Abstract
High-throughput DNA sequencing technologies have facilitated the in silico forensic analysis of human microbiome. Specific microbial species or communities obtained from the crime scene provide evidence of human contacts and their body fluids. The microbial community is influenced by geographic, ethnic, lifestyle, and environmental factors such as urbanization. An understanding of the effects of these external stressors on the human microbiome and determination of stable and changing elements are important in selecting appropriate targets for investigation. In this study, the Forensic Microbiome Database (FMD) (http://www.fmd.jcvi.org) containing the microbiome data of various locations in the human body in 35 countries was used. We focused on skin, saliva, vaginal fluid, and stool and found that the microbiome distribution differed according to the body part as well as the geographic location. In the case of skin samples, Staphylococcus species were higher than Corynebacterium species among Asians compared with Americans. Holdemanella and Fusobacterium were specific in the saliva of Koreans and Japanese populations. Lactobacillus was found in the vaginal fluids of individuals in all countries, whereas Serratia and Enterobacter were endemic to Bolivia and Congo, respectively. This study is the first attempt to collate and describe the observed variation in microbiomes from the forensic microbiome database. As additional microbiome databases are reported by studies worldwide, the diversity of the applications may exceed and expand beyond the initial identification of the host.
Keywords: human microbiome, geography, ethnicity, body fluid, sequencing platform, forensic investigation
Introduction
Microorganisms are ubiquitous on all terrestrial and aquatic environments. Microbes have played an important role in medicine, fermentation, and food industry for millennia but have not generally been exploited in forensic medicine (Alan and Sarah, 2012). However, molecular biology tools such as DNA fingerprinting, whole genome sequencing, and microarray analysis have significantly advanced the field of microbial forensics over the last two decades (Cummings and Relman, 2002; Li et al., 2012; Kuiper, 2016; Schmedes and Budowle, 2019). Massively parallel sequencing (MPS) was applied to the routine analysis of microbial forensic evidence (Turnbaugh et al., 2007; Human Microbiome Project Consortium, 2012). MPS, which is also known as next-generation sequencing (NGS), enables the detection of low levels of microorganisms and unknown pathogens even in mixed samples (Schmedes et al., 2016; Singh and Kaur, 2020). With the reduction in sequencing costs and the continued development of bioinformatics analysis via increased throughput, MPS has been used to characterize the microbial community for forensic applications (Hachem et al., 2020).
Forensic analysis using microbiome data obtained from suspects, victims, and the environment has the potential to support crime scene investigations (Clarke et al., 2017). The microbiome data can be used as potential evidence for human identification (Woerner et al., 2019), detection of body fluids (Dobay et al., 2019), and geographic location of individuals (Grantham et al., 2019). The human microbiome can be collected from locations such as human skin (Schmedes et al., 2018) and body fluids (Dobay et al., 2019), houses (Song et al., 2013), dorm rooms (Luongo et al., 2017), classrooms (Kembel et al., 2014), offices (Chase et al., 2016), bathrooms, (Flores et al., 2011), and personal belongings like shoes (Lax et al., 2015), keyboards (Lee et al., 2015), fabrics (Lee et al., 2016), and cellphones (Lax et al., 2015). Body fluids and skin swabs collected from individual populations exhibit specific characteristics. The present review shows descriptions of forensic indicator sites, including skin, vaginal fluid, stool, and saliva from populations in diverse geographical locations. Sequencing of the microbiome obtained from diverse locations demonstrated that bacterial DNA can be used to indicate individual lifestyle and behavioral patterns (Clarke et al., 2017). Thus, microbiomes provide geographic, ethnic, or lifestyle information that can be used by forensic scientists to identify the origin of unknown samples during criminal investigation. Also, evidence suggests that the combination of microbiology, molecular biology, and forensic investigations may enable the identification of the suspect and the victim based on human microbiome samples found at a crime scene, especially when traditional sources of human DNA are insufficient (Richardson et al., 2019; Phan et al., 2020).
The differences between individuals can be determined based on single sample studies; however, the forensic examination is limited by the sample size and scope, such as the availability of only one metadata variable or location (Singh et al., 2021). In this study, the Forensic Microbiome Database (FMD) (http://www.fmd.jcvi.org) was used to obtain the comprehensive microbiome data of various body sites. FMD represents a database containing the microbiomes from 35 countries (139 cities) and links publicly available 16S rRNA gene-derived taxa with their geographic origins, often at the level of the city (Clarke et al., 2017). Since an individual’s microbiome is in part influenced by geography, diet, ethnicity, and degree of urbanization, the knowledge of specific microbiome composition in a population and/or environment can facilitate the utilization of such data in determining the host’s geographical location. In addition, since crime can be transnational, the geographical location determined using the microbiome data can facilitate forensic investigations. Our review represents a timely contribution to state-of-the-art forensic investigatory tools used to analyze crime scene investigations.
Forensic Applications of Microbiome
Microbiome analysis has been utilized in human health and disease as well as forensic science aided by advanced computational tools in bioinformatics analysis, using refined methodology, sampling, and library preparation (Robinson et al., 2021). The use of microbiome analysis from different sets of body sites and diverse environments, specifically in the forensic field, can be used to establish the geographic location of criminal events and determine the cause of death and the identity of individuals (Clarke et al., 2017; García et al., 2020). For instance, skin microbiomes are unique to individuals, and thus used to determine personal identity. Also, the distinct microbiome of individual body sites provides valuable evidence in criminal investigations, such as sexual assaults (Schmedes et al., 2017). Meanwhile, specific microorganisms such as Helicobacter pylori can be linked to a host or environment since they are functionally unique with a distinct composition based on geographic location (Kersulyte et al., 2010; Nagasawa et al., 2013). Thus, the diverse location across different climate zones can also be accurately explained by the microbiome and can be used to predict the suspect’s identity (Chase et al., 2016). A recent study even identified the urban microbiomes and antimicrobial resistance genes, which reflect the significant features of cities, and highlighted their forensic applications (Danko et al., 2021).
The field application of forensic microbiome analysis requires the evaluation of its effectiveness and validation of its stability and accuracy. In addition, many basic studies are needed to demonstrate the potential role of microbial community profiles in the forensic application. Therefore, forensic microbiome analysis is indispensable as microevidence although additional studies and evaluation of techniques used in forensic microbiome analysis are required, under circumstances where criminals leave no fingerprints, blood, saliva, semen, or other bodily fluids that can be analyzed using the extracted DNA samples (Eom, 2018).
Accordingly, the stability of the microbial community over time or with translocation is a distinct advantage that cannot be overlooked in microbial forensics. Indeed, the forensic application of microbiome is strongly linked to the stability of the microbiome (Tozzo et al., 2020). The microbiome collected by swab remains stable until 2 weeks under typical indoor conditions (Fierer et al., 2010). Individuals can be distinguished based on their microbial profiles, especially gut microbiome even up to a year (Franzosa et al., 2015). Analysis of bacterial translocation has been mostly used to define uncertain causes of death. The swift translocation of intestinal bacteria is triggered immediately after death and can be used to distinguish contaminants from established microbiota. Heimesaat et al. suggested that the kinetics of Enterobacteriaceae and Enterococcus can serve as an indicator to interpret the time of death (Heimesaat et al., 2012). Also, the translocation of Clostridium in the internal organs of humans postmortem was identified (Tuomisto et al., 2013; Javan et al., 2016; García et al., 2020). The critical role of forensic microbiology in the prevention of unresolved issues and analysis of evidence beyond a reasonable doubt will increase. In this review, we will discuss the five most researched themes: skin, saliva, vaginal fluid, stool, and sequencing platforms.
The Forensic Microbiome Database
This study was accomplished using the FMD described at http://www.fmd.jcvi.org and reported in a recent study (Singh et al., 2021). In brief, the FMD contains publicly available 16S rRNA gene sequence data obtained from multiple body sites (Clarke et al., 2017). The FMD sequence data are derived from samples obtained from healthy adults (≥18 years) across multiple body sites based on 95 projects with 79 PubMed references. Also, the FMD project additionally incorporated oral and stool samples derived from a total of 161 women from Barbados, Santiago, Pretoria, and Bangkok. Sequence data were also downloaded from public websites including NCBI SRA (https://www.ncbi.nlm.nih.gov/sra), EBI (https://www.ebi.ac.uk/), and MG-RAST (https://www.mg-rast.org/), which were all accessed on July 13, 2021 and then analyzed (Singh et al., 2021). The collected data were analyzed in the following order. Using the UPARSE pipeline, the taxonomic population distribution for each sequence from the public dataset was analyzed with the FMD. Using machine learning techniques, the bacterial taxa, which can be best distinguished by geographical location, were identified. In summary, the FMD data include body site location, microbial count, and geographic information (country, subdivision, i.e., the state or department or province, and city). A complete list of available data, including studies, populations, and counts, is found online (http://www.fmd.jcvi.org). The publicly available FMD will attract additional data. The forensic microbiome analyses will be strengthened by the inclusion of further microbiome data from various studies worldwide. A review of FMD based on other studies included in this review may motivate other investigators interested in using the FMD data and expanding the database.
Skin
The healthy skin microbiome exhibits substantial taxonomic diversity, which is dependent on both geography and individuality (Oh et al., 2014). Skin bacteria are affected by age, gender, and external environmental factors, including moisture, temperature, and geographical location. Skin microbial signatures may remain for protracted periods on surfaces or objects in contact with humans (Gupta et al., 2017). Thus, the skin, which is the largest organ of the human body, contains significantly higher bacterial, viral, and fungal diversity than in other locations such as the gut, blood, or saliva (Oh et al., 2014). The diversity of skin microbiome depends on the taxonomic depth of the bioinformatics analysis and the discriminating power. Thus, while it is possible to define the various populations based only on the bacterial phylum, the analysis of microbial community is comparable with DNA-based analysis if it is performed at the level of genus and species (Tozzo et al., 2020). Accordingly, the structure of the human skin microbiome is unique to the individual (Flores et al., 2014). Furthermore, it was suggested that the skin microbiome can be obtained readily from the crime scene providing an alternative line of investigation (Neckovic et al., 2020).
We analyzed four skin samples from four different countries listed in the FMD (Table 1), and 16 samples from 10 countries, which were not included in the FMD (Table 2). A previous study reported the relative abundance of taxa on the hand microbiome and identified four main phyla (Firmicutes, Actinobacteria, Proteobacteria, and Bacteroidetes) (Edmonds-Wilson et al., 2015). They also reported that most individuals carried between eight and 24 families of bacteria dwelling on their hands, including the major taxa (Staphylococcaceae, Corynebacteriaceae, Propionibacteriaceae, and Streptococcaceae) (Edmonds-Wilson et al., 2015). Above all, Propionibacteriaceae, which is relatively higher in abundance, and Cutibacterium acnes (formerly Propionibacterium acnes), are both established commensal bacteria found in the skin, similar to Staphylococcus. Also, C. acnes resides at all body sites, with relative abundances ranging from 35% to 89% (Schmedes et al., 2017). These data suggest that C. acnes may play an informative role in forensic investigation using skin microbiomes. Even though a core microbiome was observed on the hand, pronounced intra- and interindividual differences in bacterial community composition were observed (Siqueira et al., 2012). Based on FMD data, Propionibacterium and Streptococcus dominate the microbial community in the United States, representing typical hand microbial clusters (Figure 1). However, although expected to be ubiquitous among humans, Propionibacterium was not detected in most hand microbial samples in South Korea and Yokohama (Japan) (Table 1).
Table 1.
Distribution of dominant bacteria (over 10%) by countries of four body areas including skin, saliva, vagina, and stool from the Forensic Microbiome Database (FMD).
| Sample | Country | Dominant bacteria | Note | Sample size (N) |
|---|---|---|---|---|
| Skin | Belgium | Corynebacterium, Staphylococcu, Moraxella | Ghent | 9 |
| Japan | Bifidobacterium | Yokohama | 29 | |
| USA | Streptococcus, Propionibacterium | Boulder | 76 | |
| Canada | Bacillus, Streptococcus, Propionibacterium | Waterloo | 1 | |
| Saliva | India | Streptococcus | Paderu | 9 |
| Streptococcus | Guwahati | 10 | ||
| Streptococcus, Veillonella, Prevotella | Jammu | 12 | ||
| Streptococcus, Porphyromonas | Dhanbad | 9 | ||
| Streptococcus, Veillonella, Prevotella | Tamil Nadu | 10 | ||
| Streptococcus, Porphyromonas | Telangana | 12 | ||
| Streptococcus, Prevotella | Uttarakhand | 11 | ||
| Streptococcus | West Bengal | 14 | ||
| Italy | Streptococcus, Prevotella, Neisseria | Trento | 13 | |
| Japan | Fusobacterium, Collinsella | Yokohama | 23 | |
| Collinsella, Granulicatella | Fukuoka | 177 | ||
| Bifidobacterium, Blautia | Hisayama | 2,370 | ||
| South Korea | Holdemanella, Streptococcus, Neisseria, Bifidobacterium | Sudogwon | 103 | |
| USA | Haemophilus, Streptococcus, Veillonella, Campylobacter | Minnesota_Mankato | 3 | |
| Streptococcus, Veillonella | Missouri | 116 | ||
| Streptococcus | Ohio_Columbus | 25 | ||
| Streptococcus, Veillonella, Prevotella 7 | Oklahoma_Tulsa | 1 | ||
| Haemophilus, Streptococcus, Neisseria | Texas_Austin | 5 | ||
| Haemophilus, Streptococcus, Veillonella | Texas_Houston | 240 | ||
| Vagina | Australia | Bifidobacterium | 67 | |
| Italy | Bifidobacterium | 16 | ||
| South Africa | Bifidobacterium | 131 | ||
| Vaginal introitus-mid Vagina | USA | Lactobacillus | Minnesota | 2 |
| Lactobacillus | Missouri | 88 | ||
| Lactobacillus | Oklahoma | 2 | ||
| Lactobacillus | Texas | 105 | ||
| Stool | Argentina | Bacteroides | Rosario | 39 |
| Australia | Bacteroides | Brisbane | 39 | |
| Belgium | Bacteroides | Ghent | 45 | |
| Burkina Faso | Streptococcus | Boulpon | 2 | |
| Canada | Bacteroides | Montreal | 35 | |
| Bacteroides | Nunavut | 49 | ||
| Chile | Bacteroides, Prevotella | Santiago | 109 | |
| China | Bacteroides, Bifidobacterium, Faecalibacterium | Beijing | 27 | |
| Bacteroides, Bifidobacterium | Lanzhou | 23 | ||
| Prevotella, Phascolarctobacterium | Baise | 15 | ||
| Phascolarctobacterium, Blautia | Nanning | 16 | ||
| Phascolarctobacterium, Blautia | Harbin | 21 | ||
| Phascolarctobacterium | Zhengzhou | 18 | ||
| Bacteroides, Phascolarctobacterium | Hohhot | 19 | ||
| Bacteroides, Phascolarctobacterium | Xilinguole | 21 | ||
| Phascolarctobacterium | Wuxi | 18 | ||
| Phascolarctobacterium, Blautia, Megamonas | Chengdu | 11 | ||
| Phascolarctobacterium, Prevotella | Lhasa | 10 | ||
| Phascolarctobacterium, Prevotella | Nagqu | 26 | ||
| Phascolarctobacterium | Altay | 3 | ||
| Phascolarctobacterium | Urumqi | 13 | ||
| Phascolarctobacterium, Megamonas | Yili Prefecture | 6 | ||
| Phascolarctobacterium, Megamonas | Dali Bai Autonomous Prefecture | 250 | ||
| Megamonas, Prevotella | Kunming | 12 | ||
| Gambia | Prevotella, Subdoligranulum | Banjul | 125 | |
| Germany | Akkermansia, Bacteroides | Goettingen | 15 | |
| Ghana | Akkermansia, Bacteroides, Subdoligranulum, Ruminococcus | Eikwe | 5 | |
| India | Prevotella | Baksa | 5 | |
| Prevotella | Golaghat | 5 | ||
| Prevotella | Karbi anglong | 5 | ||
| Prevotella | Tinsukia | 10 | ||
| Prevotella | Imphal East | 1 | ||
| Prevotella | Imphal West | 3 | ||
| Prevotella | Senapati | 5 | ||
| Prevotella | Ukhrul | 5 | ||
| Prevotella | East Sikkim | 8 | ||
| Prevotella | North Sikkim | 1 | ||
| Prevotella | South Sikkim | 4 | ||
| Faecalibacterium | West Sikkim | 1 | ||
| Prevotella | Vellore | 35 | ||
| Prevotella | Adilabad | 9 | ||
| Prevotella, Succinivibrio | Khammam | 7 | ||
| Indonesia | Prevotella | Bali | 19 | |
| Prevotella | Yogjakarta | 28 | ||
| Clostridium sensu stricto | Medan | 6 | ||
| Ireland | Bacteroides, Bifidobacterium | Cork | 981 | |
| Italy | Bifidobacterium, Faecalibacterium, Subdoligranulum | Bologna | 16 | |
| Bacteroides | Rome | 39 | ||
| Bifidobacterium, Faecalibacterium | Milan | 54 | ||
| Pseudobutyrivibrio | Florence | 1 | ||
| Japan | Bifidobacterium | Aichi | 19 | |
| Bifidobacterium | Mie | 11 | ||
| Bifidobacterium | Shizuoka | 17 | ||
| Bifidobacterium | Ehime | 34 | ||
| Bifidobacterium | Okayama | 14 | ||
| Bifidobacterium | Hokkaido | 38 | ||
| Bifidobacterium | Kanakawa | 45 | ||
| Bifidobacterium, Porphyromonas, Bacteroides | Yokohama | 11 | ||
| Bifidobacterium | Hyogo | 10 | ||
| Bifidobacterium | Kyoto | 13 | ||
| Bifidobacterium | Osaka | 25 | ||
| Bifidobacterium, Succinivibrio | Chiba | 33 | ||
| Bifidobacterium, Fusicatenibacter | Gunma | 13 | ||
| Bifidobacterium | Ibaraki | 21 | ||
| Bifidobacterium | Kanagawa | 34 | ||
| Bifidobacterium | Saitama | 35 | ||
| Bifidobacterium, Fusicatenibacter | Tochigi | 19 | ||
| Bifidobacterium | Tokyo | 362 | ||
| Bifidobacterium | Fukuoka | 59 | ||
| Bifidobacterium | Kagoshima | 20 | ||
| Bifidobacterium, Fusicatenibacter | Nagasaki | 13 | ||
| Bifidobacterium | Saga | 51 | ||
| Bifidobacterium | Miyagi | 40 | ||
| Malawi | Bacteroides | Mbiza | 1,038 | |
| Philippines | Bifidobacterium, Faecalibacterium | Baybay | 11 | |
| Bifidobacterium, Faecalibacterium | Ormoc | 14 | ||
| Spain | Bacteroides, Prevotella | Barcelona | 123 | |
| Sweden | Prevotella, Blautia, Faecalibacterium | Stockholm | 2 | |
| Faecalibacterium, Bifidobacterium | Uppsala | 2 | ||
| Taiwan | Bacteroides, Bifidobacterium | Taipei | 46 | |
| Tanzania | Faecalibacterium | Dedauko | 15 | |
| Faecalibacterium | Sengele | 5 | ||
| Thailand | Bacteroides, Prevotella | Bangkok | 93 | |
| Prevotella, Faecalibacterium | Khon Kaen | 25 | ||
| Uganda | Bifidobacterium, Prevotella, Bacteroides | Kampala | 50 | |
| UK | Bacteroides | London | 2,644 | |
| Bacteroides, Faecalibacterium | Newcastle | 20 | ||
| USA | Bacteroides | Los Angeles | 9 | |
| Bacteroides | Pal Alto | 21 | ||
| Blautia, Ruminococcus | Boulder | 1 | ||
| Bacteroides, Faecalibacterium, Prevotella | Boston | 15 | ||
| Bacteroides | Canbridge | 169 | ||
| Bacteroides | Mankato | 4 | ||
| Bacteroides | Columbia | 2 | ||
| Bifidobacterium, Ruminococcus | St. Louis | 1,674 | ||
| Bifidobacterium | New York | 32 | ||
| Bacteroides, Prevotella | Lancaster | 606 | ||
| Bacteroides, Parabacteroides | Philadelphia | 1 | ||
| Bacteroides | Austin | 5 |
Table 2.
Summary of country in non-FMD studies assessing microbial composition of the skin, saliva, vaginal fluid, and stool.
| Sample | Country | Dominant bacteria | Sample size | Demographics (race, gender, age) | Sampling collection | Medical history | Statistical analysis | Note | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Skin | South Korea | Ochrobactrum, Propionibacterium | 3 | Over 20 | Fabric | Healthy | Chao 1, Shannon diversity index, XOR analysis | Hand | Lee et al. (2016) |
| South Asia | Staphylococcus, Acinetobacter, Corynebacetrium | 20 | India, mean of age = 37.9 | Swab | Healthy | Linear discriminant analysis effect size (LEfSe), UCLUST | Arm | Perez et al. (2016) | |
| Staphylococcus, Corynebacterium, Anaerococcus | 20 | India, mean of age = 37.9 | Swab | Healthy | LEfSe, UCLUST | Axilla | Perez et al. (2016) | ||
| Germany | Cutibacterium, Staphylococcus | 7 | Males, between 18 and 60 years | Swab | Healthy | Bioconductor workflow | Antecubital fossa | Francuzik et al. (2018) | |
| USA | Corynebacteria, Staphylococcus | 10 | Males and females, 20–41 years | Swab | Healthy | Shannon diversity index, distance-based OTU and richness (DOTUR) | Antecubital fossa | Grice et al. (2009) | |
| USA | Propionibacterium | 4 | 28–55 years | Swab | Healthy | Chao 1, UCLUST, UniFrac | Forearm | Blaser et al. (2013) | |
| USA | Firmicutes, Actinobacteria | 15 (13 were white and of European ancestry, and 2 were Chinese-American) | Females, graduate student | Glove juice method | Healthy | UniFrac (principal coordinate analyses (PCoA)) | Hand | Hospodsky et al. (2014) | |
| Caucasian America (US borne, of European ancestry) | Corynebacterium, Staphylococcus, Streptococcus, Lactobacillus | 16 | Mean of age = 27.6 | Swab | Healthy | LEfSe, UCLUST | Arm | Perez et al. (2016) | |
| Corynebacterium, Staphylococcus | 16 | Mean of age = 27.6 | Swab | Healthy | LEfSe, UCLUST | Axilla | Perez et al. (2016) | ||
| African America | Corynebacterium, Staphylococcus | 18 | Mean of age = 25.9 | Swab | Healthy | Linear discriminant analysis effect size (LEfSe), UCLUST | Arm | Perez et al. (2016) | |
| Corynebacterium, Staphylococcus | 18 | Mean of age = 25.9 | Swab | Healthy | LEfSe, UCLUST | Axilla | Perez et al. (2016) | ||
| Latin America | Acinetobacter, Corynebacterium, Staphylococcus | 20 (Ecuador = 15 and Mexico = 5) | Ecuador and Mexico | Swab | Healthy | LEfSe, UCLUST | Arm | Perez et al. (2016) | |
| Staphylococcus | 20 (Ecuador = 15 and Mexico = 5) | Ecuador and Mexico | Swab | Healthy | LEfSe, UCLUST | Axilla | Perez et al. (2016) | ||
| Venezuela (Amerindian) | Staphylococcus, Proteobacteria | 72 | 2 months–80 years | Swab | Healthy | Chao 1, UCLUST, UniFrac | Forearm | Blaser et al. (2013) | |
| Tanzania | Proteobacteria, Actinobacteria | 29 | Females, graduate student | Glove juice method | Healthy | UniFrac | Hand | Hospodsky et al. (2014) | |
| Egypt | Proteobacteria, Firmicutes | 5 | Swab | Healthy | Antecubital fossa | Ramadan et al. (2016) | |||
| Saliva | South Korea | Neisseria | 543 (198 males, 345 females) | Males and females, 40–79 years | Chew gum and stimulated saliva samples | Orally healthy | UCLUST, UniFrac, Shannon diversity index | Takeshita et al. (2014) | |
| Japan | Prevotella, Veillonella | 2,272 (1,011 males, 1,261 females) | Males and females, 40–79 years | Chew gum and stimulated saliva samples | Orally healthy | UCLUST, UniFrac, Shannon diversity index | Takeshita et al. (2014) | ||
| China | Haemophilus, Prevotella | 29 | Males and females, 20–40 years | 5 ml of spontaneous, whole, unstimulated saliva | Orally healthy, Normal weight group (BMI = 18.5–20) | RDP classifier, Chao 1, Shannon diversity index, UniFrac, Kurskal–Wallis test | Wu et al. (2018) | ||
| Qatari | Prevotella | 997 (442 males and 555 females) | Males and females, 18≤ years | 5 ml of spontaneous, whole, unstimulated saliva | Healthy and unhealthy | QIIME, LEfSe | Murugesan et al. (2020) | ||
| Thailand | Streptococcus, Veillonella | 50 (27 males and 23 females) | Males and females, 7–15 years | Chew gum and stimulated saliva samples | Orally healthy | Shannon diversity index, UCLUST, UniFrac | Mashima et al. (2019) | ||
| Swiss | Streptococcus, Neisseria, Prevotella | 2 | Over 20 | 5 ml of spontaneous, whole, unstimulated saliva | Orally healthy | CD-HIT-EST, DESeq | Leake et al. (2016) | ||
| Germany | Firmicutes, Proteobacteria, Bacteroidetes | 10 | 20–40 years | Volunteer spit up to 2 ml of saliva into tubes containing 2 ml lysis buffer | Orally healthy | Shannon diversity index, Sørensen index, UniFrac | Li et al. (2014) | ||
| Bolivia | Serratia | 10 | Males and females | Volunteer spit up to 2 ml of saliva into tubes containing 2 ml lysis buffer | Orally healthy | DISTLM, UniFrac, SeqMatch | Nasidze et al. (2009a) | ||
| Congo | Enterobacter | 10 | Males and females | Volunteer spit up to 2 ml of saliva into tubes containing 2 ml lysis buffer | Orally healthy | DISTLM, UniFrac, SeqMatch | Nasidze et al. (2009a) | ||
| Africa | Firmicutes, Proteobacteria | 66 (Democratic Republic of Congo = 15, Sierra Leone = 13, Uganda = 38) | Democratic Republic of Congo, Sierra Leone, Uganda, males and females, 20–40 years | Volunteer spit up to 2 ml of saliva into tubes containing 2 ml lysis buffer | Orally healthy | UniFrac, Shannon diversity index, Sørensen index | Li et al. (2014) | ||
| Alaska | Firmicutes, Proteobacteria, Bacteroidetes, Actinobacteria | 76 | Four native Alaskan communities, males and females, 20–40 years | Volunteer spit up to 2 ml of saliva into tubes containing 2 ml lysis buffer | Orally healthy | UniFrac, Shannon diversity index, Sørensen index | Li et al. (2014) | ||
| Vaginal fluid | South Korea | L. crispatus, L. fermentum, L. jensenii, L. salivariusm | 110 | Vaginal swab | Healthy | Clustal X, TreeExplorer | Jin et al. (2007) | ||
| Japan | L. crispatus, L. iners | 73 | 3 age groups (18–25, 26–34, and 35–45 years) | Vaginal swab | Zhou et al. (2010) | ||||
| India | L. jensenii | 199 | Vaginal swab | Healthy | Southwest | Pramanick et al. (2017) | |||
| L. reuteri, L. fermentum, L. salivarius | 80 | 18–45 years | Vaginal swab | Healthy | RAPD analysis | Central | Garg et al. (2009) | ||
| L. crispatus, L. gasseri, L. jensenii | 132 | 18–45 years | Vaginal swab | Healthy | SAS Institute | South | Madhivanan et al. (2015) | ||
| L. mucosae, E. faecalis | 69 | 18–35 years | Vaginal swab | Healthy pregnant and nonpregnant | ClustalX, BLASTn, EZ-Taxon | Northeast | Das Purkayastha et al. (2019) | ||
| Belgium | L. crispatus, L. iners, L. jensenii, B. xylanisolvens, B. thetaiotaomicron, B. fragilis | 19 | 25–39 years | Brush™ IUMC Endometrial Sampler | Healthy premenopausal | PRIMER | Verstraelen et al. (2016) | ||
| Swiss | L. crispatus, L. iners, L. gasseri, L. jensenii | 23 | Vaginal swab | Healthy | RAPD analysis | Vasquez et al. (2002) | |||
| Europe | L. crispatus, L. iners | 416 | 18–44 years | Vaginal swab | Healthy | Inverse Simpson’s index, Bray–Curtis method, LEfSe | Fettweis et al. (2014) | ||
| North America | L. iners | 396 | 12–45 years | Vaginal swab | Healthy and not pregnant | RDP Naïve Bayesian Classifier | Ravel et al. (2011) | ||
| African America | L. iners, G. vaginalis | 1,268 | 18–44 years | Vaginal swab | Healthy | Inverse Simpson’s index, Bray–Curtis method, LEfSe | Fettweis et al. (2014) | ||
| Uganda | L. reuteri, L. crispatus, L. vaginalis, L. gasseri | 250 | Vaginal swab | Healthy | Clustal X, TreeExplorer | Jin et al. (2007) | |||
| Republic of South Africa | L. crispatus | 40 | 18–44 years | Vaginal swab | Healthy (premenopausal and HIV uninfected) | GraphPad Prism 4 software | Pendharkar et al. (2013) | ||
| Stool | South Korea | Bacteroidetes, Firmicutes, Proteobacteria | 8 | Females, over 65 | Healthy | Ion Reporter™ | Shin et al. (2016) | ||
| Japan | Actinobacteria, Bifidobacterium, Clostridium | 13 | Healthy | TaxCollector, UPGMA, UniFrac | Nam et al. (2011) | ||||
| China | Bacteroides | 1 | Healthy | TaxCollector, UPGMA, UniFrac | Nam et al. (2011) | ||||
| India | Prevotella, Faecalibacterium | 80 | Males and females, 18–55 years | Healthy and whose body mass index (18.50 to 30.00 kg/m2) with minimum of 45 kg weight | West | Tandon et al. (2018) | |||
| Turkey | Prevotella | 436 (212 males and 224 females) | Males and females, 18–70 years | Healthy | Shannon diversity index, Simpson, Chao | Deschasaux et al. (2018) | |||
| Himalaya | Treponema, Prevotella | 56 | Males and females, 18≤ years | Healthy and unhealthy | Shannon diversity index, Simpson, phyloseq, SparCC | Jha et al. (2018) | |||
| Netherlands | Clostridiales | 1,328 (695 males and 633 females) | Males and females, 18–70 years | Healthy | Shannon diversity index, Simpson, Chao | Deschasaux et al. (2018) | |||
| USA | Firmicutes | 41 | Healthy | TaxCollector, UPGMA, UniFrac | Nam et al. (2011) | ||||
| Morocco | Prevotella | 605 (324 males and 281 females) | Males and females, 18–70 years | Healthy | Shannon diversity index, Simpson, Chao | Deschasaux et al. (2018) | |||
| African Surinamese, South Asian Surinamese | Bacteroides | 1,703 (731 males and 972 females) | Males and females, 18–70 years | Healthy | Shannon diversity index, Simpson, Chao | Deschasaux et al. (2018) |
The vaginal samples were obtained from women only, and stool samples were collected with stool collection tubes of participants, respectively.
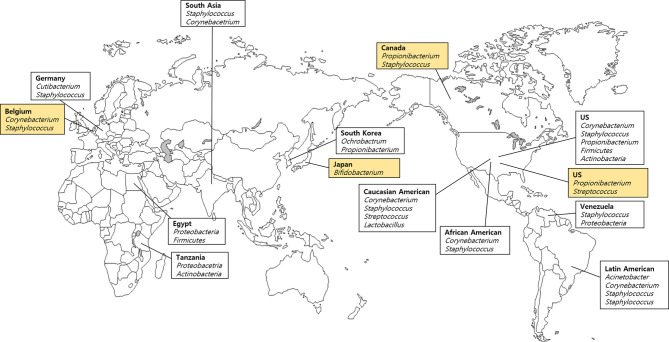
Figure 1.
Geographical variation of skin bacteria based on the Forensic Microbiome Database (FMD) (www.fmd.jcvi.org) data and non-FMD. The yellow-colored box is from the FMD data, and only bacteria which account for over 10% are shown. Four countries from the FMD and 10 results from previous studies were included, and the microbiome profiling was generated using 16S rRNA gene sequencing.
Additionally, in a comparative analysis of taxa associated with hand microbiomes in Americans and South Koreans, the American sample showed the highest proportion of Propionibacterium (31.8%) and Streptococcus (18.3%) (Table 1). In contrast, in South Korea, Lee et al. (2016) reported a preponderance of Ochrobactrum (40.0%) and Propionibacterium (30.4%) (Table 2). The samples also revealed Staphylococcus (7.1%). The palm of hand microbiome in Canada was predominantly composed of Bacillus (27.7%), Streptococcus (22.5%), and Propionibacterium (12.7%) (Table 1). Apparent differences existed between North American and Asian populations. Ochrobactrum was not detected in American and Canadian populations, whereas only a few species of Streptococcus were found in South Korean samples, which were even higher in American and Canadian samples. Hospodsky et al. (2014) analyzed American and Tanzanian hand microbiomes and reported differences compared with the US hand microbiome data (FMD). Propionibacteriaceae, Staphylococcaceae, and Streptococceaceae families were predominantly detected in American hand samples, and soil-associated Rhodobacteraceae and Nocardiodaceae were the most dominant on Tanzanian hands. At the phylum level, Firmicutes and Proteobacteria were the most abundant in American and Tanzanian hand microbiomes, respectively (Hospodsky et al., 2014).
Byrd et al. (2018) described skin microbial communities at four body sites, including forehead, antecubital fossa, forearm, and foot in the American samples. They reported that forearms exhibited an abundance of Propionibacterium and Staphylococcus. However, Propionibacterium and Staphylococcus were not the dominant genera on the Japanese forearms. According to the FMD, Japan (Yokohama) showed Bifidobacterium (27.7%) predominantly in the forearm microbiome. Two cities (Yokohama and Tokyo) with forearm microbiome data were mentioned in the FMD, and fewer proportions of bacteria were detected in Tokyo (Streptococcus 6.52% and Moraxella 3.49%). Also, the two cities did not share bacteria. Blaser et al. (2013) reported differences in the forearm microbiota between the American and the two groups of Amerindians in Venezuela (Blaser et al., 2013). Staphylococcus was the dominant genus, followed by Propionibacterium, Streptococcus, Pseudomonas, and Corynebacterium. Accordingly, they proposed a profound association between microbiota and modernization, ancestry, and biogeography (Blaser et al., 2013).
Furthermore, Perez et al. (2016) analyzed the forearm microbiome of six ethnic groups (Caucasian-American, African-American, African, Latin American, East Asian, and South Asian) living in the USA. In that study, most groups comprised Actinobacteria and Firmicutes at the phylum level and Staphylococcus and Corynebacterium at the genus level. Among the six groups, Latin Americans, in particular, showed a relative abundance of Proteobacteria and Acinetobacter (Perez et al., 2016). Notable differences in microbiome were found between ethnicities in line with Blaser et al. (2013).
In the case of the antecubital fossa, no apparent differences were found between left and right antecubital fossa in the American samples (Table 1). Propionibacterium dominated both left and right antecubital fossae (41.1% and 44.1%, respectively), followed by Staphylococcus (12.9% and 11.8%, respectively). Other studies reported that Corynebacteria species dominated the antecubital fossa, as well as moist sites such as nares (inside the nostril) and axillary vault, although Staphylococci species were sparsely detected in the American population (Grice et al., 2009; Byrd et al., 2018). However, Egyptians differed slightly from the Americans in that Proteobacteria were the predominant phyla in all Egyptian samples of the antecubital fossa (57%), followed by Firmicutes (36%) and Actinobacteria (4%) (Ramadan et al., 2016). In addition, the antecubital fossa microbiome in healthy Germans contained C. acnes and Staphylococcus epidermidis as the dominant bacteria (46.9% and 10.5%, respectively) (Francuzik et al., 2018). Each country exhibited characteristic microorganisms in the antecubital fossa compared with other body sites.
Based on these studies, it is conceivable that skin microbial communities vary even within the same country, based on topography and lifestyle. However, the diversity may be attributed to analytical differences or a combination of analytical differences and environmental factors. Nonetheless, the data are promising and suggest that microbiomes can be distinguished based on geographical location, ethnicity (perhaps largely affected by geography), and lifestyle factors. A more standardized protocol is required to characterize the skin microbiomes from diverse population samples to determine the factors contributing to the observed variation and the occasional differences within the population.
Saliva
Saliva is another body fluid found at crime scenes (Virkler and Lednev, 2009). Saliva can be found on a variety of specimens left behind at the crime scene, including cigarettes, vaping tools, bottles, cups, victim’s skin, bite marks, lip prints, and drug paraphernalia (Kapoor and Chowdhry, 2018). Accepted, presumptive, and confirmatory tests of forensic samples based on saliva are available (Old et al., 2009; Virkler and Lednev, 2009). However, some of these tests, particularly the presumptive tests, are often not specific to saliva. Other tests cannot differentiate vaginal fluid from saliva which may serve as a significant piece of evidence in reconstructing cases related to sexual assault. In addition, saliva may be detected in trace levels and the protein of interest for these tests may not be adequate enough. However, saliva contains microorganisms, which facilitates forensic investigations when combined with longstanding salivary biomarkers (Leake et al., 2016).
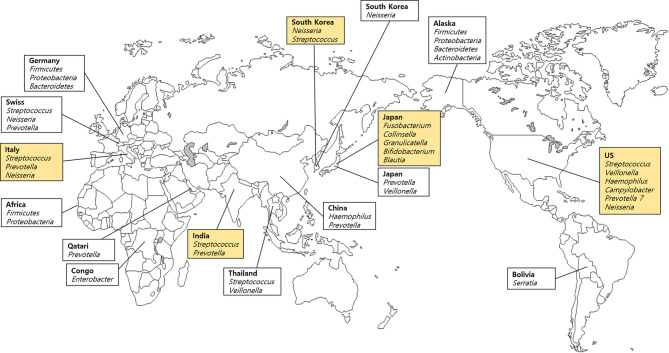
We included 19 samples of saliva obtained from five different countries listed in the FMD (Table 1), and 11 samples from each of the 11 countries, which are not included in the FMD (Table 2). Different geographical and environmental factors, such as diet, elements of hygiene, humidity, climate, temperature and oral disease, can affect the composition of microbial communities (Li et al., 2014). Several studies of saliva have demonstrated a potential geographic signature involving the oral microbiome (Nasidze et al., 2009a; Clarke et al., 2017). Saliva samples obtained from India, Italy, Japan, South Korea, and the USA were used in the FMD data. Streptococcus were the main bacteria in samples derived from most countries (Figure 2). Rothia was the highest in prevalence among Japanese; Prevotella was the highest in India and Italy; and Neisseria was the predominant salivary microbe among Italians and South Koreans. Takeshita et al. (2014) analyzed healthy South Koreans and Japanese and found that the salivary microbiome of Koreans harbored higher proportions of Neisseria, Haemophilus, and Porphyromonas and lower proportions of Prevotella and Veillonella compared with those in the Japanese population. Additionally, the geographical location had a remarkable effect on salivary microbiota, more than age, gender, or smoking, although the smoking status had a significant effect on the microbiome. In China, the salivary microbiome was dominated by 12 genera: Streptococcus, Neisseria, Haemophilus, Prevotella, Porphyromonas, Veillonella, Gemella, Rothia, Granulicatella, Fusobacterium, Actinomyces, and Alloprevotella. Among them, Haemophilus and Prevotella were the most abundant genera in healthy individuals (Wu et al., 2018).
Figure 2.
Dominant bacteria in saliva samples based on the Forensic Microbiome Database (www.fmd.jcvi.org) data and non-FMD studies. The yellow-colored box is from the FMD data, and only bacteria which account for over 10% are shown. Five countries from the FMD and 11 countries from previous studies were included, and the microbiome profiling was generated using 16S rRNA gene sequencing.
In Yokohama in Japan, the FMD data showed that Fusobacterium was the main genus (Table 1). However, when the Japanese state or city was excluded, the main genus was Rothia. Mashima et al. (2019) analyzed the salivary microbiome in rural Thai children grouped into five clusters (east, west, south, north, and central) based on economic, food, and lifestyle factors and reported significant differences between Veillonella and Prevotella among the geographical regions (p < 0.05). In addition to their abundance, the presence of V. parvula, R. aeria, and R. dentocariosa indicated potential deterioration in oral hygiene, which also relates to dental caries history. Accordingly, individuals residing in Yokohama may exhibit comparatively better dental hygiene than the other populations. Also, Prevotella was the most common and abundant bacterium in East Asia (South Korea, Japan, China, and Thailand). Leake et al. (2016) reported the preponderance of eight major genera in the saliva samples of Swiss population: Streptococcus, Neisseria, Prevotella, Haemophilus, Veillonella, Porphyromonas, Rothia, and Fusobacterium. However, despite the geographic proximity between Germany and Switzerland, no common genera except Fusobacterium were found (Li et al., 2014). In contrast, Italians carried common genera (Neisseria and Prevotella).
Li et al. (2014) performed a comparative microbiome analysis of Alaskans, Germans, and Africans, including the Democratic Republic of Congo (n = 15), Sierra Leone (n = 13), and Uganda (n = 38) and revealed more similarities between Alaskans and Germans than Africans at the genus and OTU levels (Li et al., 2014). Both native Alaskans and Germans shared 13 common genera, while Alaskans and Africans shared only six genera (Neisseria, Campylobacter, Granulicatella, Megasphaera, Selenomonas, Actinomyces) and Germans and Africans carried three common genera (Actinobacillus, Aggregatibacter, and Capnocytophaga). Also, all of the foregoing populations shared only three genera (Streptococcus, Fusobacterium, and Leptotrichia) in common. Nasidze et al. (2009a) reported considerable differences in the diversity of the saliva microbiome between African populations, which were attributed to subsistence and dietary patterns. A study involving Sierra Leone and Congo, which are geographically distant but have similar dietary patterns, showed a higher degree of similarity with each other than with Batwa (Nasidze et al., 2009a).
Although no significant geographical signature of the salivary microbiome was detected in various populations, a frequency variation in the specific genera was found. For example, significant fluctuations in the frequency of Enterobacter were seen. Enterobacter constitutes approximately 28% of the sequences obtained from the Congo but not California, China, Germany, Poland, or Turkey. Furthermore, Serratia showed a relatively high frequency among Bolivians (Nasidze et al., 2009a). Murugesan et al. (2020) characterized the salivary microbiome of the Qatari population, which was associated with gender, aging, oral health, smoking status, and coffee or tea consumption. They found that Bacteroidetes, Firmicutes, Actinobacteria, and Proteobacteria are the common phyla, with Bacteroidetes being the predominant phylum, and at the genus level Prevotella, Porphyromonas, Streptococcus, and Veillonella (mean values of males and females, 54.3%, 8.1%, 6.6%, and 6.22%, respectively) were the most abundant in Qatari saliva samples. These results indicate that Qatar differed from countries where Firmicutes was the dominant phylum such as Bangladesh, UK, Japan, South Korea, and Brazil. Further sampling of various populations is required to demonstrate the unique geographic differences of each region.
Vaginal Fluid
Identification of vaginal fluid in sexual assault cases is desirable for some forensic investigations. In particular, mixed samples containing vaginal fluid mixed with semen may suggest vaginal intercourse in sexual assault cases (Akutsu et al., 2012). Bacterial markers have been suggested to play a role in vaginal fluid identification based on the presence of Lactobacillus (87.5%), Lachnospiraceae (2.3%), Prevotella (1.1%), Alcaligenaceae (1.0%), Erysipelatoclostridium (0.9%), Corynebacterium (0.7%), Peptoniphilus (0.6%), Bifidobacterium (0.6%), Anaerococcus (0.5%), and Staphylococcus (0.5%) (Dobay et al., 2019). Also, Subdoligranulum (2.3%), Blautia (1.7%), Escherichia-Shigella (0.5%), Anaerostipes (0.4%), and Stenotrophomonas (0.3%) were found in the exposed vaginal fluid (Dobay et al., 2019). Lactobacilli play an important role in protecting the host from the urinary tract and genital infections and in maintaining the vaginal microbial balance; they occur predominantly in the vaginal microenvironment of healthy women (Boris et al., 1998; Mc and Rosenstein, 2000; Witkin et al., 2007). The abundance of Lactobacillus promotes acidic vaginal pH, which is the signature of Lactobacillus colonization, and is attributed primarily to the metabolism of glycogen to lactic acid (Mirmonsef et al., 2016; Das Purkayastha et al., 2019). However, vaginal pH and Lactobacillus diversity and dominance differ with individual lifestyles (Das Purkayastha et al., 2019). Recent studies and the human microbiome project reported nearly 60 vaginal microbiomes including four dominant species in the urinogenital tract: L. crispatus, L. iners, L. gasseri, and L. jensenii (Pavlova et al., 2002; Verhelst et al., 2004; Kroon et al., 2018).
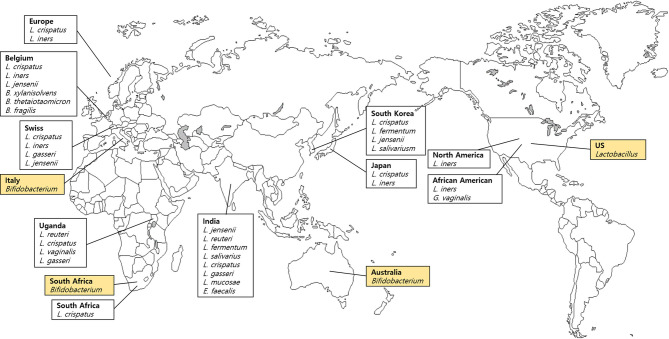
We analyzed seven vaginal fluid samples from four countries listed in FMD (Table 1) and 13 samples from 10 countries that are not included in the FMD (Table 2). Three body sites related to the vagina were listed in the FMD. Bacteria comprising less than 10% from vaginal introitus and mid-vagina from the USA were not listed. Lactobacillus, Gardnerella and Shuttleworthia were found in the vaginal fluid sample obtained from the United States. Surprisingly, Lactobacilli dominated the vaginal microenvironment and the Bifidobacterium was predominant in three countries (Australia, Italy, and South Africa), whereas Holdemanella constituted only a small proportion (Figure 3). Other species were not conspicuous in the UK. Bifidobacterium colonization occurred in the gastrointestinal tract, oral cavity, and vagina (Turroni et al., 2008; Arboleya et al., 2016; Gomez-Gallego et al., 2016).
Figure 3.
Dominant bacteria in vaginal fluid samples based on the Forensic Microbiome Database (www.fmd.jcvi.org) data and non-FMD studies. The yellow-colored box is from the FMD data, and only bacteria which account for over 10% are shown. Four countries from the FMD and 10 countries from previous studies were included, and the microbiome profiling was generated using 16S rRNA gene sequencing.
Ravel et al. (2011) demonstrated the apparent differences in vaginal microbiome among the ethnic groups and reported that the vaginal microbiome of North American women (Caucasian, African American, Hispanic, and Asian) was dominated by Lactobacillus (59%–89%) (Ravel et al., 2011). Among four groups (Caucasian, African American, Hispanic, and Asian), the relative abundance of Lactobacillus among Hispanic and African American women was only 59.6% and 61.9%, respectively; however, it accounted for 80.2% of Asians and 89.7% among Caucasian women (Ravel et al., 2011). In addition, Hispanic and African American women carried an abundance of strictly anaerobic bacteria and L. iners was the predominant Lactobacillus. Another study of Caucasian and African American women from North America showed patterns similar to those reported by Ravel et al. (2011) (Zhou et al., 2010). In that study, vaginal communities of Caucasian and African American women were dominated by Lactobacillus, including L. crispatus, L. iners, L. gasseri, and L. jensenii. Furthermore, the prevalence of Lactobacillus was lower in African American women compared with Caucasian women (Zhou et al., 2010).
Verstraelen et al. (2016) analyzed 13 women from Belgium and showed similarities in microbiome composition involving B. xylanisolvens, B. thetaiotaomicron, and B. fragilis and an undetermined Pelomonas taxon constituted up to one-third of the endometrial microbiome. Also, six of 13 participants showed an abundance of L. crispatus or L. iners, characterized by the presence of the Bacteroides core (Verstraelen et al., 2016). Other studies of African Americans reported a number of G. vaginalis and bacterial vaginosis-associated bacterium-1 (BVAB1), and the diversity of the endometrial microbiome was significantly greater than in European women (Fettweis et al., 2014). The most prevalent species in African American women was L. iners, followed by G. vaginalis, BVAB1, and L. crispatus. In contrast, the most ubiquitous species in European females was L. crispatus, followed by L. iners and G. vaginalis. BVAB1, which was common in African American women, was only found in five of 416 European samples (Fettweis et al., 2014). Lactobacillus population in the healthy vaginas of Swedish women was studied by Vasquez et al. (Vasquez et al., 2002). The most predominant species were L. crispatus, L. iners, L. gasseri, and L. jensenii. Ugandans and South Africans showed similar trends in vaginal microbiome composition compared with Swedish (Jin et al., 2007; Pendharkar et al., 2013).
Studies investigating vaginal Lactobacillus composition were carried out among various Asian populations. In a comparative study of Korean and Ugandan women, five common genera were isolated: Lactobacillus, Leuconostoc, Pediococcus, Streptococcus, and Weissella. L. fermentum was detected only in Korean women, and Pediococcus was more common in Korean women (Jin et al., 2007). Furthermore, in Korean women, the most abundant species was L. crispatus, followed by L. fermentum, L. jensenii, L. salivariusm, Pediococcus acidilactici, and Weissella kimchi. The combination of L. fermentum, P. acidilactici, and W. kimchi was characteristic of the Korean population (Jin et al., 2007). In another study, L. gasseri was the predominant microbe among Chinese women, and with a higher prevalence in fertile women than in postmenopausal women (Zhang et al., 2012). Among the Japanese communities with Lactobacillus prevalence, L. crispatus was the predominant species, followed by L. iners, similar to Caucasian and African Americans in North America (Zhou et al., 2010). In India, several studies reported a geographic variation in vaginal microbiomes (Garg et al., 2009; Pramanick et al., 2017; Das Purkayastha et al., 2019): L. jensenii in the southwest region; L. reuteri, L. fermentum, and L. salivarius in the central region; and L. crispatus, L. gasseri, and L. jensenii in south India. Interestingly, the rare vaginal microbes L. mucosae and Enterococcus faecalis was found to be prevalent in Northeast India (Das Purkayastha et al., 2019). Das Purkayastha et al. (2019) reported a distinct and diverse vaginal microenvironment in various groups, ethnicities, and regions.
Stool
The gut microbiota constitute the largest number of microbes compared with other body sites, and various microbiome studies have focused on the gut using the fecal samples (Lloyd-Price et al., 2016; Gupta et al., 2017). The composition and diversity of the gut microbiome are unique to individuals and are influenced by physical activity, diet, geographical environment, genetics, lifestyle, and ethnicity (Falony et al., 2016; Findley et al., 2016; Lynch and Pedersen, 2016; Zhernakova et al., 2016). Gut microbiota dysbiosis is attributed to metabolic and inflammatory diseases such as obesity, diabetes, and cancer (Adlerberth and Wold, 2009; Armougom et al., 2009). The most predominant phyla in the human gut are Bacteroides and/or Firmicutes, constituting more than 80% of the total microbiome (Lay et al., 2005). Dietary changes alter the composition of Bacteroides and Firmicutes (Clarke et al., 2012; Scott et al., 2013).
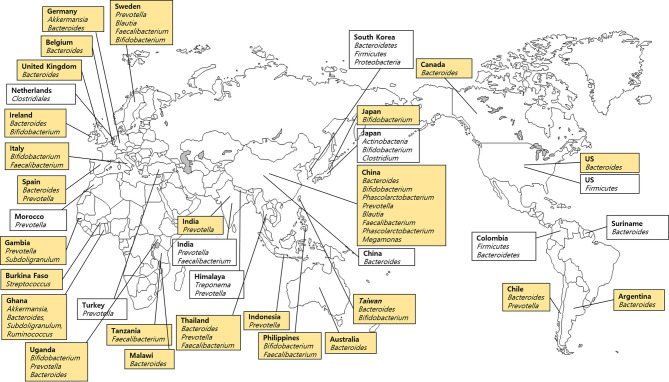
Stool samples including 99 samples from 25 countries listed in the FMD (Table 1) and 10 samples from each of the 10 countries not included in the FMD were analyzed (Table 2). We divided the stool microbiome data reported in the FMD studies into five groups to facilitate analysis based on region and abundance (Figure 4). Bifidobacterium species were predominant in East Asia (Japan, China, Taiwan, and Philippines), Prevotella in South Asia (India, Thailand, and Indonesia), and Bacteroides was the most prevalent in West Europe (Germany, Belgium, UK, Ireland, and Spain), North and South America (USA, Canada, Chile, and Argentina) and Australia. The microbiome composition was inconsistent across African countries including Gambia, Ghana, and Tanzania.
Figure 4.
Dominant bacteria in stool samples based on the Forensic Microbiome Database (www.fmd.jcvi.org) data and non-FMD studies. The yellow-colored box is from the FMD data, and only bacteria which account for over 10% are shown. Twenty-five countries from the FMD and 11 countries from previous studies were included, and the microbiome profiling was generated using 16S rRNA gene sequencing.
In contrast, Koreans carried three phyla, which were predominantly found in stool microbiomes (Bacteroidetes, Firmicutes, and Proteobacteria) and accounted for a mean value of 99% of sequences (Shin et al., 2016). Also, Shin et al. (2016) reported that Butyricimonas of the phylum Bacteroidetes was predominant in Seoul (mainland), while Catenibacterium from the Firmicutes phylum was dominant in Jeju (an island). In addition, Japanese, Korean, and Chinese communities carried an abundance of Firmicutes, Actinobacteria, and Bacteroidetes, respectively (Nam et al., 2011). Japanese harbored predominantly Bifidobacterium and Clostridium, whereas Koreans carried Prevotella and Faecalibacterium, and Chinese had Bacteroides in their gastrointestinal microbiomes (Nam et al., 2011). Zhang et al. (2015) reported that Phascolarctobacterium of Firmicutes was the most abundant microbe in Chinese. In an Indian study, Tandon et al. (2018) analyzed urban cohorts derived from Western India and reported clear taxonomic differences in the microbiomes of American, Chinese, and Japanese populations. More than 80% of the sequences of gut microbiome derived from stool samples in the western Indian cohort belonged to five genera: Prevotella, Faecalibacterium, Alloprevotella, Roseburia, and Bacteroides (Tandon et al., 2018). These results were in line with FMD data which showed that Prevotella was the most predominant in the Indian urban population. A characteristic gut microbiome profile of the Himalayan population exhibited an increased abundance of Treponema and Prevotella and a decreased abundance of Bacteroides and Bifidobacterium (Jha et al., 2018). The difference across studies in the same country is attributed to sequencing methods, differences in sample preparation, bioinformatics, and cohorts from different ethnicities and provinces, especially in China.
The distribution of the microbial community in the stool samples is explained by the composition of the gastrointestinal microbiome. Compared with other samples, the stool samples tend to be affected by the habitat and dietary patterns. Gupta et al. (2017) reviewed the variation in the gastrointestinal microbiome based on the degree of urbanization. Models involved hunter-gatherers (Hadza of Tanzania, Pygmies of Central Africa, the Matses of Peru, and Amerindians of Venezuela), traditional farmers and fishermen (Bantus of Africa, the Tunapuco of the Andean highlands, or the rural Malawian communities), and urban industrialized populations (the USA and European) (Gupta et al., 2017). The findings suggest that the higher the transition from hunter-gatherers to urban population, the lower was the microbial diversity along with depletion of Prevotella and proliferation of Bacteroides. From a forensic perspective, access to the gastrointestinal microbiome may provide clues to the whereabouts of suspects based on a comparative analysis of the aforementioned studies involving the composition and diversity of stool samples and microbial clusters.
Sequencing Platforms
It is important that the sample processing methods, data generation, and data analysis should be standardized for comparing microbiome data. Therefore, future investigations should reduce the differences in methodology and focus on standardizing analyses to ensure a better interpretation of the observed variation in these studies. Two sequencing approaches including 16S rRNA gene sequencing and whole genome shotgun sequencing have been used in the human-associated microbial analysis (Kuczynski et al., 2012). In this section, the strengths and limitations of 16S rRNA gene amplicons and shotgun metagenomics are reviewed.
Microbiome analysis based on 16S rRNA gene amplicons entails PCR amplification and sequencing of a variable region (or multiple regions). In contrast, shotgun metagenomics entails fragmentation and amplification of the microbiome genome, followed by sequencing (Ranjan et al., 2016). The primary microbial target is the 16S rRNA gene, which has been widely used. It is appropriate in the human/host DNA background (e.g., a skin swab) because the 16SrRNA primers only amplify the bacteria and archaea domains of life. The PCR step is appropriate for low biomass samples as it is inexpensive. However, single amplicon sequencing is limited by the differences in the relative abundances measured by bacterial community sequencing compared with the true relative abundances, and the primers used for amplification may misalign with the target region in some species. Human microbiome shotgun metagenomics can overcome these limitations, and theoretically, the entire genome of a microbe is amplified due to the additional targets for analysis. Furthermore, no amplification bias is detected since it bypasses primer-dependent PCR amplification. However, shotgun metagenomics entails higher sequencing costs and stochastic effects and generates a large number of uninformative reads that are not variable between taxa, which is a waste of sequencing efforts.
In contrast, recently, Schmedes et al. (2018) reported the use of hidSkinPlex, a panel of informative targets derived from skin microbiomes, which could be used with machine learning tools in human forensic applications. Clade-specific markers were investigated based on 286 bacterial (and phage) family-, genus-, species-, and subspecies-level markers, which were derived from publicly available datasets generated by human microbiome shotgun metagenomics (Oh et al., 2016). Also, the hidSkinPlex markers were used to classify skin microbiomes including three body sites (foot, hand, and manubrium). Based on supervised learning, all samples were correctly classified and the body site origin was estimated up to 86% accuracy. This preliminary approach yielded additional targets for analysis and interpretation after amplification. Furthermore, they provided insight into the utility of skin microbiome for human identification and may represent an appropriate balance between single-target and shotgun sequencing approaches.
Studies continue to examine and analyze samples more precisely. Recent studies have reported microbial community sequencing, including amplicon and shotgun metagenomic sequencing, for vaginal microbiome analysis (Berman et al., 2020). It is important to distinguish the different microbial species, since Lactobacillus and Gardnerella are the predominant genera in most of the vaginal microbiome in normal healthy women, especially when vaginal fluid is used as evidence (Ghemrawi et al., 2021). Likewise, microbial communities in each sample exhibit a characteristic distribution. Ethnic groups can be distinguished at the level of phylum or genus. Therefore, the accurate forensic application requires analysis at the taxonomic level based on the characteristic distribution of the microbial communities in each sample for optimal comparison. Whole microbial genomes obtained via shotgun metagenomic sequencing provide insight into functional genes and pathways of the microbiome, which cannot be obtained via 16S rRNA gene amplicon sequencing (Avershina et al., 2018; Berman et al., 2020). Furthermore, the poor resolution of 16S rRNA gene amplicon sequencing may not allow the identification of the location or ensure individualization of WGS (Gloor et al., 2017; Berman et al., 2020). In summary, although both methods have their pros and cons, shotgun metagenomics is more valuable in forensic applications, as differences between individuals might only be detected at the subspecies and strain level.
Conclusion
This review provides insight into the differences in microbiome diversity and abundance among different samples of forensic interest (i.e., skin, saliva, vaginal fluid, and stool). The human microbiome, as a potential forensic biomarker(s), carries individual-specific information such as ethnicity or population affiliation, geographic location, lifestyle, as well as the microbiome origin in the tissue or body fluid, and human identification. Although the information provided by the FMD is relatively small, a comparative analysis with other studies yielded similar trends. In addition, previous studies revealed common bacterial species or phyla shared by microbiomes in nearby countries.
In the case of skin samples, Staphylococcus species were predominantly higher than Corynebacterium species in Asians compared with Americans. Based on both FMD and non-FMD studies, Propionibacterium was also found higher in North America. Acinetobacter and Proteobacteria were detected in Latin America. Holdemanella and Fusobacterium were characteristic of Korean and Japanese saliva samples, respectively. However, Veillonella and Prevotella were common bacteria shared by all Asians. Veillonella was unique and found only in Asians. However, the saliva microbiome of the Swiss population was more similar to the microbiome of Italians. According to Li et al. (2014), Bacteroides were not detected in 12 countries (Germany, Poland, Turkey, Georgia, China, Philippines, South Africa, Congo, Argentina, Bolivia, Louisiana, California) but was found only in native Alaskans (Table 2 and Figure 2) (Nasidze et al., 2009b). Serratia and Enterobacter are vaginal fluid microbes endemic to Bolivia and Congo, respectively. L. crispatus and L. iners are predominant in all of the European countries in this study. L. iners and L. crispatus were dominant in America and Africa but not in Asia. Finally, in the case of the stool microbiome, which is the most abundant in the FMD, Bacteroidetes was detected in most countries. Prevotella was predominant in South Asian countries such as India, Himalaya, Turkey, Thailand, and Indonesia, whereas Bacteroides and Bifidobacterium were prevalent in East Asia including South Korea, Japan, and China. However, European, American, or African microbiomes showed no distinguishing features (Table 2; Figure 4).
Approximately equal numbers of bacterial and human cells are found in the human body, which provides novel and unique identifiable markers specific to the individual, even in identical twins (Voreades et al., 2014; Zhu et al., 2015). The variation in microbiome structure and composition may provide forensic evidence based on host lifestyle and pharmaceutical use (Gonzalez et al., 2016; Kuntz and Gilbert, 2017). A recent study highlighted the potential forensic applications based on geographic classification utilizing city-specific microbiomes (Danko et al., 2021). However, in reality, the available microbial databases are inadequate under various conditions (Hampton-Marcell et al., 2017). Also, several studies cannot provide a rationale supporting the use of microbial sequencing in forensic studies, probably because the microbiome changes over time in an individual (Oh et al., 2016). Therefore, while human microbial fingerprinting can never replace established traditional DNA profiling techniques, it is possible that in the future, it can reinforce the currently available tools used by forensic investigators. Further investigations are still required to demonstrate that microbial fingerprints can be used as effective evidence.
This review is the first study collating the available evidence and the observed variation in microbiomes from forensically relevant sources of human microbiome. As the FMD was also analyzed with 16S rRNA gene amplicon sequencing, we compared the signals under different geographies in different studies, which used the same technology/data type. However, if the 16S rRNA gene primer regions targeting each study differed, it is clearly a limitation in the comparison of microbiome studies. Also, these studies are likely to have different downstream bioinformatics pipelines with varying degrees of distribution at the genus level. Thus, comparative analyses are associated with limitations, which in turn have implications for comparisons involving body parts, body fluids, and geographic locations internationally.
Despite these limitations, we described the use of the FMD repository that contains human microbiome data from subjects across multiple countries. Additionally, we incorporated sequencing data from other independently published studies to understand the effects of body sites and geography on the human microbiome. Furthermore, the geographic location of microbial communities is critical to the development of innovative forensic techniques in similar comparative analyses using FMD and technologies based on 16S rRNA gene primers. Further studies are needed to determine the power and resolution of the microbial diversity and the abundance of robust forensic biomarkers using geographic location and lifestyle indicators. Therefore, it is anticipated that studies involving not only samples from skin, stool, vaginal fluid, and saliva but also urogenital, airway, ocular, and breast milk specimens will be analyzed to build a more representative cohort in Asians and Africans as well as Europeans and Americans.
Author Contributions
H-WCho and Y-BEom participated in the design of the study, contributed to data reduction/analysis and interpretation of the results. H-WCho contributed to data analysis and interpretation of the results. All authors contributed to manuscript writing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Soonchunhyang University Research Fund and a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) [NRF-2020R1F1A1071977].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thankfully acknowledge Dr. Bruce Budowle and Dr. August Woerner for their contribution in providing continuous feedback and valuable advisement regarding the content of this review.
References
- Adlerberth I., Wold A. E. (2009). Establishment of the Gut Microbiota in Western Infants. Acta Paediatr. 98, 229–238. 10.1111/j.1651-2227.2008.01060.x [DOI] [PubMed] [Google Scholar]
- Akutsu T., Motani H., Watanabe K., Iwase H., Sakurada K. (2012). Detection of Bacterial 16S Ribosomal RNA Genes for Forensic Identification of Vaginal Fluid. Leg. Med. 14, 160–162. 10.1016/j.legalmed.2012.01.005 [DOI] [PubMed] [Google Scholar]
- Alan G., Sarah J. P. (2012). Microbes as Forensic Indicators. Trop. Biomed. 29, 311–330. [PubMed] [Google Scholar]
- Arboleya S., Watkins C., Stanton C., Ross R. P. (2016). Gut Bifidobacteria Populations in Human Health and Aging. Front. Microbiol. 7, 1204. 10.3389/fmicb.2016.01204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armougom F., Henry M., Vialettes B., Raccah D., Raoult D. (2009). Monitoring Bacterial Community of Human Gut Microbiota Reveals an Increase in Lactobacillus in Obese Patients and Methanogens in Anorexic Patients. PLoS One 4, e7125. 10.1371/journal.pone.0007125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avershina E., Angell I. L., Simpson M., Storrø O., Øien T., Johnsen R., et al. (2018). Low Maternal Microbiota Sharing Across Gut, Breast Milk and Vagina, as Revealed by 16S rRNA Gene and Reduced Metagenomic Sequencing. Genes 9, 231. 10.3390/genes9050231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman H. L., McLaren M. R., Callahan B. J. (2020). Understanding and Interpreting Community Sequencing Measurements of the Vaginal Microbiome. Int. J. Obstet. Gynecol. 127, 139–146. 10.1111/1471-0528.15978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Dominguez-Bello M. G., Contreras M., Magris M., Hidalgo G., Estrada I., et al. (2013). Distinct Cutaneous Bacterial Assemblages in a Sampling of South American Amerindians and US Residents. ISME J. 7, 85–95. 10.1038/ismej.2012.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boris S., Suarez J. E., Vazquez F., Barbes C. (1998). Adherence of Human Vaginal Lactobacilli to Vaginal Epithelial Cells and Interaction With Uropathogens. Infect. Immun. 66, 1985–1989. 10.1128/IAI.66.5.1985-1989.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd A. L., Belkaid Y., Segre J. A. (2018). The Human Skin Microbiome. Nat. Rev. Microbiol. 16, 143–155. 10.1038/nrmicro.2017.157 [DOI] [PubMed] [Google Scholar]
- Chase J., Fouquier J., Zare M., Sonderegger D. L., Knight R., Kelley S. T., et al. (2016). Geography and Location Are the Primary Drivers of Office Microbiome Composition. mSystems 1, e00022–16. 10.1128/mSystems.00022-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke T. H., Gomez A., Singh H., Nelson K. E., Brinkac L. M. (2017). Integrating the Microbiome as a Resource in the Forensics Toolkit. Forensic Sci. Int. Genet. 30, 141–147. 10.1016/j.fsigen.2017.06.008 [DOI] [PubMed] [Google Scholar]
- Clarke S. F., Murphy E. F., Nilaweera K., Ross P. R., Shanahan F., O'Toole P. W., et al. (2012). The Gut Microbiota and Its Relationship to Diet and Obesity: New Insights. Gut Microbes 3, 186–202. 10.4161/gmic.20168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings C. A., Relman D. A. (2002). Genomics and Microbiology - Microbial Forensics - "Cross-Examining Pathogens". Science 296, 1976. 10.1126/science.1073125 [DOI] [PubMed] [Google Scholar]
- Danko D., Bezdan D., Afshin E. E., Ahsanuddin S., Bhattacharya C., Butler D. J., et al. (2021). A Global Metagenomic Map of Urban Microbiomes and Antimicrobial Resistance. Cell 184, 3376–3393. 10.1016/j.cell.2021.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Purkayastha S., Bhattacharya M. K., Prasad H. K., Upadhyaya H., Lala S. D., Pal K., et al. (2019). Contrasting Diversity of Vaginal Lactobacilli Among the Females of Northeast India. BMC Microbiol. 19, 198. 10.1186/s12866-019-1568-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschasaux M., Bouter K. E., Prodan A., Levin E., Groen A. K., Herrema H., et al. (2018). Depicting the Composition of Gut Microbiota in a Population With Varied Ethnic Origins But Shared Geography. Nat. Med. 24, 1526–1531. 10.1038/s41591-018-0160-1 [DOI] [PubMed] [Google Scholar]
- Dobay A., Haas C., Fucile G., Downey N., Morrison H. G., Kratzer A., et al. (2019). Microbiome-Based Body Fluid Identification of Samples Exposed to Indoor Conditions. Forensic Sci. Int. Genet. 40, 105–113. 10.1016/j.fsigen.2019.02.010 [DOI] [PubMed] [Google Scholar]
- Edmonds-Wilson S. L., Nurinova N. I., Zapka C. A., Fierer N., Wilson M. (2015). Review of Human Hand Microbiome Research. J. Dermatol. Sci. 80, 3–12. 10.1016/j.jdermsci.2015.07.006 [DOI] [PubMed] [Google Scholar]
- Eom Y.-B. (2018). Microbial Forensics: Human Identification. Biomed. Sci. Lett. 24, 292–304. 10.15616/BSL.2018.24.4.292 [DOI] [Google Scholar]
- Falony G., Joossens M., Vieira-Silva S., Wang J., Darzi Y., Faust K., et al. (2016). Population-Level Analysis of Gut Microbiome Variation. Science 352, 560–564. 10.1126/science.aad3503 [DOI] [PubMed] [Google Scholar]
- Fettweis J. M., Brooks J. P., Serrano M. G., Sheth N. U., Girerd P. H., Edwards D. J., et al. (2014). Differences in Vaginal Microbiome in African American Women Versus Women of European Ancestry. Microbiology 160, 2272–2282. 10.1099/mic.0.081034-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierer N., Lauber C. L., Zhou N., McDonald D., Costello E. K., Knight R. (2010). Forensic Identification Using Skin Bacterial Communities. Proc. Natl. Acad. Sci. 107, 6477–6481. 10.1073/pnas.1000162107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findley K., Williams D. R., Grice E. A., Bonham V. L. (2016). Health Disparities and the Microbiome. Trends Microbiol. 24, 847–850. 10.1016/j.tim.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores G. E., Bates S. T., Knights D., Lauber C. L., Stombaugh J., Knight R., et al. (2011). Microbial Biogeography of Public Restroom Surfaces. PLoS One 6, e28132. 10.1371/journal.pone.0028132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores G. E., Caporaso J. G., Henley J. B., Rideout J. R., Domogala D., Chase J., et al. (2014). Temporal Variability Is a Personalized Feature of the Human Microbiome. Genome Biol. 15, 531. 10.1186/s13059-014-0531-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francuzik W., Franke K., Schumann R. R., Heine G., Worm M. (2018). Propionibacterium Acnes Abundance Correlates Inversely With Staphylococcus Aureus: Data From Atopic Dermatitis Skin Microbiome. Acta Derm. Venereol. 98, 490–495. 10.2340/00015555-2896 [DOI] [PubMed] [Google Scholar]
- Franzosa E. A., Huang K., Meadow J. F., Gevers D., Lemon K. P., Bohannan B. J., et al. (2015). Identifying Personal Microbiomes Using Metagenomic Codes. Proc. Natl. Acad. Sci. 112, 2930–2938. 10.1073/pnas.1423854112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García M. G., Pérez-Cárceles M. D., Osuna E., Legaz I. (2020). Impact of the Human Microbiome in Forensic Sciences: A Systematic Review. Appl. Environ. Microbiol. 86, 01451–01420. 10.1128/AEM.01451-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg K. B., Ganguli I., Das R., Talwar G. P. (2009). Spectrum of Lactobacillus Species Present in Healthy Vagina of Indian Women. Indian J. Med. Res. 129, 652–657. [PubMed] [Google Scholar]
- Ghemrawi M., Torres A. R., Duncan G., Colwell R., Dadlani M., McCord B. (2021). The Genital Microbiome and Its Potential for Detecting Sexual Assault. Forensic Sci. Int. Genet. 51, 102432. 10.1016/j.fsigen.2020.102432 [DOI] [PubMed] [Google Scholar]
- Gloor G. B., Macklaim J. M., Pawlowsky-Glahn V., Egozcue J. J. (2017). Microbiome Datasets Are Compositional: And This Is Not Optional. Front. Microbiol. 8, 2224. 10.3389/fmicb.2017.02224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Gallego C., Garcia-Mantrana I., Salminen S., Collado M. C. (2016). The Human Milk Microbiome and Factors Influencing Its Composition and Activity. Semin. Fetal Neonatal Med. 21, 400–405. 10.1016/j.siny.2016.05.003 [DOI] [PubMed] [Google Scholar]
- Gonzalez A., Hyde E., Sangwan N., Gilbert J. A., Viirre E., Knight R. (2016). Migraines Are Correlated With Higher Levels of Nitrate-, Nitrite-, and Nitric Oxide-Reducing Oral Microbes in the American Gut Project Cohort. MSystems 1, e00105–16. 10.1128/mSystems.00023-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham N. S., Reich B. J., Laber E. B., Pacifici K., Dunn R. R., Fierer N., et al. (2019). Global Forensic Geolocation With Deep Neural Networks. arXiv 1905, 11765. abs/1905.11765 [Google Scholar]
- Grice E. A., Kong H. H., Conlan S., Deming C. B., Davis J., Young A. C., et al. (2009). Topographical and Temporal Diversity of the Human Skin Microbiome. Science 324, 1190–1192. 10.1126/science.1171700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V. K., Paul S., Dutta C. (2017). Geography, Ethnicity or Subsistence-Specific Variations in Human Microbiome Composition and Diversity. Front. Microbiol. 8, 1162. 10.3389/fmicb.2017.01162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachem M., Ahmad H., Pilankar I., Abdelrahim I., ALFalasi F., Asif Z. (2020). Advances in Human Microbiome as an Emerging Tool in Forensics. Int. J. Emerg. Technol. Learn. 11, 70–76. 10.1111/1751-7915.12699 [DOI] [Google Scholar]
- Hampton-Marcell J. T., Lopez J. V., Gilbert J. A. (2017). The Human Microbiome: An Emerging Tool in Forensics. Microb. Biotechnol. 10, 228–230. 10.1111/1751-7915.12699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimesaat M. M., Boelke S., Fischer A., Haag L.-M., Loddenkemper C., Kühl A. A., et al. (2012). Comprehensive Postmortem Analyses of Intestinal Microbiota Changes and Bacterial Translocation in Human Flora Associated Mice. PLoS One 7, e40758. 10.1371/journal.pone.0040758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hospodsky D., Pickering A. J., Julian T. R., Miller D., Gorthala S., Boehm A. B., et al. (2014). Hand Bacterial Communities Vary Across Two Different Human Populations. Microbiology 160, 1144–1152. 10.1099/mic.0.075390-0 [DOI] [PubMed] [Google Scholar]
- Human Microbiome Project Consortium (2012). Structure, Function and Diversity of the Healthy Human Microbiome. Nature 486, 207–214. 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javan G. T., Finley S. J., Can I., Wilkinson J. E., Hanson J. D., Tarone A. M. (2016). Human Thanatomicrobiome Succession and Time Since Death. Sci. Rep. 6, 1–9. 10.1038/srep29598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan D. B. (2003). Investigation of Bioterrorism-Related Anthrax, United States 2001: Epidemiologic Findings. Emerg. Infect. Dis. 8, 1019–1028. 10.3201/eid0810.020353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha A. R., Davenport E. R., Gautam Y., Bhandari D., Tandukar S., Ng K. M., et al. (2018). Gut Microbiome Transition Across a Lifestyle Gradient in Himalaya. PLoS Biol. 16, e2005396. 10.1371/journal.pbio.2005396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L., Tao L., Pavlova S. I., So J. S., Kiwanuka N., Namukwaya Z., et al. (2007). Species Diversity and Relative Abundance of Vaginal Lactic Acid Bacteria From Women in Uganda and Korea. J. Appl. Microbiol. 102, 1107–1115. 10.1111/j.1365-2672.2006.03147.x [DOI] [PubMed] [Google Scholar]
- Kapoor P., Chowdhry A. (2018). Salivary Signature in Forensic Profiling: A Scoping Review. J. Forensic Dent. Sci. 10, 123–127. 10.4103/jfo.jfds_30_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kembel S. W., Meadow J. F., O'Connor T. K., Mhuireach G., Northcutt D., Kline J., et al. (2014). Architectural Design Drives the Biogeography of Indoor Bacterial Communities. PLoS One 9, e87093. 10.1371/journal.pone.0087093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersulyte D., Kalia A., Gilman R. H., Mendez M., Herrera P., Cabrera L., et al. (2010). Helicobacter Pylori From Peruvian Amerindians: Traces of Human Migrations in Strains From Remote Amazon, and Genome Sequence of an Amerind Strain. PLoS One 5, e15076. 10.1371/journal.pone.0015076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon S. J., Ravel J., Huston W. M. (2018). Cervicovaginal Microbiota, Women's Health, and Reproductive Outcomes. Fertil. Steril. 110, 327–336. 10.1016/j.fertnstert.2018.06.036 [DOI] [PubMed] [Google Scholar]
- Kuczynski J., Lauber C. L., Walters W. A., Parfrey L. W., Clemente J. C., Gevers D., et al. (2012). Experimental and Analytical Tools for Studying the Human Microbiome. Nat. Rev. Genet. 13, 47–58. 10.1038/nrg3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper I. (2016). Microbial Forensics: Next-Generation Sequencing as Catalyst: The Use of New Sequencing Technologies to Analyze Whole Microbial Communities Could Become a Powerful Tool for Forensic and Criminal Investigations. EMBO Rep. 17, 1085–1087. 10.15252/embr.201642794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz T. M., Gilbert J. A. (2017). Introducing the Microbiome Into Precision Medicine. Trends Pharmacol. Sci. 38, 81–91. 10.1016/j.tips.2016.10.001 [DOI] [PubMed] [Google Scholar]
- Lax S., Hampton-Marcell J. T., Gibbons S. M., Colares G. B., Smith D., Eisen J. A., et al. (2015). Forensic Analysis of the Microbiome of Phones and Shoes. Microbiome 3, 21. 10.1186/s40168-015-0082-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay C., Sutren M., Rochet V., Saunier K., Dore J., Rigottier-Gois L. (2005). Design and Validation of 16S rRNA Probes to Enumerate Members of the Clostridium Leptum Subgroup in Human Faecal Microbiota. Environ. Microbiol. 7, 933–946. 10.1111/j.1462-2920.2005.00763.x [DOI] [PubMed] [Google Scholar]
- Leake S. L., Pagni M., Falquet L., Taroni F., Greub G. (2016). The Salivary Microbiome for Differentiating Individuals: Proof of Principle. Microbes Infect. 18, 399–405. 10.1016/j.micinf.2016.03.011 [DOI] [PubMed] [Google Scholar]
- Lee S.-Y., Woo S.-K., Choi G.-W., Hong Y.-J., Eom Y.-B. (2015). Microbial Forensic Analysis of Bacterial Fingerprint by Sequence Comparison of 16S rRNA Gene. J. Forensics Res. 6, 1. 10.4172/2157-7145.1000297 [DOI] [Google Scholar]
- Lee S.-Y., Woo S.-K., Lee S.-M., Eom Y.-B. (2016). Forensic Analysis Using Microbial Community Between Skin Bacteria and Fabrics. J. Toxicol. Environ. 8, 263–270. 10.1007/s13530-016-0284-y [DOI] [Google Scholar]
- Li K., Bihan M., Yooseph S., Methe B. A. (2012). Analyses of the Microbial Diversity Across the Human Microbiome. PLoS One 7, e32118. 10.1371/journal.pone.0032118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Quinque D., Horz H. P., Li M., Rzhetskaya M., Raff J. A., et al. (2014). Comparative Analysis of the Human Saliva Microbiome From Different Climate Zones: Alaska, Germany, and Africa. BMC Microbiol. 14, 316. 10.1186/s12866-014-0316-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Price J., Abu-Ali G., Huttenhower C. (2016). The Healthy Human Microbiome. Genome Med. 8, 51. 10.1186/s13073-016-0307-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luongo J. C., Barberan A., Hacker-Cary R., Morgan E. E., Miller S. L., Fierer N. (2017). Microbial Analyses of Airborne Dust Collected From Dormitory Rooms Predict the Sex of Occupants. Indoor Air 27, 338–344. 10.1111/ina.12302 [DOI] [PubMed] [Google Scholar]
- Lynch S. V., Pedersen O. (2016). The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 375, 2369–2379. 10.1056/NEJMra1600266 [DOI] [PubMed] [Google Scholar]
- Madhivanan P., Alleyn H. N., Raphael E., Krupp K., Ravi K., Nebhrajani R., et al. (2015). Identification of Culturable Vaginal Lactobacillus Species Among Reproductive Age Women in Mysore, India. J. Med. Microbiol. 64, 636. 10.1099/jmm.0.000070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashima I., Theodorea C. F., Thaweboon B., Thaweboon S., Vichayanrat T., Scannapieco F. A., et al. (2019). Characterization of the Salivary Microbiome in Healthy Thai Children. Asian Pac. J. Trop. Med. 12, 163. 10.4103/1995-7645.257116 [DOI] [Google Scholar]
- Mc L. N., Rosenstein I. J. (2000). Characterisation and Selection of a Lactobacillus Species to Re-Colonise the Vagina of Women With Recurrent Bacterial Vaginosis. J. Med. Microbiol. 49, 543–552. 10.1099/0022-1317-49-6-543 [DOI] [PubMed] [Google Scholar]
- Mirmonsef P., Hotton A. L., Gilbert D., Gioia C. J., Maric D., Hope T. J., et al. (2016). Glycogen Levels in Undiluted Genital Fluid and Their Relationship to Vaginal pH, Estrogen, and Progesterone. PLoS One 11, e0153553. 10.1371/journal.pone.0153553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugesan S., Al Ahmad S. F., Singh P., Saadaoui M., Kumar M., Al Khodor S. (2020). Profiling the Salivary Microbiome of the Qatari Population. J. Transl. Med. 18, 127. 10.1186/s12967-020-02291-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa S., Motani-Saitoh H., Inoue H., Iwase H. (2013). Geographic Diversity of Helicobacter Pylori in Cadavers: Forensic Estimation of Geographical Origin. Forensic Sci. Int. 229, 7–12. 10.1016/j.forsciint.2013.02.028 [DOI] [PubMed] [Google Scholar]
- Nam Y.-D., Jung M.-J., Roh S. W., Kim M.-S., Bae J.-W. (2011). Comparative Analysis of Korean Human Gut Microbiota by Barcoded Pyrosequencing. PLoS One 6, e22109. 10.1371/journal.pone.0022109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasidze I., Li J., Quinque D., Tang K., Stoneking M. (2009. a). Global Diversity in the Human Salivary Microbiome. Genome Res. 19, 636–643. 10.1101/gr.084616.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasidze I., Quinque D., Li J., Li M., Tang K., Stoneking M. (2009. b). Comparative Analysis of Human Saliva Microbiome Diversity by Barcoded Pyrosequencing and Cloning Approaches. Anal. Biochem. 391, 64–68. 10.1016/j.ab.2009.04.034 [DOI] [PubMed] [Google Scholar]
- Neckovic A., AH van Oorschot R., Szkuta B., Durdle A. (2020). Challenges in Human Skin Microbial Profiling for Forensic Science: A Review. Genes 11, 1015. 10.3390/genes11091015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J., Byrd A. L., Deming C., Conlan S., Program N. C. S., Kong H. H., et al. (2014). Biogeography and Individuality Shape Function in the Human Skin Metagenome. Nature 514, 59–64. 10.1038/nature13786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J., Byrd A. L., Park M., Program N. C. S., Kong H. H., Segre J. A. (2016). Temporal Stability of the Human Skin Microbiome. Cell 165, 854–866. 10.1016/j.cell.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old J. B., Schweers B. A., Boonlayangoor P. W., Reich K. A. (2009). Developmental Validation of RSID™-Saliva: A Lateral Flow Immunochromatographic Strip Test for the Forensic Detection of Saliva. J. Forensic Sci. 54, 866–873. 10.1111/j.1556-4029.2009.01055.x [DOI] [PubMed] [Google Scholar]
- Pavlova S. I., Kilic A. O., Kilic S. S., So J. S., Nader-Macias M. E., Simoes J. A., et al. (2002). Genetic Diversity of Vaginal Lactobacilli From Women in Different Countries Based on 16S rRNA Gene Sequences. J. Appl. Microbiol. 92, 451–459. 10.1046/j.1365-2672.2002.01547.x [DOI] [PubMed] [Google Scholar]
- Pendharkar S., Magopane T., Larsson P. G., de Bruyn G., Gray G. E., Hammarstrom L., et al. (2013). Identification and Characterisation of Vaginal Lactobacilli From South African Women. BMC Infect. Dis. 13, 43. 10.1186/1471-2334-13-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez G. I. P., Gao Z., Jourdain R., Ramirez J., Gany F., Clavaud C., et al. (2016). Body Site Is a More Determinant Factor Than Human Population Diversity in the Healthy Skin Microbiome. PLoS One 11, e0151990. 10.1371/journal.pone.0151990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K., Barash M., Spindler X., Gunn P., Roux C. (2020). Retrieving Forensic Information About the Donor Through Bacterial Profiling. Int. J. Leg. Med. 134, 21–29. 10.1007/s00414-019-02069-2 [DOI] [PubMed] [Google Scholar]
- Pramanick R., Parab S., Mayadeo N., Warke H., Aranha C. (2017). Diversity of Cultivable Vaginal Microbiota in Asymptomatic Women of Reproductive Age in Mumbai, India. Can. J. Biotechnol. 1, 129. 10.24870/cjb.2017-a115 [DOI] [Google Scholar]
- Ramadan M., Solyman S., Taha M., Hanora A. (2016). Preliminary Characterization of Human Skin Microbiome in Healthy Egyptian Individuals. Cell. Mol. Biol. 62, 21–27. 10.14715/cmb/2016.62.8.4 [DOI] [PubMed] [Google Scholar]
- Ranjan R., Rani A., Metwally A., McGee H. S., Perkins D. L. (2016). Analysis of the Microbiome: Advantages of Whole Genome Shotgun Versus 16S Amplicon Sequencing. Biochem. Biophys. Res. Commun. 469, 967–977. 10.1016/j.bbrc.2015.12.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel J., Gajer P., Abdo Z., Schneider G. M., Koenig S. S., McCulle S. L., et al. (2011). Vaginal Microbiome of Reproductive-Age Women. Proc. Natl. Acad. Sci. 108, 4680–4687. 10.1073/pnas.1002611107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson M., Gottel N., Gilbert J. A., Lax S. (2019). Microbial Similarity Between Students in a Common Dormitory Environment Reveals the Forensic Potential of Individual Microbial Signatures. MBio 10, e01054–e01019. 10.1128/mBio.01054-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M., Pasternak Z., Mason C. E., Elhaik E. (2021). Forensic Applications of Microbiomics: A Review. Front. Microbiol. 11, 608101. 10.3389/fmicb.2020.608101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmedes S., Budowle B. (2019). Microbial Forensics. Encyclopedia Microbiol. 134–135. 10.1016/B978-0-12-801238-3.02483-1 [DOI] [Google Scholar]
- Schmedes S. E., Sajantila A., Budowle B. (2016). Expansion of Microbial Forensics. J. Clin. Microbiol. 54, 1964–1974. 10.1128/JCM.00046-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmedes S. E., Woerner A. E., Budowle B. (2017). Forensic Human Identification Using Skin Microbiomes. Appl. Environ. Microbiol. 83, e01672–17. 10.1128/AEM.01672-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmedes S. E., Woerner A. E., Novroski N. M. M., Wendt F. R., King J. L., Stephens K. M., et al. (2018). Targeted Sequencing of Clade-Specific Markers From Skin Microbiomes for Forensic Human Identification. Forensic Sci. Int. Genet. 32, 50–61. 10.1016/j.fsigen.2017.10.004 [DOI] [PubMed] [Google Scholar]
- Scott K. P., Gratz S. W., Sheridan P. O., Flint H. J., Duncan S. H. (2013). The Influence of Diet on the Gut Microbiota. Pharmacol. Res. 69, 52–60. 10.1016/j.phrs.2012.10.020 [DOI] [PubMed] [Google Scholar]
- Shin J. H., Sim M., Lee J. Y., Shin D. M. (2016). Lifestyle and Geographic Insights Into the Distinct Gut Microbiota in Elderly Women From Two Different Geographic Locations. J. Physiol. Anthropol. 35, 31. 10.1186/s40101-016-0121-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Clarke T., Brinkac L., Greco C., Nelson K. E. (2021). Forensic Microbiome Database: A Tool for Forensic Geolocation Meta-Analysis Using Publicly Available 16s rRNA Microbiome Sequencing. Front. Microbiol. 12, 644861. 10.3389/fmicb.2021.644861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J., Kaur E. (2020). Microbial Forensics: A New Boon to Legal Justice. Microbial Diversity Interventions Scope 399–408. 10.1007/978-981-15-4099-8_22 [DOI] [Google Scholar]
- Siqueira J. F., Jr., Fouad A. F., Rocas I. N. (2012). Pyrosequencing as a Tool for Better Understanding of Human Microbiomes. J. Oral Microbiol. 4. 10.3402/jom.v4i0.10743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S. J., Lauber C., Costello E. K., Lozupone C. A., Humphrey G., Berg-Lyons D., et al. (2013). Cohabiting Family Members Share Microbiota With One Another and With Their Dogs. Elife 2, e00458. 10.7554/eLife.00458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita T., Matsuo K., Furuta M., Shibata Y., Fukami K., Shimazaki Y., et al. (2014). Distinct Composition of the Oral Indigenous Microbiota in South Korean and Japanese Adults. Sci. Rep. 4, 6990. 10.1038/srep06990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon D., Haque M. M., R S., Shaikh S., P S., Dubey A. K., et al. (2018). A Snapshot of Gut Microbiota of an Adult Urban Population From Western Region of India. PLoS One 13, e0195643. 10.1371/journal.pone.0195643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzo P., D’Angiolella G., Brun P., Castagliuolo I., Gino S., Caenazzo L. (2020). Skin Microbiome Analysis for Forensic Human Identification: What Do We Know So Far? Microorganisms 8, 873. 10.3390/microorganisms8060873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomisto S., Karhunen P. J., Vuento R., Aittoniemi J., Pessi T. (2013). Evaluation of Postmortem Bacterial Migration Using Culturing and Real-Time Quantitative PCR. J. Forensic Sci. 58, 910–916. 10.1111/1556-4029.12124 [DOI] [PubMed] [Google Scholar]
- Turnbaugh P. J., Ley R. E., Hamady M., Fraser-Liggett C. M., Knight R., Gordon J. I. (2007). The Human Microbiome Project. Nature 449, 804–810. 10.1038/nature06244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turroni F., Ribbera A., Foroni E., van Sinderen D., Ventura M. (2008). Human Gut Microbiota and Bifidobacteria: From Composition to Functionality. Anton. Leeuw. Int. J. G. 94, 35–50. 10.1007/s10482-008-9232-4 [DOI] [PubMed] [Google Scholar]
- Vasquez A., Jakobsson T., Ahrne S., Forsum U., Molin G. (2002). Vaginal Lactobacillus Flora of Healthy Swedish Women. J. Clin. Microbiol. 40, 2746–2749. 10.1128/jcm.40.8.2746-2749.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhelst R., Verstraelen H., Claeys G., Verschraegen G., Delanghe J., Van Simaey L., et al. (2004). Cloning of 16S rRNA Genes Amplified From Normal and Disturbed Vaginal Microflora Suggests a Strong Association Between Atopobium Vaginae, Gardnerella Vaginalis and Bacterial Vaginosis. BMC Microbiol. 4, 16. 10.1186/1471-2180-4-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraelen H., Vilchez-Vargas R., Desimpel F., Jauregui R., Vankeirsbilck N., Weyers S., et al. (2016). Characterisation of the Human Uterine Microbiome in Non-Pregnant Women Through Deep Sequencing of the V1-2 Region of the 16S rRNA Gene. PeerJ 4, e1602. 10.7717/peerj.1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virkler K., Lednev I. K. (2009). Analysis of Body Fluids for Forensic Purposes: From Laboratory Testing to non-Destructive Rapid Confirmatory Identification at a Crime Scene. Forensic Sci. Int. 188, 1–17. 10.1016/j.forsciint.2009.02.013 [DOI] [PubMed] [Google Scholar]
- Voreades N., Kozil A., Weir T. L. (2014). Diet and the Development of the Human Intestinal Microbiome. Front. Microbiol. 5, 494. 10.3389/fmicb.2014.00494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin S. S., Linhares I. M., Giraldo P. (2007). Bacterial Flora of the Female Genital Tract: Function and Immune Regulation. Best Pract. Res. Clin. Obstet. Gynaecol. 21, 347–354. 10.1016/j.bpobgyn.2006.12.004 [DOI] [PubMed] [Google Scholar]
- Woerner A. E., Novroski N. M., Wendt F. R., Ambers A., Wiley R., Schmedes S. E., et al. (2019). Forensic Human Identification With Targeted Microbiome Markers Using Nearest Neighbor Classification. Forensic Sci. Int. Genet. 38, 130–139. 10.1016/j.fsigen.2018.10.003 [DOI] [PubMed] [Google Scholar]
- Wu Y., Chi X., Zhang Q., Chen F., Deng X. (2018). Characterization of the Salivary Microbiome in People With Obesity. PeerJ 6, e4458. 10.7717/peerj.4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Daroczy K., Xiao B., Yu L., Chen R., Liao Q. (2012). Qualitative and Semiquantitative Analysis of Lactobacillus Species in the Vaginas of Healthy Fertile and Postmenopausal Chinese Women. J. Med. Microbiol. 61, 729–739. 10.1099/jmm.0.038687-0 [DOI] [PubMed] [Google Scholar]
- Zhang J., Guo Z., Xue Z., Sun Z., Zhang M., Wang L., et al. (2015). A Phylo-Functional Core of Gut Microbiota in Healthy Young Chinese Cohorts Across Lifestyles, Geography and Ethnicities. ISME J. 9, 1979–1990. 10.1038/ismej.2015.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhernakova A., Kurilshikov A., Bonder M. J., Tigchelaar E. F., Schirmer M., Vatanen T., et al. (2016). Population-Based Metagenomics Analysis Reveals Markers for Gut Microbiome Composition and Diversity. Science 352, 565–569. 10.1126/science.aad3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Hansmann M. A., Davis C. C., Suzuki H., Brown C. J., Schutte U., et al. (2010). The Vaginal Bacterial Communities of Japanese Women Resemble Those of Women in Other Racial Groups. FEMS Immunol. Med. Microbiol. 58, 169–181. 10.1111/j.1574-695X.2009.00618.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu A., Sunagawa S., Mende D. R., Bork P. (2015). Inter-Individual Differences in the Gene Content of Human Gut Bacterial Species. Genome Biol. 16, 1–13. 10.1186/s13059-015-0646-9 [DOI] [PMC free article] [PubMed] [Google Scholar]