the activating potential of v-Rel in a manner similar to that seen upon deletion of the C-terminal envelope-derived amino acids.
While the activation region of v-Rel does not show any significant homology with classical activation motifs, its high serine content is reminiscent of that of the CREB, TCF/Elk-1, c-Jun, and Stat transcription factors (reviewed in reference 36). These proteins belong to an emerging class of transcriptional regulators whose subcellular localization, DNA-binding, or transcriptional activities are modulated by phosphorylation (reviewed in reference 36). Consistent with the model described above, defined Ser-to-Ala substitutions significantly decreased κB site-dependent transactivation by v-Rel (mutants A6, A7, A10, A6.7, and A6.7.10). Conversely, phosphomimetic aspartate residues fully restored its function. This finding indicates an important role for serines 398, 399, 402, 438, and 439 in v-Rel-mediated transcription.
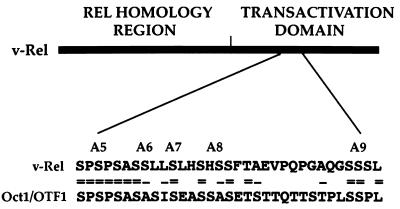
All of the Ser-to-Ala mutants that we analyzed exhibited wild-type DNA-binding activity. It therefore appears that the transcriptional defects of mutants A6, A7, A10, A6.7, and A6.7.10 did not result from changes in protein conformation incompatible with dimer formation and/or DNA contact. Rather, the data suggest that residues 398, 399, 402, 438, and 439 may be necessary for gene activation, perhaps by allowing interactions with specific cellular factors. In this respect, it is noteworthy that the v-Rel sequences defined here show 54% homology with a serine/threonine-rich region in human transcription factor Oct1/OTF1 (Fig. 7). This region is found 3′ to the POU domain of Oct1 and contains determinants important for the specificity of promoter activation (67). This region in Oct1 was suggested to participate in selective protein-protein interactions, possibly involving a subset of RNA polymerase II initiation complexes (67). Based on these observations, it is tempting to speculate that the v-Rel sequences defined here perhaps contribute to the activation of specific cellular genes.
FIG. 7.
Homology between the v-Rel transactivation domain and a portion of a serine-rich domain in human transcription factor Oct1/OTF1. Double lines indicate identical residues; single lines indicate conserved residues.
Mechanism for v-Rel-mediated gene activation.
Activator proteins promote gene expression through specific protein-protein interactions. This mechanism includes contacts with components of the cellular transcriptional machinery or with coactivators that bridge the interaction of activator proteins with basal initiation factors (28, 30). In agreement with this model, the transactivation domains of v-Rel, c-Rel, and p65/RelA were shown to associate with the basal transcription factors TBP and TFIIB (60, 82). However, the association of v-Rel with TBP and TFIIB may not be sufficient for gene activation, as transactivation-defective Ser-to-Ala mutants of v-Rel showed wild-type interactions with these factors (14). This result suggests that other protein-protein interactions may be compromised by these mutations. Recent reports indicating the functional interaction of the v-Rel homolog p65/RelA with TAFII250 and with the transcriptional coactivator CBP/p300 agree with this hypothesis (29, 55). The v-Rel activation mutants that we generated will thus be useful in future studies for identifying protein-protein interactions essential for v-Rel transcriptional, antiapoptotic, and transforming activities.
Several lines of evidence support an important role for phosphorylation in the control of Rel protein activity (reviewed in references 44 and 71). For instance, phosphorylation of the inhibitor IκBα promotes its degradation and leads to the activation of Rel/NF-κB factors. In addition, transcription by p65/RelA is upregulated by its direct phosphorylation (60, 84, 85). While this modification was reported to enhance RelA DNA binding (52), others found a specific increase in transcriptional activity (60, 84, 85). Consistent with the latter findings, RelA phosphorylation at a consensus protein kinase A site within its RHD was recently shown to promote its interaction with CBP/p300 (84, 85). Although this consensus protein kinase A phosphorylation site is conserved within the RHD of v-Rel, its mutation to alanine had no significant effect on the DNA-binding, transcriptional, and transforming activities of v-Rel (49, 50, 73). Based on the observation that v-Rel is phosphorylated in vivo (25, 48, 50, 63, 70, 81), our results lead us to postulate that phosphorylation of the v-Rel activation domain may promote its transcriptional activity. Since the serines that we identified do not belong to any known kinase recognition motif, future studies are required to identify the phosphoacceptor sites in v-Rel and the kinases and phosphatases responsible for its regulation. These assays will also help to reveal the mechanisms that modulate the functional interaction of v-Rel with the transcriptional machinery.
Antiapoptotic and transforming activities of v-Rel.
Several models were proposed to account for the transforming activity of v-Rel. Accumulating evidence suggests that its transactivating function is important for cell transformation and for the inhibition of apoptosis (40, 50, 54, 59, 73, 75, 86, 87). Our demonstration that v-Rel point mutants defective for transactivation were also defective for cell transformation agrees with these findings and reveals sequences essential for the biological function of v-Rel. Importantly, the serine residues that we identified map within the two C-terminal regions of v-Rel that were previously shown to be important for cell transformation (amino acids 389 to 432 and 437 to 503) (59). Our finding that v-Rel-mediated transactivation is necessary for the biological activity of v-Rel is also consistent with a recent report showing that the ability of v-Rel to modulate the expression of the early-response genes c-fos and c-jun is important for oncogenesis (38).
Our data showing that the substitution of serines 398, 399, and 402 with aspartate fully restored the oncogenic activity of v-Rel are the first to suggest that the phosphorylation of v-Rel may be required for its biological activity. A notable exception, however, was the mutant D10, in which serines 438 and 439 were changed to aspartate. In contrast to transformation-competent mutants D6 and D7, mutant D10 failed to transform primary lymphoid cells and did not confer resistance to TNF-α-induced apoptosis. These results demonstrated an absolute correlation between the antiapoptotic activity of v-Rel mutants and their oncogenic potential.
The lack of biological activity of mutant D10 was surprising, since this mutant activated the expression of the pIL6κBCAT reporter plasmid as efficiently as wild-type v-Rel in transient transfection assays. A possible explanation for this defect is that the transcriptional activity of v-Rel alone is not sufficient for malignant cell transformation. Consistent with this model, the transforming activity of v-Rel was abolished upon substitution of its C-terminal half with a heterologous VP16 transactivation domain or with sequences derived from pBluescript that activated transcription when fused to the N terminus of v-Rel (23, 26).
The D10 mutation may compromise specific protein-protein interactions essential for cell death inhibition and cell transformation. In this scenario, phospho groups on serines 438 and 439 of v-Rel may be necessary to recruit factors that can block the apoptotic cascade. Alternatively, D10 may be unable to activate the entire collection of genes that wild-type v-Rel activates, failing to upregulate some that are necessary for cell death inhibition and cell transformation. It is possible that phospho groups at these positions enable v-Rel to participate in defined protein-protein interactions that dictate the activation of specific target genes essential for cell death inhibition and cell transformation. Although the mechanism by which v-Rel transforms cells remains to be clarified, our studies identified a serine-rich domain that is critical for its oncogenicity. v-Rel is the only member of the Rel/NF-κB family that is acutely transforming. Future studies will help to decipher the extent to which phosphorylation of the transactivation domain of v-Rel contributes to its unique biological activity and to elucidate the mechanisms involved.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant CA-54999 from the National Cancer Institute to C.G. and by the New Jersey Commission on Science and Technology. C.C. was supported by a postdoctoral fellowship from the New Jersey Commission on Cancer Research and by the Foundation of UMDNJ. F.A. was supported by a postdoctoral fellowship from the Association pour la Recherche sur le Cancer (ARC).
We thank H. Bujard (Zentrum für Molekulare Biologie der Universität Heidelberg, Heidelberg, Germany) for the generous gifts of pUHD10-3 and HtTA-1 cells and J. Sodroski (Dana-Farber Cancer Institute, Boston, Mass.) for pUHD10-3 ENV. We are grateful to Y. Hu for assistance with initial mutagenesis studies. We thank J. Bash, I. Luque, and W.-X. Zong for fruitful discussions during the course of this work and are grateful to J. Bash, S. Crespo, L. Edelstein, B. Rayet, A. Rabson, and W.-X. Zong for helpful comments on the manuscript.
REFERENCES
- 1.Baeuerle P A, Baltimore D. A 65-kD subunit of active NF-κB is required for inhibition of NF-κB by IκB. Genes Dev. 1989;3:1689–1698. doi: 10.1101/gad.3.11.1689. [DOI] [PubMed] [Google Scholar]
- 2.Baeuerle P A, Henkel T. Function and activity of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin A S., Jr The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 4.Ballard D W, Walker W H, Doerre S, Sista P, Molitor J A, Dixon E P, Peffer N J, Hannink M, Greene W C. The v-rel oncogene encodes a κB enhancer binding protein that inhibits NF-κB function. Cell. 1990;63:803–814. doi: 10.1016/0092-8674(90)90146-6. [DOI] [PubMed] [Google Scholar]
- 5.Beug H, Muller H, Doederlein G, Graf T. Hematopoietic cells transformed in vitro by REV-T avian reticuloendotheliosis virus express characteristics of very immature lymphoid cells. Virology. 1981;115:295–309. doi: 10.1016/0042-6822(81)90112-4. [DOI] [PubMed] [Google Scholar]
- 6.Bhat G, Temin H M. Mutational analysis of v-rel, the oncogene of reticuloendotheliosis virus strain T. Oncogene. 1990;5:625–634. [PubMed] [Google Scholar]
- 7.Boehmelt G, Madruga J, Dorfler P, Briegel K, Schwartz H, Enrietto P J, Zenke M. Dendritic cell progenitor is transformed by a conditional v-Rel estrogen receptor fusion protein v-RelER. Cell. 1995;80:341–352. doi: 10.1016/0092-8674(95)90417-4. [DOI] [PubMed] [Google Scholar]
- 8.Bose H R., Jr The Rel family: models for transcriptional regulation and oncogenic transformation. Biochim Biophys Acta. 1992;1114:1–17. doi: 10.1016/0304-419x(92)90002-g. [DOI] [PubMed] [Google Scholar]
- 9.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 10.Bushdid P, Brantley D, Yull F, Blaeuer G, Hoffman L, Niswander L, Kerr L. Inhibition of NF-κB activity results in disruption of the apical ectodermal ridge and aberrant limb morphogenesis. Nature. 1998;392:615–618. doi: 10.1038/33435. [DOI] [PubMed] [Google Scholar]
- 11.Capobianco A J, Chang D, Mosialos G, Gilmore T D. p105, the NF-κB p50 precursor protein, is one of the cellular proteins complexed with the v-Rel oncoprotein in transformed chicken spleen cells. J Virol. 1992;66:3758–3767. doi: 10.1128/jvi.66.6.3758-3767.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capobianco A J, Simmons D L, Gilmore T D. Cloning and expression of a chicken c-rel cDNA: unlike p59v-rel, p68c-rel is a cytoplasmic protein in chicken embryo fibroblasts. Oncogene. 1990;5:257–265. [PubMed] [Google Scholar]
- 13.Carrasco D, Risso C A, Dorfman K, Bravo R. The v-rel oncogene promotes malignant T-cell leukemia/lymphoma in transgenic mice. EMBO J. 1996;15:3640–3650. [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, C., and C. Gélinas. Unpublished data.
- 15.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis J N, Bargmann W, Bose H R., Jr Identification of protein complexes containing the c-rel proto-oncogene product in avian hematopoietic cells. Oncogene. 1990;5:1109–1115. [PubMed] [Google Scholar]
- 17.Diehl J A, McKinsey T A, Hannink M. Differential pp40IκB-β inhibition of DNA binding by rel proteins. Mol Cell Biol. 1993;13:1769–1778. doi: 10.1128/mcb.13.3.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dougherty J P, Temin H M. High mutation rate of a spleen necrosis virus-based retrovirus vector. Mol Cell Biol. 1986;6:4387–4395. doi: 10.1128/mcb.6.12.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dougherty J P, Wisniewski R, Yang S, Rhode B W, Temin H M. New retrovirus helper cells with almost no nucleotide sequence homology to retrovirus vectors. J Virol. 1989;63:3209–3212. doi: 10.1128/jvi.63.7.3209-3212.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franklin R B, Maldonado R L, Bose H R. Isolation and characterization of reticuloendotheliosis virus transformed bone marrow cells. Intervirology. 1974;3:342–352. doi: 10.1159/000149771. [DOI] [PubMed] [Google Scholar]
- 21.Garson K, Percival H, Kang Y C. The N-terminal env-derived amino acids of v-Rel are required for full transforming activity. Virology. 1990;177:106–115. doi: 10.1016/0042-6822(90)90464-3. [DOI] [PubMed] [Google Scholar]
- 22.Gélinas C, Temin H M. The v-rel oncogene encodes a cell-specific transcriptional activator of certain promoters. Oncogene. 1988;3:349–355. [PubMed] [Google Scholar]
- 23.Gilmore, T. D. Personal communication.
- 24.Gilmore T D, Koedood M, Piffat K A, White D W. Rel/NF-κB/IκB proteins and cancer. Oncogene. 1996;13:1367–1378. [PubMed] [Google Scholar]
- 25.Gilmore T D, Temin H M. Different localization of the product of the v-rel oncogene in chicken fibroblasts and spleen cells correlates with transformation by Rev-T. Cell. 1986;44:791–800. doi: 10.1016/0092-8674(86)90845-7. [DOI] [PubMed] [Google Scholar]
- 26.Gilmore T D, White D W, Sarkar S, Sif S. Malignant transformation of cells by the v-Rel oncoprotein. In: Cooper G M, Greenberg Temin R, Sugden B, editors. The DNA provirus: Howard Temin’s scientific legacy. Washington, D.C: American Society for Microbiology; 1995. pp. 109–128. [Google Scholar]
- 27.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenblatt J. Riding high on the TATA box. Nature. 1992;360:16–17. doi: 10.1038/360016a0. [DOI] [PubMed] [Google Scholar]
- 29.Guermah M, Malik S, Roeder R G. Involvement of TFIID and USA components in transcriptional activation of the human immunodeficiency virus promoter by NF-κB and Sp1. Mol Cell Biol. 1998;18:3234–3244. doi: 10.1128/mcb.18.6.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hahn S. Structure(?) and function of acidic transcription activators. Cell. 1993;72:481–483. doi: 10.1016/0092-8674(93)90064-w. [DOI] [PubMed] [Google Scholar]
- 31.Hrdličková R, Nehyba J, Humphries E H. In vivo evolution of c-rel oncogenic potential. J Virol. 1994;68:2371–2382. doi: 10.1128/jvi.68.4.2371-2382.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inoue J-I, Kerr L D, Ransone L J, Bengal E, Hunter T, Verma I M. c-rel activates but v-rel suppresses transcription from κB sites. Proc Natl Acad Sci USA. 1991;88:3715–3719. doi: 10.1073/pnas.88.9.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishikawa H, Asano M, Kanda T, Kumar S, Gélinas C, Ito Y. Two novel functions associated with the Rel oncoproteins: DNA replication and cell-specific transcriptional activation. Oncogene. 1993;8:2889–2896. [PubMed] [Google Scholar]
- 34.Kamens J, Richardson P, Mosialos G, Brent R, Gilmore T. Oncogenic transformation by v-Rel requires an amino-terminal activation domain. Mol Cell Biol. 1990;10:2840–2847. doi: 10.1128/mcb.10.6.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanegae Y, Tavares A, Belmonte J, Verma I. Role of Rel/NF-κB transcription factors during the outgrowth of the vertebrate limb. Nature. 1998;392:611–614. doi: 10.1038/33429. [DOI] [PubMed] [Google Scholar]
- 36.Karin M. Signal transduction from the cell surface to the nucleus through the phosphorylation of transcription factors. Curr Opin Cell Biol. 1994;6:415–424. doi: 10.1016/0955-0674(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 37.Kochel T, Mushinski J F, Rice N R. The v-rel and c-rel proteins exist in high molecular weight complexes in avian and murine cells. Oncogene. 1991;6:615–626. [PubMed] [Google Scholar]
- 38.Kralova J, Liss A, Bargmann W, Bose H. AP-1 factors play an important role in transformation induced by the v-rel oncogene. Mol Cell Biol. 1998;18:2997–3009. doi: 10.1128/mcb.18.5.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kralova J, Schatzle J D, Bargmann W, Bose H R., Jr Transformation of avian fibroblasts overexpressing the c-rel proto-oncogene and a variant of c-rel lacking 40 C-terminal amino acids. J Virol. 1994;68:2073–2083. doi: 10.1128/jvi.68.4.2073-2083.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar S, Rabson A B, Gélinas C. The RxxRxRxxC motif conserved in all Rel/κB proteins is essential for the DNA-binding activity and redox regulation of the v-Rel oncoprotein. Mol Cell Biol. 1992;12:3094–3106. doi: 10.1128/mcb.12.7.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lewis R B, McClure J, Rup B, Niesel D W, Garry R F, Hoelzer J D, Nazerian K, Bose H R., Jr Avian reticuloendotheliosis virus: identification of the hematopoietic target cell for transformation. Cell. 1981;25:421–431. doi: 10.1016/0092-8674(81)90060-x. [DOI] [PubMed] [Google Scholar]
- 42.Lillie J W, Green M R. Transcription activation by the adenovirus E1A protein. Nature. 1989;338:39–44. doi: 10.1038/338039a0. [DOI] [PubMed] [Google Scholar]
- 43.Luque I, Gélinas C. Rel/NF-κB and IκB factors in oncogenesis. Semin Cancer Biol. 1997;8:103–111. doi: 10.1006/scbi.1997.0061. [DOI] [PubMed] [Google Scholar]
- 44.Maniatis T. Catalysis by a multiprotein IkappaB kinase complex. Science. 1997;278:818–819. doi: 10.1126/science.278.5339.818. [DOI] [PubMed] [Google Scholar]
- 45.McDonnell P C, Kumar S, Rabson A B, Gélinas C. Transcriptional activity of Rel family proteins. Oncogene. 1992;7:163–170. [PubMed] [Google Scholar]
- 46.Miyamoto S, Verma I M. Rel/NF-κB/IκB story. Adv Cancer Res. 1995;66:255–293. [PubMed] [Google Scholar]
- 47.Morrison L E, Boehmelt G, Enrietto P J. Mutations in the rel-homology domain alter the biochemical properties of v-Rel and render it transformation defective in chicken fibroblasts. Oncogene. 1992;7:1137–1147. [PubMed] [Google Scholar]
- 48.Morrison L E, Kabrun N, Mudri S, Hayman M J, Enrietto P J. Viral Rel and cellular Rel associate with cellular proteins in transformed and normal cells. Oncogene. 1989;4:677–683. [PubMed] [Google Scholar]
- 49.Mosialos G, Gilmore T D. v-Rel and c-Rel are differentially affected by mutations at a consensus protein kinase recognition sequence. Oncogene. 1993;8:721–730. [PubMed] [Google Scholar]
- 50.Mosialos G, Hamer P, Capobianco A J, Laursen R A, Gilmore T D. A protein kinase A recognition sequence is structurally linked to transformation by p59v-rel and cytoplasmic retention of p68c-rel. Mol Cell Biol. 1991;11:5867–5877. doi: 10.1128/mcb.11.12.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakayama K, Shimizu H, Mitomo K, Watanabe T, Okamoto S-I, Yamamoto K-I. A lymphoid cell-specific nuclear factor containing c-Rel-like proteins preferentially interacts with interleukin-6 κB-related motifs whose activities are repressed in lymphoid cells. Mol Cell Biol. 1992;12:1736–1746. doi: 10.1128/mcb.12.4.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Naumann M, Scheidereit C. Activation of NF-κB in vivo is regulated by multiple phosphorylations. EMBO J. 1994;13:4597–4607. doi: 10.1002/j.1460-2075.1994.tb06781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nehyba J, Hrdličková R, Bose H R., Jr Differences in κB DNA-binding properties of v-Rel and c-Rel are the result of oncogenic mutations in three distinct functional regions of the Rel protein. Oncogene. 1997;14:2881–2897. doi: 10.1038/sj.onc.1201150. [DOI] [PubMed] [Google Scholar]
- 54.Neiman P E, Thomas S J, Loring G. Induction of apoptosis during normal and neoplastic B-cell development in the bursa of Fabricius. Proc Natl Acad Sci USA. 1991;88:5857–5861. doi: 10.1073/pnas.88.13.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perkins N D, Felzien L K, Betts J C, Leung K, Beach D H, Nabel G J. Regulation of NF-κB by cyclin-dependent kinases associated with the p300 coactivator. Science. 1997;275:523–527. doi: 10.1126/science.275.5299.523. [DOI] [PubMed] [Google Scholar]
- 56.Ptashne M, Gann A. Transcriptional activation by recruitment. Nature. 1997;386:569–577. doi: 10.1038/386569a0. [DOI] [PubMed] [Google Scholar]
- 57.Resnitzky D, Gossen M, Bujard H, Reed S I. Acceleration of the G1/S-phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Richardson P M, Gilmore T D. v-Rel is an inactive member of the Rel family of transcriptional activating proteins. J Virol. 1991;65:3122–3130. doi: 10.1128/jvi.65.6.3122-3130.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sarkar S, Gilmore T D. Transformation by the v-Rel oncoprotein requires sequences carboxy-terminal to the Rel homology domain. Oncogene. 1993;8:2245–2252. [PubMed] [Google Scholar]
- 60.Schmitz M L, Stelzer G, Altmann H, Meisterernst M, Baeuerle P A. Interaction of the COOH-terminal transactivation domain of p65 NF-κB with TATA-binding protein, transcription factor IIB, and coactivators. J Biol Chem. 1995;270:7219–7226. doi: 10.1074/jbc.270.13.7219. [DOI] [PubMed] [Google Scholar]
- 61.Shibuya T, Chen I, Howatson A, Mak T W. Morphological, immunological, and biochemical analyses of chicken spleen cells transformed in vitro by reticuloendotheliosis virus strain T. Cancer Res. 1982;42:2722–2728. [PubMed] [Google Scholar]
- 62.Sif S, Gilmore T D. NF-κB p100 is one of the high-molecular-weight proteins complexed with the v-Rel oncoprotein in transformed chicken spleen cells. J Virol. 1993;67:7612–7617. doi: 10.1128/jvi.67.12.7612-7617.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simek S, Rice N R. pp59v-rel, the transforming protein of reticuloendotheliosis virus, is complexed with at least four other proteins in transformed chicken lymphoid cells. J Virol. 1988;62:4730–4736. doi: 10.1128/jvi.62.12.4730-4736.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smardova J, Walker A, Morrison L E, Kabrun N, Enrietto P J. The role of the carboxy terminus of v-Rel in transformation and activation of endogenous gene expression. Oncogene. 1995;10:2017–2026. [PubMed] [Google Scholar]
- 65.Stephens R M, Rice N R, Hiebsch R R, Bose H R, Gilden R V. Nucleotide sequence of v-rel: the oncogene of reticuloendotheliosis virus. Proc Natl Acad Sci USA. 1983;80:6229–6233. doi: 10.1073/pnas.80.20.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tanaka M, Herr W. Differential transcriptional activation by Oct-1 and Oct-2: interdependent activation domains induce Oct-2 phosphorylation. Cell. 1990;60:375–386. doi: 10.1016/0092-8674(90)90589-7. [DOI] [PubMed] [Google Scholar]
- 67.Tanaka M, Lai J-S, Herr W. Promoter-selective activation domains in Oct-1 and Oct-2 direct differential activation of an snRNA and mRNA promoter. Cell. 1992;68:755–767. doi: 10.1016/0092-8674(92)90150-b. [DOI] [PubMed] [Google Scholar]
- 68.Thanos D, Maniatis T. NF-κB: a lesson in family values. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 69.Theilen G, Zeigel R, Tweihaus M. Biological studies with RE virus (strain T) that induces reticuloendotheliosis in turkeys, chickens, and Japanese quails. J Natl Cancer Inst. 1966;37:747–749. [PubMed] [Google Scholar]
- 70.Tung H Y L, Bargmann W, Lim M Y, Bose H R. The v-rel oncogene product is complexed to a 40-kDa phosphoprotein in transformed lymphoid cells. Proc Natl Acad Sci USA. 1988;85:2479–2483. doi: 10.1073/pnas.85.8.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Verma I M, Stevenson J. IkappaB kinase: beginning, not the end. Proc Natl Acad Sci USA. 1997;94:11758–11760. doi: 10.1073/pnas.94.22.11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Verma I M, Stevenson J K, Schwarz E M, Van Antwerp D, Miyamoto S. Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 73.Walker W H, Stein B, Ganchi P A, Hoffman J A, Kaufman P A, Ballard D W, Hannink M, Greene W C. The v-rel oncogene: insights into the mechanism of transcriptional activation, repression, and transformation. J Virol. 1992;66:5018–5029. doi: 10.1128/jvi.66.8.5018-5029.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Watanabe S, Temin H M. Construction of a helper cell line for avian reticuloendotheliosis virus cloning vectors. Mol Cell Biol. 1983;3:2241–2249. doi: 10.1128/mcb.3.12.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.White D W, Roy A, Gilmore T D. The v-Rel oncoprotein blocks apoptosis and proteolysis of IκB-α in transformed chicken spleen cells. Oncogene. 1995;10:857–868. [PubMed] [Google Scholar]
- 76.White E, Sabbatini P, Debbas M, Wold W S M, Kusher D I, Gooding L R. The 19-kilodalton adenovirus E1B transforming protein inhibits programmed cell death and prevents cytolysis by tumor necrosis factor α. Mol Cell Biol. 1992;12:2570–2580. doi: 10.1128/mcb.12.6.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilhelmsen K C, Eggelton K, Temin H M. Nucleic acid sequences of the oncogene v-rel in reticuloendotheliosis virus strain T and its cellular homolog, the proto-oncogene c-rel. J Virol. 1984;52:172–182. doi: 10.1128/jvi.52.1.172-182.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wong T C, Lai M M C. Avian reticuloendotheliosis virus contains a new class of oncogene of turkey origin. Virology. 1981;111:289–293. doi: 10.1016/0042-6822(81)90674-7. [DOI] [PubMed] [Google Scholar]
- 79.Xu X, Gélinas C. The v-Rel oncoprotein complexes with new Rel- and RelA-related proteins in transformed cells. Virology. 1995;207:362–368. doi: 10.1006/viro.1995.1095. [DOI] [PubMed] [Google Scholar]
- 80.Xu X, Gélinas C. A mutant Rel homology domain promotes transcription by p50/NF-κB1. Oncogene. 1997;14:1521–1530. doi: 10.1038/sj.onc.1200985. [DOI] [PubMed] [Google Scholar]
- 81.Xu, X., and C. Gélinas. Unpublished data.
- 82.Xu X, Prorock C, Ishikawa H, Maldonado E, Ito Y, Gélinas C. Functional interaction of the v-Rel and c-Rel oncoproteins with the TATA-binding protein and association with transcription factor IIB. Mol Cell Biol. 1993;13:6733–6741. doi: 10.1128/mcb.13.11.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang J, Winoto A. A mouse Fas-associated protein with homology to the human Mort1/FADD protein is essential for Fas-mediated apoptosis. Mol Cell Biol. 1996;16:2756–2763. doi: 10.1128/mcb.16.6.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S. The transcriptional activity of NF-κB is regulated by the IκB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell. 1997;89:413–424. doi: 10.1016/s0092-8674(00)80222-6. [DOI] [PubMed] [Google Scholar]
- 85.Zhong H, Voll R, Ghosh S. Phosphorylation of NF-κB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell. 1998;1:661–671. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]
- 86.Zong W-X, Farrell M, Bash J, Gélinas C. v-Rel prevents apoptosis in transformed lymphoid cells and blocks TNFα-induced cell death. Oncogene. 1997;15:971–980. doi: 10.1038/sj.onc.1201266. [DOI] [PubMed] [Google Scholar]
- 87.Zong W-X, Bash J, Gélinas C. Rel blocks both anti-Fas- and TNFα-induced apoptosis and an intact Rel transactivation domain is essential for this effect. Cell Death Differ. 1998;5:963–972. doi: 10.1038/sj.cdd.4400441. [DOI] [PubMed] [Google Scholar]