Abstract
In the current study, Achillea santolinoides and Achillea aleppica aeral parts and root were extracted with ethyl acetate, methanol, and water. Detailed phytochemical profiles were obtained using UHPLC-MS, yielding the identification of hydroxybenzoic and hydroxycinnamic acids, phenolic acid glycosides and sugar esters, acylquinic acids, O-glycosyl flavones and flavonols, and flavonoid aglycons, among others. The antioxidant properties and enzyme inhibitory activities of the extracts were assayed with in vitro tests. The phenolic content of the water extracts was significantly higher as compared to the ethyl acetate and methanol ones. A. aleppica aerial parts methanol extract possessed highest flavonoid content (49.18 mg rutin equivalent/g). Antioxidant properties assessment revealed that the methanol extract of A. santolinoides roots actively scavenged DPPH (54.11 mg TE/g) and ABTS radicals (112.53 mg TE/g) and possessed highest reducing potential (183.55 and 129.92 mg TE/g, for CUPRAC and FRAP, respectively). The ethyl acetate extracts of aerial parts and roots of both species showed highest inhibition against BuCHE (6.07–6.76 mg GALAE/g). The ethyl acetate extract of A. santolinoides aerial part showed highest inhibition against tyrosinase (73.00 mg KAE/g). These results showed that the tested Achillea species might represent novel phytotherapeutic avenues for the management of Alzheimer’s disease and epidermal hyperpigmentation conditions, which are both associated with oxidative stress. This paper could shed light into future potential industrial applications using the tested Achillea species.
Keywords: medicinal plants, biopharmaceuticals, hyperpigmentation, phenolics
1. Introduction
The Achillea genus, one of the most important genera of the Asteraceae family with ethnopharmacological significance, consists of approximately 85 species mainly distributed in Middle East regions, such as Iran, Turkey, and Serbia and Eastern regions of Europe [1]. Achillea species have been reported to possess highly bioactive compounds and were rich in flavones and other flavonoids [2], non-saturated carboxylic acids [3], phenolic glycosides [4], guaianolides [5], lignans [6], phthalate derivatives [7], piperidine amides, proazulenes [8], sesquiterpenes [9], sesquiterpene lactone-diol [10], sesquiterpene lactones [11], polyacetylenes [12], spirodepressolide [13], tannins [14], and triterpene alkamide [15]. An ethnobotanical survey conducted by Mohammadnoseini and colleagues (2017) highlighted the use of several Achillea species in traditional medicine for the management of several ailments [16]. In addition, pharmacological studies have demonstrated that various Achillea species possess biological activities, such as antioxidant, antibacterial, antispasmodic, and anti-inflammatory [1].
Traditionally Achillea wilhelmsii C. Koch (new name: A. santolinoides subsp. wilhelmsii) flowers powder was sprinkled on wound to promote wound healing, the decoction of the plant was used as abortifacient, against stomach pain, fever, motion of children and jaundice while teas made from young shoots were used to manage stomach disorders [17]. The use of A. wilhelmsii also vary according to different locations, as such, A. wilhelmsii is used for its antihypertensive and antihyperlipidemic properties in Iran, to treat gastrointestinal disorders in Italy, hemorrhoids in Turkey, stomachache, diabetes, gastric, and obesity in Pakistan, and detoxification, hemostasia and acesodyne in China [16]. A. wilhelmsii rich in flavonoids and sesquiterpene lactones, have been reported to exhibit antiproliferative and apoptotic effects in PC3 cell line by suppressing the expression of oncogene hTERT in PCa [18]; essential oil of A. wilhelmsii showed anxiolytic effects in rats [19]; a clinical trial conducted on 120 randomly selected men and women, aged 40–60 years, revealed that treatment with hydroalcoholic extract of A. wilhelmsii significantly decreased triglycerides, total cholesterol, and LDL-cholesterol levels and decreased diastolic and systolic blood pressure [18,19,20]. Although A. aleppica subsp. aleppica has been reported to be used in traditional medicine, no record of the subspecies zederbaueri was found. Baris and colleagues reported the antioxidant and antimicrobial activities of A. aleppica subsp. zederbaueri ethanol extract [21].
Some enzymatic activities are considered valuable targets for drugs in the management or treatment of different serious diseases. In this regard, some enzymes are great of importance in the pharmaceutical area. For example, cholinesterases are related to manage Alzheimer disease and their inhibition could increase the level of acetycholine in the synaptic cleavage and improving memory function in Alzheimer’s patients [22]. α-amylase and α-glucosidase are main enzymes in the hydrolysis of starch and the blood glucose level can be controlled with their inhibition [23]. Tyrosinase is main enzyme in the synthesis of melanin and thus its inhibition could be valuable for controlling hyperpigmentation problems [24]. In the light of these facts, the discovery of new and effective enzyme inhibitors, especially from natural sources, is gaining great interest in the scientific platform [25,26,27].
This work attempts to comparatively assess the biological activity of the different extracts obtained from A. santolinoides subsp. wilhelmsii and A. aleppica subsp. zederbaueri aerial parts and roots. To study differences due to extraction procedures different organic solvents were used, namely ethyl acetate and methanol operating with maceration at room temperature. Furthermore, water extracts of plant materials were obtained using boiling water as mimic of traditional preparations as infusion that use boiling water. Data obtained from the chemical investigations as well as the in vitro bioassays were then combined using multivariate data approaches to evaluate possible correlations between the observed effects and the different chemical composition of the studied extracts.
2. Materials and Methods
2.1. Plant Collection and Extract Preparation
Achillea santolinoides subsp. wilhelmsii (K. Koch) Greuter and Achillea aleppica subsp. zederbaueri (Hayek) Hub.-Mor. were collected around Konya in June 2020. The aerial parts and roots were carefully separated and then dried in a shaded and well-ventilated environment at room temperature. After drying (about 10 days), the plant materials were powdered using a laboratory mill.
The powdered plant samples were extracted by different solvents, namely ethyl acetate, methanol and water. To obtain ethyl acetate and methanol extracts, the plant samples (10 g) were macerated with 200 mL of these solvents for 24 h in room temperature. Then, the extracts were filtered and evaporated to dryness. Regarding water extracts, the plant materials (10 g) were kept in 200 mL of boiled water for 15 min, this to mimic traditional preparation that in general use hot and boiled water to prepare extraction. The water extracts were filtered and then lyophilized. Obtained extracts were stored at 4 °C until experimentation.
2.2. Total Phenolic and Flavonoid Content
Spectrophotometric methods were used to determine total phenolic and flavonoid content as conducted in earlier papers. Standard equivalents (gallic acid equivalent: GAE for phenolic and rutin equivalent: RE for flavonoid) were used to explain the contents in the plant extracts [28,29].
2.3. Ultra-High Performance Liquid Chromatography Coupled with High Resolution Mass Spectrometry (UHPLC-HRMS)
UHPLC-HRMS analysis was performed as described elsewhere (Ak et al., 2021). Briefly, the separation was carried out on a reversed phase column Waters Cortecs C18 (2.7 µm, 2.1 × 100 mm) column maintained at 40°C. The binary mobile phase consisted of 0.1% formic acid in water (A) and B: 0.1% formic acid in acetonitrile (B). The gradient program began at 5% B for one min, gradually turned to 30% B over 19 min, increased gradually to 50% B over 5min, increased gradually to 70% B over 5 min, increased gradually to 95% over 3 min and finally the system was then turned to the initial condition of 5% B, and equilibrated over 4 min. The flow rate and the injection volume were set to 300 µL/min and 1 µL, respectively. Samples were prepared as follows: methanol and aqueous extracts were dissolved in methanol-water (1:1, v/v) by ultrasound (20 μg/mL), while for the etylacetate extracts methanol was used preparing sample at the same concentrations. The solutions were filtered thought syringe filters 0.22 μm (Filtratech, France) and injected into chromatographic system.
Mass spectrometry analyses were carried out on a Q Exactive Plus mass spectrometer (ThermoFisher Scientific, Inc., Waltham, MA, USA) equipped with a heated electrospray ionization (HESI-II) probe (ThermoScientific, Waltham, MA, USA). The tune parameters were as follows: spray voltage −2.5 kV; sheath gas flow rate 38; auxiliary gas flow rate 12; spare gas flow rate 0; capillary temperature 320 °C; probe heater temperature 320 °C and S-lens RF level 50. Acquisition was acquired at Full scan MS and Data Dependent-MS2 modes. Full scan spectra over the m/z range 100 to 1500 were acquired in negative ionization mode at a resolution of 70,000. Other instrument parameters for Full MS mode were set as follows: automatic gain control (AGC) target 3 × 106, maximum injection time (IT) 100 ms, number of scan ranges one. For DD-MS2 mode, instrument parameters were as follows: microscans 1, resolution 17,500, AGC target 1 × 105, maximum IT 50 ms, MSX count 1, Top5, isolation window 2.0 m/z, stepped normalized collision energy (NCE) 10, 20, 60 eV. Data acquisition and processing were carried out with Xcalibur 4.0 software (ThermoScientific, Waltham, MA, USA). All chromatograms and MS/MS data for each identified compound including fragmentation patterns are given in Supplemental Materials (Figures S1–S9).
2.4. Determination of Antioxidant and Enzyme Inhibitory Effects
Antioxidant protocols included reducing power (cupric reducing antioxidant capacity (CUPRAC) and ferric reducing power (FRAP)), metal chelating, phosphomolybenum and free radical scavenging (2,2-diphenyl-1-picrylhydrazyl (DPPH) and 3-ethylbenzothiazoline-6-sulphonic acid (ABTS)) activities. Trolox and ethylenediaminetetraacetic acid (EDTA) were used as standards in the antioxidant assays and the results were expressed as the equivalents of these standards. Experimental details were given in our previous paper [30].
Inhibitory effects of the extracts were tested against different enzymes (tyrosinase, α-amylase, α-glucosidase and cholinesterases (AChE and BuChE). Several compounds were used as standards (galatamine for cholinesterases; kojic acid for tyrosinase; acarbose for α- amylase and α-glucosidase) and the results were expressed as the equivalents of these standards. The enzyme inhibitory assays were performed as done in our earlier paper [31].
2.5. Statistical Analysis
Relative quantitative data of extracts molecules obtained from UHPLC-MS analysis was submitted to principal component analysis and Clustered Image Maps successively, for viewing the differential expression of molecules among extracts. Afterward, for biological, One-way ANOVA following by Tukey’s test were performed to determine any differences between the extracts of each studied species. p < 0.05 were assigned to be statistically significant. Then, for comparison both species extracts biological activities, principal component analysis (PCA) and Clustered Image Maps was subsequently achieved. For both realized Clustered Image Maps, “Wards” and “Euclidean” were use as linkage rule and similarity measure, respectively. The relationship between metabolites and biological activities was investigated using partial least squared regression analysis. The goodness of the model was measured through the estimation of the cumulative modeled variation in the metabolite matrix R2X(cum) and the cumulative modeled variation in the biological activities matrix R2Y(cum). All statistical procedures were performed using R software v. 3.6.1.
2.6. Bioinformatics Analysis
To investigate the genes targeted by the sesquiterpene lactones and derivatives and some phenolic compounds, the datasets for mRNA of DIGEP-Pred web-sever [32] was employed. The compounds were artabsin, dehydroleucodin, dihydrosantamarin, leucodin, matricin, tanaparthin peroxide, neochlorogenic acid, chlorogenic acid, homoorientin, vitexin and isovitexin. Only the genes with Pa (probability “to be active”) higher than 0.5 were retained. Then for KEGG pathway analysis, the obtained up-regulated and down-regulated mRNA data were submitted to Enrichr websever [33].
3. Results and Discussion
3.1. Chemical Profile
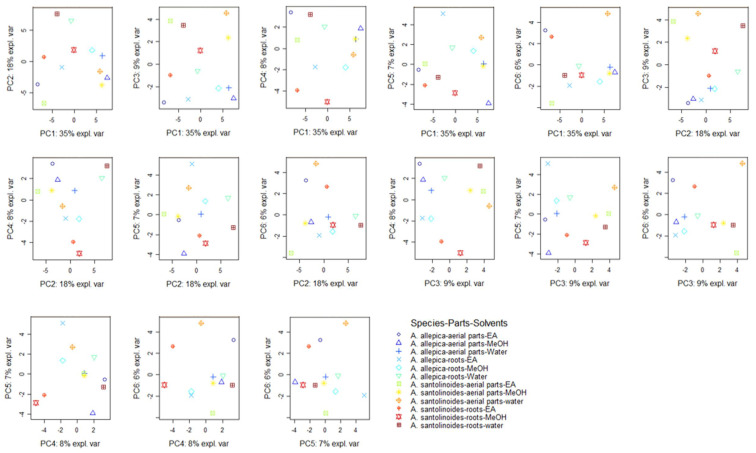
After the qualitative screening of metabolites profiles in the different extracts of the species, unsupervised principal component analysis (PCA) was carried out on the relative intensities of metabolites peak area obtained through UHPLC-MS analysis in order to screen the molecules variation between both species’ samples. Before PCA processing, metabolites profiles were log transformed and autoscaled to ensure an equal contribution of variables in prediction outcomes. From the extracted principal components (PCs), only the first six showed eigenvalue above one. In addition, they displayed a cumulative proportion explained variance higher than 80%, therefore there were used as recommended by Kaiser [34]. The molecules strongly associated with each of them were summarized in Table S1. Overall, 18, 9, 5, 5, 10, and eight molecules had the highest contribution scores on PC1, PC2, PC3, PC4, PC5, and PC6, respectively.
Afterwards, looking at the different score plot displayed in Figure 1, a considerable difference between the samples was observed. On the other hand, despite some samples seemed have common characteristics, it was difficult to clearly identify the different samples. For this purpose, an additional analysis i.e., Clustered Image Maps was performed from the coordinates of the samples derived from PCA. The samples can be split into three main clusters, the cluster I and III comprised on five samples respectively and the cluster II was represented by two samples (Figure 2). Of these three clusters, the samples of the clusters I were remarkably rich in several molecules. Hence most of the molecules were occurred predominantly in the methanol and water extracts obtained from both species the aerial parts as well as the methanol extract of A. aleppica subsp. zederbaueri roots. This finding reflects the polar character of the molecules present in these two species.
Figure 1.
Score plots of principal component analysis on the relative quantitative metabolites data of Achillea species.
Figure 2.
Clustered Image Map on the relative quantitative metabolites data obtained through UHPLC-MS analysis (Red color: High concentration, Blue color: low concentration). (C1) protocatechuic acid-O-hexoside, (C2) caffeoylgluconic acid, (C3) protocatechuic acid, (C4) p-hydroxyphenylacetic acid-O-hexoside, (C5) protocatechuic acid-O-hexoside isomer, (C6) syringic acid 4-O-hexoside (C7) neochlorogenic (3-caffeoylquinic) acid, (C8) caffeoylgluconic acid isomer, (C9) caffeic acid-O-hexoside, (C10) gentisic acid-O-hexoside, (C11) vanillic acid 4-O-hexoside, (C12) caffeoylgluconic acid isomer, (C13) O-caffeoyl hexose isomer, (C14) 4-hydroxybenzoic acid, (C15) 4-hydroxybenzoic acid-hexoside, (C16) p-hydroxyphenylacetic acid O-hexoside, (C17) quinic acid, (C18) chlorogenic (5-caffeoylquinic) acid, (C19) p-coumaric acid, (C20) 3-feruloylquinic acid, (C21) p-hydroxyphenylacetic acid, (C22) caffeic acid, (C23) gentisic acid, (C24) 5-p-coumaroylquinic acid, (C24a) 1,3-dicaffeoylquinic acid, (C25) caffeic acid-O-hexoside isomer, (C26) 5-feruloylquinic acid, (C27) m-coumaric acid, (C28) 5-p-coumaroylquinic acid isomer, (C29) 4-feruloylquinic acid, (C30) vanillic acid, (C31) o-coumaric acid, (C32) vanillic acid-4-O-(6-O-caffeoyl)-hexoside, (C33) 3,4-dicaffeoylquinic acid, (C34) 1,5-dicaffeoylquinic acid, (C35) 3,5-dicaffeoylquinic acid, (C36) dicaffeoyl-tetrahydroxy-pentanoic acid, (C37) 4,5-dicaffeoylquinic acid, (C38) shikimic acid, (C39) salicylic acid, (C40) 3-feruloyl-4-caffeoylquinic acid, (C41) 3-p-coumaroyl-5-caffeoylquinic acid, (C42) caffeic acid-O-(salicyl)-hexoside, (C43) 3-feruloyl-5-caffeoylquinic acid, (C44) 4-p-coumaroyl-5-caffeoylquinic acid, (C45) 1-caffeoyl-3-feruloylquinic acid, (C46) 3,4,5-tricaffeoylquinic acid, (C47) 6, 8-diC-hexosidyl-luteolin, (C48) O,C-dihexosyl-luteolin, (C49) diC-hexosyl-apigenin, (C50) 6-C-hexosyl-8-C-pentosyl-luteolin, (C51) 2″-O-pentosyl-6-C-hexosyl-luteolin, (C52) homoorientin, (C53) 6-C-hexosyl-8-C-pentosyl apigenin, (C54) orientin (luteolin-8-C-glucoside), (C55) C-hexosyl-C-pentosyl methylluteolin, (C56) rutin, (C57) vitexin, (C58) isovitexin, (C59) 2″-O-pentosyl-6-C-hexosyl-methylluteolin, (C60) Luteolin-7-O-glucosidea, (C61) chrysoeriol-6-C-hexoside, (C62) nepetin-O-hexuronide, (C63) 6-methoxykaempferol-O-hexoside, (C64) nepetin-O-hexoside, (C65) kaempferol-3-O-glucoside, (C66) isorhamnetin 3-O-glucoside, (C67) apigenin-7-O-glucoside, (C68) cirsiliol-O-hexoside, (C69) chrysoeriol-O-hexuronide, (C70) jaceosidin-O-hexuronide, (C71) luteolin, (C72) quercetin, (C73) patuletin (6-methoxyquercetin), (C74) axillarin, (C75) apigenin, (C76) kaempferol, (C77) hispidulin (scutellarein-6-methyl ether), (C78) chrysoeriol, (C79) cirsiliol, (C80) quercetagetin-3,6,3′(4′)-trimethyl ether, (C81) cirsimaritin (6-hydroxyapigenin-6,7-dimethyl ether), (C82) santin/eupatilin, (C83) acacetin, (C84) tanaparthin-peroxide, (C85) achillicin/matricin, (C86) dehydroachillin/dehydroleucodin, (C87) achillin/leucodin, (C88) artabsin, (C89) dihydrosantamarin, (C90) tetradecenoic acid amide, (C91) linolenamide, (C92) linoleamide, (C93) palmitamide, (C94) oleamide.
The total phenolic and flavonoid contents were determined using Folin Ciocalteau and aluminum chloride colorimetric methods, respectively. In A. alleppica extracts, water extract of root possessed the highest level of total phenolic (43.24 mg GAE/g), while the methanol extract of root contained the highest amounts of total phenolic (52.07 mg GAE/g) in A. santolinoides extracts. On the other hand, methanol extract of A. aleppica aerial part and ethyl acetate extract of A. santolinoides aerial part were found to have the highest flavonoid content respectively (49.18 and 19.58 mg RE/g) (Table 1).
Table 1.
Extraction yields (%), total bioactive compounds and total antioxidant capacity (by phosphomolybdenum assays) of the tested extracts *.
| Species | Parts | Solvents | Yields | TPC (mg GAE/g) | TFC (mg RE/g) | PBD (mmol TE/g) |
|---|---|---|---|---|---|---|
| A. aleppica | Aerial parts | EA | 4.61 | 20.77 ± 0.83 e | 13.23 ± 0.52 c | 2.33 ± 0.09 a |
| MeOH | 10.01 | 41.41 ± 0.88 b | 49.18 ± 0.98 a | 1.92 ± 0.04 b | ||
| Water | 9.88 | 36.56 ± 0.01 c | 16.62 ± 0.17 b | 1.45 ± 0.05 d | ||
| Roots | EA | 1.88 | 22.41 ± 0.59 d | 3.95 ± 0.11 e | 1.65 ± 0.04 c | |
| MeOH | 2.85 | 23.83 ± 0.24 d | 5.93 ± 0.04 d | 1.27 ± 0.03 e | ||
| Water | 4.58 | 43.24 ± 0.19 a | 4.12 ± 0.05 e | 1.57 ± 0.04 cd | ||
| A. santolinoides | Aerial parts | EA | 7.40 | 20.69 ± 0.32 f | 19.58 ± 0.32 a | 1.95 ± 0.05 a |
| MeOH | 16.04 | 32.20 ± 0.22 d | 8.42 ± 0.63 d | 1.90 ± 0.09 ab | ||
| Water | 19.05 | 44.97 ± 0.49 c | 18.08 ± 0.23 b | 1.33 ± 0.03 c | ||
| Roots | EA | 1.41 | 26.27 ± 0.90 e | 5.07 ± 0.23 e | 1.73 ± 0.10 b | |
| MeOH | 6.92 | 52.07 ± 1.58 a | 11.09 ± 0.18 c | 1.93 ± 0.12 ab | ||
| Water | 4.91 | 47.39 ± 0.05 b | 3.59 ± 0.27 f | 1.88 ± 0.04 ab |
* Values are reported as mean ± SD. EA: Ethyl acetate; MeOH: Methanol; TPC: Total phenolic content; TFC: Total flavonoid content; PBD: Phosphomolybdenum; GAE: Gallic acid equivalent; RE: Rutin equivalent; TE: Trolox equivalent. Different letters in same column indicate significant differences for each Achillea species (p < 0.05).
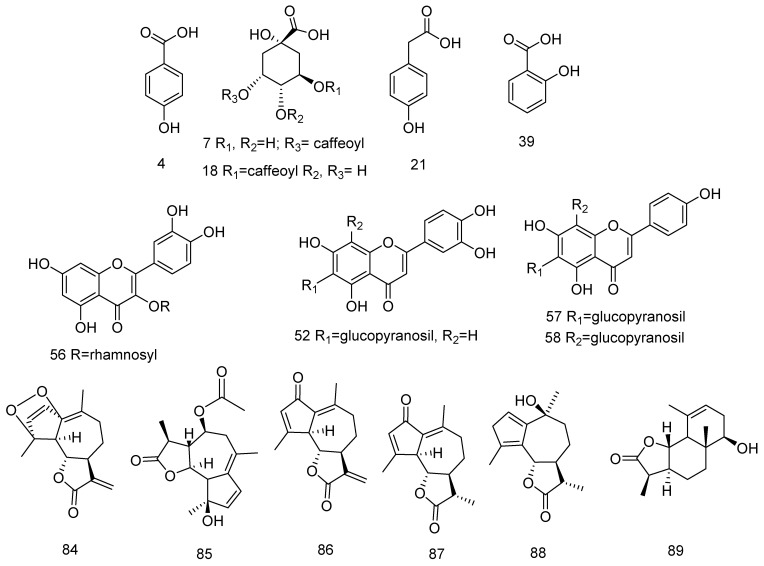
To identify the metabolites present in the studied extracts, non-targeted profiling was performed by ultra-high-performance liquid chromatography-quadrupol-Orbitrap high resolution mass spectrometry (UHPLC-HRMS). Under the conditions of Full scan-ddMS2/Top 5, the mass range for survey full scan was set at m/z 100–1200 and the MS/MS analyses were acquired by stepped higher energy collision-induced dissociation (hcd) at 10, 20, and 60 eV for data dependent (dd) MS2scans. The key points in the compounds annotation/dereplication were the accurate masses in Full MS and ddMS2, MS/MS fragmentation patterns, relative abundance of the precursor and fragment ions, elemental composition, matching with the simulated monoisotopic peak profiles, and consistence with the retentions times and fragmentation spectra of reference standards and literature data [35,36,37]. The chemical structures of main components are depicted in Figure 3.
Figure 3.
The main components in the tested Achillea extracts (for the compound numbers see Table 2).
A variety of metabolites were identified and tentatively elucidated in the assayed extracts, including, 14 hydroxybenzoic and hydroxycinnamic acids together with 12 phenolic acid glycosides and sugar esters, 18 acylquinic acids, 11 C-glycosyl flavones, 2 C, O-glycosyl flavones, 11 O-glycosyl flavones and flavonols, and 12 flavonoid aglycons, six sesquiterpene lactons, and five fatty acid amides (Table 2, Figure S1–S4). All compounds are reported for the first time in the studied Achillea sp.
Table 2.
Secondary metabolites in the studied Achillea extracts by UHPLC-ESI-MS/MS. Compound distribution is reported in the last column and different part and extracts are numberedas follows: 1. A. allepica-Aerial parts-EA; 2. A. allepica-Aerial parts-MEOH; 3. A. allepica-Aerial parts-WATER, 4. A. allepica-Roots-EA, 5. A. allepica-Roots-MEOH, 6. A. allepica-Roots-WATER, 7. A. santolinoides-Aerial parts-EA, 8. A. santolinoides-Aerial parts-MEOH, 9. A. santolinoides-Aerial parts-WATER, 10. A. santolinoides-Roots-EA, 11. A. santolinoides-Roots-MEOH, 12. A. santolinoides-Roots-WATER. a,b compound identity is confirmed by comparison with reference standards.
| No. | Identified/Tentatively Annotated Compound | Molecular Formula | Exact Mass [M − H]− |
Fragmentation Pattern In (−) ESI-MS/MS | tR (Min) | Δ ppm | Distribution |
|---|---|---|---|---|---|---|---|
| Hydroxybenzoic, hydroxycinnamic and acylquinic acids, and derivatives | |||||||
| 1 | protocatechuic acid-O-hexoside | C13H16O9 | 315.0722 | 315.0725 (100), 153.0179 (30.5), 109.0279 (99.3) | 1.71 | 0.385 | 2,3,4,5,6,7,8,9,10,11,12 |
| 2 | caffeoylgluconic acid | C15H18O10 | 357.0827 | 357.0827 (8.1), 195.0503 (100), 179.0340 (27.2), 177.0397 (18.2), 135.0440 (25.0), 87.0073 (3.6), 59.0121 (11.1) | 2.01 | −0.020 | 2,3,8,9 |
| 3 | protocatechuic acid a | C7H6O4 | 153.0182 | 153.0180 (14.6), 123.0435 (100), 109.0278 (40.8) | 2.03 | −1.362 | 1,2,3,4,5,6,7,8,9,10,11,12 |
| 4 | p-hydroxyphenylacetic acid-O-hexoside | C14H18O8 | 313.0929 | 313.0923 (2.7), 151.0387 (100), 123.0071 (0.9) | 2.67 | −0.591 | 1,2 |
| 5 | protocatechuic acid-O-hexoside isomer | C13H16O9 | 315.0722 | 315.0723 (100), 153.0180 (60.3), 123.0437 (17.1), 109.0279 (75.9) | 2.14 | 0.145 | 2,3,4,5,6,8,9,10,11,12 |
| 6 | syringic acid 4-O-hexoside | C15H20O10 | 359.0984 | 359.0984 (9.1), 197.0445 (100), 182.0210 (19.2), 166.9974 (7.6), 153.0544 (14.8), 138.0307 (28.5), 123.0072 (32.0) | 2.28 | −0.010 | 1,2,3,4,5,6,9,10 |
| 7 | neochlorogenic (3-caffeoylquinic) acid a | C16H18O9 | 353.0867 | 353.0879 (42.4), 191.0551 (100), 179.0339 (60.4), 173.0444 (3.7), 161.0236 (4.2), 135.0437 (53.1), 127.0387 (2.4), 93.0331 (4.9), 85.0277 (9.9) | 2.31 | 0.115 | 2,3,4,5,6,7,8,9,10,11,12 |
| 8 | caffeoylgluconic acid isomer | C15H18O10 | 357.0827 | 357.0810 (4.8), 195.0500 (72.1), 179.0338 (100), 177.0395 (7.3), 161.0234 (1.1), 135.0437 (77.2), 129.0177 (2.2), 87.0070 (5.6), 59.0124 (2.4) | 2.40 | −1.730 | 2,3,5,8,9 |
| 9 | caffeic acid-O-hexoside | C15H18O9 | 341.0867 | 341.0880 (5.0), 179.0338 (100), 135.0436 (62.0) | 2.40 | 0.195 | 2,3,4,5,6,8,9 |
| 10 | gentisic acid-O-hexoside | C13H16O9 | 315.0722 | 315.0724 (33.5), 153.0180 (70.9), 135.0072 (4.3), 109.0279 (100), 91.0171 (0.4) | 2.58 | 0.205 | 2,3,4,5,6,7,8,9,11,12 |
| 11 | vanillic acid 4-O-hexoside | C14H18O9 | 329.0878 | 329.0878 (27.1), 197.0446 (100), 182.0210 (15.5), 167.0335 (5.5), 153.0544 (28.7), 123.0073 (19.0), | 2.69 | -0.035 | 2,3,4,5,6,7,8,9,11,12 |
| 12 | caffeoylgluconic acid isomer | C15H18O10 | 357.0827 | 357.0828 (23.9), 339.0726 (11.2), 195.0500 (100), 179.0339 (18.9), 177.0392 (16.9), 161.0235 (3.4), 135.0437 (22.5), 129.0174 (9.7), 87.0071 (10.6), 59.0124 (1.4) | 2.81 | 0.044 | 2,3,5,6,8,9,12 |
| 13 | O-caffeoyl hexose isomer | C15H18O9 | 341.0867 | 341.0869 (23.7), 281.0665 (94.7), 251.0557(54.2), 221.0448 (44.0), 179.0339 (100), 161.0231 (56.9), 135.0437 (72.4) | 2.82 | −0.955 | 2,5,8,9 |
| 14 | 4-hydroxybenzoic acid a | C7H6O3 | 137.0244 | 137.0229 (12.6), 108.0208 (0.1), 93.0329 (100) | 2.86 | −1.527 | 1,2,3,4,5,6,7,8,9,10,11,12 |
| 15 | 4-hydroxybenzoic acid-hexoside | C13H16O8 | 299.0772 | 299.0773 (1.5), 137.0230 (100), 93.0330 (54.3) | 3.00 | 0.029 | 1,2,3,4,5,6,7,8,9,10,11,12 |
| 16 | p-hydroxyphenylacetic acid O-hexoside | C14H18O8 | 313.0929 | 313.0932 (13.4), 151.0386 (100), 123.0070 (0.9) | 3.00 | 0.309 | 2,5,11 |
| 17 | quinic acid | C7H12O6 | 191.0561 | 191.0550 (100), 173.0444 (2.0), 155.0332 (0.3), 127.0386 (4.0), 111.0436 (1.6), 93.0330 (6.2), 85.0279 (19.1) | 3.16 | −1.101 | 2,3,4,5,6,8,9,11,12 |
| 18 | chlorogenic (5-caffeoylquinic) acid a | C16H18O9 | 353.0867 | 353.0857 (1.9), 191.0550 (100), 161.0230 (1.5), 93.0331 (1.5), 85.0278 (8.8) | 3.19 | 0.835 | 1,2,3,4,5,6,7,8,10,11,12 |
| 19 | p-coumaric acid a | C9H8O3 | 163.0389 | 163.0385 (12.4), 135.0438 (4.2), 119.0487 (100) | 3.35 | −1.527 | 2,3,9 |
| 20 | 3-feruloylquinic acid b | C17H20O9 | 367.1035 | 367.1035 (22.0), 193.0496 (100), 191.0550 (2.6), 173.0443 (3.9), 134.0358 (64.7), 93.0329 (1.4), 85.0281 (0.9) | 3.44 | −0.005 | 2,3,5,6,8,10,11,12 |
| 21 | p-hydroxyphenylacetic acid a | C8H8O3 | 151.0401 | 151.0387 (100), 107.0486 (1.4), 136.0154 (1.5), 123.0072 (4.2) | 3.48 | 1.397 | 2,3,4,5,6,7,8,9,10,12 |
| 22 | caffeic acid a | C9H8O4 | 179.0338 | 179.0339 (3.6), 135.0437 (100), 151.0754 (2.4), 107.0489 (1.6) | 3.56 | −1.092 | 1,2,3,4,5,6,7,8,10,11,12 |
| 23 | gentisic acid a | C7H6O4 | 153.0182 | 153.0180 (73.8), 135.0073 (31.4), 109.0279 (100), 91.0173 (6.3) | 3.87 | −1.372 | 1,23,5,6,8,9,10,11,12 |
| 24 | 5-p-coumaroylquinic acid | C16H18O8 | 337.0929 | 337.0933 (9.3), 191.0550 (100), 173.0444 (6.8), 163.0388 (6.1), 161.0229 (0.2), 127.0385 (1.2), 119.0487 (5.3), 93.0329 (17.9), 85.0278 (5.1) | 3.96 | 0.369 | 1,2,3,4,5,6,8,9,11,12 |
| 24a | 1,3-dicaffeoylquinic acid | C25H24O12 | 515.1195 | 115.1199 (78.8), 353.0873 (36.6), 335.0779 (10.9), 191.0550 (100), 179.0338 (73.2), 173.0452 (5.6), 161.0232 (9.9), 135.0435 (58.7), 111.0434 (1.7) | 4.13 | 0.401 | 1,2,3,4,5,6,8,9,11,12 |
| 25 | caffeic acid-O-hexoside isomer | C15H18O9 | 341.0867 | 341.0830 (5.5), 179.0335 (6.2), 161.0230 (39.2), 135.0436 (63.6) | 4.34 | −4.765 | 2,3,5,6,8,9,10,12 |
| 26 | 5-feruloylquinic acid | C17H20O9 | 367.1035 | 367.1035 (18.5), 193.0498 (8.3), 191.0552 (100), 173.0444 (24.1), 134.0359 (12.2), 127.0382 (1.0), 111.0436 (5.0), 93.0329 (30.0), 85.0278 (6.0) | 4.42 | −0.015 | 2,3,4,5,7,9,10,11,12 |
| 27 | m-coumaric acid a | C9H8O3 | 163.0389 | 163.0387 (9.0), 135.0434 (1.8), 119.0486 (100) | 4.57 | −1.367 | 2,3,4,5,6,8,9,10,11 |
| 28 | 5-p-coumaroylquinic acid isomer | C16H18O8 | 337.0929 | 337.0934 (7.0), 191.0550 (100), 173.0444 (1.9), 163.0387 (2.2), 127.0385 (2.2), 119.0487 (1.6), 111.0434 (1.3), 93.0332 (5.0), 85.0278 (8.1) | 4.62 | 0.489 | 2,3,5,6,8,9,12 |
| 29 | 4-feruloylquinic acid | C17H20O9 | 367.1035 | 367.1034 (89.4), 193.0497 (9.8), 173.0443 (63.1), 155.0338 (4.1), 134.0358 (21.8), 111.0436 (14.7), 93.0329 (100), 85.0276 (0.5) | 4.66 | −0.055 | 2,3,4,5,6,10,11,12 |
| 30 | vanillic acid a | C8H8O4 | 167.0350 | 167.0338 (100), 152.0101 (27.8), 123.0071 (4.8), 95.0124 (3.4) | 4.79 | −1.232 | 2,3,4,5,6,7,8,9,11,12 |
| 31 | o-coumaric acid a | C9H8O3 | 163.0389 | 163.0387 (19.5), 135.0441 (4.1), 119.0487 (100) | 4.84 | −1.367 | 2,3,6 |
| 32 | vanillic acid-4-O-(6-O-caffeoyl)-hexoside b | C23H24O12 | 491.1195 | 491.1209 (100.0), 323.0774 (23.3), 221.0458 (4.6), 179.0343 (10.6), 167.0338 (16.2), 161.0231 (38.9), 152.0101 (18.3), 135.0437 (14.8), 123.0436 (1.2) | 5.52 | 0.928 | 2,3,4,5,6,8,9,11 |
| 33 | 3,4-dicaffeoylquinic acid a | C25H24O12 | 515.1195 | 515.1179 (94.1), 353.0875 (62.2), 335.0771 (6.7), 299.0573 (13.6), 203.0339 (41.1), 191.0548 (32.6), 179.0339(76.0), 173.0444 (100), 161.0233 (13.6), 135.0437 (77.0), 111.0436 (4.2), 93.0330 (38.4), 85.0278 (3.9) | 5.60 | −1.579 | 2,3,4,5,6,8,9,10,11,12 |
| 34 | 1,5-dicaffeoylquinic acid a | C25H24O12 | 515.1195 | 515.1189 (15.1), 353.0878 (33.0), 335.0774 (2.2), 191.0550 (100), 179.0338(6.2), 173.0446 (3.1), 161.0231 (5.0), 135.0436 (6.6), 127.0382 (1.8), 111.0433 (1.1), 93.0331 (4.5), 85.0278 (7.6) | 5.70 | −0.599 | 1,2,3,4,5,6,7,8,9,10,11,12 |
| 35 | 3,5-dicaffeoylquinic acid a | C25H24O12 | 515.1195 | 515.1204 (22.6), 353.0878 (100), 191.0551 (96.5), 179.0338 (53.1), 173.0441 (5.3), 161.0229 (7.9), 135.0437 (52.7), 111.0433 (1.1), 93.0328 (4.7), 85.0279 (9.1) | 5.86 | 0.921 | 1,2,3,4,5,6,7,8,9,10,11,12 |
| 36 | dicaffeoyl-tetrahydroxy-pentanoic acid | C23H22O12 | 489.1038 | 489.1030 (43.3), 327.0720 (40.6), 165.0392 (100), 179.0341 (17.2), 161.0231 (3.2), | 6.12 | −0.849 | 2,3,5,8,9 |
| 37 | 4,5-dicaffeoylquinic acid a | C25H24O12 | 515.1195 | 515.1204 (84.6), 353.0877 (76.6), 191.0549 (50.2), 179.0338 (72.4), 173.0443 (100), 161.0232 (8.0), 111.0435 (2.0), 93.0330 (27.1), 85.0278 (4.2) | 6.23 | 0.901 | 1,2,3,4,5,6,7,8,9,10,11,12 |
| 38 | shikimic acid | C7H10O5 | 173.0455 | 173.0444 (100), 155.0335 (2.0), 137.0232 (1.4), 127.0390 (0.5), 111.0437 (10.0), 93.0330 (68.4) | 6.23 | −6.453 | 2,3,5,8,9,11 |
| 39 | salicylic acid a | C7H6O3 | 137.0244 | 137.0228 (15.2), 109.0279 (0.7), 93.0330 (100) | 6.29 | −1.467 | 1,2,3,4,5,6,7,8,9,10,11,12 |
| 40 | 3-feruloyl-4-caffeoylquinic acid | C26H26O12 | 529.1351 | 529.1339 (100), 335.075 (9.3), 193.0498 (60.1), 191.0558 (7.6), 179.0336 (29.5), 173.0441 (29.3), 161.0231 (8.7), 135.0434 (32.1), 134.0357 (49.5), 93.0330 (6.8) | 6.49 | −1.299 | 2,3,5,6,9,11,12 |
| 41 | 3-p-coumaroyl-5-caffeoylquinic acid | C25H24O11 | 499.1246 | 499.1388 (44.0), 353.0885 (5.4), 337.0932 (83.6), 191.0553 (31.7), 173.0443 (7.0), 163.0388 (100.0), 135.0429 (1.7), 93.0326 (7.6), 85.0278 (1.8) | 6.53 | 14.205 | 2,3,4,5,8,9,11 |
| 42 | caffeic acid-O-(salicyl)-hexoside | C22H21O11 | 461.1089 | 461.1093 (49.1), 371.0756 (0.5), 341.0656 (1.9), 323.0774 (24.5), 299.0767 (1.6), 179.0340 (5.1), 161.0231 (23.1), 137.0229 (100), 93.0330 (61.3) | 6.56 | 0.405 | 1,2,3,4,5,6,7,8,9,11,12 |
| 43 | 3-feruloyl-5-caffeoylquinic acid | C26H26O12 | 529.1351 | 529.1355 (17.3), 367.1033(3.0), 353.2703 (1.6), 335.0754 (1.5), 193.0496 (100), 191.0554 (8.0), 173.0450 (9.0), 161.0230 (10.50), 134.0359 (74.4), 93.0331 (2.6) | 6.82 | 0.351 | 2,3,4,5,6,8,9,10,12 |
| 44 | 4-p-coumaroyl-5-caffeoylquinic acid | C25H24O11 | 499.1246 | 499.1218 (-0.871), 337.0932 (61.7), 179.0339 (9.0), 173.0442 (100), 163.0390 (21.2), 135.0437 (4.7), 119.0487 (8.7), 111.0437 (2.8) | 6.90 | −2.755 | 2,3,4,5,6,8,9,11 |
| 45 | 1-caffeoyl-3-feruloylquinic acid | C26H26O12 | 529.1351 | 529.1355 (17.3), 367.1033(3.0), 353.2703 (1.6), 335.0754 (1.5), 193.0496 (100), 191.0554 (8.0), 173.0450 (9.0), 161.0230 (10.50), 134.0359 (74.4), 93.0331 (2.6) | 7.23 | 0.651 | 2,4,5,10 |
| 46 | 3,4,5-tricaffeoylquinic acid | C34H30O15 | 677.1512 | 677.1509 (100). 515.1186 (32.4), 353.0879 (40.3), 335.0752 (13.7), 191.0552 (36.9), 179.0338 (70.6), 173.0444 (69.4), 161.0231 (19.8), 135.0437 (74.8), 111.0442 (3.6), 93.0330 (17.5) | 7.80 | −0.253 | 2,5,8,9,11 |
| Flavonoids | |||||||
| 47 | 6, 8-diC-hexosidyl-luteolin | C27H30O16 | 609.1461 | 609.1467 (100), 519.1136 (4.1), 489.1045 (14.6), 471.0941 (0.9), 429.0831 (6.1), 399.0722 (24.4), 369.0617 (26.2), 339.0507 (3.5), 311.0547 (5.2), 175.0387 (1.2), 133.0283 (6.7) | 3.64 | 0.622 | 2,3,5,6,8,9,10,11,12 |
| 48 | O,C-dihexosyl-luteolin | C27H30O16 | 609.1461 | 609.1469 (100), 447.0930 (24.2), 387.0808 (1.1), 369.0595 (1.6), 357.0616 (16.3), 327.0509 (54.5), 299.0557 (10.2), 298.0480 (6.7), 297.0403 (5.5), 175.0386 (1.6), 133.0283 (6.8) | 3.87 | 0.742 | 2,3,5,6,8,9,12 |
| 49 | diC-hexosyl-apigenin | C27H30O15 | 593.1512 | 593.1518 (100), 503.1205 (3.9), 473.1089 (14.9), 455.0996 (1.5), 413.0878 (1.9), 383.0773 (14.7), 353.0667 (31.6), 325.0723 (2.4), 309.0763 (1.8), 297.0769 (12.4), 175.0389 (1.5), 117.0331 (4.4) | 4.03 | 0.597 | 2,3,4,5,6,8,9,10,11,12 |
| 50 | 6-C-hexosyl-8-C-pentosyl-luteolin | C26H28O15 | 579.1355 | 579.1362 (100), 519.1219 (1.4), 489.1044 (10.7), 471.0909 (2.3), 459.0936 (9.1), 441.0836 (4.1), 429.0844 (7.1), 411.0721 (2.6), 399.0720 (26.7), 381.0613 (2.3), 369.0617 (24.6), 339.0504 (4.7), 311.0559 (4.3), 298.0483 (4.5), 175.0390 (0.9), 133.0280 (5.5) | 4.12 | 0.627 | 1,2,3,4,5,7,8,9,10,11,12 |
| 51 | 2”-O-pentosyl-6-C-hexosyl-luteolin | C26H28O15 | 579.1355 | 579.1362 (100), 459.0923 (9.6), 429.0820 (3.7), 399.0729 (1.4), 369.0618 (3.7), 357.0618 (25.8), 327.0514 (48.0), 309.0394 (7.2), 299.0558 (6.5), 298.0485 (17.0), 297.0400 (10.8), 175.0392 (1.4), 133.0278 (9.7) | 4.47 | 0.687 | 2,3,4,5,7,8,9,10,11 |
| 52 | Homoorientin a | C21H20O11 | 447.0933 | 447.0935 (100), 429.0830 (3.6), 411.0712 (0.9), 399.0707 (1.1), 387.0720 (0.5), 381.0609 (0.5), 369.0618 (2.8), 357.0617 (47.4), 327.0511 (68.9), 299.0559 (11.8), 298.0477 (9.1), 297.0403 (12.0), 285.0402 (8.6), 269.0449 (1.8), 199.0391 (1.5), 133.0280 (15.0), 107.0119 (0.7) | 4.53 | 0.225 | 1,2,3,4,5,6,8,9,10,11 |
| 53 | 6-C-hexosyl-8-C-pentosyl apigenin | C26H28O14 | 563.1406 | 563.1412 (100), 503.1214 (3.6), 473.1079 (6.7), 443.0990 (7.5), 425.0868 (2.9), 413.0889 (2.9), 383.0774 (20.1), 365.0665 (2.0), 353.0668 (25.9), 325.0715 (2.1), 324.0595 (0.4), 323.0562 (1.4), 297.0765 (9.3), 283.0611 (2.2), 175.0393 (1.3), 135.0434 (2.0), 117.0330 (3.1) | 4.53 | 0.541 | 1,2,3,4,5,6,7,8,9,10,11,12 |
| 54 | orientin (luteolin-8-C-glucoside) | C21H20O11 | 447.0933 | 447.0935 (89.2), 369.0621 (3.2), 357.0616 (31.4), 327.0511 (100), 299.0560 (10.7), 298.0476 (7.0), 297.0404 (13.5), 285.0397 (6.4), 269.0457 (1.6), 133.03 (19.4), 119.0485 (1.4), 151.022 (0.7), 107.0121 (0.6) | 4.68 | 1,2,4,5,6,8,9,10,11 | |
| 55 | C-hexosyl-C-pentosyl methylluteolin | C27H30O15 | 593.1512 | 593.1520 (100), 503.1203 (9.8), 473.1092 (11.1), 443.0963 (2.7), 425.0835 (1.0), 413.0881 (14.7), 395.0765 (0.6), 383.0775 (24.8), 341.0677 (1.1), 323.0550 (1.1), 313.0683 (1.9), 312.0639 (18.5), 299.0557 (0.5), 298.0476 (2.4), 283.0614 (1.8) | 4.75 | 0.787 | 1,2,3,4,5,6,7,8,9,10,11,12 |
| 56 | Rutin a | C27H30O16 | 609.1461 | 609.1464 (100), 301.0347 (39.7), 300.0274 (70.1), 271.0247 (39.2), 255.0297 (18.2), 243.0294 (9.3), 227.0342 (2.7), 211.0394 (0.9), 178.9977 (3.2), 163.0027 (1.6), 151.0024 (7.1), 121.0278 (1.1), 107.0121 (2.2) | 5.06 | 0.512 | 2,3,4,5,7,8,9,10,11,12 |
| 57 | Vitexin a | C21H20O10 | 431.0984 | 431.0986 (95.1), 341.0666 (0.5), 311.0562 (100), 293.0452 (2.0), 283.0610 (30.3), 117.0330 (15.0) | 5.15 | 0.200 | 1,2,3,4,5,6,7,8,9,10,11,12 |
| 58 | Isovitexin a | C21H20O10 | 431.0984 | 431.0986 (100), 413.0880 (1.7), 341.0666 (32.8), 311.0562 (69.1), 283.0610 (22.1), 269.0447 (4.1), 239.0706 (1.3), 117.0330 (8.7) | 5.30 | 0.200 | 1,2,4,5,6,7,9,10,11 |
| 59 | 2”-O-pentosyl-6-C-hexosyl-methylluteolin | C27H30O15 | 593.1512 | 593.1500 (100), 473.1140 (1.7), (443.0968 (6.1), 383.0750 (7.7), 371.0761 (16.5), 341.0664 (36.4), 323.0566 (20.7), 308.0315 (6.7), 299.0526 (2.7), 298.0486 (15.9) | 5.36 | 0.002 | 1,2,3,5,6,7,8,9,10,11,12 |
| 60 | Luteolin-7-O-glucoside a | C21H20O11 | 447.0933 | 447.0935 (100), 285.0404 (82.4), 133.0283 (11.8) | 5.39 | 0.437 | 2,4,5,8 |
| 61 | chrysoeriol-6-C-hexoside | C22H22O11 | 461.1078 | 461.1095 (100), 371.0774 (24.0), 341.0667 (73.2), 298.0481 (44.3), 296.0324 (0.9), 297.0403 (14.6) | 5.43 | 0.535 | 2 |
| 62 | nepetin-O-hexuronide | C22H20O13 | 491.0832 | 491.0829 (72.9), 315.0511 (100), 300.0275 (54.3), 272.0326 (8.6), 243.0297 (0.9), 227.0347 (0.5), 133.0284 (2.1) | 5.47 | 0.335 | 2,3,8 |
| 63 | 6-methoxykaempferol-O-hexoside | C22H22O12 | 477.1042 | 477.1041 (100), 315.0512 (56.5), 300.0272 (16.4), 299.0197 (18.2), 271.0247 (52.2), 243.0292 (0.7), 227.0344 (0.4), 151.0020 (1.6), 107.0122 (0.3) | 5.48 | 0.251 | 1,2,3,4,5,6,7,8,9,10,11,12 |
| 64 | nepetin-O-hexoside | C22H22O12 | 477.1038 | 477.1033 (100), 315.0486 (29.7), 300.0269 (15.9), 299.0197 (20.5), 271.0244 (3.6), 255.0307 (1.8), 243.0303 (2.6), 227.0344 (3.5), 165.8804 (0.5), 136.9889 (1.7), 133.0279 (10.0) | 5.67 | −0.549 | 1,2,4,5,7,8,9,11,12 |
| 65 | kaempferol-3-O-glucoside a | C21H20O11 | 447.0933 | 447.0935 (100), 285.0397 (22.0), 284.0324 (55.3), 255.0294 (41.8), 227.0341 (35.0), 151.0023 (1.6) | 5.86 | 0.195 | 2,3,4,5,8,9 |
| 66 | isorhamnetin 3-O-glucoside a | C22H22O12 | 477.1042 | 477.1041 (100), 315.0493 (10.1), 314.0433 (49.0), 300.0279 (3.1), 299.0212 (4.6), 271.0245 (18.5), 255.0300 (0.8), 243.0291 (19.6), 227.0347 (2.8), 215.0350 (3.8), 151.0022 (2.42) | 6.02 | 0.251 | 1,2,3,4,5,6,8,9 |
| 67 | apigenin-7-O-glucoside a | C21H20O10 | 431.0984 | 431.0986 (100), 269.0450 (27.7), 151.0019 (1.0), 107.0121 (1.4) | 6.06 | 0.200 | 2,3,5,8,9,11 |
| 68 | cirsiliol-O-hexoside | C23H24O12 | 491.1184 | 491.1198 (0.3), 476.0963 (26.6), 461.0726 (9.7), 329.0664 (5.0), 314.0425 (5.5), 313.0355 (13.1), 299.0197 (4.3), 285.0402 (11.8), 271.0245 (9.6), 243.0292 (9.0), | 6.31 | 0.311 | 2 |
| 69 | chrysoeriol-O-hexuronide | C23H22O12 | 475.0882 | 475.0884 (83.8), 299.0560 (100), 284.0325 (65.6), 256.0373 (6.5), 227.0347 (1.1), 175.0237 (15.3), 151.0024 (3.3), 113.0228 (37.6), 85.0278 (22.7), | 6.34 | 0.181 | 2,3,4,5,6,8,9 |
| 70 | jaceosidin-O-hexuronide | C23H22O13 | 505.0988 | 505.0993 (88.3), 329.0667 (100), 314.0432 (18.7), 299.0197 (36.3), 271.0247 (36.7), 243.0290 (0.6), 227.0342 (0.6), 175.0237 (13.2), 161.0229 (0.6), 113.0227 (34.1), 85.0278 (22.6) | 6.34 | 0.566 | 2,3,5 |
| 71 | Luteolin a | C15H10O6 | 285.0405 | 285.0403 (100), 241.0975 (21.4), 226.075 (8.4) | 7.59 | −0.181 | 1,2,3,4,5,6,7,8,9,10,11 |
| 72 | Quercetin a | C15H10O7 | 301.0354 | 301.0353 (100), 273.0405 (1.5), 178.9975 (22.7), 151.0023 (51.2), 121.0281 (12.7), 107.0123 (13.4) | 7.62 | −0.036 | 1,2,3,4,5,7,8,9 |
| 73 | patuletin (6-methoxyquercetin) b | C16H12O8 | 331.0464 | 331.0458 (100), 316.0024 (65.9), 287.0190 (14.1), 271.0246 (3.5), 259.0238 (3.1), 243.0285 (2.7), 181.0132 (7.1),165.9885 (19.2), 139.0023 (11.2), 109.9994 (9.6) | 7.72 | −0.161 | 1,2,7,8,9 |
| 74 | axillarin | C17H14O8 | 345.0616 | 345.0615 (99.2), 330.0381 (100), 315.0147 (48.0), 287.0196 (12.3), 243.0227 (2.6), 231.0295 (5.8), 215.0342 (4.1), 165.9897 (4.9), 149.0230 (1.2), 139.0385 (4.2), 136.9861 (1.3), 121.0280 (1.6) | 8.24 | −0.101 | 1,2,4,7,8,9 |
| 75 | Apigenin a | C15H10O5 | 269.0457 | 269.0453 (100), 225.0553 (1.6), 201.0546 (0.5), 151.0023 (5.4), 149.0239 (4.4), 117.0331 (18.4), 107.0124 (4.8) | 8.62 | 0.870 | 2,7,8,10,11 |
| 76 | Kaempferol a | C15H10O6 | 285.0405 | 285.0402 (100), 178.9938 (0.9), 151.0026 (1.0), 107.0121 (1.4) | 8.83 | −0.161 | 1,2,3,4,5,8 |
| 77 | hispidulin (scutellarein-6-methyl ether) a | C16H12O6 | 299.0563 | 299.0559 (62.4), 284.0324 (100), 255.0303 (1.3), 227.0471 (3.4), 212.0471 (3.2), 211.0389 (2.6), 164.9812 (2.0), 163.0005 (0.3), 149.9963 (1.1), 136.9865 (14.6), 117.0324 (1.5) | 8.92 | −.201 | 1,2,3,4,5,7,8,9,10,11 |
| 78 | Chrysoeriol a | C16H12O6 | 299.0562 | 299.0560 (93.1), 284.0324 (100), 256.0372 (6.4), 227.0344 (3.3), 211.0392 (1.8), 151.0024 (5.2), 133.0280 (1.6), 107.0122 (4.6) | 8.97 | −0.141 | 1,2,3,4,5,7,8,9,10,11 |
| 79 | cirsiliol | C17H14O7 | 329.0677 | 329.0667(100), 314.0432 (32.6), 299.0160 (21.2), 271.0248 (7.2), 255.0294 (1.0), 243.0294 (2.7), 230.1474 (11.8), 227.0344 (2.3), 163.0024 (2.0), 136.9874 (0.4), 135.0074 (1.4), 133.0282 (8.0) | 9.16 | 0.034 | 1,2,3,4,7,8,9,10,11 |
| 80 | quercetagetin-3,6,3’(4’)-trimethyl ether | C18H16O8 | 359.0772 | 359.0773 (100), 344.0536 (90.3), 329.0304 (49.3), 314.0068 (7.9), 301.0343 (3.5), 286.0118 (34.9), 258.0168 (10.9), 230.0214 (8.3), 202.0263 (10.1), 164.9807 (1.6), 148.0146 (1.6), 136.9854 (0.4), | 9.74 | 0.059 | 2,7,8,9,10 |
| 81 | cirsimaritin (6-hydroxyapigenin-6,7-dimethyl ether) | C17H14O6 | 313.0719 | 313.0822 (100), 298.0481 (56.4), 283.0246 (57.8), 269.0455 (2.8), 255.0299 (17.8), 227.0333 (5.8), 211.0333 (2.6), 163.0024 (19.5), 117.0326 (10.6) | 10.38 | −0.411 | 1,2,3,4,5,6,7,8,9,10,11,12 |
| 82 | santin/eupatilin | C18H16O7 | 343.0812 | 343.0822 (76.5), 328.0588 (100), 313.0355 (23.9), 298.0119 (19.4), 285.0402 (7.2), 270.0168 (24.2), 257.0085 (1.8), 254.0224 (0.7), 242.0218 (3.1), 226.0267 (1.6), 214.0266 (3.3), 198.0314 (2.3), 165.9895 (1.1), 164.9812 (0.3), 163.0020 (0.2), 136.9866 (1.9), 132.0201 (1.4) | 10.68 | −0.086 | 1,2,3,4,5,6,7,8,9,10,11 |
| 83 | acacetin | C16H12O5 | 283.0612 | 283.0610 (100), 268.0375 (72.4), 240.0425 (5.4), 239.0342 (4.8), 151.0026 (5.2), 107.0122 (3.1) | 11.44 | 1.036 | 2,7,8,9 |
| Tentatively Annotated Compound | Molecular Formula |
Exact Mass
[M + H]+ |
Fragmentation Pattern in (+) ESI-MS/MS | tR (min) | Δ ppm | Distribution | |
| Sesquiterpene lactones and derivatives | |||||||
| 84 | tanaparthin-peroxide | C15H18O5 | 279.1226 | 279.1213 (2.82), 261.1115 (39.19), 237.1117 (100), 243.1015 (12.03), 233.1169 (38.41), 221.0806 (77.86), 215.1064 (29.10), 203.0699 (79.07), 193.0857 (48.82), 187.1112 (16.07), 175.0752 (85.47), 165.0909 (47.60), 147.0802 (45.29), 123.0441 (37.47), 105.0701 (37.62), 91.0547 (36.27), 79.0548 (21.43), 67.0550 (13.10) | 6.41 | −0.395 | 3,4,7,8,9 |
| 85 | achillicin/matricin | C17H22O5 | 307.1537 | 307.1530 (56.90), 265.1427 (14.78), 247.1324 (100), 229.1220 (34.74), 219.1376 (28.47), 201.1272 (41.37), 173.0956 (29.40), 147.0802 (52.01), 131.0852 (31.79), 105.0700 (25.38), 91.0545 (21.60), 79.0549 (16.19) | 8.04 | −0.913 | 1,2,3,4,5,6,8 |
| 86 | dehydroachillin/ dehydroleucodin |
C15H16O3 | 245.1170 | 245.1166 (100), 227.1064 (6.46), 209.0956 (6.71), 199.1115 (18.76), 181.1010 (4.52), 156.0932 (3.66), 143.0852 (3.98), 123.0804 (5.57), 105.0701 (4.23), 91.0548 (3.25), 79.0548 (1.97), 69.0341 (10.05) | 9.37 | −0.860 | 1,2,3,5,7,8,11 |
| 87 | achillin/leucodin | C15H18O2 | 247.1326 | 247.1323 (100), 229.1213 (1.49), 219.1374 (4.62), 201.1272 (5.13), 191.1426 (4.81), 173.0959 (33.47), 158.0725 (5.02), 145.1009 (9.93), 135.0803 (3.08), 117.0699 (1.99), 107.0858 (3.94), 97.0651 (2.56), 79.0547 (2.01), 69.0341 (6.12), 55.0550 (0.66) | 9.55 | −1.412 | 1,2,3,4,5,6,7,8,9,10,11,12 |
| 88 | artabsin | C15H20O3 | 249.1482 | 249.1479 (73.40), 231.1375 (57.58), 221.1530 (6.61), 213.1268 (8.55), 203.1428 (100), 185.1322 (37.65), 175.1116 (75.95), 161.0958 (11.74), 157.1010 (78.89), 147.1166 (52.45), 133.1012 (32.49), 119.0857 (54.68), 10.0702 (59.52), 93.0703 (28.58), 81.0704 (10.70), 67.0550 (5.91), 55.0551 (8.37) | 11.30 | −1.409 | 1,2,3,4,5,6,7,9,10,11,12 |
| 89 | dihydrosantamarin | C15H22O3 | 251.1638 | 251.1635 (31.06), 233.1530 (42.34), 215.1428 (5.23), 205.1584 (100), 187.1478 (52.95), 177.1272 (47.19), 159.1165 (68.27), 147.1166 (20.87), 133.1010 (16.22), 119.0856 (16.77), 105.0701 (27.07), 97.0652 (17.16), 81.0704 (17.26), 67.0549 (5.80) | 12.64 | −1.597 | 1,2,3,7,8 |
| Fatty acids amides | |||||||
| 90 | tetradecenoic acid amide | C14H25NO | 224.2006 | 224.2004 (100), 196.2052 (0.09), 182.1537 (0.24), 168.1380 (6.51), 151.1115 (6.61), 123.1168 (2.56), 109.1014 (3.28), 95.0495 (6.80), 81.0340 (9.65), 69.0705 (11.84), 57.0707 (14.36) | 15.09 | −1.387 | 1,2,3,4,5,6,8,10,11,12 |
| 91 | linolenamide | C18H31NO | 278.2473 | 278.2472 (100), 261.2202 (0.89), 243.2098 (1.08), 219.1740 (0.69), 167.1302 (18.76), 152.1069 (6.86), 135.1169 (1.06), 109.1009 (4.05), 95.0859 (6.54), 81.0703 (9.21), 67.0549 (15.81) | 19.82 | −1.801 | 1,3,4,5,6,12 |
| 92 | linoleamide | C18H33NO | 280.2631 | 280.2628 (100), 263.2361 (82.19), 245.2258 (64.41), 221.2253 (3.56), 189.1632 (4.79), 179.1793 (9.84), 165.1634 (15.59), 147.1167 (8.77), 133.1011 (15.10), 123.1167 (23.89), 109.1013 (44.22), 95.0859 (71.60), 81.0704 (66.64), 69.0705 (50.93), 57.0706 (23.67) | 20.43 | −1.432 | 1,2,3,4,5,6,7,8,9,10,11,12 |
| 93 | palmitamide | C16H33NO | 256.2631 | 256.2627 (100), 214.2169 (0.26), 130.1227 (0.29), 116.1070 (1.43), 102.0916 (4.15), 88.0710 (0.33), 74.0607 (2.39) | 21.33 | −1.683 | 1,2,3,4,5,6,7,8,9,10,11,12 |
| 94 | oleamide | C18H35NO | 282.2786 | 282.2784 (100), 265.2520 (29.11), 247.2415 (25.90), 226.2156 (1.06), 212.2007 (3.29), 191.1790 (5.05), 177.1636 (4.54), 163.1478 (7.65), 149.1321 (12.53), 135.1167 (15.92), 121.1013 (14.04), 97.1015 (34.81), 83.0860 (35.84), 69.0706 (48.28) | 21.75 | −2.059 | 1,2,3,4,5,6,7,8,9,10,11,12 |
3.1.1. Hydroxybenzoic, Hydroxycinnamic and their Glycosides, and Sugar Esters
Based on the fragmentation patterns and retention times of reference standards, five hydroxybenzoic acids (3, 14, 23, 30, 39) and four hydroxycinnamic acids (19, 22, 27, 31) together with p-hydroxyphenylacetic acid (21) were identified in the extracts (Table 2, Figure S1). In addition, 7 hydroxybenzoic and hydroxycinnamic acids hexosides (1, 4–6, 9–11, 15, 16, 25) together with a sugar ester O-caffeoyl-hexose (13) were tentatively elucidated (Ak et al., 2021). MS/MS spectra of 3 caffeoylgluconic isomers 2, 8, and 12 ([M-H]− at m/z 357.084) were obtained (Table 2, Figure S1). They yielded a base peak at m/z 195.050 (C6H11O7−) corresponding to the [gluconic acid-H]− supported by the fragment ions at m/z 177.040 [GA-H-H2O]−, 87.007 [GA-H-C3H8O4]− and 59.012 [GA-H-C3H8O4-CO]− (Table 2).
Vanillic acid-4-O-(6-caffeoyl)-hexoside (32) was deduced from the loss of vanillic acid (168 Da) at m/z 323.077 and a subsequent transition 323.077→221.046 [M-H-102]− arising from the hexose cross ring cleavage (0,4X). The latter ion points out to the caffeoyl moiety at Hex C-6. Regarding 42, the prominent ion at m/z 323.077 [M-H-C7H6O3]− and a base peak at m/z 137.023 [salicylic acid-H]− together with m/z 93.033 [salicylic acid-H-CO2]− were in accordance with caffeic acid-O-(salicyl)-hexoside (Table 2). Both 32 and 42 were annotated in Tanacetum vulgare [35].
Among the compounds of the group, phenolic acid-hexosides 5, 15, and 16 were the major compounds in the aerial parts of both species (Figure S1A,B), especially protocatechuic acid- and 4-hydroxybenzoic acid-hexoside in A. santolinoides. Syringic acid-hexoside (6) was presented mainly in A. allepica roots (Table 2, Figure S1D). In addition, quinic acid was commonly found in all samples.
3.1.2. Acylquinic Acids
Overall, 7 monoAQA, 10 diAQA and 1 triAQA were identified in the studied extracts, mostly in the methanol and water extracts (Table 2, Figure S2). Their recognition was based on the fragmentation patterns and diagnostic ions for different subclasses AQA reported elsewhere [35,36]. Thus, 18, 24, 26, and 28 were assigned to 5-AQA as suggested a base peak at m/z 191.055 [quinic acid-H]−, while 7 and 20 were identified as 3-AQA.
Five peaks 24, 33–35, and 37 ([M-H]− at m/z 515.119) afforded prominent ions at m/z 353.088 and 191.055 indicating the subsequent losses of a caffeoyl moiety (Table 2). The vicinal diCQA 33 and 37 were witnessed by the “dehydrated” ion of quinic acid at m/z 173.044 (100%) supported by the diagnostic ions at m/z 335.0771 [CQA-H-H2O]− and 135.044 [caffeic acid-H-CO2]− in 3,4-diCQA (33) (Table 2). The second isomer was assigned to 4,5-diCQA as suggested by the lack of ion at m/z 335 and the chromatographic behavior on the reverse phase (the most lipophilic diCQA isomer). The base peak at m/z 191.055 evidenced 1,3-diCQA (24a), 1,5-diCQA (34) and 3,5-diCQA (35) supported by the relative abundance of the ions at m/z 179.034 and m/z 135.044: 73.2% and 58.7% (24a), 6.2% and 6.6% (34), and 53.1% and 52.7% (35), respectively.
Two p-coumaroyl-caffeoylquinic acids (p-CoCQA) isomers 41 and 44 at m/z 499.122 (C25H23O11) were deduced from the distinctive fragments at m/z 337.093 [M-H-caffeoyl]−, m/z 163.039 [p-CoA-H]− and m/z 119.049 [p-CoA-H-CO2]− for p-coumaric acid (Table 2). Compound 41 afforded an abundant ion m/z 337.093 (83.6%) indicating a loss of caffeoyl residue before the p-coumaroyl one. This assignment was also supported by the base peak at m/z 163.039 as was registered in 3-p-CoQA [35]. Thus, 41 was identified as 3-p-Co-5CQA, while vic 4-p-Co-5-CQA was supported by the abundant ions at m/z 337.093 (61.7%) and 173.044 (100%).
Three peaks 40, 43, and 45 yielded a precursor ion at m/z 529.136 (C26H25O12) along with prominent fragments at m/z 367.103 [M-H-caffeoyl]− and m/z 353.270 [M-H-feruloyl]− for feruloyl-caffeolylquinic acids (FCQA). The fragment ion at m/z 335.0754 [M-H-FA]− accompanied by the “dehydrated” form of quinic acid suggested 3F-4CQA (40) [36]. The assignment of 3F-5CQA was witnessed by the base peak at m/z 193.050 together with the abundant ion at m/z 134.036 (74.4%) as was registered in 3-FQA (Table 2). 1C-3FQA (45) was discernible by the base peak at m/z 161.023 [CA-H-H2O]− accompanied by the abundant ions at m/z 179.034 [CA-H]− (42.3%) and 367.104 (34.1%) [36]. The MS/MS spectrum of 46 was consistent with 3,4,5-triCQA [35].
Clorogenic acid (18) was the main monoAQA in the aerial parts and roots of both Achillea sp. diCQA were dominated by 3,5-diCQA (35) (Figure S2) except for A. santolinoides roots where 1,3-diCQA (24a) was a major compound of the group (Figure S2C).
3.1.3. Flavonoids
C-, C,O- and O-Flavonoid Glycosides
MS/MS spectra of the C-glycosyl flavones 52, 54, 57, and 58 were acquired (Table 2, Figure S3). In the (−) ESI mode 54 and 57 yielded a base peak 0,2X− [(M-H)-120]− at m/z 327.051 (54) and 311.056 (57) supported by the relevant ions at m/z 299.056 0,2X/CO− [(M-H)-120–28]− and m/z 283.061, respectively. This fragmentation pathway was consistent with C-8 hexosyl luteolin/apigenin [38]. In contrast, corresponding C-6 hexosyl isomers 52 and 58 was shown by the ions at m/z 447.094 [M-H]− (100%) and 431.099, as well as 0,3X− at m/z 357.062 and 341.067, and 0,2X− at m/z 327.051 and 311.056. The aglycones luteolin (52, 54) and apigenin (57, 58) were discernable by the RDA ions 1,3A− (m/z 151.022), 0,4A− (m/z 107.012), 1,3B− at m/z 133.028 (52, 54) and 117.033 (57, 58). Based on the comparison with reference standards, compounds 52, 54, 57, and 58 were identified as homoorientin, orientin, vitexin, and isovitexin, respectively.
Three isobars species 49, 55, and 59 shared the same [M-H]− at m/z 593.152 (Table 2, Figure S3). Concerning 49, typical ions of the C-glycosyl flavon pathway were produced at m/z 473.109 [(M-H)-120]−, 383.077 [(M-H)-90–120]− and 353.067 [(M-H)-2 × 120]− suggesting the presence of two C-hexosyl moieties on the flavonoid skeleton [35]. Considering that the C glycosylation appears exclusively at C-6 and 8 of flavones, compound 47 was assigned as 6, 8-diC-hexosyl-apigenin. C-hexosyl-C-pentosyl methylluteolin (55) was discernible by the prominent ions [0,3X0/0,2X1]− at m/z 413.088 [(M-H)-60–120]− and [0,1X0/0,1X1]− at 323.057 [(M-H)-120-150]− suggesting the presence of both C-pentosyl (X0) and C-hexosyl (X1) moieties. Additionally, methylluteolin was assigned on the basis of specie at m/z 299.560 [MeLu-H]− and 298.048 Y0/0,2X1/•CH3/CO [38]. On the other hand, compound 59 yielded prominent ions at m/z 323.057 ([(M-H)-(132 + H2O)-120]− and 443.097 ([(M-H)-(132 + H2O)]− suggesting O-pentosyl unit at 2″ of the primary hexose [38,39]. Diagnostic ions at m/z 308.032 (Z1−/0,2X0/•CH3) and 298.049 (Y1−/0,2X0/•CH3/CO) allowed for the annotation of methylluteolin. Thus, compound 59 was identified as 2″-O-pentosyl-6-C-hexosyl-methylluteolin.
Among the isobar species indicted as 47, 48, and 56 with [M-H]− at 609.147, 47 was annotated as 6, 8-diC-hexosyl-luteolin, while 48 was assigned to O, C-dihexosyl-luteolin. The latter structure was shown by a series of diagnostic ions at m/z 447.093 [M-H-Hex]−, 357.062 [M-H-Hex-90]− and 327.051 [M-H-Hex-120]−. Additionally, ions at m/z 298.048 (Y1−/0,2X0/CHO•), 175.039 (1,3A−/H2O−) and 133.028 1,3A− indicated luteolin. The sugar chain of 56 was consistent with rutinose (308 Da); aglycone quercetin was witnessed by a series of fragments including RDA ions at m/z 178.998 [1,2A-H]−, 163.003 [0,2A-H]−, 151.002 [1,3A]−, 121.028 [1,2B]−, 107.012 [0,4A]−. Based on comparison with reference standard, 56 (rutin), 60 (luteolin-7-glucoside), 65 (kaempferol-3-glucoside), 66 (isorhamnetin-3-glucoside), 67 (apigenin-7-glucoside), luteolin (50), quercetin (72), apigenin (75), kaempferol (77) and chrysoeriol (78) were unambiguously identified (Table 2).
Compounds 62, 69, and 70 presented similar fragmentation patterns yielding base peaks at m/z 315.051 (61), 299.056 (69, 70) and 329.067 (71) [(M-H)-HexA]−, respectively, indicating flavonoid hexuronides (Table 2).
Nepetin-O-hexuronide (62) was deduced from the fragment ions at m/z 243.030 [(M-H)-HexA-CH3-HCO•-CO]−, 227.035 [(M-H)-HexA-CH3-HCO•-CO2]− as well as RDA ions at m/z 133.028 (1,3B−). Compound 69 was ascribed to chrysoeriol-O-hexuronide (1,3A− at m/z 151.002, 0,4A− at m/z 107.013), while 70 was consistent with jaceosidin-O-hexuronide [40]. It should be noted that in both Achillea species the predominant compounds among the flavonoid glycosides were C-glycosyl flavons homoorientin (52) and vitexin (57) together with C-pentosyl-C-hexosyl-apigenin/methylluteolin (53, 55) (Table 2, Figure 3). Despite the similarity of the composition, compound 57 was mostly produced by the A. wilhemsii aerial parts.
6-Methoxyflavonoids
6-Methoxyflavonoids annotation was based on the characteristic fragment ions delineated in the previous studies on Tanacetum sp. [35,36].
Compound 79 ([M-H]− at m/z 329.067 (C17H14O7) could be used to illustrate the fragmentation pattern of 6-methoxylated flavones (Table 2, Figure 4). In (−) ESI-MS/MS 79 yielded fragment ions at m/z 314.043 [M-H-•CH3]−, 299.016 [M-H-2•CH3]−, 271.025 [M-H-2•CH3-CO]−, 255.029 [M-H-2•CH3-CO2]−, 243.029 [M-H-2•CH3-2CO]−, 230.147 [M-H-2•CH3-2CO-CHO•]− and 227.034 [M-H-2•CH3-CO-CO2]−. Consistent with the Orbitrap-based approach for the recognition of methoxylated flavonoids, RDA ions were registered at m/z 163.002 (1,3A−-H2O-CH2), 136.987 (1,3A−-CO-2•CH3), and 135.007 (1,3A−-H2O-CO-CH2) [35,36]. On the other hand, 1,3B− at m/z 133.028 indicated two hydroxyl groups in the ring B. Thus, compound 79 was assigned as 6-hydroxyluteolin-6, 7-dimethyl ether (cirsiliol), previously reported in Achillea sp. [41].
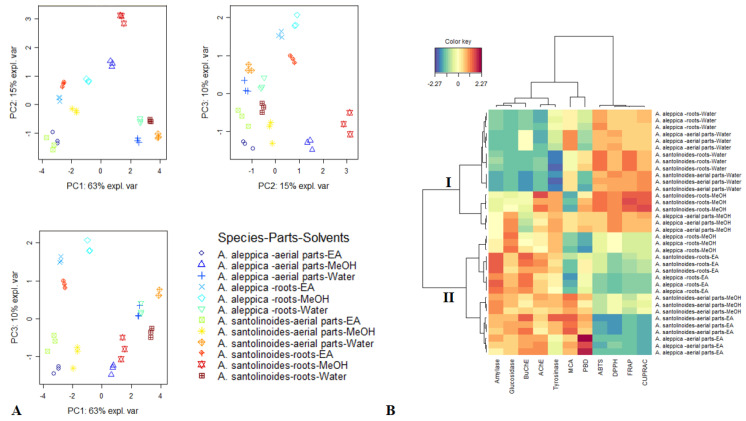
Figure 4.
Exploratory multivariate analysis on biological activities of Achillea species. (A) Score plots of principal component analysis; (B) Clustered Image Map (Red color: High activity, Blue color: low activity.
In addition, quercetagetin-3, 6-dimethyl ether (axillarin) (74) was deduced from the typical losses from RDA ion (1,3A−) at m/z 165.990 (1,3A−-•CH3), 139.039 (1,3A−-CO-CH2), 136.986 (1,3A−-CO-CH4) and 1,2 B− at m/z 121.028. Within this group, compounds 73 (patuletin), 74 (axillarin), and quercetagetin-3,6,3′(4′)-trimethyl ether (80) were quercetagetin derivatives, while compounds 77 (hispidulin) and 81 (cirsimaritin) were scutellarein derivatives (Table 2, Figure S4). In the (−) ESI mode compound 82 gave consequent losses of 3 methyl radicals at m/z 328.059, 313.036 and 298.012. Despite the lack of the initial RDA, a series of low abundant 1,3A− ions were generated at m/z 165.990 (1,3A−-•CH3), 164.981 (1,3A—CH4), 163.002 (1,3A−-H2O), 136.987 (1,3A−-CO-CH4). Moreover, (1,3B−-•CH3-CH2) at m/z 132.020 indicated 2 methoxy groups either in C-3, C-4′ or C-3′, C-4′, as was observed in santin and eupatilin, respectively [35,42].
Overall, flavonoid aglycones fingerprints of both A. allepica and A. santolinoides aerial parts extracts were dominated by cirsimaritin (81) and santin/eupatilin (82) (Figure S4).
Sesquiterpene Lactones (STLs)
The dereplication of STLs was based on the fragmentation patterns and diagnostic ions in positive ion mode as more informative for this class of natural compounds [36,43]. Based on accurate masse in Full MS, MS/MS fragmentation patterns, relative abundance of precursor and fragment ions, and elemental composition, 6 STLs were tentatively annotated in Achillea extracts.
MS/MS spectrum of 84 [M + H]+ at m/z 279.1226, yielded a fragment ions at m/z 261.111 [M + H-H2O]+ and 243.101 [M + H-2H2O]+ and a base peak at m/z 237.111 [M + H-H2O-CH2]+. This fragmentation pathway could be associated with the presence of peroxide group and 84 was tentatively ascribed to tanaparthin-peroxide, previously isolated from Achillea nobilis (Table 2) [44]. Compound 85 [M + H]+ at m/z 307.153 gave a base peak at m/z 247.132 [M + H-CH3COOH]+ which is in accordance with the structure of achillicin/matricin. Compound 87 differs from 85 for 60 Da (CH3COOH) and revealed the same fragmentation patterns as 85. Thus, compound 87 was tentatively annotated as achillin/leucodin (Table 2). Similarly, 86 [M + H]+ at m/z 245.117 was related to dehydroachillin/dehydroleucodin. Based on MS/MS fragmentation pathway, including characteristic ions corresponded to the loss of H2O (−18 Da), 2xH2O (−36 Da), CO (−28 Da), as well as concomitant loss of H2O + CO (−46 Da), 2H2O + CO (−64 Da), 88 and 89 were ascribed to artabsin and dihydrosantamarin, respectively, and were previously isolated from Achillea collina [45].
Fatty Acids Amides
The peak at 92 afforded a precursor ion at m/z 280.263 (C18H33NO) together with distinctive fragments at m/z 263.236 [M + H-NH3]+ and m/z 245.225 [M + H-NH3-H2O]+, suggesting amide of octadecadienoic acid. Additionally, the suggested structure was supported by the fragments at m/z 81.070 (C6H9), 69.070 (C5H9), 57.070 (C6H9) (Table 2). Thus, 92 was assigned as linoleamide [46]. Similarly, 90, 91, 93, and 94 were related to tetradecenoic acid amide, linolenamide, palmitamide and oleamide, respectively (Table 2) [46].
3.2. Antioxidant Effects
The total antioxidant capacity of the extracts was determined using the phosphomolybdenum assay. As shown in Table 1, for both species, the aerial part ethyl acetate extracts (2.33 and 1.95 mmol TE/g) showed the highest activity. Further antioxidant assays, free radical scavenging (DPPH and ABTS), reducing power (FRAP and CUPRAC), and metal chelating were conducted in order to obtain a comprehensive understanding of the antioxidant potential of the extracts and results were presented in Table 3. The ability of the extracts to scavenge free radicals was summarized in Table 3. Methanol extracts of A. aleppica aerial parts (55.15 mg TE/g) and A. santolinoides roots (54.11 mg TE/g) showed highest scavenging activity against DPPH. In contrast A. aleppica roots water extract (101.88 mg TE/g) and A. santolinoides roots methanol extract (112.53 mg TE/g) were most potent in scavenging ABTS. Protocatechuic acid and its derivatives identified in the A. aleppica roots water extract, A. aleppica aerial parts methanol extract, and A. santolinoides roots methanol extract, has been reported to exhibit radical scavenging activity [47,48]. Neochlorogenic (3-caffeoylquinic) acid also identified in these extracts was previously reported to exhibit scavenging activity against DPPH [49]. The reducing capacity of the extracts to donate electron and thus act as reducing agents is commonly assessed using two widely used methods, namely FRAP (ferric ion) and CUPRAC (cupric ion) assays. Similar to the DPPH assay, methanol extracts of A. aleppica aerial parts and A. santolinoides roots showed highest reducing capabilities (Table 3). The chelating capacity of the extracts was also evaluated. The water extract of the aerial parts of A. aleppica (25.37 mg EDTAE/g) and ethyl acetate and water extract of the aerial parts of A. santolinoides (27.37 and 26.06 mg EDTAE/g), respectively possessed strong chelating ability. Caffeic acid, chlorogenic acid, and protocatechuic acid were identified in aerial parts of A. aleppica water and A. santolinoides ethyl acetate extracts. Interestingly, a study conducted by Andjelković, et al. [50] has assessed the metal chelating potential of these phenolic compounds and reported that caffeic acid and chlorogenic acid were the strongest metal chelators. It can also be suggested that the presence of these metal chelators created a synergistic effect, therefore enhancing the metal chelating properties of these extracts. The hydroalcoholic extract of A. santolinoides was previously reported to possess antioxidant effect on brain tissues in pentylenetetrazole-induced seizures Wistar rat models [51]. The essential oil of A. santolinoides was also found to exhibit antioxidant potential against DPPH radical (IC50 = 129–372 mg/mL) [52].
Table 3.
Antioxidant properties of the tested extracts *.
| Species | Parts | Solvents | DPPH (mg TE/g) | ABTS (mg TE/g) | CUPRAC (mg TE/g) | FRAP (mg TE/g) | MCA (mg EDTAE/g) |
|---|---|---|---|---|---|---|---|
| A. aleppica | Aerial parts | EA | 13.83 ± 0.07 e | 22.04 ± 1.31 e | 50.49 ± 3.22 e | 27.29 ± 0.21 e | 23.55 ± 1.37 ab |
| MeOH | 55.15 ± 0.05 a | 88.93 ± 0.79 b | 151.21 ± 5.64 a | 101.38 ± 1.79 a | 21.51 ± 0.09 bc | ||
| Water | 49.71 ± 1.17 b | 90.83 ± 0.12 b | 138.34 ± 1.94 b | 95.19 ± 0.62 b | 25.37 ± 0.33 a | ||
| Roots | EA | 12.44 ± 0.14 e | 30.38 ± 1.31 d | 66.55 ± 3.46 d | 34.75 ± 1.12 d | 10.28 ± 1.37 d | |
| MeOH | 35.66 ± 0.29 d | 56.23 ± 0.79 c | 88.69 ± 0.57 c | 54.62 ± 1.10 c | 12.03 ± 0.76 d | ||
| Water | 43.44 ± 0.35 c | 101.88 ± 0.98 a | 143.53 ± 0.75 ab | 93.79 ± 0.99 b | 20.25 ± 0.52 c | ||
| A. santolinoides | Aerial parts | EA | 6.57 ± 0.15 f | 15.31 ± 0.96 f | 51.59 ± 0.11 f | 25.96 ± 0.39 f | 27.37 ± 0.46 a |
| MeOH | 30.49 ± 0.30 d | 42.06 ± 0.40 e | 104.45 ± 3.32 d | 50.42 ± 1.61 d | 26.06 ± 1.20 a | ||
| Water | 51.90 ± 0.67 b | 95.34 ± 1.15 c | 164.05 ± 1.57 b | 105.24 ± 1.07 c | 21.33 ± 0.16 b | ||
| Roots | EA | 15.93 ± 0.07 e | 50.47 ± 1.33 d | 85.86 ± 2.57 e | 43.33 ± 3.63 e | 12.25 ± 1.70 d | |
| MeOH | 54.11 ± 0.03 a | 112.53 ± 0.18 a | 183.55 ± 1.68 a | 129.92 ± 3.18 a | 10.72 ± 0.42 d | ||
| Water | 47.59 ± 0.07 c | 109.04 ± 0.20 b | 151.23 ± 0.28 c | 118.50 ± 0.41 b | 17.59 ± 0.08 c |
* Values are reported as mean ± SD. EA: Ethyl acetate; MeOH: Methanol; TE: Trolox equivalent; EDTAE: EDTA equivalents. Different letters in same column indicate significant differences for each Achillea species (p < 0.05).
3.3. Enzyme Inhibitory Effects
The inhibitory ability of extracts prepared from the aerial parts and roots of the selected Achillea species against enzymes targeted in the management of diabetes mellitus type II, Alzheimer’s disease, and skin hyperpigmentation problems was investigated. Alzheimer’s disease has escalated to epidemic proportions and the need for complementary therapeutic agents to effectively manage this debilitating condition is of paramount importance. From Table 4, A. aleppica aerial parts ethyl acetate extract and A. santolinoides roots methanol exhibited highest inhibition against AChE. A previous molecular docking study confirmed the interaction of orientin with AChE which showed least binding energy and highest binding affinity [53]. Vitexin also identified in these extracts was previously reported to bind effectively with AChE through strong hydrogen bonding [54]. Acacetin was previously reported to exhibit moderate to potential AChE inhibitory properties [55]. However, in the present study, acacetin was not identified in extracts showing more potent inhibitory activity against AChE. Santin/eupatilin identified in the ethyl acetate extracts of A. aleppica roots and A. santolinoides aerial parts was previously reported to inhibit BuChE in an in silico study. On the other hand, the ethyl acetate extracts of A. aleppica aerial parts and roots (6.07 and 6.73 mg GALAE/g) and as well as that of A. santolinoides aerial parts (6.76 and 6.70 mg GALAE/g) were most active against BuChE. The inhibition of BuChE has been advocated in the later stage of Alzheimer’s disease. During the progression of the disease, BuChE level increases, exacerbating the conditions of the patient [56]. The ability of the extracts to inhibit enzymes targeted in the management of diabetes type II, namely α-amylase and α-glucosidase, was presented in Table 4. A low inhibition against both enzymes was noted, suggesting that the different extracts of A. aleppica and A. santolinoides aerial parts and roots possessed weak anti-diabetic properties. Tyrosinase, a rate limiting enzyme responsible for the biosynthesis of melanin, is considered to be a key therapeutic strategy for the management of skin hyperpigmentation conditions. In the present study, methanol extracts of A. aleppica aerial parts and roots showed the highest inhibitory activity against tyrosinase. In other side, ethyl acetate and methanol extracts of both studied parts of A. santolinoides displayed strongest anti-tyrosinase activity. Hispidulin, isolated from Phyla nodiflora and identified in extracts which actively inhibited tyrosinase was previously reported to exhibit inhibitory action against tyrosinase with an IC50 value of 146 µM [57].
Table 4.
Enzyme inhibitory effects of the tested extracts *.
| Species | Parts | Solvents | AChE (mg GALAE/g) | BuChE (mg GALAE/g) | Tyrosinase (mg KAE/g) | Amylase (mmol ACAE/g) | Glucosidase (mmol ACAE/g) |
|---|---|---|---|---|---|---|---|
| A. aleppica | Aerial parts | EA | 2.63±0.03 a | 6.07±0.14 a | 57.63±1.17 cd | 0.29±0.04 b | 0.64±0.01 d |
| MeOH | 2.01±0.21 bc | 2.12±0.25 c | 71.22±0.57 a | 0.22±0.01 c | 0.78±0.01 b | ||
| Water | 0.48±0.04 d | 3.83±0.01 b | 54.86±2.16 d | 0.07±0.01 d | na | ||
| Roots | EA | 2.21±0.10 b | 6.73±0.25 a | 63.26±0.93 b | 0.37±0.02 a | 0.70±0.04 c | |
| MeOH | 1.83±0.04 c | 3.92±0.54b | 70.36±0.30 a | 0.24±0.01 c | 0.85±0.01 a | ||
| Water | 0.50±0.02 d | 1.25±0.04 d | 58.83±0.74 c | 0.10±0.01 d | na | ||
| A. santolinoides | Aerial parts | EA | 2.02±0.18 c | 6.76±0.77 a | 73.00±4.87 a | 0.30±0.01 c | 0.74±0.02 a |
| MeOH | 2.32±0.23 bc | 4.74±0.41 b | 69.02±0.86 a | 0.35±0.01 b | 0.66±0.08 ab | ||
| Water | 0.55±0.04 d | na | 40.32±1.40 b | 0.04±0.01 f | na | ||
| Roots | EA | 2.61±0.04 ab | 6.70±0.72 a | 66.99±1.98 a | 0.40±0.01 a | 0.60±0.01 b | |
| MeOH | 2.83±0.32 a | 3.28±0.17 c | 72.60±0.34 a | 0.19±0.01 d | 0.38±0.07 c | ||
| Water | 0.70±0.07 d | 0.78±0.02 d | 39.23±0.78 b | 0.10±0.01 e | na |
* Values are reported as mean ± SD. EA: Ethyl acetate; MeOH: Methanol; GALAE: Galatamine equivalent; KAE: Kojic acid equivalent; ACAE: Acarbose equivalent; na: not active. Different letters in same column indicate significant differences for each Achillea species (p < 0.05).
3.4. Data Mining
Subsequent to comparison of the bioactivities of the samples of each species, principal component analysis (PCA) was used in order to uncover the similarities/differences among the extracts of both species, in light of assessed antioxidant and enzyme inhibitory activities. The results of PCA were displayed in Figure 4. 88% variability of the data were captured by the first three Principal components (PCs) which each exhibited eigenvalue greater than 1. Therefore, these PCs were retained according to the method outlined by Kaiser [34]. By Referring to Sup 2, the first PC had higher correlation with more bioactivities, notably ABTS, DPPH, FRAP, CUPRAC, BuChE, amylase and glucosidase. The second PC was predominated by MCA, AChE and tyrosinase while the third PC was dominated by PBD and MCA. From the three score plots summarized in Figure 4A, a tendency to differentiate certain groups was noted. Hence, in PC1 vs. PC3 and PC2 vs PC3, extracts from A. aleppica roots EA and MeOH and A. santolinoides roots EA were grouped together. Similarly, in PC1 vs PC2 and PC1 vs. PC3, A. A. santolinoides roots MeOH and A. aleppica aerial parts MeOH were close together. Following PCA, a hierarchical classification was done to obtain a clearer picture of the different group. Based on the scores of samples on the three PCs, the hierarchical analysis revealed two principal clusters, each of which was divided into two sub-clusters (Figure 4B). The samples of the first cluster (A. aleppica roots water, A. aleppica aerial parts water, A. santolinoides roots water, A. santolinoides aerial parts water, A. santolinoides roots MeOH and A. aleppica aerial parts MeOH) were characterized by higher antioxidant activity while samples of the second cluster (A. aleppica roots MeOH, A. A. santolinoides roots EA, A. wilhelmsii, A. aleppica roots EA, A. A. santolinoides aerial parts MeOH, A. A. santolinoides aerial parts EA and A. aleppica aerial parts EA) were marked by stronger enzyme inhibitory activity.
The relationship between the metabolites and biological activities Partial was assessed and result was reported in Figure 5. As can be observed, the different biological activities were related to the synergetic action of various metabolites. In depth examination, antioxidant activities of the species could be result from the synergetic action of metabolites such as (C12) caffeoylgluconic acid isomer, (C15) 4-hydroxybenzoic acid-hexoside, (C20) 3-feruloylquinic acid, (C24a) 1,3-dicaffeoylquinic acid, (C25) caffeic acid-O-hexoside isomer, (C28) 5-p-coumaroylquinic acid isomer, (C40) 3-feruloyl-4-caffeoylquinic acid, (C47) 6, 8-diC-hexosidyl-luteolin, (C48) O,C-dihexosyl-luteolin, (C53) 6-C-hexosyl-8-C-pentosyl apigenin, (C55) C-hexosyl-C-pentosyl methylluteolin, (C66) isorhamnetin 3-O-glucoside while tyrosinase inhibitory activity of the species may be from (C16) p-hydroxyphenylacetic acid O-hexoside, (C26) 5-feruloylquinic acid, (C41) 3-p-coumaroyl-5-caffeoylquinic acid, (C45) 1-caffeoyl-3-feruloylquinic acid, (C46) 3,4,5-tricaffeoylquinic acid, (C51) 2″-O-pentosyl-6-C-hexosyl-luteolin, (C56) rutin, (C60) Luteolin-7-O-glucoside. Similarly, anti-amylase, anti-AChE and anti-BChE activities were probably arise from the action of (C14) 4-hydroxybenzoic acid, (C72) quercetin, (C74) axillarin, (C76) kaempferol, (C77) hispidulin (scutellarein-6-methyl ether), (C78) chrysoeriol, (C81) cirsimaritin (6-hydroxyapigenin-6,7-dimethyl ether), (C82) santin/eupatilin. Partial least-squares regression model resumed about 0.88% and 0.87% of the total variation in metabolites (R2X) and biological activities (R2Y) respectively, indicating the good performance of the model.
Figure 5.
The loading plot obtained from Partial least squared regression describing relationship between chemical molecules and biological activities. (C1) protocatechuic acid-O-hexoside, (C2) caffeoylgluconic acid, (C3) protocatechuic acid, (C4) p-hydroxyphenylacetic acid-O-hexoside, (C5) protocatechuic acid-O-hexoside isomer, (C6) syringic acid 4-O-hexoside (C7) neochlorogenic (3-caffeoylquinic) acid, (C8) caffeoylgluconic acid isomer, (C9) caffeic acid-O-hexoside, (C10) gentisic acid-O-hexoside, (C11) vanillic acid 4-O-hexoside, (C12) caffeoylgluconic acid isomer, (C13) O-caffeoyl hexose isomer, (C14) 4-hydroxybenzoic acid, (C15) 4-hydroxybenzoic acid-hexoside, (C16) p-hydroxyphenylacetic acid O-hexoside, (C17) quinic acid, (C18) chlorogenic (5-caffeoylquinic) acid, (C19) p-coumaric acid, (C20) 3-feruloylquinic acid, (C21) p-hydroxyphenylacetic acid, (C22) caffeic acid, (C23) gentisic acid, (C24) 5-p-coumaroylquinic acid, (C24a) 1,3-dicaffeoylquinic acid, (C25) caffeic acid-O-hexoside isomer, (C26) 5-feruloylquinic acid, (C27) m-coumaric acid, (C28) 5-p-coumaroylquinic acid isomer, (C29) 4-feruloylquinic acid, (C30) vanillic acid, (C31) o-coumaric acid, (C32) vanillic acid-4-O-(6-O-caffeoyl)-hexoside, (C33) 3,4-dicaffeoylquinic acid, (C34) 1,5-dicaffeoylquinic acid, (C35) 3,5-dicaffeoylquinic acid, (C36) dicaffeoyl-tetrahydroxy-pentanoic acid, (C37) 4,5-dicaffeoylquinic acid, (C38) shikimic acid, (C39) salicylic acid, (C40) 3-feruloyl-4-caffeoylquinic acid, (C41) 3-p-coumaroyl-5-caffeoylquinic acid, (C42) caffeic acid-O-(salicyl)-hexoside, (C43) 3-feruloyl-5-caffeoylquinic acid, (C44) 4-p-coumaroyl-5-caffeoylquinic acid, (C45) 1-caffeoyl-3-feruloylquinic acid, (C46) 3,4,5-tricaffeoylquinic acid, (C47) 6, 8-diC-hexosidyl-luteolin, (C48) O,C-dihexosyl-luteolin, (C49) diC-hexosyl-apigenin, (C50) 6-C-hexosyl-8-C-pentosyl-luteolin, (C51) 2″-O-pentosyl-6-C-hexosyl-luteolin, (C52) homoorientin, (C53) 6-C-hexosyl-8-C-pentosyl apigenin, (C54) orientin (luteolin-8-C-glucoside), (C55) C-hexosyl-C-pentosyl methylluteolin, (C56) rutin, (C57) vitexin, (C58) isovitexin, (C59) 2″-O-pentosyl-6-C-hexosyl-methylluteolin, (C60) Luteolin-7-O-glucosidea, (C61) chrysoeriol-6-C-hexoside, (C62) nepetin-O-hexuronide, (C63) 6-methoxykaempferol-O-hexoside, (C64) nepetin-O-hexoside, (C65) kaempferol-3-O-glucoside, (C66) isorhamnetin 3-O-glucoside, (C67) apigenin-7-O-glucoside, (C68) cirsiliol-O-hexoside, (C69) chrysoeriol-O-hexuronide, (C70) jaceosidin-O-hexuronide, (C71) luteolin, (C72) quercetin, (C73) patuletin (6-methoxyquercetin), (C74) axillarin, (C75) apigenin, (C76) kaempferol, (C77) hispidulin (scutellarein-6-methyl ether), (C78) chrysoeriol, (C79) cirsiliol, (C80) quercetagetin-3,6,3′(4′)-trimethyl ether, (C81) cirsimaritin (6-hydroxyapigenin-6,7-dimethyl ether), (C82) santin/eupatilin, (C83) acacetin, (C84) tanaparthin-peroxide, (C85) achillicin/matricin, (C86) dehydroachillin/dehydroleucodin, (C87) achillin/leucodin, (C88) artabsin, (C89) dihydrosantamarin, (C90) tetradecenoic acid amide, (C91) linolenamide, (C92) linoleamide, (C93) palmitamide, (C94) oleamide.
3.5. KEGG Analysis
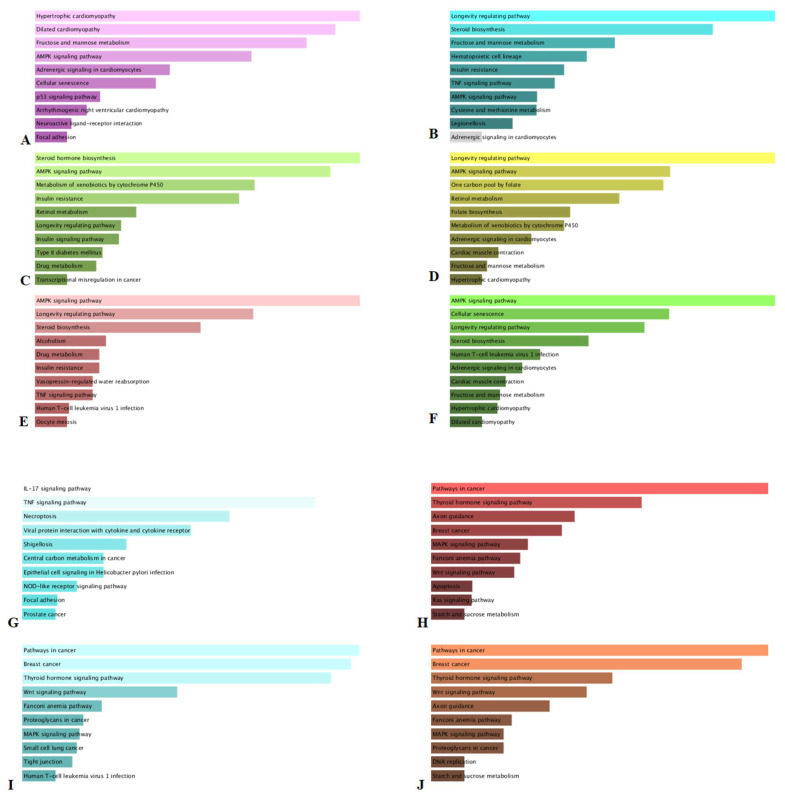
After the phytochemical screening and in vitro evaluation of biological properties of the samples, we have been engaged in the investigation of KEGG pathway enrichment analysis of identified sesquiterpene lactones and derivatives and five of the main phenolics of Achillea species. In respect of the genes modulation, 73, 122, 280, 122, 113, 122, 57, 57, 254, 287 and 272 mRNA were found to be up-regulated and down-regulated by artabsin, dehydroleucodin, dihydrosantamarin, leucodin, matricin, tanaparthin peroxide, neochlorogenic acid, chlorogenic acid, homoorientin, vitexin and isovitexin respectively (Table S3). As regards the first enriched pathway, “hypertrophic cardiomyopathy”, “longevity regulating pathway”, “steroid hormone biosynthesis”, “AMPK signaling pathway”, “IL-17 signaling pathway” and “pathways in cancer” were found to be modulated by the mRNA targeted by artabsin, dehydroleucodin, dihydrosantamarin, leucodin, matricin, tanaparthin peroxide, neochlorogenic acid, chlorogenic acid, homoorientin, vitexin and isovitexin respectively (Figure 6). Structure of these compounds are reported in Figure 3. Moreover, it is worth noting that “AMPK signaling pathway” was predicted to be regulated by nine compounds except neochlorogenic acid and chlorogenic acid. AMP-activated protein kinase (AMPK) is one of the central mediators of cellular and organismal metabolism. It has key roles in promoting catabolic pathways to produce more ATP and in inhibiting anabolic pathways [58]. Once activated, AMPK leads to a concomitant activation of ATP-producing catabolic pathways, such as glycolysis and fatty acid oxidation and inhibition of energy-consuming biosynthetic pathways, such as fatty acid, protein, and glycogen synthesis. Otherwise, AMPK is a known target for treating type-2 diabetes and metabolic syndrome and for reducing the incidence of cancer [59]. Sesquiterpenes lactones have been reported to induce an anticancer actions through an impact on multiple signaling pathways as well as a changes in the redox cell balance [60]. These effects lead to the increase in apoptotic factors and the reduction of metastasis, cellular invasion and anti-apoptotic factors. Illustratively, earlier study demonstrated the potentiality of matricin to significantly exert anti-proliferative and apoptosis-inducing effects in non-small cell lung cancer cells via activation of MAPK pathway [61]. Additionally, expression of anti-apoptotic proptein Bcl-2 was significantly decreased while the level of pro-apoptosis protein Bax as well as the activity of apoptosis marker enzymes caspase-9, caspase-8 and caspase-3 were significantly increased. Similarly, in a study of the anti-alcoholic liver disease activity of leucodin isolated from Artemisia capillaries, it has been demonstrated that leucodin dose dependently enhances phosphorylation of AMPK in alcohol-exposed HepG2 cells [62]. Furthermore, homoorientin has been demonstrated to have anti-pancreatic cancer activity via the AMPK signaling pathways [63]. While the literature has reported multiple biological mechanism, notably the regulation of AMPK pathway, to explain the pharmacological activities of vitexin and isovitexin [64]. This finding demonstrated that homoorientin, vitexin, isovitexin and both sesquiterpene lactones compounds can modulate AMPK signaling pathway. Hence, the nine compounds present in the different parts of both studied species could serve as AMPK activators and could be a promising candidate for the prevention and treatment of cancer. However, further studies on purified compounds will be necessary to confirm the conclusions of the present bioinformatics study.
Figure 6.
Top 10 KEGG pathway enrichment for the selected compounds. The bars in the panels represents the p-values computed using the Fisher exact test. The longer bars and lighter colored bars mean that the term is more significant. (A) artabsin; (B) dehydroleucodin; (C) dihydrosantamarin; (D) leucodin; (E) matricin; (F) tanaparthin peroxide; (G) neochlorogenic acid and chlorogenic acid; (H) homoorientin; (I) vitexin; (J) isovitexin.
4. Conclusions
This study allowed obtaining a detailed phytochemical fingerprint of A. aleppica and A. santolinoides roots and aerial part. Chlorogenic acid was the main derivative in aerial parts of both the species. 3,5-diCQA was the most important diCQA derivative in A. aleppica while 1,3diCQA was the most significant in A santolinoides. Sesquiterpene lactone and fatty acid amides have been also detected showing large chemical diversity in the constituents of the plant. The extraction with ethyl acetate, methanol and water allowed to prepare samples with different composition that were used to assess their in vitro bioactivity on several antioxidant and enzyme inhibition assays. The methanol extract of A. santolinoides roots possessed significant antioxidant activities. The ethyl acetate extracts of the aerial parts and roots of both Achillea species showed significant inhibition against butyrylcholinesterase while the ethyl acetate extract of A. santolinoides aerial part actively inhibited tyrosinase. The detailed phytochemical investigation, the evaluation of in vitro bioactivity, of the two Achillea species indicate these plants as valuable starting point for potential future studies and possible applications extracts in cosmetic, pharmaceuticals and nutraceuticals products. KEGG mapping using some of the phenolics and sesquiterpenes of the plants allowed to predict some of the possible molecular targets for significant bioactivities. This information opens new opportunities of research and application for A. aleppica and A. santolinoides extracts and isolated compounds.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antiox10081180/s1, Table S1. Percentages of explained variances, eigenvalues and contribution of metabolites on the first six components of PCA. Table S2. Percentages of explained variances, eigenvalues and contribution of biological activities on the first three components of PCA. Table S3. Upregulated and downreagulated mRNA by the six sesquiterpene lactones and some phenolic compounds. Figure S1: Extracted ion chromatogram of hydroxybenzoic and hydroxycinamic acids and derivatives in negative ion mode of methanolic extracts from Achillea wilhemsii aerial parts (A), A. allepica aerial parts (B), A. wilhemsii roots (C), A. allepica roots (D) (for the compound numbers see Table 2). Figure S2: Extracted ion chromatogram of acylquinic acids in negative ion mode of methanolic extracts from Achillea wilhemsii aerial parts (A), A. allepica aerial parts (B), A. wilhemsii roots (C), A. allepica roots (D) (for the compound numbers see Table 2). Figure S3: Extracted ion chromatogram of flavonoid glycosides in negative ion mode of methanolic extracts from Achillea wilhemsii aerial parts (A), A. allepica aerial parts (B), A. wilhemsii roots (C), A. allepica roots (D) (for the compound numbers see Table 2). Figure S4: Extracted ion chromatogram of flavonoid aglycones in negative ion mode of methanolic extracts from Achillea wilhemsii aerial parts (A), A. allepica aerial parts (B), A. wilhemsii roots (C), A. allepica roots (D) (for the compound numbers see Table 2). Figure S5: MS/MS spectra of hydroxybenzoic, hydroxycinnamic acids and their glycosides, and sugar esters (for the compound numbers see Table 2). Figure S6: MS/MS spectra of acylquinic acids (for the compound numbers see Table 2). Figure S7: MS/MS spectra of C-, C,O- and O-flavonoid glycosides(for the compound numbers see Table 2). Figure S8: MS/MS spectra of 6 methoxyflavonoids (aglycones and glycosides) (for the compound numbers see Table 2). Figure S9: MS/MS spectra of sesquiterpene lactones (for the compound numbers see Table 2).
Author Contributions
Conceptualization, R.G., G.Z. and D.Z.-D.; methodology, R.G., G.Z., K.I.S., E.Y. and D.Z.-D.; software, K.I.S.; validation, G.Z., C.P.-A., M.F.M. and S.D. formal analysis, G.Z.; investigation, G.Z.; resources, R.G., G.Z. and S.D.; data curation, D.Z.-D.; writing—original draft preparation, R.G., G.Z., C.P.-A., M.F.M. and S.D writing—review and editing, M.F.M. and S.D. viualization, M.I.; supervision, G.Z.; project administration, G.Z.; funding acquisition, S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cam M.E., Cesur S., Taskin T., Erdemir G., Kuruca D.S., Sahin Y.M., Kabasakal L., Gunduz O. Fabrication, characterization and fibroblast proliferative activity of electrospun Achillea lycaonica-loaded nanofibrous mats. Eur. Polym. J. 2019;120:109239. doi: 10.1016/j.eurpolymj.2019.109239. [DOI] [Google Scholar]
- 2.Kaczorová D., Karalija E., Dahija S., Bešta-Gajević R., Parić A., Ćavar Zeljković S. Influence of extraction solvent on the phenolic profile and bioactivity of two Achillea species. Molecules. 2021;26:1601. doi: 10.3390/molecules26061601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuropka G., Glombitza K.W. Further polyenic and polyynic carboxamides and sesamin from Achillea ptarmica. Planta Med. 1987;53:440–442. doi: 10.1055/s-2006-962768. [DOI] [PubMed] [Google Scholar]
- 4.Yassa N., Saeidnia S., Pirouzi R., Shafiee A. Three phenolic glycosides and immunological properties of Achillea millefolium from Iran, population of Golestan. DARU J. Pharm. Sci. 2007;1:49–52. [Google Scholar]
- 5.Glasl S., Presser A., Gunbilig D., Werner I., Narantuya S., Haslinger E., Jurenitsch J., Kubelka W. Highly hydroxylated guaianolides of Achillea asiatica and Middle European Achillea species. Phytochemistry. 2001;58:1189–1194. doi: 10.1016/S0031-9422(01)00281-3. [DOI] [PubMed] [Google Scholar]
- 6.Trifunovic S., Vlatka V., Tešević V., Dejan D., Slobodan M. Lignans from the plant species Achillea lingulata. J. Serb. Chem. Soc. 2003;68:277–280. doi: 10.2298/JSC0305277T. [DOI] [Google Scholar]
- 7.Manayi A., Kurepaz-Mahmoodabadi M., Gohari A.R., Ajani Y., Saeidnia S. Presence of phthalate derivatives in the essential oils of a medicinal plant Achillea tenuifolia. DARU J. Pharm. Sci. 2014;22:78. doi: 10.1186/s40199-014-0078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah R.M., Patel T., Tettamanzi C.M., Rajan J., Shah M., Peethambaran B. Isolation of a novel piperidide from Achillea moonshine using bioactivity guided fractionation for the treatment of acne. J. Med. Plant. Res. 2016;10:495–504. [Google Scholar]
- 9.Farhadi N., Babaei K., Farsaraei S., Moghaddam M., Pirbalouti A.G. Changes in essential oil compositions, total phenol, flavonoids and antioxidant capacity of Achillea millefolium at different growth stages. Ind. Crop. Prod. 2020;152:6. doi: 10.1016/j.indcrop.2020.112570. [DOI] [Google Scholar]
- 10.Konovalov D., Chelombit’ko V. Sesquiterpene lactones from Achillea millefolium. Chem. Nat. Compd. 1991;27:640–641. doi: 10.1007/BF00630380. [DOI] [Google Scholar]
- 11.Li H.L., Li J., Liu M.Q., Xie R.R., Zang Y., Li J., Aisa H.A. Guaianolide sesquiterpene lactones from Achillea millefolium L. Phytochemistry. 2021;186:9. doi: 10.1016/j.phytochem.2021.112733. [DOI] [PubMed] [Google Scholar]
- 12.Konovalov D. Polyacetylene compounds of plants of the Asteraceae Family (Review) Pharm. Chem. J. 2015;48:36–53. doi: 10.1007/s11094-014-1159-7. [DOI] [Google Scholar]
- 13.Todorova M.N., Tsankova E.T., Mustakerova E.I. Spirodepressolide: An unusual bis-norsesquiterpene lactone from Achillea depressa Janka. Nat. Prod. Res. 2004;18:461–464. doi: 10.1080/14786410310001643849. [DOI] [PubMed] [Google Scholar]
- 14.Asghari B., Mafakheri S., Zengin G., Dinparast L., Bahadori M.B. In-depth study of phytochemical composition, antioxidant activity, enzyme inhibitory and antiproliferative properties of Achillea filipendulina: A good candidate for designing biologically-active food products. J. Food Meas. Charact. 2020;14:2196–2208. doi: 10.1007/s11694-020-00466-5. [DOI] [Google Scholar]
- 15.Si X.T., Zhang M.L., Shi Q.W., Kiyota H. Chemical constituents of the plants in the genus Achillea. Chem. Biodiv. 2006;3:1163–1180. doi: 10.1002/cbdv.200690119. [DOI] [PubMed] [Google Scholar]
- 16.Mohammadhosseini M., Sarker S.D., Akbarzadeh A. Chemical composition of the essential oils and extracts of Achillea species and their biological activities: A review. J. Ethnopharmacol. 2017;199:257–315. doi: 10.1016/j.jep.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Ali N., Shah S.W., Ahmed G., Shah I., Shoaib M., Junaid M., Ali W. Acute toxicity and antispasmodic activities of Achillea wilhelmsii C. Koch. Pak. J. Pharm. Sci. 2014;27:309–315. [PubMed] [Google Scholar]
- 18.Ashtiani M., Nabatchian F., Galavi H.R., Saravani R., Farajian-Mashhadi F., Salimi S. Effect of Achillea wilhelmsii extract on expression of the human telomerase reverse transcriptase mRNA in the PC3 prostate cancer cell line. Biomed. Rep. 2017;7:251–256. doi: 10.3892/br.2017.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Majnooni M.B., Mohammadi-Farani A., Gholivand M.B., Nikbakht M.R., Bahrami G.R. Chemical composition and anxiolytic evaluation of Achillea wilhelmsii C. Koch essential oil in rat. Res. Pharm. Sci. 2013;8:269–275. [PMC free article] [PubMed] [Google Scholar]
- 20.Asgary S., Naderi G.H., Sarrafzadegan N., Mohammadifard N., Mostafavi S., Vakili R. Antihypertensive and antihyperlipidemic effects of Achillea wilhelmsii. Drugs Exp. Clin. Res. 2000;26:89–93. [PubMed] [Google Scholar]
- 21.Barış D., Kızıl M., Aytekin Ç., Kızıl G., Yavuz M., Çeken B., Ertekin A.S. In vitro antimicrobial and antioxidant activity of ethanol extract of three Hypericum and three Achillea species from Turkey. Int. J. Food Prop. 2011;14:339–355. doi: 10.1080/10942910903189256. [DOI] [Google Scholar]
- 22.Marucci G., Buccioni M., Ben D.D., Lambertucci C., Volpini R., Amenta F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology. 2021;190:108352. doi: 10.1016/j.neuropharm.2020.108352. [DOI] [PubMed] [Google Scholar]
- 23.Papoutsis K., Zhang J., Bowyer M.C., Brunton N., Gibney E.R., Lyng J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem. 2021;338:128119. doi: 10.1016/j.foodchem.2020.128119. [DOI] [PubMed] [Google Scholar]
- 24.Mukherjee P.K., Biswas R., Sharma A., Banerjee S., Biswas S., Katiyar C.K. Validation of medicinal herbs for anti-tyrosinase potential. J. Herb. Med. 2018;14:1–16. doi: 10.1016/j.hermed.2018.09.002. [DOI] [Google Scholar]
- 25.Bernardo J., Malheiro I., Videira R.A., Valentão P., Santos A.C., Veiga F., Andrade P.B. Trichilia catigua and Turnera diffusa extracts: In vitro inhibition of tyrosinase, antiglycation activity and effects on enzymes and pathways engaged in the neuroinflammatory process. J. Ethnopharmacol. 2021;271:113865. doi: 10.1016/j.jep.2021.113865. [DOI] [PubMed] [Google Scholar]
- 26.Spínola V., Castilho P.C. Evaluation of Asteraceae herbal extracts in the management of diabetes and obesity. Contribution of caffeoylquinic acids on the inhibition of digestive enzymes activity and formation of advanced glycation end-products (in vitro) Phytochemistry. 2017;143:29–35. doi: 10.1016/j.phytochem.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Suliman S., Yagi S., Elbashir A.A., Mohammed I., Hussein A., Ak G., Zengin G., Orlando G., Ferrante C. Phenolic profile, enzyme inhibition and antioxidant activities and bioinformatics analysis of leaf and stem bark of Ficus sycomorus L. Process Biochem. 2021;101:169–178. doi: 10.1016/j.procbio.2020.11.011. [DOI] [Google Scholar]
- 28.Slinkard K., Singleton V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Viticul. 1977;28:49–55. [Google Scholar]
- 29.Zengin G., Nithiyanantham S., Locatelli M., Ceylan R., Uysal S., Aktumsek A., Selvi P.K., Maskovic P. Screening of in vitro antioxidant and enzyme inhibitory activities of different extracts from two uninvestigated wild plants: Centranthus longiflorus subsp. longiflorus and Cerinthe minor subsp. auriculata. Eur. J. Integ. Med. 2016;8:286–292. doi: 10.1016/j.eujim.2015.12.004. [DOI] [Google Scholar]
- 30.Uysal S., Zengin G., Locatelli M., Bahadori M.B., Mocan A., Bellagamba G., De Luca E., Mollica A., Aktumsek A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017;8:290. doi: 10.3389/fphar.2017.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zengin G. A study on in vitro enzyme inhibitory properties of Asphodeline anatolica: New sources of natural inhibitors for public health problems. Ind. Crop. Prod. 2016;83:39–43. doi: 10.1016/j.indcrop.2015.12.033. [DOI] [Google Scholar]
- 32.Lagunin A., Ivanov S., Rudik A., Filimonov D., Poroikov V. DIGEP-Pred: Web service for in silico prediction of drug-induced gene expression profiles based on structural formula. Bioinformatics. 2013;29:2062–2063. doi: 10.1093/bioinformatics/btt322. [DOI] [PubMed] [Google Scholar]
- 33.Xie Z., Bailey A., Kuleshov M.V., Clarke D.J.B., Evangelista J.E., Jenkins S.L., Lachmann A., Wojciechowicz M.L., Kropiwnicki E., Jagodnik K.M., et al. Gene Set Knowledge Discovery with Enrichr. Curr. Prot. 2021;1:e90. doi: 10.1002/cpz1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaiser H.F. The application of electronic computers to factor analysis. Educ. Psychocol. Meas. 1960;20:141–151. doi: 10.1177/001316446002000116. [DOI] [Google Scholar]
- 35.Ak G., Gevrenova R., Sinan K.I., Zengin G., Zheleva D., Mahomoodally M.F., Senkardes I., Brunetti L., Leone S., Di Simone S.C. Tanacetum vulgare L. (Tansy) as an effective bioresource with promising pharmacological effects from natural arsenal. Food Chem. Toxicol. 2021;153:112268. doi: 10.1016/j.fct.2021.112268. [DOI] [PubMed] [Google Scholar]
- 36.Gevrenova R., Zheleva-Dimitrova D., Balabanova V., Voynikov Y., Sinan K.I., Mahomoodally M.F., Zengin G. Integrated phytochemistry, bio-functional potential and multivariate analysis of Tanacetum macrophyllum (Waldst. & Kit.) Sch. Bip. and Telekia speciosa (Schreb.) Baumg.(Asteraceae) Ind. Crop. Prod. 2020;155:112817. [Google Scholar]
- 37.Zheleva-Dimitrova D., Sinan K.I., Etienne O.K., Zengin G., Gevrenova R., Mahomoodally M.F., Lobine D., Mollica A. Chemical composition and biological properties of Synedrella nodiflora (L.) Gaertn: A comparative investigation of different extraction methods. Process. Biochem. 2020;96:202–212. doi: 10.1016/j.procbio.2020.06.002. [DOI] [Google Scholar]
- 38.Zheleva-Dimitrova D., Zengin G., Balabanova V., Voynikov Y., Lozanov V., Lazarova I., Gevrenova R. Chemical characterization with in vitro biological activities of Gypsophila species. J. Pharm. Biomed. Anal. 2018;155:56–69. doi: 10.1016/j.jpba.2018.03.040. [DOI] [PubMed] [Google Scholar]
- 39.Ferreres F., Gil-Izquierdo A., Andrade P.B., Valentão P., Tomás-Barberán F. Characterization of C-glycosyl flavones O-glycosylated by liquid chromatography—Tandem mass spectrometry. J. Chrom. A. 2007;1161:214–223. doi: 10.1016/j.chroma.2007.05.103. [DOI] [PubMed] [Google Scholar]
- 40.Ren D., Ran L., Yang C., Xu M., Yi L. Integrated strategy for identifying minor components in complex samples combining mass defect, diagnostic ions and neutral loss information based on ultra-performance liquid chromatography-high resolution mass spectrometry platform: Folium Artemisiae Argyi as a case study. J. Chrom. A. 2018;1550:35–44. doi: 10.1016/j.chroma.2018.03.044. [DOI] [PubMed] [Google Scholar]
- 41.Bakr R.O., Arafa R.K., Al-Abd A.M., Elshishtawy H.M. Phenolics of Achillea fragrantissima growing in Egypt and its cytotoxic activity. J. Med. Plant Res. 2014;8:763–771. [Google Scholar]
- 42.Abd-Alla H.I., Shalaby N.M., Hamed M.A., El-Rigal N.S., Al-Ghamdi S.N., Bouajila J. Phytochemical composition, protective and therapeutic effect on gastric ulcer and α-amylase inhibitory activity of Achillea biebersteinii Afan. Arch. Pharm. Res. 2016;39:10–20. doi: 10.1007/s12272-014-0544-9. [DOI] [PubMed] [Google Scholar]
- 43.Yao D., Li Z., Huo C., Wang Y., Wu Y., Zhang M., Li L., Shi Q., Kiyota H., Shi X. Identification of in vitro and in vivo metabolites of alantolactone by UPLC-TOF-MS/MS. J. Chrom. B. 2016;1033:250–260. doi: 10.1016/j.jchromb.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 44.Kastner U., Breuer J., Glasl S., Baumann A., Robien W., Jurenitsch J., Rücker G., Kubelka W. Guaianolide-endoperoxide and monoterpene-hydroperoxides from Achillea nobilis. Planta Med. 1995;61:83–85. doi: 10.1055/s-2006-958010. [DOI] [PubMed] [Google Scholar]
- 45.Todorova M., Trendafilova A., Mikhova B., Vitkova A., Duddeck H. Chemotypes in Achillea collina based on sesquiterpene lactone profile. Phytochemistry. 2007;68:1722–1730. doi: 10.1016/j.phytochem.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 46.Castillo-Peinado L., López-Bascón M., Mena-Bravo A., de Castro M.L., Priego-Capote F. Determination of primary fatty acid amides in different biological fluids by LC–MS/MS in MRM mode with synthetic deuterated standards: Influence of biofluid matrix on sample preparation. Talanta. 2019;193:29–36. doi: 10.1016/j.talanta.2018.09.088. [DOI] [PubMed] [Google Scholar]
- 47.Semaming Y., Pannengpetch P., Chattipakorn S.C., Chattipakorn N. Pharmacological properties of protocatechuic Acid and its potential roles as complementary medicine. Evid.-Based Complement. Altern. Med. 2015;2015:593902. doi: 10.1155/2015/593902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kakkar S., Bais S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014;2014:952943. doi: 10.1155/2014/952943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X., Li K., Xie H., Xie Y., Li Y., Zhao X., Jiang X., Chen D. Antioxidant and cytoprotective effects of the Di-O-Caffeoylquinic acid family: The mechanism, structure-activity relationship, and conformational effect. Molecules. 2018;23:222. doi: 10.3390/molecules23010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andjelković M., Van Camp J., De Meulenaer B., Depaemelaere G., Socaciu C., Verloo M., Verhe R. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006;98:23–31. doi: 10.1016/j.foodchem.2005.05.044. [DOI] [Google Scholar]
- 51.Hosseini M., Harandizadeh F., Niazamand S., Soukhtanloo M., Mahmoudabady M. Antioxidant effect of Achillea wilhelmsii extract on pentylenetetrazole (seizure model)—Induced oxidative brain damage in Wistar rats. Ind. J. Physiol. Pharmacol. 2013;57:418–424. [PubMed] [Google Scholar]
- 52.Saeidi K., Moosavi M., Lorigooini Z., Maggi F. Chemical characterization of the essential oil compositions and antioxidant activity from Iranian populations of Achillea wilhelmsii K.Koch. Ind. Crop. Prod. 2018;112:274–280. doi: 10.1016/j.indcrop.2017.12.007. [DOI] [Google Scholar]
- 53.Rasool M., Malik A., Waquar S., Tul-Ain Q., Jafar T.H., Rasool R., Kalsoom A., Ghafoor M.A., Sehgal S.A., Gauthaman K., et al. In-silico characterization and in-vivo validation of albiziasaponin-a, iso-orientin, and salvadorin using a rat model of Alzheimer’s disease. Front. Pharmacol. 2018;9:730. doi: 10.3389/fphar.2018.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheeja Malar D., Beema Shafreen R., Karutha Pandian S., Pandima Devi K. Cholinesterase inhibitory, anti-amyloidogenic and neuroprotective effect of the medicinal plant Grewia tiliaefolia—An in vitro and in silico study. Pharm. Biol. 2017;55:381–393. doi: 10.1080/13880209.2016.1241811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu H.R., Men X., Gao X.H., Liu L.B., Fan H.Q., Xia X.H., Wang Q.A. Discovery of potent and selective acetylcholinesterase (AChE) inhibitors: Acacetin 7-O-methyl ether Mannich base derivatives synthesised from easy access natural product naringin. Nat. Prod. Res. 2018;32:743–747. doi: 10.1080/14786419.2017.1340280. [DOI] [PubMed] [Google Scholar]
- 56.Toublet F.-X., Lalut J., Hatat B., Lecoutey C., Davis A., Since M., Corvaisier S., Freret T., Sopková-de Oliveira Santos J., Claeysen S., et al. Pleiotropic prodrugs: Design of a dual butyrylcholinesterase inhibitor and 5-HT6 receptor antagonist with therapeutic interest in Alzheimer’s disease. Eur. J. Med. Chem. 2021;210:113059. doi: 10.1016/j.ejmech.2020.113059. [DOI] [PubMed] [Google Scholar]
- 57.Lin F.-J., Yen F.-L., Chen P.-C., Wang M.-C., Lin C.-N., Lee C.-W., Ko H.-H. HPLC-Fingerprints and antioxidant constituents of Phyla nodiflora. Sci. World J. 2014;2014:528653. doi: 10.1155/2014/528653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mihaylova M.M., Shaw R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Umezawa S., Higurashi T., Nakajima A. AMPK: Therapeutic target for diabetes and cancer prevention. Curr. Pharm. Des. 2017;23:3629–3644. doi: 10.2174/0929867324666170713150440. [DOI] [PubMed] [Google Scholar]
- 60.Babaei G., Aliarab A., Abroon S., Rasmi Y., Aziz S.G.-G. Application of sesquiterpene lactone: A new promising way for cancer therapy based on anticancer activity. Biomed. Pharmacother. 2018;106:239–246. doi: 10.1016/j.biopha.2018.06.131. [DOI] [PubMed] [Google Scholar]
- 61.Bai X., Wang W., Wang Y., Li J. Anti-proliferative and apoptosis-inducing effects of matricin on human non-small cell lung cancer H1299 cells via MAPK pathway activation. Eur. J. Inflamm. 2020;18:2058739220942335. doi: 10.1177/2058739220942335. [DOI] [Google Scholar]
- 62.Shang Y., Li X.F., Jin M.J., Li Y., Wu Y.L., Jin Q., Zhang Y., Li X., Jiang M., Cui B.W., et al. Leucodin attenuates inflammatory response in macrophages and lipid accumulation in steatotic hepatocytes via P2x7 receptor pathway: A potential role in alcoholic liver disease. Biomed. Pharmacother. 2018;107:374–381. doi: 10.1016/j.biopha.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 63.Ye T., Su J., Huang C., Yu D., Dai S., Huang X., Chen B., Zhou M. Isoorientin induces apoptosis, decreases invasiveness, and downregulates VEGF secretion by activating AMPK signaling in pancreatic cancer cells. OncoTargets Ther. 2016;9:7481–7492. doi: 10.2147/OTT.S122653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He M., Min J.W., Kong W.L., He X.H., Li J.X., Peng B.W. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia. 2016;115:74–85. doi: 10.1016/j.fitote.2016.09.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article and Supplementary Materials.