Abstract
To investigate the contribution that ERK/mitogen-activated protein kinase signalling makes to cell cycle progression and gene expression, we have constructed cell lines to express an inducible version of activated MEK1. Using these cells, we show that activation of MEK leads to the expression of Fra-1 and Fra-2 but not c-Fos. Treatment of Ras-transformed cells with the MEK inhibitor PD098059 blocks expression of Fra-1 and Fra-2, showing that in Ras transformation ERK signalling is responsible for Fra-1 and Fra-2 expression. Activation of MEK1 in growth-arrested cells leads to DNA synthesis; however, ERK activation alone is insufficient because the induction of DNA synthesis is blocked by inhibition of phosphatidylinositol 3-kinase (PI3-kinase). Activation of PI3-kinase is indirect, perhaps through autocrine growth factors, and is required for the induction of cyclin D1.
The ERK/mitogen-activated protein kinase (MAP kinase) pathway has been identified as a major signalling pathway activated through p21Ras (12). This signalling pathway has been shown to be required for growth factors to stimulate cell proliferation via Ras (10, 15, 47). However much evidence has accumulated demonstrating that activation of ERKs is only one of the effector pathways of Ras signalling. At least 10 proteins have been identified which are candidates for effectors of Ras signalling because they interact with Ras only in the active GTP-bound form. These candidate effector proteins include Ras GTPase-activating proteins (1, 38), Raf family protein kinases (60, 64, 70), phosphatidylinositol 3-kinases (PI3-kinases) (51), guanine nucleotide exchange factors for Ral family GTPases (24, 29, 37, 59, 67), protein kinase C-zeta (14), MEKK-1 (53), and proteins of unknown function (61). The use of effector mutants of Ras which selectively interrupt interactions with some of these proteins has strongly suggested roles for Raf-1 (65), PI3-kinase (52), and the Ral GTPase activator Ral-GDS (66) in cell transformation. Similarly, effector mutants have been used to show that activation of multiple signalling pathways by Ras is required to stimulate DNA synthesis (28, 65). In contrast to these studies with Ras effector mutants, the observation that activated forms of Raf and MEK act as oncoproteins suggests that activation of the ERK pathway may be sufficient to stimulate DNA synthesis (10, 35).
Signalling through the ERK/MAP kinase pathway is thought to depend at least in part on the activation of gene expression. ERK/MAP kinases have been shown to phosphorylate transcription factors of the ELK/SAP family as well as the Ets family and STAT family (23). Phosphorylation of Elk-1 by ERK/MAP kinases has been shown to be required for transcriptional activation of the immediate-early gene product c-Fos (36). Ras-transformed cells have been shown to have elevated levels of Fra-1, Fra-2, c-Jun, and JunB, which contribute to elevated AP-1 activity (41). However, c-Fos expression is not elevated in Ras-transformed cells even though ERK/MAP kinase activity is raised (41). Blocking of AP-1 activity in Ras-transformed cells has been shown to suppress transformation (34). The signalling pathways that result in elevated Fra-1, Fra-2, c-Jun, and JunB expression have not been elucidated, so the mechanism by which these Fos and Jun family members are upregulated in Ras-transformed cells is not clear.
To address these issues, we have constructed inducible forms of activated MEK1 to see whether induction of MEK activity is sufficient to permit entry into DNA synthesis and induction of Fra-1, Fra-2, c-Jun, and JunB. Ras-mediated activation of Raf activates MEK (13, 25, 31), which is the immediate upstream activator of ERK1 and ERK2 (45). To date, no substrates of MEK other than ERKs have been identified. Inducible versions of activated signalling components have an advantage over constitutive expression in that long-term effects of expression of an activated signalling molecule can be avoided and the kinetics of responses can be monitored. Using cells with an inducible activated MEK, we show that MEK activation induces DNA synthesis in quiescent NIH 3T3 cells. However, induction of DNA synthesis in this system also requires participation of the PI3-kinase pathway. Activation of MEK appears to act as a switch which not only directly activates the ERK intracellular signalling pathway but also may indirectly activate other signalling pathways.
MATERIALS AND METHODS
Construction of the MEK/ΔNEE-ER fusion gene expression vector.
Rabbit MEK1 cDNA, which contains the mutations that lead to replacement of serine by glutamic acid at positions 218 and 222 (2), was digested with StuI and subsequently religated to generate the activating deletion in the amino terminus (amino acids 32 to 51) (35). The construct was then linearized with BamHI and partially digested with EagI. The BamHI-EagI MEK1 cDNA fragment and an EagI-SalI fragment encoding the mutant hormone binding domain of the mouse estrogen receptor (ER) were ligated into BamHI- and SalI-digested pBABEpuro (43).
Cell culture and transfection.
NIH 3T3 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% donor calf serum (DCS) (Gibco-Life Sciences), and v-Ras-transformed NIH 3T3 cells were grown in DMEM containing 5% DCS. For transfection with Lipofectamine (Gibco-Life Sciences), 1.5 × 105 cells were plated into 30-mm-diameter tissue culture dishes. On the following day the cells were washed with serum-free medium, and DNA-Lipofectamine complexes (0.4 μg of DNA and 5 μl of Lipofectamine) were added to the cells in 1 ml of serum-free medium. The cells were incubated for 6 h in the presence of the DNA-Lipofectamine complexes, washed, and incubated in 10% DCS–DMEM. After 24 h, the cells were trypsinized and plated at low densities in the presence of 2.5 μg of puromycin (Sigma) per ml. Cell clones were picked with sterile cotton buds and characterized for MEK/ΔNEE-ER expression by Western blot analysis and immunofluorescence.
4-Hydroxytamoxifen (4-OHT) (Research Biochemicals International) was made up as a 1 mM stock solution in ethanol and stored at −20°C. PD098059 was provided by A. Saltiel (Parke-Davis), made up as a 1 mM stock solution in dimethyl sulfoxide, and stored at −20°C. LY294002 was purchased from Biomol Research Laboratories, made up as a 50 mM stock solution in ethanol, and stored at −20°C.
Antibodies.
ERK2 polyclonal rabbit antiserum no. 122 was generated against a C-terminal ERK2 peptide (32), MEK1 polyclonal rabbit antiserum no. 179 was raised against recombinant glutathione S-transferase–MEK1 (2). Mouse monoclonal antibodies against MEK1 and p27Kip1 were from Transduction Laboratories. Anti-RSK1 was a gift from P. Cohen, Dundee, United Kingdom. Anti-cFos, anti-FosB, anti-Fra-1, anti-Fra-2, anti-cJun, and anti-JunB were purchased from Santa Cruz Biotechnology. Phospho-specific anti-protein kinase B (PKB)/Akt rabbit polyclonal antiserum (against serine 473) was kindly provided by J. Downward, Imperial Cancer Research Fund, London, United Kingdom. Anti-cyclin D1 antibody was from G. Peters, ICRF. Anti-CDK2 and anti-CDK4 were from Santa Cruz Biotechnology or from C. Sherr, Memphis, Tenn.
Preparation of cell extracts and analysis by Western blotting.
Cells were washed twice in ice-cold phosphate-buffered-saline (PBS) and lysed in 20 mM Tris (pH 8.0)–40 mM sodium pyrophosphate–50 mM sodium fluoride–5 mM MgCl2–100 mM sodium vanadate–10 mM EGTA–1% Triton X-100–0.5% sodium deoxycholic acid–20 mg of leupeptin per ml–20 mg of aprotinin per ml–1 mM phenylmethylsulfonyl fluoride. Cell debris was removed by centrifugation at 12,000 rpm in an Eppendorf microcentrifuge and protein concentrations were determined by the Bradford protein assay (Bio-Rad). Cell extracts (50 μg) were electrophoresed through sodium dodecyl sulfate (SDS)-polyacrylamide gels and Western blotted onto nitrocellulose. Western blots were probed with the appropriate dilutions of primary antibodies for at least 1 h at room temperature.
DNA synthesis assay.
DNA synthesis was determined by uptake and incorporation of bromodeoxyuridine (BrdU) (Amersham Life Sciences) from the culture medium. Cells were made quiescent either by incubation for 48 h in serum-free DMEM or by contact inhibition in the presence of 5% DCS. After 20 to 24 h of exposure to 10 μM BrdU, cells were fixed and permeabilized for immunofluorescence as described below. BrdU incorporation into DNA was detected by incubating the fixed cells with a mouse monoclonal antibody against BrdU (Boehringer Mannheim) at 5 μg/ml in the presence of 1 mg of DNase I per ml for 1 h at 37°C.
Immunofluorescence.
Cells were fixed in 4% formaldehyde in PBS for 15 min, washed in several changes of PBS for 30 min, permeabilized in 0.2% Triton X-100 in PBS for 15 min, and blocked by incubation in 10% fetal bovine serum in PBS for 30 min. Primary antibody incubations were performed for 1 h at room temperature; after a 15-min wash in PBS, the cells were incubated with appropriate fluorescent second antibodies (Jackson Immunoresearch Laboratories) for 1 h at room temperature. Stained preparations were mounted under glass coverslips and examined with a Bio-Rad MRC 1000 or 1024 confocal imaging system in conjunction with a Nikon Diaphot epifluorescence microscope.
ERK2 assay.
Fifty micrograms of cell lysate was diluted to a volume of 200 μl with lysis buffer and immunoprecipitated for 90 min with 5 μl of ERK2 antiserum (no. 122) applied to 20 μl of protein A-agarose (Bio-Rad) on a revolving wheel at 4°C. The reaction mixture was washed twice with lysis buffer, once with 30 mM Tris (pH 8.0), and once with kinase buffer (30 mM Tris [pH 8.0], 20 mM MgCl2, 2 mM MnCl). The washed immunoprecipitates were drained of excess liquid and incubated with 30 μl of kinase buffer containing 10 μM ATP (Sigma), 0.25 mg of myelin basic protein (Sigma), and 66 μCi of [γ-32P]ATP (Amersham) per ml at 30°C for 30 min. The reaction was stopped by the addition of SDS sample buffer (80 mM Tris [pH 6.8], 2% SDS, 10% glycerol, 100 μM dithiothreitol, 0.02% bromophenol blue), boiled for 5 min, and analyzed on an SDS–15% polyacrylamide gel. The gel was blotted onto nitrocellulose which was subsequently autoradiographed and analyzed on a Molecular Dynamics PhosphorImager, using ImageQuant software.
RSK assay.
Two hundred micrograms of cell lysate was immunoprecipitated for 90 min with 2.7 μg of RSK1 antiserum applied to 20 μl of protein G-Sepharose (Sigma) on a revolving wheel at 4°C. The reaction mixture was washed twice with lysis buffer, once with 50 mM MOPS (morpholinepropanesulfonic acid) (pH 7.0), and once with kinase buffer (50 mM MOPS [pH 7.0], 10 mM MgCl2, 0.1 mM EGTA). The washed immunoprecipitates were drained of excess liquid and incubated with 50 μl of kinase buffer containing 50 μM ATP (Sigma), 30 μM peptide KKKNRTLSVA, and 50 μCi of [γ-32P]ATP (Amersham) per ml at 30°C for 15 min. The reactions were terminated by spotting 40 μl of the reaction mixture onto P81 paper (Whatman), which was subsequently washed five times with 75 mM orthophosphoric acid. RSK1 activity was quantified by counting the radioactivity on the P81 paper in a Cerenkov counter.
Assays for CDK activity.
Serum-starved cells were lysed in 100 mM HEPES (pH 7.0)–500 mM NaCl–10 mM EDTA–20 mM β-glycerophosphate–20 mM sodium fluoride–2 mM sodium vanadate–0.5 mM dithiothreitol–0.2% Triton X-100–20 μg of aprotinin per ml–20 μg of leupeptin per ml–1 mM phenylmethylsulfonyl fluoride. Lysates were centrifuged at 12,000 rpm to remove insoluble material, and protein concentrations were determined with the Bradford assay (Bio-Rad). Five hundred micrograms of cleared extract was immunoprecipitated with antibodies coupled to protein G-Sepharose (Sigma) for 90 min at 4°C. Immune complexes were washed three times with lysis buffer, once with 50 mM HEPES (pH 7.4), and then once with kinase buffer (50 mM HEPES, 10 mM MgCl2, 10 mM MnCl2, 10 mM β-glycerophosphate, 1 mM dithiothreitol, 20 μg of aprotinin per ml, 20 μg of leupeptin per ml, and 1 mM phenylmethylsulfonyl fluoride). CDK4-associated kinase activity was measured by using as a substrate 0.5 μg of a glutathione S-transferase fusion protein containing amino acids 763 to 928 of human pRb (a generous gift of S. Mittnacht) in 20 μl of kinase buffer with 50 μM ATP and 5 μCi of [γ-32P]ATP. For CDK2 assays the substrate was 2 μg of histone H1 (Boehringer Mannheim). After 15 min of incubation at 30°C, reactions were stopped by addition of SDS sample buffer, and the mixtures were boiled and resolved by SDS-polyacrylamide gel electrophoresis. After electrophoresis, proteins were transferred to polyvinylidene difluoride membranes (Immobilon; Millipore), and radioactivity was detected with a PhosphorImager (Molecular Dynamics). Radioactivity was quantitated by the ImageQuant program.
RESULTS
Morphological transformation induced by regulatable MEK1.
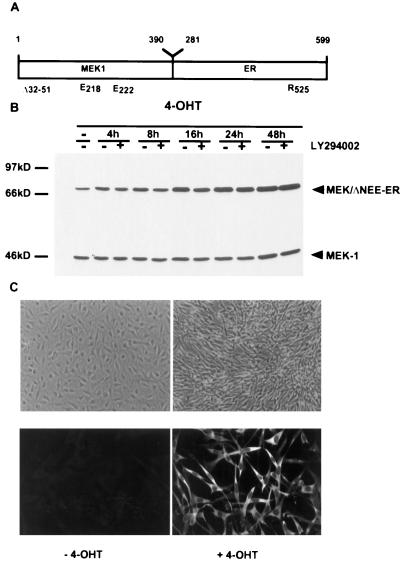
In order to analyze the role of MEK1 in cell transformation and cell cycle regulation, we established a conditionally activated MEK1 by fusion to the hormone binding domain of a mutant mouse ER which responds to 4-OHT but not to estradiol (33). A variety of constructs were generated, in which different activating mutations were combined with the ER either amino terminal or carboxy terminal to MEK1. Placement of the hormone binding domain of the ER at the C terminus of an activated MEK1 generated by mutation of the amino acids serine 218 and 222 to glutamic acid in the catalytic domain, which mimics the activating phosphorylations (2, 10), and deletion of a region in the amino terminus (amino acids 32 to 51) which has been described to enhance further the kinase activity of the mutant MEK (16, 35) created the most effective version (Fig. 1A). Deletion of amino acids 32 to 51 also removes the nuclear export signal of MEK1 (17); however, the MEK/ΔNEE-ER fusion protein was present only in the cytoplasm (Fig. 1C), presumably as a consequence of fusion to the ER.
FIG. 1.
4-OHT-dependent morphological transformation of NIH 3T3 cells expressing inducible activated MEK1 (MEK/ΔNEE-ER). (A) Activated MEK1 was generated by replacement of serines 218 and 222 with glutamic acid in the catalytic domain in combination with the activating deletion in the amino terminus (amino acids 32 to 51) and fused to the mutant hormone binding domain of the murine ER (amino acids 281 to 599). (B and C) NIH 3T3 cells were stably transfected with the MEK/ΔNEE-ER fusion gene construct, and individual cell clones were selected. The expression of the MEK/ΔNEE-ER fusion protein was analyzed by Western blotting (B) and immunofluorescence (C [bottom panels] with a monoclonal antibody against MEK1. In the absence of 4-OHT, the cells are flat and untransformed; induction with 4-OHT leads to morphological transformation (C [top panels]).
We established NIH 3T3 cell clones stably expressing this conditionally active form of MEK1 (NIH:MEK/ΔNEE-ER cells). Western blotting analysis and immunofluorescence staining showed that there was a significant increase in the level of the fusion protein detectable after 4-OHT induction over time (Fig. 1B and C). Similar observations showing an increase in the levels of an ER fusion protein with time were described by Samuels et al. with a Raf-ER fusion protein (55).
Individual NIH:MEK/ΔNEE-ER cell clones were analyzed for their morphology. In the absence of 4-OHT, the cells are flat and untransformed, but the induction of the MEK1-ER fusion protein by addition of 4-OHT results in a refractile phenotype (Fig. 1C) and disruption of actin stress fibers (data not shown). The first morphological changes are detectable about 16 h after addition of 4-OHT; after 24 to 30 h, the transformed phenotype is complete. MEK1-induced transformation occurs in the presence and absence of serum with similar kinetics. The morphological transformation induced by MEK/ΔNEE-ER was blocked with the MEK-specific inhibitor PD098059 (15) in a concentration-dependent manner. Treatment of the cells with 30 μM PD098059 completely prevented MEK-induced transformation (data not shown).
Activated MEK1 induces low levels of ERK and RSK activities.
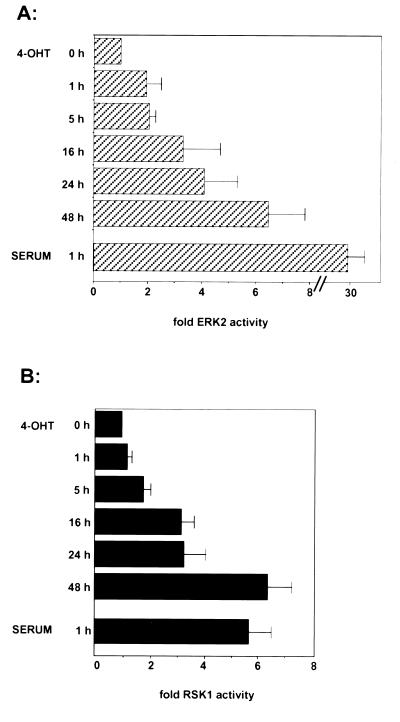
In order to characterize the downstream events after induction of activated MEK, we analyzed the activity of the MEK substrate ERK2 after stimulation of MEK/ΔNEE-ER with 4-OHT (Fig. 2A). Between 1 and 5 h after 4-OHT addition, endogenous ERK2 activity was induced twofold; at 16 to 24 h after induction, ERK2 activity was increased to three- to fourfold. After 48 h, the level of ERK2 activity was further increased to approximately sixfold. In comparison, the induction of ERK2 activity after stimulation with 10% serum for 1 h was about 30-fold. Thus, activation of inducible MEK1 with 4-OHT leads to a sustained but very weak activation of ERK2. Similar results were obtained for ERK1 activation (data not shown).
FIG. 2.
Activation of ERK2 and RSK1 by inducible MEK. After serum deprivation, cells were stimulated with 1 μM 4-OHT or 10% serum for the indicated times. (A) Endogenous ERK2 was immunoprecipitated from equal amounts of protein and assayed for myelin basic protein kinase activity. (B) In a parallel assay, the lysates were immunoprecipitated with RSK1 antibody, and the immunoprecipitates were assayed for their kinase activity by using an RSK-specific peptide as the substrate. The averages from three independent experiments are shown; error bars represent standard deviations.
Because the induction of endogenous ERK activity by activated MEK1 was so low, we wished to determine whether it led to activation of downstream signalling events. Figure 2B shows that the ERK activity is sufficient to lead to the subsequent activation of the ERK substrate RSK1. Between 1 and 5 h after addition of 4-OHT, the kinase activity of endogenous RSK1 was stimulated about twofold. At 16 to 24 h after 4-OHT induction, RSK activity was induced approximately threefold, and it was further increased up to sixfold after 48 h. The level of endogenous RSK kinase activity after induction of the MEK/ΔNEE-ER fusion protein was low compared to that after induction with 10% serum for 1 h (sixfold). However, these results show that the low level of ERK2 activity after induction of the MEK/ΔNEE-ER fusion protein with 4-OHT is sufficient to activate the downstream target RSK1. Analysis of several other cell clones expressing the MEK/ΔNEE-ER fusion protein gave similar results (data not shown).
Inducible activated MEK1 stimulates DNA synthesis.
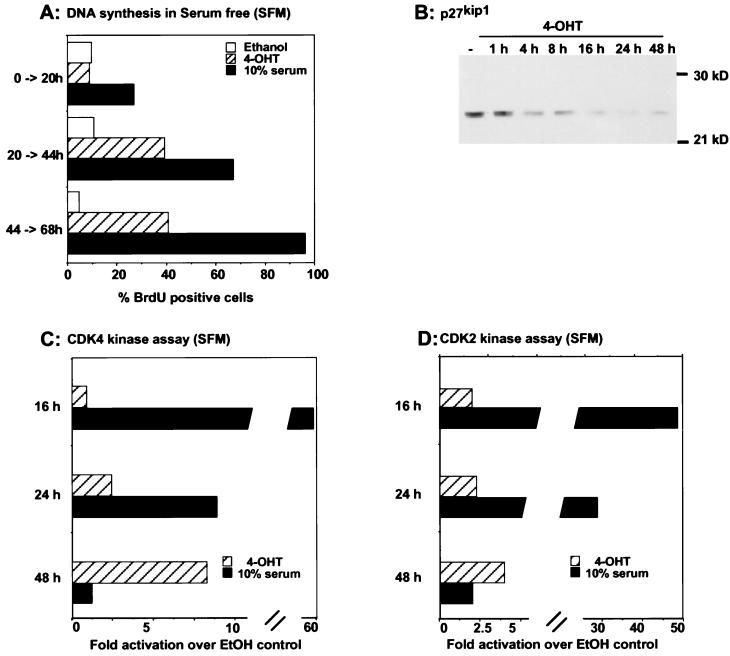
Given that induction of MEK activates endogenous ERK only two- to sixfold, it was of interest to determine if this was sufficient to induce DNA synthesis. When passaged in the absence of 4-OHT, MEK/ΔNEE-ER-expressing cells, like wild-type NIH 3T3 cells, enter proliferation arrest when they are grown to confluency in 5% serum or incubated in serum-free medium. In order to analyze whether the induction of activated MEK1 leads to cell cycle progression, quiescent MEK/ΔNEE-ER cells were induced with 1 μM 4-OHT. DNA synthesis was measured by the incorporation of BrdU after different labelling periods following 4-OHT induction (Fig. 3A). Activation of MEK/ΔNEE-ER with 4-OHT was mitogenic, leading to approximately 40% of the cells incorporating BrdU over the time course of stimulation under serum-free conditions. When cells were arrested by contact inhibition in the presence of serum, activation of MEK/ΔNEE-ER cells led to 60% of the cells beginning DNA synthesis, which is comparable to serum stimulation of uninduced cells (data not shown). In the presence of the solvent control, ethanol, approximately 5 to 15% of the NIH:MEK/ΔNEE-ER cells incorporated BrdU. Consistent with the start of DNA synthesis, Fig. 3B shows that induction of MEK/ΔNEE-ER led to a decrease in the level of the cyclin-dependent kinase inhibitor p27Kip1, degradation of which is associated with reentry into the cell cycle (48).
FIG. 3.
Activated MEK induces DNA synthesis. NIH:MEK/ΔNEE-ER cells were grown to confluency and then serum starved (SFM) for 48 h. Cells were treated with 1 μM 4-OHT, 10% serum, or ethanol (EtOH) and incubated with BrdU for three different labelling periods as indicated. (A) DNA synthesis was assessed by the incorporation of BrdU. Three separate experiments gave similar results. (B to D) In parallel experiments cell lysates were prepared at the time points indicated. After standardization for protein amounts, lysates were analyzed by Western blotting for expression of p27Kip1 (B) or assayed for CDK4 (C) and CDK2 (D) kinase activity.
Our finding that DNA synthesis is induced following induction of MEK is in contrast to the conclusions of a recent study that showed, for a different inducible MEK system, that activation of MEK is insufficient to stimulate DNA synthesis in quiescent cells (7). However, Cheng et al. (7) analyzed the effects of MEK activity only up to 20 h after induction. In our system DNA synthesis occurred only after 20 h of induction of MEK activity (Fig. 3A). Supporting the observation that stimulation of DNA synthesis by the inducible MEK/ΔNEE-ER fusion protein was a delayed event, increases in CDK4 and CDK2 kinase activities were apparent only at later time points, whereas serum treatment of uninduced cells activated CDK4 and CDK2 within 16 h (Fig. 3C and D). While the level of CDK4 and CDK2 activity following activation of inducible MEK was much less than that produced by serum stimulation, it was of a magnitude similar to that associated with cell cycle reentry resulting from activation of an inducible B-Raf–ER construct (68).
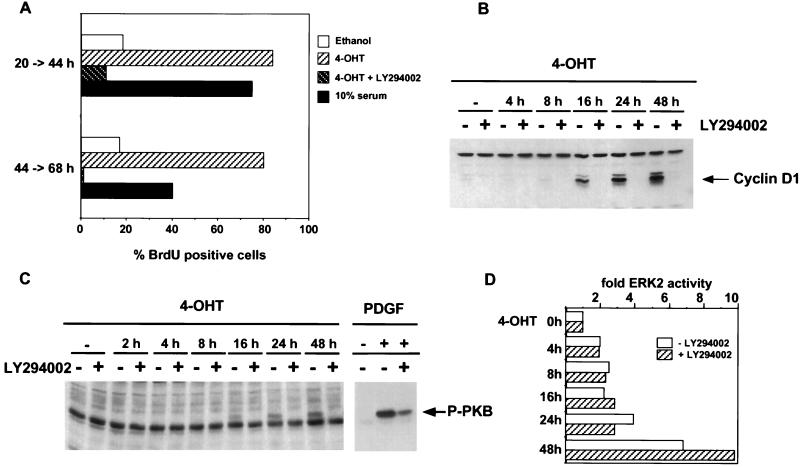
Previous work by McCarthy et al. has shown that induction of Raf activity can lead to the indirect stimulation of other signalling pathways through the induction of autocrine growth factors (40); therefore, it was possible that activation of MEK in our system was leading to the activation of other signalling pathways. Since stimulation of DNA synthesis by some growth factors has been shown to be blocked by inhibition of PI3-kinase (50), we investigated whether induction of DNA synthesis by inducible MEK was blocked by treatment with the PI3-kinase inhibitor LY294002 (63). Figure 4A shows that treatment with LY294002 blocked induction of DNA synthesis, demonstrating that signalling pathways in addition to ERK activation are required for activated MEK to induce DNA synthesis. Figure 4B shows that PI3-kinase activity was required for the earliest step in cell cycle progression: the induction of cyclin D1 expression following activation of MEK. To investigate the kinetics of induction of PI3-kinase, we monitored the phosphorylation of PKB, a downstream signalling event involving PI3-kinase (11). While platelet-derived growth factor (PDGF) stimulation of PKB in a PI3-kinase-dependent manner occurs within 5 min of treatment, phosphorylation of PKB following induction of MEK becomes apparent only at 16 h following induction. This phosphorylation of PKB was dependent on PI3-kinase, as shown by its sensitivity to LY294002 (Fig. 4C), and parallels the induction of cyclin D1 expression. The effect of LY294002 on cyclin D1 expression and DNA synthesis was not due to downregulation of expression of MEK/ΔNEE-ER (Fig. 1B) or suppression of ERK2 activation by the inducible MEK (Fig. 4D). The slow kinetics of PKB phosphorylation following the 4-OHT induction of MEK suggest that this is indirect. Evidence for indirect activation of PI3-kinase via autocrine production following ERK activation is provided by the observation that MEK/ΔNEE-ER-induced PKB phosphorylation is partially blocked by treatment with suramin (4), an inhibitor of autocrine pathways (data not shown). Consistent with the delay before PI3-kinase activation, entry into DNA synthesis following activation of MEK was delayed compared to that for cells stimulated with serum.
FIG. 4.
MEK-induced DNA synthesis is PI3-kinase dependent. (A) NIH:MEK/ΔNEE-ER cells were arrested in serum and induced with 1 μM 4-OHT in the absence or presence of 10 μM LY294002, 10% serum, or ethanol. Cells were incubated with BrdU for two different labelling periods as indicated and DNA synthesis was assessed by the incorporation of BrdU. (B) Serum-deprived NIH:MEK/ΔNEE-ER cells were induced with 1 μM 4-OHT for different lengths of time in the presence or absence of 10 μM LY294002 as indicated. Equal amounts of protein extracts were separated by SDS-polyacrylamide gel electrophoresis, and the induction of cyclin D1 was determined by Western blotting. (C) After serum deprivation, NIH:MEK/ΔNEE-ER cells were induced with 1 μM 4-OHT for the indicated times or for 5 min with PDGF (50 ng/ml) in presence or absence of 10 μM LY294002. Activated PKB/Akt (P-PKB) was detected by Western blotting with a phospho-specific PKB/Akt antibody. The Western blot showing PDGF-induced PKB/Akt phosphorylation is a shorter exposure than that for PKB/Akt phosphorylation induced by activated MEK1. (D) Serum-starved NIH:MEK/ΔNEE-ER cells were induced as described for panel C, and endogenous ERK2 was immunoprecipitated from equal amounts of protein and assayed for MBP kinase activity.
Induction of AP-1 components by inducible MEK1.
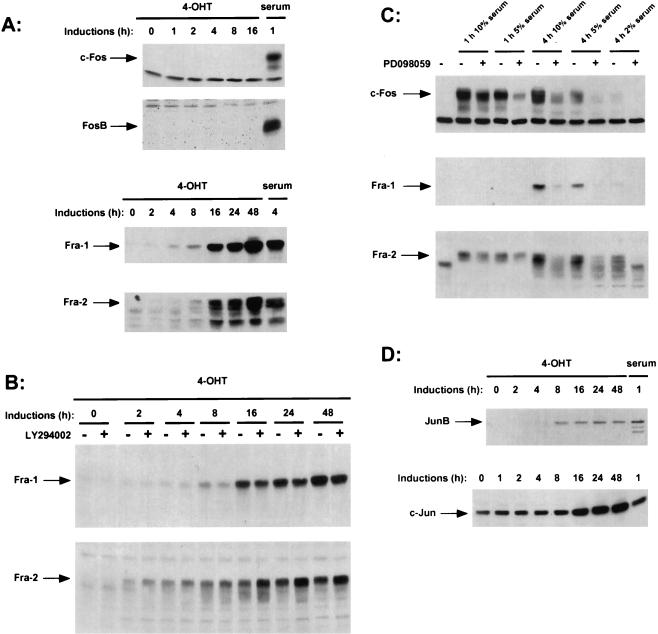
The transcription factor AP-1 has been described to be involved in cell proliferation. Dysregulation of the AP-1 component c-Fos or c-Jun leads to cell transformation, suggesting that AP-1 plays an important role in the process of transformation. The ERK/MAP kinase pathway has been described as being involved in the regulation of the expression of c-Fos (23). In order to analyze whether the activation of MEK1 is sufficient to induce members of the Fos and Jun families, we induced serum-starved NIH:MEK/ΔNEE-ER cells with 4-OHT for different lengths of time and analyzed the expression of endogenous AP-1 components by Western blotting (Fig. 5). The c-Fos gene product was undetectable at all time points we analyzed. However, using the same cell clone, we were able to detect c-Fos expression after induction with 10% serum for 1 h. Furthermore, induction of activated MEK1 did not suppress the ability of serum to induce c-Fos. We then examined the expression of the other Fos family members: FosB, Fra-1, and Fra-2. Previous studies demonstrated that serum stimulation leads to a distinct pattern of expression of the individual Fos proteins. c-Fos and FosB are expressed transiently with similar kinetics, whereas the expression of Fra-1 and Fra-2 is delayed but the proteins are expressed for a prolonged time period (9, 30, 46). Activation of MEK1 did not lead to the induction of FosB; however, the Fos-related gene products Fra-1 and Fra-2 were induced between 4 and 8 h after induction of the MEK/ΔNEE-ER fusion protein with 4-OHT, and their expression was sustained (Fig. 5A). Thus, activation of the ERK/MAP kinase pathway induces expression of the Fos-related gene products Fra-1 and Fra-2 but not that of c-Fos and FosB. The elevated expression of Fra-1 and Fra-2 following activation of the inducible MEK did not require PI3-kinase activity, because it was unaffected by treatment with LY294002 (Fig. 5B). These results demonstrated that activation of MEK1 and thereby ERK1 and ERK2 leads to expression of Fra-1 and Fra-2. Consistent with the lack of a requirement for PI3-kinase activity for Fra-1 and Fra-2 expression, elevated levels of Fra-1 and Fra-2 could be detected at 4 to 8 h, before PKB activation or cyclin D1 expression could be detected (16 h). To determine whether MEK activation is required for growth factor-mediated activation of Fra-1 and Fra-2, we used the MEK inhibitor PD098059. Serum-induced expression of Fra-1 and Fra-2 was blocked by treatment with PD098059 (Fig. 5C). We could also detect a drastic decrease in c-Fos expression when MEK1 was blocked with the inhibitor. We therefore conclude that the induction of Fra-1 and Fra-2 and also c-Fos by serum requires ERK activation but that c-Fos induction requires additional signals.
FIG. 5.
Induction of AP-1 components by inducible MEK. (A) After serum deprivation, NIH:MEK/ΔNEE-ER cells were induced with 1 μM 4-OHT or 10% serum for the indicated times. Equal amounts of protein extracts were separated by SDS-polyacrylamide gel electrophoresis, and the induction of Fos family members was analyzed by Western blotting with the appropriate antisera. (B) Fra-1 and Fra-2 expression was determined in NIH:MEK/ΔNEE-ER cell extracts from cells induced with 4-OHT for the indicated times in presence or absence of 10 μM LY294002. (C) Serum-deprived wild-type NIH 3T3 cells were induced with 10, 5, or 2% serum for the indicated times in the presence or absence of PD098059. Expression of c-Fos, Fra-1 and Fra-2 was analyzed by Western blotting. (D) Cell extracts were prepared as described for panel A and Western blotted for the expression of JunB and c-Jun.
The induction of MEK1 led to a significant mobility shift of Fra-2 (Fig. 5A), which has been shown to result from phosphorylation (19). When we blocked the ERK/MAP kinase pathway in serum-stimulated cells with the MEK inhibitor, we observed a drastic decrease of the slower-migrating forms of Fra-2 and also c-Fos (Fig. 5C). These results indicate that the phosphorylation of c-Fos and Fra-2 is at least partially mediated by the ERK/MAP kinase pathway.
All four Fos family members can heterodimerize with members of the Jun family to form the transcription factor AP-1. We examined the role of MEK1 in regulation of the induction of Jun family members. Like that of Fra-1 and Fra-2, expression of JunB became apparent between 4 and 8 h after induction of the MEK/ΔNEE-ER fusion protein with 4-OHT. Even in quiescent cells c-Jun was apparent, but after 16 h of 4-OHT treatment the level of c-Jun increased (Fig. 5D). Although this delayed elevation of c-Jun expression may reflect an indirect effect, unlike the expression of cyclin D1 it is not via PI3-kinase, as LY294002 had no effect (data not shown). By the specific activation of the ERK/MAP kinase pathway with an inducible MEK1 protein, we can show that activation of the MAP kinase pathway leads to the induction of the Fos family members Fra-1 and Fra-2 and the Jun family members c-Jun and JunB.
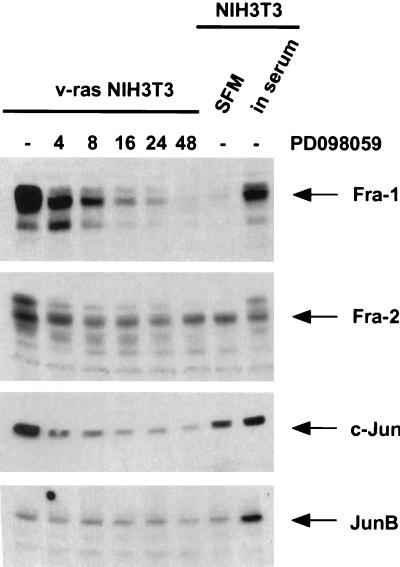
It has been shown that Ras-transformed cells contain elevated AP-1 activity but that only certain AP-1 components are upregulated by Ras transformation. Mechta et al. (41) have demonstrated that c-Jun, JunB, Fra-1, and Fra-2, but not c-Fos, are upregulated in Ras-transformed cells. Since we could show that the activation of MEK induces the expression of Fos and Jun family members, we analyzed whether the upregulation of these AP-1 components in Ras-transformed cells is also mediated by the ERK/MAP kinase pathway. Figure 6 shows that the expression of Fra-1, Fra-2, and c-Jun is elevated in v-Ras-transformed cells. Treatment of these cells with the MEK inhibitor PD098059 for different lengths of time led to the downregulation of these AP-1 components to levels comparable to those in serum-starved wild-type NIH 3T3 cells. JunB is not upregulated in these cells; therefore, treatment with the MEK inhibitor has no effect. At 24 h after addition of PD098059, the Ras- transformed cells were almost completely morphologically reverted (data not shown).
FIG. 6.
A MEK inhibitor blocks expression of AP-1 components in Ras-transformed NIH 3T3 cells. After serum deprivation, v-Ras-transformed NIH 3T3 cells were either not treated or treated with 30 μM PD098059 for the indicated times. The expression of AP-1 components was determined by Western blotting with the appropriate antisera. The expression levels of these AP-1 components in serum-deprived (SFM) or cycling (in serum) wild-type NIH 3T3 cells are shown for comparison.
DISCUSSION
We have established NIH 3T3 cell clones expressing a conditionally regulatable version of activated MEK1. Induction of the MEK/ΔNEE-ER fusion protein by 4-OHT leads to morphological transformation and stimulates cell cycle entry. These results show that activation of MEK1 results in cell cycle progression in quiescent NIH 3T3 cells. Interestingly, induction of MEK/ΔNEE-ER with 4-OHT activates ERK at only low levels (two- to sixfold) (Fig. 2), indicating that this low level of ERK activation is sufficient to permit cell cycle entry. This finding is consistent with the observation that cell cycle progression by a conditionally active form of Raf (ΔRaf-ER) is dependent on the level of Raf activity. High levels of Raf activity induce cell cycle arrest by the induction of the cyclin-dependent kinase inhibitor p21Waf1/Cip1 (57, 68).
While we show that activation of inducible MEK in quiescent cells results in DNA synthesis, direct MEK signalling is itself insufficient for cell cycle entry. Inhibition of PI3-kinase blocks DNA synthesis following induction of MEK activity, because PI3-kinase activity is required for expression of cyclin D1. This activation of PI3-kinase appears to be indirect, because phosphorylation of PKB as a measure of PI3-kinase signalling was detectable only at 16 h, whereas ERK activity was elevated at 1 to 2 h postactivation of inducible MEK. A possible explanation of this delayed activation of PI3-kinase is that it is a consequence of signalling through autocrine growth factors induced by ERK signalling. Previous work by McCarthy et al. has shown that inducible Raf indirectly activates Jnks as a consequence of Raf signalling leading to the expression of autocrine heparin-binding epidermal growth factor (40). Treatment of the inducible MEK/ΔNEE-ER-expressing cells with suramin, which blocks autocrine signalling (4), partially blocked MEK-induced PKB phosphorylation (data not shown), indicating that activation of PI3-kinase is at least in part indirect and mediated by autocrine growth factors.
In related studies using a different inducible MEK system, Cheng et al. (7) also concluded that MEK signalling alone was insufficient for DNA synthesis. However, in their system activation of MEK did not lead to DNA synthesis unless cyclin D1 and CDK4 were overexpressed to titrate out p27Kip1 (7). In contrast to the studies of Cheng et al. (7), we detect downregulation of p27Kip1 (Fig. 3B). Furthermore, while Cheng et al. (7) monitored induction of DNA synthesis up to 20 h, we have found that DNA synthesis is induced only at later time points when the PI3-kinase pathway has been activated. Therefore, the differences between the studies reported here and those of Cheng et al. (7) reflect in part the time period in which DNA synthesis was examined but may also reflect differences in ERK activation or culture conditions between the two inducible systems. In our system ERK activation was low (a maximum of sixfold), whereas persistent high levels of ERK activity are known to elevate levels of p21Waf1/Cip1 such that DNA synthesis is inhibited (57, 68). In the system described here, p21Waf1/Cip1 was induced to similar levels by MEK/ΔNEE-ER and serum (data not shown).
In order to delineate transcription factor induction mediated by MEK activation, we investigated whether activation of the ERK/MAP kinase pathway is sufficient to induce AP-1 components. Although the ERK/MAP kinase pathway signalling cascade has been described as being involved in the induction of the immediate-early gene product c-Fos via phosphorylation and activation of the ternary complex factor ELK1 (36), we could not detect any induction of endogenous c-Fos protein after activation of MEK1. FosB, another Fos family member, which is induced with kinetics similar to those of c-Fos, was also not induced by the ERK/MAP kinase pathway. However, the expression of the immediate-early gene products c-Fos and FosB was induced by stimulation of the same NIH:MEK/ΔNEE-ER cell clone with serum (Fig. 5A), demonstrating that the lack of c-Fos and FosB induction by MEK1 was not due to a complete loss of inducibility of these genes as described for Ras-transformed cells (41). In contrast to c-Fos and FosB, the Fos-related gene products Fra-1 and Fra-2 were highly upregulated after induction of activated MEK1. Fra-1 and Fra-2 induction was rapid following MEK activation and was not blocked by inhibition of PI3-kinase, arguing that it is a direct signalling response to ERK activation, although we cannot rule out an effect of other signalling pathways induced by activated MEK.
The observation that the inducible MEK-expressing cells begin DNA synthesis without induction of c-Fos is consistent with the finding that knockout of the mouse c-Fos gene shows that c-Fos is not essential for the viability, profileration, and differentiation of most cell types (27). Correspondingly, FosB−/− mice are viable (5, 20). Furthermore, c-Fos−/− fibroblasts grow with kinetics similar to those of Fos+/+ fibroblasts (20) and can be transformed with oncogenic Ras (26), indicating again that c-Fos is not essential for cell proliferation and also not necessary for cellular transformation induced by oncogenic Ras or MEK1. Furthermore, the expression of c-Fos leads to morphological transformation independent of the cell cycle (42).
It has been suggested that the induction of Fra-1 and Fra-2 by serum growth factors may be an indirect effect via induction of c-Fos–c-Jun heterodimers acting on the TRE (3, 56, 58). However, activation of MEK1 in NIH:MEK/ΔNEE-ER cells leads to Fra-1 and Fra-2 induction in the absence of c-Fos protein. This is consistent with the observation that Fra-1 is inducible in Fos−/− fibroblasts, although to a lesser extent than in wild-type cells (6). Here we show that induction of the ERK/MAP kinase pathway leads to phosphorylation of Fra proteins, which may increase the transcriptional activity of Fra-Jun heterodimers (44) and result in the positive autoregulation of Fra-1 and Fra-2 via AP-1 sites in their promoters. Although it has been shown in some studies that Fra-1 and Fra-2 repress AP-1 activity (58, 69), these results are contradictory to the observation that Fra-1 and Fra-2 are overexpressed in Ras- and v-Src-transformed cells and lead to an increased AP-1 activity (41, 44).
All four Fos family members are able to heterodimerize with members of the Jun family to form AP-1 complexes. We show that the induction of the MEK/ΔNEE-ER fusion protein in NIH 3T3 cells induces not only the Fos family members Fra-1 and Fra-2 but also c-Jun and JunB expression. Similar to that of Fra-1 and Fra-2, c-Jun gene expression is mediated by positive autoregulation via an AP-1 binding site present in its promoter, which is recognized by c-Jun–ATF2 heterodimers (62). An AP-1-independent mechanism of regulation via ERK phosphorylation of Ets-2 may be responsible for Ras-induced JunB expression (8, 18, 39).
The transcription factor AP-1 seems to be involved in the regulation of many different processes, e.g., proliferation and differentiation. It is likely that specificity is achieved by the differential induction of individual AP-1 components and their phosphorylation status. Previous studies showed that different dimer combinations have different binding affinities which are also dependent on the specific TRE sequence and the promoter context (21, 22, 49, 54). AP-1 activity is increased in Ras-transformed cells, and dominant negative Jun has been shown to inhibit Ras transformation. Mechta et al. (41) have shown that the elevated AP-1 activity in Ras-transformed cells is a consequence of elevated expression of Fra-1 and Fra-2 together with c-Jun and JunB; however, the identity of the Ras-dependent signalling pathway leading to elevated AP-1 activity has not been clear. Our data obtained by using inducible MEK and treatment of Ras-transformed cells with the MEK inhibitor PD098059 show that ERK activation is the major Ras-dependent signalling pathway leading to elevated AP-1 activity.
ACKNOWLEDGMENTS
I.T. was supported by a European Union Human Capital and Mobility Fellowship, and C.J.M. is a Gibb Life Fellow of the Cancer Research Campaign.
We thank S. Mittnacht for helpful discussions and P. Cohen, J. Downward, G. Peters, and C. Sherr for antibody reagents.
REFERENCES
- 1.Adari H, Lowy D R, Willumsen B M, Der C J, McCormick F. Guanosine triphosphatase activating protein (GAP) interacts with the p21 ras effector binding domain. Science. 1988;240:518–521. doi: 10.1126/science.2833817. [DOI] [PubMed] [Google Scholar]
- 2.Alessi D R, Saito Y, Campbell D G, Cohen P, Sithanandam G, Rapp U, Ashworth A, Marshall C J, Cowley S. Identification of the sites in MAP kinase kinase-1 phosphorylated by p74raf-1. EMBO J. 1994;13:1610–1619. doi: 10.1002/j.1460-2075.1994.tb06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergers G, Graninger P, Braselmann S, Wrighton C, Busslinger M. Transcriptional activation of the fra-1 gene by AP-1 is mediated by regulatory sequences in the first intron. Mol Cell Biol. 1995;15:3748–3758. doi: 10.1128/mcb.15.7.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betsholtz C, Johnsson A, Heldin C H, Westermark B. Efficient reversion of simian sarcoma virus-transformation and inhibition of growth factor-induced mitogenesis by suramin. Proc Natl Acad Sci USA. 1986;83:6440–6444. doi: 10.1073/pnas.83.17.6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown J R, Ye H, Bronson R T, Dikkes P, Greenberg M E. A defect in nurturing in mice lacking the immediate early gene fosB. Cell. 1996;86:297–309. doi: 10.1016/s0092-8674(00)80101-4. [DOI] [PubMed] [Google Scholar]
- 6.Brusselbach S, Mohle-Steinlein U, Wang Z-Q, Schreiber M, Lucibello F C, Muller R, Wagner E F. Cell proliferation and cell cycle progression are not impaired in fibroblasts and ES cells lacking c-Fos. Oncogene. 1995;10:79–86. [PubMed] [Google Scholar]
- 7.Cheng M, Sexl V, Sherr C J, Roussel M F. Assembly of cyclin D-dependent kinase and titration of p27Kip1 regulated by mitogen-activated protein kinase kinase (MEK1) Proc Natl Acad Sci USA. 1998;95:1091–1096. doi: 10.1073/pnas.95.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffer P, de Jonge M, Mettouchi A, Binetruy B, Ghysdael J, Kruijer W. junB promoter regulation: Ras mediated transactivation by c-Ets-1 and c-Ets-2. Oncogene. 1994;9:911–921. [PubMed] [Google Scholar]
- 9.Cohen D R, Curran T. fra-1: a serum-inducible, cellular immediate-early gene that encodes a Fos-related antigen. Mol Cell Biol. 1988;8:2063–2069. doi: 10.1128/mcb.8.5.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowley S, Paterson H, Kemp P, Marshall C J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 11.Cross D A, Alessi D R, Cohen P, Andjelkovich M, Hemmings B A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 12.Denhardt D T. Signal-transducing protein phosphorylation cascades mediated by Ras/Rho proteins in the mammalian cell: the potential for multiplex signalling. Biochem J. 1996;318:729–747. doi: 10.1042/bj3180729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dent P, Haser W, Haystead T A J, Vincent L A, Roberts T M, Sturgill T W. Activation of mitogen-activated protein kinase kinase by v-Raf in NIH 3T3 cells and in vitro. Science. 1992;257:1404–1406. doi: 10.1126/science.1326789. [DOI] [PubMed] [Google Scholar]
- 14.Diaz-Meco M T, Lozano J, Municio M M, Berra E, Frutos S, Sanz L, Moscat J. Evidence for the in vitro and in vivo interaction of Ras with protein kinase C zeta. J Biol Chem. 1994;269:31706–31710. [PubMed] [Google Scholar]
- 15.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukuda M, Gotoh I, Adachi M, Gotoh Y, Nishida E. A novel regulatory mechanism in the mitogen-activated protein (MAP) kinase cascade. J Biol Chem. 1997;272:32642–32648. doi: 10.1074/jbc.272.51.32642. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda M, Gotoh I, Gotoh Y, Nishida E. Cytoplasmic localization of mitogen-activated protein kinase kinase directed by its NH2 terminal, leucine-rich short amino acid sequence, which acts as a nuclear export signal. J Biol Chem. 1996;271:20024–20028. doi: 10.1074/jbc.271.33.20024. [DOI] [PubMed] [Google Scholar]
- 18.Galang C K, Der C J, Hauser C A. Oncogenic Ras can induce transcriptional activation through a variety of promoter elements including tandem c-Ets-2 binding sites. Oncogene. 1994;9:2913–2921. [PubMed] [Google Scholar]
- 19.Gruda M C, Kovary K, Metz R, Bravo R. Regulation of Fra-1 and Fra-2 phosphorylation differs during the cell cycle of fibroblasts and phosphorylation in vitro by MAP kinase affects DNA binding activity. Oncogene. 1994;9:2537–2547. [PubMed] [Google Scholar]
- 20.Gruda M C, Vanamsterdam J, Rizzo C A, Durham S K, Lira A, Bravo R. Expression of FosB during mouse development—normal development of FosB knockout mice. Oncogene. 1996;12:2177–2185. [PubMed] [Google Scholar]
- 21.Hai T, Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci USA. 1991;88:3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halazonetis T D, Georgopoulos K, Greenberg M E, Leder P. c-jun dimerizes with itself and with c-fos forming complexes with different binding affinities. Cell. 1988;55:917–924. doi: 10.1016/0092-8674(88)90147-x. [DOI] [PubMed] [Google Scholar]
- 23.Hill C S, Treisman R. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell. 1995;80:199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- 24.Hofer F, Fields S, Schneider C, Martin G S. Activated Ras interacts with the Ral guanine nucleotide dissociation stimulator. Proc Natl Acad Sci USA. 1994;91:11089–11093. doi: 10.1073/pnas.91.23.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howe L R, Leevers S J, Gómez N, Nakielny S, Cohen P, Marshall C J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992;71:335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- 26.Hu E, Mueller E, Oliviero S, Papaioannou V E, Johnson R, Spiegelmann B M. Targeted disruption of the c-fos gene demonstrates c-fos-dependent and -independent pathways for gene expression stimulated by growth factors or oncogenes. EMBO J. 1994;13:3094–3103. doi: 10.1002/j.1460-2075.1994.tb06608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson R S, Spiegelman B M, Papaioannou V. Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell. 1992;71:577–586. doi: 10.1016/0092-8674(92)90592-z. [DOI] [PubMed] [Google Scholar]
- 28.Joneson T, White M A, Wigler M H, Bar-Sagi D. Stimulation of membrane ruffling and MAP kinase activation by distinct effectors of Ras. Science. 1996;271:810–812. doi: 10.1126/science.271.5250.810. [DOI] [PubMed] [Google Scholar]
- 29.Kikuchi A, Demo S D, Ye Z H, Chen Y W, Williams L T. ralGDS family members interact with the effector loop of Ras p21. Mol Cell Biol. 1994;14:7483–7491. doi: 10.1128/mcb.14.11.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kovary K, Bravo R. Expression of different Jun and Fos proteins during the G0-to-G1 transition in mouse fibroblasts: in vitro and in vivo associations. Mol Cell Biol. 1991;11:2451–2459. doi: 10.1128/mcb.11.5.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kyriakis J M, App H, Zhang X F, Banerjee P, Brautigan D L, Rapp U R, Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992;358:417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- 32.Leevers S J, Marshall C J. Activation of extracellular signal-regulated kinase, ERK2, by p21ras oncoprotein. EMBO J. 1992;11:569–574. doi: 10.1002/j.1460-2075.1992.tb05088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Littlewood T D, Hancock D C, Danielian P S, Parker M G, Evan G I. A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic Acids Res. 1995;23:1686–1690. doi: 10.1093/nar/23.10.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lloyd A C, Yancheva N, Wasylyk B. Transformation suppressor activity of a Jun transcription factor lacking its activation domain. Nature. 1991;352:635–638. doi: 10.1038/352635a0. [DOI] [PubMed] [Google Scholar]
- 35.Mansour S J, Matten W T, Hermann A S, Candia J M, Rong S, Fukasawa K, Vande Woude G F, Ahn N G. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 36.Marais R, Wynne J, Treismann R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993;73:381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- 37.Martelli P, Lapetina E G, Der C J, White G C. Identification of a novel RalGDS-related protein as a candidate effector for Ras and Rap1. J Biol Chem. 1996;271:29903–29908. doi: 10.1074/jbc.271.47.29903. [DOI] [PubMed] [Google Scholar]
- 38.Martin G A, Viskochil D, Bollag G, McCabe P C, Crosier W, Haubruck H, Conroy L, Clark R, O’Connel P, Cawthorn R M, Innis M A, McCormick F. The GAP-related domain of the neurofibromatosis type 1 gene product interacts with ras p21. Cell. 1990;63:843–849. doi: 10.1016/0092-8674(90)90150-d. [DOI] [PubMed] [Google Scholar]
- 39.McCarthy S A, Chen D, Yang B S, Garcia R J, Cherwinski H, Chen X R, Klagsbrun M, Hauser C A, Ostrowski M C, McMahon M. Rapid phosphorylation of Ets-2 accompanies mitogen-activated protein kinase activation and the induction of heparin-binding epidermal growth factor gene expression by oncogenic Raf-1. Mol Cell Biol. 1997;17:2401–2412. doi: 10.1128/mcb.17.5.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarthy S A, Samuels M L, Pritchard C A, Abraham J A, McMahon M. Rapid induction of heparin-binding epidermal growth factor/diphtheria toxin receptor expression by Raf and Ras oncogenes. Genes Dev. 1995;9:1953–1964. doi: 10.1101/gad.9.16.1953. [DOI] [PubMed] [Google Scholar]
- 41.Mechta F, Lallemand D, Pfarr C M, Yaniv M. Transformation by ras modifies AP1 composition and activity. Oncogene. 1997;14:837–847. doi: 10.1038/sj.onc.1200900. [DOI] [PubMed] [Google Scholar]
- 42.Miao G G, Curran T. Cell transformation by c-Fos requires an extended period of expression and is independent of the cell cycle. Mol Cell Biol. 1994;14:4295–4310. doi: 10.1128/mcb.14.6.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morgenstern J P, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murakami M, Sonobe M H, Ui M, Kabuyama Y, Watanabe H, Wada T, Handa H, Iba H. Phosphorylation and high level expression of Fra-2 in v-src transformed cells: a pathway of activation of endogenous AP-1. Oncogene. 1997;14:2435–2444. doi: 10.1038/sj.onc.1201077. [DOI] [PubMed] [Google Scholar]
- 45.Nakielny S, Cohen P, Wu J, Sturgill T W. MAP kinase activator from insulin-stimulated skeletal muscle is a protein threonine/tyrosine kinase. EMBO J. 1992;11:2123–2129. doi: 10.1002/j.1460-2075.1992.tb05271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishina H, Hironori S, Takeshi S, Moriyuki S, Iba H. Isolation and characterization of fra-2, an additional member of the fos gene family. Proc Natl Acad Sci USA. 1990;87:3619–3623. doi: 10.1073/pnas.87.9.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pagès G, Lenormand P, L’Allemain G, Chambard J C, Meloche S, Pouysségur J. Mitogen-activated protein kinase p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci USA. 1993;90:8319–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polyak K, Kato J Y, Solomon M J, Sherr C J, Massague J, Roberts J M, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:8–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 49.Rauscher F J, III, Voulalas P J, Franza B R, Jr, Curran T. Fos and Jun bind cooperatively to the AP-1 site: reconstitution in vitro. Genes Dev. 1988;2:1687–1699. doi: 10.1101/gad.2.12b.1687. [DOI] [PubMed] [Google Scholar]
- 50.Roche S, Koegl M, Courtneidge S A. The phosphatidylinositol 3-kinase alpha is required for DNA synthesis induced by some, but not all, growth factors. Proc Natl Acad Sci USA. 1994;91:9185–9189. doi: 10.1073/pnas.91.19.9185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodriguez V P, Warne P H, Dhand R, Vanhaesebroeck B, Gout I, Fry M J, Waterfield M D, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez V P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 53.Russell M, Lange C C, Johnson G L. Direct interaction between Ras and the kinase domain of mitogen-activated protein kinase kinase kinase (MEKK1) J Biol Chem. 1995;270:11757–11760. doi: 10.1074/jbc.270.20.11757. [DOI] [PubMed] [Google Scholar]
- 54.Ryseck R P, Bravo R. c-Jun, JunB and JunD differ in their binding affinities to AT-1 and CRE consensus sequences: effect of Fos proteins. Oncogene. 1991;6:533–542. [PubMed] [Google Scholar]
- 55.Samuels M L, Weber M J, Bishop M, McMahon M. Conditional transformation of cells and rapid activation of the mitogen-activated protein kinase cascade by an estradiol-dependent human Raf-1 protein kinase. Mol Cell Biol. 1993;13:6241–6252. doi: 10.1128/mcb.13.10.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schreiber M, Poirier C, Franchi A, Kurzbauer R, Guenet J-L, Carle G F, Wagner E. Structure and chromosomal assignment of the mouse fra-1 gene, and its exclusion as a candidate gene for oc (osteosclerosis) Oncogene. 1997;15:1171–1178. doi: 10.1038/sj.onc.1201460. [DOI] [PubMed] [Google Scholar]
- 57.Sewing A, Wiseman B, Lloyd A C, Land H. High-intensity Raf signal causes cell cycle arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5588–5597. doi: 10.1128/mcb.17.9.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sonobe M H, Yoshida T, Murakami M, Kameda T, Iba H. fra-2 promoter can respond to serum-stimulation through AP-1 complexes. Oncogene. 1995;10:689–696. [PubMed] [Google Scholar]
- 59.Spaargaren M, Bischoff J R. Identification of the guanine nucleotide dissociation stimulator for Ral as a putative effector molecule of R-ras, H-ras, K-ras, and Rap. Proc Natl Acad Sci USA. 1994;91:12609–12613. doi: 10.1073/pnas.91.26.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Aelst L, Barr M, Marcus S, Polverino A, Wigler M. Complex formation between Ras and Raf and other protein kinases. Proc Natl Acad Sci USA. 1993;90:6213–6217. doi: 10.1073/pnas.90.13.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Aelst L, White M, Wigler M H. Ras partners. Cold Spring Harbor Symp Quant Biol. 1994;59:181–186. doi: 10.1101/sqb.1994.059.01.022. [DOI] [PubMed] [Google Scholar]
- 62.Van Dam H, Duyndam M, Pottier P, Bosch A, De Vries-Smits L, Herrlich P, Zantema A, Angel P, van der Eb A. Heterodimer formation of c-Jun and ATF-2 is responsible for induction of c-jun by the 243 amino acid adenovirus E1A protein. EMBO J. 1993;12:479–487. doi: 10.1002/j.1460-2075.1993.tb05680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vlahos C J, Matter W F, Hui K Y, Brown R F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 64.Vojtek A B, Hollenberg S M, Cooper J A. Mammalian Ras interacts directly with the serine threonine kinase Raf. Cell. 1993;74:205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- 65.White M A, Nicolette C, Minden A, Polverino A, Van A L, Karin M, Wigler M H. Multiple Ras functions can contribute to mammalian cell transformation. Cell. 1995;80:533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- 66.White M A, Vale T, Camonis J H, Schaefer E, Wigler M H. A role for the Ral guanine nucleotide dissociation stimulator in mediating Ras- induced transformation. J Biol Chem. 1996;271:16439–16442. doi: 10.1074/jbc.271.28.16439. [DOI] [PubMed] [Google Scholar]
- 67.Wolthuis R M, Bauer B, van’t Veer L J, de Vries-Smits A M, Cool R H, Spaargaren M, Wittinghofer A, Burgering B M, Bos J L. RalGDS-like factor (Rlf) is a novel Ras and Rap 1A-associating protein. Oncogene. 1996;13:353–362. [PubMed] [Google Scholar]
- 68.Woods D, Parry D, Cherwinski H, Bosch E, Lees E, McMahon M. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5598–5611. doi: 10.1128/mcb.17.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoshioka K, Deng T, Cavigelli M, Karin M. Antitumor promotion by phenolic antioxidants: inhibition of AP-1 activity through induction of Fra expression. Proc Natl Acad Sci USA. 1995;92:4972–4976. doi: 10.1073/pnas.92.11.4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang X F, Settleman J, Kyriakis J M, Takeuchi-Suzuki E, Elledge S J, Marshall M S, Bruder J T, Rapp U R, Avruch J. Normal and oncogenic p21ras proteins bind to the amino-terminal regulatory domain of c-Raf-1. Nature. 1993;364:308–313. doi: 10.1038/364308a0. [DOI] [PubMed] [Google Scholar]