Abstract
Cyclosporine A’s (CsA) immunosuppressive effect makes it an ideal drug for organ transplantation. However, CsA’s uses are restricted due to its side effects. We investigated the effects of avocado seed (AvS) powder on CsA-induced nephrotoxicity and immunosuppression in rats. The injection of CsA (5 mg/kg, subcutaneously, for 10 days) increased serum levels of creatinine, uric acid, and urea, and the renal levels of the malondialdehyde. It decreased creatinine clearance and the renal activity of antioxidant enzymes (superoxide dismutase, catalase, and glutathione peroxidase) and Na+/K+ ATPase. The administration of CsA also significantly downregulated the renal expression of interferon-gamma, tumor necrosis factor-alpha, interleukin 1 beta, monocyte chemotactic protein 1, intercellular adhesion molecule-1, and vascular cell adhesion molecule 1 genes, and increased renal DNA damage. Histopathological examination confirmed the biochemical and molecular alterations that accompanied CsA nephrotoxicity. All CsA-induced deleterious effects, except immunosuppression, were ameliorated by feeding rats on a basal diet supplemented with 5% AvS powder for 4 weeks. Importantly, AvS also maximized CsA’s immunosuppressive effect. These findings suggest a potential ameliorative effect of AvS on CsA-induced nephrotoxicity, and AvS enhances CsA’s immunosuppressive effect. Therefore, AvS might be used in combination with CsA in transplantation treatment to relieve the CsA-induced nephrotoxicity.
Keywords: avocado seeds, cyclosporine A, nephrotoxicity, immunosuppression, oxidative stress
1. Introduction
Cyclosporine (CsA) is an antibiotic extracted from Tolypocladium inflatum gams fungus that possesses powerful immunosuppressive properties which make it an ideal drug for solid organ transplantations (particularly for the kidneys) [1]. Administration of CsA to patients after solid organ transplantation improves survival statistically [2,3]. However, CsA’s prolonged use results in oxidative-stress-dependent side effects in many organs, including severe nephrotoxicity [4,5,6]. Indeed, some patients who underwent heart transplantations and were post-surgically treated with CsA for a long time suffered from nephrotoxicity [7]. Specifically, CsA can induce oxidative-stress-dependent nephrotoxicity by triggering endoplasmic reticulum stress and boosting free radical release from mitochondria [8]. Another mechanism by which CsA could cause nephrotoxicity is the induction of apoptosis in renal cells [9]. Due to its importance after transplantation surgeries, there is an urgent need for researchers to find out safe adjuvants to reduce CsA’s side effects without inhibiting its immunosuppressive potential.
The avocado (Persea americana Mill.), a member of the Lauraceae flowering plant family, has very high levels of unsaturated oils and high nutritional value [10]. Avocado pulp is rich in many health promoting constituents, such as carotenoids (especially lutein); monounsaturated fatty acids (particularly oleic acid); and phenolic compounds, such as hydroxybenzoic acid, hydrocinnamic acid, and catechin [11,12]. These bioactive constituents have antioxidant, anti-inflammatory, anticancer, antimicrobial, antidiabetic, and hypolipidemic properties [13]. In decades past, the avocado seed (AvS), which represents 13–18% of the fruit, was considered a waste product and was discarded during the processing of the pulp, causing serious ecological concern [14]. In the last decade, researchers investigated the chemical structure of the AvS and found several health-promoting bioactive compounds, including phytosterols, triterpenes, fatty acids, furanoic acids, proanthocyanidins, and abscisic acid [15]. Moreover, the AvS has higher amounts of phenolic compounds (such as hydrocinnamic acid, catechin, epicatechin, and leucoanthocyanidins) than the pulp [16]. These health-promoting bioactive compounds give the AvS many medicinal properties, including antioxidant, anticancer, antimicrobial, antifungal, anthelmintic, hypotensive, antidiabetic, and hypolipidemic properties [17,18,19]. Among the extracts of 15 fruit wastes investigated for their antioxidant properties, the AvS showed the most potent activity [20]. For their health-promoting and nutritious properties, AvS extracts could serve as an ingredient in functional foods. A recent study by Saad [21] showed that feeding rats on a basal diet supplemented with 5% AvS powder can improve immunity following treatment with CsA. In this study, the author used a very high dose of CsA (50 mg/kg, subcutaneously, for 10 days) instead of the optimal dose (5 mg/kg). Moreover, Saad’s study lacked a molecular approach to confirm their hematological and biochemical findings.
Based on the aforementioned properties of the AvS, it seems to have much ecological and economic potential, and might be able to be used in the treatment of oxidative stress and inflammatory-related diseases and toxicities. However, the AvS’s effects on CsA-induced nephrotoxicity and immunosuppression remained elusive prior to our study’s commencement. Therefore, this study aimed to explore the ameliorative potential of the AvS for CsA-induced nephrotoxicity by investigating the modulations of oxidative stress, apoptosis, and inflammation associated with CsA-induced nephrotoxicity.
2. Materials and Methods
2.1. Plant and Chemicals
Avocados used in this study were obtained from a local market. Cyclosporine-A (Sandimun Neoral®) was purchased in form of capsules (25 mg/capsule) from Novartis Pharma Co., Plantation, FL, USA.
2.2. Diet Preparation
The basal diet of the rats was prepared following the laboratory animal diet guidelines as previously described [22]. The avocado seeds (AvS) were dried in an oven (Contherm Thermotec 2000, Taipei, Taiwan) at 40 °C for 48 h until achieving a moisture level of 4%. The small pieces were then crushed into powder with a Retsch laboratory mill and passed through a 40 mesh screen to obtain avocado seed powder. Then the powder was stored in plastic packs at 5 °C for further analyses. Treatment diets were prepared by incorporating 50 g of AvS powder into each kilogram of food to get a concentration of 5%. The formulations of the basal and treatment diets are shown in Table 1.
Table 1.
Formulations of the diets—basal or supplemented with 5% avocado seed powder.
| Ingredients (g/Kg) | Basal Diet | Basal Diet with 5% Avocado Seed Powder |
|---|---|---|
| Casein (>85% protein) | 120.0 | 120.0 |
| Corn oil | 70.0 | 70.0 |
| Mineral mix | 35.0 | 35.0 |
| Vitamin mix | 10.0 | 10.0 |
| L- cystine | 3.0 | 3.0 |
| Choline bitartrate | 2.5 | 2.5 |
| Wheat bran | 50.0 | 50.0 |
| Sucrose | 100.0 | 100.0 |
| Corn starch | 609.5 | 559.5 |
| Avocado seeds powder | -- | 50.0 |
2.3. Detection of Bioactive Compounds by GC-MS
Bioactive compounds in AvS powder were detected by gas chromatography coupled to mass spectrometry (GC-MS) by using an ISQ LT single quadrupole mass spectrometer (Thermo Scientific TRACE 1310 Gas Chromatograph, Walham, MA, USA). The ethanolic extract of the AvS was used for the GC-MS analysis.
2.4. Experimental Design
Twenty-eight Sprague–Dawley male rats weighing 120–160 g which were 10 to 12 weeks old were purchased from the Vaccine and Immunity Organization, Ministry of Health, Egypt. The animals were housed in cages supplied with a light/dark system of 12 h/12 h at 23 ± 2 °C. They were fed a basal diet ad libitum and had free access to water. The animals were acclimatized for one week; then they were randomized into four groups. Each group contained seven animals. In the first group (control group; Cnt), rats were given a basal diet for 4 weeks and were subcutaneously injected with 200 µL/rat/daily of olive oil as a vehicle for 10 days (from D1 to D10). In the second group (avocado seeds—AvS), rats were fed on a basal diet supplemented with 5% AvS powder for 4 weeks. In the third group (cyclosporine A—CsA), rats were subcutaneously injected with 5 mg/kg/day CsA in olive oil for 10 days and were fed on a basal diet for 4 weeks. Each CsA capsule (25 mg) was dissolved in 5 mL of olive oil, and each animal was injected with 200 µL of this mixture as previously described [22]. In the fourth group (CsA + AvS), rats received CsA at a dose of 5 mg/kg/day subcutaneously for 10 days and were fed on a basal diet supplemented with 5% AvS powder for 4 weeks. Ethical approval from the Animal Ethical Committee of Kafrelsheikh University, license number KFS-127/29, was obtained to perform this experiment.
2.5. Growth-Related Parameters
Feed intake (FI) was recorded weekly. Growth-related parameters, including the final body weight gain (FBWG) and feed efficiency ratio (FER), were determined using the following equations: FBWG (g) = final BW (g) − initial BW (g); FER = FBWG (g)/total FI (g).
2.6. Sampling
At the end of the experiment, rats were placed in metabolic cages individually to collect the 24 h urine totals to determine creatinine clearance. The animals were fasted overnight before being euthanized by exsanguination. Blood samples were collected from jugular veins and sera were separated by centrifugation at 4000 rpm for 10 min. The kidneys were immediately removed, washed with isotonic saline, and weighed. The kidney was split into 4 parts: the first part was frozen at −80 °C for isolation of total RNA and further molecular analysis, the second part was fixed in 10% formalin for histopathological examination, the third part was freshly used for the comet assay, and the fourth part was homogenized for antioxidant analysis.
2.7. Biochemical Analysis
Serum levels of uric acid, urea, and creatinine were determined according to methods described by Fossati et al. [23], Patton and Crouch [24], and Bartels et al. [25], respectively. Creatinine clearance was estimated from the levels of both serum and urine creatinine in addition to the 24 h urinary volumes. Kidney specimens from all groups were homogenized (1 g tissue + 9 mL homogenization buffer) and centrifuged (1000 rpm for 20 min), and the obtained sediment was used for estimation of Na + K + ATPase using the method described by Bontin [26]. However, the supernatant was used for estimating the levels of the lipid peroxidation marker malondialdehyde (MDA) and the activities of the antioxidant enzymes (superoxide dismutase (SOD; EC 1.15.1.1), catalase (CAT; EC 1.11.1.6), and glutathione peroxidase (GPx; EC 1.11.1.9)). MDA renal levels were assessed as previously described [27]. This method depends on the measurement of thiobarbituric acid reactive substance content (TBARS), which reacts with MDA to produce thiobarbituric acid reactive products, which were measured at the absorbance of 530 nm. SOD activity was assessed as previously described [28]. The method involved blocking epinephrine’s oxidation into adrenochrome, which is hampered by SOD. CAT activity was determined spectrophotometrically at 240 nm by calculating the degradation rate of H2O2 [29]. GPx activity was determined as previously described [30].
2.8. Comet Assay
Comet assays were performed to determine DNA damage in kidneys following different treatments. This assay was carried out as previously described [31]. To obtain separate whole renal cells, fragments of renal tissue (5 mm cubes) were minced using a pair of scissors in chilled PBS supplemented with 54 mM EDTA, and the mixture was filtered using a nylon mesh with a diameter of 150 µm. Based on this assay, DNA damage was quantitatively and qualitatively assessed in a total of 100 cells/sample with the aid of a fluorescence microscope (at 40×), Komet 5 image analysis software (Kinetic Imaging, Ltd., Liverpoo1, UK), and GelRed stain. The DNA damage parameters included the percentage of damaged DNA that migrated away from the intact DNA and tail moment, in addition to the comet tail length, which was measured from the middle of the intact DNA to the end of the tail.
2.9. Molecular Analysis by Real-Time PCR
Real-time PCR (qRT-PCR) was used to detect the renal expression levels of immunomodulatory and proinflammation-related genes (interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNFa), interleukin 1 beta (IL1β), monocyte chemotactic protein 1 (MCP1), intercellular adhesion molecule-1 (ICAM1), and vascular cell adhesion molecule 1 (VCAM1)). To perform qRT-PCR, we first extracted total RNA from kidney tissue using GeneJET RNA Purification Kit following the manufacturer’s protocol (Thermo Scientific, #K0731, Walham, MA, USA). Following the assessment of RNA integrity and purity, a reverse transcriptase (RevertAid H Minus, Thermo Scientific, #EP0451, Walham, MA, USA) was used to convert RNA into cDNA that was used as a template for qRT-PCR reaction in the presence of Syber green master mix (2× Maxima, Thermo Scientific, #K0221, Walham, MA, USA) and gene-specific primers (Table 2). The relative expression was expressed as fold change against the housekeeping GAPDH gene using the 2−ΔΔCt method.
Table 2.
Primers used for real-time PCR.
| Gene | Forward Primer | Reverse Primer | Accession Number |
|---|---|---|---|
| TNFα | GCATGATCCGCGACGTGGAA | AGATCCATGCCGTTGGCCAG | NM_012675.3 |
| IL1β | CACCTCTCAAGCAGAGCACAG | GGGTTCCATGGTGAAGTCAAC | NM_031512.2 |
| MCP1 | TCGCTTCTGACACCATGCA | TGCTACAGGCAGCAAATGTGA | M57441.1 |
| IFN-γ | GATCCAGCACAAAGCTGTCA | GACTCCTTTTCCGCTTCCTT | AF010466.1 |
| ICAM1 | AGATCACATTCACGGTGCTG | CTTCAGAGGCAGGAAACAGG | NM_012967.1 |
| VCAM1 | TGCACGGTCCCTAATGTGTA | TGCCAATTTCCTCCCTTAAA | NM_012889.2 |
| GAPDH | CAACTCCCTCAAGATTGTCAGCAA | GGCATGGACTGTGGTCATGA | NM_017008.4 |
2.10. Histopathology Examinations
Following fixation in 10% formalin, kidney specimens were histologically prepared according to standard histological procedures. Sections 4–6 μm thick were stained with hematoxylin and eosin. The kidney damage score in the renal corpuscle involved checking for widened Bowman’s capsules, and shrunken and branched glomeruli; tubular dilatation, pyknotic nuclei, interstitial, and perivascular edema in renal tubules; and hyaline cast in the lumen. This damage score was blindly determined in 5 randomly chosen fields per slide using X400. The score grades were 0 (no damage), 1 (≤10% damage), 2 (11–25% damage), 3 (26–45% damage), 4 (46–75% damage), and 5 (≥76% damage) [32,33].
2.11. Statistical Analysis
The obtained results were statistically analyzed using one-way ANOVA with GraphPad Prism-8 followed by Tukey’s honestly significant difference. Data are presented as mean ± standard error of mean (SEM) and significance was declared at p < 0.05.
3. Results
3.1. GC-MS Analysis of Avocado Seed Extract
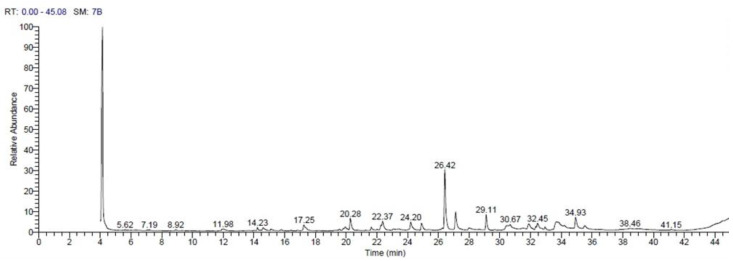
The GC-MS revealed the presence of 21 compounds in the ethanolic extract of the avocado seeds (Table 3, Figure 1). Most of these compounds were terpenes and esters of fatty acid derivatives (dodecanoic acid, tetradecanoic acid, 9,12-octadecadienoic acid (z, z), 9-octadecenoic acid (z), heptadecanoic acid, 16-methyl,-methyl ester, 9,12-octadecadienoic acid, hexadecadienoic acid, and oleic acid), which are phytochemical agents in various plants.
Table 3.
Chemical compounds, retention time (RT), and constituents of the avocado seed as detected by GC-MS.
| RT | Compound Name | Area % | Molecular Formula | Molecular Weight |
|---|---|---|---|---|
| 14.23 | 7-Hexadecene | 1.41 | C16H32 | 224 |
| 14.60 | 10-Undecenal | 2.32 | C11H20O | 168 |
| 17.25 | 13-Oxabicyclo[1 0.1.0]tridecan | 4.58 | C12H22O | 182 |
| 19.94 | Tridecanal | 2.22 | C13H26O | 198 |
| 20.28 | Dodecanoic acid, methyl ester | 5.53 | C13H26O2 | 214 |
| 21.64 | 5Oxatricyclo[8.2.0.0(4,6)]dodecane, 12-trimethyl-9-methylene | 1.32 | C15H24O | 220 |
| 22.27 | cis-9-Hexadecenal | 1.71 | C16H30O | 238 |
| 22.37 | Tetradecanal | 4.34 | C14H28O | 212 |
| 24.20 | Cyclopentadecanone | 3.33 | C15H28O | 224 |
| 24.90 | Tetradecanoic acid, methyl ester | 3.37 | C15H30O2 | 242 |
| 26.42 | Z,Z-4,15-Octadecadien-1-ol acetate | 26.06 | C20H36O2 | 308 |
| 27.12 | Aromadendrene oxide-(1) | 8.63 | C15H24O | 220 |
| 29.11 | Hexadecanoic acid, methyl ester | 7.28 | C17H34O2 | 270 |
| 30.67 | Oleic Acid | 1.31 | C18H34O2 | 282 |
| 31.88 | 9-Octadecenoic acid (z) | 3.47 | C18H34O2 | 282 |
| 32.33 | 9,12-Octadecadienoic acid (z,z)-, methyl ester | 1.13 | C19H34O2 | 294 |
| 32.45 | 9-Octadecenoic acid (Z)-, methyl ester | 3.77 | C19H36O2 | 296 |
| 32.94 | Heptadecanoic acid, 16-methyl-, methyl ester | 1.26 | C19H38O2 | 298 |
| 33.67 | 9,12-Octadecadienoic acid | 9.32 | C18H32O2 | 280 |
| 34.92 | 7-Methyl-Z-tetradecen-1-ol acetate | 5.71 | C17H32O2 | 268 |
| 35.53 | Hexadecadienoic acid, methyl ester | 1.94 | C17H30O2 | 266 |
Figure 1.
The GC-MS chromatogram of the ethanolic avocado seed extract shows the structures of various phytochemicals at retention times (RT) 4–44 min.
3.2. Growth-Related Parameters and Biochemical Analysis
The CsA group showed significantly (p < 0.05) less final body weight gain (FBWG), a significantly lower total feed intake (FI), and a significantly lower feed efficiency ratio (FER) than the control group (Table 4). In contrast, the AvS + CsA group exhibited significantly (p < 0.05) higher FBWG, FI, and FER than the CsA group. Surprisingly, rats fed a diet supplemented with 5% AvS powder (AvS group) showed significantly (p < 0.05) higher FBWG and FER than the other groups.
Table 4.
Effects of avocado seed powder on growth-related parameters.
| Groups | FBWG (g) | Total FI (g) | FER |
|---|---|---|---|
| Cnt | 60.00 ± 3.28 b | 501.00 ± 12.79 a | 0.120 ± 0.006 b |
| AvS | 73.33 ± 3.19 a | 475.00 ± 10.43 a | 0.154 ± 0.002 a |
| CsA | 30.33 ± 2.03 d | 351.67 ± 8.67 c | 0.086 ± 0.007 d |
| AvS + CsA | 47.00 ± 2.61 c | 437.00 ± 11.89 b | 0.108 ± 0.004 c |
Means with different superscript letters (a–d) in the same column are significantly different at p < 0.05. Data are presented as mean ± SEM (n = 7 animals in each group). Cnt: control group; AvS: avocado seed powder-treated group; CsA: cyclosporine A-treated group; AvS + CsA: cyclosporine A and avocado seed powder-cotreated group. FBWG, final body weight gain; FI, feed intake; FER, feed efficiency ratio.
Importantly, the AvS group showed significantly lower serum levels of kidney function biomarkers (uric acid, urea, and creatinine) relative to the control (Cnt) group (Table 5). In contrast, rats in the CsA group exhibited significantly (p < 0.05) higher serum levels of these biomarkers compared to Cnt and AvS groups. After treatment with AvS (AvS + CsA group), their levels were significantly (p < 0.05) declined in comparison to the CsA group.
Table 5.
Effects of avocado seed powder on the serum levels of kidney function biomarkers in rats.
| Groups | Uric Acid (mg/dL) | Urea (mg/dL) | Creatinine (mg/dL) |
|---|---|---|---|
| Cnt | 2.61 ± 0.10 c | 17.63 ± 0.76 c | 0.72 ± 0.03 c |
| AvS | 2.13 ± 0.12 d | 14.31 ± 0.63 d | 0.64 ± 0.02 d |
| CsA | 6.56 ± 0.21 a | 74.23 ± 2.18 a | 1.46 ± 0.06 a |
| AvS + CsA | 3.62 ± 0.11 b | 32.35 ± 1.06 b | 0.93 ± 0.04 b |
Means with different superscript letters (a–d) in the same column are significantly different at p < 0.05. Data are presented as mean ± SEM (n = 7/group).
Rats in the AvS group had significantly lower levels of the lipid peroxidation biomarker MDA and significantly higher levels of GPx in their kidneys when compared with the control (Cnt) group (Table 6). On the other hand, in the CsA group, the levels of MDA were significantly increased, and the activities of the antioxidant enzymes (SOD, CAT, and GPx) were significantly decreased as compared to Cnt and AvS groups. After administration of AvS (AvS + CsA group), MDA levels were significantly reduced and the activities of the antioxidant of enzymes were significantly increased compared to the AvS group.
Table 6.
Effects of avocado seed powder on oxidant/antioxidant status of kidney.
| Groups | MDA (nmol/g Tissue) |
SOD (U/g Tissue) |
CAT (U/g Tissue) |
GPx (U/g Tissue) |
|---|---|---|---|---|
| Cnt | 11.37 ± 0.73 c | 3.75 ± 0.24 a | 3.04 ± 0.19 a | 115.75 ± 7.51 b |
| AvS | 9.70 ± 0.54 d | 3.94 ± 0.28 a | 3.15 ± 0.17 a | 138.11 ± 7.82 a |
| CsA | 35.68 ± 2.72 a | 1.21 ± 0.09 c | 1.46 ± 0.08 c | 37.30 ± 2.36 d |
| AvS + CsA | 18.24 ± 0.91 b | 2.45 ± 0.14 b | 2.25 ± 0.09 b | 92.73 ± 6.00 c |
Means with different superscript letters (a–d) in the same column are significantly different at p < 0.05. Data are presented as mean ± SEM (n = 7/group).
The CsA group showed significant decreases in creatinine clearance rate in urine, renal Na+/K+ ATPase activity, and kidney weight compared to control and AvS groups (Table 7). In contrast, the AvS cotreated (AvS + CsA) group significantly increased creatinine clearance, renal Na+/K+ ATPase, and kidney weight relative to the CsA group. The AvS group exhibited significantly lower creatinine clearance and significantly higher Na+/K+ ATPase than the AvS + CsA group.
Table 7.
Effects of avocado seed powder on creatinine clearance, Na+/K+ ATPase, and kidney weight.
| Groups | Creatinine Clearance (mL/min/100 g Body wt) |
Na+/K+ ATPase (ng/mg) |
kidney Weight (g) |
|---|---|---|---|
| Cnt | 1.6 ± 0.04 a | 0.85 ± 0.02 a | 1.66 ± 0.05 bc |
| AvS | 1.14 ± 0.02 c | 0.79 ± 0.005 b | 1.93 ± 0.02 a |
| CsA | 0.82 ± 0.03 d | 0.14 ± 0.004 d | 1.26 ± 0.05 d |
| AvS + CsA | 1.25 ± 0.04 b | 0.55 ± 0.03 c | 1.60 ± 0.03 c |
Means with different superscript letters (a–d) in the same column are significantly different at p < 0.05. Data are presented as mean ± SEM (n = 7/group).
3.3. Comet Assay Results
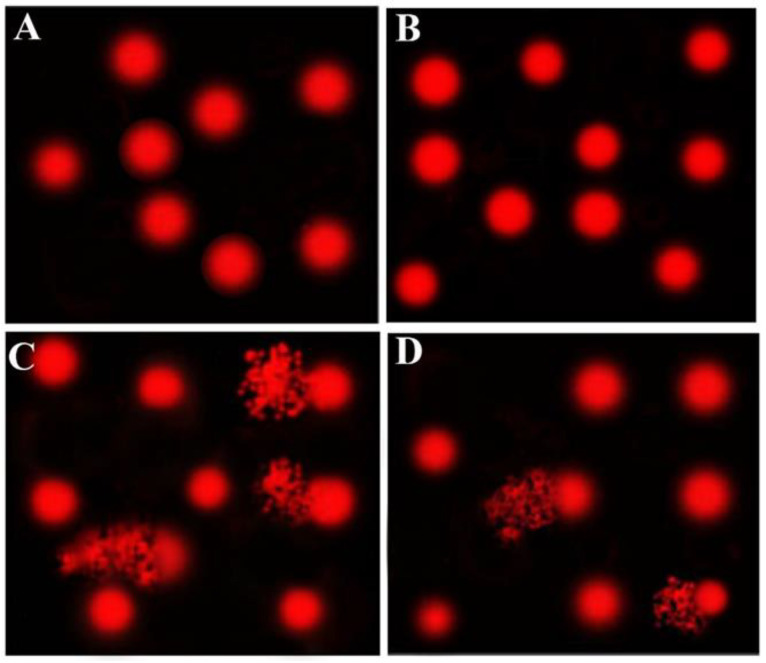
Comet assays were performed to assess the DNA damage in the kidneys after various treatments. The results of the comet assays are shown in Table 8 and Figure 2. The CsA group showed significantly (p < 0.05) higher DNA damage in renal cells, as indicated by increases in the tail length, tail DNA%, and tail moment, compared to control and AvS groups. This elevated DNA damage was decreased following cotreatment with AvS (AvS + CsA group). However, no significant difference was noticed between the control and AvS groups.
Table 8.
Effects of AvS powder on DNA damage, as detected by comet assay in rat kidneys.
| Group | Tailed % | Untailed % | Tail Length (µm) | Tail DNA% | Tail Moment * |
|---|---|---|---|---|---|
| Cnt | 2 | 98 | 1.65 ± 0.11 c | 1.10 | 1.82 |
| AvS | 4 | 96 | 1.89 ± 0.12 c | 1.75 | 3.31 |
| CsA | 18 | 82 | 5.63 ± 0.28 a | 4.50 | 25.34 |
| AvS + CsA | 11 | 89 | 3.95 ± 0.19 b | 3.24 | 12.80 |
Data are expressed as mean ± SEM (n = 7/group). Different superscript letters (a–c) in the same column of tail length show significance differences at p < 0.05. * Tail moment is the tail DNA (%) × tail length (µm).
Figure 2.
The effects of AvS and/or CsA on the DNA damage of renal cells as detected by comet assay. Comet assays were performed in three independent experiments, and the images here are representative. (A) Cnt, (B) AvS, (C) CsA, (D) AvS + CsA. Degraded DNA appears as fragmented patches behind the intact DNA (circle), as seen in (C,D).
3.4. Molecular Analysis
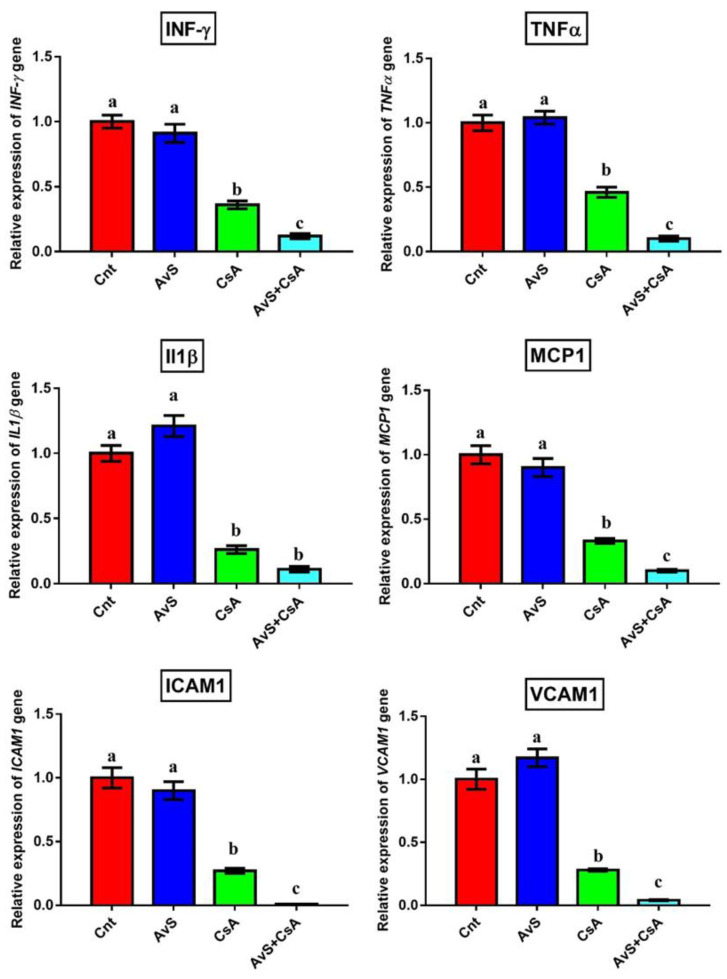
To investigate whether AvS could modulate the immunosuppressive effect of CsA, we detected the changes in the expression of immunomodulatory and inflammatory genes (IFN-γ, TNFα, IL1β, MCP1, ICAM1, and VCAM1) in the kidney following treatment with AvS and/or CsA using qRT-PCR. We found significant downregulations in gene expression after treatment with CsA alone or in combination with AvS. The lowest expression levels of such genes were in the co-treated group (AvS + CsA) (Figure 3). On the other hand, no significant difference was found between the control and AvS groups.
Figure 3.
The effects of AvS and/or CsA treatment on the levels of IFN-γ, TNFα, IL1β, MCP1, ICAM1, and VCAM1 in rat kidneys, as detected by qRT-PCR. Data are presented as fold change (mean) ± SEM (n = 7/group). Columns with different letters (a–c) are significantly different at p < 0.05.
3.5. Histopathological Examination
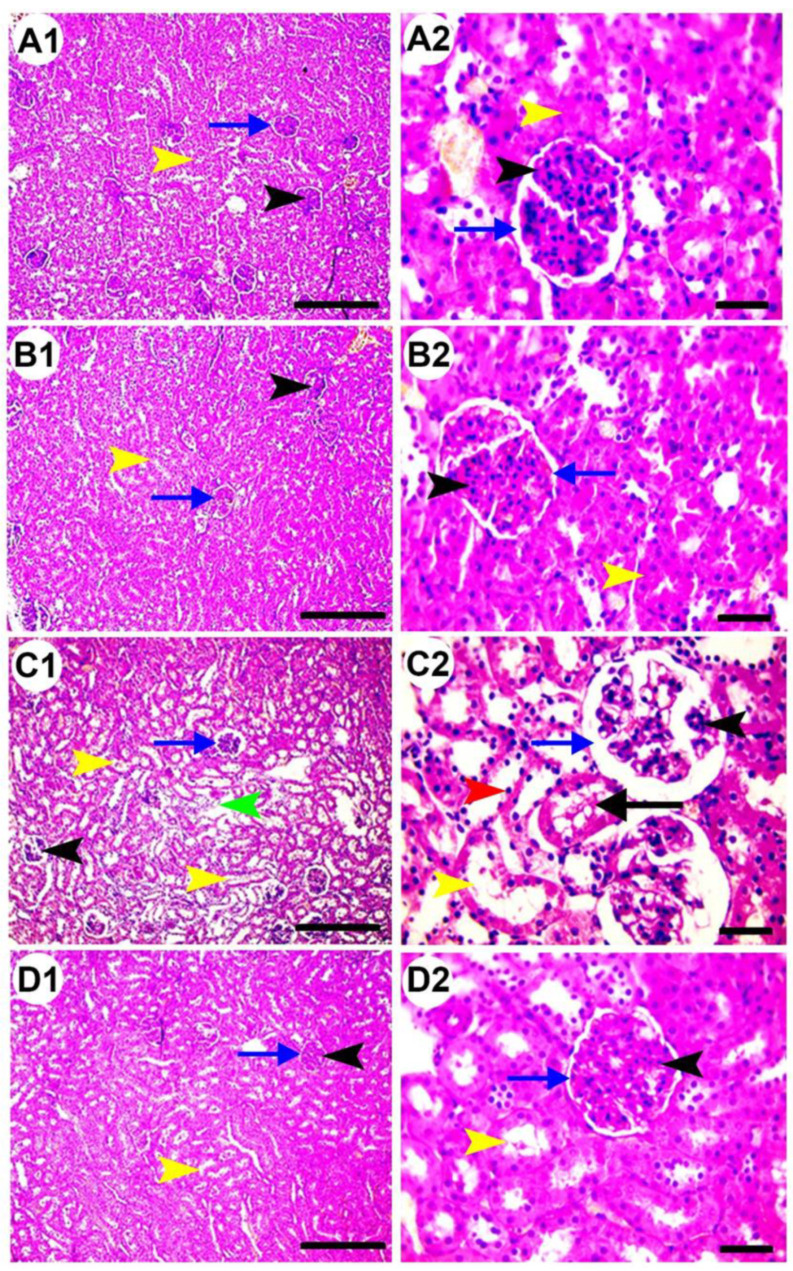
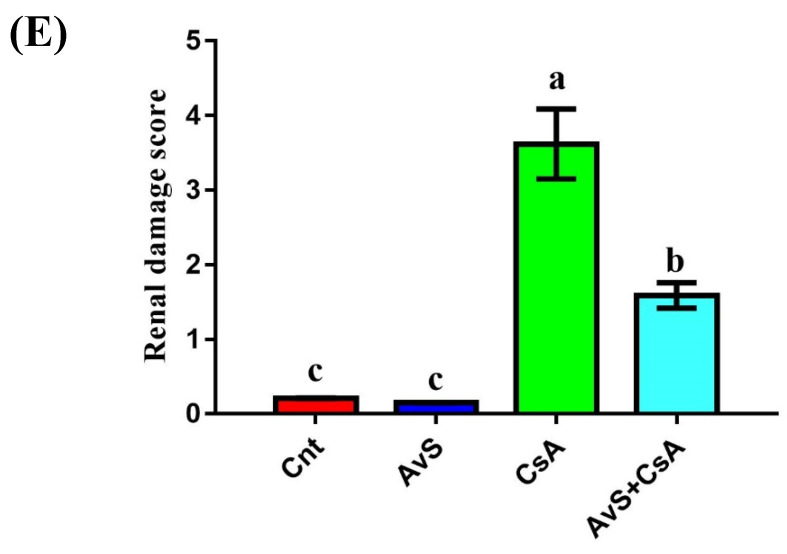
Histological examination of renal tissue sections of control and AvS groups revealed the presence of normal glomeruli (black arrowheads), Bowman’s capsules (blue arrows), and tubules (yellow arrowheads) (Figure 4A,B). In contrast, the administration of CsA resulted in notable histopathological changes in renal corpuscles and tubules (Figure 4C). These changes included shrunken and split glomeruli (black arrowheads), widened Bowman’s space (blue arrows), interstitial and perivascular edema (green arrowhead), tubular dilation (yellow arrowheads), pyknotic nuclei in tubal lining epithelia (red arrowhead), and the accumulation of hyaline cast in the tubal lumen (black arrow). Co-treatment with AvS (AvS + CsA group) reduced the renal damage induced by CsA, resulting in only mild tubular dilation (yellow arrowheads, Figure 4D). The highest renal damage score was given to the CsA group, and the score of the AvS + CsA group was significantly lower (Figure 4E).
Figure 4.
(A–D) Microscopic pictures of H&E-stained renal sections showing normal glomeruli, tubules, and interstitial tissue from the control (A1,2) and AvS (B1,2) groups; tubular dilation, perivascular edema (green arrowhead), hyaline cast (black arrow), pyknotic nuclei (red arrowhead), and widened Bowman’s space from the CsA group (C1,2); and mild tubular dilation from the AvS + CsA group (D1,2). Black arrowheads: glomeruli; blue arrows: Bowman’s space; yellow arrowheads: renal tubules. Scale bars: 100 μm for (A1,B1,C1,D1); and 50 μm for the remaining images. (E) Renal damage score; values are mean ± SEM (n = 7/group). Columns with different letters (a–c) are significantly different at p < 0.05.
4. Discussion
For its powerful immunosuppressive properties, CsA is commonly used after organ transplantations. However, its prolonged uses cause many adverse effects, including oxidative-stress-dependent nephrotoxicity [1,2,3,5]. On the other hand, antioxidants are known to protect against oxidative renal damage induced by CsA [34]. The AvS conatins several health-promoting bioactive compounds with antioxidant and anti-inflammatory properties [15,16,17,18,19] that can antagonize the detrimental effects of free radicals and subsequently protect renal cells from damage. Therefore, in this study, we investigated the ameliorative effect of AvS on the nephrotoxicity induced by CsA. We also assessed whether AvS would alter CsA’s immunosuppressive effect. To the best of our knowledge, this is the first study to report that AvS could reduce nephrotoxicity and increase the immunosuppression induced by CsA through, at least in part, modulation of oxidative stress and inflammation and immunity-related markers. We reached this overall conclusion based on the following evidence: co-treatment with AvS resulted in (1) increases in final body weight gain and feed efficiency ratio, (2) reductions in the serum levels of renal function parameters (uric acid, urea, and creatinine), (3) increases in creatinine clearance and renal Na+/K+ ATPase, (4) a decrease in the renal level of an oxidative stress marker (MDA), (5) increases in the activity of renal antioxidant enzymes (SOD, CAT, GPx), (6) a reduction in DNA damage in renal cells, (7) the downregulation of immunomodulatory and inflammatory genes (IFN-γ, TNFα, IL1β, MCP1, ICAM1, and VCAM1), and (8) improved renal histopathology.
In agreement with Saad [21] and Zizhang et al. [35], treatment with CsA resulted in significant reductions in feed intake (FI), final body weight gain (FBWG), and feed efficiency ratio (FER). Loss of appetite could be attributed to CsA’s ability to penetrate the blood–brain barrier and the subsequent inhibition of the appetite center [36]. CsA and AvS co-treatment improved FI, FBWG, and FER in animals. Interestingly, animals administered AvS alone showed significantly increased FBWG and FER as compared to the control animals. In contrast, Saad [21] found decreases in FBWG and FER following administration of AvS powder to rats. In support to our findings, many other studies have reported a growth-promoting effect for AvS and attributed this effect to the nutritious properties of these seeds, which are rich in vitamins, essential minerals, and amino acids, and several other health-promoting bioactive compounds [15,16,20]. This growth-promoting effect denotes the importance of AvS and its ingredients as a value-added food.
In the present study, treatment with CsA alone resulted in elevated serum levels of renal function biomarkers (uric acid, urea, and creatinine), suggesting severe renal damage. These results are in agreement with many previous reports [37,38]. CsA impairs kidney function by reducing glomerular filtration rate (GFR) and/or inducing tubular damage [34,39]. Creatinine clearance is used as an indicator of plasma renal flow. We found significant decreases in creatinine clearance in urine, Na+/K+ ATPase activity in kidney, and kidney weight. In accordance with our findings, previous studies also showed similar effects for CsA [40,41]. This functional impairment was followed by structural damage via atrophied glomeruli, widened Bowman’s space, interstitial and perivascular edema, and tubular dilation. Similar histopathological changes were reported by other studies [42,43].
Our results revealed that CsA administration significantly increased the levels of lipid peroxidation biomarker (MDA) and reduced the activities of antioxidant enzymes (SOD, CAT, GPx) in the kidney. Similar results were obtained by Capasso et al. [44]. Oxidative stress commences when the release of reactive oxygen species (ROS) overrides the activities of endogenous antioxidant enzymes, causing damage to various cellular macromolecules, including proteins, lipids, and DNA [31,45,46]. Elevation of renal MDA levels indicates oxidative damage to renal cell membranes, which are rich in lipids [33,47]. Previous studies have shown that CsA can trigger oxidative-stress-dependent nephrotoxicity via induction of endoplasmic reticulum stress and boosting free radical release from mitochondria [8]. The histopathological alterations induced by CsA could be attributed to ROS overproduction, which could cause detrimental effects on the glomerular endothelium and subsequent endothelial dysfunction [48]. Besides, accumulation of hyaline cast within the renal tubules in CsA-treated animals may be due to ROS and lipid peroxidation. The latter can induce cell membrane damage with subsequent shedding of cytoplasmic contents into the lumen that further extend the hyaline cast [49].
We found that co-treatment with AvS powder improves kidney function, structure, and antioxidant status, and inhibits oxidative stress damage in the kidney. Hence, the results suggest that consumption of AvS might have some therapeutic effects for kidney injuries induced by CsA in rats. These results agree with Rao et al. [50] and Abdel Moneim et al. [51], who found that the avocado can act as a nephroprotective agent by inhibiting renal oxidative stress. The AvS’s antioxidant properties could be attributed to its phytochemical ingredients such as phytosterols, triterpenes, fatty acids, furanoic acids, proanthocyanidins, and abscisic acid; and phenolic compounds such as hydrocinnamic acid, catechin, epicatechin, and leucoanthocyanidins [15,16]. Based on GC-MS analysis, we found 21 compounds in the ethanolic extract of the AvS. Most of them were terpenes and esters of fatty acid derivatives (dodecanoic acid, tetradecanoic acid, 9,12-octadecadienoic acid (z, z), 9-octadecenoic acid (z), heptadecanoic acid, 16-methyl,-methyl ester, 9,12-octadecadienoic acid, hexadecadienoic acid, and oleic acid). Richard et al. [52] stated that unsaturated fatty acids can function as antioxidants. Avocado byproducts (seeds and peels) are a good source of phenolic compounds that can inhibit lipid and protein oxidation [53]. Avocado flavonoids could prevent the formation of renal calculi by suppressing the release of ROS, thereby protecting renal cells from oxidative stress damage [54]. Avocado has antioxidant, anti-inflammatory, hypocholesterolemic, and antidiabetic effects [55].
CsA could also induce nephrotoxicity by triggering apoptosis in renal cells [9]. The comet assay results indicate induction of DNA fragmentation in renal cells following treatment with CsA, suggesting a role in apoptosis. Consistently, previous studies also showed that CsA could induce DNA fragmentation, thereby causing chronic organ diseases [56,57]. In contrast, co-treatment with AvS resulted in a significant reduction in DNA damage. The avocado seed extract was shown to have no genotoxic activity [15].
In the present study, we found significant downregulation in the expression levels of immunomodulatory and inflammatory genes (IFN-γ, TNFa, IL1β, MCP1, ICAM1, and VCAM1) following treatment with CsA. Consistently with those findings, Rincón et al. [58] also reported significant reductions in IFN-γ and TNFa after treatment with CsA. In contrast, Saad [21] found an increase in TNFα levels in renal tissues after administration of a very high dose of CsA (50 mg/kg). These contradictory results may be attributed to the methods used for quantification of TNFα (Elisa vs. real-time PCR) and the CsA dose (50 mg/kg vs. 5 mg/kg). Therefore, further experiments, including Western blots, will be required to check whether the molecular changes in inflammatory genes are consistent with changes in their proteins. Moreover, the IL1β and MCP1 downregulation by CsA agree with Daull et al. [59,60]. ICAM1 and VCAM1 modulate the interaction between inflammation and immunity [61], and their expression is inhibited by CsA [62]. On the other hand, we found that co-treatment with AvS and CsA maximizes the immunosuppressive effect of CsA, as revealed by lower expression levels of IFN-γ, TNFa, IL1β, MCP1, ICAM1, and VCAM1 in the renal tissues. This implies that AvS has not only antioxidant properties, but also has an anti-inflammatory effect. Indeed, previous studies reported anti-inflammatory effects for avocado and AvS, as revealed by a reduction in cytokine production [13,63]. In contrast, Saad [21] reported an immunostimulant effect for 5% AvS powder following treatment with an overdose of CsA (50 mg/kg).
Avocado seeds, as a waste product, are produced in large amounts during industrial processing, and their unmanageable disposal may cause environmental problems. These seeds are available in bulk at zero cost. Thus, modern approaches are needed to obtain benefits from this waste. For example, these seeds and their ingredients can be used as natural food additives (due to their growth-promoting effect), and in medicine to alleviate CsA’s side effects (due to their antioxidant and anti-inflammatory effects).
The present study lacked results on the effects of different treatments on the concentrations of immunoglobulins in blood, the expression of immunity-related markers for adaptive and innate immunity, and appropriate rejection models of renal transplantation in animals. Moreover, an appropriately designed clinical trial in transplant patients treated with cyclosporin should be carried out to confirm our results in a relevant human model before translation to the clinic.
5. Conclusions
Many natural products relieved CsA’s oxidative stress effects, but it is still unknown whether these natural products could modulate CsA’s immunosuppressive effect. To the best of our knowledge, this is the first study to report that AvS treatment not only reduces the oxidative-stress-dependent nephrotoxicity induced by CsA, but also modulates the expression of inflammation and immunity-related genes, which could improve CsA’s immunosuppressive effect. This was accomplished by decreasing oxidative stress, apoptosis, and inflammation, all of which were manifested in the enhanced biochemical parameters and diminished renal damage scores. AvS could therefore potentially be a therapeutic choice for patients with CsA-induced nephrotoxicity. However, further experiments are required to unveil the exact molecular pathways by which AvS exerts this beneficial effect, and to determine whether AvS is clinically applicable.
Author Contributions
Conceptualization, A.M.E., M.A.E.-M. and A.M.G.Z.; methodology, A.M.E. and A.M.G.Z.; software, H.I.G. and M.Y.A.; validation, M.A.E.-M. and N.S.Z.; formal analysis, A.M.E., M.A.E.-M. and A.M.G.Z.; investigation, M.A.E.-M., H.I.G., M.Y.A. and N.S.Z.; resources, H.I.G., M.Y.A. and N.S.Z.; data curation, A.M.E., M.A.E.-M. and A.M.G.Z.; writing—original draft preparation, A.M.E. and A.M.G.Z.; writing—review and editing, M.A.E.-M.; visualization, H.I.G., M.Y.A. and N.S.Z.; supervision, M.A.E.-M.; project administration, H.I.G., M.Y.A. and N.S.Z.; funding acquisition, H.I.G., M.Y.A. and N.S.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Group Research Project under grant number G.R.P1/211-42.
Institutional Review Board Statement
The study was approved by the Animal Ethical Committee of Kafrelsheikh University with the license number KFS-127/29.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the present findings are contained within the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Türk G., Ateşşahin A., Sönmez M., Yüce A., Ceribaşi A.O. Lycopene protects against cyclosporine a-induced testicular toxicity in rats. Theriogenology. 2007;67:778–785. doi: 10.1016/j.theriogenology.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Zimmermann S., Sekula P., Venhoff M., Motschall E., Knaus J., Schumacher M., Mockenhaupt M. Systemic immunomodulating therapies for stevens-johnson syndrome and toxic epidermal necrolysis: A systematic review and meta-analysis. JAMA Dermatol. 2017;153:514–522. doi: 10.1001/jamadermatol.2016.5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tedesco D., Haragsim L. Cyclosporine: A review. J. Transplant. 2012;2012:230386. doi: 10.1155/2012/230386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Türk G., Sönmez M., Ceribaşi A.O., Yüce A., Ateşşahin A. Attenuation of cyclosporine a-induced testicular and spermatozoal damages associated with oxidative stress by ellagic acid. Int. Immunopharmacol. 2010;10:177–182. doi: 10.1016/j.intimp.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Ghazipour A.M., Shirpoor A., Ghiasi R., Pourheydar B., Khalaji N., Naderi R. Cyclosporine a induces testicular injury via mitochondrial apoptotic pathway by regulation of mir-34a and sirt-1 in male rats: The rescue effect of curcumin. Chem. Biol. Interact. 2020;327:109180. doi: 10.1016/j.cbi.2020.109180. [DOI] [PubMed] [Google Scholar]
- 6.Josephine A., Amudha G., Veena C.K., Preetha S.P., Varalakshmi P. Oxidative and nitrosative stress mediated renal cellular damage induced by cyclosporine a: Role of sulphated polysaccharides. Biol. Pharm. Bull. 2007;30:1254–1259. doi: 10.1248/bpb.30.1254. [DOI] [PubMed] [Google Scholar]
- 7.da Silva J.B., de Melo Lima M.H., Secoli S.R. Influence of cyclosporine on the occurrence of nephrotoxicity after allogeneic hematopoietic stem cell transplantation: A systematic review. Rev. Bras. Hematol. Hemoter. 2014;36:363–368. doi: 10.1016/j.bjhh.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Q., Wang X., Nepovimova E., Wang Y., Yang H., Kuca K. Mechanism of cyclosporine a nephrotoxicity: Oxidative stress, autophagy, and signalings. Food Chem. Toxicol. 2018;118:889–907. doi: 10.1016/j.fct.2018.06.054. [DOI] [PubMed] [Google Scholar]
- 9.Rao S.R., Sundararajan S., Subbarayan R., Murugan Girija D. Cyclosporine-a induces endoplasmic reticulum stress and influences pro-apoptotic factors in human gingival fibroblasts. Mol. Cell. Biochem. 2017;429:179–185. doi: 10.1007/s11010-017-2945-9. [DOI] [PubMed] [Google Scholar]
- 10.Chanderbali A.S., Albert V.A., Ashworth V.E., Clegg M.T., Litz R.E., Soltis D.E., Soltis P.S. Persea americana (avocado): Bringing ancient flowers to fruit in the genomics era. BioEssays. 2008;30:386–396. doi: 10.1002/bies.20721. [DOI] [PubMed] [Google Scholar]
- 11.Rodríguez-Carpena J.G., Morcuende D., Andrade M.J., Kylli P., Estévez M. Avocado (Persea americana mill.) phenolics, in vitro antioxidant and antimicrobial activities, and inhibition of lipid and protein oxidation in porcine patties. J. Agric. Food Chem. 2011;59:5625–5635. doi: 10.1021/jf1048832. [DOI] [PubMed] [Google Scholar]
- 12.Segovia F.J., Corral-Pérez J.J., Almajano M.P. Avocado seed: Modeling extraction of bioactive compounds. Ind. Crop. Prod. 2016;85:213–220. doi: 10.1016/j.indcrop.2016.03.005. [DOI] [Google Scholar]
- 13.Plaza L., Sánchez-Moreno C., de Pascual-Teresa S., de Ancos B., Cano M.P. Fatty acids, sterols, and antioxidant activity in minimally processed avocados during refrigerated storage. J. Agric. Food Chem. 2009;57:3204–3209. doi: 10.1021/jf900541r. [DOI] [PubMed] [Google Scholar]
- 14.Ortiz M.A., Dorantes A.L., Gallndez M.J., Cardenas S.E. Effect of a novel oil extraction method on avocado (Persea americana mill) pulp microstructure. Plant Foods Hum. Nutr. 2004;59:11–14. doi: 10.1007/s11130-004-0032-3. [DOI] [PubMed] [Google Scholar]
- 15.Padilla-Camberos E., Martínez-Velázquez M., Flores-Fernández J.M., Villanueva-Rodríguez S. Acute toxicity and genotoxic activity of avocado seed extract (Persea americana mill., c.V. Hass) Sci. World J. 2013;2013:245828. doi: 10.1155/2013/245828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W., Bostic T.R., Gu L. Antioxidant capacities, procyanidins and pigments in avocados of different strains and cultivars. Food Chem. 2010;122:1193–1198. doi: 10.1016/j.foodchem.2010.03.114. [DOI] [Google Scholar]
- 17.Jiménez-Arellanes A., Luna-Herrera J., Ruiz-Nicolás R., Cornejo-Garrido J., Tapia A., Yépez-Mulia L. Antiprotozoal and antimycobacterial activities of Persea americana seeds. BMC Complementary Altern. Med. 2013;13:109. doi: 10.1186/1472-6882-13-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abubakar A.N.F., Achmadi S.S., Suparto I.H. Triterpenoid of avocado (Persea americana) seed and its cytotoxic activity toward breast mcf-7 and liver hepg2 cancer cells. Asian Pac. J. Trop. Biomed. 2017;7:397–400. doi: 10.1016/j.apjtb.2017.01.010. [DOI] [Google Scholar]
- 19.Uchenna U.E., Shori A.B., Baba A.S. Inclusion of avocado (Persea american) seeds in the diet to improve carbohydrate and lipid metabolism in rats. Rev. Argent. Endocrinol. Metab. 2017;54:140–148. [Google Scholar]
- 20.Dao P.T.A., An N.T.H., Thuy N.T.T., Tuyet N.T.A., Truc T.T.M. Screening on Antioxidant Activities of by-Products from Vegetables and Fruits in Tay Nguyen Region and Applying for Shrimp cold Storage; Proceedings of the 2016 3rd International Conference on Green Technology and Sustainable Development (GTSD); Kaohsiung, Taiwan. 24–25 November 2016; pp. 93–97. [Google Scholar]
- 21.Saad S.A. The antioxidant activity of avocado seeds on immunosuppression induced by cyclosporine in rats. Home Econ. J. 2020;36:93–109. [Google Scholar]
- 22.Reeves P.G., Rossow K.L., Lindlauf J. Development and testing of the ain-93 purified diets for rodents: Results on growth, kidney calcification and bone mineralization in rats and mice. J. Nutr. 1993;123:1923–1931. doi: 10.1093/jn/123.11.1923. [DOI] [PubMed] [Google Scholar]
- 23.Fossati P., Prencipe L., Berti G. Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin. Chem. 1980;26:227–231. doi: 10.1093/clinchem/26.2.227. [DOI] [PubMed] [Google Scholar]
- 24.Patton C.J., Crouch S.R. Spectrophotometric and kinetics investigation of the berthelot reaction for the determination of ammonia. Anal. Chem. 1977;49:464–469. doi: 10.1021/ac50011a034. [DOI] [Google Scholar]
- 25.Bartels H., Böhmer M., Heierli C. Serum creatinine determination without protein precipitation. Clin. Chim. Acta Int. J. Clin. Chem. 1972;37:193–197. doi: 10.1016/0009-8981(72)90432-9. [DOI] [PubMed] [Google Scholar]
- 26.Bontin S. Presence of enzyme system of mammalian tissue. Wiley Inter. Sci. 1970;197:257–363. [Google Scholar]
- 27.Mesbah L., Soraya B., Narimane S., Jean P.F. Protective effect of flavonides against the toxicity of vinblastine cyclophosphamide and paracetamol by inhibition of lipid-peroxydation and increase of liver glutathione. Haematology. 2004;7:59–67. [Google Scholar]
- 28.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. doi: 10.1016/S0021-9258(19)45228-9. [DOI] [PubMed] [Google Scholar]
- 29.Aebi H. Methods in Enzymology. 3rd ed. Volume 105. Lippincott-Raven Publishers; Philadelphia, PA, USA: 1984. Catalase in Vitro; pp. 121–126. [DOI] [PubMed] [Google Scholar]
- 30.Hafeman D.G., Sunde R.A., Hoekstra W.G. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J. Nutr. 1974;104:580–587. doi: 10.1093/jn/104.5.580. [DOI] [PubMed] [Google Scholar]
- 31.El-Demerdash F.M., El-Magd M.A., El-Sayed R.A. Panax ginseng modulates oxidative stress, DNA damage, apoptosis, and inflammations induced by silicon dioxide nanoparticles in rats. Environ. Toxicol. 2001;36:362–1374. doi: 10.1002/tox.23132. [DOI] [PubMed] [Google Scholar]
- 32.Chang C.L., Sung P.H., Chen K.H., Shao P.L., Yang C.C., Cheng B.C., Lin K.C., Chen C.H., Chai H.T., Chang H.W., et al. Adipose-derived mesenchymal stem cell-derived exosomes alleviate overwhelming systemic inflammatory reaction and organ damage and improve outcome in rat sepsis syndrome. Am. J. Transl. Res. 2018;10:1053–1070. [PMC free article] [PubMed] [Google Scholar]
- 33.Zahran R., Ghozy A., Elkholy S.S., El-Taweel F., El-Magd M.A. Combination therapy with melatonin, stem cells and extracellular vesicles is effective in limiting renal ischemia–reperfusion injury in a rat model. Int. J. Urol. 2020;27:1039–1049. doi: 10.1111/iju.14345. [DOI] [PubMed] [Google Scholar]
- 34.Ciarcia R., Damiano S., Florio A., Spagnuolo M., Zacchia E., Squillacioti C., Mirabella N., Florio S., Pagnini U., Garofano T., et al. The protective effect of apocynin on cyclosporine a-induced hypertension and nephrotoxicity in rats. J. Cell. Biochem. 2015;116:1848–1856. doi: 10.1002/jcb.25140. [DOI] [PubMed] [Google Scholar]
- 35.Ouyang Z., Cao W., Zhu S., Liu X., Zhong Z., Lai X., Deng H., Jiang S., Wang Y. Protective effects of 2-deoxy-d-glucose on nephrotoxicity induced by cyclosporine a in rats. Int. J. Clin. Exp. Pathol. 2014;7:4587–4595. [PMC free article] [PubMed] [Google Scholar]
- 36.Bellwon P., Culot M., Wilmes A., Schmidt T., Zurich M.G., Schultz L., Schmal O., Gramowski-Voss A., Weiss D.G., Jennings P., et al. Cyclosporine a kinetics in brain cell cultures and its potential of crossing the blood-brain barrier. Toxicol. Vitr. 2015;30:166–175. doi: 10.1016/j.tiv.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Haleagrahara N., Yee T., Chakravarthi S., Lee N. Protective effect of n-acetylcysteine on cyclosporine a-induced changes in lipid hydroperoxide levels and renal dysfunction in rats. Arch. Med. Sci. 2009;5:16–22. [Google Scholar]
- 38.Helmy M.M., Helmy M.W., El-Mas M.M. Upregulation of cystathionine-γ-lyase/hydrogen sulfide pathway underlies the celecoxib counteraction of cyclosporine-induced hypertension and renal insult in rats. Prostaglandins Other Lipid Mediat. 2019;141:1–10. doi: 10.1016/j.prostaglandins.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Kim J.S., Yang J.W., Han B.G., Kwon H.J., Kim J.H., Choi S.O. Protective role of apelin against cyclosporine-induced renal tubular injury in rats. Transplant. Proc. 2017;49:1499–1509. doi: 10.1016/j.transproceed.2017.03.080. [DOI] [PubMed] [Google Scholar]
- 40.Adekunle I.A., Imafidon C.E., Oladele A.A., Ayoka A.O. Ginger polyphenols attenuate cyclosporine-induced disturbances in kidney function: Potential application in adjuvant transplant therapy. Pathophysiology. 2018;25:101–115. doi: 10.1016/j.pathophys.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Deppe C.E., Heering P.J., Tinel H., Kinne-Saffran E., Grabensee B., Kinne R.K. Effect of cyclosporine a on na+/k(+)-atpase, na+/k+/2cl- cotransporter, and h+/k(+)-atpase in mdck cells and two subtypes, c7 and c11. Exp. Nephrol. 1997;5:471–480. [PubMed] [Google Scholar]
- 42.Yoon H.E., Yang C.W. Established and newly proposed mechanisms of chronic cyclosporine nephropathy. Korean J. Intern. Med. 2009;24:81–92. doi: 10.3904/kjim.2009.24.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee J. Use of antioxidants to prevent cyclosporine a toxicity. Toxicol. Res. 2010;26:163–170. doi: 10.5487/TR.2010.26.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Capasso G., Di Gennaro C.I., Della Ragione F., Manna C., Ciarcia R., Florio S., Perna A., Pollastro R.M., Damiano S., Mazzoni O., et al. In vivo effect of the natural antioxidant hydroxytyrosol on cyclosporine nephrotoxicity in rats. Nephrol. Dial. Transplant. 2008;23:1186–1195. doi: 10.1093/ndt/gfm784. [DOI] [PubMed] [Google Scholar]
- 45.Abdelhady D.H., El-Magd M.A., Elbialy Z.I., Saleh A.A. Bromuconazole-induced hepatotoxicity is accompanied by upregulation of pxr/cyp3a1 and downregulation of car/cyp2b1 gene expression. Toxicol. Mech. Methods. 2017;27:544–550. doi: 10.1080/15376516.2017.1333555. [DOI] [PubMed] [Google Scholar]
- 46.Abu Gazia M., El-Magd M.A. Effect of pristine and functionalized multiwalled carbon nanotubes on rat renal cortex. Acta Histochem. 2018;121:207–217. doi: 10.1016/j.acthis.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 47.El-Magd M.A., Kahilo K.A., Nasr N.E., Kamal T., Shukry M., Saleh A.A. A potential mechanism associated with lead-induced testicular toxicity in rats. Andrologia. 2016;49:e12750. doi: 10.1111/and.12750. [DOI] [PubMed] [Google Scholar]
- 48.Singh A., Ramnath R.D., Foster R.R., Wylie E.C., Friden V., Dasgupta I., Haraldsson B., Welsh G.I., Mathieson P.W., Satchell S.C. Reactive oxygen species modulate the barrier function of the human glomerular endothelial glycocalyx. PLoS ONE. 2013;8:e55852. doi: 10.1371/journal.pone.0055852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao Y., Roursgaard M., Danielsen P.H., Moller P., Loft S. Carbon black nanoparticles promote endothelial activation and lipid accumulation in macrophages independently of intracellular ros production. PLoS ONE. 2014;9:e106711. doi: 10.1371/journal.pone.0106711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rao U.S.M., Ponnusamy K., Naidu J., Sundaram S. Modulatory influence of avocado on renal oxido -lipidemic stress and mrna expression of nos in renal artery studied in nephropathy induced rats. Int. Med. J. 2014;21:1–7. [Google Scholar]
- 51.Abdel Moneim A., Ahmed O., Fahim H., Mohamed E. The preventive effects of avocado fruit and seed extracts on cardio-nephrotoxicity induced by diethylnitrosamine/2-acetylaminoflurine in wistar rats. Basic Sci. Med. 2017;6:4–13. [Google Scholar]
- 52.Richard D., Kefi K., Barbe U., Bausero P., Visioli F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008;57:451–455. doi: 10.1016/j.phrs.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 53.Rosero J.C., Cruz S., Osorio C., Hurtado N. Analysis of phenolic composition of byproducts (seeds and peels) of avocado (Persea americana mill.) cultivated in colombia. Molecules. 2019;24:3209. doi: 10.3390/molecules24173209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anshar A., Bahar M.A., Ikliptikawati D. The effect of avocado to the profile of blood urea nitrogen (bun) and creatinine in rats (rattus norvegicus) induced with meloxicam. J. Ris. Vet. Indones. (J. Indones. Vet. Res.) 2018;2:1–7. doi: 10.20956/jrvi.v2i1.3802. [DOI] [Google Scholar]
- 55.Lara-Márquez M., Báez-Magaña M., Raymundo-Ramos C., Spagnuolo P.A., Macías-Rodríguez L., Salgado-Garciglia R., Ochoa-Zarzosa A., López-Meza J.E. Lipid-rich extract from mexican avocado (Persea americana var. Drymifolia) induces apoptosis and modulates the inflammatory response in caco-2 human colon cancer cells. J. Funct. Foods. 2020;64:103658. doi: 10.1016/j.jff.2019.103658. [DOI] [Google Scholar]
- 56.Tu H.P., Chen Y.T., Chiu H.C., Chin Y.T., Huang S.M., Cheng L.C., Fu E., Chiang C.Y. Cyclosporine a enhances apoptosis in gingival keratinocytes of rats and in oecm1 cells via the mitochondrial pathway. J. Periodontal Res. 2009;44:767–775. doi: 10.1111/j.1600-0765.2008.01189.x. [DOI] [PubMed] [Google Scholar]
- 57.Øzbay L.A., Smidt K., Mortensen D.M., Carstens J., Jørgensen K.A., Rungby J. Cyclosporin and tacrolimus impair insulin secretion and transcriptional regulation in ins-1e beta-cells. Br. J. Pharmacol. 2011;162:136–146. doi: 10.1111/j.1476-5381.2010.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rincón J., Parra G., Quiroz Y., Benatuil L., Rodríguez-Iturbe B. Cyclosporin a reduces expression of adhesion molecules in the kidney of rats with chronic serum sickness. Clin. Exp. Immunol. 2000;121:391–398. doi: 10.1046/j.1365-2249.2000.01251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Daull P., Barabino S., Feraille L., Kessal K., Docquier M., Parsadaniantz S.M., Baudouin C., Garrigue J.S. Modulation of inflammation-related genes in the cornea of a mouse model of dry eye upon treatment with cyclosporine eye drops. Curr. Eye Res. 2019;44:476–485. doi: 10.1080/02713683.2018.1563197. [DOI] [PubMed] [Google Scholar]
- 60.Satonaka H., Suzuki E., Nishimatsu H., Oba S., Takeda R., Goto A., Omata M., Fujita T., Nagai R., Hirata Y. Calcineurin promotes the expression of monocyte chemoattractant protein-1 in vascular myocytes and mediates vascular inflammation. Circ. Res. 2004;94:693–700. doi: 10.1161/01.RES.0000118250.67032.5E. [DOI] [PubMed] [Google Scholar]
- 61.Pflugfelder S.C., Stern M., Zhang S., Shojaei A. Lfa-1/icam-1 interaction as a therapeutic target in dry eye disease. J. Ocul. Pharmacol. Ther. 2017;33:5–12. doi: 10.1089/jop.2016.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang W., Smith C., Monette R., Hutchison J., Stanimirovic D.B. Indomethacin and cyclosporin a inhibit in vitro ischemia-induced expression of icam-1 and chemokines in human brain endothelial cells. Acta Neurochirurgica Suppl. 2000;76:47–53. doi: 10.1007/978-3-7091-6346-7_10. [DOI] [PubMed] [Google Scholar]
- 63.Dabas D., Elias R.J., Ziegler G.R., Lambert J.D. In vitro antioxidant and cancer inhibitory activity of a colored avocado seed extract. Int. J. Food Sci. 2019;2019:6509421. doi: 10.1155/2019/6509421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the present findings are contained within the manuscript.