Abstract
p90rsk is a distal member of the mitogen-activated protein kinase signaling pathway. It has been cloned from a variety of species including Xenopus laevis, mouse, chicken, rat, and human. The clone p90rsk-mo-1, isolated by others from a mouse library, contains a unique 33-nucleotide deletion not found in the p90rsk clones from any other species that have been examined. When p90rsk-mo-1 was expressed in Cos-7 cells that were subsequently stimulated with epidermal growth factor, the immunoprecipitated p90rsk-mo-1 protein showed no measurable kinase activity toward the ribosomal protein S6 peptide. By comparison, expression of rat p90rsk-1 resulted in significant kinase activity. Deletion of the 33-nucleotide region missing in the p90rsk-mo-1 clone from the p90rsk-rat-1 cDNA abolished kinase activity in the resulting protein. When these 33 nucleotides were introduced into the p90rsk-mo-1 cDNA, the expressed protein showed significant kinase activity. Reverse transcription-PCR and direct sequencing of mRNA isolated from several mouse tissues indicated the presence of the full-length form of p90rsk-1 in the mouse and showed no conclusive evidence for a deletion-containing form. This study indicates the presence of a full-length p90rsk-1 mRNA in mouse tissues that is homologous to that identified in other species and suggests that the deletion in p90rsk-mo-1 may be a cloning artifact. The findings provide additional support for the conclusion that the first catalytic domain of p90rsk is responsible for its enzymatic activity toward ribosomal protein S6.
p90rsk is a distal member of the MAP kinase signaling pathway that is directly phosphorylated and activated by MAP kinases in response to cellular stimulation by growth factors. p90rsk is a serine/threonine kinase that contains two putative catalytic domains (8). Studies indicate that both domains may be functional. The amino-terminal domain appears to be responsible for substrate phosphorylation, whereas the carboxyl-terminal domain may play some role in autophosphorylation (2, 6). The details of the mechanism by which the two catalytic domains of p90rsk are regulated are an ongoing area of research.
A variety of in vitro and in vivo p90rsk substrates have been identified, including ribosomal protein S6, c-jun, c-fos, nur77, CREB, CREB-binding protein, serum response factor, SOS, and glycogen synthase kinase 3 (3–5, 10–13). The principal physiological substrate(s) of p90rsk, and hence its primary function in the cell, is not known.
Three isoforms of p90rsk have been identified in vertebrates. The p90rsk-1 isoform has been cloned from a variety of species, including Xenopus laevis, mouse, chicken, rat, and human. These molecules show a high degree of homology; for example, X. laevis and human p90rsk-1 have 85% amino acid identity. The one striking difference among all the p90rsk-1 genes is a 33-nucleotide deletion found only in the mouse gene (p90rsk-mo-1). This deletion results in the loss of 11 amino acids just C terminal to the first catalytic domain of p90rsk.
As part of a study investigating the substrate specificity of the two p90rsk catalytic domains, we observed that expressed p90rsk-mo-1 protein lacked measurable kinase activity. In the present study, we investigated this observation, and here we discuss the physiological relevance of the findings.
MATERIALS AND METHODS
Abbreviations.
BSA, bovine serum albumin; EGF, epidermal growth factor; HA, influenza virus hemagglutinin epitope; PCR, polymerase chain reaction; PKI, peptide inhibitor of cAMP-dependent protein kinase; PVDF, polyvinyldifluoride; RT-PCR, coupled reverse transcription and PCR; SDS, sodium dodecyl sulfate.
Materials.
EGF was purchased from Upstate Biotechnology Incorporated, Lake Placid, N.Y. Mouse anti-HA antibody (12CA5) was purchased from Boehringer Mannheim, Indianapolis, Ind.
Epitope tagging.
p90rsk-mo-1 was epitope tagged at the amino terminus with the HA epitope (YPYDVPDYA), utilizing the pBluescript-HA vector kindly provided by Tomas Kirchhausen (Harvard Medical School, Boston, Mass.). The p90rsk-mo-1 coding sequence was obtained from pBS-rskmo-1 (kindly provided by Raymond Erikson, Harvard University, Cambridge, Mass.) (1).
Construction of rat-1-Δ and mouse-1 vectors.
pMT2-HA-rskrat-1-Δ was constructed by using PCR. The coding sequence of pMT2p85.epi (generously provided by Joseph Avruch) (7) was removed by digestion with EcoRI and transferred into pBSBX, a modified pBluescript vector in which the BamHI site had been destroyed by digesting with BamHI, end-filling with the Klenow fragment, and religating. The 33 nucleotides that are missing in the mouse cDNA clone were deleted from the rat cDNA by using two rounds of amplification. Round one amplified two adjacent fragments (R and S) so that the overlapping region between the two contained the mutation. Fragment R was generated by using primers 1 (CAAGGATCCTTTGGCAAAG) and 21 (CTTCCGGTCCTTCCCCATCAACACCCCATA), where the underlined sequence indicates a BamHI site. Fragment S was generated by using primers 20 (TATGGGGTGTTGATGGGGAAGGACCGGAAG) and 19 (GAAACTGGGGCATGCCTAG), where the underlined sequence indicates an SphI site. The second round of amplification contained a mixture of fragments R and S as the template and primers 1 and 19 to amplify a fragment containing the deletion and flanked by unique restriction enzyme sites. The PCR product was digested with BamHI and SphI, gel purified, and ligated in place of the wild-type sequence in pBSBX-HA-rat-1 to generate the deletion mutation (pBSBX-HA-rskrat-1-Δ). This coding sequence was transferred to the EcoRI site in the pMT2 vector for expression in mammalian cells.
To generate the full-length rskmouse-1 construct, the 33 nucleotides of interest were amplified by RT-PCR from mouse spleen RNA and inserted into the rskmo-1 clone. Specifically, PCR was performed with primers 1 and 19, following RT of mouse spleen RNA by using oligo(dT) as the RT primer. This product was digested with BamHI and SphI, gel purified, and ligated in place of the corresponding sequence in the pCMV-HA-rskmo-1 vector. All PCR-generated constructs were sequenced to confirm that no unintentional mutations had been generated.
Transfection and stimulation of Cos-7 cells.
A total of 1 × 105 to 2 × 105 Cos-7 cells were plated onto 60-mm dishes and transfected with 3 μg of plasmid and 15 μl of Lipofectin (Gibco BRL, Gaithersburg, Md.). After 6 h, the cells were rinsed with phosphate-buffered saline and fed with Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum. Approximately 32 h posttransfection, the cells were switched to Dulbecco’s modified Eagle’s medium containing 0.1% fetal bovine serum and incubated for an additional 14 h. Cells were stimulated with 50 ng of EGF per ml for 5 min, rinsed with ice-cold phosphate-buffered saline, and lysed by sonication in buffer H (50 mM β-glycerophosphate [pH 7.3], 1.5 mM EGTA, 1 mM dithiothreitol, 0.15 mM sodium orthovanadate, 1 mM benzamidine, 10 μg of aprotinin per ml, 10 μg of leupeptin per ml, 2 μg of pepstatin A per ml). The lysates were centrifuged at 100,000 × g for 20 min to obtain a high-speed-centrifugation cell extract. Kinase assays were performed on the day of harvest.
Kinase assays.
Cell extracts were immunoprecipitated with antibodies to p90rsk (anti-rsk antibody) and to the HA epitope (anti-HA antibody 12CA5) for 1 to 2 h on ice, followed by the addition of protein A-Sepharose for 1 to 2 h. The immunoprecipitates were collected by centrifugation, rinsed twice with ice-cold buffer H, and resuspended in 40 μl of 1× assay mixture (25 mM β-glycerophosphate [pH 7.3], 1.25 mM EGTA, 1 mM dithiothreitol, 10 mM MgCl2, 0.15 mM sodium orthovanadate, 2 μM PKI, 10 μM calmidizolium, 1 mg of BSA per ml, 100 μM ATP, 0.5 μCi of [γ-32P]ATP per ml [3,000 Ci/mmol]). Twenty-one microliters of the suspension was added in duplicate to 4 μl of 1.5 mM S6 peptide (RRLSSLRA) and incubated at 30°C for 20 min in a thermal mixer. Twenty microliters of each sample was spotted on P-81 chromatography paper (Whatman, Maidstone, England) and washed extensively in 150 mM phosphoric acid. The assay papers were rinsed in ethanol, air dried, and soaked in scintillation fluid before being analyzed in a scintillation counter.
RT-PCR.
Five micrograms of total RNA from various mouse tissues and NIH 3T3 cells was reverse transcribed with oligo(dT) as the primer. Two microliters of this cDNA (10% of the total) was used for DNA amplification in a 50-μl reaction mixture containing 1× cloned Pfu buffer (20 mM Tris-HCl [pH 8.75], 10 mM KCl, 10 mM [NH4]2SO4, 2 mM MgSO4, 0.1% Triton X-100, 0.1 mg of BSA per ml), 250 μM each deoxynucleoside triphosphate, 200 ng of the appropriate primers, 1.25 U of Pfu DNA polymerase (Stratagene, La Jolla, Calif.), and 10% dimethyl sulfoxide. DNA was amplified in a Perkin-Elmer Cetus thermal cycler (model 2400) by using the following conditions: 95°C for 4 min; 30 cycles of 94°C for 45 s, 55°C for 45 s, and 72°C for 45 s; 72°C for 5 min. A second round of PCR was performed without dimethyl sulfoxide, by using 2 μl of the first-round product as the template for the second round of amplification. The reaction products were resolved on a 2.5% agarose gel comprised of a 50:50 mix of SeaKem LE and NuSieve GTG agarose (FMC BioProducts, Rockland, Maine) including 1 μg of a 100-bp DNA ladder (Gibco BRL) as a molecular weight standard. The primers used were 13 (GCCATAGACCATGAGAAG), 25 (CGTCAGCATCTCAAACAT), 28 (GCCATTGACCACGAAAAG), and 29 (TTCCGGTCCTTCCCCATC). Primer 28 is the rat-specific version of primer 13.
Sequencing.
RT-PCR of 5 μg of total RNA from mouse heart, brain, spleen, and testis generated a 220-bp fragment in each sample. These fragments were gel purified on low-melting-point agarose and sequenced by using the fmol DNA sequencing system (Promega, Madison, Wis.). Approximately 4 fmol of PCR product and 40 fmol of control plasmids were sequenced with primer 19, by using the following amplification conditions: 95°C for 2 min and then 30 cycles of 94°C for 30 s, 42°C for 30 s, and 70°C for 1 min. Sequencing reaction products were resolved on 8% acrylamide–urea sequencing gels, dried, and exposed to film overnight.
RESULTS
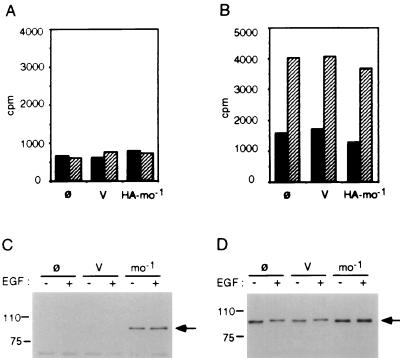
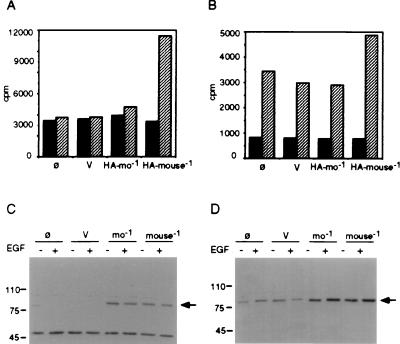
The p90rsk mouse clone (pCMV-rskmo-1) was tagged at the amino terminus with the HA epitope, as described in Materials and Methods, and transiently expressed in Cos-7 cells to generate HA-tagged p90rsk-mo-1. The cells were stimulated with EGF for 5 min, lysed, and centrifuged at 100,000 × g to recover the high-speed-centrifugation cell extract. The transiently expressed p90rsk-mo-1 was separated from the endogenous p90rsk in the cell extract by immunoprecipitation with a monoclonal antibody against the HA epitope. We observed that this immunoprecipitate was routinely devoid of kinase activity against the S6 peptide substrate (Fig. 1A). Kinase assays of immunoprecipitates of endogenous p90rsk with anti-rsk antibody confirmed the presence of significant kinase activity in EGF-stimulated Cos-7 cells (Fig. 1B). Furthermore, expression of exogenous HA-p90rsk-mo-1 was confirmed by Western blot analysis of anti-HA immunoprecipitates (Fig. 1C). Although HA-tagged p90rsk-mo-1 protein was expressed in substantial quantity and could be immunoprecipitated with anti-HA antibody, significant kinase activity was never observed.
FIG. 1.
Kinase assay of mouse p90rsk-mo-1. Cos-7 cells were mock transfected without DNA (ø) or transiently transfected with pCMV-neo vector control (V) or with pCMV-HA-rskmo-1 (HA-mo-1) as described in Materials and Methods. Cells were stimulated for 5 min with EGF (hatched bars) or with carrier BSA (solid bars) and harvested by sonication in buffer H. Sixty micrograms of the cell extracts obtained by centrifugation at 100,000 × g was immunoprecipitated with either anti-HA (A) or anti-rsk (B) antibody. After two washes, the immunoprecipitates were assayed for S6 kinase activity as described in Materials and Methods. Anti-HA immunoprecipitates (C) or 12 μg of total cell extracts (D) was resolved by SDS-polyacrylamide gel electrophoresis and transferred to PVDF membranes for analysis. Membrane blots were probed with rabbit anti-rsk antibody to confirm protein expression.
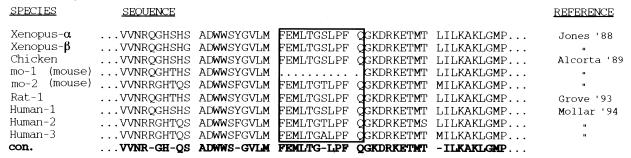
A comparison of p90rsk-1 nucleotide sequences from various species reveals a high degree of homology. In species as divergent as X. laevis and human, the sequence identity is 85%. One striking difference among the p90rsk sequences identified thus far is the omission of 33 nucleotides (11 amino acids) in the sequence cloned from mouse (rskmo-1) (1). This corresponds to nucleotides 757 to 790 (where nucleotide 1 is the A of the first ATG codon), coding for a region just C-terminal to the first catalytic domain of p90rsk (Fig. 2). We hypothesized that this deletion could be responsible for the lack of measurable kinase activity in p90rsk-mo-1.
FIG. 2.
Comparison of p90rsk sequences from various species. A partial sequence alignment of the p90rsk sequences cloned from various species. The 11-amino-acid region of interest is boxed. Con., consensus.
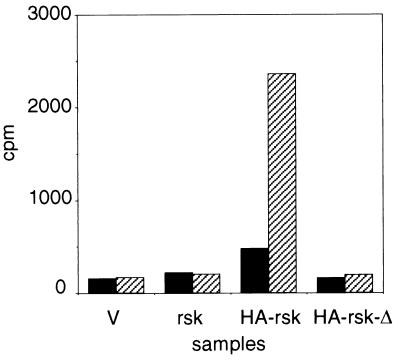
An HA-tagged rat clone of p90rsk (pMT2p85.epi) was expressed in EGF-stimulated Cos-7 cells and was found to possess significant activity toward S6 peptide. When this construct was mutated to delete the 33 nucleotides corresponding to those missing in the rskmo-1 clone (generating pMT2-HA-rskrat-Δ), the resulting protein had no measurable kinase activity (Fig. 3), although a significant amount of HA-p90rsk-rat-1-Δ was expressed and immunoprecipitates of endogenous p90rsk contained activated protein (data not shown).
FIG. 3.
Comparison of p90rsk-rat-1 and p90rsk-rat-Δ kinase activities. Cos-7 cells were transiently transfected with pMT2 vector control (V), pMT2-rskrat-1 (rsk), pMT2p85.epi (HA-rsk), or pMT2-rskrat-Δ (HA-rsk-Δ) as described in Materials and Methods. Cells were stimulated for 5 min with EGF (hatched bars) or with carrier BSA (solid bars) and harvested by sonication in buffer H. Sixty micrograms of the cell extracts obtained by centrifugation at 100,000 × g was immunoprecipitated with anti-HA antibody. After two washes, the immunoprecipitates were assayed for S6 kinase activity as described in Materials and Methods.
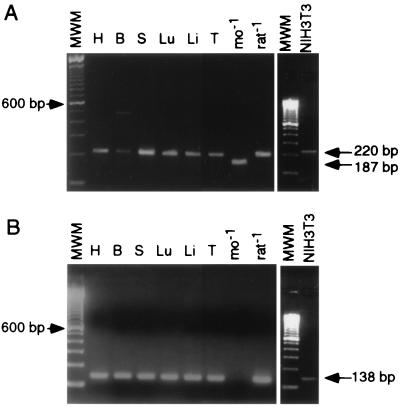
The p90rsk-mo-1 clone was originally generated from a mouse expression library, specifically a modified NIH 3T3 cell line (1). To determine whether the deletion-containing form of p90rsk is expressed in normal mouse tissue, samples of total RNA from several mouse tissues (heart, brain, spleen, lung, liver, and testis) were used as templates for RT-PCR analysis. A set of primers flanking the region of interest was used (primers 13 and 19) so that a 220-bp fragment would be generated from a full-length sequence and a 187-bp fragment would result from amplification of the deleted form. Following amplification of the mouse samples, a 220-bp product was generated exclusively, consistent with the presence of a full-length p90rsk sequence in the mouse tissues. The same was true for RT-PCR from NIH 3T3 cell RNA (Fig. 4A). The 187-bp product was successfully amplified only from the pBS-rskmo-1 control plasmid.
FIG. 4.
RT-PCR of p90rsk from mouse tissues and NIH 3T3 cells. Five micrograms of RNA obtained from mouse heart (H), brain (B), spleen (S), lung (Lu), liver (Li), and testis (T) were reverse transcribed as described in Materials and Methods. (A) cDNA was amplified with a pair of primers which flanked the 33-nucleotide region of interest so that the presence of a deletion would result in a smaller product. (B) Amplification with a primer pair specific for the full-length p90rsk sequence. pBS-rskmo-1 (mo-1) and pMT2p85.epi (rat-1) were included as control templates for each set of amplifications. MWM, molecular weight markers.
RT-PCR experiments were also performed with sequence-specific primers that overlapped the 33-nucleotide region. A primer corresponding to the full-length sequence (primer 25) and a primer which straddled the deleted sequence (primer 29) were used for RT-PCR analysis of the mouse RNA samples. RT-PCR analysis with primers 13 (or 28 for rat) and 25 did not generate a product from the pBS-rskmo-1 control plasmid but did lead to amplification of a 138-bp fragment from the rskrat-1 control plasmid as well as from the mouse RNA samples and NIH 3T3 cells (Fig. 4B), consistent with the presence of full-length message in these samples. A similar analysis with primers 13 (or 28 for rat) and 29 gave no reproducible amplification of a 137-bp product, except with the rskmo-1 control (data not shown), consistent with the absence of the deletion-containing form in normal mouse tissues or NIH 3T3 cells. If a small amount of the deletion-containing form of p90rsk-1 has escaped detection in this assay and does exist in mouse tissues, it is unlikely that it plays a significant role in regulating the activity of the full-length kinase, since overexpression of inactive p90rsk-mo-1 does not affect the activity of endogenous p90rsk in Cos-7 cells (Fig. 1B).
The 220-bp mouse PCR products (Fig. 4A) from heart, brain, spleen, and testis were gel purified and sequenced to confirm that they resulted from p90rsk mRNA. The sequences of the mouse PCR products were identical to the sequence for rat p90rsk-1 within the 33 nucleotides of interest. The sequencing samples were not contaminated with p90rsk-rat-1 DNA, since the remainder of the p90rsk sequence, where divergent, was that of mouse rather than rat (accession no. AF084468).
Finally, the 33 nucleotides of interest were recovered from mouse spleen by using PCR and transferred into the pCMV-HA-rskmo-1 vector by using unique BamHI and SphI restriction enzyme sites, generating the pCMV-HA-rskmouse-1 vector. Expression of this construct in Cos-7 cells generated HA-tagged p90rsk displaying significant kinase activity following EGF stimulation (Fig. 5). These results indicate that normal mouse tissues and NIH 3T3 cells contain a full-length p90rsk and that transfer of a 33-nucleotide region from its coding sequence to p90rsk-mo-1 resulted in a kinase that could be activated by EGF stimulation in Cos-7 cells.
FIG. 5.
Kinase assay of p90rsk-mo-1 versus p90rsk-mouse-1. Cos-7 cells were mock transfected without DNA (ø) or transiently transfected with pCMV-neo vector control (V), pCMV-HA-rskmo-1 (HA-mo-1), or pCMV-HA-rskmouse-1 (HA-mouse-1) in which the missing 33 nucleotides have been inserted into the p90rsk-mo-1 construct. Cells were stimulated for 5 min with EGF (hatched bars) or with carrier BSA (solid bars) and harvested by sonication in buffer H. Sixty micrograms of the cell extracts obtained by centrifugation at 100,000 × g was immunoprecipitated with either anti-HA (A) or anti-rsk (B) antibody. After washing, the immunoprecipitates were assayed for S6 kinase activity as described in Materials and Methods. Anti-HA immunoprecipitates (C) or 12 μg of total cell extracts (D) was resolved by SDS-polyacrylamide gel electrophoresis and transferred to PVDF membranes for analysis. Membrane blots were probed with rabbit anti-rsk antibody to confirm protein expression.
DISCUSSION
In this study, we have shown that p90rsk-mo-1 is devoid of kinase activity toward S6 peptide substrate. This lack of activity is due to the deletion of 33 nucleotides from the cDNA, which results in the in-frame deletion of 11 amino acids from p90rsk. One explanation for the observed inactivity of p90rsk-mo-1 could be the absence of an essential threonine or serine within the 11-amino-acid region of interest (Fig. 2). However, mutations of threonine-257 and serine-259 to alanines in HA-rskrat-1 did not result in the loss of p90rsk kinase activity (data not shown), suggesting that these amino acids are not critical for activation. Furthermore, the region missing in rskmo-1 corresponds to approximately one α-helical turn of the F helix and a significant portion of loop G (where the nomenclature is taken from cyclic AMP-dependent protein kinase structural designations [9]). The F helix is one of the more highly conserved elements of kinase structure. Thus, we postulate that it is the loss of part of this element that results in an inactive kinase, most likely due to improper folding.
Our experience with this mutated protein confirms the findings of Bjørkæk et al. (2) and Fisher and Blenis (6) that the first catalytic domain of p90rsk is responsible for activity toward S6 peptide, since the rskmo-1 deletion is found in the amino-terminal domain of p90rsk. The deletion-containing form of p90rsk does not appear to exist in normal mouse tissue, as determined by RT-PCR and direct sequencing, whereas a full-length message for p90rsk was identified in those samples. Thus, the original omission of the 33 nucleotides in the p90rsk-mo-1 gene was most likely a cloning artifact.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant R01-DK42528 from the National Institutes of Health and by a grant from the Muscular Dystrophy Association. D.J.S. is an American Diabetes Association Mentor-based Fellow.
We thank Lauri Aicher for preparing RNA from mouse tissues for this study. We thank Ray Erikson for reading the manuscript and for his helpful comments. Insightful discussions with members of this laboratory were greatly appreciated.
REFERENCES
- 1.Alcorta D A, Crews C M, Sweet L J, Bankston L, Jones S W, Erikson R L. Sequence and expression of chicken and mouse rsk: homologs of Xenopus laevis ribosomal S6 kinase. Mol Cell Biol. 1989;9:3850–3859. doi: 10.1128/mcb.9.9.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjørkæk C, Zhao Y, Moller D E. Divergent functional roles for p90rsk kinase domains. J Biol Chem. 1995;270:18848–18852. doi: 10.1074/jbc.270.32.18848. [DOI] [PubMed] [Google Scholar]
- 3.Chen R-H, Abate C, Blenis J. Phosphorylation of the c-fos transrepression domain by mitogen-activated protein kinase and 90-kDa ribosomal S6 kinase. Proc Natl Acad Sci USA. 1993;90:10952–10956. doi: 10.1073/pnas.90.23.10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis I J, Hazel T G, Chen R-H, Blenis J, Lau L F. Functional domains and phosphorylation of the orphan receptor nur77. Mol Endocrinol. 1993;7:953–964. doi: 10.1210/mend.7.8.8232315. [DOI] [PubMed] [Google Scholar]
- 5.Douville E, Downward J. EGF induced SOS phosphorylation in PC12 cells involves p90RSK-2. Oncogene. 1997;15:373–383. doi: 10.1038/sj.onc.1201214. [DOI] [PubMed] [Google Scholar]
- 6.Fisher T L, Blenis J. Evidence for two catalytically active kinase domains in pp90rsk. Mol Cell Biol. 1996;16:1212–1219. doi: 10.1128/mcb.16.3.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grove J R, Price D J, Banerjee P, Balasubramanyam A, Ahmad M F, Avruch J. Regulation of an epitope-tagged recombinant rsk-1 S6 kinase by phorbol ester and erk/MAP kinase. Biochemistry. 1993;32:7727–7738. doi: 10.1021/bi00081a018. [DOI] [PubMed] [Google Scholar]
- 8.Jones S W, Erikson E, Blenis J, Maller J L, Erikson R L. A Xenopus ribosomal protein S6 kinase has two apparent kinase domains that are each similar to distinct protein kinases. Proc Natl Acad Sci USA. 1988;85:3377–3381. doi: 10.1073/pnas.85.10.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knighton D R, Zheng J, Ten Eyck L F, Ashford V A, Xuong N-H, Taylor S S, Sowadski J M. Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991;253:407–414. doi: 10.1126/science.1862342. [DOI] [PubMed] [Google Scholar]
- 10.Moxham C M, Tabrizchi A, Davis R J, Malbon C C. jun N-terminal kinase mediates activation of skeletal muscle glycogen synthase by insulin in vivo. J Biol Chem. 1996;271:30765–30773. doi: 10.1074/jbc.271.48.30765. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima T, Fukamizu A, Takahashi J, Gage F H, Fisher T, Blenis J, Montminy M R. The signal-dependent coactivator CBP is a nuclear target for pp90rsk. Cell. 1996;86:465–474. doi: 10.1016/s0092-8674(00)80119-1. [DOI] [PubMed] [Google Scholar]
- 12.Rivera V M, Miranti C K, Misra R P, Ginty D D, Chen R-H, Blenis J, Greenberg M E. A growth factor-induced kinase phosphorylates the serum response factor at a site that regulates its DNA-binding activity. Mol Cell Biol. 1993;13:6260–6273. doi: 10.1128/mcb.13.10.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sweet L J, Alcorta D A, Jones S W, Erikson E, Erikson R L. Identification of mitogen-responsive ribosomal protein S6 kinase pp90rsk, a homolog of Xenopus S6 kinase II, in chicken embryo fibroblasts. Mol Cell Biol. 1990;10:2413–2417. doi: 10.1128/mcb.10.5.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]