Abstract
Oral breast cancer prevention medications entail systemic exposure, limiting acceptance by high risk women. Delivery through the breast skin, although an attractive alternative, requires demonstration of drug distribution throughout the breast. We conducted a randomized double-blind, placebo-controlled Phase II clinical trial comparing telapristone acetate (TPA), a progesterone receptor (PR) antagonist, administered orally (12 mg/day) or transdermally (12 mg/breast) for 4±1 weeks to women planning mastectomy. Plasma and tissue concentrations, measured at five locations in the mastectomy specimen using LC-MS/MS, were compared. In 60 evaluable subjects, median drug concentration (ng/g tissue) was 103 (IQR: 46.3–336) in the oral vs 2.82 (IQR: 1.4–5.5) in the transdermal group. Despite poor dermal permeation, within-breast drug distribution pattern was identical in both groups (R2=0.88, p=0.006), demonstrating that transdermally and orally delivered drug is distributed similarly through the breast, and is strongly influenced by tissue adiposity (p<0.0001). Other skin-penetrant drugs should be tested for breast cancer prevention.
Keywords: Local transdermal delivery, breast tissue, drug distribution, telapristone acetate
Introduction
Breast cancer prevention is a high-priority health need for women at increased risk of breast cancer. Large randomized trials have shown that endocrine agents (selective estrogen receptor modulators (1), and aromatase inhibitors (2, 3) are efficacious for this purpose. However, the acceptance of these drugs by women who are likely to benefit from them has been low (4, 5). Reasons include quality of life impairments, health risks, and reluctance by healthy women to take oral medication for prevention. Importantly, breast cancer prevention requires only that the breast be exposed to the drug; systemic exposure is both unnecessary and harmful (6, 7). Prevention strategies that target the breast locally, with low systemic exposure and minimal systemic toxicity, may overcome these barriers. Proposed methods of local therapy to the breast include local transdermal delivery through the breast skin (8), and intra-ductal delivery through a catheter cannulating the ductal orifice (9, 10). Of these, the far simpler alternative is transdermal delivery of drugs through the breast skin; this avoids fast hepatic metabolism, is noninvasive and self-administered, without costly devices. Local transdermal therapy (LTT) to the breast is widely applicable, and is likely to improve the tolerability and the acceptance of pharmacological cancer prevention regimens by women (11).
In a previous pilot study, we have tested a transdermal gel formulation of 4-hydroxytamoxifen (4-OHT), an active metabolite of tamoxifen (12). Women with ductal carcinoma in situ (DCIS) of the breast were randomized to 4-OHT gel or oral tamoxifen, used for 6–10 weeks during the pre-surgical window. A similar, significant decrease in cell proliferation (Ki67 labeling) following therapy occurred in both groups, replicating results from a previous, similar trial that enrolled postmenopausal women with estrogen receptor positive breast cancer (13). Building on these encouraging findings, we intended to continue clinical investigations with 4-OHT gel, but this became unavailable due to decisions by the manufacturer.
We therefore looked for other agents with biologic plausibility for breast cancer prevention, and suitable for transdermal delivery (lipophilic, with low molecular weight). Given extensive epidemiological and preclinical data supporting the role of progesterone and progestin exposure as breast cancer risk factors (14–17), we selected a selective progesterone receptor modulator (SPRM) as a promising candidate. SPRMs are of interest because of the specificity of PR blockade, and a safety profile that has allowed telapristone acetate (TPA) and ulipristal acetate to be tested for uterine fibroids (18–21). Data from rodent mammary carcinogenesis models also support the development of SPRMs for breast cancer prevention (22, 23).
We evaluated the dermal permeation of TPA using human skin in vitro, with oleic acid as a permeation enhancer (24), and then in nude rats in vivo, where application of the TPA gel to the mammary skin produced significantly higher concentrations in the treated mammary glands than in untreated mammary glands and plasma (8). Based on these results, Repros Therapeutics agreed to formulate a hydroalcoholic TPA gel formulation, and perform preclinical testing. First, they tested the toxicity limit in mini pigs (daily dose 129 mg in 1mL) for four months, preparing a fresh batch of colloidal suspension every 2 weeks because stability was poor at this high concentration. The pig study revealed higher tissue concentrations of TPA in the treated mammary gland than in the untreated glands, with low plasma concentration. Next, the TPA dose was lowered to a suitable range for the human protocol (10 mg), and photosafety was demonstrated in rabbits. The dose was selected following discussions with Repros (personal communications with Ronald Wiehle PhD) and based on transvaginal delivery trials, which showed that a 12 mg vaginal dose was efficacious for women with symptomatic uterine fibroids (NCT02323646 and NCT01451424), while a 24 mg dose was not more effective and but the drug tended to precipitate out of solution (NCT01451424 and NCT01631903). Thus the final transdermal formulation delivered 12 mg/day in a 1 mL volume. Stability testing of this indicated no significant changes in product potency or purity when stored in applicators at 2–8 °C or 25 °C, for up to 2 months. Based on this preclinical safety, permeability, and stability data, we obtained an FDA IND #123864 for a randomized double-blind, placebo-controlled Phase II clinical trial, designed to demonstrate that breast tissue concentrations achieved with TPA delivered through the breast skin are at least 50% of those seen with oral delivery. Our main secondary endpoint was to compare the intramammary distribution of transdermally and orally delivered drug, an important aspect of local delivery systems, which has not been systematically examined in the past.
Methods
Clinical trial design
Women planning unilateral or bilateral mastectomy for clinical care were recruited during the interval between surgical consultation and surgery (Institutional Review Board [IRB] STU00100531). Mastectomy indications included therapy for Stage 0-II breast cancer, or surgical risk reduction (for pathogenic variants in cancer causing genes, or lobular carcinoma in situ). Women with skin conditions such as psoriasis or eczema on the breast were excluded. Consenting women were randomized 1:1 to transdermal TPA gel 24 mg daily, 12 mg per breast (with oral placebo) or oral TPA 12 mg daily (with gel placebo), used for 4 ± 1 weeks. A negative pregnancy test was required for women with child-bearing potential. Subjects with breast cancer began study medication within 8 weeks of core needle biopsy (CNB) diagnosis. Subjects undergoing risk-reducing surgery underwent an optional research CNB within the same time period. The gel was dispensed in a metered dose canister; a single pump delivered the prescribed dose, which was applied to the entire skin envelope of each breast. The last dose was delivered on the day prior to surgery.
Compliance was defined as consumption of 80% of the total dose, including at least 2 of the 3 last doses. It was assessed through study diaries, capsule counts and weighing of gel dispensers at the conclusion of therapy. Noncompliant participants were not evaluated for study endpoints other than toxicity. Study sites were the Breast Centers of Northwestern Medicine, Memorial Sloan Kettering Cancer Center, and Cedars-Sinai Medical Center. The protocol was approved by the IRB of each institution.
Study agents
Repros Therapeutics formulated a transdermal alcoholic colloidal suspension of TPA, modified from a commercially available transdermal formulation for testosterone (AndroGel®, AbbVie Inc., Chicago, IL). Sodium hydroxide was replaced with tromethamine to improve gel formation, and butylated hydroxytoluene, 0.02% (w/w), was added as antioxidant to improve stability. The final gel product contained 1.4% drug TPA, 60% ethanol, 20% benzyl benzoate, 4% isopropyl miristate, 0.8% tromethamine, 12.5% water, 2% carbopol 980, and 0.02% butylated hydroxytoluene. Oral capsules were provided by Repros Therapeutics, as were TPA gel and placebo gel. TPA capsules were supplied as 12 mg of TPA powder in Size 3 hard gelatin capsules. Placebo contained no active ingredient but microcrystalline cellulose and magnesium stearate. Oral capsules and gel products of TPA and placebo were identical in appearance.
Tissue for analysis: mastectomy specimen processing
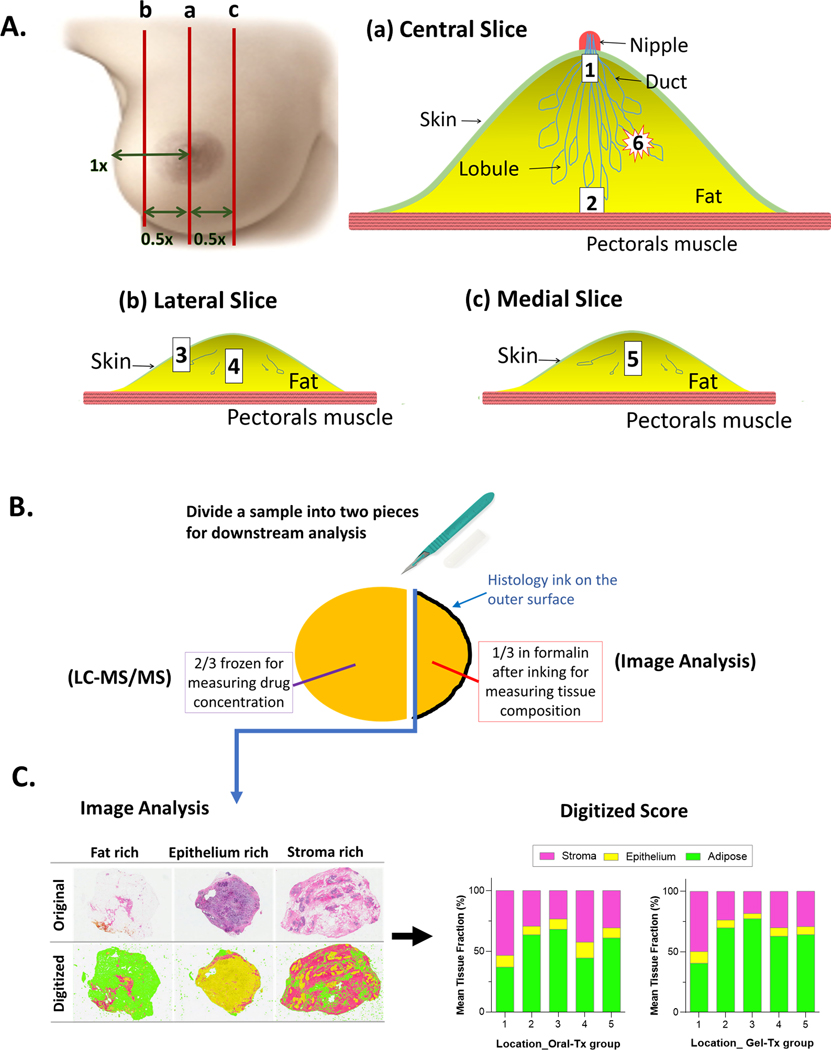
Mastectomy was performed with or without use of tumescence solution (25), according to surgeon preference; its use was recorded. The breast was delivered immediately to the gross room, weighed, measured, and bread-sliced. In accordance with our protocol-specified sampling method (Figure 1A), three slices (central, medial, lateral) were sampled for drug quantitation from 5 locations: 1) retroareolar 2) deepest central location in the breast, 3) superficial fat from the lateral slice, 4) middle of lateral slice 5) middle of medial slice. These were used for the primary endpoint of determining breast tissue drug concentration, and the major secondary endpoint of within-breast drug distribution. Two additional locations were sampled when possible: 6) tumor and 7) axillary lymph node. Each tissue sample was split: two-thirds was flash-frozen for drug measurement and one-third was formalin-fixed and paraffin-embedded (FFPE) for evaluation of tissue components (Figure 1B). FFPE sections were stained with hematoxylin and eosin and digitized at the NU pathology core. Images were transmitted to the University of North Carolina and scored for evaluation of proportional tissue area of adipose, epithelium, stroma) (Figure 1C), as previously described (26).
Figure 1.
Processing of mastectomy specimen for drug quantitation and evaluation of tissue composition. A. the distance from nipple to lateral edge and to the medial edge of the breast was measured and the half way point was marked between nipple and each edge of breast. Slice (a) was the central slice, through the nipple. Slice (b) was half way between nipple and lateral edge; e.g. if distance from nipple to lateral edge was 10 cm, slice (b) was taken 5 cm lateral to nipple. Slice (c) was half way from nipple to medial edge of breast. The samples were collected as follow: Lay slice (a) flat and collect sample #1 from the area behind the nipple, about 1 cm deep to the nipple papilla and sample #2 from the deepest location in the center of the breast close to the pectoral fascia, but avoiding the retromammary fat. Lay slice (b) flat, collect sample #3 from the subcutaneous fat, and sample #4 from the center of the slice, equidistant between superficial and deep surfaces. Lay slice (c) flat and collect sample #5 from its center, equidistant between superficial and deep surfaces. If tumor is present, lay the slice with the tumor flat and collect sample #6 from the tumor. Slice number and distance from surface were recorded for each sample. If an axillary lymph node was present and there was no known tumor in the breast (i.e. the mastectomy was for risk reduction), the axillary node was taken as sample #7. This was possible in only 5 subjects. B. Each sample was divided into two pieces: 2/3 for drug quantitation and 1/3 for tissue composition analysis. The smaller sample was inked on the outer surface (black color) and fixed in formalin for 6–24 hours. The inner surface of this sample (without ink, facing drug quantitation sample) was embedded face down in paraffin (blue line with arrow). C. This inner surface was sectioned and stained for H&E. Whole slide images of original H&E slides were taken with 20X magnification in Aperio system (left panel of C, upper row of images) and scored digitally for analysis of tissue composition (left panel of C, lower row of images). Digitized images by tissue composition were color coded: green, yellow, magenta for adipose tissue, epithelium, and stroma, respectively. Digitized score was summarized as tissue composition per sampling location per sample (right panel).
Drug and serum hormone concentration assays
Plasma and breast tissue concentration of TPA (CDB-4124) and its active monodemethylated metabolite (CDB-4453) were determined using Liquid Chromatography - Tandem Mass Spectrometry, performed at the Illinois Institute of Technology Research Institute as previously described (27), and detailed in supplementary methods. Serum concentrations of estradiol, progesterone, and follicle stimulating hormone (FSH) were measured using a validated protocol at Ligand Assay and Analysis Core Laboratory, University of Virginia. Estradiol assay were done with enzyme-linked immunosorbent assay kit (Cat # ES180S, Calbiotech Inc.) Progesterone and FSH assays were performed with Immulite technology (Cat # L2KPW2 and L2KFS2 (Siemens Corp.). Each hormone assay was performed in duplicate and mean value was reported for each sample.
Statistical Methods
The primary objective was to compare breast tissue TPA concentration between the oral and transdermal arms, to test the hypothesis that concentrations are equivalent between groups. Among women who contributed two breasts, one side was designated as “primary”: tumor-containing breast among cancer patients, and the breast with the more complete sample set for women undergoing risk-reducing surgery. Equivalence was defined as the mean whole-breast concentration in the transdermal group being within 50% of the mean in the oral group. A sample size of 30 women per group would achieve 80.3% power with 5% significance level for a test of equivalence, assuming mean concentration in the oral group is 45.3% (SD=30%) and the true difference is 0. Power was calculated using PASS software (Kaysville Utah). Assuming 15% attrition rate, we planned to randomize 35 subjects per group using the method of permuted blocks.
An important secondary endpoint was comparison of drug distribution across sampling locations in the breast. We explored potential predictors of tissue drug concentration: use of tumescence solution during surgery, bra cup size, body mass index (BMI), and percent adiposity as measured by histologic assessment. TPA concentration was log transformed to satisfy the normality assumption, and a value of 0.01 was added before log transformation to account for 0 concentrations. For each predictor, univariable linear mixed effects models were fitted, with log(concentration) as the outcome variable, predictor as the fixed effect, and subject as a random effect to account for 5 locations in each “primary” breast. Within-subject correlation between multiple locations was assumed to have the compound symmetry structure. Separate models were fitted for each treatment arm, and arms were not compared since the difference in drug concentration between arms was extremely large. Multivariable mixed models were also fitted to determine whether baseline clinical characteristics (BMI and cup size) were associated with concentration after accounting for tissue characteristics (% adipose). Models were fitted using SAS PROC MIXED.
Other secondary endpoints included 1) comparison of concurrently-obtained plasma drug levels to those in breast tissue, 2) serum progesterone, 3) tumor cell proliferation at baseline and after treatment, 4) changes in gene expression before and after treatment,5) tolerability of treatment, using the patient-reported Breast Eight Symptom Scale (BESS), administered before and after the treatment. Continuous variables were compared between groups using the Wilcoxon rank-sum test, and categorical variables were compared between groups using Fisher’s exact test. BESS scores were analyzed as described by Cella et. al. (28)
Results
Participant characteristics
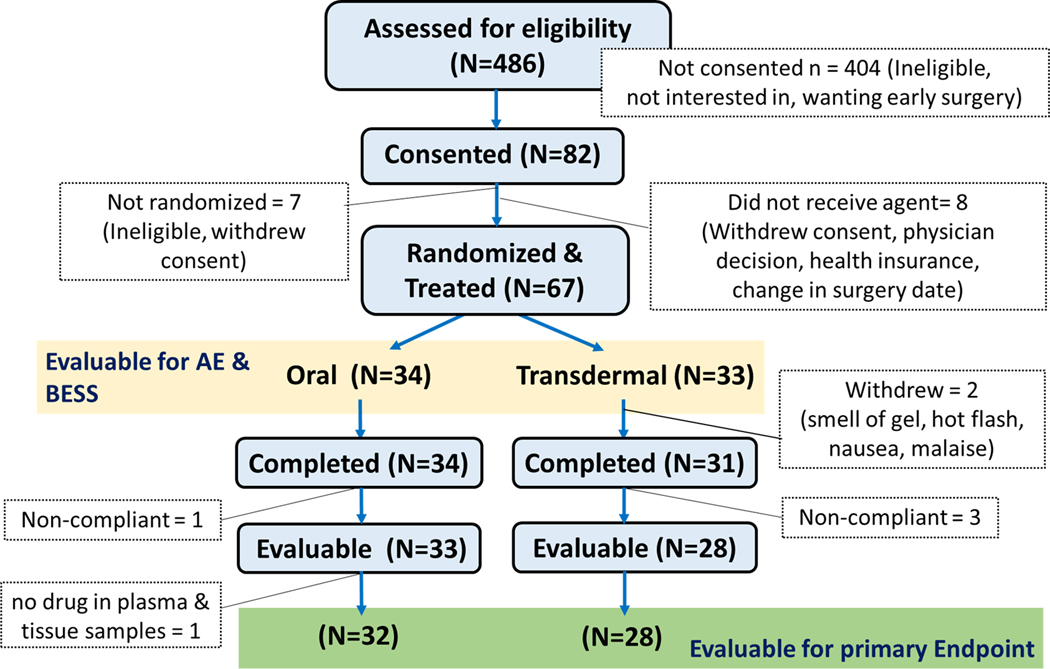
Between October 2015 and November 2017, 67 women were enrolled (Figure 2); the trial was terminated because the transdermal agent had reached the end of its shelf life. Among these, 32 and 28 were evaluable in the oral and transdermal arms. Demographic and tumor characteristics are described in Table 1. Two-thirds of the participants (40/60) were required mastectomy for a diagnosis of cancer, and the remainder were women at high risk for breast cancer, and had opted for risk-reducing mastectomy. Of the 40 cancer cases, 83% of tumors (33/40) were ER positive and 72% (29/40) were both ER and PR positive. HER2 status was not determined for DCIS patients.
Figure 2.
CONSORT diagram showing participant flow through various stages of enrollment and participation.
Table 1.
Participant characteristics.
| Oral (N=32) | Transdermal (N=28) | |
|---|---|---|
| Age (years)1 | 50 ± 14 | 48 ± 9.9 |
| Menopause | ||
| Pre | 18 (56%) | 18 (64%) |
| Post | 14 (44%) | 10 (36%) |
| Race | ||
| European | 25 (78%) | 24 (86%) |
| Non- European | 7 (22%) | 4 (14%) |
| Indication for mastectomy | ||
| Invasive cancer | 13 (41%) | 17 (61%) |
| DCIS | 8 (25%) | 2 (7%) |
| Non-cancer | 11 (34%) | 9 (32%) |
| Mastectomy with Tumescence | 18 (56%) | 19 (68%) |
| Breast size (cup size) | ||
| Small (A&B) | 15 (47%) | 11 (39%) |
| Medium/Large (≥C) | 15 (47%) | 16 (57%) |
| Not available | 2 (4%) | 1 (4%) |
| BMI | ||
| Normal (<25) | 17 (53%) | 10 (36%) |
| Overweight (25–29.9) | 6 (19%) | 10 (36%) |
| Obese (≥30) | 8 (25%) | 8 (29%) |
| Not available | 1 (3%) | 0 (0%) |
| Tumor markers, clinical assays | (N=21) | (N=19) |
| ER status | ||
| Positive | 18 (86%) | 15 (79%) |
| Negative (<1%) | 3 (14%) | 2 (16%) |
| Not available | 0 (0%) | 2 (11%) |
| PR status | ||
| Positive | 15 (71%) | 14 (73%) |
| Negative (<1%) | 5 (24%) | 3 (11%) |
| Not available | 1 (5%) | 2 (11%) |
| HER-2 status | ||
| Positive | 0 (0%) | 2 (10.5%) |
| Negative | 13 (62%) | 15 (79 %) |
| Not available2 | 8 (38%) | 2 (10.5%) |
| Tumor size (cm)1 | 1.5 ± 1.1 | 2.2 ± 1.7 |
Age and tumor size are reported as mean ± SD. Tumor size was not available for one subject in transdermal group (N=18).
HER-2 status was not available for DCIS cases.
Tissue drug concentrations and distribution through the breast
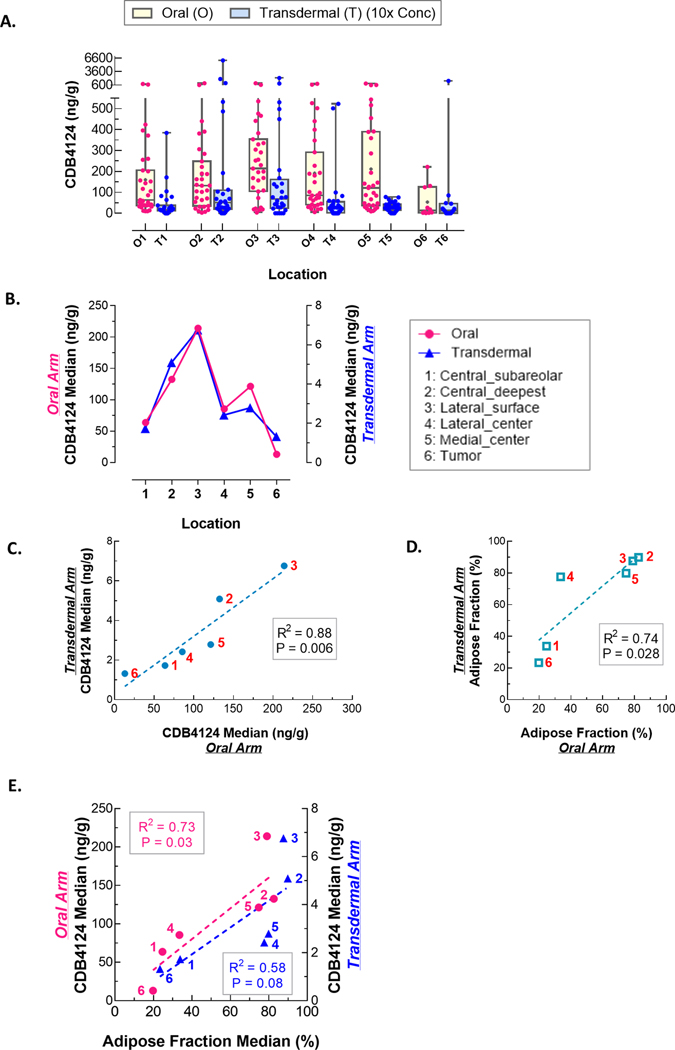
Of the 67 enrolled subjects, 60 were considered evaluable. Drug concentration values were not normally distributed; therefore, we report group and per-breast median and interquartile range (IQR) concentrations. The permeation of the transdermal formulation was poor, as demonstrated by the group-median concentration in the transdermal (2.82; IQR: 1.4–5.5 ng/g) compared to the oral group (103; IQR: 46.3–336 ng/g). This was also true of the median drug concentration at each of the five locations per breast (Table 2 and Figure 3A). However, the relative drug distribution across sampling sites was similar between treatment groups (Figure 3B), and the locations ranked exactly the same for the two treatment groups, with the subcutaneous location 3 having the highest concentration, and the deepest (location 2) having the next highest. We also examined the correlation in drug concentration across locations in the two groups (Figure 3C), and again observed a striking similarity in relative concentrations across the sites, that was highly significant (R2 = 0.88). Notably, the deepest location in the breast, which was farthest from the skin (location 2) showed the second-highest concentration in both oral and gel groups.
Table 2.
Median concentration of TPA in tissue and plasma and % adipose tissue according to treatment groups.1
| Tissue concentration (ng/g) |
Adipose tissue area (%) |
|||||
|---|---|---|---|---|---|---|
| Grossing Location | Oral (n=31) 2 | Transdermal (n=28) | P3 | Oral (n=31) | Transdermal (n=28) | P3 |
| 1 | 63.7 (37.7, 205) | 1.73 (1.15, 3.61) | <0.001 | 24.7 (7.7, 59.7)4 | 33.9 (16.7, 54.7) | 0.43 |
| 2 | 133 (34.9, 248) | 5.09 (2.12, 10.8) | <0.001 | 82.6 (18.6, 89.8) | 89.9 (40.5, 94.4) | 0.11 |
| 3 | 214 (104, 354) | 6.76 (2.55, 15.5) | <0.001 | 79.1 (45.3, 92.1) | 87.6 (63.5, 94.6) | 0.13 |
| 4 | 85.5 (40.6, 292) | 2.42 (0.42, 5.2) | <0.001 | 33.6 (9.2, 87) | 77.6 (26.4, 91.3) | 0.054 |
| 5 | 121 (37.9, 389) | 2.79 (1.44, 4.27) | <0.001 | 74.8 (31.1, 89.7) | 79.9 (36.7, 91) | 0.48 |
| Median per breast 5 | 103 (46.3, 336) | 2.82 (1.37, 5.47) | <0.001 | 64.9 (25, 85.2) | 77.8 (45.3, 88.5) | 0.19 |
| 6 (Tumor) |
13 (2.48, 124) (n=10) |
1.32 (0, 4.62) (n=15) |
0.008 | 19.8 (10.5, 53.1) (n=11) |
23.4 (5.43, 32.5) (n=14) |
0.96 |
| Plasma | 69.0 (32.8, 138) | 0.93 (0.39, 1.41) | ||||
| (ng/mL) | (n=32) | (n=28) | <0.0001 | |||
| Ratio of Tissue median to Plasma | ||||||
| 1.44 (0.74,2.98) (n=31) |
2.73 (1.78,5.50) (n=24)6 |
0.02 | ||||
Tissue drug concentration is reported as median with interquartile range.
Breast tissue samples for one evaluable subject were not available for analysis (failure to collect at time of surgery) but her plasma sample was submitted.
Wilcoxon rank-sum test.
n=30 for location 1 in the oral treatment arm (one sample for this location was missing).
locations 6 (tumor) was not included in the calculation of median values of breast concentration and adipose tissue fraction since the per-protocol primary objective was based on locations 1–5. In addition, there were missing values for subjects without cancer.
Ratios for four patients could not be computed, as their plasma values were 0.
Figure 3.
Drug distribution in the breast by study arm. A. TPA (CDB4124) concentrations varied by sampling location, regardless treatment arms: oral arm (O) and transdermal arm (T). 10x drug concentration of transdermal arm was shown in graph. B. Although tissue concentration in the transdermal arm was significantly lower than in the oral arm, concentrations varied similarly by location in both treatment arms: the highest in location 2 and 3; the lowest in location 1 and 6 (tumor). C. There was significant correlation of drug distribution between treatment arms. The solid blue circles along the regression line indicate the median tissue drug concentration at each location, and the red numerals indicate the sampling location. D. The adipose fraction of the breast tissue samples was also similar between arms; the open blue squares indicate the % adipose tissue in each sample, and the red numerals indicate the location. E. Drug concentration of both treatment arms were plotted against adipose% of tissue sample in each arm (blue transdermal, pink oral). The slopes are similar across the study arms.
We then evaluated factors that may have influenced tissue drug concentrations. These included the use of tumescence solution during mastectomy, breast size (using bra cup size as a surrogate), BMI, and the adipose and epithelial area of the tissue sample used for drug concentration measurement. The group-median breast concentrations were similar by cup size for the oral arm (138.9 ng/mL vs. 71.1 ng/ml; p = 0.63), and were marginally higher for larger cup sizes in the transdermal arm (2.9 ng/mL vs. 1.7 ng/mL; p = 0.08). The strongest effect on tissue drug concentrations was related to the adipose area of the tissue sample used for drug concentration assay, and was very similar across the two arms (Figure 3D). The measured drug concentration had an essentially identical relationship with tissue adiposity across the five sampling sites (Table 3 and Figure 3D and 3E). Thus, the samples with the highest adipose area demonstrated the highest values in both transdermal and oral groups. Relationships of these factors with tissue drug concentration were evaluated in mixed effects models that accounted for multiple within-subject measurements, shown in Table 3. In univariable models, the use of tumescence did not substantially influence the measured tissue drug concentrations in either arm. In the transdermal arm, there were significant positive associations with larger cup size, greater tissue adiposity, and higher BMI; and a borderline negative association with epithelial area. In the multivariable model, only adipose fraction was statistically significant in both arms.
Table 3.
Determinants of breast tissue drug concentration, univariable and multivariable analysis.
| Oral (N=32) | Transdermal (N=28) | |||||
|---|---|---|---|---|---|---|
| Variables | N1 | Parameter Estimates (95% CI) | p-value | N1 | Parameter Estimates (95% CI) | p-value |
| Univariable models | ||||||
| BMI | 153 | 0.07 (−0.01, 0.15) | 0.08 | 140 | 0.14 (0.03, 0.24) | 0.009 |
| Cup size2 | 143 | −0.01 (−0.95, 0.93) | 0.98 | 135 | 2.16 (0.73, 3.60) | 0.005 |
| Tumescence | 153 | −0.05 (−0.94, 0.84) | 0.91 | 140 | 0.80 (−0.88, 2.49) | 0.33 |
| Adipose fraction3 | 152 | 0.11 (0.07, 0.14) | <0.0001 | 139 | 0.27 (0.17, 0.38) | <0.0001 |
| Epithelium3 | 152 | −0.06 (−0.19, 0.07) | 0.37 | 139 | −0.51 (−1.00, −0.26) | 0.04 |
| Multivariable models | ||||||
| BMI | 142 | 0.06 (−0.03, 0.15) | 0.19 | 130 | 0.08 (−0.03, 0.18) | 0.16 |
| Cup size2 | 142 | −0.34 (−1.32, 0.63) | 0.47 | 130 | 1.03 (−0.53, 2.59) | 0.19 |
| Adipose fraction3 | 142 | 0.09 (0.05, 0.12) | <0.0001 | 130 | 0.23 (0.13, 0.34) | <0.0001 |
The number of samples, reflecting the observations used in the mixed effects model accounting for multiple log-transformed breast tissue drug concentration measurements taken for each subject. These mixed effects models were fitted in both univariable and multivariable analyses.
The breast cup sizes were used to compare effect of breast sizes (medium/large vs. small size).
Parameter estimates for adipose or epithelium reflect the coefficient pertaining to 10% increase in adipose or epithelium.
Tissue and Plasma concentrations of TPA
The median tissue and plasma concentrations of TPA are summarized in Table 2. Of 60 participants, 32 women in the oral group and 24 women in transdermal group presented detectable plasma concentrations of TPA (>0.2ng/mL). The median plasma concentration of the transdermal group was 1.3% that of the oral group (0.93 ng/mL vs. 69 ng/mL, respectively). Although transdermal permeation of TPA with the current gel formulation was poor, we observed that the ratio of median drug concentration (tissue:plasma) was higher in the transdermal group than in the oral group (2.73 in transdermal group vs. 1.44 in oral group, p=0.02). Furthermore, the ratio of median tumor drug concentration to plasma was approximately 7 times higher in transdermal group than in oral group (1.5 in transdermal group vs. 0.22 in oral group).
Change in serum hormones and missed periods in premenopausal women associated with the intervention
Since TPA is known to suppress ovulation in premenopausal women (27, 29), we measured serum concentrations of estradiol, progesterone and FSH prior to and following intervention. The median concentration of each hormone is summarized in Table 4. Although these values are not adjusted for menstrual cycle phase, we observed a significant decline of estradiol and progesterone concentrations (−23.7 pg/mL, p=0.007 for estradiol, and −2.36 ng/mL, p=0.004 for progesterone) in the oral treatment group, but changes in the transdermal group were not significant. There were no significant changes in FSH concentrations of both groups.
Table 4.
Changes in serum sex hormone concentrations of premenopausal women according to the treatments.
| Oral (N=18) | Transdermal (N= 18) | |||
|---|---|---|---|---|
| Hormones | Median (IQR) | P1 | Median (IQR) | P1 |
| Estradiol (pg/mL) | ||||
| Baseline | 122 (100, 172) | 95.9 (75.1, 135) | ||
| Post-treatment | 89.7 (78.5, 104) | 76.4 (49.7, 91.9) | ||
| Changes from baseline | −23.7 (−97.5, −1.70) | 0.007 | −5.30 (−29.4, 3.90) | 0.23 |
| Progesterone (ng/mL) | ||||
| Baseline | 2.63 (0.16, 10.3) | 0.42 (0, 1.43) | ||
| Post-treatment | 0.27 (0.12, 0.37) | 0.66 (0.2, 1.52) | ||
| Changes from baseline | −2.36 (−10.1, 0) | 0.004 | 0.19 (−0.12, 1.51) | 0.74 |
| FSH (mIU) | ||||
| Baseline | 4.60 (2.33, 5.75) | 7.30 (3.76, 10.6) | ||
| Post-treatment | 3.79 (2.44, 5.73) | 7.20 (5.86, 12.0) | ||
| Changes from baseline | 0.30 (−2.84, 2.21) | 0.79 | 1.91 (−0.40, 4.28) | 0.14 |
Paired Wilcoxon signed-rank test between baseline and post-treatment value within a treatment group.
Patient reported symptoms and adverse events with oral and transdermal administration
Quality of life parameters assessed by the BESS questionnaire at study entry and on the day prior to surgery were analyzed. Of 67 participants, 63 completed their BESS questionnaire at both time points. The results are summarized as described previously by Cella and colleagues (28) in Table S1. Following treatment, three symptom clusters (cognitive, gastrointestinal, and bladder) were significantly decreased in the oral group (p < 0.05), whereas the proportion reporting vaginal symptoms was significantly decreased in the transdermal group. The mean severity of body pain and gastrointestinal symptoms was decreased in oral group, whereas vasomotor symptoms were decreased in transdermal group. There was no other significant change in the proportion of participants who reported symptoms, or in the mean severity of symptoms.
A total of 67 randomized and treated participants were eligible for evaluation of adverse effects (AEs). AEs that were possibly, probably, or definitely related to study agent are summarized in Table S2. Most were Grade 1 events, consisting of commonly experienced minor symptoms. Three participants reported Grade 2 events: arthralgia (oral group), upper respiratory infection, and nausea (both in the transdermal group). One participant described a Grade 3 headache. Seven (20.6%) oral-group participants reported 11 gel site application AEs (burning, redness, acne, warmth), whereas four (12.1%) transdermal group participants experienced 8 application site AEs (burning, dryness, itchiness, rash, redness). Regardless of group, all site application AEs were grade 1, either possibly or probably related, and all but one resolved during the study without discontinuation of study drug. Overall, there was no significant between-group difference in AEs.
Discussion
Breast cancer prevention requires effective preventive medication that is acceptable to women at increased risk, but acceptance has been a barrier to orally delivered agents. LTT through the skin envelope of the breast is a promising alternative approach. The unique features of the breast predict the success of LTT: the embryological origin of the breast as a skin appendage (30, 31), a well-developed internal lymphatic circulation (32), and the presence of a subcutaneous and retromammary fatty envelope. Although early phase trials have shown that biologically effective drug concentrations can be achieved in the breast (12, 13) there are gaps in knowledge regarding the in-breast drug distribution with LTT. Our trial was designed to address several of these gaps. We hypothesized that the fatty envelope of the breast may serve as a drug reservoir for prolonged distribution to the breast, aided by the intramammary lymphatic circulation, so that transdermally delivered drugs are disseminated throughout the breast. However, no previous study has evaluated the uniformity of drug delivery throughout the breast. We therefore designed a randomized Phase II trial which included an oral therapy arm. Ideally, we would have utilized 4-OHT, an agent with known permeation and biological effect when delivered transdermally. However, this was not available at the time and future availability was unclear. We therefore turned to a plausible alternative that appeared to have good permeability in preclinical testing, both in vitro experiments using human skin (33), and using animal models that have been shown to be predictive of permeation though human skin (nude rat, minipig) (8, 34, 35). Thus, the poor permeation of the modified transdermal formulation tested in our trial was surprising, and points to the need for planned interim analyses and Bayesian adaptive design in trials of novel agents to confirm permeation even when preclinical data are encouraging. Nevertheless, we were able to address several questions pertinent to the feasibility of LTT to the breast: does it distribute to the deepest portions of the breast? What is the effect of breast tissue composition? What is the effect of breast size?
Despite the low concentrations of TPA observed in the breast with transdermal delivery, our study illustrates that the pattern of distribution of the drug within the breast is remarkably similar to orally delivered agent. Of note, we chose the deepest location in the breast, in line with the nipple, and furthest from breast skin as one of our sampling sites (location 2 in Figure 1), along with four other sites, one of which was subcutaneous. We observed variability in drug concentration in both oral and gel groups, but the rank-order of drug concentration was almost exactly similar: the location behind the nipple was the lowest, the subcutaneous location was the highest, with the deepest location being second-highest. The correlation of drug concentration values when plotted by location was 0.88 (p=0.006). These findings are extremely encouraging vis a vis the ability of transdermally delivered drugs to reach through the breast in the same pattern as those delivered orally.
A strength of our study was the careful evaluation of the composition of each sample that was used for drug concentration assay. We utilized a validated method (26), using digitized tissue sections, to measure the percent area of adipose, epithelial, or stromal cells. As expected for a lipophilic drug [cLogP =4.8 calculated by ACD/Labs software V11.02], the adipose proportion was a highly significant contributor to the tissue drug concentration in both groups (p<0.0001), in multivariable models that included bra cup size and BMI. In univariable models however, the transdermal group demonstrated higher drug concentrations when the breast size was larger (p=0.005) and when the BMI was higher (p<0.009), contrary to our a priori concern that concentrations would be lower when these features were present. However, in light of the associations observed between drug concentration and tissue composition, the patterns for breast size and BMI may reflect the proportion of adipose in the breast tissue. Our results are encouraging in terms of the applicability of transdermal delivery to women with larger breast sizes and higher body mass index, but need to be verified with drug formulations that have better penetrance.
The strength of the association between drug concentration and adipose fraction of the tissue is striking, and is seen with both oral and transdermal delivery. Prior studies have reported concentrations of tamoxifen metabolites in breast cancer and benign tissue (13, 36), but have not quantitated fractions of adipose and non-adipose tissue. Nevertheless, it is likely that other lipophilic drugs, including tamoxifen, are also enriched in adipose tissue. The known biologic efficacy of tamoxifen suggests that the high concentration in adipose tissue may ensure continuous drug exposure to interspersed non-adipose tissue. If so, prolonged retention in breast adipose tissue and slow release to neighboring non-adipose tissue may allow longer dosing intervals for transdermally delivered drugs.
Prior studies of oral tamoxifen show that plasma concentrations of 4-OHT 5-fold (12) to 10-fold (13) higher than with transdermal delivery of 4-OHT. We observed a far greater difference with TPA (69 ng/mL vs. 0.93 ng/mL), which is likely attributable to the poor penetration of this formulation. The breast:plasma concentration ratio of TPA is also of interest, and was higher in the transdermal than in the oral arm, as expected (2.73 vs. 1.44, p=0.02). A more permeable drug formulation will likely have a more favorable ratio, but this remains to be demonstrated with new formulations of this or other drugs. Of note, the low circulating levels of TPA when delivered transdermally did not cause changes in serum hormone concentrations in premenopausal women; whereas oral delivery resulted in significant decreases in serum estradiol and progesterone concentrations.
We measured parameters designed to evaluate drug efficacy (reduction in Ki67, changes in gene expression); we did not observe significant changes in tumor Ki67 or gene expression in either the oral or the transdermal group (data not shown). We have recently reported a pre-operative window study of oral TPA in Stage I-II breast cancer patients, where a one-third reduction in Ki67 post-therapy was accompanied by a significant decrease in cell-cycle related genes (27). In the present study, where the primary endpoint was drug permeation, we included women without cancer. This inclusive design limits our ability to reach conclusions regarding biologic effects of TPA by either route of administration. Since the clinical development of TPA has been discontinued, its biologic effects are only of general interest for this class of drugs.
The effect of breast radiation on transdermal delivery, an important question in the DCIS setting, is being addressed in our ongoing clinical trial using 4-OHT gel, which has demonstrated permeation into the breasts [12, 13]. Here, we are enrolling women who have had prior unilateral breast radiotherapy for DCIS or invasive cancer, while the contralateral breast is non-radiated (NCT04009044). This trial will provide some information on the possible effect of breast tissue rearrangement procedures on transdermal drug delivery, which will also be evaluated in future studies.
In summary, our results, although disappointing with regard to the transdermal permeation of the TPA formulation, are highly encouraging with regard to the important question as to whether distribution of drug within the breast is similar with oral and transdermal delivery. This finding is of general interest for drug distribution, and relevant to the development of new transdermal agents for the breast.
Supplementary Material
Study Highlights.
What is the current knowledge on the topic?
Despite increasing interest in the transdermal delivery of breast cancer prevention drugs through the breast skin, there are no data addressing within-breast distribution of transdermally delivered drugs.
What question did this study address?
1) Does a new formulation of telapristone acetate penetrate the breast skin in sufficient quantity for biologic effect; 2) is in-breast drug distribution similar by oral and transdermal routes? 3) What is the effect of tissue composition?
What does this study add to our knowledge?
This formulation of telapristone acetate displayed poor transdermal permeation. Nevertheless, the in-breast drug distribution pattern was remarkably similar with oral and transdermal administration. In all locations, concentrations were influenced by the adipose fraction of the tissue sample.
How might this change clinical pharmacology or translational science?
Local drug delivery to the breast may obviate concerns regarding systemic adverse events of oral drugs. This study proves the important principle that transdermally-delivered drug reaches throughout the breast, with in-breast variability that is similar to the oral route. Our findings therefore advance the science of transdermal drug delivery.
Acknowledgements:
We thank Dr. Mark Sherman for critical input into study design, and Ning Sun at IIT Research Institute for contribution to the drug concentration assays. We thank Repros Therapeutics for drug supply for the study.
Funding information: This work was funded by National Cancer Institute/Division of Cancer Prevention HHSN261201200035I/HHSN26100003.
Footnotes
Conflict of Interest statement: The authors declared no competing interests for this work.
References
- (1).Cuzick J. et al. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet 381, 1827–34 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Goss PE et al. Exemestane for breast-cancer prevention in postmenopausal women. NEnglJMed 364, 2381–91 (2011). [DOI] [PubMed] [Google Scholar]
- (3).Cuzick J. et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet 383, 1041–8 (2014). [DOI] [PubMed] [Google Scholar]
- (4).Port ER, Montgomery LL, Heerdt AS & Borgen PI Patient reluctance toward tamoxifen use for breast cancer primary prevention. AnnSurgOncol 8, 580–5 (2001). [DOI] [PubMed] [Google Scholar]
- (5).Hackett J. et al. Uptake of breast cancer preventive therapy in the UK: results from a multicentre prospective survey and qualitative interviews. Breast Cancer Res Treat 170, 633–40 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Fisher B. et al. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. JNatlCancer Inst 90, 1371–88 (1998). [DOI] [PubMed] [Google Scholar]
- (7).Day R, Ganz PA, Costantino JP, Cronin WM, Wickerham DL & Fisher B. Health-related quality of life and tamoxifen in breast cancer prevention: a report from the National Surgical Adjuvant Breast and Bowel Project P-1 Study. JClinOncol 17, 2659–69 (1999). [DOI] [PubMed] [Google Scholar]
- (8).Lee O. et al. Local transdermal therapy to the breast for breast cancer prevention and DCIS therapy: preclinical and clinical evaluation. Cancer Chemother Pharmacol 76, 1235–46 (2015). [DOI] [PubMed] [Google Scholar]
- (9).Stearns V. et al. Preclinical and clinical evaluation of intraductally administered agents in early breast cancer. Sci Transl Med 3, 106ra8 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Chun YS et al. Intraductal administration of a polymeric nanoparticle formulation of curcumin (NanoCurc) significantly attenuates incidence of mammary tumors in a rodent chemical carcinogenesis model: Implications for breast cancer chemoprevention in at-risk populations. Carcinogenesis 33, 2242–9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Karavites LC, Allu S, Khan SA & Kaiser K. Awareness of preventive medication among women at high risk for breast cancer and their willingness to consider transdermal or oral tamoxifen: a focus group study. BMC cancer 15, 878 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Lee O. et al. A randomized phase II presurgical trial of transdermal 4-hydroxytamoxifen gel versus oral tamoxifen in women with ductal carcinoma in situ of the breast. Clin Cancer Res 20, 3672–82 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Rouanet P. et al. Neoadjuvant percutaneous 4-hydroxytamoxifen decreases breast tumoral cell proliferation: a prospective controlled randomized study comparing three doses of 4-hydroxytamoxifen gel to oral tamoxifen. JClinOncol 23, 2980–7 (2005). [DOI] [PubMed] [Google Scholar]
- (14).Brisken C. Progesterone signalling in breast cancer: a neglected hormone coming into the limelight. NatRevCancer 13, 385–96 (2013). [DOI] [PubMed] [Google Scholar]
- (15).Trabert B, Sherman ME, Kannan N. & Stanczyk FZ Progesterone and breast cancer. Endocrine reviews, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Temkin SM, Mallen A, Bellavance E, Rubinsak L. & Wenham RM The role of menopausal hormone therapy in women with or at risk of ovarian and breast cancers: Misconceptions and current directions. Cancer 125, 499–514 (2019). [DOI] [PubMed] [Google Scholar]
- (17).D’Alonzo M, Bounous VE, Villa M. & Biglia N. Current Evidence of the Oncological Benefit-Risk Profile of Hormone Replacement Therapy. Medicina (Kaunas) 55, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Ioffe OB, Zaino RJ & Mutter GL Endometrial changes from short-term therapy with CDB-4124, a selective progesterone receptor modulator. Mod Pathol 22, 450–9 (2009). [DOI] [PubMed] [Google Scholar]
- (19).Spitz IM Clinical utility of progesterone receptor modulators and their effect on the endometrium. Curr Opin Obstet Gynecol 21, 318–24 (2009). [DOI] [PubMed] [Google Scholar]
- (20).Melis GB et al. Pharmacokinetic evaluation of ulipristal acetate for uterine leiomyoma treatment. Expert opinion on drug metabolism & toxicology 8, 901–8 (2012). [DOI] [PubMed] [Google Scholar]
- (21).Levens ED et al. CDB-2914 for uterine leiomyomata treatment: a randomized controlled trial. ObstetGynecol 111, 1129–36 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Lee O, Choi MR, Christov K, Ivancic D. & Khan SA Progesterone receptor antagonism inhibits progestogen-related carcinogenesis and suppresses tumor cell proliferation. Cancer letters 376, 310–7 (2016). [DOI] [PubMed] [Google Scholar]
- (23).Wiehle R, Lantvit D, Yamada T. & Christov K. CDB-4124, a progesterone receptor modulator, inhibits mammary carcinogenesis by suppressing cell proliferation and inducing apoptosis. Cancer PrevRes(Phila) 4, 414–24 (2011). [DOI] [PubMed] [Google Scholar]
- (24).Lee O, Ivancic D, Shidfar A, Wiehle R. & Khan SA In vitro and in vivo transdermal delivery of CDB4124, a progesterone receptor modulator: a potential method for breast cancer prevention. Cancer Research 73, (2013). [Google Scholar]
- (25).Staradub VL & Morrow M. Modified radical mastectomy with knife technique. Arch Surg 137, 105–10 (2002). [DOI] [PubMed] [Google Scholar]
- (26).Sandhu R, Chollet-Hinton L, Kirk EL, Midkiff B. & Troester MA Digital histologic analysis reveals morphometric patterns of age-related involution in breast epithelium and stroma. Hum Pathol 48, 60–8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Lee O. et al. Selective progesterone receptor modulators in early stage breast cancer: a randomized, placebo-controlled Phase II window of opportunity trial using telapristone acetate. Clin Cancer Res, (2019). [DOI] [PubMed] [Google Scholar]
- (28).Cella D. et al. Symptom measurement in the Breast Cancer Prevention Trial (BCPT) (P-1): psychometric properties of a new measure of symptoms for midlife women. Breast Cancer Res Treat 109, 515–26 (2008). [DOI] [PubMed] [Google Scholar]
- (29).Nelson AL Investigational hormone receptor agonists as ongoing female contraception: a focus on selective progesterone receptor modulators in early clinical development. Expert Opin Investig Drugs 24, 1321–30 (2015). [DOI] [PubMed] [Google Scholar]
- (30).Mikkola ML & Millar SE The mammary bud as a skin appendage: unique and shared aspects of development. J MammaryGlandBiolNeoplasia 11, 187–203 (2006). [DOI] [PubMed] [Google Scholar]
- (31).Ackerman AB, Kessler G, Gyorfi T, Tsou HC & Gottlieb GJ Contrary view: the breast is not an organ per se, but a distinctive region of skin and subcutaneous tissue. AmJDermatopathol 29, 211–8 (2007). [DOI] [PubMed] [Google Scholar]
- (32).Suami H, Pan WR, Mann GB & Taylor GI The lymphatic anatomy of the breast and its implications for sentinel lymph node biopsy: a human cadaver study. AnnSurgOncol 15, 863–71 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Lee O, Ivancic D, Chatterton RT Jr., Rademaker AW & Khan SA In vitro human skin permeation of endoxifen: potential for local transdermal therapy for primary prevention and carcinoma in situ of the breast. Breast Cancer (Dove Med Press) 3, 61–70 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Yoshimatsu H, Ishii K, Konno Y, Satsukawa M. & Yamashita S. Prediction of human percutaneous absorption from in vitro and in vivo animal experiments. Int J Pharm 534, 348–55 (2017). [DOI] [PubMed] [Google Scholar]
- (35).Mitra A. et al. Use of minipig skin biopsy model as an innovative tool to design topical formulation to achieve desired pharmacokinetics in humans. Journal of pharmaceutical sciences 104, 1701–8 (2015). [DOI] [PubMed] [Google Scholar]
- (36).Pujol H. et al. Phase I study of percutaneous 4-hydroxy-tamoxifen with analyses of 4-hydroxy-tamoxifen concentrations in breast cancer and normal breast tissue. Cancer ChemotherPharmacol 36, 493–8 (1995). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.