Abstract
Eight analogues of the bioherbicides macrocidin A (1) and Z (2) with structural variance in the size of the macrocycle, its para- or meta-cyclophane character, and its functional groups were synthesized on two modular routes and tested for herbicidal, antibiotic, and antibiofilm activities. Apart from the lead compounds 1 and 2, the structurally simplified dihydromacrocidin Z (3) and normacrocidin Z (4) showed high herbicidal activity in either thistles, dandelions or in both. The derivatives 2, 3, and dibromide 9 also inhibited the growth of Staphylococcus aureus biofilms by ca 70% when applied at subtoxic concentrations as low as ca 20 µM, which are unlikely to induce bacterial resistance. They also led to the dispersion of preformed biofilms of S. aureus, exceeding a similar effect by microporenic acid A, a known biofilm inhibitor. Compounds 3 and 9 showed no noticeable cytotoxicity against human cancer and endothelial cells at concentrations below 50 µM, making them conceivable candidates for application as anti-biofilm agents in a medicinal context.
Keywords: macrocidin, polycyclic tetramate macrolactams, 3-acyltetramic acids, antibiotics, biofilms
1. Introduction
Macrocidins are polycyclic tetramic acid macrolactams (PTMs). Macrocidin A (1; Figure 1) was first isolated from the fungus Phoma macrostoma Montagne in 2003 by a Dow AgroSciences group headed by Graupner [1]. It was found to induce chlorosis in broadleaf weeds by a unique mode of action implying an interference with the phytoene synthase and desaturase in the chlorophyll and carotenoid biosynthesis [2]. So far, only two total syntheses of macrocidin A (1) have been published [3,4]. Macrocidin Z (2), which carries an E-alkene in lieu of the expoxide, was isolated from Phoma macrostoma cultures and synthesised in parallel by us only recently [5]. Although concentrated Phoma macrostoma cultures, formulated as broadcast granules, are being used as bioherbicides for environment-friendly weed management in the US and Canada, efforts towards the synthesis of simplified macrocidin derivatives with improved herbicidal properties were sporadically made (e.g., by Graupner’s group [6] and Syngenta [7]), albeit without disclosing details. As 3-acyltetramic acids from a broad range of sources were found to have antibiotic or biofilm inhibitory effects [8,9,10,11,12] (e.g., by us in the case of macrocidin Z (2) [5]), we now synthesised analogues of the natural macrocidins with variation of the structural key features such as the size of the macrocycle, its para- or meta-cyclophane character, and its decoration with functional groups other than epoxide. We tested them for herbicidal, antibacterial, and antibiofilm activities, and for cytotoxicity to human cells.
2. Results
2.1. Chemistry
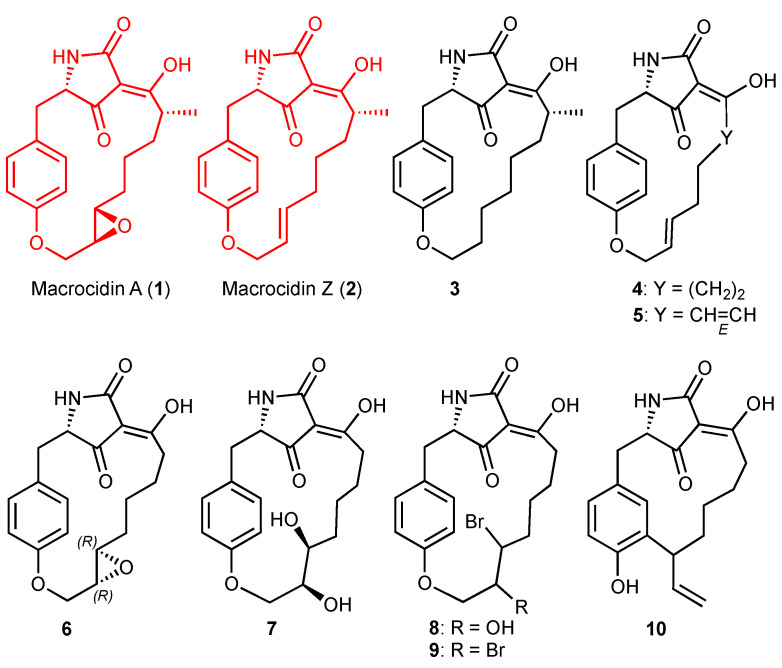
Figure 1 depicts eight new derivatives 3–10 that were prepared on two efficient modular routes. They share the same tetramic acid derived from l-tyrosine, yet differ in their degree of resemblance to the lead macrocidins (1) and (2). The dihydro derivate 3 of macrocidin Z is the only one retaining the methyl group at an R-configured stereocenter. Like macrocidin Z (2), the derivatives 4 and 5 feature E-alkenes in lieu of the epoxide. Normacrocidin A (6) lacks the methyl group while retaining the epoxide, albeit with a non-natural R,R configuration. In compound 7, the epoxide is replaced by a vicinal diol, in derivative 8 by a bromohydrin, and in 9 by a vicinal dibromide. Furthest from the natural leads 1 and 2 (in terms of structure) is derivative 10, which has a 13-membered instead of a 17-membered macrocycle comprising a meta- rather than a para-cyclophane.
Figure 1.
Structures of the natural lead compounds macrocidin A (1) and macrocidin Z (2) and structural variants 3–10.
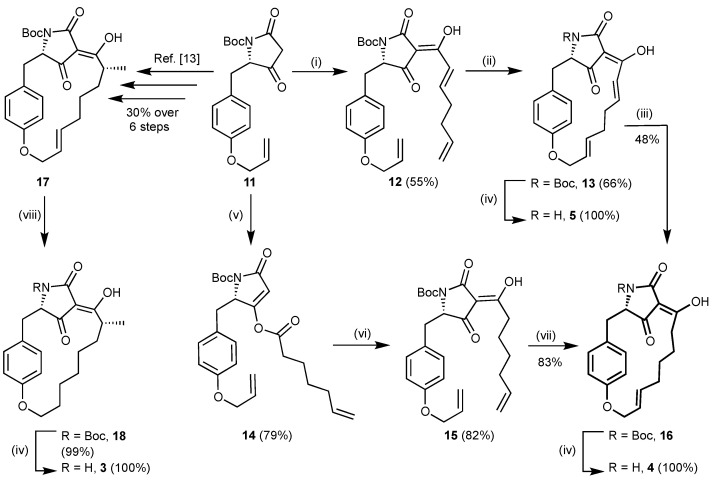
In Scheme 1, two alternative synthetic approaches to key intermediate 16, an N-Boc-protected normacrocidin Z, are illustrated. The known l-tyrosine derived tetramic acid 11 [4] was furnished with a 3-(hepta-2,6-dienoyl) residue by a one-pot reaction first with ketenylidentriphenylphosphorane, Ph3PCCO, to give the corresponding 3-acyl ylide (not shown), followed by a Wittig olefination of the latter with 4-pentenal to leave 3-acyltetramic acid 12 in 55% yield [13]. An E-selective ring-closing metathesis reaction with a Grubbs type-II catalyst gave macrocycle 13 in 66% yield as a single stereoisomer. Its reduction with Wilkinson’s catalyst and triethylsilane to afford a silyl enol ether (not shown), followed by its cleavage with KF, gave N-Boc-protected normacrocidin Z 16 in a 48% yield. Removal of the N-Boc-protecting group from compounds 16 and 13 using TFA left test candidates 4 and 5 as pure stereoisomers. For the synthesis of larger quantities of 16, another route was developed also starting from tetramic acid 11 in analogy to our syntheses of macrocidins A and Z [4,5]. 3-Acyltetramic acid 15 was built up by 4-O-acylation of 11 with 6-heptenoic acid and subsequent rearrangement of tetramate 14. Ring-closing metathesis of 15 with Grubbs type-II catalyst afforded key intermediate 16 in 54% total yield over three steps. Enantiopure dihydromacrocidin Z (3) was obtained from N-Boc-protected macrocidin Z 17 by hydrogenation and removal of the N-Boc-protecting group from 18 in 99% yield over two steps [5].
Scheme 1.
Syntheses of key intermediate 16 and of macrocidin derivatives 3, 4, and 5. Reagents and conditions: (i) Ph3PCCO, THF, reflux, 2 h; then KOtBu, THF, reflux, 20 min; then 4-pentenal, THF, reflux → rt, 21 h; (ii) Grubbs II catalyst, CH2Cl2, reflux, 18 h; (iii) Rh(PPh3)3Cl, Et3SiH, CH2Cl2, reflux, 19 h; then KF, MeOH, −15 °C, 27 h; (iv) TFA, CH2Cl2, rt, 15 min; (v) EDC HCl, DMAP, 6-heptenoic acid, CH2Cl2, 0 °C → rt, 4 h; (vi) NEt3, DMAP, CH2Cl2, rt, 24 h; (vii) Grubbs II catalyst, CH2Cl2, reflux, 24 h; (viii) H2 (1 atm), Pd/C, EtOAc, rt, 31 h.
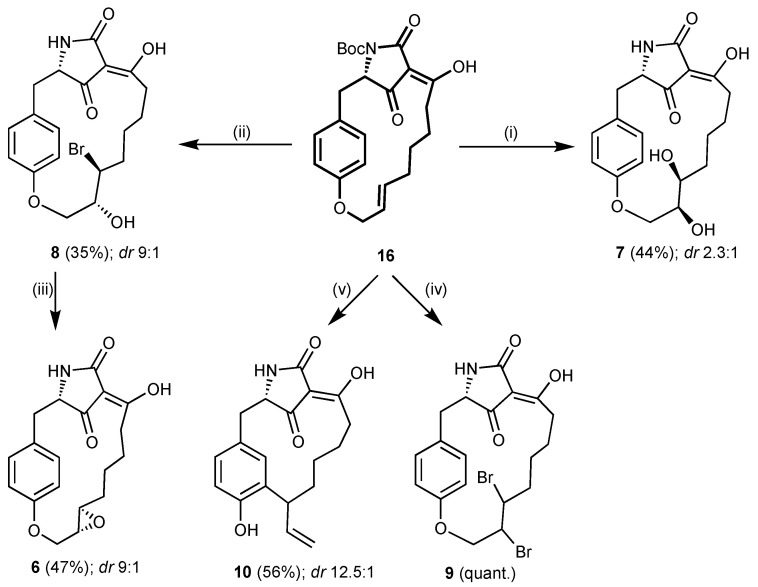
Key intermediate 16 was also used to introduce further functionalizations, formally replacing the epoxide in macrocidin A (1). Its hydroxylation with AD-mix α afforded, after deprotection, diol 7 as an inseparable 2.3:1 mixture of two diastereomers (Scheme 2 shows major diastereomer). Alkene 16 was also converted to an inseparable mixture of two diastereomeric bromohydrins 8 with NBS and H2O in DMSO [14,15]. A side product (16%) of this reaction, carrying an additional bromo residue next to the enol group, could be separated. Upon treatment with KOtBu, the bromohydrins 8 were converted in 47% yield to a 9:1 mixture of expoxides 6 as indicated by 1H NMR spectra. The configuration of the major isomer of 6 (shown in Scheme 2) was assigned by comparison with a mixture of isomers 6 obtained on a different route. Hence, we assume that the dr of precursor bromohydrins 8 was also 9:1 as for the epoxides 6. After futile attempts by Ramana et al. [16] and our group at direct epoxidation of alkenes such as 4, 16, or 17, this was the first time the epoxide function could be installed in the preformed macrocycle of a macrocidin precursor. Alkene 16 could also be brominated with bromine in CCl4 to give vicinal dibromide 9 as a mixture of two diastereomers with trans-positioned bromo residues as to NMR spectra. Finally, when heated in diethylaniline in a sealed tube, the para-cyclophane 16 underwent a Claisen rearrangement to afford meta-cyclophane 10 in 56% yield and as a 12.5:1 mixture of diastereomers according to NMR spectra. Like the stereopure derivatives 3–5, the diastereomeric mixtures of derivatives 6–10 were tested for bioactivity, although in the case of diol 7 the two diastereomers, present in similar proportions, might dilute or cancel each other out in terms of biological activities. It should be noted, that most derivatives shown in Scheme 1 and Scheme 2 were difficult to purify and analyze. Sometimes, only multiple reversed-phase column chromatography runs led to the desired degree of purity. Their NMR spectra (cf. Supporting Information, SI) are rather complex due to the tautomerization of the 3-acyltetramic acid moiety [17].
Scheme 2.
Modular synthesis of macrocidin derivatives 6–10 starting from key intermediate 16. Reagents and conditions: (i) AD-mix α, tBuOH/H2O, 7 °C, 9 d; then TFA, CH2Cl2, rt, 15 min; (ii) NBS, H2O, DMSO, 8 °C → rt, 22 h; then TFA, CH2Cl2, rt, 15 min; (iii) KOtBu, THF, 0 °C → rt, 4 d; (iv) Br2, CCl4, 80 °C, 30 h; (v) diethylaniline, sealed tube, 190 °C, 42 h.
2.2. Herbicidal Activity
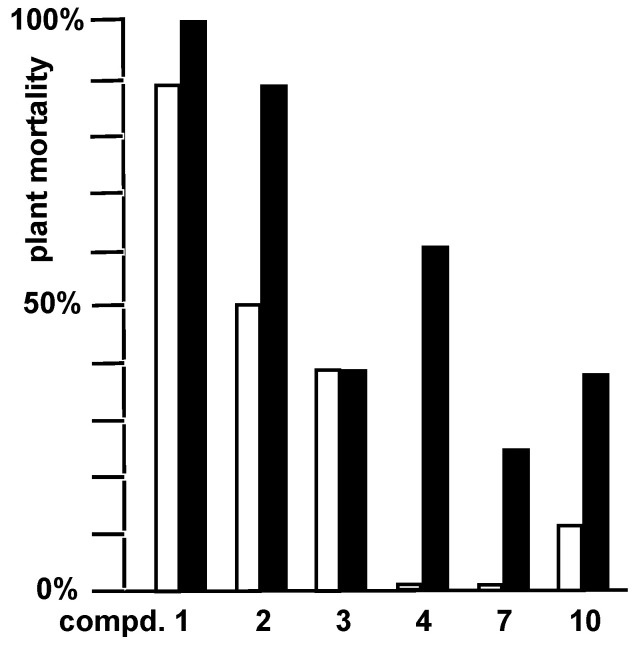
Prior to screening them for antimicrobial effects, the new derivatives were tested for herbicidal activity against thistles and dandelions which had been found to be susceptible to the chlorosis-inducing natural macrocidins [2,6]. For both species, they were applied as max. 150 mM solutions to four pots with two plants each, and their bleaching, withering, and necrotizing effects were assessed after two and then after three to six weeks. None of the compounds reached the efficiency of the synthetic commercial herbicide diflufenican, which was used as a positive control. In line with literature, the lead compound macrocidin A (1) exhibited the highest maximum herbicidal efficiency of all tested compounds, causing 88% mortality of dandelions and 100% of thistles, three weeks after application of a 100 mM solution in a mixture of isopropanol/water = 1:1 + 0.25% Tween 20 (Figure 2). Interestingly, the epoxide appeared not to be crucial for herbicidal activity, since macrocidin Z (2) still displayed a high efficiency of 88% mortality in thistles and of 50% in dandelions after 42 days at 100 mM. Contrary to an earlier assumption by Graupner, Bailey et al. [6], even derivatives with saturated backbones may show herbicidal efficiency, e.g., dihydromacrocidin Z (3) (38% mortality in thistles and dandelions). The α-methyl group seemed to be important, apparent from the lower figures for normacrocidin Z (4) when compared to 2 (dandelions: 0%, thistles: 63% mortality, 35 days after treatment with 150 mM) and for S,R,R-normacrocidin A (6) which was virtually inactive against both plants. The 13-membered macrocyclic meta-cyclophane 10 exerted a maximum herbicidal efficiency with 38% mortality in thistles, yet only 13% in dandelions after four weeks at 150 mM. Normacrocidin Z (4) and diol 7 also displayed a distinct specificity for thistles over dandelions (for pictures of treated plants cf. Figure S68 in the SI).
Figure 2.
Percentage of final mortality of dandelions (white columns) and thistles (black columns) treated with 0.2 mL/plant of 100–150 mM solutions of active macrocidin derivatives after two to six weeks. Mortality by diflufenican was 100% for either plant species.
2.3. Antimicrobial Activity
As 3-acyltetramic acids were frequently shown to have antimicrobial effects [8,9,12,18,19,20], the new macrocidin derivatives were tested for activity against three different bacteria, namely the Gram-positive strain Staphylococcus aureus (SH1000) and the Gram-negative strains Acinetobacter baumannii, Escherichia coli with the wild-type strain K12 and the ΔTolC mutant (JW5503), which lacks the AcrAB−TolC efflux system. None of the macrocidin analogues displayed activity against the wild-type strain of E. coli. Weak activities against E. coli ΔTolC were found only for dibromide 9 (IC50 = 75 ± 15 µM), dihydromacrocidin Z (3) (IC50 = 82 ± 15 µM), and macrocidin Z (2) (IC50 ca 100 µM). These derivatives were also similarly active against S. aureus (cf. Table S1 for IC50 values and Figures S69–S70 for growth curves in the SI). In comparison, the clinically established antibiotic vancomycin was active with an IC50 of ca 12 µM against S. aureus, and the antibiotic erythromycin with a nanomolar IC50 value. None of the macrocidin derivatives showed a clear antibiotic effect on A. baumannii. Even when applied at the highest concentration of 100 µM, the compounds could not prevent the cultures from reaching an OD600 of at least 60–70% of the maximum value (cf. Figure S71 in the SI). Overall, the toxicity of macrocidin Z (2) and its new synthetic analogues 3–10 against bacteria is weak.
2.4. Antibiofilm Activity
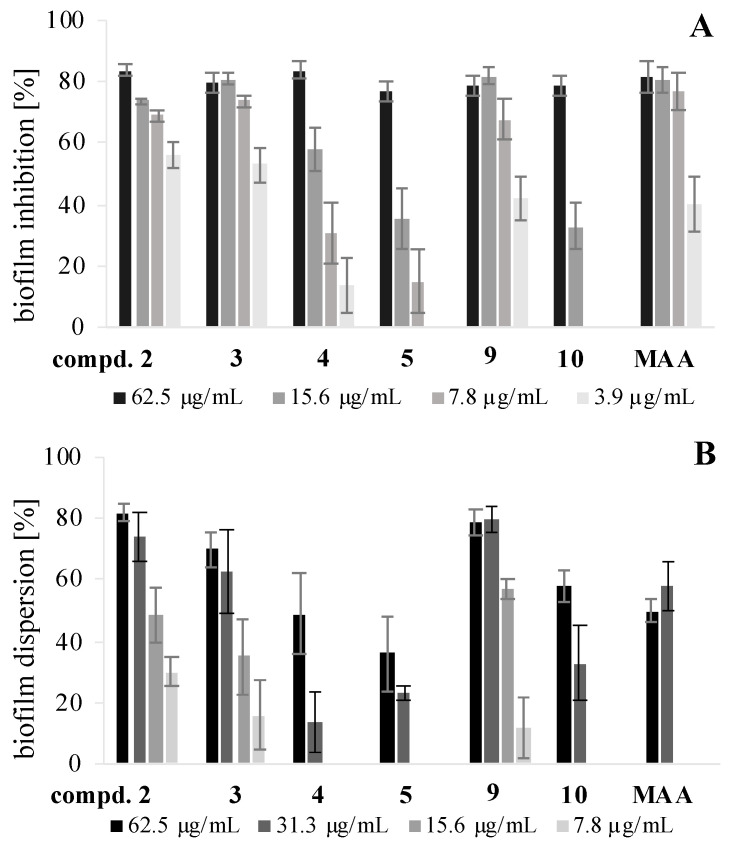
The macrocidinoids 2–10 were tested for inhibitory effects on the formation of biofilms by Staphylococcus aureus and Pseudomonas aeruginosa bacteria, as well as for dispersive effects on preformed biofilms of S. aureus and the fungal species Candida albicans (cf. SI for data Table S2). While all compounds 2–6 and 8–10 inhibited the formation of S. aureus biofilms by at least 75% relative to untreated controls (=0%) at the highest tested concentration of 250 µg/mL, only macrocidin Z (2), its dihydro derivative 3 and dibromide 9 caused a distinct biofilm inhibition of ca 70% when applied at subtoxic concentrations as low as 7.8 µg/mL (corresponding to 16 µM and 23 µM, respectively). These activities matched or even exceeded that of microporenic acid A (MAA), the known biofilm inhibitor [21] used as a positive control (Figure 3A). A second group of moderately active inhibitors, comprising normacrocidin Z (4), diene 5 and phenol 10, led to an inhibition of biofilm formation of more than 30% when applied at a concentration of 15.6 µg/mL (corresponding to 43–48 µM). The derivatives 7 and 8 had little inhibitory effect at concentrations below 250 µg/mL.
Figure 3.
Effects of various concentrations of compounds 2–5, 9, and 10 on the formation of (A) and the dispersion of preformed (B) biofilms of S. aureus; positive control: microporenic acid A (MAA); error bars indicate SD of two repeats with duplicates.
The dispersive effects on preformed biofilms of S. aureus were generally slightly less pronounced (Figure 3B). Derivatives 6–8 were inactive at all concentrations up to 250 µg/mL. The most distinct effects of at least 35% dispersion over the concentration range from 250 µg/mL down to 15.6 µg/mL were again observed for derivatives 2, 3, and 9, which clearly outperformed MAA. The latter was active only at the highest two concentrations, such as the compounds 4, 5, and 10. In the case of C. albicans, compounds 7 and 10 proved inactive, whereas the other compounds, including MAA, showed weak dispersive effects at very high concentrations (250–125 µg/mL) with derivate 5 being the only one active at a lower concentration of 62.5 µg/mL (192 µM) (cf. Figure S72 in the SI). None of the tested derivatives inhibited the formation of P. aeruginosa biofilms.
This study demonstrated that macrocidin analogues may interfere with the formation and persistence of bacterial and fungal biofilms, depending on their structure and polarity. The strongest effects against S. aureus were found for the lipophilic and structurewise “simple” derivatives 2–5 and 9. Interestingly, the activity against biofilms decreased, or even disappeared, when hydroxy groups were introduced into the molecule, as the derivatives 7 and 8 exemplify.
2.5. Cytotoxicity
The macrocidinoids 2, 3, and 6–10 were submitted to provisional MTT tests for cytotoxicity/antiproliferative effects against human 518A2 melanoma cells, colon carcinoma cells HCT-116wt and HCT-116p53−/−, and KBV cervix carcinoma cells as well as hybrid endothelial EaHy cells. Gratifyingly, none of the compounds but 2 caused signs of toxicity or inhibition of proliferation in the tested cells when applied at concentrations as high as 50 µM (cf. Table S3 in the SI). This is far outside of any clinically relevant range and would bode well for a potential future application as biofilm interfering agents in a medicinal context. Even macrocidin Z (2) which was antiproliferative with IC50 concentrations of ca. 15 to 30 µM in cells of colon carcinoma HCT-116 and cervical carcinoma KBV warrants a more in-depth study of its applicability as an anti-biofilm agent.
3. Discussion
We synthesised eight derivatives of the natural tetramic acids macrocidin A (1) and Z (2) on two efficient modular routes, which allow the introduction of various functionalities and scaffold modifications on a few key intermediates. The double bonds of intermediates 13 and 16 were converted in good yields to bromides, diols, bromohydrins and saturated bonds using standard reactions. A thermal Claisen rearrangement opened an easy access to a 13-membered macrocycle featuring a meta-cyclophane motif. For the first time, we could introduce the epoxide in a macrocidin precursor with a preformed macrocycle. The derivatives and the natural lead compound macrocidin Z (2) were tested for herbicidal and antimicrobial activity, as well as for biofilm interference. The incentive for this extended bioscreening were the frequent reports on the high incidence of antimicrobial and antifungal effects by 3-acyltetramic acids in general. Interestingly, the structurally simple compounds macrocidin Z (2) and dihydromacrocidin Z (3) showed a high herbicidal and antibiofilm activity. Normacrocidin Z (4) was selectively herbicidal against thistles, and dibromide 9 displayed an S. aureus biofilm dispersing effect surpassing even that of the known biofilm inhibitor microporenic acid A. With the exception of diol 7, which was moderately herbicidal against thistles, hydroxy groups on the alkyl backbone of the macrocycle, appear to be generally detrimental to both herbicidal and antibiofilm effects. Contrary to suppositions in the scant literature on macrocidinoids, the epoxide function is obviously not crucial to either activity. The observed distinct and strain-specific effects of the active macrocidinoids 2, 3, and 9 on the biofilms of S. aureus are all the more interesting, as their direct antibacterial activities (i.e., toxicities against bacteria) are rather limited. Thus, their application as biofilm inhibitors would probably not induce bacterial resistance. The at best marginal cytotoxicities of compounds 3 and 9 in human cancer and endothelial cells indicate that these compounds would presumably be well tolerated also by higher organisms. Compound 2 might indeed pose a toxicity problem which should be clarified prior to further tests as a biofilm inhibitor.
4. Materials and Methods
4.1. General Information
IR spectra were recorded with a PerkinElmer Spectrum 100 FT-IR spectrophotometer (PerkinElmer, Rodgau, Germany) with ATR sampling unit. Optical rotations were measured at 589 nm (Na-D line) on a PerkinElmer 241 polarimeter (PerkinElmer, Rodgau, Germany); [α]D values are given in 10−1 deg cm2 g−1. High resolution mass spectra were obtained with a UPLC/Orbitrap MS system in ESI mode (ThermoFisher Scientific, Bremen, Germany). NMR spectra were recorded with a Bruker Avance III HD 500 spectrometer (1H NMR: 500 MHz and 13C NMR: 125 MHz) (Bruker, Karlsruhe, Germany). Chemical shifts are given in parts per million, relative to the residual solvent peak as an internal standard, and coupling constants (J) are quoted in Hz. Most tetramic acids were measured in CDCl3 and also in CD3OD where they usually exist as a single (enol) tautomer. Quaternary C-atoms of tetramic acids were sometimes difficult to spot in JMOD or 13C NMR spectra. For these, more signals cropped up in HMBC and/or HSQC correlation spectra and were considered for peak assignment. In CDCl3 solution, signals of virtually all C-atoms of tetramic acids were visible yet split up in multiple, difficult to assign sets for individual tautomers both in 1H and JMOD/13C NMR spectra. In line with literature, we assume the tautomers with exocyclic C–C double bond as drawn for the 3-acyltetramic acids in Figure 1, to be the major tautomer [17]. For the purification of synthetic products, chromatography silica gel 60 (40–63 μm) or silica gel RP18 (40–63 μm) were used. Analytical thin layer chromatography (TLC) was carried out using Merck silica gel 60 F254 pre-coated aluminum-backed plates. Analytical HPLC was performed on a Shimadzu Nexera XR (Shimadzu GmbH, Duisburg, Germany) using a Knauer Eurospher II C18-column (150 × 4 mm) (Knauer GmbH, Berlin, Germany). Chiral HPLC was performed on a Beckmann System Gold Programmable Solvent Modul 126 using a Phenomenex Lux® Amylose-1-HPLC-column (100 × 4.6 mm) (Phenomenex Ltd., Aschaffenburg, Germany). All air- and water-sensitive reactions were carried out under a dry argon atmosphere.
4.2. Compounds
(S,Z)-tert-Butyl-2-(4-(allyloxy)benzyl)-4-((E)-1-hydroxyhepta-2,6-dien-1-ylidene)-3,5-dioxopyrrolidine-1-carboxylate (12). Tetramic acid 11 [4] (1.90 g, 5.50 mmol, 1.10 eq) in dry THF (305 mL) was treated with ketenylidenetriphenylphosphorane (1.66 g, 5.50 mmol, 1.10 eq) in dry THF (140 mL) over 20 min while refluxing. After stirring for 2 h, KOtBu (0.62 g, 5.50 mmol, 1.10 eq) was added. The solution was stirred for a further 20 min, before 4-pentenal (0.42 g, 5.00 mmol, 1.00 eq) in dry THF (65 mL) was added over a period of 15 min. Stirring at reflux was continued for 4 h and for 17 h at room temperature. The solvent was concentrated under reduced pressure and the crude product was dissolved in CH2Cl2 (300 mL). It was washed with sat. NH4Cl solution (200 mL). The aqueous phase was extracted with CH2Cl2 (3 × 100 mL), the combined organic phases were washed with brine (300 mL) and dried over Na2SO4. Removal of the solvent and purification by column chromatography on reversed phase silica gel (RP18, 40% MeCN in H2O + 0.1% HCOOH → 60% MeCN in H2O + 0.1% HCOOH → 70% MeCN in H2O + 0.1% HCOOH → 80% MeCN in H2O + 0.1% HCOOH → 100% MeCN + 0.1% HCOOH) afforded 3-acyltetramic acid 12 (1.24 g, 2.74 mmol, 55%). Rf = 0.88 (10% MeOH in CH2Cl2); −95.0° (c 1.00, MeOH); Major tautomer: 1H NMR (500 MHz, CD3OD) δ 7.29 (dt, J = 15.6, 6.9 Hz, 1H), 7.12 (d, J = 15.6 Hz, 1H), 6.90 (m, 2H), 6.76 (m, 2H), 6.01 (ddt, J = 17.3, 10.7, 5.3 Hz, 1H), 5.83 (ddt, J = 17.0, 10.3, 6.7 Hz, 1H), 5.34 (dq, J = 17.3, 1.6 Hz, 1H), 5.20 (dq, J = 10.7, 1.6 Hz, 1H), 5.08 (dq, J = 17.0, 1.4 Hz, 1H), 5.02 (dq, J = 10.3, 1.6 Hz, 1H), 4.53 (m, 1H), 4.47 (dt, J = 5.3, 1.6 Hz, 2H), 3.35 (dd, J = 14.0, 5.5 Hz, 1H), 3.20 (dd, J = 14.0, 2.6 Hz, 1H), 2.45 (q, J = 6.9 Hz, 2H), 2.27 (q, J = 6.9 Hz, 2H), 1.62 (s, 9H) ppm; Significant signals minor tautomer: 1H NMR (500 MHz, CD3OD) δ 5.00 (m, 2H), 4.60 (m, 1H), 4.43 (m, 2H), 3.39 (m, 1H), 3.07 (m, 1H), 2.10 (m, 2H) ppm; 13C NMR (125 MHz, CD3OD) δ 159.3, 153.9, 138.2, 134.9, 131.8, 127.6, 122.1, 117.4, 116.2, 115.6, 69.7, 35.9, 35.7, 33.7, 33.0, 28.4 ppm; Major tautomer: 1H NMR (500 MHz, CDCl3) δ 7.30–7.11 (m, 2H), 6.92 (m, 2H), 6.75 (m, 2H), 6.01 (ddt, J = 17.2, 10.5, 5.3 Hz, 1H), 5.78 (ddt, J = 17.0, 10.3, 6.5 Hz, 1H), 5.37 (dq, J = 17.2, 1.6 Hz, 1H), 5.25 (dq, J = 10.5, 1.6 Hz, 1H), 5.11–4.91 (m, 2H), 4.46 (dt, J = 5.3, 1.6 Hz, 2H), 4.38 (m, 1H), 3.40–3.30 (m, 1H), 3.25 (m, 1H), 2.45 (q, J = 6.7 Hz, 2H), 2.27 (q, J = 6.7 Hz, 2H), 1.61 (s, 9H) ppm; Significant signals minor tautomer: 1H NMR (500 MHz, CDCl3) δ 4.60 (m, 1H), 3.38 (m, 1H), 3.20 (m, 1H), 2.40 (m, 2H), 2.24 (m, 2H) ppm; Major tautomer: 13C NMR (125 MHz, CDCl3) δ 192.5, 176.3, 173.8, 157.7, 152.3, 149.0, 136.8, 133.3, 130.84, 126.5, 121.7, 117.8, 116.0, 114.7, 100.7, 84.1, 68.8, 65.7, 35.1, 32.7, 32.1, 28.2 ppm; Significant signals minor tautomer: 13C NMR (125 MHz, CDCl3) δ 201.0, 178.4, 164.6, 157.8, 153.2, 150.2, 130.8, 126.4, 121.4, 114.8, 102.9, 83.4, 63.2, 34.9, 32.8, 32.0, 28.3 ppm; IR νmax 2981 (w), 2937 (w), 2367 (w), 1769 (w), 1712 (m), 1642 (m), 1610 (m), 1578 (m), 1511 (m), 1414 (w), 1396 (w), 1370 (m), 1349 (m), 1304 (s), 1248 (m), 1150 (m), 1028 (w), 996 (w) cm–1; HRMS (ESI) m/z [M + Na]+ calcd. for C26H31NO6Na+ 476.20436, found 476.20380.
(3S,6Z,8E,12E)-4-Aza-N-(tert-butoxycarbonyl)-7-hydroxy-15-oxa-5,21-dioxo-tricyclo-[14.2.2.13,6]henicosa-1(18),6,8,12,16(17),19-hexaene (13). 3-Acyltetramic acid 12 (207 mg, 456 μmol, 1.00 eq) in dry, degassed CH2Cl2 (90 mL) was treated with 2nd generation Grubbs catalyst (39 mg, 46 μmol, 10 mol%). The solution was stirred at reflux for 18 h. The solvent was removed under reduced pressure and the crude product was purified by column chromatography on reversed phase silica gel (RP18, 40% MeCN in H2O + 0.1% HCOOH → 50% MeCN in H2O + 0.1% HCOOH → 60% MeCN in H2O + 0.1% HCOOH → 70% MeCN in H2O + 0.1% HCOOH → 80% MeCN in H2O + 0.1% HCOOH → 100% MeCN + 0.1% HCOOH) to afford 13 as pale brown resin (128 mg, 301 µmol, 66%). Rf = 0.75 (5% MeOH in CH2Cl2); −19.6° (c 1.00, CHCl3); 1H NMR (500 MHz, CD3OD) δ 6.90 (dt, J = 15.6, 7.6 Hz, 1H), 6.80 (d, J = 8.5 Hz, 2H), 6.74–6.61 (m, 2H), 6.58 (d, J = 15.6 Hz, 1H), 5.54 (dt, J = 15.3, 7.6 Hz, 1H), 5.41 (dt, J = 15.3, 5.7 Hz, 1H), 4.63–4.48 (m, 3H), 3.29 (dd, J = 13.8, 3.2 Hz, 1H), 3.04 (dd, J = 13.8, 3.2 Hz, 1H), 2.52–2.26 (m, 4H), 1.63 (s, 9H) ppm; Major tautomer: 13C NMR (125 MHz, CD3OD) δ 174.7 (HMBC correlation), 158.4, 153.1, 134.1, 131.1, 128.3, 126.6, 122.9, 118.0, 117.6, 115.3, 84.9, 67.7, 66.0, 37.0, 33.6, 31.9, 28.4 ppm; Significant signals minor tautomer: 13C NMR (125 MHz, CD3OD) δ 159.9, 132.3, 117.2, 114.9 ppm; Major tautomer: 1H NMR (500 MHz, CDCl3) δ 6.95–6.48 (m, 6H), 5.50 (m, 1H), 5.35 (m, 1H), 4.62–4.39 (m, 3H), 3.27 (dd, J = 13.6, 3.6 Hz, 1H), 3.07 (m, 1H), 2.57–2.41 (m, 2H), 2.33–2.12 (m, 2H), 1.63 (s, 9H) ppm; Significant signals minor tautomer: 1H NMR (500 MHz, CDCl3) δ 3.38 (dd, J = 13.6, 3.6 Hz, 1H) ppm; C2, C4 and C3 not observed; IR νmax 2973 (w), 2931 (w), 2934 (m), 1764 (m), 1713 (m), 1644 (s), 1608 (w), 1579 (s), 1508 (m), 1394 (w), 1369 (m), 1350 (s), 1306 (s), 1274 (m), 1254 (m), 1222 (m), 1159 (s), 1141 (m), 1109 (w), 978 cm–1; HRMS (ESI) m/z [M + Na]+ calcd. for C24H27NO6Na+ 448.17306, found 448.17270.
(3S,6Z,8E,12E)-4-Aza-7-hydroxy-15-oxa-5,21-dioxo-tri-cyclo [14.2.2.13,6]henicosa-1(18),6,8,12,16(17),19-hexaene (5). Tetramic acid 13 (245 mg, 576 μmol, 1.00 eq) in dry CH2Cl2 (11 mL) was treated with TFA (1.10 mL) and stirred for 15 min at room temperature. Toluene (75 mL) was added and the solvent was concentrated under reduced pressure. This was repeated once to yield 5 as a pale brown foam (187 mg, 576 µmol, quant.). Rf = 0.63 (10% MeOH in CH2Cl2 + 0.1% HCOOH); +92.9° (c 1.00, MeOH); 1H NMR (500 MHz, CD3OD): Diastereotopic H-atoms indicated as a, b: δ 7.03–6.41 (m, 6H, OCHC=CHCH2, CHAr), 5.57 (m, 1H, OCH2HC=CH), 5.35 (m, 1H, OCH2HC=CH), 4.60/4.48 (m, 2H, ArOCH2), 4.05 (brs, 1H, CHN), 3.01a (dd, J = 13.6, 3.9 Hz, 1H), 2.85b (dd, J = 13.6, 2.0 Hz, 1H, ArCH), 2.53–2.13 (m, 4H, OCH2HC=CH(CH2)2) ppm; 13C NMR (125 MHz, CD3OD) δ 172.8 (HNCO), 157.9 (OCq,Ar), 149.8 (OCHC=CHCH2), 132.8 (OCH2HC=CH), 131.2 (CH2CCHAr), 128.0 (OCH2HC=CH), 126.5 (CH2Cq,Ar), 123.2 (OCHC=CHCH2), 117.6 (OCCHAr), 67.4 (ArOCH2), 38.0 (ArCH2), 33.5 (OCHC=CHCH2), 32.5 (OCH2HC=CHCH2) ppm; Major tautomer: 1H NMR (500 MHz, CDCl3): δ 7.07–6.42 (m, 6H), 5.57–5.31 (m, 2H), 4.55 (m, 2H), 4.10 (m, 1H), 3.13 (dd, J = 13.8, 4.1 Hz, 1H), 2.84 (m, 1H), 2.55–2.11 (m, 4H) ppm; Significant signals minor tautomer: 1H NMR (500 MHz, CDCl3) δ 4.20 (m, 1H), 2.90 (m, 1H) ppm; Major tautomer: 13C NMR (125 MHz, CDCl3) δ 195.6, 176.1, 171.8, 156.5, 148.6, 133.5, 132.2, 130.0, 126.8, 125.0, 122.3, 117.0, 111.6, 100.9, 66.5, 62.2, 37.6, 32.6, 31.7 ppm; Significant signals minor tautomer: 13C NMR (125 MHz, CDCl3) δ 203.7, 172.7, 170.2, 157.1, 149.7, 131.6, 130.8, 127.1, 125.5, 122.0, 115.7, 115.4, 104.0, 67.5, 60.2, 38.1, 32.1, 30.4 ppm; IR νmax 3303 (m), 2927 (w), 2934 (m), 2070 (w), 1643 (s), 1576 (s), 1507 (m), 1428 (m), 1369 (w), 1338 (w), 1254 (m), 1219 (m), 1177 (m), 1115 (m), 975 (s) cm–1; HRMS (ESI) m/z [M + H+] calcd. for C19H20NO4+ 326.13868, found 326.13785.
(3S,6Z,12E)-4-Aza-N-(tert-butoxycarbonyl)-7-hydroxy-15-oxa-5,21-dioxo-tricyclo-[14.2.2.13,6]henicosa-1(18),6,12, 16(17),19-pentaene (16). Tetramic acid 13 (77.0 mg, 181 μmol, 1.00 eq) and Wilkinson’s catalyst (17 mg, 18 μmol, 10 mol%) in dry CH2Cl2 (2.5 mL) were treated with Et3SiH (144 μL, 905 μmol, 5.00 eq). The solution was stirred for 19 h under reflux and the solvent was removed under reduced pressure. The crude product was dissolved in dry MeOH (2.6 mL) and KF (26.3 mg, 453 μmol, 2.50 eq) was added. After stirring for 20 h at −15 °C, more KF (26.3 mg, 453 μmol, 2.50 eq) was added and stirring was continued for a further 7 h at −15 °C. Chilled H2O (50 mL) and chilled brine (20 mL) were added. The aqueous phase was extracted with EtOAc (3 × 50 mL), and the combined organic phases were washed with 0.5M H2SO4 (40 mL) and dried over Na2SO4. Removal of the solvent and purification by column chromatography on reversed phase silica gel (RP18, 30% MeCN in H2O + 0.1% HCOOH → 40% MeCN in H2O + 0.1% HCOOH → 50% MeCN in H2O + 0.1% HCOOH → 60% MeCN in H2O + 0.1% HCOOH) gave 16 as a colourless foam (37 mg, 86.6 µmol, 48%). Rf = 0.83 (10% MeOH in CH2Cl2); +12.4° (c 1.00, MeOH); Major tautomer: 1H NMR (500 MHz, CD3OD) δ 6.90–6.60 (m, 4H), 5.53 (dt, J = 15.4, 7.9 Hz, 1H), 5.40 (dt, J = 15.4, 5.3 Hz, 1H), 4.64–4.49 (m, 3H), 3.41 (dd, J = 14.1, 4.5 Hz, 1H), 3.10 (dd, J = 14.1, 2.9 Hz, 1H), 2.36 (m, 2H), 2.09–1.90 (m, 2H), 1.64 (s, 9H), 1.30 (m, 2H), 1.08 (m, 2H) ppm; Significant signals minor tautomer: 1H NMR (500 MHz, CDCl3) δ 3.29 (dd, J = 14.1, 4.5 Hz, 1H), 3.03 (dd, J = 14.1, 2.9 Hz, 1H), 2.91 (m, 2H) ppm; Major tautomer: 13C NMR (125 MHz, CD3OD) δ 158.0, 150.8, 135.8, 131.7, 127.1, 127.0, 117.9, 84.7, 68.0, 35.7, 33.6, 33.2, 29.6, 28.4, 28.0 ppm; Significant signals minor tautomer: 13C NMR (125 MHz, CD3OD) δ 158.3, 128.2, 118.5, 67.7, 37.0, 33.4, 29.8, 28.3, 27.7 ppm; 1H NMR (500 MHz, CDCl3) δ 7.02–6.42 (m, 4H), 5.56–5.26 (m, 2H), 4.69–4.37 (m, 3H), 3.49–3.34 (dd, J = 14.0, 4.9 Hz, 1H), 3.29 (m, 1H), 3.15–3.03 (m, 1H), 2.48–1.86 (m, 3H), 1.63 (s, 9H), 1.47–0.99 (m, 4H) ppm; Major tautomer: 13C NMR (125 MHz, CDCl3) δ 197.4, 196.8, 164.3, 156.6, 149.8, 134.5, 131.9, 125.8, 125.70, 118.4, 102.2, 83.5, 67.6, 62.1, 35.0, 32.7, 32.22, 28.7, 28.3, 26.9 ppm; Significant signals minor tautomer: 13C NMR (125 MHz, CD3OD) δ 192.0, 191.8, 156.1, 136.6, 130.0, 125.75, 114.0, 105.5, 84.4, 67.0, 65.8, 34.7, 34.5, 32.24, 28.4, 27.6, 26.7, 26.0 ppm; IR νmax 2978 (w), 2935 (w), 2863 (w), 1778 (m), 1744 (m), 1712 (s), 1662 (m), 1607 (s), 1509 (s), 1475 (w), 1456 (w), 1440 (m), 1423 (w), 1395 (w), 1366 (m), 1350 (s), 1305 (s), 1272 (m), 1258 (s), 1217 (s), 1150 (s), 1111 (m), 1081 (w), 1017 (w), 971 (m) cm–1; HRMS (ESI) m/z [M + Na+] calcd. for C24H29NO6Na+ 450.18871, found 450.18776.
(S)-tert-Butyl-2-(4-(allyloxy)benzyl)-3-(hept-6-enoyloxy)-5-oxo-2,5-dihydro-1H-pyrrole-1-carboxylate (14). 6-Heptenoic acid (2.11 mL, 15.6 mmol, 1.00 eq) in dry CH2Cl2 (78 mL) was treated with EDC∙HCl (3.59 g, 18.7 mmol, 1.20 eq) and DMAP (0.38 g, 3.12 mmol, 0.20 eq) at 0 °C. The solution was stirred for 20 min, before tetramic acid 11 (5.93 g, 17.2 mmol, 1.1 eq) was added at room temperature. After stirring for 4 h, the reaction was quenched with 0.5M H2SO4 (250 mL). The organic phase was separated and the aqueous phase was extracted with EtOAc (3 × 150 mL). The combined organic phases were washed with brine (200 mL) and dried over Na2SO4. After removal of the solvent under reduced pressure, the crude product was purified by column chromatography (silica gel 60, 10% EtOAc in hexanes → 15% EtOAc in hexanes → 20% EtOAc in hexanes → 25% EtOAc in hexanes) to obtain 14 as an orange resin (5.64 g, 12.4 mmol, 79%). Rf = 0.48 (30% EtOAc in hexanes); +107.5° (c 1.00, MeOH); 1H NMR (500 MHz, CDCl3) δ 6.93–6.83 (m, 2H), 6.81–6.71 (m, 2H), 6.03 (m, 1H), 5.88 (s, 1H), 5.79 (ddt, J = 17.0, 10.3, 6.7 Hz, 1H), 5.40 (m, 1H), 5.28 (m, 1H), 5.06–4.94 (m, 2H), 4.77 (dd, J = 6.0, 2.8 Hz, 1H), 4.48 (m, 2H), 3.29 (dd, J = 14.3, 6.2 Hz, 1H), 3.14 (dd, J = 14.3, 2.8 Hz, 1H), 2.48 (td, J = 7.4, 1.9 Hz, 2H), 2.09 (m, 2H), 1.74–1.65 (m, 2H), 1.60 (s, 9H), 1.46 (qn, J = 7.7 Hz, 2H) ppm; 13C NMR (125 MHz, CDCl3) δ 168.8, 168.2, 165.3, 157.9, 149.5, 138.0, 133.3, 130.5, 126.2, 117.9, 115.3, 114.8, 108.3, 83.3, 68.9, 60.7, 35.0, 34.3, 33.4, 28.4, 28.2, 23.9 ppm; IR νmax 3075 (w), 2975 (w), 2939 (w), 2863 (w), 1777 (s), 1744 (s), 1712 (s), 1633 (m), 1611 (w), 1582 (w), 1514 (s), 1478 (w), 1457 (w), 1424 (w), 1392 (w), 1370 (m), 1356 (m), 1320 (s), 1248 (s), 1226 (m), 1172 (s), 1158 (s), 1115 (m), 1064 (s), 1032 (m), 996 (m) cm–1; HRMS (ESI) m/z [M + Na+] calcd. for C26H33O6NNa+ 478.22001, found 478.21968.
(S,Z)-tert-Butyl-2-(4-(allyloxy)benzyl)-4-(1-hydroxyhept-6-en-1-ylidene)-3,5-dioxopyrrolidine-1-carboxylate (15). 4-O-acyltetramic acid 14 (5.54 g, 12.2 mmol, 1.0 eq) in dry CH2Cl2 (122 mL) was treated with dry NEt3 (2.04 mL, 14.6 mmol, 1.2 eq) at room temperature and stirred for 10 min. DMAP (743 mg, 6.1 mmol, 0.5 eq) was added and the solution was stirred for a further 24 h. NaHCO3 (200 mL) was added and the aqueous phase was extracted with EtOAc (2 × 150 mL). The combined organic phases were washed with brine (200 mL) and dried over Na2SO4. Removal of the solvent under reduced pressure and purification by column chromatography on reversed phase silica gel (RP18, 40% MeCN in H2O + 0.1% HCOOH → 60% MeCN in H2O + 0.1% HCOOH → 80% MeCN in H2O + 0.1% HCOOH → 100% MeCN + 0.1% HCOOH) afforded 15 as an orange resin (4.54 g, 9.97 mmol, 82%). Rf = 0.91 (10% MeOH in CH2Cl2); −31.8° (c 1.00, MeOH); 1H NMR (500 MHz, CD3OD) δ 6.90 (m, 2H), 6.77 (m, 2H), 6.01 (m, 1H), 5.78 (ddt, J = 17.1, 10.5, 7.2 Hz, 1H), 5.35 (dd, J = 17.1, 1.5 Hz, 1H), 5.21 (m, 1H), 5.01 (m, 1H), 4.94 (m, 1H), 4.58 (s, 1H), 4.46 (d, J = 5.2 Hz, 2H), 3.38 (dd, J = 14.2, 5.4 Hz, 1H), 3.18 (dd, J = 14.2, 2.3 Hz, 1H), 2.75 (t, J = 6.4 Hz, 2H), 2.04 (q, J = 7.2 Hz, 2H), 1.62 (s, 9H), 1.57–1.45 (m, 2H), 1.34 (m, 2H) ppm; 13C NMR (125 MHz, CD3OD) δ 195.2 (HMBC correlation), 159.3, 139.5, 134.9, 131.9, 127.4, 117.4, 115.5, 115.3, 84.8, 69.7, 64.6 (HMBC correlation), 35.8, 34.4, 29.4, 28.4, 26.2 ppm; Major tautomer: 1H NMR (500 MHz, CDCl3) δ 6.92 (m, 2H), 6.75 (m, 2H), 6.02 (m, 1H), 5.85–5.70 (m, 1H), 5.38 (m, 1H), 5.27 (m, 1H), 5.07–4.91 (m, 2H), 4.39 (dd, J = 5.4, 2.7 Hz, 1H), 4.46 (d, J = 5.3 Hz, 2H), 3.33 (dd, J = 14.1, 5.8 Hz, 1H), 3.24 (dd, J = 14.1, 2.6 Hz, 1H), 2.93–2.61 (m, 2H), 2.07 (q, J = 7.1 Hz, 2H), 1.61 (s, 9H), 1.60–1.29 (m, 4H) ppm; Significant signals minor tautomer: 1H NMR (500 MHz, CDCl3) δ 7.10 (m, 2H), 6.87 (m, 2H), 4.64 (dd, J = 5.4, 2.7 Hz, 1H), 4.52 (d, J = 5.3 Hz, 2H), 3.41 (dd, J = 14.1, 5.8 Hz, 1H), 3.21 (dd, J = 14.1, 2.6 Hz, 1H), 2.01 (q, J = 7.1 Hz, 2H) ppm; Major tautomer: 13C NMR (125 MHz, CDCl3) δ 196.5, 192.4, 164.4, 157.8, 149.1, 138.3, 133.32, 130.9, 126.4, 117.8, 115.1, 114.7, 102.5, 84.2, 68.82, 65.8, 35.0, 33.4, 32.9, 28.3, 28.2, 25.5 ppm; Significant signals minor tautomer: 13C NMR (125 MHz, CDCl3) δ 157.9, 150.0, 138.4, 133.30, 130.8, 126.0, 117.9, 115.3, 114.9, 105.6, 83.5, 68.80, 61.9, 34.9, 34.8, 32.7, 28.5, 28.4, 24.7 ppm; IR νmax 3075 (w), 2978 (w), 2932 (w), 2860 (w), 1770 (w), 1744 (m), 1716 (s), 1667 (m), 1640 (m), 1604 (s), 1510 (s), 1457 (w), 1421 (m), 1395 (w), 1366 (m), 1349 (s), 1301 (s), 1241 (s), 1223 (s), 1172 (s), 1151 (s), 1025 (m), 996 (m), 967 (m), 913 (s) cm–1; HRMS (ESI) m/z [M + Na+] calcd. for C26H33O6NNa+ 478.22001, found 478.21944.
(3S,6Z,12E)-4-Aza-N-(tert-butoxycarbonyl)-7-hydroxy-15-oxa-5,21-dioxo-tricyclo[14.2.2.13,6]henicosa-1(18),6,12,16(17),19-pentaene (16) from 15. 3-Acyltetramic acid 15 (2.30 g, 5.04 mmol, 1.00 eq) in dry, degassed CH2Cl2 (1.00 L) was treated with 2nd generation Grubbs catalyst (428 mg, 504 μmol, 10 mol%). The solution was stirred at reflux for 24 h. The solvent was removed under reduced pressure and the crude product was purified by column chromatography on reversed phase silica gel (RP18, 40% MeCN in H2O + 0.1% HCOOH → 60% MeCN in H2O + 0.1% HCOOH → 80% MeCN in H2O + 0.1% HCOOH → 100% MeCN + 0.1% HCOOH) to yield 16 as a pale brown foam (1.78 g, 4.16 mmol, 83%). For analytical data see above.
(3S,6Z,12E)-4-Aza-7-hydroxy-15-oxa-5,21-dioxo-tricyclo[14.2.2.13,6]henicosa- 1(18),6,12,16(17),19-pentaene (4). Carbamate 16 (926 mg, 2.17 mmol, 1.00 eq) in dry CH2Cl2 (43 mL) was treated with TFA (4.3 mL) and stirred for 15 min at room temperature. Toluene (100 mL) was added and the solvent was concentrated under reduced pressure. This was repeated once to yield 4 as a pale brown foam (709 mg, 2.17 mmol, quant.). Rf = 0.64 (10% MeOH in CH2Cl2 + 0.01% HCOOH); +52.2° (c 1.00, MeOH); 1H NMR (500 MHz, CD3OD): Diastereotopic H-atoms indicated as a, b: δ 7.09–6.68 (m, 4H, CHAr), 5.58 (dt, J = 15.1, 7.8 Hz, 1H, OCH2HC=CH), 5.33 (m, 1H, OCH2HC=CH), 4.64a (dd, J = 13.9, 7.7 Hz, 1H, ArOCH), 4.53b (m, 1H, ArOCH), 4.15 (t, J = 3.3 Hz, 1H, CHN), 3.11–2.67a (brs, 1H, OCCH), 3.07a (dd, J = 14.1, 3.8 Hz, 1H, ArCH), 2.91b (dd, J = 14.1, 3.5 Hz, 1H, ArCH), 2.42–1.86b (brs, 1H, OCCH), 2.05a (m, 1H, HC=CHCH), 1.94b (m, 1H, HC=CHCH), 1.34–1.05 (m, 4H, HC=CHCH2(CH2)2) ppm; 13C NMR (125 MHz, CD3OD) δ 157.5 (OCq,Ar), 127.4 (OCH2CH=CH), 126.9 8 (CH2Cq,Ar), 118.3 (OCCHAr), 67.8 (ArOCH2), 36.6 (ArCH2), 33.6 (OCCH2), 33.4 (HC=CHCH2), 29.8 (HC=CHCH2(CH2)2), 28.5 (HC=CHCH2(CH2)2) ppm; Major tautomer: 1H NMR (500 MHz, CDCl3) δ 7.10–6.58 (m, 4H), 5.54 (m, 1H), 5.39 (m, 1H), 4.60 (m, 2H), 4.16 (m, 1H), 3.28–3.16 (m, 2H), 2.87 (dd, J = 14.1, 1.7 Hz, 1H), 2.12–1.84 (m, 3H), 1.40–1.04 (m, 4H) ppm; Significant signals minor tautomer: 1H NMR (500 MHz, CDCl3) δ 5.49 (m, 1H), 5.32 (m, 1H), 4.29 (s, 1H), 2.87 (m, 1H) ppm; Major tautomer: 13C NMR (125 MHz, CDCl3) δ 194.5, 188.6, 175.8, 156.0, 136.8, 132.0, 130.3, 125.7, 125.6, 117.9, 114.1, 101.5, 67.0, 62.4, 36.2, 32.43, 32.41, 28.7, 27.6 ppm; Significant signals minor tautomer: 13C NMR (125 MHz, CDCl3) δ 201.2 (HMBC correlation), 192.4, 170.3, 156.5, 134.9, 131.2, 118.2, 104.7, 67.6, 59.8, 36.4, 33.1, 32.1, 28.5, 27.1 ppm; IR νmax 3255 (w), 2929 (w), 2857 (w), 1770 (w), 1646 (s), 1608 (s), 1508 (s), 1433 (m), 1367 (w), 1305 (w), 1259 (m), 1214 (s), 1176 (s), 1159 (s), 1113 (m), 1075 (m), 1062 (m), 1015 (m), 973 (s) cm–1. HRMS (ESI) m/z [M + H+] calcd. for C19H22NO4+ 328.15433, found 328.15343.
(3S,6Z,12S,13S)-4-Aza-7,12,13-trihydroxy-15-oxa-5,21-dioxo-tricyclo[14.2.2.13,6]henicosa-1(18),6,16(17),19-tetraene (7). AD-mix α (2.86 g, 1.4 g/mmol) in H2O (10.3 mL) was treated with alkene 16 (874 mg, 2.04 mmol, 1.00 eq) in tBuOH (10.3 mL) at 0 °C. The two-phase mixture was stirred at 7 °C for 5 d, before more AD-mix α (1.43 g, 0.7 g/mmol) was added. After stirring for a further 4 d, the mixture was treated with Na2SO3 (3.60 g, 28.6 mmol, 14.0 eq) and stirred for 2 h at room temperature. H2O was added to dissolve the precipitate. The aqueous phase was washed with EtOAc (10 mL) and the organic phase was extracted with H2O (10 mL). The solvent was removed under reduced pressure and the crude product was suspended in MeOH. The precipitate was filtered off and washed with MeOH. Concentration of the filtrate and purification of the residue by column chromatography on reversed phase silica gel (RP18, H2O + 0.1% HCOOH → 20% MeCN in H2O + 0.1% HCOOH → 40% MeCN in H2O + 0.1% HCOOH → 60% MeCN in H2O + 0.1% HCOOH) gave a mixture of Boc-protected and deprotected tetramic acid 7. This was dissolved in dry CH2Cl2 (25 mL) and treated with TFA (1.5 mL). After stirring for 15 min at room temperature, toluene (100 mL) was added. The solvent was concentrated under reduced pressure and more toluene (100 mL) was added. Removal of the solvent under reduced pressure gave diol 7 as a yellowish foam and as a mixture of two diastereomers according to HPLC and NMR spectra. Yield: 323 mg (894 µmol, 44%, dr 2.3:1). Rf = 0.63 (10% MeOH in CH2Cl2 + 0.1% HCOOH); 1H NMR (500 MHz, CD3OD): Signals of major diastereomer marked as A, signals of minor diastereomer marked as B; diastereotopic H-atoms indicated as a, b: δ 7.13–6.75 (m, 4H, CHAr), 4.26 (m, 1H, ArOCHa), 4.22 (m, 1H, CHN), 4.07 (dd, J = 11.7, 8.8 Hz, 1H, ArOCHb,B), 4.05 (m, 1H, J = 11.8, 8.9 Hz, 1H, ArOCHb,A), 3.80 (m, 1H, ArOCH2CHOHA), 3.60 (m, 2H, ArOCH2CHOHB, ArOCH2CHOHCHOHB), 3.46 (dt, J = 9.6, 2.7, 3.9 Hz, 1H, ArOCH2CHOHCHOHA), 3.10 (dt, J = 14.0, 3.8 Hz, 1H, ArCHa), 2.97 (dt, J = 14.0, 3.2 Hz, 1H, ArCHb), 1.67–1.17 (m, 4H, (CH2)2), 0.88–0.58 (m, 2H, OCCH2CH2) ppm; OCCH2 not observed; Major diastereomer: 13C NMR (125 MHz, CD3OD): δ 189.0 (CHO, HMBC correlation), 157.8 (OCq,Ar), 131.1 (CH2CCHAr, HMBC correlation), 127.2 (CH2Cq,Ar, HMBC correlation), 118.4 (OCCHAr), 113.7 (OCCHAr), 69.9 (ArOCH2CHOHCHOH), 67.5 (ArOCH2), 67.4 (ArOCH2CHOH, HSQC correlation), 36.6 (ArCH2), 34.5 (ArOCH2(HCOH)2CH2), 32.9 (OCCH2), 27.3 (ArOCH2(HCOH)2CH2CH2), 25.4 (OCCH2CH2) ppm; Minor diastereomer 13C NMR (125 MHz, CD3OD): δ 131.9 (HMBC correlation), 126.5 (HMBC correlation), 119.4, 115.2, 69.7, 68.5, 32.8, 25.9 ppm; IR νmax 3354 (m), 2933 (m), 1646 (s), 1607 (s), 1508 (s), 1433 (w), 1338 (w), 1255 (m), 1216 (m), 1177 (w), 1113 (w), 1016 (w) cm–1; HRMS (ESI) m/z [M + H+] calcd. for C19H24NO4+ 362.15981, found 362.15894.
(3S,6Z)-4-Aza-12-bromo-7,13-dihydroxy-15-oxa-5,21-dioxo-tricyclo[14.2.2.13,6]henicosa-1(18),6,16(17),19-tetraene (8). Alkene 16 (660 mg, 1.54 mmol, 1.00 eq) in DMSO (8 mL) was treated with H2O (41.7 μL, 2.32 mmol, 1.50 eq) and NBS (412 mg, 2.23 mmol, 1.50 eq) at 8 °C. After stirring the solution for 22 h at room temperature, sat. NaHCO3 solution (50 mL) was added. The aqueous phase was extracted with EtOAc (3 × 50 mL) and the combined organic phases were dried over Na2SO4. The solvent was removed under reduced pressure and the crude mixture of products was purified by column chromatography on reversed phase silica gel (RP18, 20% MeCN in H2O + 0.1% HCOOH → 30% MeCN in H2O + 0.1% HCOOH → 40% MeCN in H2O + 0.1% HCOOH → 50% MeCN in H2O + 0.1% HCOOH → 60% MeCN in H2O + 0.1% HCOOH → 80% MeCN in H2O + 0.1% HCOOH). Bromohydrins 8 and a side product with an additional bromo substituent were obtained separately and only partially deprotected. The mixture of bromohydrin 8 and its N-Boc-protected derivative was dissolved in dry CH2Cl2 (9 mL) and treated with TFA (900 μL). The solution was stirred for 15 min at room temperature and toluene (100 mL) was added. The mixture was concentrated under reduced pressure and toluene (50 mL) was added again. Removal of the solvent under reduced pressure gave 8 as a yellowish foam and as a mixture of two inseparable diastereomers of initially unknown dr according to 13C and 1H NMR spectra. Yield (8): 196 mg (462 µmol, 30%). Rf = 0.41 (10% MeOH in CH2Cl2); 1H NMR (500 MHz, CD3OD): Signals of major diastereomer marked as A, signals of minor diastereomer marked as B; diastereotopic H-atoms indicated as a, b: δ 7.24–6.75 (m, 4H, CHAr), 4.67–4.19 (m, 4H, CHN, ArOCH2, CHBr), 3.73 (m, 1H, CHOH), 3.14 (m, 2H, ArCHa, OCCHa,A), 2.96 (dd, J = 14.0, 3.9 Hz, 1H, ArCHb), 1.60–0.91 (m, 5H, CBrCH2CHaCH2), 0.76–0.26 (m, 1H, CBrCH2CHbCH2) ppm; 1H NMR (500 MHz, CDCl3) δ 7.20–6.72 (m, 4H), 4.60–4.11 (m, 4H), 3.78 (m, 1H), 3.60–3.20 (m, 2H), 2.94 (m, 1H), 2.20–2.00 (m, 1H), 1.63–1.33 (m, 3H), 1.30–0.96 (m, 2H), 0.66–0.30 (m, 1H) ppm; Major tautomer: 13C NMR (125 MHz, CDCl3): Signals of major diastereomer marked as A, signals of minor diastereomer marked as B: δ 194.3B (CO), 194.2A (CO), 188.2 (COH), 175.6B (HNCO),175.5A (HNCO), 157.1 (OCq,Ar), 131.4B (CH2CCHAr), 130.1A (CH2CCHAr), 127.6 (CH2Cq,Ar), 117.0 (OCCHAr), 101.90B (NCOCCO), 101.88A (NCOCCO), 73.0 (CHOH), 69.6 (ArOCH2), 62.0B (HCNH), 61.9A (HCNH), 55.9 (CHBr), 36.2 (ArCH2), 34.2 (CHBrCH2), 33.1 (OCCH2), 27.4 (CHBrCH2), 26.0 (OCCH2CH2), 24.6 (CHBrCH2CH2) ppm; Significant signals minor tautomer: 13C NMR (125 MHz, CDCl3): Signals of major diastereomer marked as A, signals of minor diastereomer marked as B: δ 202.6B, 202.5A, 189.03B, 189.00A, 169.1B, 169.0A, 156.3, 133.3A, 132.4B, 127.5, 116.2A, 115.3B, 106.0, 67.7, 59.6B, 59.5A, 53.7, 36.6, 33.4, 31.4, 24.6 ppm; IR νmax 3356 (m), 2936 (m), 1652 (s), 1609 (s), 1508 (s), 1462 (w), 1374 (w), 1254 (m), 1217 (m), 1177 (w), 1114 (w), 1043 (w) cm–1; HRMS (ESI) m/z [M + H+] calcd. for C19H23NO5Br+ 424.07451, found 424.07521.
(3S,6E)-4-Aza-13,16-dioxa-5,22-dioxo-tetracyclo[15.2.2.13,6.012,14]docosa-1(19),6,17(18),20-tetraene (6). Bromohydrin 8 (248 mg, 473 μmol, 1.00 eq) in dry THF (1.5 mL) was treated with KOtBu (86.7 mg, 709 μmol, 1.50 eq) at 0 °C. The suspension was stirred for 4 d at room temperature and then more KOtBu (58 mg, 473 μmol, 1.00 eq) was added. Stirring was continued for 1 d and H2O (5 mL) as well as EtOAc (5 mL) were added. The organic phase was separated and extracted with H2O (5 mL). The combined aqueous phases were concentrated under reduced pressure. The crude product was purified by column chromatography on reversed phase silica gel (RP18, 20% MeCN in H2O + 0.1% HCOOH → 30% MeCN in H2O + 0.1% HCOOH → 40% MeCN in H2O + 0.1% HCOOH → 50% MeCN in H2O + 0.1% HCOOH → 80% MeCN in H2O + 0.1% HCOOH) to yield a virtually pure product. Another column chromatography on reversed phase silica gel (RP18, 0% MeCN in H2O + 0.1% HCOOH → 20% MeCN in H2O + 0.1% HCOOH → 30% MeCN in H2O + 0.1% HCOOH → 40% MeCN in H2O + 0.1% HCOOH → 50% MeCN in H2O + 0.1% HCOOH→ 80% MeCN in H2O + 0.1% HCOOH) afforded epoxide 6 as a pale brown foam and as a 9:1 mixture of two diastereomers according to 13C and 1H NMR spectra. Yield: 76.0 mg (221 µmol, 47%). Rf = 0.28 (10% MeOH in CH2Cl2); 1H NMR (500 MHz, CD3OD): Signals of major diastereomer marked as A, signals of minor diastereomer marked as B¸ diastereotopic H-atoms indicated as a, b: δ 7.05 (m, 2H, OC(CH)2), 6.77 (m, 2H, CH2C(CH)2), 4.55A,a (dd, J = 14.0, 3.1 Hz, 1H, ArOCH), 4.40B,a (m, 1H, ArOCH), 4.21 (t, J = 3.2 Hz, 1H, CHN), 4.03A,b (dd, J = 14.0, 3.1 Hz, 1H, ArOCH), 3.95B,b (m, 1H, ArOCH), 3.14a (dd, J = 14.1, 3.5 Hz, 1H, ArCH), 3.22–2.69a (brs, 1H, OCCH), 3.05 (m, 1H, ArOCH2CHO), 2.93b (dd, J = 14.1, 3.5 Hz, 1H, ArCH), 2.65 (m, 1H, ArOCH2CHOCH), 1.71a (m, 1H, ArOCH2CHOCHCH), 1.63–1.15 (m, 3H, OCCHb, ArOCH2CHOCHCH2CH2), 1.15–0.41 (m, 3H, ArOCH2CHOCHCHb, OCCH2CH2) ppm; 13C NMR (125 MHz, CD3OD): Signals of major diastereomer marked as A, signals of minor diastereomer marked as B: δ 187.5 (HMBC correlation), 157.7 (OCq,Ar), 132.7A(CH2CCHAr), 132.5B (CH2CCHAr), 128.2 (CH2Cq,Ar), 116.5B (OCCHAr), 116.0A (OCCHAr), 113.2A (OCCHAr), 66.6B (ArOCH2), 66.4A (ArOCH2), 62.4 (HCNH, HSQC correlation), 58.5 (OCH2CHOCH), 58.4 (OCH2CHOCH), 36.3 (CH2Ar), 33.3 (ArOCH2CHOCHCH2), 31.9 (OCCH2), 27.3 (ArOCH2CHOCHCH2CH2), 24.8A (OCCH2CH2), 24.7B (OCCH2CH2) ppm; 1H NMR (500 MHz, CDCl3) δ 7.25–6.43 (m, 5H), 4.53 (t, J = 11.7 Hz, 1H), 4.44–3.83 (m, 2H), 3.40 (m, 1H), 3.24 (m, 1H), 3.12–2.80 (m, 2H), 2.71 (m, 1H), 2.21 (m, 1H), 1.92–1.42 (m, 3H), 1.19–0.35 (m, 3H) ppm; Major tautomer: 13C NMR (125 MHz, CDCl3): Signals of major diastereomer marked as A, signals of minor diastereomer marked as B: δ 194.6, 187.6, 175.3, 156.2, 131.5, 126.65, 115.1, 103.5, 65.5, 62.4, 57.2, 57.08, 35.7, 32.1, 30.6, 26.0, 23.5 ppm; Significant signals minor tautomer: 13C NMR (125 MHz, CDCl3): Signals of major diastereomer marked as A, signals of minor diastereomer marked as B: δ 202.2, 190.0, 169.2, 156.5, 131.8, 126.5, 115.2, 106.3, 66.2, 59.5, 58.0, 57.12, 36.1, 32.4, 31.4, 26.6, 23.4 ppm; IR νmax 3283 (w), 2931 (m), 1706 (s), 1654 (s), 1609 (s), 1509 (s), 1436 (m), 1373 (m), 1306 (w), 1239 (s), 1221 (s), 1180 (m), 1113 (w), 1072 (w), 1044 (m), 915 (m) cm–1; HRMS (ESI) m/z [M + H+] calcd. for C19H22NO5+ 344.14925, found 344.14904.
(3S,6Z)-4-Aza-12,13-dibromo-7-hydroxy-15-oxa-5,21-dioxo-tricyclo-[14.2.2.13,6] henicosa-1(18),6,16(17),19-tetraene (9). A solution of alkene 16 (500 mg, 1.17 mmol, 1.00 eq) in CCl4 (5.3 mL) was treated with bromine (90.3 μL, 1.75 mmol, 1.50 eq) and stirred in a sealed tube for 30 h at 80 °C. The solvent was removed under reduced pressure and the remainder was purified by column chromatography on reversed phase silica gel (RP18, 30% MeCN in H2O + 0.1% HCOOH → 35% MeCN in H2O + 0.1% HCOOH → 40% MeCN in H2O + 0.1% HCOOH → 45% MeCN in H2O + 0.1% HCOOH → 50% MeCN in H2O + 0.1% HCOOH → 100% MeCN in H2O + 0.1% HCOOH) to afford dibromide 9 as a yellow foam and as a mixture of two diastereomers of unknown dr. Yield: 228 mg (468 µmol, 40%). Rf = 0.62 (10% MeOH in CH2Cl2); 1H NMR (500 MHz, CDCl3) δ 8.67/8.42/8.17 (s, 1H, NH), 7.24–6.72 (m, 4H, CHAr), 4.82–3.83 (m, 5H, CH2CHNH, ArOCH2, (CHBr)2), 3.39–2.94 (m, 2H, ArCH2), 2.41–0.75 (m, 8H, OCCH2, CHBr(CH2)3) ppm; Major tautomer: 13C NMR (125 MHz, CDCl3) δ 198.3 (CO), 193.3 (COH), 168.5 (HNCO), 157.5 (OCq,Ar), 133.0 (CH2CCHAr), 130.4 (CH2CCHAr), 126.8 (CH2Cq,Ar), 115.6 (OCCHAr), 101.6 (NCOCCO, HMBC correlation), 70.0 (ArOCH2), 62.5 (HCNH), 59.6 ((CHBr)2), 54.3 ((CHBr)2), 38.2 (CH2CHBr), 34.9 (CH2Ar), 27.0, 21.9 (HOCCH2, CHBrCH2(CH2)2) ppm; Significant signals minor tautomer: 13C NMR (125 MHz, CDCl3): δ 168.7, 132.7, 130.9, 62.4, 61.9, 55.0, 26.4/25.5 ppm; IR νmax 3241 (m), 2929 (m), 1693 (s), 1652 (s), 1605 (s), 1507 (s), 1460 (m), 1431 (m), 1350 (w), 1299 (w), 1250 (m), 1215 (s), 1177 (m), 1112 (w), 1112 (w), 1040 (w), 1016 (m), 925 (w), 906 (m), 858 (m), 811 (w), 769 (w), 733 (s) cm–1; HRMS (ESI) m/z [M + H+] calcd. for C19H22Br2NO4+ 487.98896, found 487.98884.
((3S,6Z)-4-Aza-7,14-dihydroxy-5,18-dioxo-12-vinyl-tricyclo[11.3.1.13,6]octadeca-1(17),6,13(14),15-tetraene (10). A solution of alkene 16 (500 mg, 1.17 mmol, 1.00 eq) in degassed diethylaniline (4.7 mL) was stirred in a sealed tube at 190 °C for 42 h. The solution was diluted with EtOAc (50 mL). The organic phase was washed with 2M HCl (2 × 75 mL) and the aqueous phase was extracted with EtOAc (30 mL). The combined organic phases were dried over Na2SO4 and the solvent was removed under reduced pressure. Purification of the crude product by column chromatography on reversed phase silica gel (RP18, 30% MeCN in H2O + 0.1% HCOOH → 40% MeCN in H2O + 0.1% HCOOH → 50% MeCN in H2O + 0.1% HCOOH → 60% MeCN in H2O + 0.1% HCOOH → 100% MeCN in H2O + 0.1% HCOOH) afforded 10 as a colourless foam and as an inseparable mixture of two diastereomers. Yield: 218 mg (666 µmol, 57%, dr 12.5:1). Rf = 0.28 (10% MeOH in CH2Cl2); 1H NMR (500 MHz, CD3OD): Signals of major diastereomer marked as A, signals of minor diastereomer marked as B; diastereotopic H-atoms indicated as a, b: δ 6.88–6.72 (m, 2H, CHArCCH2), 6.60A (m, 1H, CHArCOH), 6.56B (m, 1H, CHArCOH), 6.36B (m, 1H, C=CH), 5.95A (ddd, J = 16.0, 10.4, 6.2 Hz, 1H, C=CH), 5.00–4.90 (m, 2H, CH2=C), 4.00A (t, J = 3.4 Hz, 1H, CH2CHNH), 3.96B (t, J = 3.4 Hz, 1H, CH2CHNH), 3.71 (m, 1H, CHC=C), 3.58a (m, 1H, HOCCH), 3.14A,a (dd, J = 14.0, 3.5 Hz, ArCH), 3.08B,a (dd, J = 14.0, 3.5 Hz, ArCH), 2.78b (dd, J = 13.7, 3.5 Hz, 1H, ArCH), 2.13b (dt, J = 14.0, 4.0 Hz, 1H, HOCCH), 1.99a (m, 1H, OCCH2CH), 1.67a (m, 1H, CHCH2CH), 1.55b (m, 1H, CHCH2CH), 1.45–1.26 (m, 2H, OC(CH2)2CH2), 0.89B,b (m, 1H, OCCH2CH), 0.44A,b (m, 1H, OCCH2CH) ppm; 13C NMR (125 MHz, CD3OD): Signals of major diastereomer marked as A, signals of minor diastereomer marked as B: δ 198.9 (CO), 188.8 (COH), 176.2 (HNCO), 155.4 (OCq,Ar), 144.1A (C=CH), 143.1B (C=CH), 131.1 (OCCHCHAr), 130.9 (CHCq,Ar), 130.7A (CCHArC), 129.9B (CCHArC), 126.2 (CH2Cq,Ar), 115.8B (OCCHAr), 115.2A (OCCHAr), 113.2B (C=CH2), 113.0A (C=CH2), 105.4 (NCOCCO), 63.9A (HCNH), 63.4B (HCNH), 40.7 (CHC=C), 37.4A (ArCH2), 37.3B (ArCH2), 35.4A (CHCH2CH2), 32.1B (CHCH2CH2), 29.3A (HOCCH2), 25.9B (HOCCH2), 24.9A, 24.8A (OC(CH2)2CH2, OCCH2CH2), 23.6B, 23.4B (OC(CH2)2CH2, OCCH2CH2) ppm; IR νmax 3294 (m), 2931 (m), 2863 (w), 1969 (w), 1656 (s), 1694 (s), 1511 (m), 1435 (m), 1336 (w), 1252 (m), 1169 (w), 1100 (w), 911 (m) cm–1; HRMS (ESI) m/z [M + H+] calcd. for C19H22NO4+ 328.15433, found 328.15411.
(3S,6Z,8R,12E)-4-Aza-N-(tert-butoxycarbonyl)-7-hydroxy-8-methyl-15-oxa-5,21-dioxo-tricyclo[14.2.2.13,6]henicosa-1(18),6,12,16(17),19-pentaene (18). A solution of alkene 17 (263 mg, 596 μmol, 1.00 eq) in EtOAc (6 mL) was treated with Pd on charcoal (26.3 mg, 10 wt%). The resulting suspension was stirred under a H2-atmosphere for 31 h at room temperature. The solid was filtered off over Celite® and washed with EtOAc. The combined filtrates were concentrated under reduced pressure to give 18 as an orange foam. Yield: 261 mg (588 µmol, 99%). Rf = 0.93 (10% MeOH in CH2Cl2); +38.8° (c 0.75, MeOH); Major tautomer: 1H NMR (500 MHz, CD3OD) δ 7.02–6.57 (m, 4H), 4.50 (m, 1H), 4.26–4.06 (m, 2H), 3.41 (dd, J = 14.4, 3.0 Hz, 1H), 3.37 (m, 1H), 3.08 (dd, J = 14.8, 3.0 Hz, 1H), 1.63 (s, 9H), 1.60–1.15 (m, 7H), 1.08 (d, J = 6.7 Hz, 3H), 1.03 (m, 1H), 0.73–0.42 (m, 2H) ppm; Significant signals minor tautomer: 1H NMR (500 MHz, CD3OD) δ 4.68 (m, 1H), 3.54 (m,1H) ppm¸ Major tautomer: 13C NMR (125 MHz, CD3OD) δ 193.7, 174.9, 157.4, 150.6, 131.8, 127.9, 116.1, 104.2, 85.2, 67.8, 66.4, 38.3, 35.4, 34.9, 29.4, 28.4, 27.5, 26.1, 24.7, 17.7 ppm; Significant signals minor tautomer: 13C NMR (125 MHz, CD3OD) δ 63.3, 32.8, 30.4, 28.44, 23.7, 14.3 ppm; Major tautomer: 1H NMR (500 MHz, CDCl3) δ 7.06–6.65 (m, 4H), 4.44 (m, 1H), 4.26–4.06 (m, 2H), 3.42 (m, 1H), 3.41 (dd, J = 14.6, 3.0 Hz, 1H), 3.12 (dd, J = 14.6, 4.0 Hz, 1H), 1.64 (s, 9H), 1.50–1.13 (m, 7H), 1.06 (m, 3H), 1.04 (m, 1H), 0.73–0.42 (m, 2H) ppm; Significant signals minor tautomer: 1H NMR (500 MHz, CDCl3) δ 4.62 (m, 1H), 3.55 (m, 2H), 1.60 (s, 9H) ppm; Major tautomer: 13C NMR (125 MHz, CDCl3) δ 195.6, 191.6, 174.1, 155.8, 149.0, 132.6, 129.3, 126.6, 117.6, 117.0, 102.8, 84.4, 67.0, 65.5, 36.5, 34.6, 34.1, 28.36, 28.3, 26.1, 24.9, 23.5, 17.6 ppm; Significant signals minor tautomer: 13C NMR (125 MHz, CDCl3) δ 131.1, 130.7, 116.5, 115.4, 62.2, 36.6, 34.9, 33.8, 28.4, 28.0, 26.4, 25.8, 25.4, 23.8, 16.6 ppm; IR νmax 2971 (m), 2935 (m), 2233 (w), 2078 (m), 1740 (s), 1611 (s), 1509 (m), 1440 (m), 1354 (s), 1302 (m), 1256 (w), 1228 (s), 1218 (s), 1156 (m), 1116 (s), 972 (s) cm–1; HRMS (ESI) m/z [M + Na+] calcd. for C25H33NO6Na+ 466.22001, found 466.21899.
(3S,6Z,8R)-4-Aza-7-hydroxy-8-methyl-15-oxa-5,21-dioxo-tricyclo[14.2.2.13,6] henicosa-1(18),6,16(17),19-tetraene (3). To a solution of tetramic acid 18 (227 mg, 512 μmol, 1.00 eq) in CH2Cl2 (9.5 mL) was added TFA (950 μL). After stirring for 15 min at room temperature, toluene (100 mL) was added and the solvent was removed under reduced pressure. Toluene (50 mL) was added again and the solvent was removed, which afforded 3 as an orange foam. Yield: 176 mg (512 µmol, quant.). Rf = 0.51 (10% MeOH in CH2Cl2); –17.7° (c 0.74, MeOH); Major tautomer: 1H NMR (500 MHz, CD3OD): diastereotopic H-atoms indicated as a, b: δ 7.14 (m, 1H, CHArCCH2), 6.96 (m, 1H, CHArCCH2), 6.78 (m, 2H, CHArCO), 4.23–4.07 (m, 3H, CHN, ArOCH2), 3.33 (m, 1H, CHMe), 3.07a (dd, J = 14.3, 4.1 Hz, 1H, ArCH), 2.97b (dd, J = 14.4, 2.4 Hz, 1H, ArCHb), 1.52 (m, 2H, OCH2CH2), 1.44–1.16 (m, 5H, CHMeCHa, CMeCH2CH2, O(CH2)2CH2), 1.08b (m, 1H, CHMeCH), 1.05 (d, J = 6.9 Hz, 3H, CH3), 0.68–0.40 (m, 2H, CMe(CH2)2CH2) ppm; Significant signals minor tautomer: 1H NMR (500 MHz, CD3OD) δ 6.64 (m, 2H), 3.53 (m, 1H) ppm; Major tautomer: 13C NMR (125 MHz, CD3OD) δ 198.2 (CO, HMBC correlation), 190.2 (COH), 177.5 (HNCO, HMBC correlation), 157.0 (OCq,Ar), 133.3 (CHArCCH2), 130.8 (CHArCCH2), 128.1 (CH2Cq,Ar), 118.2 (OCCHAr), 117.4 (OCCHAr), 103.2 (NCOCCO), 67.6 (ArOCH2), 63.6 (CHN), 37.3 (CHMe), 36.4 (ArCH2), 35.2 (CMeCH2), 29.5 (CMeCH2CH2), 27.3 (CMe(CH2)2CH2), 25.9 (OCH2CH2), 24.7 (O(CH2)2CH2), 17.8 (CH3) ppm; Significant signals minor tautomer: 13C NMR (125 MHz, CD3OD) δ 131.9, 116.0 ppm; Major tautomer: 1H NMR (500 MHz, CDCl3) δ 7.19–7.00 (m, 2H), 6.79 (m, 2H), 4.35–4.09 (m, 3H), 3.38 (m, 1H), 3.28–2.83 (m, 2H), 1.52 (m, 2H), 1.44–1.12 (m, 5H), 1.12 (m, 1H), 1.07 (d, J = 6.8 Hz, 3H), 0.67–0.41 (m, 2H) ppm; Significant signal minor tautomer: 1H NMR (500 MHz, CD3OD) δ 3.63 (m, 1H) ppm; Major tautomer: 13C NMR (125 MHz, CDCl3) δ 194.3, 192.1, 175.9, 155.7, 132.6, 129.6, 126.4, 117.3, 116.7, 102.0, 66.8, 62.2, 36.0, 34.4, 28.4, 26.0, 24.9, 23.6, 17.6 ppm; Significant signals minor tautomer: 13C NMR (125 MHz, CDCl3) δ 130.5, 116.3, 115.9, 66.9, 63.7, 36.4, 36.1, 29.4, 25.9, 25.1, 23.9, 17.1 ppm; IR νmax 2929 (m), 2853 (m), 1654 (s), 1608 (s), 1509 (s), 1456 (m), 1340 (m), 1259 (m), 1222 (m), 1174 (m) cm–1; HRMS (ESI) m/z [M + H]+ calcd. for C20H26NO4+ 344.18563, found 344.18481.
4.3. Experimental Design and Evaluation of Herbicidal Tests
The test compounds were applied as solutions in a mixture of isopropanol/water = 1:1 + 0.25% Tween 20 to arrays of two plant species (dandelions and thistle) that are susceptible to natural macrocidins. Plants were grown in 100 cm2 pots with two individuals/pot and four replicates/treatment. Within each species there were three treatments: untreated control, the macrocidinoids (100 or 150 mM) and the commercial herbicide diflufenican (1.2 mM). Spraying was done with 0.4 mL/100 cm2. Effects were assessed after 14 days and a second time after three to six weeks when mortality was fully developed. A mortality factor, i.e., the percentage of eventually dead plants, was calculated according to the Henderson-Tilton method [22] using the formula:
| Mortality [%] = (1 − Ta × Cb/Tb × Ca) × 100 |
with Tb = number of plants before treatment (=8); Ta = number of vital, treated plants at the end of observation period; Cb = number of vital, untreated control plants at beginning (=8); Ca = number of vital, untreated control plants at the end of observation period.
4.4. Antimicrobial Activity
The antibacterial activities were determined by the so-called broth microdilution method [23]. In brief: all cultivations were done in standard microbiological media such as TSB medium (tryptic soy broth) for S. aureus (SH1000) and LB medium (lysogeny broth) for Escherichia coli (ATCC25922) and at 37 °C (only A. baumannii was cultivated at 30 °C). The overnight cultures of the bacterial test strains were diluted to an OD600 of 0.1 and further incubated until an OD600 = 0.5 was reached. These cultures were used as working cultures. They were diluted to obtain an OD600 of 0.1, determined in 45 µL of the bacterial suspension in each well of a 384-well plate, or in 90 µL of the bacterial suspension in each well of a half-area 96-well plate. Compound solutions were prepared in separate 96-well compound plates starting from 10 mM stock solutions in DMSO. The compound concentrations were adjusted so that the maximum DMSO concentrations in the assay plates were 1%, assuring no interference with growth from the solvent. The respective volumes of the compound solutions were added to the microbial suspensions with the 96-channel semi-automated pipettor CyBio Selma (Analytik Jena). The OD600 was determined directly after compound addition and subsequently after 1, 3 and 24 h using the Epoch 2 microplate reader (BioTek Instruments).
4.5. Antibiofilm Activity
Staphylococcus aureus DSM 1104 from a stock kept at −20 °C was precultured in 25 mL CASO (casein-peptone soymeal-peptone) medium in a 250 mL flask at 37 °C and shaken (100 rpm, 20 h. The OD600 of the culture solution was adjusted to 0.001 McFarland standard. The solution was incubated in 96-well microtiter plates (TPP tissue culture ref. No. 92196) for 18 h at 37 °C with 150 μL of serially diluted test compounds (250–2 μg/mL) in CASO with 4% glucose broth. Compounds showing high activities (e.g., 2, 3, 9) were diluted in the range of 10–0.3 μg/mL. The inhibition of biofilm formation was evaluated by staining with 150 μL of 0.1% crystal violet (CV; Thermo Fisher, Waltham, MA, USA) following previously established protocols [21,24]. Briefly, the supernatant of the 96-well plate was discarded and the wells were washed once with PBS buffer. The remaining biofilms were stained with 0.1% CV at room temperature for 15 min, washed three times with PBS buffer, and finally dissolved in 150 μL ethanol (95%). The absorbance of the resulting solution at 530 nm was quantified using a plate reader (Synergy 2, BioTek, Santa Clara, CA, USA). Methanol (2.5%) and microporenic acid A [21] (250–2 μg/mL) were used as negative and positive controls. Standard deviations (SD) of two repeats with duplicates were 10% or less. Effects on the biofilms and SD values are shown in Table S1 in the Supporting Information (SI).
The precultured bacterial suspension of S. aureus strain DSM 1104 was adjusted to 0.001 McFarland standard at OD600 and incubated in 96-well tissue microtiter plates for 18 h in 150 μL CASO with 4% glucose broth. The supernatant of the 96-well plate was removed and the remainder was washed with 150 μL PBS buffer. The test compounds were serially diluted in 150 μL of fresh media (CASO with 4% glucose broth) to concentrations of 250–2 μg/mL, and added to the wells. The plates were incubated for a further 24 h at 37 °C. Staining of the preformed biofilm and of the controls was carried out as described above [21,24]. The SD of two repeats with duplicates were 15% or less. SD values are shown in Table S1 in the ESI.
Candida albicans DSM 11225 was taken from a −20 °C stock and precultured in 25 mL YPED (Yeast extract Peptone Dextrose) medium in a 250 mL flask at 30 °C and shaken (100 rpm, 18 h). The OD600 of the culture solution was adjusted to 0.05 McFarland standard in RPMI 1640 medium. 150 μL of the solution was added to 96-well non-tissue microtiter plates (Falcon non-tissue plate ref. No. 351172) for 90 min at 37 °C, and shaken with 150 rpm. The supernatant was discarded and the residue washed twice with PBS buffer. The test compounds were serially diluted in 150 μL of fresh medium (RPMI 1640) to concentrations of 250–2 μg/mL and added to the wells. Methanol (2.5%) was used as the negative control. The plates were further incubated at 37 °C and shaken (150 rpm, 24 h). The supernatant of the 96-well plate was discarded, the wells were washed once with PBS buffer, and the biofilms were stained with 150 μL of 0.1% CV at room temperature for 25 min and then washed four times with PBS buffer. The biofilms were dissolved in 150 μL ethanol (95%), and the absorbance of the resulting solution at 610 nm was finally quantified using a plate reader (Synergy 2, BioTek, Santa Clara, CA, USA). SD of two repeats with duplicates each were 10% or less. Dispersal effects on preformed biofilms and SD values are shown in Table S1 (SI).
P. aeruginosa (PA 14) was cultured in 25 mL LB medium (Luria-Bertani Broth) in a 250 mL flask at 37 °C, shaken with 100 rpm, for 18 h. The OD600 of the culture solution was adjusted to 0.025 McFarland standard in LB medium. The test compounds were diluted in 100 μL bacterial solution to concentrations of 250–2 μg/mL and the resulting solutions were added to 96-well plates in an MBEC Innovatech incubator (MBEC Assay®, Edmonton, AB, Canada). The plates were incubated at 37 °C at 150 rpm for 24 h. The biofilms were established on the pegs under the growth conditions. The pegs and plates were rinsed once with PBS buffer, the biofilms on pegs were stained with 150 μL 0.1% CV at room temperature for 15 min and then rinsed twice with PBS buffer. The pegs were transferred into a new plate with 150 μL ethanol (95%) and the absorbance at 550 nm was quantified using a plate reader (Synergy 2, BioTek, Santa Clara, CA, USA). Myxovalargin A and methanol (2.5%) were used as positive and negative control.
4.6. Cytotoxicity
The cytotoxic effect upon treatment with macrocidinoids 2, 3, 6–10 for 72 h was determined by standard MTT assays [25]. The tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; abcr) is reduced by viable cells to a violet, water-insoluble formazan. Human 518A2 melanoma cells, colon carcinoma cells HCT-116wt and HCT-116p53−/−, and KBV cervix carcinoma cells as well as hybrid endothelial EaHy cells (5 × 104 cells mL−1, 100 µL/well), were seeded in 96-well tissue culture plates and cultured for 24 h at 37 °C, 5% CO2 and 95% humidity. After treatment with the test compounds (stock solutions 10 mM in DMSO and freshly diluted appropriately with sterile Milli-Q water) incubation of cells was continued for 72 h. Blank and solvent controls were treated identically. After addition of a 5 mg mL−1 MTT stock solution in phosphate buffered saline (PBS), microplates were incubated for 2 h at 37 °C, centrifuged at 300 g, 4 °C for 5 min and the supernatant was discarded. The precipitate of formazan crystals was then redissolved in a 10% (w/v) solution of sodium dodecylsulfate (SDS; Carl Roth) in DMSO containing 0.6% (v/v) acetic acid. To ensure complete dissolution of the formazan, the microplates were incubated for at least 1 h in the dark. Finally, the absorbance at λ = 570 and 630 nm (background) was measured using a microplate reader (Tecan F200). All experiments were carried out in quadruplicate and the percentage of viable cells was calculated as the mean ± SD with controls set to 100%. The determined IC50 (inhibitory concentration) values are shown in Table S2 (cf. SI).
Acknowledgments
H.Z. is grateful for a personal PhD stipend from the “Drug Discovery and Cheminformatics for New Anti-Infectives (iCA)” and is financially supported by the Ministry for Science & Culture of the German State of Lower Saxony (MWK No. 21—78904-63-5/19). We thank Blondelle Matio Kemkuignou (Dept. Microbial Drugs, HZI Braunschweig) for assistance with the biological tests, Claudia Soltendieck (WG COPS, HZI Braunschweig) for her excellent technical assistance during the antimicrobial activity evaluation, and Sofia I. Bär (University Bayreuth) for preliminary MTT cytotoxicity studies.
Abbreviations
DMAP: dimethylaminopyridine; DMSO: dimethylsulfoxide; EDC: 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide; NBS: N-bromosuccinimide; NMO: N-methylmorpholine-N-oxide; PBS: phosphate-buffered saline; TFA: trifluoracetic acid; THF: tetrahydrofuran; sat.: saturated.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics10081022/s1. Figures S1–S67: 1H and 13C NMR spectra of 3–10, 12–16, 18 and HPLC spectra of 3–10. Figure S68: herbicidal effects on pot plants. Figure S69: growth inhibitory effects of macrocidin derivatives on E. coli ΔTolC cultures; Figure S70: growth inhibitory effects of macrocidin derivatives on Staphylococcus aureus (SH1000) cultures. Figure S71: growth inhibitory effects of selected macrocidin derivatives on Actinetobacter baumannii cultures. Figure S72: dispersal effects on preformed biofilms of C. albicans. Table S1: antibacterial effects of compounds 2–10 on E. coli ΔTolC and S. aureus. Table S2: S. aureus and C. albicans biofilm growth inhibition and dispersion data. Table S3: IC50 values for human cells.
Author Contributions
Conceptualization, L.T., R.S. and M.E.-B.; methodology, L.T., C.P., M.E.-B., H.S. and U.B.; formal analysis and investigation, H.Z., H.S., S.J., A.S., M.E.-B. and U.B.; writing, R.S. and L.T.; supervision, R.S., U.B. and M.S.; project administration, R.S.; funding acquisition, R.S. and M.E.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the German Bundesministerium für Wirtschaft und Energie, grant numbers ZF4514501MD7 and ZF4513301MD7XX. This publication was funded by the German Research Foundation (DFG) and the University of Bayreuth in the funding programme Open Access Publishing.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Graupner P.R., Carr A., Clancy E., Gilbert J., Bailey K.L., Derby J.A., Gerwick B.C. The macrocidins: Novel cyclic tetramic acids with herbicidal activity produced by Phoma macrostoma. J. Nat. Prod. 2003;66:1558–1561. doi: 10.1021/np030193e. [DOI] [PubMed] [Google Scholar]
- 2.Hubbard M., Taylor W.G., Bailey K.L., Hynes R.K.W. The dominant modes of action of macrocidins, bioherbicidal metabolites of Phoma macrostoma, differ between susceptible plant species. Environ. Exp. Bot. 2016;132:80–91. doi: 10.1016/j.envexpbot.2016.08.009. [DOI] [Google Scholar]
- 3.Yoshinari T., Ohmori K., Schrems M.G., Pfaltz A., Suzuki K. Total synthesis and absolute configuration of macrocidin A, a cyclophane tetramic acid natural product. Angew. Chem. Int. Ed. 2010;49:881–885. doi: 10.1002/anie.200906362. [DOI] [PubMed] [Google Scholar]
- 4.Haase R.H., Schobert R. Synthesis of the bioherbicidal fungus metabolite macrocidin A. Org. Lett. 2016;18:6352–6355. doi: 10.1021/acs.orglett.6b03240. [DOI] [PubMed] [Google Scholar]
- 5.Matio Kemkuignou B., Treiber L., Zeng H., Schrey H., Schobert R., Stadler M. Macrooxazoles A-D, new 2,5-disubstituted oxazole-4-carboxylic acid derivatives from the plant pathogenic fungus Phoma macrostoma. Molecules. 2020;25:5497. doi: 10.3390/molecules25235497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graupner P.R., Gerwick B.C., Siddall T.L., Carr A.W., Clancy E., Gilbert J.R., Bailey K.L., Derby J.A. Chlorosis inducing phytotoxic metabolites: New herbicides from Phoma macrostoma. In: Rimando A., editor. Natural Products for Pest Management. ACS; Washington, DC, USA: 2006. pp. 37–47. (ACS Symposium Series). [DOI] [Google Scholar]
- 7.Dumas A. DMCCB Basel Symposium 2016: Macrocycles in drug- and agrochemical discovery. Chimia. 2016;70:561–562. doi: 10.2533/chimia.2016.561. [DOI] [PubMed] [Google Scholar]
- 8.Biersack B., Diestel R., Jagusch C., Rapp G., Sasse F., Schobert R. First syntheses of melophlins P, Q, R and effects of melophlins on the growth of microorganisms and tumor cells. Chem. Biodivers. 2008;5:2423–2430. doi: 10.1002/cbdv.200890207. [DOI] [PubMed] [Google Scholar]
- 9.Kempf K., Schmitt F., Bilitewski U., Schobert R. Synthesis, stereochemical assignment and bioactivity of the Penicillium metabolites penicillenols B1 and B2. Tetrahedron. 2015;71:5064–5068. doi: 10.1016/j.tet.2015.05.116. [DOI] [Google Scholar]
- 10.Wang J., Yao Q.-F., Amin M., Nong X.-H., Zhang X.-Y., Qi S.-H. Penicillenols from a deep-sea fungus Aspergillus restrictus inhibit Candida albicans biofilm formation and hyphal growth. J. Antibiot. 2017;70:763–770. doi: 10.1038/ja.2017.45. [DOI] [PubMed] [Google Scholar]
- 11.Lowery C.A., Park J., Gloeckner C., Meijler M.M., Mueller R.S., Boshoff H.I., Ulrich R.L., Barry C.L., III, Bartlett D.H., Kravchenko V.V., et al. Defining the mode of action of tetramic acid antibacterials derived from Pseudomonas aeruginosa quorum sensing signals. J. Am. Chem. Soc. 2009;131:14473–14479. doi: 10.1021/ja9056079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruckner S., Bilitewski U., Schobert R. Synthesis and antibacterial activity of four stereoisomers of the spider-pathogenic fungus metabolite torrubiellone D. Org. Lett. 2016;18:1136–1139. doi: 10.1021/acs.orglett.6b00245. [DOI] [PubMed] [Google Scholar]
- 13.Schlenk A., Diestel R., Sasse F., Schobert R. A selective 3-acylation of tetramic acids and the first synthesis of ravenic acid. Chem. Eur. J. 2010;16:2599–2604. doi: 10.1002/chem.200902544. [DOI] [PubMed] [Google Scholar]
- 14.Dalton D.R., Dutta V.P., Jones D.G. Bromohydrin formation in dimethyl sulfoxide. J. Am. Chem. Soc. 1968;90:5498–5501. doi: 10.1021/ja01022a030. [DOI] [Google Scholar]
- 15.Brizzi V., Francioli M., Brufani M., Filocamo L., Bruni G., Massarelli P. Synthesis, binding affinity and selectivity of new β1- and β2-adrenoceptor blockers. Farmaco. 1999;54:713–720. doi: 10.1016/S0014-827X(99)00077-4. [DOI] [PubMed] [Google Scholar]
- 16.Ramana C.V., Mondal M.A., Puranik V.G., Gurjar M.K. Synthetic studies toward macrocidins: An RCM approach for the construction of the central cyclic core. Tetrahedron Lett. 2006;47:4061–4064. doi: 10.1016/j.tetlet.2006.03.192. [DOI] [Google Scholar]
- 17.Royles B.J.L. Naturally occurring tetramic acids: Structure, isolation, and synthesis. Chem. Rev. 1995;95:1981–2001. doi: 10.1021/cr00038a009. [DOI] [Google Scholar]
- 18.Jeong Y.-C., Moloney M.G., Bikadi Z., Hazai E. A detailed study of antibacterial 3-acyltetramic acids and 3-acylpiperidine-2,4-diones. ChemMedChem. 2014;9:1826–1837. doi: 10.1002/cmdc.201402093. [DOI] [PubMed] [Google Scholar]
- 19.Jeong Y.-C., Anwar M., Moloney M.G., Bikadi Z., Hazai E. Natural product inspired antibacterial tetramic acid libraries with dual enzyme target activity. Chem. Sci. 2013;4:1008–1015. doi: 10.1039/C2SC21713A. [DOI] [Google Scholar]
- 20.Chen Y., Moloney J.G., Christensen K.E., Moloney M.G. Fused-ring oxazolopyrrolopyridopyrimidine systems with Gram-negative activity. Antibiotics. 2017;6:2. doi: 10.3390/antibiotics6010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chepkirui C., Yuyama K., Wanga L., Decock C., Matasyoh J., Abraham W.R., Stadler M. Microporenic acids A-G, biofilm inhibitors and antimicrobial agents from the basidiomycete Microporus sp. J. Nat. Prod. 2018;81:778–784. doi: 10.1021/acs.jnatprod.7b00764. [DOI] [PubMed] [Google Scholar]
- 22.Henderson C.F., Tilton E.W. Tests with acaricides against brown wheat mite. J. Econ. Entomol. 1955;48:157–161. doi: 10.1093/jee/48.2.157. [DOI] [Google Scholar]
- 23.Wiegand I., Hilpert K., Hancock E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008;3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 24.Yuyama K.T., Chepkirui C., Wendt L., Fortkamp D., Stadler M., Abraham W.R. Bioactive compounds produced by Hypoxylon fragiforme against Staphylococcus aureus biofilms. Microorganisms. 2017;5:80. doi: 10.3390/microorganisms5040080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosmann T.J. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.