Abstract
Antibiotic resistance in bacteria has become a major global health problem. One of the main reservoirs of antibiotic resistance genes is the human gut microbiota. To characterise these genes, a metagenomic approach was used. In this study, a comprehensive antibiotic resistome catalog was established using fecal samples from 246 healthy individuals from world’s longevity township in Jiaoling, China. In total, 606 antibiotic resistance genes were detected. Our results indicated that antibiotic resistance genes in the human gut microbiota accumulate and become more complex with age as older groups harbour the highest abundance of these genes. Tetracycline resistance gene type tetQ was the most abundant group of antibiotic resistance genes in gut microbiota, and the main carrier of antibiotic resistance genes was Bacteroides. Antibiotic efflux, inactivation, and target alteration were found to be the dominant antimicrobial resistance mechanisms. This research may help to establish a comprehensive antibiotic resistance catalog that includes extremely long-lived healthy people such as centenarians, and may provide potential recommendations for controlling the use of antibiotics.
Keywords: metagenomics, gut microbiota, antibiotic resistance genes, longevity people, cumulative effect
1. Introduction
Since the discovery of penicillin in 1929 [1], antibiotic resistance in bacteria has become an increasing threat to human health and a global health problem [2]. The emergence of antibiotic-resistant pathogens such as the New Delhi metallo-β-lactamase superbug [3], the carbapenem-resistant Klebsiella pneumoniae [4], multidrug-resistant Mycobacterium tuberculosis [5], and methicillin-resistant Staphylococcus aureus (MRSA) [6] has presented a major impact on human health. It is generally believed that the emergence and rapid spread of antibiotic resistance in the microbiota can be mainly attributed to the abuse of antibiotics by humans [7].
Antibiotics can have several effects on the human gut microbiota, which is a complex and dynamic equilibrium ecosystem [8]. When exposed to antibiotics, the microbiota not only responds through its own resistance mechanisms, but also optimises and spreads antibiotic resistance genes (ARG) through transformation, transfer, and recombination, and forms a colony with antibiotic resistance phenotype [9]. This antibiotic-induced disruption of microbiota may cause various diseases such as diabetes [10,11,12], neurological disorders [13], obesity [14,15], inflammation [16], and infections [17]. The human gut microbiota is considered to be the reservoir of ARG [18], which can quickly and easily exchange these genes to spread drug resistance [19]. This presents a high risk of increased antibiotic resistance in human pathogens [20], which has become a serious global public health problem as it renders previously reliable antibiotics ineffective.
Different methods have been used to characterise ARG in human gut microbiota, including isolation of antibiotic-resistant strains [21], polymerase chain reaction (PCR) based on specific primers, high-throughput quantitative PCR (qPCR) [22], microarray analysis [23] and metagenomics [24,25,26,27]. At present, metagenomic analysis based on high-throughput sequencing is commonly used in studying ARG due to its high efficiency and excellent characteristics [28,29]. Just as the human microbiome can constitute a mobile ARG reservoir, pathogens can use these genes to obtain antibiotic resistance through gene transfer [30]. Reference databases such as the Antibiotic Resistance Genes Database (ARDB) [31] and the Comprehensive Antibiotic Resistance Database (CARD) [32] have been developed to help researchers investigate ARG, such as those in the intestinal flora of Chinese, Danish, and Spanish populations. The difference in antibiotic resistance can be attributed to the different use and selection pressure of antibiotics in different countries [33]. However, the characteristics of ARG over time has not been clearly characterized. In particular, the relationship between the ARG in gut microbiota of longevity people and age remains unknown.
To address this problem, we analysed the ARG in the gut microbiota of 246 individuals from the world’s longevity township Jiaoling in China. Our results indicated that ARG in the human gut microbiota accumulate and become more complex with age given that older groups harbour the highest abundance of these genes. Bacteroides was found to be the main carrier of ARG in the human gut microbiota, of which tetracycline antibiotics resistance gene type tetQ was the most abundant group. Antibiotic efflux, inactivation, and target alteration were the dominant resistance mechanisms. This study will help establish a comprehensive list of antibiotic resistance in healthy and long-lived people, and provide a useful reference for the management of human ARG.
2. Materials and Methods
2.1. Study Cohort and Sample Collection
The overall research objective of the Jiaoling (world’s longevity township, Jiaoling County, Meizhou City, Guangdong Province, China) cohort is to study how ARG in the gut microbiota changes with age and how this affects health. The study was approved by the Ethics Committee of The First Affiliated Hospital/School of Clinical Medicine of Guangdong Pharmaceutical University.
From this cohort, 246 participants were randomly recruited from eight towns in June 2019. Participants must meet the following conditions: (1) born in Jiaoling; (2) have lived in Jiaoling for five consecutive years since the time of sampling; and (3) all age groups. All selected participants signed an informed consent form before the physical examination and biological material collection. To proceed to the metagenomic study, additional criteria were employed: (1) has fecal samples; (2) did not undergo antibiotic treatment within one month before the biological material was collected; and (3) no severe disease (diabetes, cancer, etc.). All participants met these requirements. Fecal samples were freshly collected from each subject and immediately frozen at −20 °C, transported to the laboratory on an ice pack, and stored at −80 °C until analysis.
2.2. DNA Extraction
Genomic DNA was extracted according to the manufacturer’s instructions (Magen, Stool DNA Kit, Guangzhou Magen Biotechnology Co., Ltd., Guangzhou, China) with some modification. Briefly, 1 mL of STL buffer was added to a 0.25–1 g sample and vortexed with glass beads for 15–20 min. It was then centrifuged at 12,000× g for 20 min, and the supernatant was transferred to a new 2-mL tube. In addition, 160 µL PS buffer and 160 µL absorbent solution were added. After centrifugation at 12,000× g for 10 min, the supernatant was transferred to a new 2-mL tube, and 650 μL GDP buffer was added. The column was used to filter the product, and then the product followed by DNA elution with 200 μL of sterile water.
2.3. Metagenomic Sequencing and Data Quality Control
The samples were sequenced using an Illumina HiSeq PE150 platform (Beijing Novogene Technology Co., Ltd., Beijing, China). The following standards were used for quality control: (1) reads were removed that contained low-quality bases (quality value ≤ 38) exceeding a certain percentage (default is 40 bp); (2) N bases were removed to reach a certain proportion of reads (default is 10 bp); (3) reads were removed whose overlap with the adapter exceeded a certain threshold (default is 15 bp); (4) if the sample had human contamination, it was compared with the human sequence to filter out the possible source of the human reads [34,35,36]. Bowtie2 software was used by default.
2.4. Metagenomic Assembly
After preprocessing, clean data were obtained and SOAPdenovo software [9] was used for assembly analysis. For the single sample assembly, parameters were: -d 1, -M 3, -R, -u, -F [37,38,39]. The assembled Scaffolds were broken from the N junction to obtain sequence fragments without N, called Scaftigs (i.e., continuous sequences within scaffolds) [40]. Bowtie2 software was used to compare the Clean Data after quality control of each sample to the assembled Scaftigs of each sample to acquire unused PE reads. The unused reads of each sample were then put together, and K-mer = 55 was selected for mixed assembly [41]. For Scaftigs generated by single sample and mixed assembly, fragments below 500 bp were filtered out [42,43,44].
2.5. Gene Catalog Construction
Starting from each sample- and mixed-assembled Scaftigs, MetaGeneMark was used for ORF (Open Reading Frame) prediction [45,46,47]. For the ORF prediction results of each sample and hybrid assembly, CD-HIT software was used for de-redundancy to obtain a non-redundant initial gene catalogue [48,49]. The clean data of each sample were compared to the initial gene catalogue using Bowtie2, and the number of reads on the gene comparison was calculated in each sample. The genes that support the number of reads ≤ 2 in each sample were filtered out, and the gene catalogue (unigenes) was obtained for subsequent analysis.
2.6. Species Annotation
(1) DIAMOND [50] software (V0.9.9, https://github.com/bbuchfink/diamond/) (accessed on 17 November 2020) was used to blast the unigenes to the sequences of Bacteria which are all extracted from the NR database (Version: 2018-01-02, https://www.ncbi.nlm.nih.gov/) (accessed on 17 November 2020) of NCBI. (2) The LCA algorithm which was applied to system classification of MEGAN [51] software was taken to make sure the species annotation information of sequences. (3) The table containing the number of genes and the abundance information of each sample in each taxonomy hierarchy (kingdom, phylum, class, order, family, genus, species) were obtained based on the LCA annotation result and the gene abundance table.
2.7. Analysis of Antibiotic Resistance Genes
The core component of the CARD database is Antibiotic Resistance Ontology (ARO), which integrates information such as sequence, antibiotic resistance, mechanism of action, and associations between AROs, and provides online interfaces between ARO and PDB, NCBI and other databases [52]. The basic steps of resistance gene annotation were as follows: (1) Resistance Gene Identifier software (RGI has built-in blastp, and uses the bitscore value to compare the results to score [53]) was used to compare unigenes; (2) Starting from the abundance of ARO, an abundance bar graph display, an abundance cluster heat map display, an abundance distribution circle graph display, and resistance gene species attribution analysis (annotate to unigenes of ARO) were performed. For part of the ARO with a long name, the first three words and an underscore were used to display the abbreviation. Finally, there were a total of 5,364,988 ORFs after the original de-redundancy, 3096 genes were compared to the CARD database, and a total of 606 types of ARO were included.
2.8. Statistical Analysis
Statistical analysis was implemented using the R platform and SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). The “ggplot2” package and GraphPad Prism 8 software (GraphPad Software Inc., San Diego, CA, USA) were used to visualize. SOAPdenovo software [9] was used for assembly analysis. DIAMOND software [50] was used to blast. MEGAN software [51] was used to make sure the species annotation information of sequences. The Wilcoxon rank-sum test was used to evaluate the significance of differences in six groups. * for p < 0.05; ** for p < 0.01; *** for p < 0.001.
3. Results
3.1. Abundance of Antibiotic Resistance Genes Is an Age-Related Cumulative Effect
We established 5.36 million human gut microbiota genes from the sequencing data of 246 individuals. The subjects were divided into six age groups, Y20 group (0–20 years old), Y40 group (21–40 years old), Y60 group (41–60 years old), Y80 group (61–80 years old), Y100 group (81–100 years old), Y120 group (100–120 years old). Of these, 3096 unique ARG were found after the original de-redundancy. These genes account for 0.057% of the total genes of the human gut microbiota. This is higher than the 0.026% reported in the previous literature in 2013 [33]. Similarly, compared with other natural environments (including soil, ocean, lake, etc.), antibiotic resistance genes are obviously very abundant in the human gut microbiota [33].
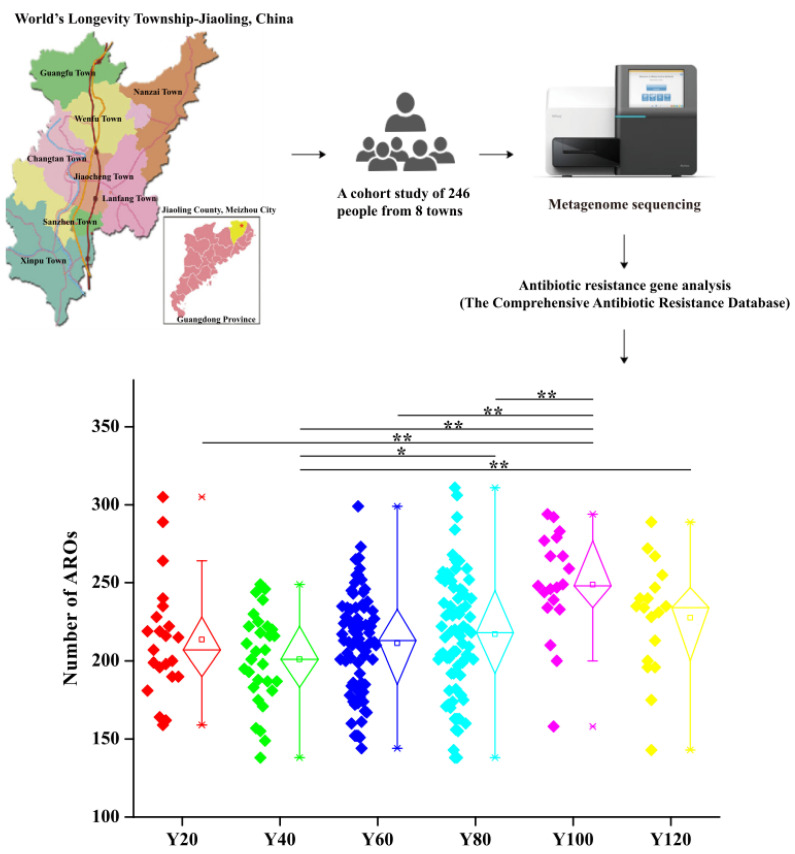
To compare the ARG abundances of different age groups, we calculated the number of ARG in each group based on sequencing coverage. The number of resistance genes in the older group (Y100) was significantly higher than that in the younger groups (Y20, Y40 and Y60; Figure 1). There is no difference between Y20–Y60 and that, from Y100, Y120, the number decreased (Figure 1). Our results indicate that ARG in the human gut microbiota accumulate and become more complex with age, with older groups harbouring the highest abundance of these genes.
Figure 1.
Comparison of the abundance of ARG in each group. The levels of significance for the Wilcoxon rank-sum test are: * for p < 0.05; ** for p < 0.01.
3.2. Representative Resistance Gene Types in Different Age Groups
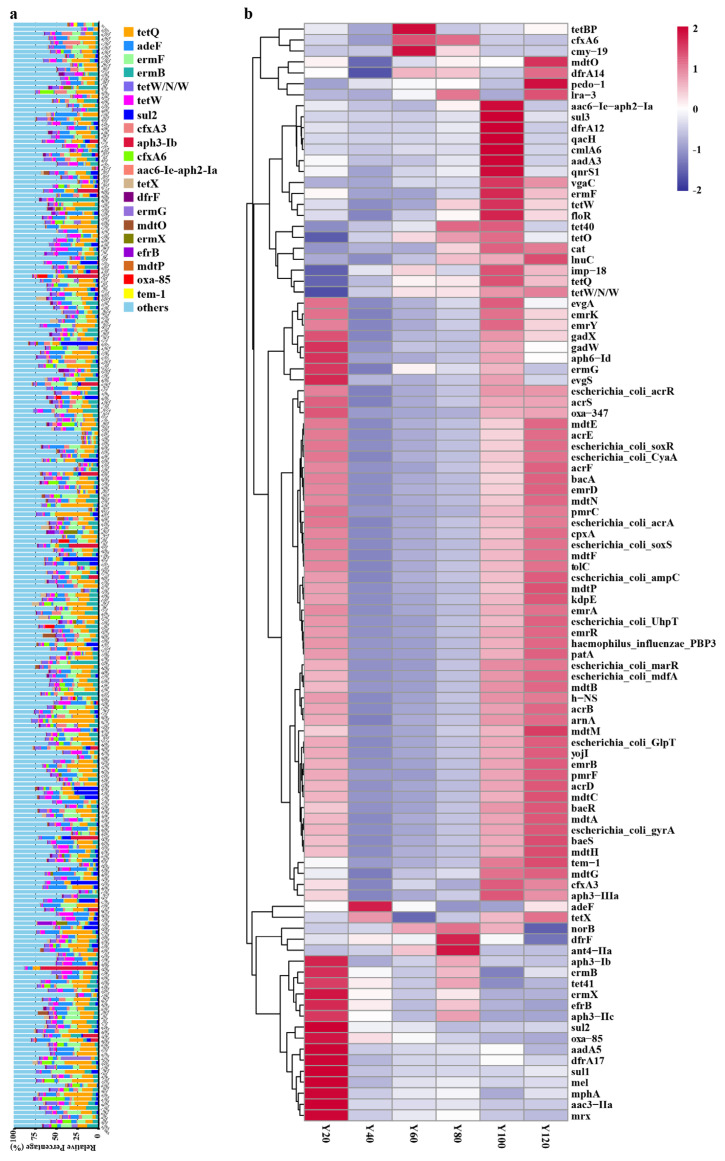
The top twenty most abundant antibiotic resistance gene types varied among the different age groups (Figure S1) with the exception of tetQ, which was the most abundant type in all groups (Figure 2a). Previous literature has shown that the prevalence of the tetQ gene in Bacteroides isolates has nearly tripled [54]. The mechanism of tetracycline resistance gene type tetQ is antibiotic target protection, and tetQ belongs to the gene family of tetracycline-resistant ribosomal protection proteins (Table 1). The second most abundant gene type was the fluoroquinolone and tetracycline (FT) resistance gene type adeF (Figure 2a). It operates through antibiotic efflux and belong to the gene family of resistance–nodulation–cell division (RND) efflux pumps (Table 1). ermF and ermB were the third and fourth most abundant the gene types, respectively (Figure 2a). They are macrolide–lincosamide–streptogramin B (MLS) resistance genes that alter the antibiotic target and belong to gene family of erm 23S ribosomal RNA methyltransferases (Table 1). We found that the mechanisms of ARG in Proteobacteria, Firmicutes, and Bacteroidetes are mainly antibiotic efflux, inactivation, and target alteration. Antibiotic efflux is mainly present in Proteobacteria, while antibiotic inactivation and target alteration mainly occur in Firmicutes (Figure S2).
Figure 2.
Comparative analysis of ARG in different age groups. (a) comparison of the top 20 most abundant ARG types in differently-aged individuals; (b) comparison of the top 100 most abundant ARG types in the different age groups.
Table 1.
Top 20 antibiotic resistance genes in the gut microbiota.
| ARO Name | Drug Class | Resistance Mechanism | AMR Gene Family |
|---|---|---|---|
| tetQ | tetracycline antibiotic | antibiotic target protection | tetracycline-resistant ribosomal protection protein |
| adeF | fluoroquinolone antibiotic; tetracycline antibiotic | antibiotic efflux | Resistance–nodulation–cell division RND antibiotic efflux pump |
| ermF | macrolide antibiotic; lincosamide antibiotic; streptogramin antibiotic | antibiotic target alteration | erm 23S ribosomal RNA methyltransferase |
| ermB | macrolide antibiotic; lincosamide antibiotic; streptogramin antibiotic | antibiotic target alteration | erm 23S ribosomal RNA methyltransferase |
| tetW/N/W | tetracycline antibiotic | antibiotic target protection | tetracycline-resistant ribosomal protection protein |
| tetW | tetracycline antibiotic | antibiotic target protection | tetracycline-resistant ribosomal protection protein |
| sul2 | sulfonamide antibiotic; sulfone antibiotic | antibiotic target replacement | sulfonamide resistant sul |
| cfxA3 | cephamycin | antibiotic inactivation | cfxA beta-lactamase |
| aph3-Ib | aminoglycoside antibiotic | antibiotic inactivation | aph3 |
| cfxA6 | cephamycin | antibiotic inactivation | cfxA beta-lactamase |
| aac6-Ie-aph2-Ia | aminoglycoside antibiotic | antibiotic inactivation | aph2; aac6 |
| tetX | glycylcycline; tetracycline antibiotic | antibiotic inactivation | tetracycline inactivation enzyme |
| dfrF | diaminopyrimidine antibiotic | antibiotic target replacement | trimethoprim resistant dihydrofolate reductase dfr |
| ermG | macrolide antibiotic; lincosamide antibiotic; streptogramin antibiotic | antibiotic target alteration | erm 23S ribosomal RNA methyltransferase |
| mdtO | nucleoside antibiotic; acridine dye | antibiotic efflux | major facilitator superfamily MFS antibiotic efflux pump |
| ermX | macrolide antibiotic; lincosamide antibiotic; streptogramin antibiotic | antibiotic target alteration | erm 23S ribosomal RNA methyltransferase |
| efrB | macrolide antibiotic; fluoroquinolone antibiotic; rifamycin antibiotic | antibiotic efflux | ATP-binding cassette ABC antibiotic efflux pump |
| mdtP | nucleoside antibiotic; acridine dye | antibiotic efflux | major facilitator superfamily MFS antibiotic efflux pump |
| oxa-85 | cephalosporin; penam | antibiotic inactivation | oxa beta-lactamase |
| tem-1 | monobactam; cephalosporin; penam; penem | antibiotic inactivation | tem beta-lactamase |
In the different age groups, different representative resistance gene types were observed (Figure 2b). aph3−Ib, ermB, tet41, ermX, efrB, aph3−IIc, sul2, oxa−85, aadA5, dfrA17, sul1, mel, mphA, aac3−IIa, and Mrx resistance gene types have the highest abundance in the Y20 group. Meanwhile, adeF, tetBP, cmy−19, dfrF, and ant4−IIa resistance gene types were the most abundant in the Y40, Y60, and Y80 groups. In the Y100 group, aac6−Ie−aph2−Ia, sul3, dfrA12, qacH, cmlA6, aadA3, qnrS1, vgaC, ermF, tetW, and floR were found to have the highest abundance. Lastly, mdtO and pedo−1 resistance gene types have the highest abundance in the Y120 group. Other categories of drugs and resistance mechanisms were listed in Supplementary Table S1.
3.3. Representative Types of Antibiotics in Different Age Groups
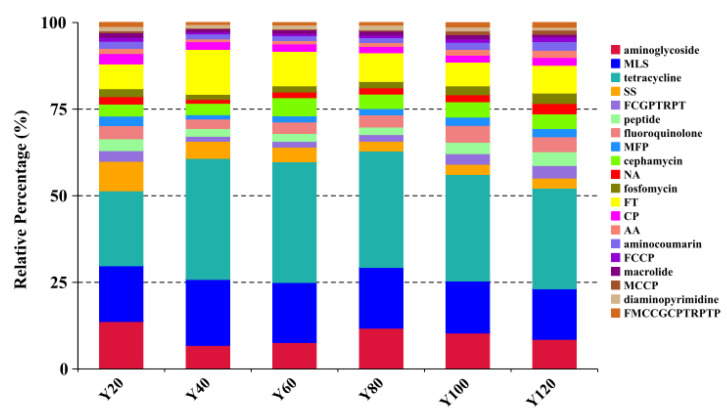
We mapped each resistance gene type to its corresponding antibiotic, and calculated the sum of the relative abundances of antibiotic types (Figure 3). The results showed that tetracycline, MLS, aminoglycoside, FT, and sulfonamide and sulfone (SS) were the top five ARG types in each of the age groups, with tetracycline being the most abundant (Figure 3, Figure S3). At the same time, the trend of antibiotics consumption gradually increased with age. This was observed for antibiotics such as aminoglycoside and aminocoumarin (AA), fluoroquinolone, cephalosporin, glycylcycline, penam, tetracycline, rifamycin, phenicol, and triclosan (FCGPTRPT), fosfomycin, and peptide (Figure S3). Abbreviations of antibiotic types are listed in Supplementary Table S1.
Figure 3.
Comparison of the top 15 most abundant antibiotic types in the different age groups. The cumulative bar graph represents the distribution of the top 20 antibiotic resistance types in the different age groups.
3.4. Representative Antibiotic Resistance Types of Different Bacterial Genera
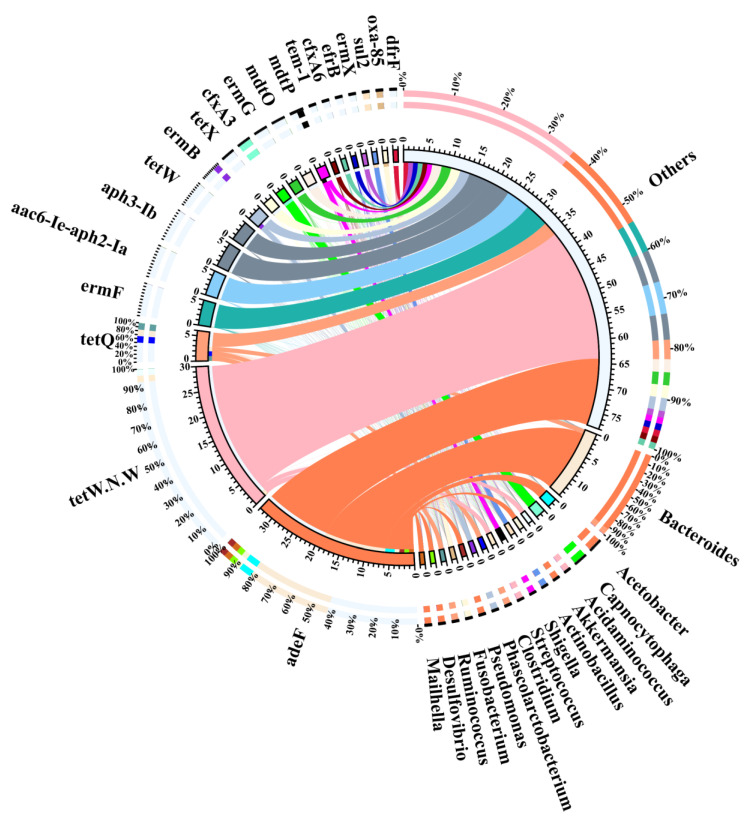
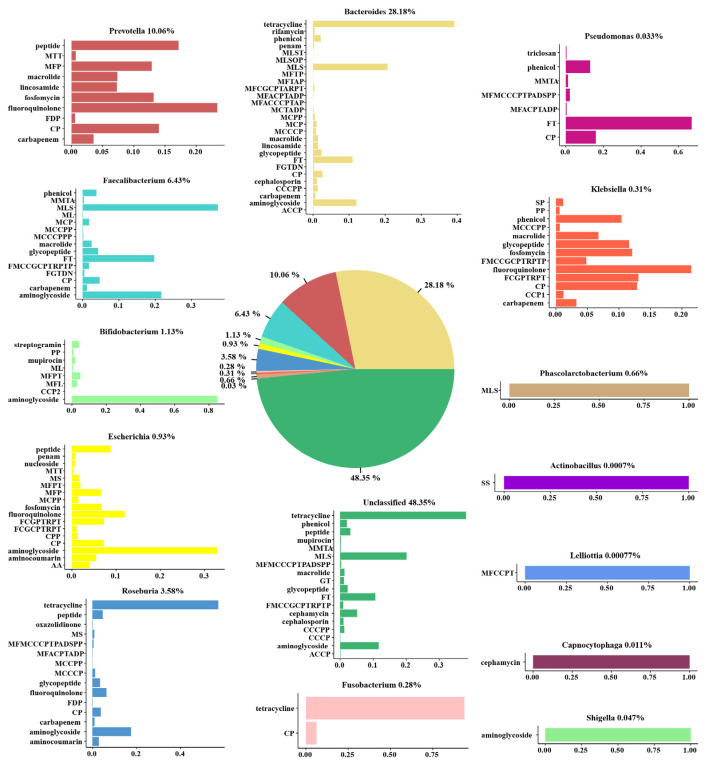
To find out which bacterial genera contributed to the ARG reservoir, we performed an association analysis of ARG and bacterial genera. We found that the most predominant genus in the gut microbiota was Bacteroides, followed by Firmicutes and Proteobacteria. However, Firmicutes was the main carrier of antibiotic resistance gene bacteria, followed by Proteobacteria and Bacteroides (Figure S4). We observed that the distribution of bacteria rich in resistance genes at the phylum level was different between ARG and gut microbiota genes. This inconsistency indicates that, compared with other genes, ARG are less likely to appear in Bacteroides, but more likely to exist in Firmicutes and Proteobacteria. Interestingly, among the top twenty most abundant antibiotic resistance gene types, Bacteroides was the main carrier (Figure 4). The top ten contributing bacteria were Bacteroides (28.18%), Prevotella (10.06%), Faecalibacterium (6.43%), Roseburia (3.58%), Bifidobacterium (1.13%), Escherichia (0.93%), Phascolarctobacterium (0.66%), Klebsiella (0.31), Fusobacterium (0.28%), and Pseudomonas (0.03%). Similarly, the main carrier of ARG was Bacteroides (Figure 5).
Figure 4.
Analysis of the main bacterial genera carrying the top 20 antibiotic resistance gene types.
Figure 5.
Major contributors to ARG. The pie chart represents the proportion of intestinal flora rich in ARG. The histograms represent the distribution of antibiotic resistance types in different bacterial genera.
We then characterised the antibiotic resistance types present in these bacteria. We observed that abundant antibiotic resistance types in Bacteroides were tetracycline resistance, followed by MLS. Similar to this, tetracycline was also the main antibiotic resistance type in Roseburia, Fusobacterium, and other unclassified bacteria (48.35%). Meanwhile, the main type of antibiotic resistance in Prevotella and Klebsiella was that of fluoroquinolone. The main type of antibiotic resistance in Faecalibacterium was MLS, followed by aminoglycoside. Remarkably, aminoglycoside was the main antibiotic resistance type in Bifidobacterium, Escherichia, and Shigella and was also widely distributed in Bacteroides, Faecalibacterium, and Roseburia. Lastly, we found that Phascolarctobacterium, Actinobacillus, Lelliottia, Capnocytophaga, and Shigella possessed only one type of antibiotic resistance (Figure 5).
4. Discussion
In this study, we characterised the reservoir of ARG in the human gut microbiota at the metagenomic level. We found that these genes were widespread in the microbiota and were more abundant and diverse in older (Y100 groups) individuals than in younger (Y20 and Y40 groups) ones. Bacteroides was revealed to be the main carrier of ARG, of which tetQ genes were the most abundant group. Antibiotic efflux, inactivation, and target alteration were the dominant mechanisms of resistance.
The detection of a large number of antibiotic resistance genes in the human gut microbiota has a technical reason that we cannot ignore. Due to the rapid development of high-throughput technology, which allows resistance genes that could not be sequenced on plasmids to be sequenced. The rapid change of bioinformatics analysis technology and the improvement of resistance gene database have made the resistance genes that were missed on the plasmids fully excavated. Therefore, the plasmid borne genes may have made a greater contribution.
The problem of microbial resistance to antibiotics can be attributed to several factors. It is an accepted fact that antibiotic abuse is the main reason for the development of resistance [55,56,57]. The difference in ARGs among the different age groups may be explained by the different selection pressures of antibiotics [58]. Based on previous studies, there is a direct correlation between antibiotic use and degree of resistance [59]. Antibiotic treatment disturbs the balance between the human host and its different microbes, leading to the emergence of antibiotic-resistant strains and related diseases [60,61]. In China, the abuse of antibiotics and the resulting problems of resistance are very serious. It is estimated that approximately 75% of seasonal flu patients take antibiotics [62]. In addition, compared with other countries, China has the fastest growth rate of resistance and the highest number and types of ARG [63,64]. It can be inferred that, due to the relatively weak supervision of antibiotics in China in the past few decades, antibiotics have been used in large quantities from childhood and accumulated over the life course. This may partly explain why the gut microbiota of the elderly in China have the largest number of ARG, although there are several other issues that need to be explored and traced, including how these resistance genes are human-related are acquired and spread.
Another contributing factor to the development of resistance are human-related activities, which may also explain how genes are acquired and spread. Antibiotics are widely used in activities such as livestock, agriculture, and aquaculture. Due to the increased demand for protein products, antibiotic use in livestock has increased significantly [65]. The persistent use of antibiotics in these contexts will increase the selective pressure for antibiotic resistance and the emergence of antibiotic-resistant strains, in which significant genetic exchange and recombination can occur and be easily transmitted to humans [66,67,68]. Bacteria from food, farm animals, and human clinical isolates can acquire antibiotic resistance through horizontal gene transfer [69], that is, antibiotic-resistant bacteria and ARG can be transmitted from animals to humans through various channels such as the food chain [70,71]. Forslund et al. demonstrated that the long-term use of antibiotics in livestock is a decisive factor for the high abundance of the ARG in the human gut microbiota [72].
The environment is also an important factor. As a variety of microorganisms are present in the environment, humans may interact with these microorganisms harbouring ARG directly or indirectly [73]. Antibiotics are not completely metabolized in the human body and may escape degradation and be excreted via urine and feces [74]. Since traditional wastewater treatment plants are not specifically designed to remove antibiotics, they are then discharged directly into the environment [75,76]. In addition, applying manure and sludge as fertilizer to soil, coupled with reclaimed water for irrigation, can promote the spread of antibiotics and ARG in the soil. Indeed, it has been shown that most of the ARG and genetic elements found in clinical isolates were also isolated in samples collected from wastewater [77,78]. Soil [79], sewage [80], and even air dust [81] may be important reservoirs involved in the spread of ARG. This suggests that the environment is a huge reservoir involved in the spread of ARG [82].
Finally, it should be noted that the problem of antibiotic resistance is not only due to external factors such as antibiotic abuse, but also internal mechanisms of bacteria that can cause antibiotics resistance—for example, enzymatic inhibition of antibiotic molecules, where bacteria can detoxify antibiotics by producing enzymes that can add specific chemical functional groups or destroy drugs through hydrolysis. Aminoglycoside-modifying enzymes can acetylate, phosphorylate, or adenylate aminoglycoside antibiotics so that they are unable to bind to bacterial ribosomal target sites [83]. Decreased antibiotic penetration can also occur [84]. For instance, a decrease in the number of porins can change the selectivity of the porin channel and limit drug uptake [85]. Efflux pumps are also an efficient antibiotic resistance mechanism as they actively remove antibiotics from the inside of the cell [86]. Lastly, target site modification can change the structure of the target such as the penicillin binding protein in the case of MRSA [87].
Together, these findings suggest that, in the human gut microbiota, the abundance of ARG is an age-related cumulative effect and different gene types are predominant in differently aged individuals. Several factors contribute to the development of ARG in the human gut microbiota; however, the extent to which these genes are affected by the factors needs to be further studied. Transformation of these factors may work synergistically with other factors, such as age, physical sex, and eating habits. Future research should seek to clarify the key role of hosts, carriers, and vectors in the transmission chain and determine the mechanisms that promote the spread of ARG between humans, the environment, and bacteria.
Acknowledgments
We sincerely thank those who contributed to this research, in particular our study subjects and Beijing Novogene Technology Co., Ltd. (Manager Jingwen Ye), without which this study would not have been possible.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics10081006/s1. Figure S1: The top twenty most abundant ARG types in the different age groups. Figure S2: Antimicrobial resistance mechanism of genes in gut microbiota. Figure S3: The box chart represents the distribution of a certain type of antibiotic resistance in the different age groups. Figure S4: Comparison of the distribution of the human gut ARG (inner cycle) and the microbiome gene set (outer cycle) at the bacterial phylum level. The ratios of genes (>1%) assigned to each phylum are shown in the pie charts. Table S1: Categories of drugs and resistance mechanisms.
Author Contributions
L.W.: Data curation, Conceptualization, Methodology, Formal analysis, Investigation, Writing—original draft, Writing–review and editing, Visualization. X.X.: Conceptualization, Methodology, Formal analysis, Investigation, Writing—review and editing. Y.L.: Conceptualization, Methodology, Formal analysis, Investigation. T.L.: Conceptualization, Methodology, Investigation. H.Z.: Conceptualization, Methodology, Formal analysis, Investigation, Visualization. J.M.: Conceptualization, Methodology, Formal analysis, Investigation, Visualization. L.Y.: Methodology, Investigation. J.Y.: Methodology, Investigation. L.L.: Methodology, Investigation. Y.X.: Methodology, Investigation. H.L.: Methodology, Investigation. J.Z.: Conceptualization, Methodology, Resources, Writing—review and editing, Funding acquisition. X.C.: Methodology, Investigation. Y.D.: Conceptualization, Methodology, Formal analysis, Writing—review and editing, Supervision, Validation. Q.W.: Conceptualization, Methodology, Resources, Writing—review and editing, Funding acquisition, Supervision, Validation. All authors have read and agreed to the published version of the manuscript.
Funding
This study was jointly supported by research grants from the Key-Area Research and Development Program of Guangdong Province (2018B020205002), the Guangdong Province Academy of Sciences Special Project for Capacity Building of Innovation Driven Development (2020GDASYL-20200301002), and the Project by the Department of Science and Technology of Guangdong Province (2019QN01N107).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of The First Affiliated Hospital/School of Clinical Medicine of Guangdong Pharmaceutical University (2021(13)).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The authors declare that all the data and the material used in this study are available within this article. All data generated or analysed during this study are available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fleming A. On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae. Br. J. Exp. Pathol. 1929;10:226. doi: 10.1093/clinids/2.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wise R., Hart T., Cars O., Streulens M., Helmuth R., Huovinen P., Sprenger M. Antimicrobial resistance: Is a major threat to public health. Brit. Med. J. 1998;317:609. doi: 10.1136/bmj.317.7159.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammerum A.M., Toleman M.A., Hansen F., Kristensen B., Lester H., Walsh T.R., Fuursted K. Global spread of new delhi metallo-β-lactamase 1. Lancet Infect. Dis. 2010;10:829–830. doi: 10.1016/S1473-3099(10)70276-0. [DOI] [PubMed] [Google Scholar]
- 4.McKenna M. Antibiotic resistance: The last resort. Nature. 2013;499:394–396. doi: 10.1038/499394a. [DOI] [PubMed] [Google Scholar]
- 5.Riccardi G., Pasca M.R., Buroni S. Mycobacterium tuberculosis: Drug resistance and future perspectives. Future Microbiol. 2009;4:597–614. doi: 10.2217/fmb.09.20. [DOI] [PubMed] [Google Scholar]
- 6.Hiramatsu K., Cui L., Kuroda M., Ito T. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2001;9:486–493. doi: 10.1016/S0966-842X(01)02175-8. [DOI] [PubMed] [Google Scholar]
- 7.Goossens H., Ferech M., Stichele R.V., Elseviers M. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet. 2005;365:579–587. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 8.Lynch S.V., Pedersen O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016;375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 9.Wright G.D. The antibiotic resistome: The nexus of chemical and genetic diversity. Nat. Rev. Microbiol. 2007;5:175–186. doi: 10.1038/nrmicro1614. [DOI] [PubMed] [Google Scholar]
- 10.Forslund K., Hildebrand F., Nielsen T., Falony G., Le C.E., Sunagawa S., Prifti E., Vieira-Silva S., Gudmundsdottir V., Pedersen H.K., et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528:262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Groot P., Nikolic T., Pellegrini S., Sordi V., Imangaliyev S., Rampanelli E., Hanssen N., Attaye I., Bakker G., Duinkerken G., et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Gut. 2021;70:92–105. doi: 10.1136/gutjnl-2020-322630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin J.J., Li Y.R., Cai Z.M., Li S.H., Zhu J.F., Zhang F., Liang S.S., Zhang W.W., Guan Y.L., Shen D.Q., et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 13.Hsiao E.Y., McBride S.W., Hsien S., Sharon G., Hyde E.R., McCue T., Codelli J.A., Chow J., Reisman S.E., Petrosino J.F., et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreira R.E., de Carvalho L.M., Reis D.C., Cassali G.D., Faria A.M.C., Maioli T.U., Brunialti-Godard A.L. Diet-induced obesity leads to alterations in behavior and gutmicrobiota composition in mice. J. Nutr. Biochem. 2021;92:108622. doi: 10.1016/j.jnutbio.2021.108622. [DOI] [PubMed] [Google Scholar]
- 15.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 16.Craven M., Egan C.E., Dowd S.E., McDonough S.P., Dogan B., Denkers E.Y., Bowman D., Scherl E.J., Simpson K.W. Inflammation drives dysbiosis and bacterial invasion in murine models of ileal Crohn’s disease. PLoS ONE. 2012;7:e41594. doi: 10.1371/journal.pone.0041594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manges A.R., Labbe A., Loo V.G., Atherton J.K., Behr M.A., Masson L., Tellis P.A., Brousseau R. Comparative metagenomic study of alterations to the intestinal microbiota and risk of nosocomial Clostridum difficile-associated disease. J. Infect. Dis. 2010;202:1877–1884. doi: 10.1086/657319. [DOI] [PubMed] [Google Scholar]
- 18.Aira A., Feher C., Rubio E., Soriano A. The intestinal microbiota as a reservoir and a therapeutic target to fight multi-drug-resistant bacteria: A narrative review of the literature. Infect. Dis. Ther. 2019;8:469–482. doi: 10.1007/s40121-019-00272-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Schaik W. The human gut resistome. Phil. Trans. R. Soc. B. 2015;370:20140087. doi: 10.1098/rstb.2014.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jernberg C., Lofmark S., Edlund C., Jansson J. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010;156:3216–3223. doi: 10.1099/mic.0.040618-0. [DOI] [PubMed] [Google Scholar]
- 21.Florez A.B., Ammor M.S., Alvarez-Martin P., Margolles A., Mayo B. Molecular analysis of tet(W) gene-mediated tetracycline resistance in dominant intestinal Bifidobacterium species from healthy humans. Appl. Environ. Microbiol. 2006;72:7377–7379. doi: 10.1128/AEM.00486-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seville L.A., Patterson A.J., Scott K.P., Mullany P., Quail M.A., Parkhill J., Ready D., Wilson M., Spratt D., Roberts A.P. Distribution of tetracycline and erythromycin resistance genes among human oral and fecal metagenomic DNA. Microb. Drug Resist. 2009;15:159–166. doi: 10.1089/mdr.2009.0916. [DOI] [PubMed] [Google Scholar]
- 23.Lu N., Hu Y.F., Zhu L.Y., Yang X., Yin Y.S., Lei F., Zhu Y.L., Du Q., Wang X., Meng Z.Q., et al. DNA microarray analysis reveals that antibiotic resistance-gene diversity in human gut microbiota is age related. Sci. Rep. UK. 2014;4:4302. doi: 10.1038/srep04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu Y., Sun S., Huang Y., Gao Q., Xie X., Wang P., Li J., Liang L., He X., Jiang Y., et al. Metagenomic analysis revealed the potential role of gut microbiome in gout. NPJ Biofilms Microbi. 2021;7:66. doi: 10.1038/s41522-021-00235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forsberg K.J., Patel S., Gibson M.K., Lauber C.L., Knight R., Fierer N., Dantas G. Bacterial phylogeny structures soil resistomes across habitats. Nature. 2014;509:612–616. doi: 10.1038/nature13377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forslund K., Sunagawa S., Coelho L.P., Bork P. Metagenomic insights into the human gut resistome and the forces that shape it. Bioessays. 2014;36:316–329. doi: 10.1002/bies.201300143. [DOI] [PubMed] [Google Scholar]
- 27.Pehrsson E.C., Tsukayama P., Patel S., Mejia-Bautista M., Sosa-Soto G., Navarrete K.M., Alderon M.C., Abrera L.C., Hoyos-Arango W., Bertoli M.T., et al. Interconnected microbiomes and resistomes in low-income human habitats. Nature. 2016;533:212–216. doi: 10.1038/nature17672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li B., Yang Y., Ma L.P., Ju F., Guo F., Tiedje J.M., Zhang T. Metagenomic and network analysis reveal wide distribution and co-occurrence of environmental antibiotic resistance genes. ISME J. 2015;9:2490–2502. doi: 10.1038/ismej.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y., Jiang X.T., Chai B.L., Ma L.P., Li B., Zhang A.N., Cole J.R., Tiedje J.M., Zhang T. ARGs-OAP: Online analysis pipeline for antibiotic resistance genes detection from metagenomic data using an integrated structured ARG-database. Bioinformatics. 2016;32:2346–2351. doi: 10.1093/bioinformatics/btw136. [DOI] [PubMed] [Google Scholar]
- 30.Sommer M.O.A., Dantas G., Church G.M. Functional characterization of the antibiotic resistance reservoir in the human microflora. Science. 2009;325:1128–1131. doi: 10.1126/science.1176950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu B., Pop M. ARDB—Antibiotic Resistance Genes Database. Nucleic Acids Res. 2009;37:D443–D447. doi: 10.1093/nar/gkn656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McArthur A.G., Waglechner N., Nizam F., Yan A., Azad M.A., Baylay A.J., Bhullar K., Canova M.J., De Pascale G., Ejim L., et al. The comprehensive antibiotic resistance database. Antimicrob. Agents Chemother. 2013;57:3348–3357. doi: 10.1128/AAC.00419-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu Y.F., Yang X., Qin J.J., Lu N., Cheng G., Wu N., Pan Y.L., Li J., Zhu L.Y., Wang X., et al. Metagenome-wide analysis of antibiotic resistance genes in a large cohort of human gut microbiota. Nat. Commun. 2013;4:2151. doi: 10.1038/ncomms3151. [DOI] [PubMed] [Google Scholar]
- 34.Karlsson F.H., Tremaroli V., Nookaew I., Bergstrom G., Behre C.J., Fagerberg B., Nielsen J., Backhed F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 35.Karlsson F.H., Fak F., Nookaew I., Tremaroli V., Fagerberg B., Petranovic D., Backhed F., Nielsen J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012;3:1245. doi: 10.1038/ncomms2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scher J.U., Sczesnak A., Longman R.S., Segata N., Ubeda C., Bielski C., Rostron T., Cerundolo V., Pamer E.G., Abramson S.B., et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202. doi: 10.7554/eLife.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin N., Yang F.L., Li A., Prifti E., Chen Y.F., Shao L., Guo J., Le Chatelier E., Yao J., Wu L.J., et al. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59–64. doi: 10.1038/nature13568. [DOI] [PubMed] [Google Scholar]
- 38.Feng Q., Liang S.S., Jia H.J., Stadlmayr A., Tang L.Q., Lan Z., Zhang D.Y., Xia H.H., Xu X.Y., Jie Z.Y., et al. Gut microbiome development along the colorectal adenoma–carcinoma sequence. Nat. Commun. 2015;6:6528. doi: 10.1038/ncomms7528. [DOI] [PubMed] [Google Scholar]
- 39.Brum J.R., Ignacio-Espinoza J.C., Roux S., Doulcier G., Acinas S.G., Alberti A., Chaffron S., Cruaud C., de Vargas C., Gasol J.M., et al. Patterns and ecological drivers of ocean viral communities. Science. 2015;348:1261498. doi: 10.1126/science.1261498. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen H.B., Almeida M., Juncker A.S., Rasmussen S., Li J.H., Sunagawa S., Plichta D.R., Gautier L., Pedersen A.G., Le Chatelier E., et al. Identification and assembly of genomes and genetic elements in complex metagenomic samples without using reference genomes. Nat. Biotechnol. 2014;32:822–828. doi: 10.1038/nbt.2939. [DOI] [PubMed] [Google Scholar]
- 41.Qin J.J., Li R.Q., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeller G., Tap J., Voigt A.Y., Sunagawa S., Kultima J.R., Costea P.I., Amiot A., Bohm J., Brunetti F., Habermann N., et al. Potential of fecal microbiota for early-stage detection of colorectal cancer. Mol. Syst. Boil. 2014;10:766. doi: 10.15252/msb.20145645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sunagawa S., Coelho L.P., Chaffron S., Kultima J.R., Labadie K., Salazar G., Djahanschiri B., Zeller G., Mende D.R., Alberti A., et al. Structure and function of the global ocean microbiome. Science. 2015;348:1261359. doi: 10.1126/science.1261359. [DOI] [PubMed] [Google Scholar]
- 44.Li J.H., Jia H.J., Cai X.H., Zhong H.Z., Feng Q., Sunagawa S., Arumugam M., Kultima J.R., Prifti E., Nielsen T., et al. An integrated catalog of reference genes in the human gut microbiome. Nat. Biotechnol. 2014;32:834–841. doi: 10.1038/nbt.2942. [DOI] [PubMed] [Google Scholar]
- 45.Mende D.R., Waller A.S., Sunagawa S., Jaervelin A.I., Chan M.M., Arumugam M., Raes J., Bork P. Assessment of metagenomic assembly using simulated next generation sequencing data. PLoS ONE. 2012;7:e31386. doi: 10.1371/journal.pone.0031386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oh J., Byrd A.L., Deming C., Conlan S., Kong H.H., Segre J.A. Biogeography and individuality shape function in the human skin metagenome. Nature. 2014;514:59–64. doi: 10.1038/nature13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu W.H., Lomsadze A., Borodovsky M. Ab initio gene identification in metagenomic sequences. Nucleic Acids Res. 2010;38:e132. doi: 10.1093/nar/gkq275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li W.Z., Godzik A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22:1658–1659. doi: 10.1093/bioinformatics/btl158. [DOI] [PubMed] [Google Scholar]
- 49.Fu L.M., Niu B.F., Zhu Z.W., Wu S.T., Li W.Z. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics. 2012;28:3150–3152. doi: 10.1093/bioinformatics/bts565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buchfink B., Xie C., Huson D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods. 2015;12:59–60. doi: 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
- 51.Huson D.H., Mitra S., Ruscheweyh H.J., Weber N., Schuster S.C. Integrative analysis of environmental sequences using MEGAN4. Genome Res. 2011;21:1552–1560. doi: 10.1101/gr.120618.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jia B.F., Raphenya A.R., Alcock B., Waglechner N., Guo P.Y., Tsang K.K., Lago B.A., Dave B.M., Pereira S., Sharma A.N., et al. CARD 2017: Expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2017;45:D566. doi: 10.1093/nar/gkw1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alcock B.P., Raphenya A.R., Lau T.T.Y., Tsang K.K., Bouchard M., Edalatmand A., Huynh W., Nguyen A.L.V., Cheng A.A., Liu S., et al. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020;48:517–525. doi: 10.1093/nar/gkz935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shoemaker N.B., Vlamakis H., Hayes K., Salyers A.A. Evidence for extensive resistance gene transfer among Bacteroides spp. and among Bacteroides and other genera in the human colon. Appl. Environ. Microbiol. 2001;67:561–568. doi: 10.1128/AEM.67.2.561-568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bansal R., Jain A., Goyal M., Singh T., Sood H., Malviya H.S. Antibiotic abuse during endodontic treatment: A contributing factor to antibiotic resistance. J. Fam. Med. Prim. Care. 2019;8:3518–3524. doi: 10.4103/jfmpc.jfmpc_768_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cars O., Molstad S., Melander A. Variation in antibiotic use in the European Union. Lancet. 2001;357:1851–1853. doi: 10.1016/S0140-6736(00)04972-2. [DOI] [PubMed] [Google Scholar]
- 57.Gasparrini A.J., Wang B., Sun X.Q., Kennedy E.A., Hernandez-Leyva A., Ndao I.M., Tarr P.I., Warner B.B., Dantas G. Persistent metagenomic signatures of early-life hospitalization and antibiotic treatment in the infant gut microbiota and resistome. Nat. Microbiol. 2019;4:2285–2297. doi: 10.1038/s41564-019-0550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bronzwaer S.L.A.M., Cars O., Buchholz U., Molstad S., Goettsch W., Veldhuijzen I.K., Kool J.L., Sprenger M.J.W., Degener J.E. A European study on the relationship between antimicrobial use and antimicrobial resistance. Emerg. Infect. Dis. 2002;8:278–282. doi: 10.3201/eid0803.010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laxminarayan R., Matsoso P., Pant S., Brower C., Rottingen J.A., Klugman K., Davies S. Access to effective antimicrobials: A worldwide challenge. Lancet. 2016;387:168–175. doi: 10.1016/S0140-6736(15)00474-2. [DOI] [PubMed] [Google Scholar]
- 60.Schwartz D.J., Langdon A.E., Dantas G. Understanding the impact of antibiotic perturbation on the human microbiome. Genome Med. 2020;12:82. doi: 10.1186/s13073-020-00782-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mannion A., Dzink-Fox J., Shen Z.L., Piazuelo M.B., Wilson K.T., Correa P., Peek R.M., Camargo M.C., Fox J.G. Helicobacter pylori antimicrobial resistance and gene variants in high- and low-gastric-cancer-risk populations. J. Clin. Microbiol. 2021;59:e03203-20. doi: 10.1128/JCM.03203-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heddini A., Cars O., Qiang S., Tomson G. Antibiotic resistance in China—A major future challenge. Lancet. 2009;373:30. doi: 10.1016/S0140-6736(08)61956-X. [DOI] [PubMed] [Google Scholar]
- 63.Zhang R.F., Eggleston K., Rotimi V., Zeckhauser R.J. Antibiotic resistance as a global threat: Evidence from China, Kuwait and the United States. Glob. Health. 2006;2:6. doi: 10.1186/1744-8603-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feng J., Li B., Jiang X.T., Yang Y., Wells G., Zhang T., Li X.Y. Antibiotic Resistome in a large-scale healthy human gut microbiota deciphered by metagenomic and network analyses. Environ. Microbiol. 2018;20:355–368. doi: 10.1111/1462-2920.14009. [DOI] [PubMed] [Google Scholar]
- 65.Schar D., Sommanustweechai A., Laxminarayan R., Tangcharoensathien V. Surveillance of antimicrobial consumption in animal production sectors of low-and middle-income countries: Optimizing use and addressing antimicrobial resistance. PLoS Med. 2018;15:e1002521. doi: 10.1371/journal.pmed.1002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laxminarayan R., Duse A., Wattal C., Zaidi A.K.M., Wertheim H.F.L., Sumpradit N., Vlieghe E., Hara G.L., Gould I.M., Goossens H., et al. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013;13:1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 67.Vanderhaeghen W., Dewulf J. Antimicrobial use and resistance in animals and human beings. Lancet Planet. Health. 2017;1:e307–e308. doi: 10.1016/S2542-5196(17)30142-0. [DOI] [PubMed] [Google Scholar]
- 68.Watts J.E.M., Schreier H.J., Lanska L., Hale M.S. The rising tide of antimicrobial resistance in aquaculture: Sources, sinks and solutions. Mar. Drugs. 2017;15:158. doi: 10.3390/md15060158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smillie C.S., Smith M.B., Friedman J., Cordero O.X., David L.A., Alm E.J. Ecology drives a global network of gene exchange connecting the human microbiome. Nature. 2011;480:241–244. doi: 10.1038/nature10571. [DOI] [PubMed] [Google Scholar]
- 70.Kumar S.B., Arnipalli S.R., Ziouzenkova O. Antibiotics in food chain: The consequences for antibiotic resistance. Antibiot. Basel. 2020;9:688. doi: 10.3390/antibiotics9100688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Founou L.L., Founou R.C., Essack S.Y. Antimicrobial resistance in the farm-to-plate continuum: More than a food safety issue. Future Sci. OA. 2021;7:FSO692. doi: 10.2144/fsoa-2020-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Forslund K., Sunagawa S., Kultima J.R., Mende D.R., Arumugam M., Typas A., Bork P. Country-specific antibiotic use practices impact the human gut resistome. Genome Res. 2013;23:1163–1169. doi: 10.1101/gr.155465.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rothschild D., Weissbrod O., Barkan E., Kurilshikov A., Korem T., Zeevi D., Costea P.I., Godneva A., Kalka I.N., Bar N., et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555:210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 74.Michael C.A., Dominey-Howes D., Labbate M. The antimicrobial resistance crisis: Causes, consequences, and management. Front. Public Health. 2014;2:145–153. doi: 10.3389/fpubh.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grenni P., Ancona V., Caracciolo A. Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J. 2018;136:25–39. doi: 10.1016/j.microc.2017.02.006. [DOI] [Google Scholar]
- 76.Stachurova T., Pikova H., Bartas M., Semerad J., Svobodova K., Malachova K. Beta-lactam resistance development during the treatment processes of municipal wastewater treatment plants. Chemosphere. 2021;280:130749. doi: 10.1016/j.chemosphere.2021.130749. [DOI] [PubMed] [Google Scholar]
- 77.Szczepanowski R., Linke B., Krahn I., Gartemann K.H., Guetzkow T., Eichler W., Puehler A., Schlueter A. Detection of 140 clinically relevant antibiotic-resistance genes in the plasmid metagenome of wastewater treatment plant bacteria showing reduced susceptibility to selected antibiotics. Microbiology. 2009;155:2306–2319. doi: 10.1099/mic.0.028233-0. [DOI] [PubMed] [Google Scholar]
- 78.Rizzo L., Manaia C., Merlin C., Schwartz T., Dagot C., Ploy M.C., Michael I., Fatta-Kassinos D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013;447:345–360. doi: 10.1016/j.scitotenv.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 79.Forsberg K.J., Reyes A., Wang B., Selleck E.M., Sommer M.O.A., Dantas G. The shared antibiotic resistome of soil bacteria and human pathogens. Science. 2012;337:1107–1111. doi: 10.1126/science.1220761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vaz-Moreira I., Nunes O.C., Manaia C.M. Bacterial diversity and antibiotic resistance in water habitats: Searching the links with the human microbiome. FEMS Microbiol. Rev. 2014;38:761–778. doi: 10.1111/1574-6976.12062. [DOI] [PubMed] [Google Scholar]
- 81.Muzslay M., Moore G., Turton J.F., Wilson A.P. Dissemination of antibiotic-resistant enterococci within the ward environment: The role of airborne bacteria and the risk posed by unrecognized carriers. Am. J. Infect. Control. 2013;41:57–60. doi: 10.1016/j.ajic.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 82.Zhuang M., Achmon Y., Cao Y.P., Liang X.M., Chen L., Wang H., Siame B.A., Leung K.Y. Distribution of antibiotic resistance genes in the environment. Environ. Pollut. 2021;285:117402. doi: 10.1016/j.envpol.2021.117402. [DOI] [PubMed] [Google Scholar]
- 83.Peterson E., Kaur P. Antibiotic resistance mechanisms in bacteria: Relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Front. Microbiol. 2018;9:2928. doi: 10.3389/fmicb.2018.02928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pages J.M., James C.E., Winterhalter M. The porin and the permeating antibiotic: A selective diffusion barrier in Gram-negative bacteria. Nat. Rev. Microbiol. 2008;6:893–903. doi: 10.1038/nrmicro1994. [DOI] [PubMed] [Google Scholar]
- 85.Kumar A., Schweizer H.P. Bacterial resistance to antibiotics: Active efflux and reduced uptake. Adv. Drug Deliv. Rev. 2005;57:1486–1513. doi: 10.1016/j.addr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 86.Rahman T., Yarnall B., Doyle D.A. Efflux drug transporters at the forefront of antimicrobial resistance. Eur. Biophys. J. 2017;46:647–653. doi: 10.1007/s00249-017-1238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pazos M., Vollmer W. Regulation and function of class A Penicillin-binding proteins. Curr. Opin. Microbiol. 2021;60:80–87. doi: 10.1016/j.mib.2021.01.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all the data and the material used in this study are available within this article. All data generated or analysed during this study are available from the corresponding authors upon reasonable request.