ABSTRACT
A diverse genetic toolkit is critical for understanding bacterial physiology and genotype-phenotype relationships. Inducible promoter systems are an integral part of this toolkit. In Burkholderia and related species, the l-rhamnose-inducible promoter is among the first choices due to its tight control and the lack of viable alternatives. To improve upon its maximum activity and dynamic range, we explored the effect of promoter system modifications in Burkholderia cenocepacia with a LacZ-based reporter. By combining the bacteriophage T7 gene 10 stem-loop and engineered rhaI transcription factor-binding sites, we obtained a rhamnose-inducible system with a 6.5-fold and 3.0-fold increases in maximum activity and dynamic range, respectively, compared to the native promoter. We then added the modified promoter system to pSCrhaB2 and pSC201, common genetic tools used for plasmid-based and chromosome-based gene expression, respectively, in Burkholderia, creating pSCrhaB2plus and pSC201plus. We demonstrated the utility of pSCrhaB2plus for gene expression in B. thailandensis, B. multivorans, and B. vietnamiensis and used pSC201plus to control highly expressed essential genes from the chromosome of B. cenocepacia. The utility of the modified system was demonstrated as we recovered viable mutants to control ftsZ, rpoBC, and rpsF, whereas the unmodified promoter was unable to control rpsF. The modified expression system allowed control of an essential gene depletion phenotype at lower levels of l-rhamnose, the inducer. pSCRhaB2plus and pSC201plus are expected to be valuable additions to the genetic toolkit for Burkholderia and related species.
IMPORTANCE Species of Burkholderia are dually recognized as being of attractive biotechnological potential but also opportunistic pathogens for immunocompromised individuals. Understanding the genotype-phenotype relationship is critical for synthetic biology approaches in Burkholderia to disentangle pathogenic from beneficial traits. A diverse genetic toolkit, including inducible promoters, is the foundation for these investigations. Thus, we sought to improve on the commonly used rhamnose-inducible promoter system. Our modifications resulted in both higher levels of heterologous protein expression and broader control over highly expressed essential genes in B. cenocepacia. The significance of our work is in expanding the genetic toolkit to enable more comprehensive studies into Burkholderia and related bacteria.
KEYWORDS: Burkholderia, rhamnose-inducible promoter, LacZ, PrhaBAD, conditional growth mutant, essential gene, inverse PCR
INTRODUCTION
There are more than one hundred species in the genus Burkholderia, with many recognized as either having enormous biotechnological potential or being causative agents of potentially lethal opportunistic infections (1). In terms of biotechnology, multiple species and strains have been found to produce bioplastics (2), promote plant growth by nitrogen-fixing nodulation (3), or act as agents for biocontrol (4, 5) and bioremediation (6). On the contrary, a subgroup of species, known as the B. cepacia complex (Bcc), are notorious opportunistic pathogens, noted for causing severe respiratory infections in patients with cystic fibrosis (CF) (7). Near-uniform resistance to cationic antimicrobials, aminoglycosides, and all but the most potent β-lactam/β-lactamase inhibitor combinations have rendered treatment difficult (8). In addition, there are no universal eradication protocols for Bcc infections of the CF lung (9). Phylogenetics alone cannot fully separate beneficial species from those with pathogenic potential (1), suggesting case-by-case analysis is needed to characterize individual genetic arsenals (10).
Pursuing Burkholderia species for biotechnology therefore necessitates genetic tools to finely dissect the genotype-phenotype relationship and explore synthetic biology applications. One approach to both heterologous protein expression and manipulating endogenous gene sets is the use of inducible promoters. In species of Burkholderia, only the use of the l-arabinose- and l-rhamnose-inducible promoter systems (both from Escherichia coli) has been reported. Additionally, Lefebre and Valvano have also suggested that the Plac and Ptac promoters are nonfunctional in several Burkholderia species (11). Originally, the arabinose-inducible promoter system was designed for plasmid-based inducible protein expression in B. cenocepacia and B. vietnamiensis, even permitting sustained expression during intracellular infection of Acanthamoeba polyphaga (11). Nonetheless, some caveats included leaky expression (12) and that the high concentrations of arabinose required for maximum induction (2 to 3%) were noted to cause an osmotic stress-like bloated cell morphology (13). In an effort to rectify these issues, we previously cloned the l-rhamnose-inducible system from E. coli into a broad-host-range vector with a pBBR1 origin (13). In an egfp reporter assay, strong induction was achieved with as little as 0.02% l-rhamnose, and virtually no fluorescence was observed in the absence of l-rhamnose, demonstrating both high sensitivity and tight regulation of gene expression in B. cenocepacia (13, 14).
The genetic arrangement and induction cascade of l-rhamnose-inducible expression of rhamnose catabolic genes of E. coli and other Enterobacteriaceae (15) are shown in Fig. 1. Upon detection of l-rhamnose, RhaR dimerizes and makes specific DNA contacts upstream of its own PrhaSR promoter to recruit the RNA polymerase and activate transcription of the AraC/XylS-family transcriptional regulators rhaS and rhaR (16). RhaS also binds l-rhamnose, dimerizes, and binds specific DNA regions to activate transcription from the PrhaBAD promoter, controlling the production of l-rhamnose catabolic genes rhaBAD (17). The RhaS dimer also activates the PrhaT promoter, controlling production of the RhaT l-rhamnose-H+ symporter (18). The DNA regions bound by dimerized RhaR and RhaS are known as rhaI half-sites and differ in both sequence and protein-binding affinity (19, 20). The gene of interest is typically placed under the control of PrhaBAD. It is important to note that the l-rhamnose catabolic pathway, including the specific l-rhamnose transporter rhaT, was not identified in species of Burkholderia (15). So far, the specific mechanism for rhamnose uptake in Burkholderia remains unknown.
FIG 1.
Activation and layout of the l-rhamnose-inducible promoter used in this study. (A) l-Rhamnose is bound by the transcription factor RhaR, which dimerizes and binds upstream elements of its own promoter. Dimerized RhaR recruits the RNA polymerase to PrhaSR. RhaS also binds l-rhamnose, dimerizes, and binds upstream elements of PrhaBAD, resulting in recruitment of the RNA polymerase and activation of the promoter (20, 69). The system is placed with PrhaBAD in front of a gene of interest (for example, yfg) to control the expression with l-rhamnose. (B) The genetic elements manipulated in this study are enclosed by dashed boxes. (C) A sequence-based view of the modified regions (shaded) in the PrhaBAD and PrhaSR promoters. Numbers below the sequence are positions related to the transcription start of rhaSR, while numbers above the line are positions relative to the transcription start of the gene placed downstream of PrhaBAD.
Despite the demonstrated utility of the l-rhamnose-inducible expression system in species of Burkholderia, efforts to control essential genes at the genome level from a single expression system are limited by the maximum activity and the dynamic range (absolute difference between the “on” and “off” state) of the chosen system (21–23). Here, we designed, constructed, and tested a variety of modifications to the l-rhamnose-inducible expression system in B. cenocepacia, using a fluorescent LacZ reporter assay. We identified a modified l-rhamnose-inducible promoter with stronger induced activity and wider dynamic range. We validate our system showing broader control of highly expressed essential genes in B. cenocepacia K56-2 with smaller amounts of inducer.
RESULTS
Single modifications yield variable effects on induced and uninduced expression.
To develop a system that would allow us to measure changes in gene expression from the rhamnose-inducible system, we cloned the full lacZ gene in pSCrhaB2 (13), a plasmid containing the native l-rhamnose-inducible system from E. coli (Table 1). The lacZ gene is naturally absent from Burkholderia, and LacZ reporter assays have been used previously to assess promoter activity in this genus (24). This reporter assay measures the formation of functional protein. While changes in mRNA stability and translation efficiency may occur, our goal was to evaluate the overall effect of each modification on the final protein product of gene expression. To measure lacZ activity driven by different constructs, we used the highly sensitive fluorogenic substrate 4-methylumbelliferyl-β-d-galactopyranoside (MUG) (25). We introduced modifications to (i) the ribosome-binding sites (RBS) (26), (ii) the 5′ untranslated region (5′ UTR) (27, 28), and (iii) the rhaI half-sites bound by RhaS (19, 20), based on previous evidence of these elements regulating protein expression. We used inverse PCR (29, 30) to introduce a series of single modifications (see descriptions in Table 2), transformed B. cenocepacia with the resulting plasmids, and then measured LacZ activity under induced and uninduced conditions in cell extracts.
TABLE 1.
Bacterial strains and plasmids used in this worka
| Strain or plasmid | Feature(s) | Source or reference |
|---|---|---|
| Burkholderia cenocepacia K56-2 | Cystic fibrosis clinical isolate from the ET12 lineage | 70 |
| Burkholderia multivorans ATCC 17616 | Soil isolate | ATCC |
| Burkholderia thailandensis E264 | Rice-field soil isolate from Thailand | DSMZ |
| Burkholderia vietnamiensis LMG 16232 | Isolated from cystic fibrosis patient’s sputum; also called CCUG 31370 | BCCM |
| E. coli DH5α | F− Φ80lacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17 (rH− mK+) phoA supE44 λ− thi-1 gyrA96 relA1 | Invitrogen |
| E. coli SY327 | F− araD Δ(lac-proAB) argE(Am) recA56 Rifr nalA λpir | 71 |
| E. coli MM294 | F− φ80 lacZΔM15 endA1 recA1 hsdR17 (rH− mK+) supE44 thi-1 ΔgyrA96 (ΔlacZYA-argF)U169 relA1 | 72 |
| Pseudomonas aeruginosa PAO1 | Human wound isolate | 73 |
| Pseudomonas aeruginosa PA14 | High-virulence human burn wound isolate | 74 |
| CGftsZ | K56-2 with pSC201-ftsZ integrated in ftsZ | This study |
| CGftsZplus | K56-2 with pSC201plus-ftsZ integrated at ftsZ | This study |
| CGrplN-O | K56-2 with pSC201-rplN-O integrated at rplN | This study |
| CGrplN-Oplus | K56-2 with pSC201plus-rplN-O integrated at rplN | This study |
| CGrpoBC | K56-2 with pSC201-rpoBC integrated at rpoBC | This study |
| CGrpoBCplus | K56-2 with pSC201plus-rpoBC integrated at rpoBC | This study |
| CGrpsF | K56-2 with pSC201-rpsF integrated at rpsF | This study |
| CGrpsFplus | K56-2 with pSC201plus-rpsF integrated at rpsF | This study |
| E. coli DH5α | F− Φ80lacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17 (rH− mK+) phoA supE44 λ− thi-1 gyrA96 relA1 | Invitrogen |
| E. coli SY327 | F− araD Δ(lac-proAB) argE(Am) recA56 Rifr nalA λpir | 71 |
| E. coli MM294 | F− φ80 lacZΔM15 endA1 recA1 hsdR17 (rH− mK+) supE44 thi-1 ΔgyrA96 (ΔlacZYA-argF)U169 relA1 | 72 |
| pAPC03 | pSCrhaB2 with lacZ and terminators (from pJPD01) ligated into KpnI and XbaI sites | This study |
| pKRJ12 (pSCrhaB2-lacZ) | pAPC03 with 6 bp between RBS and start codon; this is the parental plasmid used as a template to test the effect of other modifications | This study |
| pJPD01 | Tetr, Ω-FRT-attPMCS, hmqA, lacZ, oripMB1, intφCTX, and oriT | Gift from Eric Déziel, 65 |
| pSC201 | ori R6K rhaR rhaS PrhaB dhfr mob+ | 37 |
| pSC201-rplN-O | pSC201 with 308-bp 5′ fragment of rplN inserted into NdeI and XbaI sites | This study |
| pSC201-rpoBC | pSC201 with 293-bp 5′ fragment of rpoB inserted into NdeI and XbaI sites | This study |
| pSC201-rpsF | pSC201 with 318-bp 5′ fragment of rpsF inserted into NdeI and XbaI sites | This study |
| pSC201-ftsZ | pSC201 with 301-bp 5′ fragment of ftsZ inserted into NdeI and XbaI sites | This study |
| pSC201plus | pSC201 with modifications of pKRJ38: rhaI1-rhaI1 permutation of rhaS binding sites upstream of PrhaBAD and bacteriophage T7 gene 10 stem loop inserted upstream of native rhaBAD 5′ UTR | This study |
| pSC201plus-rplN-O | pSC201plus with 308-bp 5′ fragment of rplN inserted into NdeI and XbaI sites | This study |
| pSC201plus-rpoBC | pSC201plus with 293-bp 5′ fragment of rpoB inserted into NdeI and XbaI sites | This study |
| pSC201plus-rpsF | pSC201plus with 318-bp 5′ fragment of rpsF inserted into NdeI and XbaI sites | This study |
| pSC201plus-ftsZ | pSC201plus with 301-bp 5′ fragment of ftsZ inserted into NdeI and XbaI sites | This study |
| pSCrhaB2 | oripBBR1rhaRrhaS PrhaB dhfr mob+ | 13 |
| pSCrhaB2plus | pSCrhaB2 with modifications of pKRJ38; rhaI1-rhaI1 permutation of rhaS binding sites upstream of PrhaBAD and bacteriophage T7 gene 10 stem loop inserted upstream of native rhaBAD 5′ UTR | This study |
Plasmids with promoter system modifications used in β-galactosidase assays are listed in Table 2.
TABLE 2.
Plasmids harboring modifications of the parental l-rhamnose-inducible system
| Type of modification | Plasmid | Description | Modification reference or source |
|---|---|---|---|
| None (parental) | pKRJ12 | pSCrhaB2 with lacZ under control of PrhaBAD | This study |
| RBS | pKRJ02 | pKRJ12 with AGGAGG RBS | This study |
| pKRJ04 | pKRJ12 with AAGGAGG RBS | This study | |
| pKRJ07 | pKRJ12 with GGAGG RBS | This study | |
| pKRJ08 | pKRJ12 with AGGAAGG RBS | 75 | |
| pKRJ09 | pKRJ12 with ACGAGGG RBS | 75 | |
| pKRJ10 | pKRJ12 with ACAAAGG RBS | 75 | |
| 5′ UTR | pKRJ13 | pKRJ12 with AU-rich translational enhancer from E. coli fepB (TTAACCTTA) downstream of native rhaBAD 5′ UTR | 76 |
| pKRJ14 | pKRJ13 with added 5′ CC and 3′ AATA, resulting in CCTTAACCTTATTAATA | 27 | |
| pKRJ15 | pKRJ12 with AU-rich translational enhancer from phage T7 (TTAACTTCA) downstream of native rhaBAD 5′ UTR | 77 | |
| pKRJ16 | pKRJ15 with added 5′ CA and 3′ AATA, resulting in CATTAACTTCATTAATA | 27 | |
| pKRJ17 | pKRJ12 with AU-rich GSGV 5′ UTR GCTCTTTAACAATTTATCAT (instead of native rhaBAD 5′ UTR) | 27 | |
| pKRJ18 | pKRJ12 with AU-rich JBE1 5′ UTR GAATTCAAAAGATCTTTTAA (instead of native rhaBAD 5′ UTR) | 27 | |
| pKRJ23 | pKRJ12 with bacteriophage T7 gene 10 stem loop inserted upstream of native rhaBAD 5′ UTR | 33 | |
| rhaI half-sites | pKRJ24 | pKRJ12 with rhaI1-rhaI1 permutation of rhaS binding sites upstream of PrhaBAD | 20 |
| pKRJ25 | pKRJ12 with rhaI1-rhaI6 permutation of rhaS binding sites upstream of PrhaBAD | 20 | |
| pKRJ26 | pKRJ12 with rhaI6-rhaI1 permutation of rhaS binding sites upstream of PrhaBAD | 20 | |
| pKRJ27 | pKRJ12 with rhaI6-rhaI6 permutation of rhaS binding sites upstream of PrhaBAD | 20 | |
| 5′ UTR + rhaI half-sites | pKRJ28 | pKRJ17 with rhaI1-rhaI1 permutation of rhaS binding sites upstream of PrhaBAD | 20, 27 |
| pKRJ29 | pKRJ17 with rhaI1-rhaI6 permutation of rhaS binding sites upstream of PrhaBAD | 20, 27 | |
| pKRJ30 | pKRJ17 with rhaI6-rhaI1 permutation of rhaS binding sites upstream of PrhaBAD | 20, 27 | |
| pKRJ31 | pKRJ17 with rhaI6-rhaI6 permutation of rhaS binding sites upstream of PrhaBAD | 20, 27 | |
| pKRJ32 | pKRJ18 with rhaI1-rhaI1 permutation of rhaS binding sites upstream of PrhaBAD | 20, 27 | |
| pKRJ33 | pKRJ18 with rhaI1-rhaI6 permutation of rhaS binding sites upstream of PrhaBAD | 20, 27 | |
| pKRJ34 | pKRJ18 with rhaI6-rhaI1 permutation of rhaS binding sites upstream of PrhaBAD | 20, 27 | |
| pKRJ35 | pKRJ18 with rhaI6-rhaI6 permutation of rhaS binding sites upstream of PrhaBAD | 20, 27 | |
| rhaSR | pKRJ36 | pKRJ12 with PrhaSR promoter replaced with PJ23119 | 34 |
| Regulatory genes | pKRJ37 | pKRJ36 with near complete deletion of rhaR | 34 |
| T7 gene 10 stem loop + rhaI1-rhaI1 | pKRJ38 (pSCrhaB2plus-lacZ) | pKRJ23 with rhaI1-rhaI1 permutation of rhaS binding sites upstream of PrhaBAD | 20, 33 |
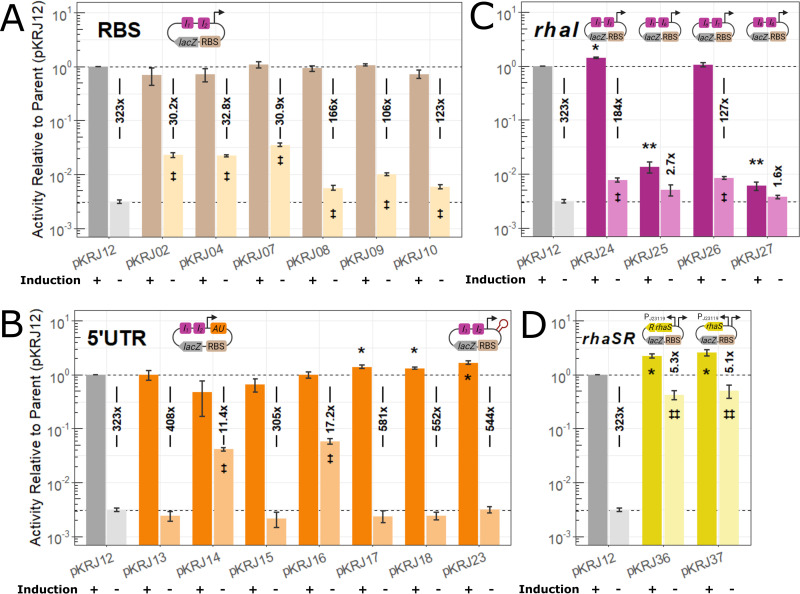
Spacing between the RBS and the start codon is important for efficient translation, with 5 to 8 bp being optimal (31, 32). To modify the native RBS of the l-rhamnose-inducible promoter (AGGA), we constructed RBS variants with differing complementarity to the B. cenocepacia anti-Shine-Dalgarno (SD) sequence (5′ CCUCCUU 3′), leaving intact the 6 bp between the RBS and the start codon (Table 2). All the RBS modifications increased the levels of uninduced LacZ activity from 1.8-fold (AGGAAGG in pKRJ08) to 11.4-fold (GGAGG in pKRJ07) without increasing activity under inducing conditions (Fig. 2A). Consequently, the dynamic range of this series decreased substantially compared to the parental promoter in pKRJ12 (Fig. 2A). We next focused on modifying the 5′ UTR of the system. To that end, we constructed seven variants with modified PrhaBAD 5′ UTRs shown previously to enhance gene expression (Table 2). One of the constructs (pKRJ23) included a stem-loop from phage T7 gene 10, shown to protect against RNase E degradation, thereby increasing mRNA abundance (33). From this series, pKRJ17, pKRJ18, and pKRJ23 demonstrated higher induced activity relative to parent pKRJ12 (1.3-, 1.4-, and 1.7-fold, respectively), while the others showed no significant change (Fig. 2B). With respect to the uninduced activity, only the AU-rich 5′ UTRs in pKRJ14 and pKRJ16 showed significantly higher activity than pKRJ12, resulting in a substantially lower dynamic range (Fig. 2B). There was no correlation between predicted mRNA folding and resulting LacZ reporter activity (see Fig. S1 in the supplemental material), suggesting that factors other than mRNA structure govern the activity of these 5′ UTRs.
FIG 2.
Characterizing activity of select single promoter system modifications by β-galactosidase assays. K56-2 bearing the parental or modified plasmids was induced for 2 h with 0.01% l-rhamnose (or not), and then lysate was used for a fluorescent β-galactosidase assay. To facilitate comparison, activity is given relative to the parent (pKRJ12) induced with l-rhamnose. The ratio between the induced and uninduced conditions was used to calculate the dynamic range for each variant. Modifications shown are to the RBS (A), 5′UTR (B), rhaI half-sites (C), and rhaSR regulatory genes (D). Insets are representations of the modifications; I1 and I2, rhaI half-sites; AU, AU-rich element. Values shown are means ± standard deviations from at least three biological replicates. Significance was assigned by one-way analysis of variance (ANOVA), followed by Dunnett’s post hoc test for pKRJ12. *, P < 0.05, and **, P < 0.005, inducing conditions; ‡, P < 0.05, and ‡‡, P < 0.005, noninducing conditions.
The rhaI half-sites bound by RhaS play important roles in transcriptional regulation (19, 20). PrhaBAD is preceded by rhaI1 and rhaI2 (Fig. 1), and PrhaT is preceded by rhaI5 and rhaI6 (20). Each half-site is half of a degenerate inverted repeat, that is, they are not the same sequence, allowing for differences in binding. Previous work has shown that rhaI1 is the strongest half-site, followed by rhaI6 (20). Using inverse PCR, we sequentially exchanged rhaI1 and rhaI2 for rhaI1 and rhaI6, resulting in four new promoter variants (Table 2). As shown in Fig. 2C, there was a clear effect of exchanging the half-sites, with variants bearing rhaI6 in the promoter-proximal site having ∼100-fold less induced activity than the parent vector. The double rhaI1 construct was the only one to have significantly higher induced activity (1.4-fold versus the parent). Exchanging the half-sites, however, appears to come at the cost of increased uninduced activity for the variants, with those bearing rhaI1 in the promoter proximal site having greater uninduced activity (1.6- to 2.8-fold versus the parent). Consequently, the dynamic range of the system is reduced compared to the parent vector between approximately 1.75-fold (pKRJ24, the double rhaI1 variant) to 200-fold (pKRJ27, the double rhaI6 variant) (Fig. 2C).
Altering how RhaS interacts with promoter DNA yielded favorable effects on induced activity, so we next modified the expression of RhaS and RhaR themselves. Kelly et al. (34) showed that constitutive expression of RhaS increased induced PrhaBAD activity without substantially increasing uninduced activity in E. coli. Therefore, we replaced the native PrhaSR with the strong constitutive PJ23119 promoter (http://parts.igem.org/Part:BBa_J23119) used previously (29) while maintaining the native RBS. The resultant vector (pKRJ36) was used as a template for near complete deletion of rhaR, which no longer served a regulatory purpose, creating plasmid pKRJ37. Upon induction with l-rhamnose, pKRJ36 and pKRJ37 both displayed significantly increased levels of LacZ activity, 2.2- and 2.6-fold, respectively, compared to the parent vector (Fig. 2D). However, without l-rhamnose, these variants both displayed well over 100-fold increases in uninduced LacZ activity. While the enhanced induced activity was enticing, the lack of regulatory control halted further exploration of these variants.
Combining modifications creates a promoter with favorable ranges of expression.
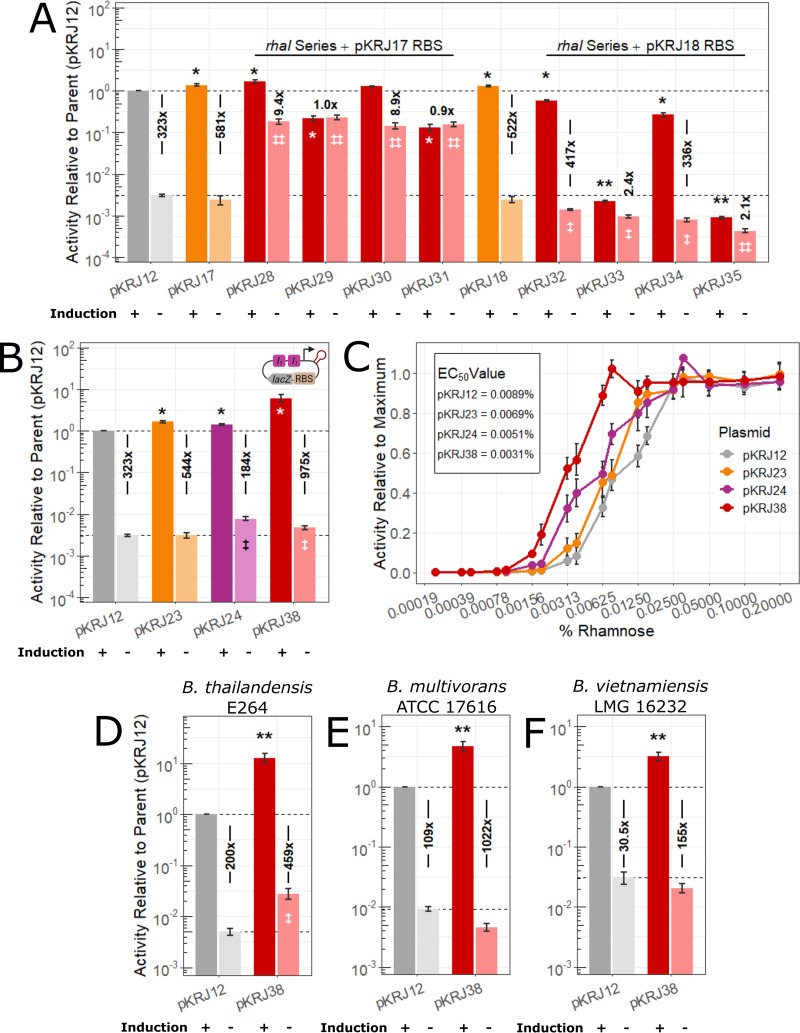
Genetic components often display nonadditive interactions that can be either beneficial or disadvantageous (35). Given this, we decided to combine some of the modifications that displayed promising effects (higher induced activity while mitigating changes to uninduced activity) (Table 2). The rhaI half-site modifications were combined with the 5′ UTRs from pKRJ17 and pKRJ18, resulting in pKRJ28-31 and pKRJ32-35, respectively (Fig. 3A). Upon measuring the l-rhamnose-induced LacZ activity of these new constructs, only pKRJ28 displayed greater activity than the parent vector (1.7-fold). All of the rhaI variants based on the pKRJ18 5′ UTR had lower induced activity than any of the single modifications on their own; however, the pattern in activity between rhaI1 and rhaI6 half-sites was the same as that for the individual half-site variants. Without l-rhamnose, the variants based on the pKRJ18 5′ UTR had up to 7.1-fold lower uninduced LacZ activity than the parent vector, suggesting this combination permits tighter repression, which may be desirable when cloning toxic proteins. On the contrary, variants based on the pKRJ17 5′ UTR showed up to 74.8-fold higher uninduced LacZ activity versus the parent vector, suggesting this combination loosens the repression of the promoter. The differences in reporter activity between the sets of combinations based on pKRJ17 (pKRJ28-31) and pKRJ18 (pKRJ32-35) were unexpected, considering pKRJ17 and pKRJ18 showed similar activity alone (Fig. 2B). While these results were not desirable, complex interactions are known to govern the effect of genetic module combinations, requiring exhaustive permutations to fully dissect (27, 35, 36). In summary, none of these combinations resulted in an expression system with high induced activity and wide dynamic range.
FIG 3.
Characterizing activity of select double promoter system modifications by β-galactosidase assays. Assays were performed and shown as described for Fig. 2. (A) Pairwise combinations of the rhaI half-site series and RBS modifications from pKRJ17 and pKRJ18. (B) Combination of the rhaI1-rhaI1 half-sites and T7 gene 10 stem-loop; the inset is a representation of the new promoter construction. In panels A and B, the ratio between activity under the induced and uninduced condition was used to calculate the dynamic range for each variant. (C) l-Rhamnose dose-response of β-galactosidase activity with the parent (pKRJ12), double modification construct pKRJ38, and constituent single modification constructs (pKRJ23 and pKRJ24). (D to F) β-Galactosidase reporter activity of pKRJ12 and pKRJ38 in B. thailandensis E264 (D), B. multivorans ATCC 17616 (E), and B. vietnamiensis LMG 16232 (F). Values shown are means ± standard deviations from at least three biological replicates. Significance was assigned by one-way ANOVA followed by Dunnett’s post hoc test to pKRJ12. *, P < 0.05, and **, P < 0.005, inducing conditions; ‡, P < 0.05, and ‡‡, P < 0.005, noninducing conditions.
We next combined the T7 gene 10 stem-loop from pKRJ23 with the double rhaI1 half-site modification from pKRJ24, resulting in pKRJ38 (Fig. 3B). The activity driven by this construct displayed a 6.1-fold increase in induced activity compared to the parent promoter, the highest change we observed thus far. Despite a 1.5-fold increase in uninduced activity, pKRJ38 demonstrated the highest dynamic range, at 975-fold, which represents a 3.0-fold increase compared to the parent system. Therefore, pKRJ38 was chosen for follow-up studies. First, to ensure our modifications did not hamper the titratable response of the promoter, we characterized LacZ activity over a wide range of l-rhamnose concentrations. Shown in Fig. 3C, alongside the parental promoter (in pKRJ12) and the constituent single modifications (the T7 gene 10 stem-loop in pKRJ23 and the rhaI1-rhaI1 modification in pKRJ24), the promoter construct in pKRJ38 was more sensitive to induction than the parental promoter, yielding a response at lower concentrations of l-rhamnose (as indicated by a lower 50% effective concentration [EC50] value). Furthermore, the promoter maintained a dynamic range, with intermediate responses observed at intermediate concentrations of l-rhamnose. Given the favorable results with pKRJ38, we added both of the modifications to pSCrhaB2, which we named pSCrhaB2plus.
To extend the utility of the modified promoter, we tested the activity in three other species of Burkholderia: B. thailandensis E264, B. multivorans ATCC 17616, and B. vietnamiensis LMG 16232. B. thailandensis is a commonly used surrogate for pathogenic members of the B. pseudomallei group of bioterror agents. While B. multivorans and B. vietnamiensis are both members of the Bcc, the former is a known cystic fibrosis pathogen, with the latter being investigated for biotechnological applications (1). Compared to the parent, the modified promoter in pKRJ38 yielded 3- to 12-fold higher levels of induced lacZ activity, with only minor changes to uninduced activity (Fig. 3D to F). Notably, the parent l-rhamnose-inducible promoter in B. vietnamiensis LMG 16232 displayed only a 30-fold change in LacZ activity upon induction, suggesting poor usability. Therefore, the modified promoter in pKRJ38 (with a 155-fold change in LacZ activity) represents the only useful form of l-rhamnose-inducible promoter in this strain.
pSC201plus facilitates recovery of mutants in highly expressed essential genes.
We have shown above how promoter system modifications were used to create pSCrhaB2plus, a better expression vector for Burkholderia. To add to the genetic toolbox, we also added the same modifications to a commonly used integrative plasmid, pSC201 (37), which we named pSC201plus. We and others have used the integrative pSC201 for physiological studies of essential genes as the plasmid facilitates the integration of the l-rhamnose-inducible promoter upstream of essential genes and the recovery of rhamnose-dependent, conditional growth (CG) mutants (Fig. S2A) (38–43).
In a previous study, we used a transposon bearing an outward-facing l-rhamnose-inducible promoter to identify the essential genome of B. cenocepacia K56-2 (44). While we identified many essential genes with the l-rhamnose promoter interrupting the native promoter, we could not recover this type of mutant for several genes. Comparison to transcriptome sequencing (RNA-seq) data (45) revealed many of the missing genes are highly expressed (e.g., genes encoding housekeeping or ribosomal proteins). Therefore, we hypothesized that the parental l-rhamnose-inducible promoter is not strong enough to yield viable mutants when placed in front of highly expressed essential genes. To test this hypothesis, we targeted four highly expressed essential genes or operons for promoter replacement (ftsZ, rpoBC, rpsF, and rplN-O) with pSC201 and pSC201plus in parallel (Fig. S2B). For selection, these genes had to pass three criteria: (i) the l-rhamnose-inducible promoter had not been identified upstream in our previous saturating transposon mutagenesis experiment; (ii) a higher-than-median reads per kilobase of transcript per million mapped reads (RPKM) value in RNA-seq data from Sass et al. (45); and (iii) the gene or operon must be essential in K56-2 (44). We should note that the RNA-seq data are from a different, but clonally isolated, strain of B. cenocepacia, J2315, as transcriptomic data for K56-2 were not available to us. Moreover, the selected genes were all related to housekeeping or central functions, reducing the influence of growth condition-specific effects. The mutants were named with the prefix “CG” followed by the name of the downstream gene(s). When we tested the conjugation efficiency of the two plasmids (Fig. S3), we observed a significant difference between the number of CG mutants recovered for the rpoBC operon with the two systems; pSC201plus yielded approximately 6-fold more exconjugants than pSC201. However, this difference was not seen for the other genes (Fig. S3).
Next, we screened the CG mutants for the presence and stability of the rhamnose-dependent growth phenotype (Table 3). While all colonies of CGftsZ, CGftsZplus, CGrpoBC, and CGrpoBCplus had l-rhamnose-dependent growth, no recovered colonies of CGrplN-O and CGrplN-Oplus had l-rhamnose-dependent growth. These colonies were grown on antibiotic selective medium at all times and passed PCR screening for the correct location of the insertion, suggesting that compensatory mutations arose either in the promoter or elsewhere in the genome. In the case of rpsF, we observed the l-rhamnose-dependent CG phenotype in 26.1% of CGrpsFplus colonies but not for any colonies of CGrpsF. This suggests that expression levels of RpsF required for viability were attained only with the modified promoter system. The fact that CG mutants were recovered in rpsF, but only using pSC201plus, suggests the higher dynamic range of this promoter system improves success rates in creating mutants in highly expressed essential genes.
TABLE 3.
Counts of insertional mutants with the CG phenotype (l-rhamnose-dependent growth)
| Interrupted gene/operon | Mutant name | Integrative plasmid | No. of colonies with CG phenotype/total no. of colonies (%CG) |
|---|---|---|---|
| ftsZ | CGftsZ | pSC201 | 22/22 (100) |
| CGftsZplus | pSC201plus | 19/19 (100) | |
| rplN-O | CGrplN-O | pSC201 | 0/30 (0) |
| CGrplN-Oplus | pSC201plus | 0/28 (0) | |
| rpoBC | CGrpoBC | pSC201 | 17/17 (100) |
| CGrpoBCplus | pSC201plus | 17/17 (100) | |
| rpsF | CGrpsF | pSC201 | 0/25 (0) |
| CGrpsFplus | pSC201plus | 6/23 (26.1) |
pSC201plus permits more sensitive control of essential gene mutant physiology.
As we determined before, the modified l-rhamnose-inducible promoter in pSC201plus is more sensitive to lower concentrations of l-rhamnose and has a higher level of maximum activity. Our next goal was to assess and compare its effects on CG mutant physiology. As the mutants have l-rhamnose-dependent growth, we determined the growth dose-response in an l-rhamnose gradient using the mutants in ftsZ and rpoBC (Fig. 4A). We observed two important findings supporting the use of pSC201plus to control essential genes. First, the modified promoter in pSC201plus furnished high levels of growth at much lower concentrations of l-rhamnose, with EC50 values 3- to 7-fold lower than those for the parental promoter. Second, wild-type levels of growth were achieved. CGftsZ requires high levels of l-rhamnose (at least 0.2%) to reach a maximum OD600, yet it does not display full wild-type growth, while CGftsZplus displays wild-type levels of growth at 0.02% l-rhamnose.
FIG 4.
Comparing CG mutants created by insertional mutagenesis with pSC201 versus pSC201plus. (A) Rhamnose dose-response curves of the CG mutants and WT K56-2. Cells were grown for 18 h, after which OD600 values were measured. (B) Micrographs of the CG mutants in ftsZ subcultured for 2 h with the l-rhamnose EC50 of CGftsZ (0.0443%) or CGftsZplus (0.006%). Scale bar is 5 μm. (C) Length distributions of 200 cells from panel B. The notches in the box around the median line indicate the 95% confidence interval. (D) Rifampin susceptibility test of the CG mutants in rpoBC. The mutants were grown in a rifampin gradient for 18 h with the l-rhamnose EC50 of CGrpoBC (0.0262%) or CGrpoBCplus (0.0088%). Values shown are means ± standard deviations from three biological replicates.
An important feature of CG mutants is the ability to tune essential gene expression and study cell physiology when depleted of an essential function. As the mutants made with pSC201plus are more responsive to l-rhamnose with respect to growth, we next tested if they also displayed depleted phenotypes at lower concentrations of l-rhamnose. For the mutants in ftsZ, we took advantage of the rapid halt in cell division that accompanies depletion of FtsZ. Multimeric FtsZ filaments form at the midcell of growing cells, guiding assembly of the divisome (46); FtsZ depletion inhibits cell division but not metabolism and cell growth, resulting in a filamentous cell morphology (47). To assess changes in morphology, we subcultured CGftsZ and CGftsZplus for 2 h with the l-rhamnose EC50 of both the mutants and then measured cell length (Fig. 4B and C). As controls, we included 0% and 0.2% l-rhamnose. We reasoned that CGftsZplus would require less l-rhamnose than CGftsZ to return to wild-type morphology. At the EC50 of CGftsZplus (0.006%), cells of the parental mutant CGftsZ showed no change in length compared to 0% l-rhamnose but rather only responded to its own EC50 (0.0443%) of l-rhamnose and above. On the contrary, cells of CGftsZplus were shorter at 0.006% l-rhamnose and had wild-type morphology at 0.0443% and above. At 0% l-rhamnose, cells of CGftsZplus were significantly shorter than those of CGftsZ, likely reflecting a higher initial abundance of FtsZ.
To validate the lower requirement of l-rhamnose to achieve a wild type-like phenotype with an unrelated gene, we used rpoBC, encoding the RNA polymerase β subunit target of rifampin (48), which allowed us to perform a hypersusceptibility assay. For many antimicrobials, depleting the target increases the susceptibility, as less inhibition is needed to cross the critical functional threshold (38, 49, 50). We reasoned that CGrpoBCplus would be sensitized to rifampin at lower l-rhamnose concentrations than CGrpoBC. At the EC50 of CGrpoBCplus (0.0088%), the parental mutant CGrpoBC displayed virtually no growth, whereas CGrpoBCplus was hypersusceptible to subinhibitory concentrations of rifampin (Fig. 4D). At the EC50 of CGrpoBC (0.0262%), CGrpoBC was made hypersusceptible to rifampin, while CGrpoBCplus displayed wild-type susceptibility. Together, these results demonstrate that the modified promoter in pSC201plus enables finer control over mutants in a broader range of essential genes with less rhamnose.
DISCUSSION
Deciphering how genetics governs physiology relies heavily upon a capable set of genetic tools (51, 52). Modern genetic engineering methods are needed especially for species of Burkholderia, which display an intriguing but also troubling duality of beneficial and pathogenic features (1). Here, we report a step forward in the optimization of the l-rhamnose-inducible promoter. We combined two modifications shown individually to enhance LacZ reporter activity, the phage T7 gene 10 mRNA stem-loop and a double rhaI1 half-site. Together, these combinations permitted greater levels of expression and control of essential genes in B. cenocepacia without substantially compromising the promoter’s hallmark tight repression. We have introduced this novel combination into two common plasmids, pSCrhaB2 and pSC201, yielding pSCrhaB2plus and pSC201plus, for plasmid-based protein expression and control of chromosomal genes, respectively. Furthermore, we also identified two combinations of a 5′ UTR and rhaI half-site modification with both tighter repression than the parental promoter and also at least moderately induced activity, which may prove useful when expressing toxic proteins.
The first of the changes we introduced were RBS variants to modify the extent of complementarity between the anti-SD site in the 16S rRNA and the mRNA transcript. While recent work has demonstrated that the RBS is not necessary for defining translation start sites, the RBS is important for efficient translation initiation (53). It is important to note that while the native RBS downstream of PrhaBAD (5′-AGGA-3′) is unmodified from E. coli, it is also recognized by the B. cenocepacia ribosome, owing to the high degree of conservation in the 3′ end of the 16S rRNA. Furthermore, although known preferences are lacking for Burkholderia, we did not alter the native 6-bp spacing between the RBS and start codon, which was shown to be optimal for translation efficiency (32, 54). Curiously, attempts to improve on the RBS resulted in no significant changes in induced reporter activity but, instead, substantial changes in uninduced activity. However, the pattern of effects suggests that at high levels of promoter induction, the steps of ribosome scanning and assembly of the translation initiation complex are at a maximum for these synthetic transcripts. When uninduced, there are many fewer synthetic transcripts, perhaps allowing the effect of increased translation initiation by a strengthened RBS to become more apparent. Alternatively, effects of a stronger RBS in the induced state may be masked by mRNA interactions of the RBS and coding region (55). However, with the variety of RBS sequences we tested, the same inhibitory RBS-coding region interactions would be unlikely.
Other groups have also reported modifications to the l-rhamnose-inducible promoter, with activity tested in E. coli and Cupriavidus necator. Wegerer et al. (35) and Alagesan et al. (14) implemented a stem-loop from phage T7 gene 10 into the 5′ UTR and found this modification greatly increases signal in their respective reporter assays. In B. cenocepacia, we found this stem-loop increased reporter activity by 1.7-fold, as it likely protects transcripts from the putative RNase E, K562_RS13765. Degradation by RNase E is hindered by structured RNA, thereby increasing mRNA abundance (33). In addition, Wegerer et al. (35) found that manipulating a variety of plasmid-based features, including replication and dimer-resolving proteins, increased reporter assay signal; however, these changes are vector specific and would not carry over when manipulating chromosomal genes. In our work, the broad-host-range pBBR1 origin of replication (13) of pSCrhaB2 was not modified in pSCrhaB2plus. Therefore, we expect both vectors to have the same plasmid copy number, estimated at 5 copies/cell (as in E. coli [56]) to 35 copies/cell (as in Cupriavidus metallidurans [57]). The l-rhamnose promoter system has also been shown to function well in Pseudomonas aeruginosa (58). However, in both PAO1 and PA14, even for the parental promoter we observed high levels of uninduced LacZ activity (data not shown), and the reasons for the discrepancy remain unknown to us.
Recently, it was shown that the l-rhamnose-inducible promoter can be synthetically reduced by constitutive expression of RhaS, as shown in E. coli (34) and Synechocystis sp. strain PCC 6803 (59). When we attempted to carry these findings over to B. cenocepacia, we observed up to 2.6-fold increased induced LacZ activity but at the cost of a well over 100-fold increase in activity in the absence of l-rhamnose, which was not previously observed (34, 59). The difference could be due to the choice of constitutive promoter for rhaS: we used the strong J23119 promoter due to a lack of characterized alternatives, while Kelly et al. (34) used the ampR promoter. The strong promoter in our construct likely drives high levels of rhaS transcription. Previously, it was shown that the DNA-binding domains of AraC, XylS, and RhaS (all members of the AraC/XylS family) are all capable of activating transcription independently of their effector molecules when overexpressed (20, 60, 61). Thus, it remains possible that weaker constitutive expression of rhaS in Burkholderia may result in a system with tighter regulation. Additionally, although our construct with the double rhaI1 half-sites had increased induced promoter activity, it also displayed increased uninduced activity. Together, these results highlight the challenges of obtaining high dynamic ranges of expression, as enhancing affinity of transcription factor binding may also affect the uninduced state.
Due to their central roles, essential genes have attracted attention from researchers in diverse topics, from cell physiology to antibiotic discovery. In studying a selection of essential genes, viable CG mutants with both the parental and modified promoters were recovered in ftsZ and rpoBC, while CG mutants were only recovered in rpsF with the modified promoter. CG mutants in rplN-O were not recovered with either promoter. Indeed, while colonies were obtained for mutants in rplN-O and rpsF (using pSC201), the mutants did not display l-rhamnose-dependent growth, suggesting a compensatory mutation arose, perhaps by removing the inducible control from the l-rhamnose promoter. Interestingly, the ability to recover CG mutants inversely correlates with the RPKM values of the genes from Sass et al. (45); given in descending order, they are rplN-O, rpsF, rpoBC, and ftsZ. The rplN-O operon is among the most highly expressed essential operons in B. cenocepacia; thus, it is not entirely surprising that even our modified l-rhamnose-inducible promoter was unable to sustain the required levels of promoter activity. Additionally, the failure to recover mutants in rplN-O highlights some of the challenges in working with essential genes. For insertional schemes such as this, the genetic locus under study is altered from the wild type. Alternatively, the targeted repression model of CRISPR interference maintains a wild-type locus when uninduced (30), with benefits evidenced by the creation of several near (essential) genome-wide libraries (50, 62). However, CRISPR/Cas tools may require organism-specific optimization (29, 63) and cannot target every gene (64) due to requirements of the PAM and other host factors. Together, the LacZ reporter assay and phenotypic data demonstrate that our enhanced l-rhamnose-inducible promoter can be used for higher levels of exogenous protein expression and more sensitive manipulation of a broader range of essential genes. We expect this promoter construct to be a valuable part of the genetic toolkit for synthetic biology approaches in B. cenocepacia and related species.
MATERIALS AND METHODS
Strains and growth conditions.
All strains and plasmids from previous studies used here can be found in Table 1. All strains were grown in LB-Lennox medium (Difco) with shaking at 230 rpm. Cultures were made to 5 ml in 5-inch glass tubes, unless otherwise stated. B. cenocepacia K56-2 was grown at 37°C, while B. thailandensis E264, B. multivorans ATCC 17616, and B. vietnamiensis LMG 16232 were grown at 30°C. The following selective antibiotics and concentrations were used: trimethoprim (100 μg/ml for Burkholderia, 300 μg/ml for P. aeruginosa PAO1, 600 μg/ml for P. aeruginosa PA14, 50 μg/ml for E. coli; Sigma), tetracycline (10 μg/ml for E. coli; Sigma), and gentamicin (50 μg/ml for Burkholderia).
Molecular biology and cloning.
The lacZ gene and transcriptional terminators were amplified from pJPD01 (65) using primers 1006 and 1007 (see Table S1 in the supplemental material) with Q5 high-fidelity polymerase (NEB). The fragment was introduced into pSCrhaB2 by restriction cloning using KpnI and XbaI to create pAPC03. Trimethoprim-resistant colonies were screened for the lacZ gene using primers 954 and 1010 with OneTaq polymerase (NEB). The ribosome-binding site placement in pAPC03 relative to the start of lacZ was refined using inverse PCR with primers 1110 and 1134. The resulting PCR product was used for blunt-end ligation as previously described (29) and transformed into E. coli DH5α, creating pKRJ12. All further modifications were constructed using inverse PCR and blunt-end ligation. Concerning the rhaI half-site modifications, the last two bases of the promoter-proximal half-site overlap the −35 hexamer and, therefore, were not changed to preserve this critical sigma factor binding site. The l-rhamnose promoter region of all plasmids was sent for Sanger sequencing to ensure the correct modification was made (Eurofins and Genome Quebec). The resulting plasmids were introduced by triparental mating into Burkholderia species as previously described (29) or were extracted by miniprep (Omega BioTek) and electroporated into P. aeruginosa per Choi et al. (66). Trimethoprim-resistant colonies were also screened by colony PCR for each modification.
Inverse PCR was also used to introduce the modifications into pSC201 and pSCrhaB2 (rhaI1-rhaI1 permutation of rhaS binding sites upstream of PrhaBAD and bacteriophage T7 gene 10 stem-loop inserted upstream of the native rhaBAD 5′ UTR). The singly modified plasmids were used as the template for a second round of inverse PCR, resulting in the doubly modified pSC201plus and pSCrhaB2plus. The l-rhamnose promoter regions of the singly and doubly modified plasmids were sent for Sanger sequencing (Eurofins and Genome Quebec).
Conditional growth mutants were constructed as previously described (38, 67). Briefly, approximately 300 bp of the 5′ region of the genes of interest was amplified from the K56-2 genome by PCR with Q5 high-fidelity polymerase (NEB) (primers are listed in Table S1). The fragments were digested with NdeI and XbaI and ligated into both pSC201 and pSC201plus. The constructs were chemically transformed into E. coli SY327. Transformants were selected with trimethoprim and screened by colony PCR. The constructs were introduced into K56-2 by triparental mating as before (integration diagram is in Fig. S2), and exconjugants were selected on medium with trimethoprim, gentamicin, and 0.2% l-rhamnose and screened by colony PCR. Additionally, the exconjugants were screened for a growth defect on medium without l-rhamnose.
β-Galactosidase assay. (i) Experimental.
The β-galactosidase assay method is based on those by Meisner and Goldberg (58) and Zhang and Bremer (68), with some modifications. The desired strains were grown overnight with antibiotic selection as described above. After 16 h, the cultures were diluted at 1:50 in 5 ml fresh medium (with antibiotics) and grown at 37°C with 230 rpm shaking until mid-log phase (optical density at 600 nm [OD600] of ∼0.6; approximately 3 h). In 96-well format, the cultures were diluted to an OD600 of 0.2 in 200 μl in the presence or absence of l-rhamnose (with antibiotics), in triplicate. The lid of the plate was coated with a Triton X-100–ethanol solution to prevent condensation. The cells were induced for 2 h at 37°C with 230-rpm shaking.
During the induction, the buffer and lysis solution were prepared. The buffer solution consisted of the following: 60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4. The buffer solution was then split into two aliquots, one for culture dilution prior to measuring β-galactosidase activity and another for preparation of the lysis solution. Near the end of the induction period, the buffer solution was modified by adding (to a final concentration of) 0.8 mg/ml cetrimonium bromide, 0.4 mg/ml sodium deoxycholate, 36 mM β-mercaptoethanol, 0.46 mg/ml egg-white lysozyme, and 300 μM methyl-umbelliferyl galactopyranoside (MUG) to make the lysis solution.
After the induction period, the OD600 of the induced cultures was measured and then diluted 1:100 in the buffer solution in a 96-well plate. In another 96-well plate, 80 μl of lysis solution was added to 120 μl of diluted induced culture. The plate was immediately put into a plate reader (BioTek) set to read fluorescence every 2 min (360/40 nm excitation, 460/40 nm emission) at 30°C.
(ii) Analysis.
Within each biological replicate, the blank-corrected fluorescence values at each time were first made relative to the OD600 of each well and then averaged across technical triplicates. Inspection of the plots of fluorescence/OD600 versus time revealed the expected curve where the change in fluorescence is initially linear, as the substrate is in excess, followed by a decrease in the rate of fluorescence change when the substrate becomes limiting. The initial period (usually between 10 and 20 min) where fluorescence linearly increases was taken for analysis. Linear regression was performed on these data for each condition and strain, and the slope was taken as a measure of β-galactosidase abundance. These values were compared to parental vectors for relative changes in induced or uninduced β-galactosidase expression or within strains to calculate fold change in expression states.
Microscopic analysis.
Cells from overnight cultures were subcultured at an OD of 0.1 in 5 ml LB medium, with 0.2% l-rhamnose and 100 μg/ml trimethoprim as appropriate. The subcultures were grown for 3 h at 37°C until early exponential phase. Cells were harvested, washed twice by centrifugation to remove excess l-rhamnose, and inoculated in fresh medium with various concentrations of l-rhamnose at an OD of 0.05. The cells were grown for 2 h at 37°C (approximately 3 generations). Without further processing, cells were harvested and immobilized on 1.5% agarose pads for imaging as previously described (38).
Rifampin hypersusceptibility testing.
Overnight cultures of the appropriate strains were washed twice by centrifugation to remove excess l-rhamnose. The strains were inoculated in 96-well format into a 2-fold rifampin (Sigma) gradient at a final OD600 of 0.01 in 200 μl. Also in the medium was a concentration of l-rhamnose to achieve approximately 50% of maximum growth (EC50), as determined by logistic regression of an l-rhamnose dose-response curve performed in the same format as the rifampin hypersensitivity tests. The 96-well plates were grown shaking at 230 rpm for 18 h at 37°C, after which the OD600 values were recorded.
Conjugation efficiency.
This assay was performed as previously reported (29), with some modifications, to permit growth of CG mutants. Briefly, after 18 h of conjugation on LB agar with 0.2% l-rhamnose, the mating spot was resuspended in LB, serially diluted, and plated on LB agar with 50 μg/ml gentamicin only (to select for total K56-2 recipients) and on LB agar with 50 μg/ml gentamicin, 100 μg/ml trimethoprim, and 0.2% l-rhamnose (to select for successful exconjugants). The conjugation efficiency is the ratio of successful exconjugants (Tpr and Gmr) to total recipients (Gmr).
Statistical testing.
All statistical tests were performed in base R version 4.0.1 with the appropriate assumptions. The Dunnett’s multiple comparison post hoc test was used from the DescTools package version 0.99.39 (https://CRAN.R-project.org/package=DescTools).
Plasmid availability.
For ease of distribution, the following plasmids are available from AddGene: pSCrhaB2plus (number 164226) and pSC201plus (number 164227).
ACKNOWLEDGMENTS
We are grateful to Eric Déziel (Institut National de la Recherche Scientifique–Institut Armand Frappier) for the gift of pJPD01 and to Mazdak Khajehpour (University of Manitoba, Canada) for helpful discussion on enzyme kinetic analysis.
We are also thankful for the financial support of this work: a Cystic Fibrosis Canada grant and an NSERC discovery grant to S.T.C.; a Cystic Fibrosis Canada studentship and Canadian Institutes of Health Research (CIHR) Vanier award to A.M.H.; a Natural Sciences and Engineering Council (NSERC) studentship to K.R.J.; and a Mitacs Globalink Research Internship to A.P.C.
We declare we have no competing financial interests.
S.T.C. and A.M.H. formulated the ideas and design of the project; K.R.J., A.M.H., and A.P.C. designed and cloned the modified promoter constructs; K.R.J. and A.M.H. performed assays of promoter function and phenotypic assays; K.R.J. and A.M.H. processed and analyzed the data; A.M.H. wrote the manuscript and edited it, together with S.T.C., K.R.J., and A.P.C.; S.T.C. provided financial support and supervised the work.
Footnotes
Supplemental material is available online only.
Contributor Information
Silvia T. Cardona, Email: silvia.cardona@umanitoba.ca.
M. Julia Pettinari, University of Buenos Aires
REFERENCES
- 1.Eberl L, Vandamme P. 2016. Members of the genus Burkholderia: good and bad guys. F1000Res 5:1007. 10.12688/f1000research.8221.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez S, Humery A, Groleau M-C, Déziel E. 2020. Quorum sensing controls both rhamnolipid and polyhydroxyalkanoate production in Burkholderia thailandensis through ScmR regulation. Front Bioeng Biotechnol 8:1033. 10.3389/fbioe.2020.01033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bournaud C, de Faria SM, dos Santos JMF, Tisseyre P, Silva M, Chaintreuil C, Gross E, James EK, Prin Y, Moulin L. 2013. Burkholderia species are the most common and preferred nodulating symbionts of the Piptadenia group (tribe mimoseae). PLoS One 8:e63478. 10.1371/journal.pone.0063478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho Y-N, Chiang H-M, Chao C-P, Su C-C, Hsu H-F, Guo C, Hsieh J-L, Huang C-C. 2015. In planta biocontrol of soilborne Fusarium wilt of banana through a plant endophytic bacterium, Burkholderia cenocepacia 869T2. Plant Soil 387:295–306. 10.1007/s11104-014-2297-0. [DOI] [Google Scholar]
- 5.Shehata HR, Lyons EM, Jordan KS, Raizada MN. 2016. Bacterial endophytes from wild and ancient maize are able to suppress the fungal pathogen Sclerotinia homoeocarpa. J Appl Microbiol 120:756–769. 10.1111/jam.13050. [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Hu X, Cao Y, Pang W, Huang J, Guo P, Huang L. 2019. Biodegradation of phenanthrene and heavy metal removal by acid-tolerant Burkholderia fungorum FM-2. Front Microbiol 10:408. 10.3389/fmicb.2019.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zlosnik JE, Zhou G, Brant R, Henry DA, Hird TJ, Mahenthiralingam E, Chilvers MA, Wilcox P, Speert DP. 2015. Burkholderia species infections in patients with cystic fibrosis in British Columbia, Canada. 30 years’ experience. Ann Am Thorac Soc 12:70–78. 10.1513/AnnalsATS.201408-395OC. [DOI] [PubMed] [Google Scholar]
- 8.Scoffone VC, Chiarelli LR, Trespidi G, Mentasti M, Riccardi G, Buroni S. 2017. Burkholderia cenocepacia infections in cystic fibrosis patients: drug resistance and therapeutic approaches. Front Microbiol 8:1592. 10.3389/fmicb.2017.01592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regan KH, Bhatt J. 2019. Eradication therapy for Burkholderia cepacia complex in people with cystic fibrosis. Cochrane Database Syst Rev 4:CD009876. 10.1002/14651858.CD009876.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mullins AJ, Murray JAH, Bull MJ, Jenner M, Jones C, Webster G, Green AE, Neill DR, Connor TR, Parkhill J, Challis GL, Mahenthiralingam E. 2019. Genome mining identifies cepacin as a plant-protective metabolite of the biopesticidal bacterium Burkholderia ambifaria. Nat Microbiol 4:996–1005. 10.1038/s41564-019-0383-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lefebre MD, Valvano MA. 2002. Construction and evaluation of plasmid vectors optimized for constitutive and regulated gene expression in Burkholderia cepacia complex isolates. Appl Environ Microbiol 68:5956–5964. 10.1128/AEM.68.12.5956-5964.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu D, Damron FH, Mima T, Schweizer HP, Yu HD. 2008. PBAD-based shuttle vectors for functional analysis of toxic and highly regulated genes in Pseudomonas and Burkholderia spp. and other bacteria. Appl Environ Microbiol 74:7422–7426. 10.1128/AEM.01369-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardona ST, Valvano MA. 2005. An expression vector containing a rhamnose-inducible promoter provides tightly regulated gene expression in Burkholderia cenocepacia. Plasmid 54:219–228. 10.1016/j.plasmid.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Alagesan S, Hanko EKR, Malys N, Ehsaan M, Winzer K, Minton NP. 2018. Functional genetic elements for controlling gene expression in Cupriavidus necator H16. Appl Environ Microbiol 84:e00878-18. 10.1128/AEM.00878-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodionova IA, Li X, Thiel V, Stolyar S, Stanton K, Fredrickson JK, Bryant DA, Osterman AL, Best AA, Rodionov DA. 2013. Comparative genomics and functional analysis of rhamnose catabolic pathways and regulons in bacteria. Front Microbiol 4:407. 10.3389/fmicb.2013.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tobin JF, Schleif RF. 1990. Transcription from the rha operon psr promoter. J Mol Biol 211:1–4. 10.1016/0022-2836(90)90003-5. [DOI] [PubMed] [Google Scholar]
- 17.Egan SM, Schleif RF. 1993. A regulatory cascade in the induction of rhaBAD. J Mol Biol 234:87–98. 10.1006/jmbi.1993.1565. [DOI] [PubMed] [Google Scholar]
- 18.Vía P, Badía J, Baldomà L, Obradors N, Aguilar J. 1996. Transcriptional regulation of the Escherichia coli rhaT gene. Microbiology 142:1833–1840. 10.1099/13500872-142-7-1833. [DOI] [PubMed] [Google Scholar]
- 19.Egan SM, Schleif RF. 1994. DNA-dependent renaturation of an insoluble DNA binding protein: identification of the RhaS binding site at rhaBAD. J Mol Biol 243:821–829. 10.1006/jmbi.1994.1684. [DOI] [PubMed] [Google Scholar]
- 20.Wickstrum JR, Skredenske JM, Kolin A, Jin DJ, Fang J, Egan SM. 2007. Transcription activation by the DNA-binding domain of the AraC family protein RhaS in the absence of its effector-binding domain. J Bacteriol 189:4984–4993. 10.1128/JB.00530-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloodworth RA, Gislason AS, Cardona ST. 2013. Burkholderia cenocepacia conditional growth mutant library created by random promoter replacement of essential genes. MicrobiologyOpen 2:243–258. 10.1002/mbo3.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Judson N, Mekalanos JJ. 2000. Transposon-based approaches to identify essential bacterial genes. Trends Microbiol 8:521–526. 10.1016/s0966-842x(00)01865-5. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Claveau D, Vaillancourt JP, Roemer T, Meredith TC. 2011. High-frequency transposition for determining antibacterial mode of action. Nat Chem Biol 7:720–729. 10.1038/nchembio.643. [DOI] [PubMed] [Google Scholar]
- 24.Asghar AH, Shastri S, Dave E, Wowk I, Agnoli K, Cook AM, Thomas MS. 2011. The pobA gene of Burkholderia cenocepacia encodes a group I Sfp-type phosphopantetheinyltransferase required for biosynthesis of the siderophores ornibactin and pyochelin. Microbiology 157:349–361. 10.1099/mic.0.045559-0. [DOI] [PubMed] [Google Scholar]
- 25.Sicard C, Shek N, White D, Bowers RJ, Brown RS, Brennan JD. 2014. A rapid and sensitive fluorimetric β-galactosidase assay for coliform detection using chlorophenol red-β-d-galactopyranoside. Anal Bioanal Chem 406:5395–5403. 10.1007/s00216-014-7935-0. [DOI] [PubMed] [Google Scholar]
- 26.Salis HM, Mirsky EA, Voigt CA. 2009. Automated design of synthetic ribosome binding sites to control protein expression. Nat Biotechnol 27:946–950. 10.1038/nbt.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mutalik VK, Guimaraes JC, Cambray G, Mai Q-A, Christoffersen MJ, Martin L, Yu A, Lam C, Rodriguez C, Bennett G, Keasling JD, Endy D, Arkin AP. 2013. Quantitative estimation of activity and quality for collections of functional genetic elements. Nat Methods 10:347–353. 10.1038/nmeth.2403. [DOI] [PubMed] [Google Scholar]
- 28.Seo SW, Yang J-S, Kim I, Yang J, Min BE, Kim S, Jung GY. 2013. Predictive design of mRNA translation initiation region to control prokaryotic translation efficiency. Metab Eng 15:67–74. 10.1016/j.ymben.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Hogan AM, Rahman ASMZ, Lightly TJ, Cardona ST. 2019. A broad-host-range CRISPRi toolkit for silencing gene expression in Burkholderia. ACS Synth Biol 8:2372–2384. 10.1021/acssynbio.9b00232. [DOI] [PubMed] [Google Scholar]
- 30.Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA. 2013. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152:1173–1183. 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Komarova AV, Tchufistova LS, Dreyfus M, Boni IV. 2005. AU-rich sequences within 5’ untranslated leaders enhance translation and stabilize mRNA in Escherichia coli. J Bacteriol 187:1344–1349. 10.1128/JB.187.4.1344-1349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ringquist S, Shinedling S, Barrick D, Green L, Binkley J, Stormo GD, Gold L. 1992. Translation initiation in Escherichia coli: sequences within the ribosome-binding site. Mol Microbiol 6:1219–1229. 10.1111/j.1365-2958.1992.tb01561.x. [DOI] [PubMed] [Google Scholar]
- 33.Lopez PJ, Dreyfus M. 1996. The lacZ mRNA can be stabilised by the T7 late mRNA leader in E. coli. Biochimie 78:408–415. 10.1016/0300-9084(96)84747-X. [DOI] [PubMed] [Google Scholar]
- 34.Kelly CL, Liu Z, Yoshihara A, Jenkinson SF, Wormald MR, Otero J, Estévez A, Kato A, Marqvorsen MHS, Fleet GWJ, Estévez RJ, Izumori K, Heap JT. 2016. Synthetic chemical inducers and genetic decoupling enable orthogonal control of the rhaBAD Promoter. ACS Synth Biol 5:1136–1145. 10.1021/acssynbio.6b00030. [DOI] [PubMed] [Google Scholar]
- 35.Wegerer A, Sun T, Altenbuchner J. 2008. Optimization of an E. coli L-rhamnose-inducible expression vector: test of various genetic module combinations. BMC Biotechnol 8:2. 10.1186/1472-6750-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urtecho G, Tripp AD, Insigne KD, Kim H, Kosuri S. 2019. Systematic dissection of sequence elements controlling σ70 promoters using a genomically-encoded multiplexed reporter assay in E. coli. Biochemistry 58:1539–1551. 10.1021/acs.biochem.7b01069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ortega XP, Cardona ST, Brown AR, Loutet SA, Flannagan RS, Campopiano DJ, Govan JR, Valvano MA. 2007. A putative gene cluster for aminoarabinose biosynthesis is essential for Burkholderia cenocepacia viability. J Bacteriol 189:3639–3644. 10.1128/JB.00153-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hogan AM, Scoffone VC, Makarov V, Gislason AS, Tesfu H, Stietz MS, Brassinga AKC, Domaratzki M, Li X, Azzalin A, Biggiogera M, Riabova O, Monakhova N, Chiarelli LR, Riccardi G, Buroni S, Cardona ST. 2018. Competitive fitness of essential gene knockdowns reveals a broad-spectrum antibacterial inhibitor of the cell division protein FtsZ. Antimicrob Agents Chemother 62:e01231-18. 10.1128/AAC.01231-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milani A, Vecchietti D, Rusmini R, Bertoni G. 2012. TgpA, a protein with a eukaryotic-like transglutaminase domain, plays a critical role in the viability of Pseudomonas aeruginosa. PLoS One 7:e50323. 10.1371/journal.pone.0050323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohamed YF, Valvano MA. 2014. A Burkholderia cenocepacia MurJ (MviN) homolog is essential for cell wall peptidoglycan synthesis and bacterial viability. Glycobiology 24:564–576. 10.1093/glycob/cwu025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moule MG, Hemsley CM, Seet Q, Guerra-Assunção JA, Lim J, Sarkar-Tyson M, Clark TG, Tan PBO, Titball RW, Cuccui J, Wren BW. 2014. Genome-wide saturation mutagenesis of Burkholderia pseudomallei K96243 predicts essential genes and novel targets for antimicrobial development. mBio 5:e00926-13. 10.1128/mBio.00926-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sass A, Everaert A, Acker HV, den Driessche FV, Coenye T. 2018. Targeting the nonmevalonate pathway in Burkholderia cenocepacia increases susceptibility to certain β-lactam antibiotics. Antimicrob Agents Chemother 62:e02607-17. 10.1128/AAC.02607-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagley S, Hemsley C, Thomas R, Moule MG, Vanaporn M, Andreae C, Robinson M, Goldman S, Wren BW, Butler CS, Titball RW. 2014. The twin arginine translocation system is essential for aerobic growth and full virulence of Burkholderia thailandensis. J Bacteriol 196:407–416. 10.1128/JB.01046-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gislason AS, Turner K, Domaratzki M, Cardona ST. 2017. Comparative analysis of the Burkholderia cenocepacia K56-2 essential genome reveals cell envelope functions that are uniquely required for survival in species of the genus Burkholderia. Microb Genom 3:e000140. 10.1099/mgen.0.000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sass AM, Van Acker H, Förstner KU, Van Nieuwerburgh F, Deforce D, Vogel J, Coenye T. 2015. Genome-wide transcription start site profiling in biofilm-grown Burkholderia cenocepacia J2315. BMC Genomics 16:775. 10.1186/s12864-015-1993-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Du S, Lutkenhaus J. 2019. At the heart of bacterial cytokinesis: the Z ring. Trends Microbiol 27:781–791. 10.1016/j.tim.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sánchez-Gorostiaga A, Palacios P, Martínez-Arteaga R, Sánchez M, Casanova M, Vicente M. 2016. Life without division: physiology of Escherichia coli FtsZ-deprived filaments. mBio 7:e01620-16. 10.1128/mBio.01620-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Campbell EA, Korzheva N, Mustaev A, Murakami K, Nair S, Goldfarb A, Darst SA. 2001. Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell 104:901–912. 10.1016/s0092-8674(01)00286-0. [DOI] [PubMed] [Google Scholar]
- 49.Johnson EO, LaVerriere E, Office E, Stanley M, Meyer E, Kawate T, Gomez JE, Audette RE, Bandyopadhyay N, Betancourt N, Delano K, Silva ID, Davis J, Gallo C, Gardner M, Golas AJ, Guinn KM, Kennedy S, Korn R, McConnell JA, Moss CE, Murphy KC, Nietupski RM, Papavinasasundaram KG, Pinkham JT, Pino PA, Proulx MK, Ruecker N, Song N, Thompson M, Trujillo C, Wakabayashi S, Wallach JB, Watson C, Ioerger TR, Lander ES, Hubbard BK, Serrano-Wu MH, Ehrt S, Fitzgerald M, Rubin EJ, Sassetti CM, Schnappinger D, Hung DT. 2019. Large-scale chemical–genetics yields new M. tuberculosis inhibitor classes. Nature 571:72–78. 10.1038/s41586-019-1315-z. [DOI] [PubMed] [Google Scholar]
- 50.de Wet TJ, Winkler KR, Mhlanga M, Mizrahi V, Warner DF. 2020. Arrayed CRISPRi and quantitative imaging describe the morphotypic landscape of essential mycobacterial genes. Elife 9:e60083. 10.7554/eLife.60083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sykes EME, Deo S, Kumar A. 2020. Recent advances in genetic tools for Acinetobacter baumannii. Front Genet 11:601380. 10.3389/fgene.2020.601380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Todor H, Silvis MR, Osadnik H, Gross CA. 2021. Bacterial CRISPR screens for gene function. Curr Opin Microbiol 59:102–109. 10.1016/j.mib.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saito K, Green R, Buskirk AR. 2020. Translational initiation in E. coli occurs at the correct sites genome-wide in the absence of mRNA-rRNA base-pairing. Elife 9:e55002. 10.7554/eLife.55002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Komarova AV, Tchufistova LS, Supina EV, Boni IV. 2002. Protein S1 counteracts the inhibitory effect of the extended Shine–Dalgarno sequence on translation. RNA 8:1137–1147. 10.1017/s1355838202029990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thiel K, Mulaku E, Dandapani H, Nagy C, Aro E-M, Kallio P. 2018. Translation efficiency of heterologous proteins is significantly affected by the genetic context of RBS sequences in engineered cyanobacterium Synechocystis sp. PCC 6803. Microb Cell Fact 17:34. 10.1186/s12934-018-0882-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jahn M, Vorpahl C, Hübschmann T, Harms H, Müller S. 2016. Copy number variability of expression plasmids determined by cell sorting and droplet digital PCR. Microb Cell Fact 15:211. 10.1186/s12934-016-0610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bi C, Su P, Müller J, Yeh Y-C, Chhabra SR, Beller HR, Singer SW, Hillson NJ. 2013. Development of a broad-host synthetic biology toolbox for Ralstonia eutropha and its application to engineering hydrocarbon biofuel production. Microb Cell Fact 12:107. 10.1186/1475-2859-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meisner J, Goldberg JB. 2016. The Escherichia coli rhaSR-PrhaBAD inducible promoter system allows tightly controlled gene expression over a wide range in Pseudomonas aeruginosa. Appl Environ Microbiol 82:6715–6727. 10.1128/AEM.02041-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kelly CL, Taylor GM, Hitchcock A, Torres-Méndez A, Heap JT. 2018. A rhamnose-inducible system for precise and temporal control of gene expression in Cyanobacteria. ACS Synth Biol 7:1056–1066. 10.1021/acssynbio.7b00435. [DOI] [PubMed] [Google Scholar]
- 60.Bustos SA, Schleif RF. 1993. Functional domains of the AraC protein. Proc Natl Acad Sci U S A 90:5638–5642. 10.1073/pnas.90.12.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaldalu N, Toots U, de Lorenzo V, Ustav M. 2000. Functional domains of the TOL plasmid transcription factor XylS. J Bacteriol 182:1118–1126. 10.1128/JB.182.4.1118-1126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hawkins JS, Silvis MR, Koo B-M, Peters JM, Osadnik H, Jost M, Hearne CC, Weissman JS, Todor H, Gross CA. 2020. Mismatch-CRISPRi reveals the co-varying expression-fitness relationships of essential genes in Escherichia coli and Bacillus subtilis. Cell Syst 11:523–535. 10.1016/j.cels.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rock JM, Hopkins FF, Chavez A, Diallo M, Chase MR, Gerrick ER, Pritchard JR, Church GM, Rubin EJ, Sassetti CM, Schnappinger D, Fortune SM. 2017. Programmable transcriptional repression in mycobacteria using an orthogonal CRISPR interference platform. Nat Microbiol 2:16274. 10.1038/nmicrobiol.2016.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fontana J, Dong C, Kiattisewee C, Chavali VP, Tickman BI, Carothers JM, Zalatan JG. 2020. Effective CRISPRa-mediated control of gene expression in bacteria must overcome strict target site requirements. Nat Commun 11:1618. 10.1038/s41467-020-15454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chapalain A, Groleau M-C, Le Guillouzer S, Miomandre A, Vial L, Milot S, Déziel E. 2017. Interplay between 4-hydroxy-3-methyl-2-alkylquinoline and n-acyl-homoserine lactone signaling in a Burkholderia cepacia complex clinical strain. Front Microbiol 8:1021. 10.3389/fmicb.2017.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Choi K-H, Kumar A, Schweizer HP. 2006. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for DNA fragment transfer between chromosomes and plasmid transformation. J Microbiol Methods 64:391–397. 10.1016/j.mimet.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 67.Gislason AS, Choy M, Bloodworth RAM, Qu W, Stietz MS, Li X, Zhang C, Cardona ST. 2017. Competitive growth enhances conditional growth mutant sensitivity to antibiotics and exposes a two-component system as an emerging antibacterial target in Burkholderia cenocepacia. Antimicrob Agents Chemother 61:e00790-16. 10.1128/AAC.00790-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang X, Bremer H. 1995. Control of the Escherichia coli rrnB P1 promoter strength by ppGpp. J Biol Chem 270:11181–11189. 10.1074/jbc.270.19.11181. [DOI] [PubMed] [Google Scholar]
- 69.Wickstrum JR, Skredenske JM, Balasubramaniam V, Jones K, Egan SM. 2010. The AraC/XylS family activator RhaS negatively autoregulates rhaSR expression by preventing cyclic AMP receptor protein activation. J Bacteriol 192:225–232. 10.1128/JB.00829-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Darling P, Chan M, Cox AD, Sokol PA. 1998. Siderophore production by cystic fibrosis isolates of Burkholderia cepacia. Infect Immun 66:874–877. 10.1128/IAI.66.2.874-877.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miller VL, Mekalanos JJ. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol 170:2575–2583. 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meselson M, Yuan R. 1968. DNA restriction enzyme from E. coli. Nature 217:1110–1114. 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- 73.Holloway BW, Zhang C. 1990. Genetic maps. In O’Brien SJ (ed), Locus maps of complex organisms, 5th ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 74.Rahme LG, Stevens EJ, Wolfort SF, Shao J, Tompkins RG, Ausubel FM. 1995. Common virulence factors for bacterial pathogenicity in plants and animals. Science 268:1899–1902. 10.1126/science.7604262. [DOI] [PubMed] [Google Scholar]
- 75.Elmore JR, Furches A, Wolff GN, Gorday K, Guss AM. 2017. Development of a high efficiency integration system and promoter library for rapid modification of Pseudomonas putida KT2440. Metab Eng Commun 5:1–8. 10.1016/j.meteno.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hook-Barnard IG, Brickman TJ, McIntosh MA. 2007. Identification of an AU-rich translational enhancer within the Escherichia coli fepB leader RNA. J Bacteriol 189:4028–4037. 10.1128/JB.01924-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Olins PO, Rangwala SH. 1989. A novel sequence element derived from bacteriophage T7 mRNA acts as an enhancer of translation of the lacZ gene in Escherichia coli. J Biol Chem 264:16973–16976. 10.1016/S0021-9258(18)71444-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1 to S3, Table S1. Download AEM.00647-21-s0001.pdf, PDF file, 0.4 MB (371.4KB, pdf)