Abstract
The synthesis of new compounds with antimicrobial and antiviral properties is a central objective today in the context of the COVID-19 pandemic. Benzimidazole and pyrazole compounds have remarkable biological properties, such as antimicrobial, antiviral, antitumor, analgesic, anti-inflammatory, anti-Alzheimer’s, antiulcer, antidiabetic. Moreover, recent literature mentions the syntheses and antimicrobial properties of some benzimidazole–pyrazole hybrids, as well as other biological properties thereof. In this review, we aim to review the methods of synthesis of these hybrids, the antimicrobial activities of the compounds, their correlation with various groups present on the molecule, as well as their pharmaceutical properties.
Keywords: benzimidazole, pyrazole, hybrids, antimicrobial, pharmaceutical properties
1. Introduction
Microbial resistance is one of the burning issues facing clinical practice and finding new effective compounds against multi-resistant pathogens is one of the major goals in current biomedical research [1]. The discovery of new antimicrobial and antiviral compounds is a major goal in the context of today’s COVID-19 pandemic [2]. It is known that both patients with severe cases and patients with moderate cases of COVID-19, with or without pneumonia, have received treatments with various antibiotics [3]. Among the heterocyclic compounds known in the literature for their antimicrobial activities are those with benzimidazole ring and those with pyrazole ring, aromatic compounds with a very wide range of medicinal properties. Benzimidazole derivatives developed a considerable interest in medical domain due to their therapeutic action as antitumor [4,5,6,7], antimicrobial [8,9,10,11,12,13,14], antihelmintic [15], antihistaminic [16,17], proton pump inhibitors [16,18], anti-inflammatory [19,20] and anti-hypertensive [21] drugs. Astemizole-related compounds demonstrated anti-prion activity for the treatment of Creutzfeldt–Jakob disease, while albendazole compounds are currently used as medication for the treatment of a variety of parasitic worm infestations. Additionally, benzimidazoles treat mitochondrial dysfunction in Alzheimer’s disease [22], possess neurotropic, psychoactive, analgesic effects [23], anticoagulant proprieties [24] and are efficient agents in Diabetes mellitus [25]. Additionally, pyrazole compounds possess a diversity of biological activities as analgesic [26,27,28], anticonvulsivant [29,30], antitumor [31,32,33,34], antidiabetic [35,36], antimicrobial [37,38,39,40,41,42,43], antipyretic [44,45], antiviral [46,47], antimalarial [48,49], local anesthetic [50] and so forth.
Moreover, the literature mentions a series of benzimidazole–pyrazole hybrids with remarkable antimicrobial properties, and not only, antiviral activities, even anti-COVID-19 [51,52,53,54], in the context of the new pandemic, which has led us to current research, to study their synthesis methods, antimicrobial properties, structure–property relationships, and their biological activities.
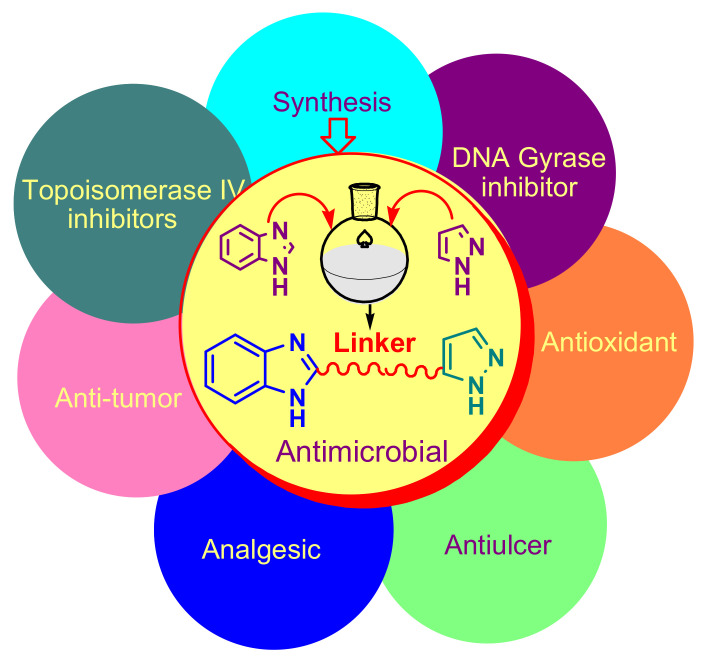
In this review, we aim to review the various methods of synthesis of benzimidazole–pyrazole hybrid compounds with antibacterial and antifungal properties, DNA-Gyrase inhibitors, topoisomerase IV inhibitors, as well as the other biological properties they possess, such as: antitumor, antioxidant, anti-inflammatory, analgesic, antiulcer (Figure 1). In order to highlight the structures of the heterocycles in the discussed compounds, we colored the benzimidazole nucleus with blue, the pyrazole with green, the linker with red, and the compounds with good biological activity are marked with a rectangle.
Figure 1.
Schematic representation of the synthesis and biological properties of benzoimidazole–pyrazole compounds.
2. Synthesis, Antimicrobial Activities of Benzimidazole–Pyrazole Compounds. Benzimidazole–Pyrazole Compounds as Potent DNA Gyrase and Topoisomerase IV Inhibitors
2.1. Benzimidazoles Substituted in the “2” Position with Pyrazole Moiety
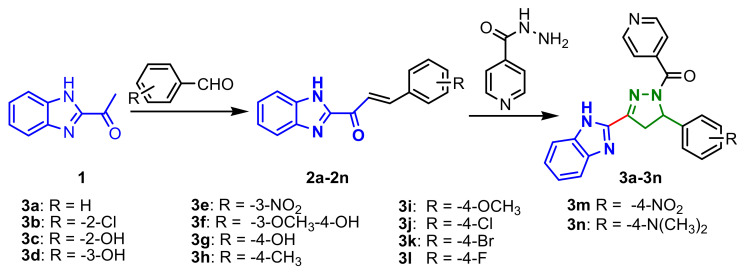
Benzimidazole chalcones 2a–2n, synthesized from 2-acetylbenzimidazole 1 and aldehydes in ethanolic KOH by a Claisen–Schmidt condensation, were cyclocondensated with izoniazide, to give (3-(1H-benzo[d]imidazol-2-yl)-5-(aryl)-4,5-dihydro-1H-pyrazol-1-yl)(pyridin-4-yl)methanones 3a–3n in good yields (Scheme 1). All compounds showed antimicrobial activity against bacterial strains E. coli, P. aeruginosa, S. aureus, S. pyogenes and fungi C. albicans, A. niger and A. clavatus. The compounds 3d, 3g, 3h, were found to be the best antibacterials, with MIC of 25 μg mL−1 against P. aeuginosa (3d) and E. coli (3g, 3h) and compound 3n the best antifungal, with MIC 25 μg mL−1 against A. niger [55].
Scheme 1.
Synthesis of benzimidazole–pyrazoles 3a–3n.
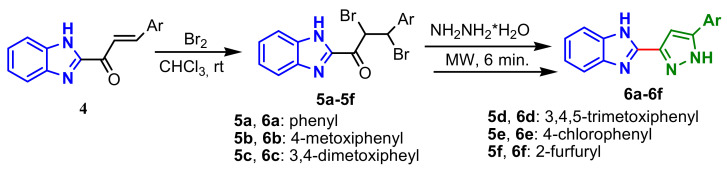
Rajora and Srivastava reported the synthesis of some 2-(1H-pyrazol-3-yl)-1H-benzo[d]imidazoles by bromination of benzimidazolyl chalcone 4, with the formation of dibrominated intermediates 5a–5f, followed by cyclization in the presence of hydrazine hydrate and dehydrobromination, with the formation of compounds 6a–6f (Scheme 2).
Scheme 2.
Synthesis of benzimidazole-pyrazole 7.
Compounds 6a–6f showed good antimicrobial activity on four bacterial strains, E. coli, P. aeruginusa, B. subtilis, K. pneumoniae and two fungi, Candida albicons and Aspergillus niger, considering ciprofoxacin and fluconazole as standard drugs [56].
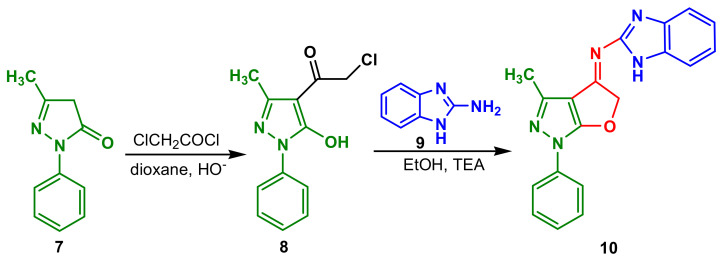
2-Chloro-1-(5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)ethanone 8 synthesized by refluxing 5-pyrazolone 7 with chloroacetyl chloride in a basic dioxane solution reacted with 2-aminobenzimidazole 9 to give N-(3-methyl-1-phenyl-1H-furo [2,3-c]pyrazol-4(5H)-ylidene)-1H-benzimidazol-2-amine 10 (Scheme 3) [57]. Compound 10 showed a very good anti-Gram-positive profile, being equivalent to chloramphenicol against B. subtilis (MIC 3.125 μg mL−1), significant activity against B. thuringiensis (MIC 6.25 μg mL−1) and also good antibacterial activities against Gram-positive bacteria, E. coli (MIC 50 μg mL−1) and P. aeruginosa (MIC 50 μg mL−1). Antifungal activity of the compound 10 was 50% lower than cycloheximide in inhibitory the growth of B. fabae and F. oxysporum (MIC 6.25 μg mL−1).
Scheme 3.
Synthesis of benzimidazole–pyrazole 10.
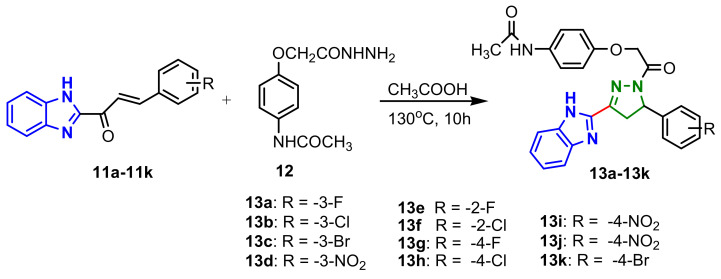
A similar condensation of chalcones 11a–11k with intermediate hydrazide 12 in acetic acid, at 130 °C afforded new benzimidazole bearing pyrazoline derivatives 13a–13k in excellent yields (Scheme 4). All compounds showed antimicrobial activity against bacteria E. coli MTCC443, P. aeruginosa MTCC1688, S. aureus MTCC 96, S. pyogenes MTCC 442 and fungi C. albicans MTCC 227, A. niger MTCC 282 and A. clavatus MTCC 1323. Compounds 13a–13d showed the highest inhibition against almost all bacteria tested with values of minimum inhibitory concentrations of 25–50 mg mL−1, while derivatives 13e–13k had antifungal activity against almost all strains tested, with similar CMI values [58]. Structure–activity relationship studies have shown that the presence of electron-withdrawing groups in the aromatic ring, like F, Cl, Br and NO2, are responsible for increasing antimicrobial activity for most microorganisms tested.
Scheme 4.
Synthesis of benzimidazole–pyrazoles 13a–13k.
Kalaria et al., reported a L-proline promoted one-pot four-component tandem reaction for synthesis of compound 16, starting from carbothioamide 14, pyrazolyl aldehyde 15, α-bromoethylacetate and malononitrile (Scheme 5) [59]. Antibacterial activity of the compounds 16 was screened against three Gram-positive bacteria (Streptococcus pneumoniae MTCC 1936, Bacillus subtilis MTCC 441 and Clostridium tetani MTCC 449) and three Gram-negative bacteria (Escherichia coli MTCC 443, Salmonella typhi MTCC 98, Vibrio cholerae MTCC 3906) using ampicillin, norfloxacin and ciprofloxacin as the standard antibacterial drugs. Compound 16 illustrated an excellent activity against Gram-positive bacteria B. subtilis (62.5 μg mL−1), being more potent than ampicillin (250 μg mL−1) and norfloxacin (100 μg mL−1) and also against C. tetani, with a CMI of 200 μg mL−1 compared with 250 μg mL−1 for ampicilin. Additionally, the structure–activity relationship (SAR) showed that the presence of benzimidazole in the fifth position in the pyrazole ring is responsible for its biological activity.
Scheme 5.
Synthesis of benzimidazole–pyrazoles 16.
Patil et al., reported two series o benzimidazole–pyrazole compounds 19a–19f and 20a–20f in two steps: a condensation between 2-benzimidazolehydrazine 17 and pyrazole 18a–18f, followed by cyclization with thioglicolic acid (Scheme 6) [60]. The compounds 19b, 19d, 20a and 20f show good activity against bacteria P. aeruginosa, S. aureus and P. vulgaris, while the others show moderate to poor activity against all pathogens. The compounds 19a and 19c exhibited good activity against fungal strains A. niger and A. flavus.
Scheme 6.
Synthesis of benzimidazole–pyrazoles 19a–19f and 20a–20f.
Reddy et al., reported the synthesis of a new class of pyrazolyl–benzimidazoles 23a–23c possessing an amide group by reaction between pyrazolones 21a–21c with 1H-benzo[d]imidazol-2-amine 9, and the oxidation of the intermediate compounds 22a–22c with chloranil (Scheme 7) [61]. It was found that the presence of electron-withdrawing substituent “Cl” on the aromatic ring increases the antimicrobial activity, compound 23c being a potent antifungal agent against A. niger considering ketoconazole as standard. Additionally, compounds 23a and 23c possess antimicrobial activity against B. subtilis and P. aeruginosa (chloramphenicol standard).
Scheme 7.
Synthesis of benzimidazole–pyrazoles 23a–23c.
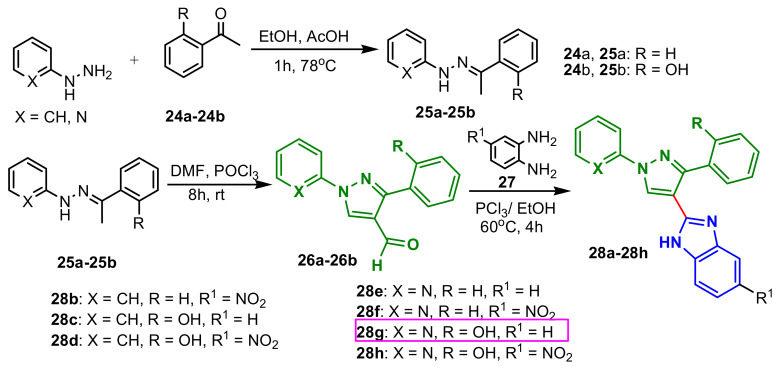
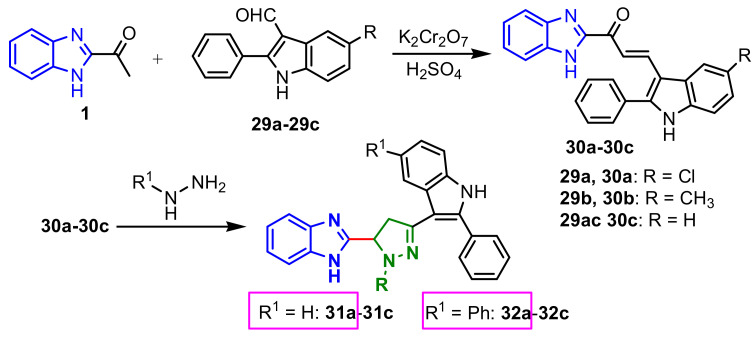
Padalkar et al., synthesized a new class of antimicrobial agents, by reaction of phenyl hydrazine with substituted acetophenones 24 to give the corresponding hydrazones 25, which on Vilsmeier–Haack reaction with POCl3–DMF gave substituted 3-aryl-4-formyl pyrazoles 26. Compounds 25a–25b were condensed with o-substituted aromatic amines 27 in the presence of PCl3 in ethanol to obtain corresponding 2-[substituted-1H-pyrazol-4-yl]-1H-benzimidazoles 28b–28i (Scheme 8) [62]. The compound 28g showed good antibacterial activity against Escherichia coli and Staphylococcus aureus, and compounds 28d, 28e, 28h exhibited weak to moderate growth inhibitory activity against both E. coli and S. aureus as revealed from their MIC values (Table 1). Compounds 28f and 28h show good inhibitory growth in the case of Candida albicans (MIC = 62.5 μg mL−1). Saundane et al., reported the synthesis of a series of benzimidazole–pyrazole compounds using a two-step strategy (Scheme 9): synthesis of intermediate chalcones 25a–25b by a condensation reaction, followed by a cyclization reaction with hydazine (compounds 31) or phenylhydrazine (compounds 32) [63]. All compounds were assessed for their in vitro antibacterial activity against four representative bacterial species E. coli (MTCC-723), S. aureus (ATCC-29513), K. pneumonia (NCTC-13368) and P. aeruginosa (MTCC-1688) using gentamycin as a reference and for their antifungal activity against A. oryzae (MTCC-3567T), A. niger (MTCC-281), A. flavus (MTCC-1973), A. terreus (MTCC-1782). Compounds 31a and 32a possess good antibacterial and antifungal activity (Table 2), against E. coli, S. aureus (MIC = 8 μg mL−1) and A. niger (MIC = 8 μg mL−1 for 31a). Additionally, all compounds possess antioxidant activity.
Scheme 8.
Synthesis of benzimidazole–pyrazoles 27a–27i.
Table 1.
Antibacterial and antifungal activities of the compounds 28a–28h indicated by MIC (μg mL−1).
| Compound | E. coli | S. aureus | C. albicans | A. niger |
|---|---|---|---|---|
| 28b | 312 | 312 | 312 | 312 |
| 28c | 187.5 | 312 | 312 | 312 |
| 28d | 250 | 62.5 | 250 | 187.5 |
| 28e | 187.5 | 62.5 | 312 | 250 |
| 28f | 312 | 187.5 | 62.5 | 312 |
| 28g | 62.5 | 62.5 | 312 | 312 |
| 28h | 125 | 62.5 | 62.5 | 125 |
| Streptomycin | 125 | 125 | - | - |
| Fluconazole | - | - | 125 | 125 |
Scheme 9.
Synthesis of benzimidazole–pyrazoles 31a–31c and 32a–32c.
Table 2.
Antibacterial and antifungal activities of the compounds 28a–28h.
| Compound | Antibacterial Activity (MIC μg mL−1) | Antifungal Activity (MIC μg mL−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| Ec | Sa | Kp | Pa | Ao | An | Af | At | |
| 31a | 8 | 8 | 16 | 16 | 16 | 8 | 16 | 8 |
| 31b | 32 | 64 | 128 | 128 | 64 | 128 | 256 | 128 |
| 31c | 128 | 512 | 512 | 256 | 512 | 512 | 256 | 128 |
| 32a | 8 | 16 | 16 | 32 | 32 | 16 | 8 | 32 |
| 32b | 32 | 32 | 16 | 128 | 128 | 128 | 256 | 128 |
| 32c | 32 | 64 | 512 | 256 | 128 | 256 | 256 | 128 |
| Gentamycin | 2 | 2 | 2 | 2 | - | - | - | - |
| Fluconazole | - | - | - | - | 2 | 2 | 2 | 2 |
Ec: Escherichia coli (MTCC-723), Sa: Staphylococcus aureus (ATCC-29513), Kp: Klebsiella pneumonia (NCTC-13368), Pa: Pseudomonas aeruginosa (MTCC-1688), Ao: Aspergillus oryzae (MTCC-3567T), An: Aspergillus niger (MTCC-281), Af: Aspergillus flavus (MTCC-1973), At: Aspergillus terreus (MTCC-1782).
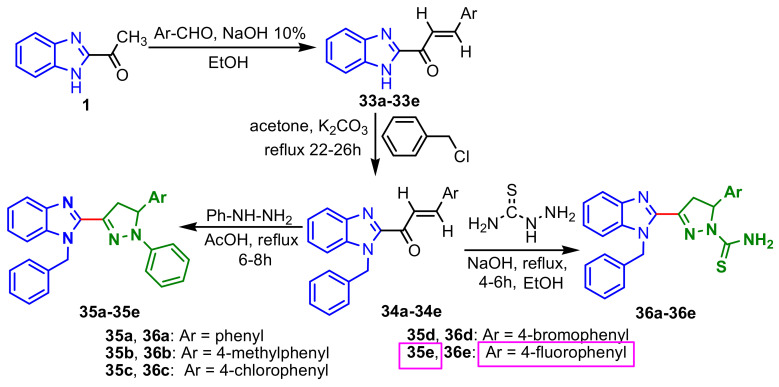
Padhy et al., synthesized two series of benzimidazole–pyrazole compounds in three steps: (i) Claisen–Schmidt condensation of 2-acetylbezimidazole 1 with substituted aromatic aldehydes in presence of NaOH, to give the intermediates chalcones 33a–33e; (ii) condensation of the chalcones 33 with benzyl chloride gave the corresponding 1-benzyl substituted compounds 34a–34e; (iii) the reaction of compounds 34 with phenylhydrazine in the presence of acetic acid afforded 1-benzyl-2-(5-aryl-1-phenyl-4,5-dihydro-1H-pyrazol-3-yl)-1H-benzimidazoles 35a–35e, while (iv) condensation with thiosemicarbazide in presence of NaOH, give 5-aryl-3-(1-benzyl-1H-benzimidazol-2-yl)-4,5-dihydro-1H-pyrazole-1-carbothioamides 36a–36e in good yields (Scheme 10). The in vitro antimicrobial activity of compounds 35–36 was tested against four bacterial strains, S. aureus, B. subtilis, E. coli, P. aeruginosa, and one fungus, C. albicans. The compounds exhibited weaker antimicrobial activities compared to those of the control drugs (Ciprofloxacin and Fluconazole), the MIC values of the compounds ranged between 64–1024 μg mL–1 for the 1-phenylpyrazolines 35a–35e and between 128–512 μg mL–1 for the pyrazoline-1-carbothioamides 36a–36e. Compound 35e showed good activity (64 μg mL–1) against all tested bacterial strains [64].
Scheme 10.
Synthesis of benzimidazole–pyrazoles 35a–35e and 36a–36e.
4-(1H-benzimidazol-2-yl)benzenamine 37, obtained by cyclization reaction of 1,2-phenylenediamine with 4-amino benzoic acid, was diazotized and treated with ethylacetoacetate to produce ethyl 2-(2-(4-(1H-benzimidazol-2-yl)phenyl) hydrazono)-3-oxobutanoate 38 through intramolecular rearrangement reaction. Dehydrative cyclisation of 38 in the with different hydrazine hydrochlorides produce corresponding benzimidazole–pyrazole 39a–39i (Scheme 11) [65]. The antitubercular and antimicrobial activity of compounds 39 was determined on four Gram-positive strains, three Gram-negative strains and 2 fungi. In Table 3 we marked in green the very good antimicrobial activities of compounds 39c and 39f, as well as of the compounds 3d and 3g, for the labeled strains, and the very good antibacterial activities of all compounds against Staphylococcus aureus. The values of the minimum inhibitory concentrations (MIC) in Table 3 showed that compounds, 39c and 39f, possess almost all MICs as good as the standards used for antitubercular and antimicrobial activities, and their antifungal activities are twice as high, compared to Ketoconazole, ie 3.9 μg mL–1 against Aspergillus niger ATCC 9029 and 1.95 μg mL–1 against Aspergillus fumigatus ATCC 46645. With the exception of compounds 39f, 39h and 39i, all compounds showed better antibacterial activity (MIC = 125 μg mL–1) than standard, Ciprofloxacin, compounds 39c and 39d being 16 times more active(MIC = 7.81 μg mL–1) than standard.
Scheme 11.
Synthesis of benzimidazole–pyrazoles 39a–39i.
Table 3.
Minimum inhibitory concentration (μg mL−1) of the compounds 39a–39i.
| Compound | Antitubercular Activity |
Antibacterial Activity | Antifungal Activity |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gram-Positive Bacteria | Gram-Negative Bacteria |
|||||||||
| Mt | Sa | Se | Ml | Bc | Ec | Pa | Kp | An | Af | |
| 39a | 125.5 | 62.5 | 62.5 | 125 | 62.5 | 62.5 | 125 | 31.25 | 62.5 | 31.25 |
| 39b | 62.5 | 31.25 | 31.25 | 62.5 | 31.25 | 62.5 | 62.5 | 31.25 | 31.25 | 15.62 |
| 39c | 3.9 | 7.81 | 1.95 | 3.9 | 7.81 | 7.81 | 7.81 | 1.95 | 3.9 | 1.95 |
| 39d | 7.81 | 7.81 | 3.9 | 7.81 | 7.81 | 7.81 | 7.81 | 3.9 | 7.81 | 3.9 |
| 39e | 62.5 | 62.5 | 31.25 | 62.5 | 62.5 | 62.5 | 62.5 | 31.25 | 62.5 | 31.25 |
| 39f | 3.9 | 125 | 3.9 | 3.9 | 7.81 | 7.81 | 3.9 | 1.95 | 3.9 | 1.95 |
| 39g | 7.81 | 15.62 | 3.9 | 7.81 | 7.81 | 15.62 | 7.81 | 3.9 | 7.81 | 3.9 |
| 39h | >125 | 125 | 125 | >125 | >125 | 125 | >125 | 62.5 | 62.5 | 62.5 |
| 39i | 125 | 125 | 125 | >125 | 125 | 62.5 | 125 | 62.5 | 62.5 | 31.25 |
| Standard | 0.97 | 125 | 1.95 | 3.9 | 7.81 | 7.81 | 3.9 | 1.95 | 7.81 | 3.9 |
Mb: Mycobacterium tuberculosis, Sa: Staphylococcus aureus ATCC 9144, Se: Staphylococcus epidermidis ATCC 155, Ml: Micrococcus luteus ATCC 4698, Bacillus cereus ATCC 11778, Ec: Escherichia coli ATCC 25922, Pa: Pseudomonas aeruginosa ATCC 2853, Kp: Klebsiella pneumoniae ATCC 11298, An: Aspergillus niger ATCC 9029, Af: Aspergillus fumigatus ATCC 46645. Standard: Isoniazid: reference standard against M. tuberculosis, Ciprofloxacin: standard for other bacteria, Ketoconazol: reference standard for fungi.
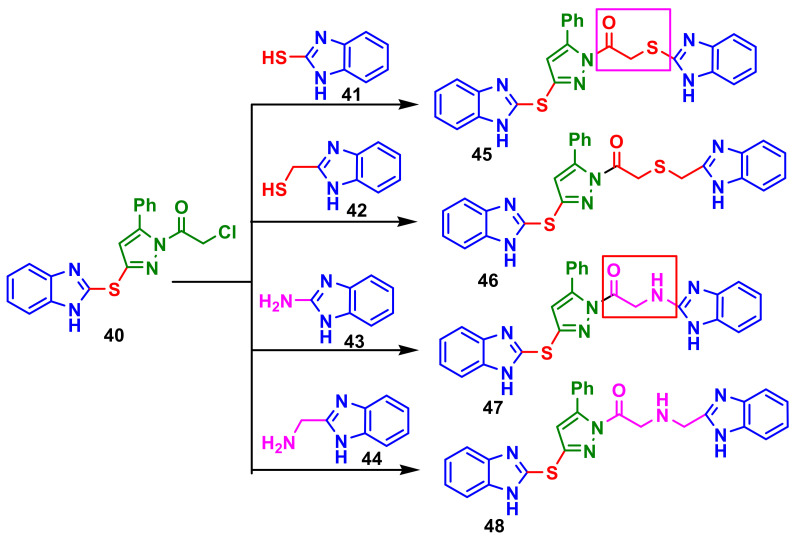
Suram et al., reported the synthesis of a series of bis(benzimidazolyl)pyrazole compounds from chlororacetylpyrazole-benzimidazole 40 and benzimidazoles 41–44, to obtain compounds 45–48 (Scheme 12) [66]. It was observed that the compound with thio ethanone linkage 45 and amino ethanone linkage 47 displayed slightly higher activity than that with methyl thio ethanone 46 and methyl amino ethanone linkage 48 on the microbial tested strains, S. aureus, B. subtilis, P. aeruginosa, K. pneumoniae, A. niger and P. chrysogenum, when compared with the standard drugs chloramphenicol and ketoconazole.
Scheme 12.
Synthesis of dibenzimidazole–pyrazoles 45–48.
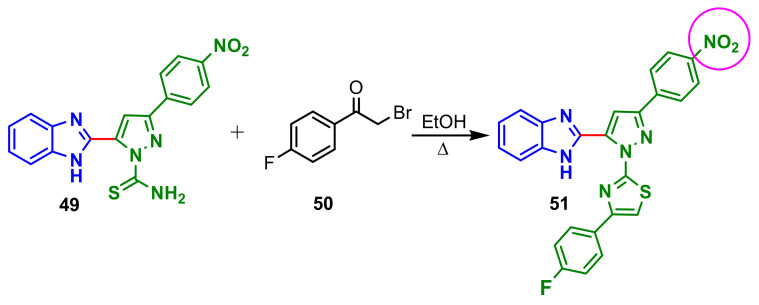
A new class of benzimidazole–pyrazoles was prepared using a Claisen–Schmidt reaction [67]. From all synthesized compounds, derivative 51, obtained by cyclocondensation reaction of thioamide 49 with 4-fluorophenacyl bromide 50 (Scheme 13), having nitro substituent on the aromatic ring showed greater antimicrobial activity particularly against Pseudomonas aeruginosa, with an inhibition zone of 34 mm at 100 μg per well, and Penicillium chrysogenum, with an inhibition zone of 41 mm at 100 μg per well.
Scheme 13.
Synthesis of dibenzimidazole–pyrazole 51.
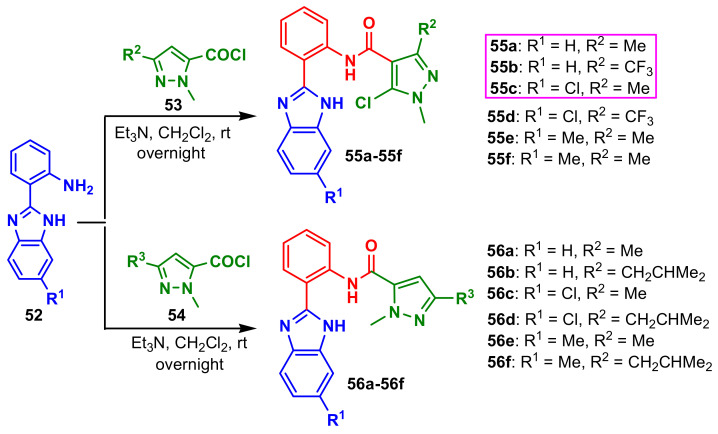
Si et al., synthesized two series of benzimidazole–pyrazoles by reaction of benzimidazole 52 with pyrazole-5-carbonyl chlorides 53 and 54 to afford the final compounds 55a–55f and 56a–56f (Scheme 14) [68]. The authors reported the antifungal activities against four fungi, B. cinerea, R. solani, F. graminearum, A. solani, considering hymexazol as the positive control at 100 μg mL −1 (Table 4). All compounds showed better inhibitory activity against B. cinerea. The inhibition rates of compounds 55a–55f exceeded 60% against R. solani and the inhibition rates of compounds 55a–55f ranged from 58.28% to 68.28% against A. solani, which were better than 55.43% of the control hymexazol. The compounds with pyrazole-4-carboxamide moiety 55a–55f showed higher activities than the target compounds with pyrazole-5-carboxamide moiety 56a–56f. Thus, the activities of 55a and 55b were better than those of 56a and 56b and the activities of 55c and 55d were better than those of 56c and 56d.
Scheme 14.
Synthesis of dibenzimidazole–pyrazole 55a–55f and 56a–56f.
Table 4.
Inhibitory rates of the compounds 55–56 against four phytopathogenic fungi at 100 ug mL−1.
| Compound | B. cinerea | R. solani | F. graminearum | A. solani |
|---|---|---|---|---|
| 55a | 65.53 | 63.34 | 39.21 | 29.71 |
| 55b | 83.11 | 64.84 | 52.37 | 34.57 |
| 55c | 72.37 | 62.34 | 51.38 | 52.57 |
| 55d | 79.68 | 69.08 | 52.63 | 58.28 |
| 55e | 85.62 | 63.34 | 45.52 | 6.00 |
| 55f | 85.39 | 62.84 | 47.37 | 68.28 |
| 56a | 43.38 | 38.65 | 21.05 | 21.42 |
| 56b | 45.66 | 30.17 | 18.42 | 29.14 |
| 56c | 44.98 | 39.90 | 22.36 | 24.57 |
| 56d | 52.28 | 36.66 | 25.79 | 34.00 |
| 56e | 52.28 | 34.91 | 17.36 | 62.57 |
| 56f | 41.55 | 45.38 | 25.00 | 30.85 |
| Hymexazol | 100.00 | 72.82 | 68.16 | 55.43 |
Jardosh et al., synthesized new pyrido[1,2-a]benzimidazoles starting chloroformilation and alkilation of 4-methyl-2-p-tolylcyclopent-3-enone 57. In the next step, a one-pot three-component reaction was used to afford final compounds 61a–61c (Scheme 15). The in vitro antimicrobial activity of 61a–61c against S. typhi, S. pneumoniae, E. coli, C. tetani, V. cholera, B. subtilis, C. albicans and A. fumigatus using broth microdilution technique was assessed. All compounds 61a–61c displayed good antimicrobial activity compared to standard drugs, as can be seen in Table 5 [69].
Scheme 15.
Synthesis of benzimidazole–pyrazole 61a–61c.
Table 5.
In vitro antimicrobial activity of benzimidazole–pyrazoles 61a–61c.
| Compounds | Microorganisms (μg mL−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| B. subtilis | C. tetanis | S. pneumoniae | E. coli | S.typhi | V. cholera | A. fumigatus | C. albicans | |
| 61a | 100 | 200 | 100 | 200 | 250 | 250 | >1000 | 250 |
| 61b | 500 | 200 | 200 | 250 | 250 | 50 | 200 | >1000 |
| 61c | 250 | 250 | 250 | 62.5 | 200 | 100 | >1000 | 250 |
| Ciprofloxacin | 50 | 100 | 50 | 25 | 25 | 25 | - | - |
| Chloramphenicol | 50 | 50 | 50 | 50 | 50 | 50 | - | - |
| Norfloxacin | 100 | 50 | 10 | 10 | 10 | 10 | - | - |
| Ampicillin | 250 | 250 | 100 | 100 | 100 | 100 | - | - |
| Griseofulvin | - | - | - | -- | - | - | 100 | 500 |
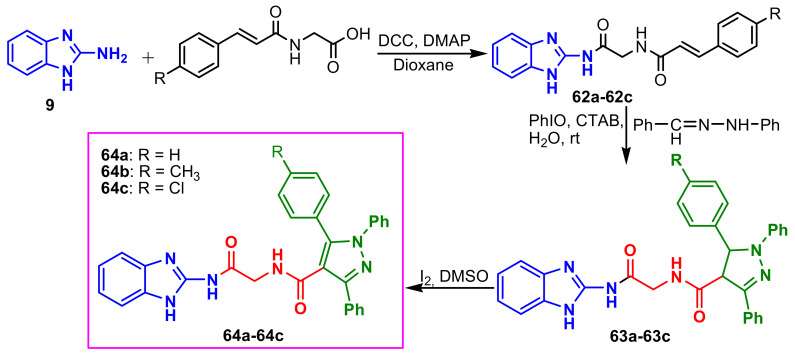
Sowdari et al., synthesized a new class of diamidomethane-linked benzazolyl–pyrazoles 64a–64c by a green approach, using the synthesis strategy indicated in Scheme 16 [70]. Compounds 64a and 64c were found to be potential antifungal agents against Aspergillus niger (MIC = 50 and 25 μg mL−1, respectively) and Penicillium chrysogenum (MIC = 12.5 and 12.5 μg mL−1, respectively) compared to the standard drug, Ketoconazole.
Scheme 16.
Synthesis of benzimidazole–pyrazole 64a–64c.
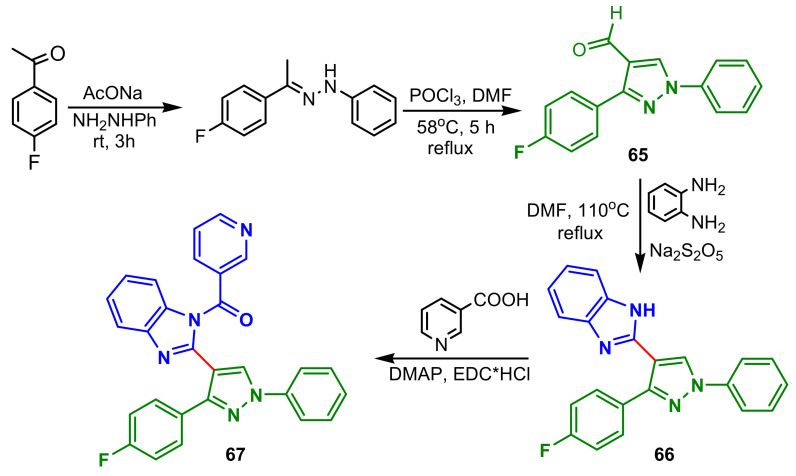
β-ketoacyl-acyl carrier protein synthase III (FabH) is an attractive target for the development of new antibacterial agents, because it catalyzes the initial step of fatty acid biosynthesis, essential for bacterial survival. Thus, Wang et al., reported the synthesis of a new series of benzimidazole–pyrazol amides with low toxicity and potent FabH inhibitory. Synthesis of compound 67 from 1-(4-fluorophenyl)ethanone is accomplished in four steps: condensation with phenylhydrazine, followed by cyclization by reflux with POCl3 in DMF for 5 h, to obtain pyrazole 65, which with 1,2-phenylenediamine and Na2S2O5 has provided benzimidazole–pyrazole intermediate 66, which by acylation with nicotinic acid, DMAP and EDC hydrochloride led to the final product 67 (Scheme 17). Compound 67 showed the most potent inhibition activity against four bacteria strains (with MIC of 0.98, 0.49, 0.98, 0.98 μg mL−1, respectively, against E. coli, P. aeruginosa, B. subtilis and S. aureus) and FabH (with IC50 of 1.22 μM). Additionally, FabH mutant Xanthomonas Campestris experiment validated that compounds binding site outcomes FabH.
Scheme 17.
Synthesis of benzimidazole–pyrazole 67.
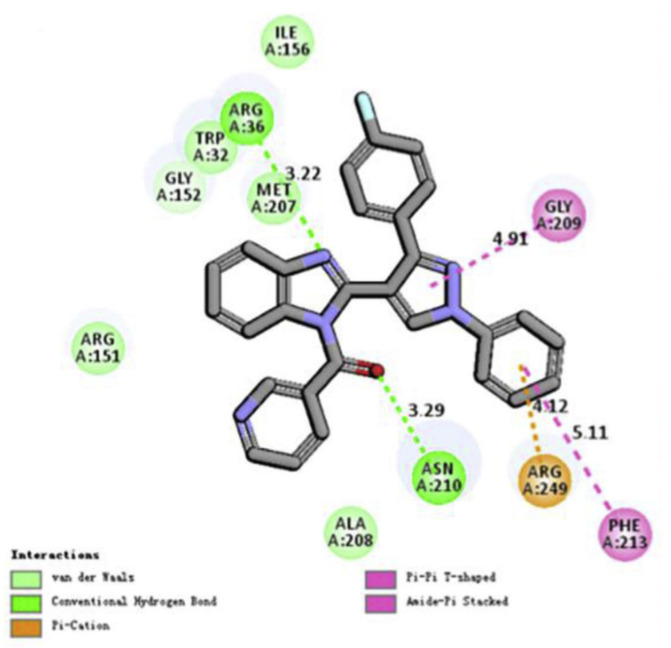
2D molecular docking modeling and surrounding residues of E. coli FabH was also performed for compound 67 (Figure 2) [71].
Figure 2.
Docking model of representative compound 67. 2D molecular docking modeling of compound 67 and surrounding residues of E. coli FabH (PDB code: 1HNJ) adapted from [71].
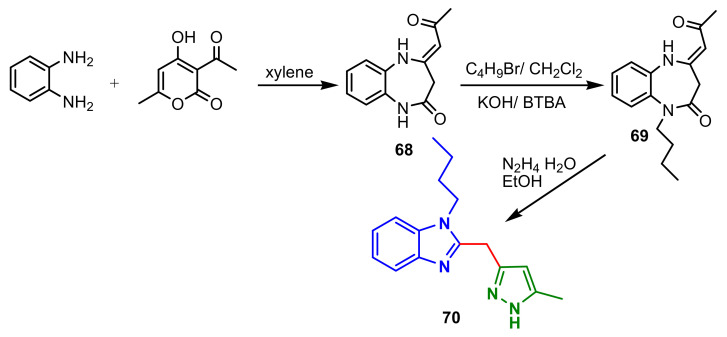
A special method for the synthesis of a benzimidazolo–pyrazole compound was reported by Chkirate et al., [72]. Thus, condensation of 1,2-phenylenediamine with dehydroacetic acid afford 68 which reacted with 1-bromobutane to give the alkylated 1,5-benzodiazepine 69. Compound 69 reacts with an excess of hydrazine monohydrate to afford the pyrazolyl–benzimidazole 70 (Scheme 18). The minimum inhibitory concentration (MIC) of 70 against S. aureus, E. coli and P. aeruginosa was evaluated at 12.5 μg mL−1, 50 μg mL−1 and 50 μg mL−1, respectively, compared to standard drug Chloramphenicol. Additionally, Co(II) and Zn(II) complexes of 70 possess remarkable antibacterial activity.
Scheme 18.
Synthesis of benzimidazole–pyrazole 70.
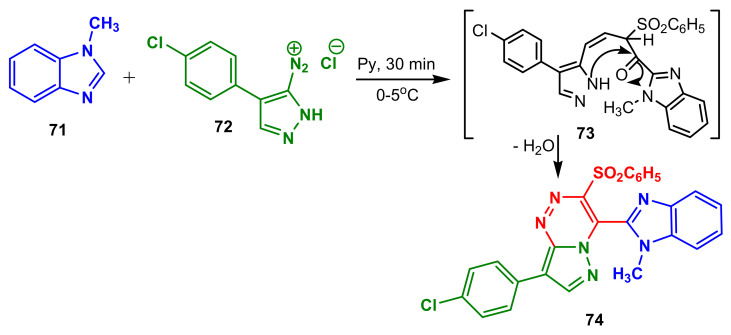
Elaziz et al., synthesized benzimidazole–pyrazole 74 from 1-methylbenzimdazole 71 and diazonium salt 72, through the intermediate 73 [73]. Compound 74 possessed better antibacterial activity than standard Cephalothin against anaerobic E. coli (16.5 μg mL−1 versus 24.3 μg mL−1), Salmonella typhimurium (13.4 μg mL−1 versus 28.5 μg mL−1), and better antibacterial activity than standard Chloramphenicol against Bacillus subtilis (23.3 μg mL−1 versus 32.4 μg mL−1, Scheme 19, Table 6).
Scheme 19.
Synthesis of benzimidazole–pyrazole 74.
Table 6.
Antibacterial activity of the compound 74 and the standards (MIC μg mL−1).
| Compound | Staphylococcus aureus | Bacillus subtilis | Escherichia coli anaerobic | Salmonella typhimurium |
|---|---|---|---|---|
| 74 | 25.3 | 23.3 | 16.5 | 13.4 |
| Chloramphenicol | 24.5 | 32.4 | - | - |
| Cephalothin | - | - | 24.3 | 28.5 |
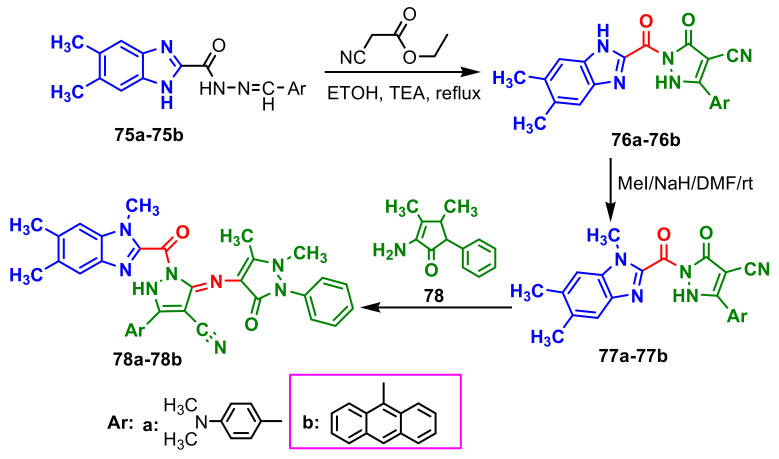
Bassyouni et al., synthesized three series of benzimidazole–pyrazoles 76–78b [74]. Compounds 76a and 76b were synthesized by the reaction of 75a and 75b with ethyl cyanoacetate in ethanol in the presence of triethylamine, respectively (Scheme 20). Methylation of 76a and 76b was achieved by their reaction with methyl iodide or DMC that yielded compounds 77a and 77b. Compounds 77a and 77b reacted with 4-aminoantipyrine in ethanol, in the presence of catalytic amounts of acetic acid to give 78a and 78b. The antibacterial activity of the compounds 76a, 76b, 77b, 78a and 78b was examined with Gram-positive bacteria Bacillus subtilis, Bacillus cereus and Staphylococcus aureus, Gram-negative bacteria Escherichia coli, Pseudomonas aeruginosa and Salmonella typhimurium. The antibacterial activity showed that compound 76a was the most active against S. typhimurium and its activity exceeded the activity of the reference antibiotic amoxicillin. Compounds 77b and 78b exhibited high antimicrobial activity against S. aureus (Table 7).
Scheme 20.
Synthesis of benzimidazole–pyrazoles 76a–76b, 77a–77b, 78a–78b.
Table 7.
The antimicrobial activity of the compounds 76a, 76b, 77b, 78a, 78b.
| Microorganism | Inhibition Zone Diameter (mm/mg Sample) | |||||
|---|---|---|---|---|---|---|
| 76a | 76b | 77b | 78a | 78b | Amoxicillin | |
| Bacillus cereus | 10 | 7 | 9 | 7 | 10 | 22 |
| Bacillus subtilis | - | - | 10 | 7 | 9 | 25 |
| Staphylococcus aureus | 10 | 10 | 18 | 10 | 18 | 16 |
| Escherichia coli | 7 | 12 | 8 | 8 | - | 22 |
| Pseudomonas aeruginosa | 10 | 9 | 11 | 11 | 13 | 30 |
| Salmonella typhimurium | 40 | - | - | - | 11 | 20 |
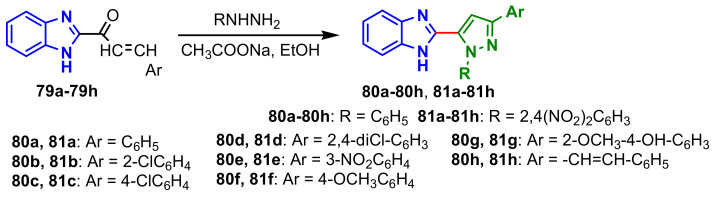
Benzimidazolo–pyrazole compounds 80a–80h and 81a–81h were synthesized from the reaction of chalcones 79a–79h with phenylhydrazine and 2,4-dinitrophenylhydrazine, respectively (Scheme 21) [75]. All compounds were screened for their antimicrobial activities against E. coli, P. aeruginosa, S. aureus, B. subtilis, C. albicans and A. niger (Table 8). The best antimicrobial activities of the compounds are marked in green in Table 8. It is observed that compounds 80b and 80h showed a good antibacterial activity against all the strains tested, and compounds with 2,4-dinitrophenylhydrazine had better antifungal activity than the antibacterial one, e.g., compounds 81b and 81f. Only compound 80a showed significant antitubercular activity at the concentration of 100μg mL−1 compared with the standard drug, Rifampicin.
Scheme 21.
Synthesis of benzimidazole–pyrazoles 80a–80h, 81a–81h.
Table 8.
The antimicrobial activity of the compounds 80a–80h and 81a–81h.
| Compounds | Zone of Inhibition at 100 μg mL−1 (in mm) | |||||
|---|---|---|---|---|---|---|
| E. coli | P. aeruginosa | S. aureus | B. subtilis | C. albicans | A. niger | |
| 80a | - | - | - | 15 | - | - |
| 80b | 14 | 13 | 14 | 16 | - | - |
| 80d | 12 | 17 | - | - | - | - |
| 80e | - | 12 | 12 | - | - | - |
| 80f | - | - | 14 | 18 | - | - |
| 80g | 13 | - | - | - | - | - |
| 80h | 19 | 16 | 18 | 15 | - | - |
| 81b | - | - | - | - | 13 | 16 |
| 81c | - | - | 14 | 13 | - | - |
| 81d | 17 | 15 | - | - | 18 | - |
| 81f | - | - | - | 12 | 14 | 17 |
| 81g | - | 13 | 15 | - | - | - |
| 81h | - | - | - | - | - | 14 |
| Gentamycin | 22 | 22 | 19 | 20 | - | - |
| Ketoconazole | - | - | - | - | 25 | 20 |
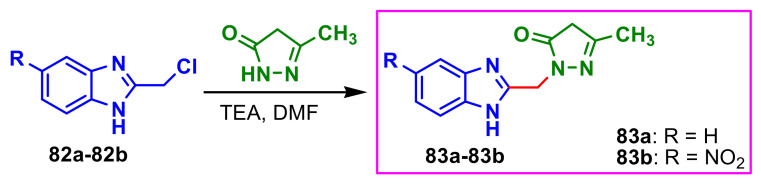
El-Gohary et al., synthesized benzimidazole–pyrazole molecules 83a–83b with antimicrobial properties, using the reaction between benzimidazoles 82a–82b and 3-methyl-1H-pyrazol-5(4H)-one in dimethylformamide (DMF), in presence of triethyl-amine (TEA) as catalyst (Scheme 22) [76]. The compounds 83a–83b showed very good antimicrobial activity against two bacteria B. cereus and S. aureus, against two fungi, C. albicans and A. fumigatus, compared to the standards used, ampicillin and fluconazole (Table 9).
Scheme 22.
Synthesis of benzimidazole–pyrazoles 83a–83b.
Table 9.
Antibacterial and antifungal activities of compounds 83a–83b.
| Compounds | MIC, μg mL−1 (mM) | |||
|---|---|---|---|---|
| B. cereus | S. aureus | C. albicans | A. fumigatus | |
| 83a | 1250 (5.48) | 156.25 (0.684) | 625 (2.74) | 312.5 (1.37) |
| 83b | 1250 (4.57) | 2500 (9.15) | 1250 (4.57) | 625 (2.29) |
| Ampicillin | 1250 (3.58) | 312.5 (0.894) | - | - |
| Fluconazole | - | - | 2500 (8.16) | - |
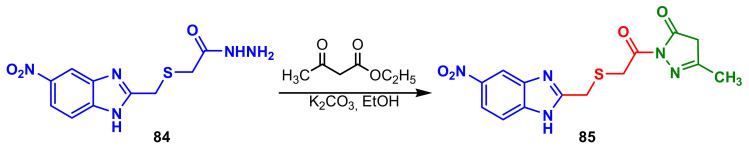
The benzimidazole–pyrazole 85 synthesized by cyclization of benzimidazole 84 in the reaction with of ethyl 3-oxobutanoate (Scheme 23), possessed good antifungal activity, against C. albicans (MIC = 2500 μg mL−1) compared with standard Fluconazole (MIC = 2500 μg mL−1) [77].
Scheme 23.
Synthesis of benzimidazole–pyrazoles 85.
2.2. Benzimidazoles Substituted in the Position “1” with Pyrazole Moiety
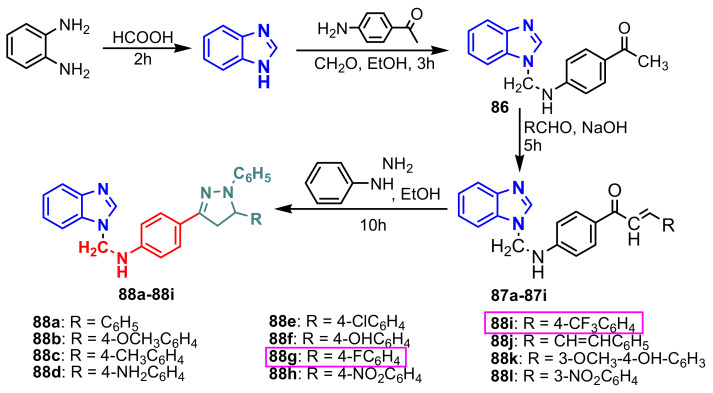
Krishnanjaneyulu et al., synthesized a series of 1-substituted benzimidazoles with pyrazole moiety through a linker using a four-step strategy: benzimidazole synthesis, N-alkylation, condensation with aldehydes with the formation of chalcones 87a–87i and cyclization with the formation of the pyrazole nucleus, in compounds 88a–88i (Scheme 24) [78]. All compounds were evaluated for their antibacterial activity against four Gram-positive bacteria: Staphylococcus aureus ATCC 9144, Staphylococcus epidermidis ATCC 155, Micrococcus luteus ATCC 4698 and Bacillus cereus ATCC 11778, and three Gram-negative bacteria: Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 2853 and Klebsiella pneumoniae ATCC 11298. The antifungal activity of the compounds 88a–88i was evaluated against two fungi, Aspergillus niger ATCC 9029 and Aspergillus fumigatus ATCC 46645 (Table 10). It was found that compounds containing an electron-withdrawing group at the phenyl group attached to C-5 of pyrazole displayed superior antimicrobial activities than compounds possessing an electron-releasing group. The unsubstituted derivatives displayed moderate antimicrobial activity. The best antimicrobial activity was shown by compounds 88g and 88i, which in Table 10 is marked in green.
Scheme 24.
Synthesis of benzimidazole–pyrazole 88a–88i.
Table 10.
Minimum inhibitory concentration in μg mL−1 of the compounds 88a–88l.
| Compounds | Sa | Se | Ml | Bc | Ec | Pa | Kp | An | Af |
|---|---|---|---|---|---|---|---|---|---|
| 88a | 31.25 | 31.25 | 15.62 | 62.5 | 31.25 | 31.25 | 15.62 | 62.5 | 31.25 |
| 88b | 125 | 62.5 | 31.25 | 125 | 62.5 | 125 | 31.25 | 125 | 125 |
| 88c | 125 | 62.5 | 125 | 125 | 62.5 | 62.5 | 31.25 | 125 | 125 |
| 88d | 62.5 | 31.25 | 31.25 | 31.25 | 62.5 | 62.5 | 31.25 | 125 | 62.5 |
| 88e | 15.62 | 15.62 | 7.81 | 15.62 | 15.62 | 7.81 | 7.81 | 31.25 | 15.62 |
| 88f | 62.5 | 31.25 | 31.25 | 31.25 | 62.5 | 125 | 31.25 | 125 | 62.5 |
| 88g | 15.62 | 7.81 | 7.81 | 7.81 | 15.62 | 7.81 | 7.81 | 31.25 | 7.81 |
| 88h | 31.25 | 15.62 | 15.62 | 15.62 | 15.62 | 15.62 | 7.81 | 31.25 | 15.62 |
| 88i | 15.62 | 7.81 | 3.9 | 7.81 | 7.81 | 15.62 | 7.81 | 15.62 | 7.81 |
| 88j | 31.25 | 31.25 | 15.62 | 31.25 | 31.25 | 62.5 | 15.62 | 125 | 31.25 |
| 88k | 62.5 | 62.5 | 31.25 | 31.25 | 62.5 | 125 | 31.25 | 125 | 125 |
| 88l | 31.25 | 15.62 | 15.62 | 15.62 | 31.25 | 15.62 | 7.81 | 31.25 | 15.62 |
| Ciprofloxacin | 15.62 | 7.81 | 7.81 | 7.81 | 15.62 | 7.81 | 3.9 | - | - |
| Ketoconazole | - | - | - | - | - | - | - | 15.62 | 7.81 |
Sa: Staphylococcus aureus ATCC 9144; Se: Staphylococcus epidermidis ATCC 155; Ml: Micrococcus luteus ATCC 4698; Bc: Bacillus cereus ATCC 11778; Ec: Escherichia coli ATCC 25922; Pa: Pseudomonas aeruginosa ATCC 2853; Kp: Klebsiella pneumoniae ATCC 11298, An: Aspergillus niger ATCC 9029; Af: Aspergillus fumigatus ATCC 46645.
Dawoud et al., reported the synthesis of a benzimidazole–pyrazole compound 90 from chalcone intermediate 89 (Scheme 25). It was found by the agar diffusion method that compound 90 possessed good antimicrobial activity against Escherichia coli, Salmonella. SP., Staphylococcus aurous and Candida albicans [79].
Scheme 25.
Synthesis of benzimidazole–pyrazole 90.
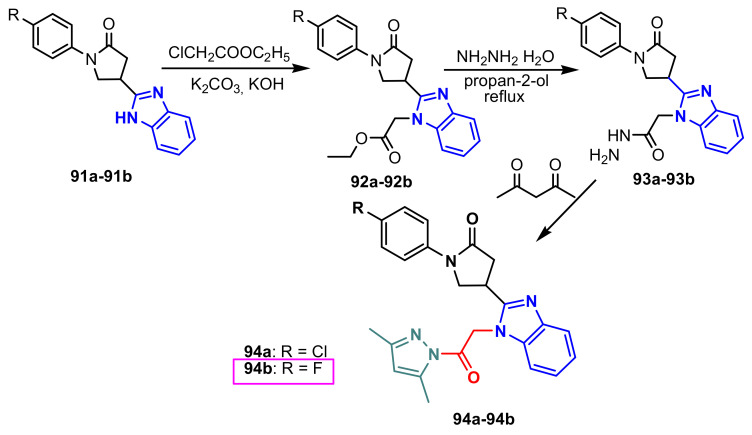
Tumosienė et al., reported the synthesis of the benzimidazole–pyrazole compounds 94a–94b, in three steps, from 2-substituted benzimidazoles 91a–91b (Scheme 26). It was found that compound 94b shows good antimicrobial activity against Staphylococcus aureus ATCC 9144 and Escherichia coli ATCC 8739 (250 μg mL−1) [80].
Scheme 26.
Synthesis of benzimidazole–pyrazoles 94a–94b.
2.3. Benzimidazoles Substituted in the Position “4” (“7”) with Pyrazole Moiety
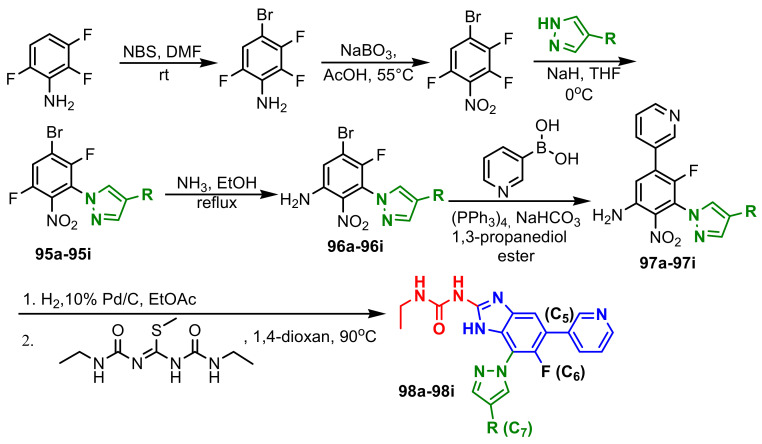
Grilot et al., reported the synthesis of second-generation antibacterial benzimidazole–pyrazoles from 2,3,6-trifluorobenzenamine in seven steps, as can be seen in Scheme 27 [81]. Compound 98a, with a 3-pyridine moiety at C5, which maintains a hydrogen bond with Arg136, showed a reasonable MIC against S. aureus (0.25 μg mL−1). It can be observed that introduction of a fluorine atom at C6 on pyrazole 98b, has no improved antibacterial potency (0.25 μg mL−1 against S. aureus), nor affinity for Gyrase B and Topoisomerase IV, as previously reported [80], but oral exposure was improved 2-fold, which led to the exclusive focus on C6-fluorobenzimidazole pyrazoles. The authors also studied the variation of MIC and the polarity of molecules with the introduction of the substituent in the “5” position, as seen in Table 11. Compound 98f showed a MIC against S. aureus of 0.125 µg mL−1 and could be improved slightly by the addition of a methyl group on the C5 substituent, as in 98g and its resolution yielded compounds 98h and 98i. The (S)-isomer 98i was 4-fold more potent than the (R)-isomer 98h against S. aureus and showed acceptable oral exposure, but compound 98h exhibited a high serum shift (16-fold).
Scheme 27.
Synthesis of benzimidazole–pyrazoles 98a–98i.
Table 11.
MIC values and SAR studies in pyrazole series 17a–17i.
| Compounds | Minimum Inhibitory Concentration μg/mL | |||||||
|---|---|---|---|---|---|---|---|---|
| C5 | C6 | C7′ | Sa | Sa + HS | E fs | E fm | S p | |
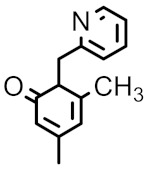
| 98a |

|
H | H | 0.25 | 4 | 0.063 | - | 0.016 |
| 98b |

|
F | H | 0.25 | 2 | 0.063 | - | 0.032 |
| 98c |
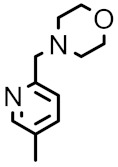
|
F | H | 0.25 | 1 | 0.125 | 0.5 | 0.016 |
| 98d |

|
F | Me | 0.063 | 0.5 | 0.063 | 0.25 | 0.016 |
| 98e |
|
F | Me | 0.016 | 0.125 | 0.016 | 0.032 | < 0.008 |
| 98f |
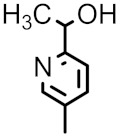
|
F | H | 0.125 | 3 | 0.125 | 0.5 | 0.032 |
| 98g |

|
F | H | 0.063 | 2 | 0.125 | 0.25 | 0.032 |
| 98h |
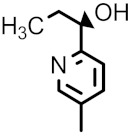
|
F | H | 0.25 | 4 | 0.063 | 0.25 | 0.016 |
| 98i |
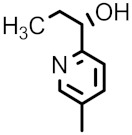
|
F | H | 0.063 | 1 | 0.063 | 0.5 | 0.063 |
Sa = S. aureus; Sa + HS = S. aureus + 50% human serum; E fs = E. faecalis; E fm = E. faecium; S p = S. pneumoniae.
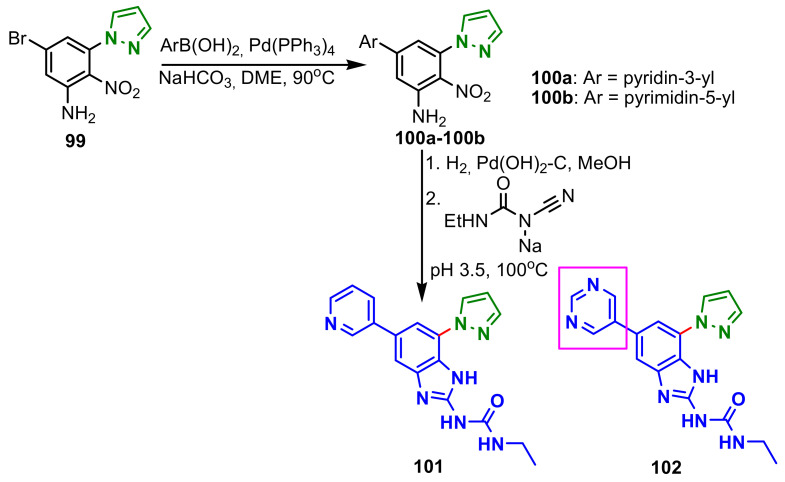
Charifson et al., reported the synthesis of benzimidazole–pyrazoles 101–102 from 5-bromo-2-nitro-3-(1H-pyrazol-1-yl)benzenamine 99, using a Suzuki coupling reaction (Scheme 28) [82]. The compounds were found to be inhibitors of DNA Gyrase and Topoisomerase IV, with potent antibacterial activity. The results of the evaluation for enzymatic inhibition and antibacterial potency of the compounds are shown in Table 12. The superior enzymatic and antibacterial inhibitory activity of compound 102 vs. 101 is observed, due to the presence of the pyrimidine nucleus in the molecule.
Scheme 28.
Synthesis of benzimidazole–pyrazoles 101 and 102.
Table 12.
Gyrase and Topoisomerase IV Inhibition and antibacterial activities of the compounds.
| Compound | Enzyme Inhibiton Data Ki (μM) | Minimum Inhibitory Concentration (μg/mL) | ||||
|---|---|---|---|---|---|---|
| S. aureus gyrase | E. coli gyrase | E. coli topoIV | S. aureus | S.pneumoniae | H. influenzae | |
| 101 | 0.015 | <0.004 | 0.046 | 0.063 | 0.008 | 1 |
| 102 | 0.016 | <0.004 | 0.058 | 0.031 | <0.008 | 0.25 |
3. Biological Activities of Antimicrobial Benzimidazole–Pyrazole Compounds
3.1. Analgesic and Anti-Inflammatory Activity of Antimicrobial Benzimidazole–Pyrazole Compounds
Benzimidazole and pyrazole/pyrazoline are important nitrogen-containing heterocyclics in anti-inflammatory research [83,84]. In recent decades, there have been various studies on the anti-inflammatory activity of benzimidazole compounds.
Chikkula et al., reported the analgesic activity [65] of the compounds 39a–39i (Scheme 11). The analgesic activity of novel benzimidazole derivatives 39a–39i varied with reaction time. The compounds displayed moderate analgesic activity at 30 min of reaction time. In the second hour, the analgesic activity reached to peak level. Additionally, the presence of phenyl ring at the N-1 atom of pyrazole ring significantly increased analgesic activity. The anti-inflammatory activity of the synthesized compounds varies similarly to their analgesic activity. Compounds 39c, 39d and 39e had the best analgesic activity.
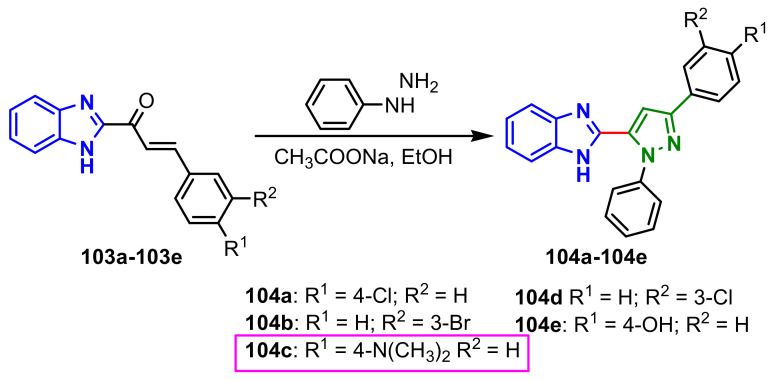
Arora et al., reported the synthesis of the compounds 104a–104e from chalcones 103a–103e (Scheme 29) [85]. The in vivo studies for analgesic activity were evaluated in albino mice by Eddy’s hot plate method. The compound 104a showed mild activity at a dose of 100 and 200 mg kg−1 in the range of 7.38 ± 0.12–7.36 ± 0.15 at 90 min when compared with standard drug Diclofenac sodium at a dose of 5 mg kg−1 in the range of 9.45 ± 0.28 at 90 min. Additionally, the compound 104a showed slightly less moderate activity in the range of 53.03 and 59.09% at a dose of 100 and 200 mg kg−1 at a time interval of 3h of carrageenan challenge when compared to compound 104c (63.63%) as well as when compared with standard Diclofenac sodium at the same time period exhibited 69% of activity, respectively, at a dose of 100 mg kg−1.
Scheme 29.
Synthesis of benzimidazole–pyrazoles 104a–104e.
3.2. Benzimidazole–Pyrazole Compounds with Antitumor Activities
Kalirajan et al., [75] reported anticancer activity of compounds 80a–80h and 81a–81h against MCF7 human breast cell line by in vitro Sulforhodamine B assay (SRB assay) method. The compounds 80b, 81a and 81b have significant activity when compared with standard drug Doxorubicin (Adriamycin, ADR), with GI50 values of 16.3 μg mL−1, 16.0 μg mL−1, and 17.1 μg mL−1, respectively (Doxorubicin with GI50 value <10 μg mL−1). El-Gohary and Shaaban [76] reported the antitumor activity of compounds 83a and 83b and the results are shown in Table 13. Compound 83a, exhibiting the highest in vitro antitumor activity, was evaluated for in vivo antitumor activity against EAC in mice. Additionally, compound 83a was assessed for in vitro cytotoxicity toward human normal lung fibroblast (W138) cell line employing MTT assay [86,87,88] and utilizing 5-fluorouracil as a standard cytotoxic drug. IC50 value for 83a was determined 0.246 mM, therefore less cytotoxic than 5-fluorouracil (IC50 = 0.051 mM). Additionally, results of the DNA-binding assay confirmed that antimicrobial and antitumor compound 83a exerts its biological activities through interaction with DNA. Bezimidazole–pyrazole 85 displayed eminent activity toward HCT-116 cell line [77] with IC50 value of 5.41 μM close to that of 5-fluorouracil of 4.00 μM and lower than that measured for the previously prepared benzimidazoles [76,89].
Table 13.
In vitro antitumor activity of the compounds 83a–83b toward Hep-G2, HCT-116 and MCF-7 cancer cell lines.
| Compound | IC50 (mM) | ||
|---|---|---|---|
| HepG2 | HCT-116 | MCF-7 | |
| 83a | 0.054 | 0.042 | 0.062 |
| 83b | 0.265 | 0.20 | 0.281 |
| 5-Fluorouracil | 0.061 | 0.041 | 0.0415 |
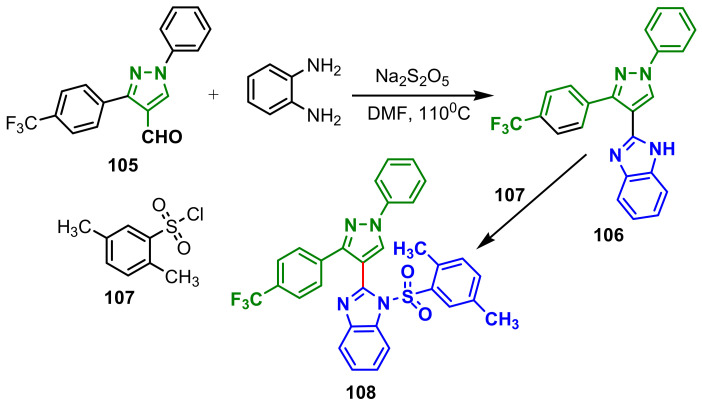
Wang et al., synthesized a series of benzimidazole grafted benzsulfamide-containing pyrazole ring derivatives using a strategy in two steps: 1. synthesis of benzimidazole–pyrazole hybrid by reaction of pirazolecarbaldehyde 105 with 1,2,-phenylenediamine and 2. reaction with sulfonyl chloride 107 with the hybrid 106 (Scheme 30) [90]. Compound 108 showed the most excellent inhibition against tubulin assembly (IC50 = 1.52 μM) and in vitro growth inhibitory activity against a panel of four human cancer cell lines, IC50 = 0.15, 0.21, 0.33 and 0.17 μM, respectively, for A549, Hela, HepG2 and MCF-7. Additionally, compound 108 validly induces A549 cell apoptosis, causes cell cycle arrest in the G2/M phase and disrupts the cellular microtubule network. Due to these promising results, along with molecular docking data, compound 108 is a potential anticancer agent.
Scheme 30.
Synthesis of benzimidazole–pyrazole 108.
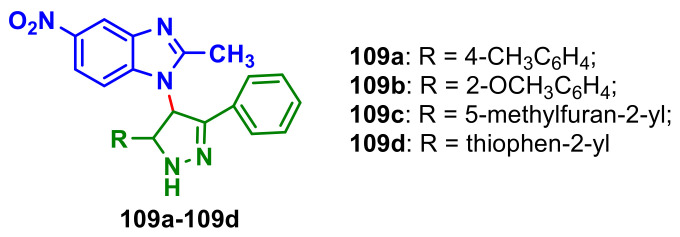
Shake et al., reported synthesis of 1-substituted benzimidazoles with pyrazole moiety 109a–109d (Figure 3), by cyclization of the corresponding chalcones with hydrazine hydrate in ethanol, and their antitumor and antiviral activities [91]. The in vitro cytotoxic screening of the compounds 109a–109d against four different cell lines is showed in Table 14. It can be seen that compound 109d has the best antitumor activity on all determined tumor lines.
Figure 3.
Benzimidazole–pyrazoles 109a–109d.
Table 14.
In vitro antitumor activity of the compounds 109a–109d toward A-549, HCT-116, Hep-G2 and MCF-7 cancer cell lines.
| Compounds | % Inhibitory Activity against Tumor Cell Lines at 300 μM | |||
|---|---|---|---|---|
| A-549 | HCT-116 | Hep-G2 | MCF-7 | |
| 109a | 7.2 | 81.1 | 40.0 | 35.1 |
| 109b | 38.3 | 65.5 | 60.8 | 49.4 |
| 109c | 0.0 | 35.3 | 41.7 | 39.5 |
| 109d | 48.8 | 49.9 | 62.4 | 59.6 |
| Doxorubicin | 84.1 ± 12.6 | 111.7 ± 20.5 | 63.6 ± 9.4 | 163.8 ± 10.1 |
3.3. Benzimidazole–Pyrazole Compounds as Antioxidants
Saundane et al., evaluated the scavenging effects of the compounds 31a–31c and 32a–32c [63] on the DPPH radical by Hatano’s method [92]. The RSA (Radical Scavenging Activity) results suggested that the compound 31a exhibited good antioxidant activity of 71.95 and 72.43 % at the concentration of 100 μg mL−1. Additionally, the reductive ability of synthesized compounds was assessed by the extent of conversion of Fe3+/ferricyanide complex to the Fe2+/ferrous form. The reductive ability results suggested that the compound 31a exhibited good reducing power ability at the concentration of 100 μg mL−1.
Bassyouni et al., reported that compounds 77a, 77b and 78b displayed mild antioxidant activity, of 227.9 μmol L−1, 412.7 μmol L−1 and 361.8 μmol L−1, respectively, using the 2,2-diphenyl-1-picrylhydrazyl radical scavenging assay [74].
Durgamma et al., reported the synthesis of amido-linked benzimidazolyl–pyrazoles 110a–110c (Figure 4) from the corresponding chalcones [93]. All compounds possess good antioxidant activity in the DPPH method, with IC50 values of 0.121 μmol mL−1, 0.127 μmol mL−1 and 0.135 μmol mL−1, respectively, compared to ascorbic acid with IC50 = 0.256 μmol mL−1.
Figure 4.
Benzimidazole–pyrazoles 109a–109d.
3.4. Benzimidazole–Pyrazole Compounds as Antiulcer Agents
Noor et al., synthesized the benzimidazole–pyrazole hybrids 113a–113f by reaction of benzimidazole–hydrazide 111 with chalcones 112a–112f in acetic acid (Scheme 31) [94]. The antiulcer activity of all the benzimidazole–pyrazole hybrids was tested in vivo by an ethanol-induced gastric ulcer model in rats, with Omeprazole (30 mg/kg) as a standard. All compounds 113a–113f exhibited an antiulcer effect.
Scheme 31.
Synthesis of benzimidazole–pyrazoles 113a–113f.
4. Conclusions
This review summarizes the syntheses of benimidazole–pyrazole compounds with antimicrobial properties, as well as their biological activities mentioned in the literature. From the data presented, it can be concluded that hybrids with pyrazole moiety in position “4” (“7”) possess the strongest antimicrobial properties. The presence of certain groups grafted on the benzimidazole and pyrazole nuclei, such as -COOCH3, -NHCO, -CHO, -CF3, -NO2, -CN, -F, -Cl, -OH, OCH3, -N(CH3)2 as well as other heterocycles in the molecule (pyridine, pyrimidine, thiazole, indole), increases the antimicrobial activity of the compounds [95]. Additionally, the binding linker between benzimidazole and pyrazole is important for their antimicrobial activity. Additionally, the antimicrobial activity is improved if the molecule contains linker groups such as carbonyl (CO), amide (NHCO), or other heteroatoms. We hope that this review will be a starting point for the synthesis of other benzimidazole–pyrazole hybrids with antimicrobial properties, which have much better bacterial and antifungal properties than those of antibiotics marketed or used in hospitals today.
Acknowledgments
The author is thankful to Department of Organic Chemistry, Biochemistry and Catalysis, for providing necessary facilities to carry out this research work.
Abbreviations
| AcOH | Acetic acid |
| AcONa | Sodium acetate |
| BTBA | Benzyltributylammonium chloride |
| CTAB | etyltrimethylammonium bromide |
| DCC | N,N′-dicyclohexylcarbodiimide |
| DMAP | N,N-dimethylpyridin-4-amine |
| DME | Dimethoxyethane |
| DMF | Dimethylformamid |
| DMSO | Dimethylsulfoxid |
| EDC*HCl | N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride |
| Et | ethyl |
| EtOH | Ethanol |
| MeOH | Methanol |
| NBS | N-Bromsuccinimid |
| PPA | polyphosphoric acid |
| Ph | phenyl |
| Py | pyridine |
| TEA | triethilamine |
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Breijyeh Z., Jubeh B., Karaman R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules. 2020;25:1340. doi: 10.3390/molecules25061340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balasubramaniam B., Prateek , Ranjan S., Saraf M., Kar P., Singh S.P., Thakur V.K., Singh A., Gupta R.K. Antibacterial and Antiviral Functional Materials: Chemistry and Biological Activity toward Tackling COVID-19-like Pandemics. ACS Pharmacol. Transl. Sci. 2021;4:8–54. doi: 10.1021/acsptsci.0c00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nasir N., Rehman F., Omair S.F. Risk factors for bacterial infections in patients with moderate to severe COVID-19 case: A case-control study. J. Med. Virol. 2021;93:4564–4569. doi: 10.1002/jmv.27000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khalifa M.E., Gobouri A.A., Kabli F.M., Altalhi T.A., Almalki A.S.A., Mohamed M.A. Synthesis, antibacterial, and anti HepG2 cell line human hepatocyte carcinoma activity of some new potentially benzimidazole-5-(aryldiazenyl)thiazole derivatives. Molecules. 2018;23:3285. doi: 10.3390/molecules23123285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onnis V., Demurtas M., Deplano A., Balboni G., Baldisserotto A., Manfredini S., Pacifico S., Liekens S., Balzarini J. Design, synthesis and evaluation of antiproliferative activity of new benzimidazolehydrazones. Molecules. 2016;21:579. doi: 10.3390/molecules21050579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheong J.E., Zaffagni M., Chung I., Xu Y., Wang Y., Jernigan F.E., Zetter B.R., Sun L. Synthesis and anticancer activity of novel water soluble benzimidazole carbamates. Eur. J. Med. Chem. 2018;144:372–385. doi: 10.1016/j.ejmech.2017.11.037. [DOI] [PubMed] [Google Scholar]
- 7.Bistrovic A., Krstulovic L., Harej A., Grbcic P., Sedic M., Kostrun S., Pavelic S.K., Bajic M., Raic-Malic S. Design, synthesis and biological evaluation of novel benzimidazole amidines as potent multi-target inhibitors for the treatment of non-small cell lung cancer. Eur. J. Med. Chem. 2018;143:1616–1634. doi: 10.1016/j.ejmech.2017.10.061. [DOI] [PubMed] [Google Scholar]
- 8.Aragón-Muriel A., Liscano Y., Upegui Y., Robledo S.M., Ramírez-Apan M.T., Morales-Morales D., Oñate-Garzón J., Polo-Cerón D. In Vitro Evaluation of the Potential Pharmacological Activity and Molecular Targets of New Benzimidazole-Based Schiff Base Metal Complexes. Antibiotics. 2021;10:728. doi: 10.3390/antibiotics10060728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karaburun A.C., Çavusoglu B.K., Çevik U.A., Osmaniye D., Saglık B.N., Levent S., Özkay Y., Atlı O., Koparal A.S., Kaplancıklı Z.A. Synthesis and antifungal potential of some novel benzimidazole-1,3,4-oxadiazole compounds. Molecules. 2019;24:191. doi: 10.3390/molecules24010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H.-Z., He S.-C., Peng Y.-J., Zhang H.-J., Gopala L., Tangadanchu V.K.R., Gan L.-L., Zhou C.-H. Design, synthesis and antimicrobial evaluation of novel benzimidazole-incorporated sulfonamide analogues. Eur. J. Med. Chem. 2017;136:165–183. doi: 10.1016/j.ejmech.2017.04.077. [DOI] [PubMed] [Google Scholar]
- 11.Ai X., Pu X., Yi Y., Liu Y., Xu S., Liang J., Shang R. Synthesis and pharmacological evaluation of novel pleuromutilin derivatives with substituted benzimidazole moieties. Molecules. 2016;21:1488. doi: 10.3390/molecules21111488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu H.-B., Gao W.-W., Tangadanchu V.K.R., Zhou C.-H., Geng R.-X. Novel aminopyrimidinyl benzimidazoles as potentially antimicrobial agents: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2018;143:66–84. doi: 10.1016/j.ejmech.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 13.Marinescu M. Chemistry and Applications of Benzimidazole and Its Derivatives. IntechOpen; London, UK: 2019. pp. 11–130. [Google Scholar]
- 14.Marinescu M., Tudorache D.G., Marton G.I., Zalaru C.M., Popa M., Chifiriuc M.C., Stavarache C.E., Constantinescu C. Density functional theory molecular modeling, chemical synthesis, and antimicrobial behaviour of selected benzimidazole derivatives. J. Mol. Struct. 2017;1130:463–471. doi: 10.1016/j.molstruc.2016.10.066. [DOI] [Google Scholar]
- 15.Márquez-Navarro A., Nogueda-Torres B., Hernández-Campos A., Soria-Arteche O., Castillo R., Rodríguez-Morales S., Yépez-Mulia L., Hernández-Luis F. Anthelmintic activity of benzimidazole derivatives against Toxocara canis second-stage larvae and Hymenolepis nana adults. Acta Trop. 2009;109:232–235. doi: 10.1016/j.actatropica.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Bansal Y., Silakari O. The therapeutic journey of benzimidazoles: A review. Bioorg. Med. Chem. 2012;20:6208–6236. doi: 10.1016/j.bmc.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Hadole C.D., Rajput J.D., Bendre R.S. Concise on some biologically important 2-substituted benzimidazole derivatives. Curr. Chem. Curr. Res. 2018;7:195. doi: 10.4172/2161-0401.1000195. [DOI] [Google Scholar]
- 18.Shin J.M., Kim N. Pharmacokinetics and pharmacodynamics of the proton pump inhibitors. J. Neurogastroenterol. Motil. 2013;19:25–35. doi: 10.5056/jnm.2013.19.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaba M., Singh S., Mohan C. Benzimidazole: An emerging scaffold for analgesic and anti-inflammatory agents. Eur. J. Med. Chem. 2014;76:495–505. doi: 10.1016/j.ejmech.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 20.Bansal Y., Kaur M., Silakari O. Benzimidazole–ibuprofen/mesalamine conjugates: Potential candidates for multifactorial diseases. Eur. J. Med. Chem. 2015;89:671–682. doi: 10.1016/j.ejmech.2014.10.081. [DOI] [PubMed] [Google Scholar]
- 21.Zhu W., Bao X., Ren H., Da Y., Wu D., Li F., Yan Y., Wang L., Chen Z. N-Phenyl indole derivatives as AT1 antagonists with anti-hypertension activities: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2016;115:161–178. doi: 10.1016/j.ejmech.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 22.Kim T.H., Yang H.Y., Park B.G., Jung S.Y., Park J.H., Park K.D., Min S.J., Tae J., Yang H., Cho S., et al. Discovery of benzimidazole derivatives as modulators of mitochondrial function: A potential treatment for Alzheimer’s disease. Eur. J. Med. Chem. 2017;125:1172–1192. doi: 10.1016/j.ejmech.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Cheretaev I.V., Korenyuk V., Nozdrachev A.D. Neurotropic, psychoactive, and analgesic properties of benzimidazole and its derivatives: Physiological mechanisms. Neurosci. Behav. Physiol. 2018;48:848–853. doi: 10.1007/s11055-018-0639-8. [DOI] [Google Scholar]
- 24.Kumbhar S.S., Choudhari P.B., Bhatia M.S. 3D QSAR and Pharmacophore modelling of selected benzimidazole derivatives as factor IXa inhibitors. Indian J. Pharm. Sci. 2017;79:813–819. doi: 10.4172/pharmaceutical-sciences.1000295. [DOI] [Google Scholar]
- 25.Aboul-Enein H.Y., El Rashedy A.A. Benzimidazole derivatives as antidiabetic agents. Med. Chem. 2015;5:318–325. doi: 10.4172/2161-0444.1000280. [DOI] [PubMed] [Google Scholar]
- 26.Domiati S., El-Mallah A., Ghoneim A., Bekhit A., Razik H.A.E. Evaluation of anti-inflammatory, analgesic activities, and side effects of some pyrazole derivatives. Inflammopharmacology. 2016;24:163–172. doi: 10.1007/s10787-016-0270-7. [DOI] [PubMed] [Google Scholar]
- 27.Karrouchi K., Radi S., Ramli Y., Taoufik J., Mabkhot Y.N., Al-aizari F.A., Ansar M. Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review. Molecules. 2018;23:134. doi: 10.3390/molecules23010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moraes P.A., Brum E.S., Brusco I., Marangoni M.A., Lobo M.M., Camargo A.F., Nogara P.A., Bonacorso H.G., Martins M.A.P., Da Rocha J.B., et al. Pyrazole-Enaminones as Promising Prototypes for the Development of Analgesic Drugs. ChemistrySelect. 2020;5:14620–14625. doi: 10.1002/slct.202004049. [DOI] [Google Scholar]
- 29.Gao M., Qu K., Zhang W., Wang X. Pharmacological activity of pyrazole derivatives as an anticonvulsant for benefit against epilepsy. Neuroimmunomodulation. 2021;28:90–97. doi: 10.1159/000513297. [DOI] [PubMed] [Google Scholar]
- 30.Mandour A.H., El-Sawy E.R., Ebaid M.S., Hassan S.M. Synthesis and potential biological activity of some novel 3-[(N-substituted indol-3-yl)methyleneamino]-6-amino-4-aryl-pyrano(2,3-c)pyrazole-5-carbonitriles and 3,6-diamino-4-(N-substituted indol-3-yl)pyrano(2,3-c)pyrazole-5-carbonitriles. Acta Pharm. 2012;62:15–30. doi: 10.2478/v10007-012-0007-0. [DOI] [PubMed] [Google Scholar]
- 31.Santos N.E., Carreira A.R.F., Silva V.L.M., Braga S.S. Natural and Biomimetic Antitumor Pyrazoles, A Perspective. Molecules. 2020;25:1364. doi: 10.3390/molecules25061364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang X., Zang J., Zhu M., Gao Q., Wang B., Xu W., Zhang Y. Design, Synthesis, and Antitumor Evaluation of 4-Amino-(1H)-pyrazole Derivatives as JAKs Inhibitors. ACS Med. Chem. Lett. 2016;7:950–955. doi: 10.1021/acsmedchemlett.6b00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du K., Xia C., Wei M., Chen X., Zhang P. Microwave-assisted rapid synthesis of sugar-based pyrazole derivatives with anticancer activity in water. RSC Adv. 2016;6:66803–66806. doi: 10.1039/C6RA05284C. [DOI] [Google Scholar]
- 34.Ismail M.F., El-sayed A.A. Synthesis and in-vitro antioxidant and antitumor evaluation of novel pyrazole-based heterocycles. J. Iran. Chem. Soc. 2019;16:921–937. doi: 10.1007/s13738-018-1566-x. [DOI] [Google Scholar]
- 35.Datar P.A., Jadhav S.R. Design and Synthesis of Pyrazole-3-one Derivatives as Hypoglycaemic Agents. Int. J. Med. Chem. 2015;2015:670181. doi: 10.1155/2015/670181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naim M.J., Alm O., Alam M.J., Shaquiquzzaman M., Alam M.M., Naidu V.G.M. Synthesis, docking, in vitro and in vivo antidiabetic activity of pyrazole-based 2,4-thiazolidinedione derivatives as PPAR-γ modulators. Arch. Pharm. 2018;351:1700223. doi: 10.1002/ardp.201700223. [DOI] [PubMed] [Google Scholar]
- 37.Cetin A., Bildirici I. A study on synthesis and antimicrobial activity of 4-acyl-pyrazoles. J. Saudi Chem. Soc. 2018;22:279–296. doi: 10.1016/j.jscs.2016.05.008. [DOI] [Google Scholar]
- 38.Harikrishna N., Arun M., Isloor A.M., Ananda K., Obaid A., Fun H.K. Synthesis, and antitubercular and antimicrobial activity of 1′-(4-chlorophenyl)pyrazole containing 3,5-disubstituted pyrazoline derivatives. New J. Chem. 2016;40:73–76. doi: 10.1039/C5NJ02237A. [DOI] [Google Scholar]
- 39.Ardiansah B. A Recent update: Antimicrobial agents containing pyrazole nucleus. Asian J. Pharm. Clin. Res. 2018;11:88–94. doi: 10.22159/ajpcr.2018.v11i12.29418. [DOI] [Google Scholar]
- 40.Metwally N.H., Ragab E.A., Mohamed M.S. Synthesis of some novel N5-sulfonylated and N1-alkyated pyrazole derivatives and their antimicrobial activity in conjunction with molecular docking study. J. Heterocycl. Chem. 2020;57:1698–1713. doi: 10.1002/jhet.3895. [DOI] [Google Scholar]
- 41.Whitt J., Duke C., Ali M.A., Chambers S.A., Khan M.M.K., Gilmore D., Alam M.A. Synthesis and antimicrobial studies of 4-[3-(3-Fluorophenyl)-4-formyl-1H-pyrazol-1-yl]benzoic Acid and 4-[3-(4-Fluorophenyl)-4-formyl-1H-pyrazol-1-yl]benzoic acid as potent growth inhibitors of drug-resistant bacteria. ACS Omega. 2019;4:14284–14293. doi: 10.1021/acsomega.9b01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Azmi A., Mahmoud H. Facile Synthesis and antimicrobial activities of novel 1,4-bis(3,5-dialkyl-4H-1,2,4-triazol-4-yl) benzene and 5-aryltriaz-1-en-1-yl-1-phenyl-1H-pyrazole-4-carbonitrile derivatives. ACS Omega. 2020;5:10160–10166. doi: 10.1021/acsomega.0c01001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zalaru C., Dumitrascu F., Draghici C., Tarcomnicu I., Tatia R., Moldovan L., Chifiriuc M.C., Lazar V., Marinescu M., Nitulescu M.G., et al. Synthesis, spectroscopic characterization, DFT study and antimicrobial activity of novel alkylaminopyrazole derivatives. J. Mol. Struct. 2018;1156:12–21. doi: 10.1016/j.molstruc.2017.11.073. [DOI] [Google Scholar]
- 44.Uamaru N., Shigematsu H., Toda A., Eyanagi R., Kitamura S., Ohta S. Design, synthesis, and pharmacological activity of nonallergenic pyrazolone-Type antipyretic analgesics. J. Med. Chem. 2010;53:8727–8733. doi: 10.1021/jm101208x. [DOI] [PubMed] [Google Scholar]
- 45.Padmini T., Kamal B.R. Synthesis, Anti-inflammatory, Analgesic and Antipyretic Activity of Novel 1,3,5-Trisubstituted Pyrazole Derivatives. Asian J. Chem. 2019;31:1225–1229. doi: 10.14233/ajchem.2019.21781. [DOI] [Google Scholar]
- 46.Desideri N., Fioravanti R., Monaco L.P., Atzori E.M., Carta A., Delogu I., Collu G., Loddo R. Design, Synthesis, Antiviral evaluation, and SAR studies of new 1-(phenylsulfonyl)-1H-pyrazol−4-yl-methylaniline derivatives. Front. Chem. 2019;7:214. doi: 10.3389/fchem.2019.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corona A., Onnis V., Deplano A., Bianco G., Demurtas M., Distinto S., Cheng Y.C., Alcaro S., Esposito F., Tramontano E. Design, synthesis and antiviral evaluation of novel heteroarylcarbothioamide derivatives as dual inhibitors of HIV-1 reverse transcriptase-associated RNase H and RDDP functions. Pathog. Dis. 2017;75:ftx078. doi: 10.1093/femspd/ftx078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pandya K.M., Patel A.H., Desai P.S. Development of Antimicrobial, Antimalarial and Antitubercular Compounds Based on a quinoline-pyrazole clubbed scaffold derived via Doebner reaction. Chem. Afr. 2020;3:89–98. doi: 10.1007/s42250-019-00096-5. [DOI] [Google Scholar]
- 49.Kumar G., Tanwar O., Kumar J., Akhter S.M., Sharma S., Pillai C.R., Alam M.M., Zama M.S. Pyrazole-pyrazoline as promising novel antimalarial agents: A mechanistic study. Eur. J. Med. Chem. 2018;149:139–147. doi: 10.1016/j.ejmech.2018.01.082. [DOI] [PubMed] [Google Scholar]
- 50.Zalaru C., Dumitrascu F., Draghici C., Iovu M., Marinescu M., Tarcomnicu I., Nitulescu G.M. Synthesis and biological screening of some novel 2-(1H-pyrazol-1-yl)-acetamides as lidocaine analogue. Indian J. Chem. B. 2014;53:733–739. [Google Scholar]
- 51.Refat M.S., Hamza R.Z., Adam A.M.A., Saad H.A., Gobouri A.A., Al-Salmi F.A., Altalhi T., El-Megharbel S.M. Synthesis of N,N′-bis(1,5-dimethyl-2-phenyl-1,2-dihydro-3-oxopyrazol-4-yl)sebacamide that ameliorate osteoarthritis symptoms and improve bone marrow matrix structure and cartilage alterations induced by monoiodoacetate in the rat model: “Suggested potent anti-inflammatory agent against COVID-19”. Hum. Exp. Toxicol. 2021;40:325–341. doi: 10.1177/0960327120945779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Masih A., Agnihotri A.K., Srivastava J.K., Pandey N., Bhat H.R., Singh U.P. Discovery of novel pyrazole derivatives as a potent anti-inflammatory agent in RAW264.7 cells via inhibition of NF-ĸB for possible benefit against SARS-CoV-2. J. Biochem. Mol. Toxicol. 2021;35:e22656. doi: 10.1002/jbt.22656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Francesconi V., Cichero E., Schenone S., Naesens L., Tonelli M. Synthesis and Biological Evaluation of Novel (thio)semicarbazone-Based Benzimidazoles as Antiviral Agents against Human Respiratory Viruses. Molecules. 2020;25:1487. doi: 10.3390/molecules25071487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Humphries F., Shmuel-Galia L., Jiang Z., Wilson R., Landis P., Ng S.L., Parsi K.M., Maehr R., Cruz J., Morales-Ramos A., et al. A diamidobenzimidazole STING agonist protects against SARS-CoV-2 infection. Sci. Immunol. 2021;6:eabi9002. doi: 10.1126/sciimmunol.abi9002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Desai N.C., Pandya D.D., Joshi V.V., Rajpara K.M., Vaghani H.V., Satodiya H.M. Synthesis, characterization and antimicrobial screening of hybrid molecules containing benzimidazole-pyrazole and pyridine nucleus. Med. Chem. Res. 2012;21:4463–4472. doi: 10.1007/s00044-012-9990-4. [DOI] [Google Scholar]
- 56.Rajora J., Srivastava Y.K. Synthesis and antimicrobial activities of some benzimidazolyl pyrazoles. Rasayan J. Chem. 2009;2:655–658. [Google Scholar]
- 57.Bondock S., Khalifa W., Fadda A.A. Synthesis and antimicrobial activity of some new 4-hetarylpyrazole and furo[2,3-c]pyrazole derivatives. Eur. J. Med. Chem. 2011;46:2555–2561. doi: 10.1016/j.ejmech.2011.03.045. [DOI] [PubMed] [Google Scholar]
- 58.Desai N.C., Pandya D.D., Kotadiya G.M., Desai P. Synthesis and characterization of novel benzimidazole bearing pyrazoline derivatives as potential antimicrobial agents. Med. Chem. Res. 2014;23:1474–1487. doi: 10.1007/s00044-013-0756-4. [DOI] [Google Scholar]
- 59.Kalaria P.K., Satasia S.P., Raval D.K. L-Proline promoted green and regioselective synthesis of a novel pyrazole based trifluoromethylated fused thiazolopyran scaffol and its biological evaluation. RSC Adv. 2014;4:32353–32362. doi: 10.1039/C4RA04283B. [DOI] [Google Scholar]
- 60.Patil S.B., Goudgon N.M. Synthesis of 3-(1–benzy-1H-benzo[d]imidazol–2-yl-amino)–2-(3–aryl-1-phenyl-1H-pyrazol-4-yl) thiazolidin-4-ones and their antimicrobial activities. Int. J. Pharm. Sci. Res. 2010;1:50–56. [Google Scholar]
- 61.Reddy L.M., Prakash T.B., Padmaja A., Padmavathi V. Synthesis and antimicrobial activity of pyrazolyl benzoxazoles, benzothiazoles and benzimidazoles. Med. Chem. Res. 2015;24:970–979. doi: 10.1007/s00044-014-1180-0. [DOI] [Google Scholar]
- 62.Padalkar V.S., Borse B.N., Gupta V.D., Phatangare K.R., Patil V.S., Sekar N. Synthesis and antimicrobial activities of novel 2-[substituted-1H-pyrazol-4-yl] benzothiazoles, benzoxazoles, and benzimidazoles. J. Heterocycl. Chem. 2016;53:1347–1355. doi: 10.1002/jhet.1506. [DOI] [Google Scholar]
- 63.Saundane A.R., Mathada K.N. Synthesis, characterization, and biological evaluation of some new chalcones containing indole moiety and their derivatives. Mon. Chem. 2016;147:1291–1301. doi: 10.1007/s00706-015-1648-8. [DOI] [Google Scholar]
- 64.Padhy G.K., Panda J., Behera A.K. Synthesis and characterization of novel benzimidazole embedded 1,3,5-trisubstituted pyrazolines as antimicrobial agents. J. Serb. Chem. Soc. 2017;82:985–993. doi: 10.2298/JSC160604089P. [DOI] [Google Scholar]
- 65.Chikkula K., Sundararajan R. Analgesic, anti-inflammatory, and antimicrobial activities of novel isoxazole/pyrimidine/pyrazole substituted benzimidazole analogs. Med. Chem. Res. 2017;26:3026–3037. doi: 10.1007/s00044-017-2000-0. [DOI] [Google Scholar]
- 66.Suram D., Thatha S., Venkatapuram P., Adivireddy P. Synthesis and Antimicrobial Activity of a New Class of Benzazolyl Pyrazoles. J. Heterocycl. Chem. 2017;54:3152–3162. doi: 10.1002/jhet.2929. [DOI] [Google Scholar]
- 67.Reddy N.B., Zyryanov G.V., Reddy G.M., Balakrishna A., Padmaja A., Padmavathi V., Reddy C.S., Garcia J.R., Sravya G. Design and synthesis of some new benzimidazole containing pyrazoles and pyrazolyl thiazoles as potential antimicrobial agents. J. Heterocycl. Chem. 2019;56:589–596. doi: 10.1002/jhet.3435. [DOI] [Google Scholar]
- 68.Si W.J., Wang X., Chen M., Wang M.Q., Lu A.M., Yang C.L. Design, synthesis, antifungal activity and 3D-QSAR study of novel pyrazole carboxamide and niacinamide derivatives containing benzimidazole moiety. New J. Chem. 2019;43:3000–3010. doi: 10.1039/C8NJ05150J. [DOI] [Google Scholar]
- 69.Jardosh H.H., Sangani C.B., Patel M.P., Patel R.G. One step synthesis of pyrido[1,2-a]benzimidazole derivatives of aryloxypyrazole and their antimicrobial evaluation. Chin. Chem. Lett. 2013;24:123–126. doi: 10.1016/j.cclet.2013.01.021. [DOI] [Google Scholar]
- 70.Sowdari J., Gudi Y., Donthamsetty S.V., Venkatapuram P., Adivireddy P. Green Approach for the Synthesis of a New Class of Diamidomethane-linked Benzazolyl Pyrazoles and Evaluation as Antifungals. J. Heterocycl. Chem. 2019;56:2080–2089. doi: 10.1002/jhet.3569. [DOI] [Google Scholar]
- 71.Wang Y.T., Shi T.Q., Fu J., Zhu H.L. Discovery of novel bacterial FabH inhibitors (Pyrazol-Benzimidazole amide derivatives): Design, synthesis, bioassay, molecular docking and crystal structure determination. Eur. J. Med. Chem. 2019;171:209–220. doi: 10.1016/j.ejmech.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 72.Chkirate K., Karrouchi K., Dege N., Sebbar N.K., Ejjoummany A., Radi S., Adarsh N.N., Talbaoui A., Ferbinteanu M., Essassi E.M., et al. Co(II) and Zn(II) pyrazolyl-benzimidazole complexes with remarkable antibacterial activity. New J. Chem. 2020;44:2210–2221. doi: 10.1039/C9NJ05913J. [DOI] [Google Scholar]
- 73.Abd Elaziz A.A.E.S., Farag A.M., Alagib I.I.A., Abdallah E.M., Mohammed N.E.A. Evaluation of some new synthesis benzothiazole and benzimidazole derivatives as potential antimicrobial and anticancer agents. Int. J. Adv. Appl. Sci. 2020;7:69–77. doi: 10.21833/ijaas.2020.02.010. [DOI] [Google Scholar]
- 74.Bassyouni F.A., Tawfik H.A., Hamed A.R., Soltan M.M., ElHefnawi M., ElRashedy A.A., Moharam M.E., Rehim M.A. Synthesis, antioxidant, and antimicrobial activities of new 2-(1,5,6-trimethyl-1H-benzo[d]imidazole-2-carbonyl)-2,3-dihydro-1H-pyrazole-4-carbonitriles, (1,3,4-oxadiazol-2-yl)-1Hbenzo[d]imidazol-5-yl)(phenyl)methanones, and (1,3,4-oxadiazol-2-yl)-1,5-dihydro-[1,2,4]triazolo[1,5-a]pyridine-8-carbonitriles: QSAR and molecular docking analysis. Egypt. Pharm. J. 2012;11:80–92. doi: 10.7123/01.EPJ.0000422113.69898.e0. [DOI] [Google Scholar]
- 75.Kalirajan R., Rathore L., Jubie S., Gowramma B., Gomathy S., Sankar S. Microwave assisted synthesis of some novel pyrazole substituted benzimidazoles and evaluation of their biological activities. Indian J. Chem. B. 2011;50:1794–1799. doi: 10.1002/chin.201216123. [DOI] [Google Scholar]
- 76.El-Gohary N.S., Shaaban M.I. Synthesis and biological evaluation of a new series of benzimidazole derivatives as antimicrobial, antiquorum-sensing and antitumor agents. Eur. J. Med. Chem. 2017;131:255–262. doi: 10.1016/j.ejmech.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 77.El-Gohary N.S., Shaaban M.I. Synthesis, antimicrobial, antiquorum-sensing and antitumor activities of new benzimidazole analogs. Eur. J. Med. Chem. 2017;137:439–449. doi: 10.1016/j.ejmech.2017.05.064. [DOI] [PubMed] [Google Scholar]
- 78.Krishnanjaneyulu I.S., Saravanan G., Vamsi J., Supriya P., Bhavana J.U., Kumar M.V.S. Synthesis, characterization and antimicrobial activity of some novel benzimidazole derivatives. J. Adv. Pharm. Technol. Res. 2014;5:21–27. doi: 10.4103/2231-4040.126983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dawoud N.T.A., Mahmoud F.F., Ismil Z.H., Lotfy D.R. Synthesis and Biological Screening of Some New Substituted 1-Acetyl Benzimidazol-2-yl Methyl Isoindoline-1,3-Dione Analogs as Anti-Microbial Agents. J. Chem. Pharm. Res. 2018;10:110–118. [Google Scholar]
- 80.Tumosienė I., Peleckis A., Jonuškienė I., Vaickelionienė R., Kantminiene K., Šiugždaitė J., Beresnevičius Z.J., Mickevičius V. Synthesis of novel 1,2- and 2-substituted benzimidazoles with high antibacterial and antioxidant activity. Mon. Chem. 2018;149:577–594. doi: 10.1007/s00706-017-2066-x. [DOI] [Google Scholar]
- 81.Grillot A.-L., Le Tiran A., Shannon D., Krueger E., Liao Y., O’Dowd H., Tang Q., Ronkin S., Wang T., Waal N., et al. Second-Generation Antibacterial Benzimidazole Ureas: Discovery of a Preclinical Candidate with Reduced Metabolic Liability. J. Med. Chem. 2014;57:8792–8816. doi: 10.1021/jm500563g. [DOI] [PubMed] [Google Scholar]
- 82.Charifson P.S., Grillot A.L., Grossman T.H., Parsons J.D., Badia M., Bellon S., Deininger D.D., Drumm J.E., Gross C.H., LeTiran A., et al. Novel Dual-Targeting Benzimidazole Urea Inhibitors of DNA Gyrase and Topoisomerase IV Possessing Potent Antibacterial Activity: Intelligent Design and Evolution through the Judicious Use of Structure-Guided Design and Stucture-Activity Relationships. J. Med. Chem. 2008;51:5243–5263. doi: 10.1021/jm800318d. [DOI] [PubMed] [Google Scholar]
- 83.Veerasamy R., Roy A., Karunakaran R., Rajak H. Structure–Activity Relationship Analysis of Benzimidazoles as Emerging Anti-Inflammatory Agents: An Overview. Pharmaceuticals. 2021;14:663. doi: 10.3390/ph14070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharma S., Kumar D., Singh G., Monga V., Kumar B. Recent Advancements in the Development of Heterocyclic Anti-Inflammatory Agents. Eur. J. Med. Chem. 2020;200:112438. doi: 10.1016/j.ejmech.2020.112438. [DOI] [PubMed] [Google Scholar]
- 85.Arora R., Kaur A., Gill N.S. Analgesic and Anti-inflammatory Activity of Some Newly Synthesized Novel Pyrazole Derivatives of Benzimidazole. Curr. Res. Chem. 2012;4:76–87. doi: 10.3923/crc.2012.76.87. [DOI] [Google Scholar]
- 86.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 87.Denizot F., Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 88.Gerlier D., Thomasset T. Use of MTT colorimetric assay to measure cell activation. J. Immunol. Methods. 1986;94:57–63. doi: 10.1016/0022-1759(86)90215-2. [DOI] [PubMed] [Google Scholar]
- 89.Madalageri P.M., Kotresh O. Synthesis, DNA protection and antimicrobial activity of some novel chloromethyl benzimidazole derivatives bearing dithiocarbamates. J. Chem. Pharm. Res. 2012;4:2697–2703. [Google Scholar]
- 90.Wang Y.T., Shi T.Q., Zhu H.L., Liu C.H. Synthesis, biological evaluation and molecular docking of benzimidazole grafted benzsulfamide-containing pyrazole ring derivatives as novel tubulin polymerization inhibitors. Bioorg. Med. Chem. 2019;27:502–515. doi: 10.1016/j.bmc.2018.12.031. [DOI] [PubMed] [Google Scholar]
- 91.Shake Y.M., Omar M.A., Mahmoud K., Elhallouty S.M., El-Senousy W.M., Ali M.M., Mahmoud A.E., Abdel-Halim A.H., Soliman S.M., El Diwani H.I. Synthesis, in vitro and in vivo antitumor and antiviral activity of novel 1-substituted benzimidazole derivatives. J. Enzym. Inhib. Med. Chem. 2015;30:826–845. doi: 10.3109/14756366.2014.979344. [DOI] [PubMed] [Google Scholar]
- 92.Saundane A.R., Kirankumar N.M. Synthesis, characterization, and biological evaluation of Schiff bases containing indole moiety and their derivatives. Mon. Chem. 2015;146:1751–1761. doi: 10.1007/s00706-015-1440-9. [DOI] [Google Scholar]
- 93.Durgamma S., Muralikrishna A., Padmavathi V., Padmaja A. Synthesis and antioxidant activity of amido-linked benzoxazolyl/benzothiazolyl/benzimidazolyl-pyrroles and pyrazoles. Med. Chem. Res. 2014;23:2916–2929. doi: 10.1007/s00044-013-0884-x. [DOI] [Google Scholar]
- 94.Noor A., Qazi N.G., Nadeem H., Khan A.U., Paracha R.Z., Ali F., Saeed A. Synthesis, characterization, anti-ulcer action and molecular docking evaluation of novel benzimidazole-pyrazole hybrids. Chem. Cent. J. 2017;11:85. doi: 10.1186/s13065-017-0314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marinescu M., Cinteza L.O., Marton G.I., Chifiriuc M.C., Popa M., Stanculescu I., Zalaru C.M., Stavarache C.E. Synthesis, density functional theory study and in vitro antimicrobial evaluation of new benzimidazole Mannich bases. BMC Chem. 2020;14:45. doi: 10.1186/s13065-020-00697-z. [DOI] [PMC free article] [PubMed] [Google Scholar]