Abstract
Background
Primary obesity surgery endoluminal (POSE) utilizes an incision-less operating platform system to create full-thickness plications in the gastric fundus and body (original POSE). Many studies have demonstrated the safety and efficacy of original POSE for the treatment of obesity.
Objective
We aimed to conduct a systematic review and meta-analysis of available literature in an attempt to evaluate the outcomes of original POSE per the ASGE task force thresholds.
Methods
Bibliographic databases were systematically searched for studies assessing the outcomes of POSE for the treatment of obesity. All randomized controlled trials (RCTs) and observational studies that assessed outcomes of POSE were included. Studies were included if they reported percent total weight loss (%TWL) or percent excess weight loss (%EWL) and the incidence of serious adverse events (SAE).
Results
A total of seven studies with 613 patients were included. Two included studies were RCTs, while the remaining were observational studies. Pooled mean %EWL at 3–6 months and 12–15 months were 42.62 (95% CI 37.56–47.68) and 48.86 (95% CI 42.31–55.41), respectively. Pooled mean %TWL at 3–6 months and 12–15 months was 13.45 (95% CI 8.93–17.97) and 12.68 (95% CI 8.13–17.23), respectively. Subgroup analysis of two RCTs showed that weight loss at 1 year was significantly higher in POSE patients (%EWL difference in means 19.45 (95% CI 4.65–34.24, p value = 0.01). The overall incidence of serious adverse events was only 2.84% and included GI bleeding, extra-gastric bleeding, hepatic abscess, severe pain, severe nausea, and severe vomiting. The mean number of total anchors placed in the fundus and body was 13.18 (95% CI 11.77–14.58), and the mean procedure time was 44.55 min (95% CI 36.44–52.65).
Conclusion
POSE, a minimally invasive endoscopic bariatric therapy, is a safe and effective modality for the treatment of obesity. The outcomes of POSE meet and surpass the ASGE joint task force thresholds. Future studies should evaluate newer versions of this procedure that emphasize gastric body plication sparing the fundus.
Keywords: Primary obesity surgery endoluminal, POSE, Endoscopic gastroplasty, Endoscopic bariatric and metabolic therapies, EBMTs, Endoscopic bariatric therapy, Weight loss
Background
More than 1.9 billion adults are overweight, and 650 million suffer from obesity globally, yet these overwhelming statistics continue to rise [1]. Lifestyle modifications are often recommended but fail to achieve sustained and significant weight loss. Bariatric surgery is an effective long-term option, but only 1–2% of eligible patients undergo surgery [2-4]. Therefore, the majority of obese patients remain untreated.
Endoscopic bariatric and metabolic therapies (EBMTs) are minimally invasive procedures developed to fill the gap between medical and surgical interventions for the treatment of obesity. Primary obesity surgery endoluminal (POSE) utilizes an incision-less operating platform system (IOP) (USGI Medical San Clemente, Calif, USA) to create full-thickness plications in the gastric fundus and body (original POSE), which leads to reduced gastric accommodation and delayed gastric emptying. Many studies have demonstrated the safety and efficacy of POSE, but these studies have shown variable outcomes. Thus, there is a need to systematically review the available POSE studies to resolve uncertainty and better inform physicians and patients about incorporating POSE in clinical practice. Previously published systematic reviews and meta-analyses have evaluated POSE together with other endoscopic gastroplasty (EG) techniques that utilize different devices and mechanisms of action [5-7].
A joint task force of the American Society for Gastrointestinal Endoscopy (ASGE) and the American Society for Metabolic and Bariatric Surgery (ASMBS) defined thresholds for an EBMT in a Preservation and Incorporation of Valuable endoscopic Innovations (PIVI) statement [8, 9]. According to these thresholds, an EBMT intended as a primary obesity intervention should achieve a mean minimum threshold of 25% excess weight loss (%EWL) measured at 12 months. In addition to the absolute threshold of weight loss, the mean %EWL difference between a primary EBT and control groups should be a minimum of 15% EWL and be statistically significant. The risk associated with EBT should equate to a 5% incidence of serious adverse events (SAE). We aimed to conduct a systematic review and meta-analysis of available literature in an attempt to evaluate the outcomes of original POSE per the ASGE task force thresholds.
Methods
Search strategy
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10]. Electronic searches were performed using Ovid Medline, Scopus, Cochrane Register of Controlled Trials, and Web of Science databases from their dates of inception to November 2019. The search strategies are detailed in Supplement 1.
All data were extracted from article texts, tables, and figures with any estimates made based on the presented data and figures. Two investigators (S.S and A.K) independently reviewed each included article, and its eligibility was determined based on predetermined inclusion and exclusion criteria. Any discrepancy resolved by discussion and re-evaluation by senior authors.
Ethics approval was not required for this research. None of the investigators collected data through intervention or interaction with the individual, and no identifiable patient information was collected.
Study selection, data extraction, and quality assessment
All RCTs and observational studies that assessed outcomes of POSE for obesity treatment were included. Studies were included if they reported %EWL or percent total weight loss (%TWL) and adverse events. Studies were excluded if; endoscopic gastroplasty techniques using devices other than the IOP were used, no of patients was < 5 patients because of the bias associated with case reports/small case series and patient in the study have undergone prior endoscopic bariatric therapy or bariatric surgery and overlapping patient cohorts.
Three investigators used a standardized data collection form to extract the following information: Study design, sample size, patient demographics, body mass index (BMI), procedure time, plication patterns, number of plications/anchors, improvement in co-morbidities, adverse events, mortality, weight loss, and other reported outcomes. Primary outcomes of interest were weight loss measured as %TWL or %EWL at follow-up, and severe adverse events (SAE) reported in the included studies.
Quality assessment of randomized controlled trials (RCTs) was done using the NIH Quality Assessment of Controlled Intervention Studies tool. For quality assessment of observational studies, the NIH Quality Assessment for Before-After (Pre-Post) Studies with No Control Group was used. The quality assessment of the studies was done by two independent authors (A.K and S.S). A disagreement on the score was discussed with seniors authors and was resolved by consensus.
Statistical analysis
All statistical analysis was conducted using Comprehensive Meta-Analysis Software Version 3 (Biostat; Englewood, NJ, USA). Mean values for %TWL and %EWL were pooled as weighted means. The pooled means were computed using the DerSimonian and Laird random-effects model. A p value of < 0.05 was considered statistically significant. The I2 statistic was used to estimate heterogeneity across studies, where values of 25, 50, and 75% correspond to cut-off points for low, moderate, and high degrees of heterogeneity. Adverse events reported in included studies were pooled and expressed as a percentage. Studies that did not report standard deviations or if standard deviations could not be calculated, then the reported mean of the study was used as an estimate of its standard deviation to include them in the meta-analysis.
Results
Study selection, characteristics, and quality of included studies
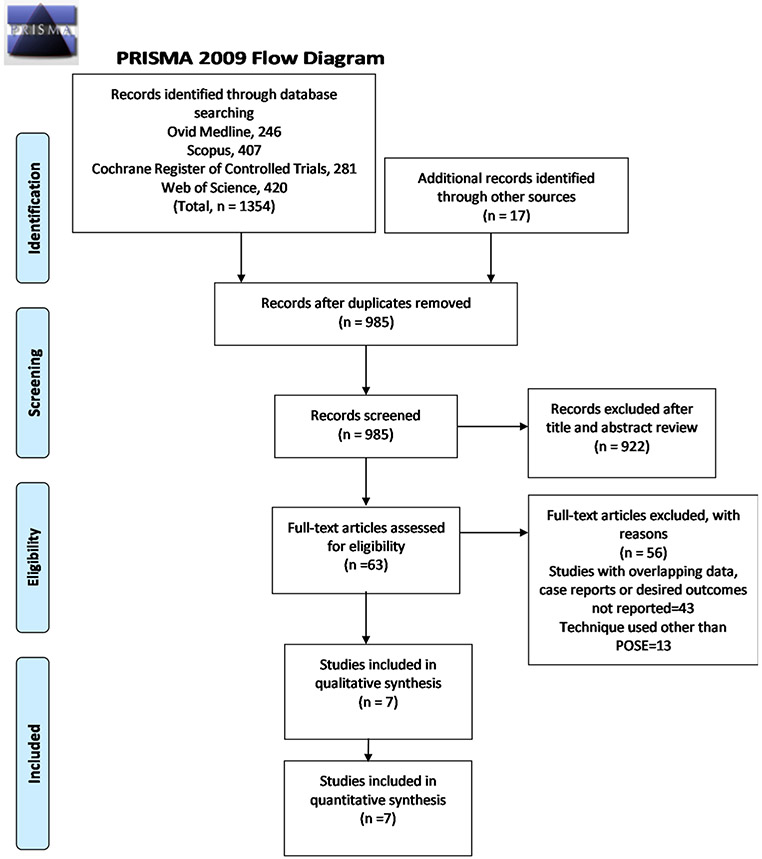
The PRISMA flow diagram for study selection is shown in Fig. 1. Out of a total of 1371 citations, seven studies with 613 patients fulfilling all inclusion criteria were finally included in the meta-analysis [11-17]. Individual study characteristics are summarized in Table 1. Two included studies were RCTs, while remaining were observational studies. Sullivan et al. [15] compared POSE (n = 221) with sham treatment group (n = 111), while Miller et al. [17] compared POSE (n = 34) with diet and exercise (n = 10). All studies were all published from 2013 to 2019. Results of the quality assessment of all included studies were considered good for analysis (Supplement 2).
Fig. 1.
PRISMA flow diagram for study selection
Table 1.
Characteristics of included studies
| Study (Year published) |
Design | Setting | Country | Inclusion criteria | Exclusion criteria |
Total patients (N) |
Gender Female (N) |
Age years Mean (S.D) |
BMI base line (S.D) |
% TWL | % EWL | Serious adverse events N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Espinós (2013) | Observational | Single-Center | Spain | Age > 20 years, obesity class I and II or class III refusing surgery | Age < 20 years, non-ambulatory patients, untreated hormonal or genetic cause of obesity, unable to follow-up | 45 | 34 | 43.4 ± 9.2 | 36.7 ± 3.8 | 15.5 ± 6.1 at 6 months | 49.4 ± 21.5 at 6 months | None |
| Lopez Nava (2015) | Observational | Single-Center | Spain | Obesity class I and II with or without co-morbid conditions, class III willing to comply with aftercare | 147 | 102 | 43.8 ± 11.0 | 38.0 ± 4.8 | 13.6 ± 6.4 at 6 months 15.1 ± 7.8 at 12 months | 42.16 ± 21.8 at 6 months 44.9 ± 24.4 at 12 months | 1 (bleeding at the suture site, managed without incident) | |
| Espinós (2016) | Observational | Single-Center | Spain | Age 21–59 years, obesity class I and II, no contraindication for general anesthesia | History of bariatric, gastric or esophageal surgery, esophageal stricture or anatomical disorder, moderate to severe GERD, active peptic ulcer or hiatal hernia > 3 cm, GI motility disorder, pancreatic insufficiency, inflammatory bowel disease, DM with HbA1c > 7.0, severe coagulopathy, hepatic insufficiency or cirrhosis, drug or alcohol use, quit smoking within last 6 months, patients taking steroids, anti-depressants, anti-psychotics and pregnancy | 18 | 14 | 39.3 ± 9.8 | 36.3 ± 2.3 | 19.1 ± 6.6 at 15 months | 63.7 ± 25.1 at 15 months | None |
| Miller (2017) | Randomized controlled trial (RCT) | Multi-Center | Three centers Austria, Spain, Netherlands | Age 20–60 years, obesity class 1 and II, failure of conservative weight loss measures, no significant weight change (± 5.0%TBWL) in the last 6 months, ASA score ≤ 2, not taken any weight loss medications for ≥ 6 months, agreed not to have additional weight loss interventions or liposuction for ≥ 30 months after study enrollment and been willing to cooperate with postoperative dietary recommendations and assessments | History of bariatric, gastric or esophageal surgery, stricture or other anatomical defect, GERD (Grade B, C, or D), pancreatic insufficiency, active peptic ulcer, pregnancy or plans to conceive within 12 months, steroid use, inflammatory gastrointestinal disease, coagulation disorders, cirrhosis, Type II DM > 2 years, HbA1C > 7, immunosuppression, recent smoking in 6 months, portal hypertension, drug or alcohol use, severe eating disorders, significant depression, psychosis or other mood or eating disorder, known hormonal or genetic cause for obesity | 34 Intervention (POSE) | 25 | 38.3 ± 10.3 | 36.2 ± 3.3 | 12.7 ± 6.2 at 6 months 13.0 ± 7.7 at 12 months | 45.5 ± 23.8 at 6 months 45.0 ± 27.4 at 12 months | 2 (minor postoperative bleeding, resolved within 24 h without sequelae) |
| 10 Control (lifestyle) | 9 | 38.5 ± 12.5 | 37.2 ± 3.7 | 04.7 ± 7.3 at 6 months 05.3 ± 7.5 at 12 months | 14.5 ± 33.2 at 6 months 18.1 ± 26.1 at 12 months | None | ||||||
| Sullivan (2017) | Randomized controlled trial (RCT) | Multi-Center | 11 centers USA | Age 22–60 years, obesity class 1 with at least one non-severe co-morbid obesity-related condition, or BMI ≥ 35 & < 40 kg/m2 without any condition, cannot opt other weight loss measures for the next 24 months | History of bariatric, gastric or esophageal surgery, stricture or other esophageal anatomical defect, severe GERD, hiatal hernia > 3 cm, inflammatory gastrointestinal disease, Type II DM > 10 years, HbA1C > 7, known hormonal or genetic cause for obesity | 221 Intervention (POSE) | 195 | 44.2 ± 8.6 | 36.0 ± 2.4 | 4.95 ± 7.0 at 12 months | 1 (extra-gastric bleeding) 1 (hepatic abscess) 3 (severe nausea) 1 (severe pain) 3 (severe vomiting) 1 SAE | |
| 111 Control (Sham) | 101 | 45.3 ± 9.1 | 36.2 ± 2.2 | 1.38 ± 5.6 at 12 months | ||||||||
| Garcia (2019) | Observational | Single-Center | Spain | History of bariatric, gastric or esophageal surgery, pregnant, psychotic disorders, drug or alcohol abuse, serious pathologies such as cancer, heart diseases, HIV, or hepatitis C | 21 (15 Original POSE + 6 POSE-18) | 18 | 40.0 | 40.3 ± 4.0 47.4 ± 4.1 (POSE-18) | 14.93 ± 5.08 at 3 months 16.87 ± 4.36 at 3 months (POSE-18) | 41.18 ± 15.06 at 3 months 35.88 ± 8.35 (POSE-18) at 3 months | None | |
| Abeid (2019) | Observational | Single-Center | Cairo | Age > 20 years, obesity class I or II with or without co-morbid conditions or class III not willing to opt surgery | 6 | 6 | 42 ± 11.3 | 35.5 ± 3.4 | 9.23 ± 5.79 at 6 months 11.59 ± 6.48 at 12 months | 33.6 ± 24.41 at 6 months 44.91 ± 32.81 at 12 months | None |
A total of 492 patients underwent the POSE procedure for weight loss in the included studies. The pooled mean age of the patients was 41.84 years (95% CI 39.87–43.82), and only 19.91% were male (Table 2). The mean pre-procedure BMI was 36.66 kg/m2, and the range was between 35.49 ± 3.36 kg/m2 and 47.23 ± 4.1 kg/m2.
Table 2.
Population, procedure characteristics, and outcomes of the included studies
| No. of POSE patients N = 492 No. of studies = 7 |
|
|---|---|
| Pooled mean age years | 41.84 (95% CI 39.87–43.82) |
| Gender male | 19.91% |
| Pooled mean BMI | 36.66 (95% CI 35.82–37.50) |
| Mean procedure time (min) | 44.55 (95% CI 36.44–52.65) |
| Total no of anchors | 13.18 (95% CI 11.77–14.58) |
| Anchors in the gastric fundus | 8.33 (95% CI 7.51–9.15) |
| Anchors in the gastric body | 5.66 (95% CI 4.23–7.10) |
| Serious adverse events | Overall 2.84% |
| Gastrointestinal bleeding 3 (0.61%) | |
| Extra-gastric bleeding 1 (0.20%) | |
| Hepatic abscess 1 (0.20%) | |
| Severe pain 1 (0.20) | |
| Severe nausea 4 (0.81%) | |
| Severe vomiting 4 (0.81%) | |
| Minor adverse events | Mild abdominal pain (43.75%) |
| Sore throat (26.47%) | |
| Nausea (20.22%) | |
| Vomiting (17.27%) | |
| Heartburn/reflux (3.65%) | |
| Chest pain (0.41%) | |
| Low-grade fever (0.20%) | |
| Post-procedure decrease in calorie intake capacity at 6 months | 404.26 (95% CI 198.00–610.52) kcal (p < 0.001) (2 studies) |
| %TWL at 3–6 months | 13.45 (95% CI 8.93–17.97) |
| %TWL at 12–15 months | 12.68 (95% CI 8.13–17.23) |
| %EWL at 3–6 months | 42.62 (95% CI 37.56–47.68) |
| %EWL at 12–15 months | 48.86 (95% CI 42.31–55.41) |
Weight loss
%EWL
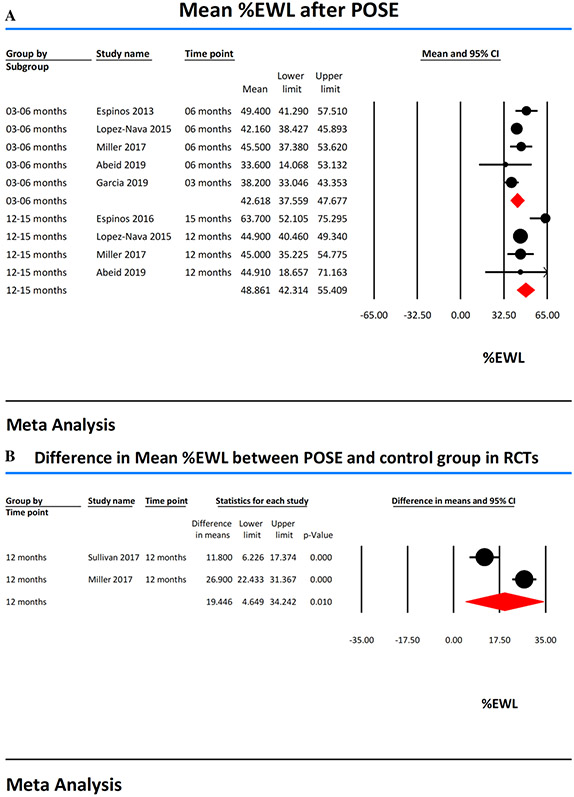
The %EWL was reported at 3 months in two studies [12, 13] and 6 months in four studies [11, 13, 14, 17]. Based on these studies, the pooled mean %EWL at 3–6 months was 42.62 (95% CI 37.56–47.68, I2 = 40). The %EWL at 12 months was reported in 3 studies [13, 14, 17], while one study [16] reported %EWL at 15 months. The mean %EWL at 12–15 months was 48.86 (95% CI 42.31–55.41, I2 = 67) (Fig. 2a). Two RCTs [15, 17] reported the difference in mean %EWL between the treatment (POSE procedure N = 255) and control groups (lifestyle modification or sham procedure N = 121). The mean %EWL difference between POSE procedure group and control groups in RCTs at 12 months follow-up was 19.45 (95% CI 4.65–34.24, I2 = 94) and was statistically significant (p value 0.01) (Fig. 2b).
Fig. 2.
A Forest plots showing percent excess weight loss (%EWL) achieved with POSE. B Forest plots showing the difference in mean percent excess weight loss (%EWL) in RCTs
%TWL
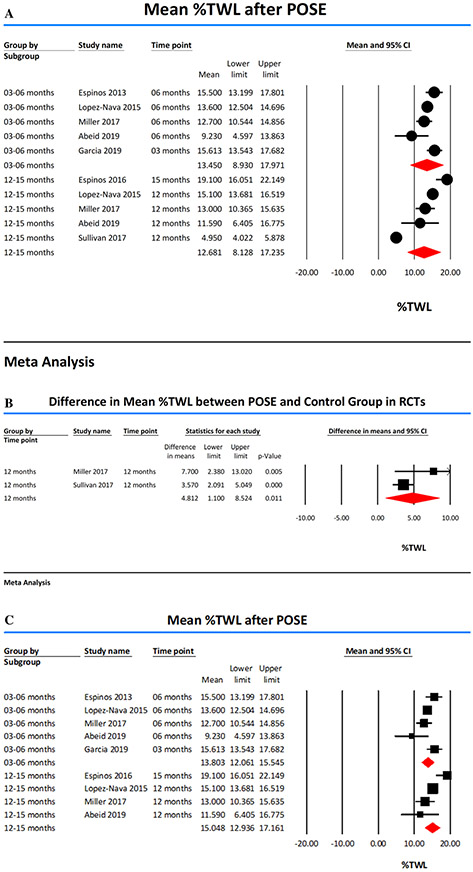
The %TWL was reported at 3 months in 2 studies [12, 13], 6 months in 4 studies [11, 13, 14, 17], 12 months in 4 studies [13-15, 17] and 15 months in one study [16]. Pooled mean %TWL at 3–6 months and 12–15 months was 13.45 (95% CI 8.93–17.97, I2 = 59) and 12.68 (95% CI 8.13–17.23, I2 = 98), respectively (Fig. 3a). Subgroup analysis of 2 RCTs [15, 17] showed that %TWL was significantly higher in POSE group (difference in means 4.81 (95% CI 1.10–8.52, p value = 0.01, I2 = 53) (Fig. 3b).
Fig. 3.
A Forest plots showing Percent Total Weight Loss (%TWL) achieved with POSE. B Forest plots showing the difference in mean percent total weight loss (%TWL) in RCTs. C Forest plots showing Percent Total Weight Loss (%TWL) achieved with POSE after exclusion of study by Sullivan et al [15] which was found to be substantially heterogeneous
Sensitivity analysis and meta-regression
We studied the influence of a single study on the %TWL by removing one study at a time. The exclusion of the study by Sullivan et al. [15] showed an increase in %TWL at 12 months to 15.05 (95% CI 12.94–17.16); otherwise, there was no clinically significant difference in the results indicating that the results were statistically reliable except for the study by Sullivan et al. [15]. Multiple analyses were performed to identify potential sources of heterogeneity. Excluding the study of Sullivan et al. [15] decreased the heterogeneity (I2) of %TWL estimates at 12 months to 72%. Study design RCT with sham control [15] did explain substantial study heterogeneity on meta-regression analysis (Supplementary Fig. 1). Therefore, we removed this clinically and statistically heterogeneous study and reported %TWL at 12 months as 15.05 (95% CI 12.94–17.16, I2 = 72) (Fig. 3c).
Adverse events
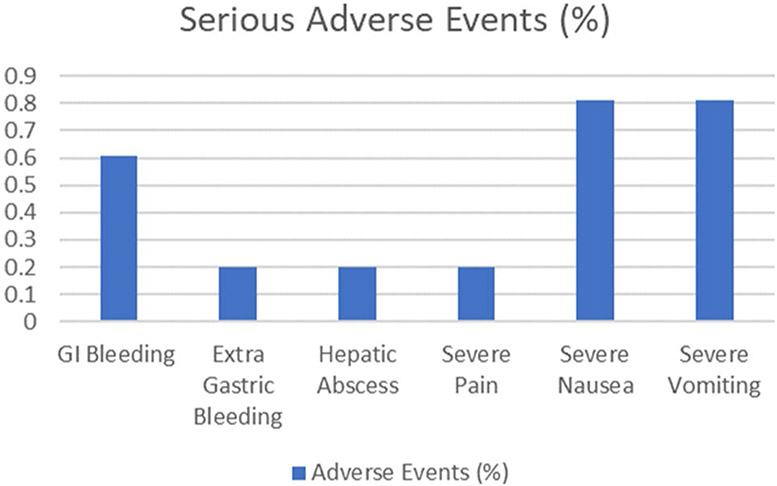
All studies reported the incidence of SAE. There was no mortality reported. The overall incidence of SAE was 2.84%. SAE reported were gastrointestinal (GI) bleeding in 3 (0.61%) [13, 17], extra-gastric bleeding in 1 (0.20%) [15], hepatic abscess in 1 (0.20%) [15], severe pain in 1 (0.20%) [15], severe nausea in 4 (0.81%) [15] and severe vomiting in 4 (0.81%) [15] patients. Gastrointestinal (GI) bleeding was described as minor bleeding at the suture site in one patient [13], which was managed without incident. GI bleeding in two other patients [17] was reported minor postoperative bleeding, which resolved within 24 h without any sequelae. The extra-gastric bleed likely occurred from a blood vessel on the external surface of the stomach, the patient underwent laparoscopy, but the bleeding stopped without any additional intervention. The patient with hepatic abscess was hospitalized and treated with intravenous antibiotics and interventional radiology drainage. Hepatic abscess resolved without any sequelae (Fig. 4).
Fig. 4.
Incidence of serious adverse events
The most common minor adverse events reported were post-procedure abdominal pain (43.75%), sore throat (26.47%), nausea (20.22%), and vomiting (17.27%) [11, 14, 15]. These symptoms resolved resolve quickly on their own or with supportive therapy. Other minor adverse events were Heartburn/reflux in 18 patients [15], chest pain in 2 patients [11], low-grade fever in 1 patient [11], and low hematocrit requiring observation in 1 patient.
Co-morbidities, gastric emptying, and satiety
Only one study [15] reported changes in co-morbidities after POSE. Improvement of diabetes occurred in significantly more patients after POSE than sham procedure at 12 months (56.25% vs. 10.00%, p value 0.036). Trends of improvement in hyperlipidemia (35.71%) and hypertension (19.39%) were also reported [15]. One other study [16] reported improvement in glucose/insulin ratio (p < 0.05) and postprandial decrease in ghrelin (p = 0.03), as well as a postprandial increase in PYY (p = 0.001) after POSE.
One study [16] reported a significant delay in gastric emptying rate after the POSE procedure (p < 0.05) at 2 months. However, the gastric emptying function returned to baseline at 6 months. Two studies [16, 17] performed satiety testing. Caloric intake capacity significantly decreased by 404.26 (95% CI 198.00–610.52) kcal (p < 0.001) at 6 months. In one study [17], caloric intake capacity decreased by 607.8 (95% CI 329.9–885.7) kcal (p < 0.01) at 12 months. Significant reductions in volume of liquid ingested was also reported at 6 months (351.0 (95% CI 224.4–477.6), p = 0.001) and 12 months (378.2 (95% CI 216.6–539.8), p = 0.001) after POSE [17].
Procedure technique and characteristics
The POSE procedure involves the use of the IOP system to create full-thickness plications in the stomach to induce weight loss. The IOP consists of transport, which is a flexible, steerable, multi-lumen access device for passage of ultraslim endoscope for visualization, a g-Lix for tissue manipulation, and a g-Prox for the placement of snowshoe-shaped tissue anchors [13, 18]. The anchors hold plicated tissue permitting serosal approximation to reduce the stomach volume. The original POSE procedure involved the placement of anchors in the fundus and distal body. The average number of anchors placed in the fundus ranged from 7.5 [13] to 9.7 [16] and the pooled mean was 8.33 (95% CI 7.51–9.15). All included studies on an average placed 3.0 [11] to 4.2 [17] anchors in the stomach body, except one study [12] where 18 anchors were placed in the stomach body in a subgroup (POSE-18) of 6 higher BMI patients. The mean total number of anchors placed was 13.18 (95% CI 11.77–14.58). The POSE procedure time ranged between 20 and 69 min. The pooled mean procedure time was 44.55 min (95% CI 36.44–52.65).
Discussion
We report the results of a meta-analysis evaluating the efficacy, safety, and procedural details of POSE. Our analysis suggests that the POSE procedure is safe and effective for the treatment of obesity. The %EWL and %TWL at 12–15 months was 48.86 (95% CI 42.31–55.41) and 12.68 (95% CI 8.13–17.23), respectively. In addition to the absolute threshold of weight loss, the mean % EWL difference between POSE and control groups in RCTs at 12 months was 19.45 and was statistically significant. The overall incidence of severe adverse event rate was low (2.84%), and there was no mortality. The outcomes of POSE surpass the ASGE joint task force thresholds defined in the PIVI statement [8] and thus meets these criteria to be incorporated into clinical practice.
The magnitude of weight loss after the POSE procedure was superior to that achieved with intensive lifestyle interventions. In a head-to-head RCT [17] comparing POSE with intensive diet and exercise, POSE resulted in significantly higher weight loss at 12 months (%EWL 45.0 vs. 18.1). Bariatric surgeries are associated with more substantial and durable weight loss [19, 20], whereas long-term data with POSE is lacking. Only one study reported 15 months of follow-up weight loss after POSE, and weight regain is possible on a longer follow-up. Although bariatric surgery benefits outweigh the risk of adverse events and small mortality [21], many patients do not undergo bariatric surgery due to their invasive nature, the stigma of altered anatomy, perceived risk of adverse events, or lack of insurance coverage [4, 22]. POSE is a safe, minimally invasive therapy with quick recovery time and does not require abdominal incisions. POSE produces remodeling of the stomach but does not significantly alter the anatomy. After POSE, the stomach remains intact with its innervation and blood supply, and there is potential for revision or conversion to bariatric surgery. Therefore, EBMT like POSE, can be an attractive therapy for not only individuals with class I and II obesity but also for patients who are not surgical candidates or do not wish to undergo surgery. However, currently, EBMTs are available at selected centers and are performed on a limited number of patients. There are several barriers, and many areas need to be addressed for the widespread adoption of EBMTs. Standardized EBMT training and credentialing systems are needed. EBMTs are mostly a self-pay procedure in the USA, and insurance coverage remains a significant barrier to widespread adoption. Randomized control trials, long-term outcomes, and data on the improvement of obesity-related co-morbidities after EBMTs will be needed.
The safety profile of POSE was very favorable; the overall incidence of SAE was only 2.84%. Included studies reported these events as SAE, but according to ASGE Quality Task Force recommendations, most of these can be classified as mild to moderate adverse events [23]. The majority of these SAE were related to severe nausea (0.81%), vomiting (0.81%), and pain (0.20%) reported in only one study [15], whereas these symptoms were only mild in other studies. Protocol in this RCT [15] did not allow for prescribing pain or nausea medications to subjects upon discharge. Post-procedure symptoms of abdominal pain, nausea, and vomiting usually resolve within 1 week of discharge and can be controlled in most patients with routine use of antiemetics and pain medications. GI bleeding (0.61%) was minor and managed conservatively without incident. Extra-gastric bleeding was reported likely due to improper plication placement technique [15], and after retraining of investigators on proper technique did not recur. The only case of the hepatic abscess was likely related to translocation of gut microbial flora outside of the gastroluminal space and can be prevented with judicious use of antibiotics.
All observational POSE studies reported excellent weight loss ranging from 44.9 to 63.7%EWL at 12–15 months. POSE was also evaluated in two RCTs providing the highest level of evidence. Miller et al. compared POSE with diet and exercise and reported excellent weight loss (difference in mean %EWL at 12 months of 26.9). Sullivan et al. [15] compared POSE with the sham procedure and showed significantly higher weight loss (difference in mean %EWL at 12 months of 11.8); however, the co-primary endpoint of super superiority margin was not met. The likely explanation of the lower spectrum of weight loss seen in this study was that subjects received low-intensity lifestyle therapy and follow-up in contrast to other EBMT studies that incorporated higher-intensity lifestyle therapy and other interventions. Secondly, the sham control design also contributed to lower weight loss. ASGE PIVI [8] recommends that EBMT is best evaluated when compared to a second treatment, rather than a sham since sham groups in bariatric trials have proven to be unreliable with considerable variability in weight loss [24, 25].
Original POSE procedure was performed in all included studies. Original POSE involved the placement of approximately 7–9 anchors in the gastric fundus to decrease fundal volume and limit gastric fundal accommodation in response to a meal [13], and only 3–4 additional anchors are placed in the distal stomach body to prolong gastric emptying. Espinós et al. [16] reported delayed gastric emptying after POSE, and the delay in gastric emptying was associated with more and sustained weight loss at 15-month. However, the gastric emptying was normal 6 months after POSE. Normalization of gastric emptying was possibly related to the small number of anchors placed in the distal body. García et al. [12] successfully demonstrated safety and feasibility of placing 18 anchors in the stomach body in addition to the fundal anchors in a small subgroup of severely obese patients resulting in effective weight loss (16.87%TWL at 3 months). Jirapinyo et al. [18] suggested that focusing on the gastric body may have a more significant effect on gastric motility and, consequently, higher weight loss. They described distal POSE through a belt-and-suspenders approach, a novel POSE technique focusing on placement of anchors solely in the gastric body and sparing the fundus. Distal POSE resulted in excellent weight loss (27.6%TWL and 56.0%EWL at 6 months) in the only reported case [18]. We believe distal POSE can further refine the original POSE technique and can be a more effective treatment for obesity that merits further extensive studies.
Studies comparing EBMTs are lacking. Intragastric balloons (IGB), a space-occupying device, is the most common EBMT [26], but one major limitation is the weight regain after removal of the balloon at the end of 6 months [27-30]. In our analysis of POSE studies, weight regain was not seen at 12–15 months (%EWL 48.86) compared to 3–6 months (%EWL 42.62) follow-up. The incidence of SAE and early removal reported in the literature after IGB [27, 28] was higher than the SAE for POSE in our analysis. Weight loss with POSE seems durable with fewer adverse events compared with IGB.
ESG is another endoscopic gastroplasty procedure that has gained momentum worldwide as a promising EBMT [31, 32]. While several observational studies have shown that ESG is an effective and safe option for weight loss, RCTs are still lacking, and only one retrospective controlled study is available [33]. Cheskin et al. [33] retrospectively compared ESG with high-intensity diet and lifestyle therapy and found a difference in mean %TWL of 6.3 (%TWL ESG 20.6 versus 14.3 lifestyle cohort). These results were comparable to POSE RCT by Miller et al., which showed a difference in mean %TWL of 7.7 between POSE and lifestyle modification. A meta-analysis [6] indirectly comparing observational uncontrolled ESG studies with POSE (RCTs and observational studies) reported a mean difference in %EWL at 12 months of 7.84 in favor of ESG. While ESG procedure places suture on the greater curvature of the stomach to form a sleeve, the original POSE studies focused on the stomach fundus. Distal POSE technique focusing on the stomach body rather than the fundus can potentially achieve superior weight loss similar to seen in observational ESG studies. SAE profile of POSE in our analysis is comparable to that reported with ESG [22]. The POSE procedure time in our analysis was shorter than reported for ESG [31]. ESG also appears to have a longer learning curve. Saumoy et al. [34] showed that 38 ESG procedures by a single operator are required to attain efficiency and mastery was attained after 55 procedures, whereas García et al. [12] reported the original POSE and POSE-18 (fundus plus 18 plications in the body) average plication time of 20 min and 25 min, respectively, in a study with only 21 patients.
Ours is the first comprehensive systematic review and meta-analysis to evaluate the effectiveness, safety, and procedural technique, specifically for POSE. We included recent POSE studies [12, 14] not included in the previous meta-analysis [6, 7]. Despite our rigorous review, our study has several limitations. The quality of our systematic review and meta-analysis is inherently limited by the quality of the included studies. Only two studies were RCTs, and the rest were observational studies without controls of variable sample size. Length of follow-up varied among studies, and the longest follow-up time available was 15 months; hence, future studies with longer follow-up are needed. Most of the studies did not report the impact of POSE on obesity-related co-morbidities. A high degree of statistical heterogeneity was found in some of our estimates. Omitting the study from Sullivan et al. partially reduced the heterogeneity. This study varied from other studies by the sham control group and low-intensity lifestyle and follow-up. Other reasons for heterogeneity could be variability in the POSE procedure, operator, and patient characteristics.
In conclusion, POSE is a minimally invasive endoscopic bariatric therapy with effective weight loss outcomes and a favorable safety profile. The outcomes of the original POSE meet and surpass the ASGE joint task force thresholds. Future studies should evaluate newer versions of this procedure that emphasize gastric body plication sparing the fundus.
Supplementary Material
Acknowledgements
The authors acknowledge Anna Crawford (Librarian, West Virginia University Health Sciences Library) for conducting the literature searches.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
Footnotes
Disclosures Drs. Shailendra Singh, Ahmad Najdat Bazarbashi, Ahmad Khan, Monica Chowdhry, Mohammad Bilal, Diogo Turiani Hourneaux de Moura, and Shyam Thakkar have no conflicts of interest or financial ties to disclose. Dr. Pichamol Jirapinyo has the following disclosures: Apollo Endosurgery (research support), Endogastric Solutions (consultant), Fractyl (research support), GI Dynamics (consultant/research support). Dr. Christopher C. Thompson has the following disclosures: Apollo Endosurgery—Consultant/Research Support (Consulting fees/Institutional Research Grants), Aspire Bariatrics—Research Support (Institutional Research Grant), BlueFlame Healthcare Venture Fund—General Partner, Boston Scientific—Consultant (Consulting fees), Covidien/Medtronic—Consultant (Consulting Fees), EnVision Endoscopy (Board Member), Fractyl—Consultant/Advisory Board Member (Consulting Fees), GI Dynamics—Consultant (Consulting Fees)/Research Support (Institutional Research Grant), GI Windows—Ownership interest, Olympus/Spiration—Consultant (Consulting Fees).
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00464-020-08267-z.
References
- 1.WHO. WHO ∣ Overweight and obesity. WHO [Google Scholar]
- 2.Ju T, Rivas L, Arnott S et al. (2019) Barriers to bariatric surgery: factors influencing progression to bariatric surgery in a U.S. metropolitan area. Surg Obes Relat Dis. 10.1016/j.soard.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 3.Funk LM, Jolles S, Fischer LE, Voils CI (2015) Patient and referring practitioner characteristics associated with the likelihood of undergoing bariatric surgery a systematic review. JAMA Surg. 10.1001/jamasurg.2015.1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchwald H, Oien DM (2013) Metabolic/bariatric surgery worldwide 2011. Obes Surg 23:427–436 [DOI] [PubMed] [Google Scholar]
- 5.Cohen RV, Oliveira da Costa MV, Charry L, Heins E (2019) Endoscopic gastroplasty to treat medically uncontrolled obesity needs more quality data: a systematic review. Surg Obes Relat Dis 10.1016/j.soard.2019.03.016 [DOI] [PubMed] [Google Scholar]
- 6.Khan Z, Khan MA, Hajifathalian K et al. (2019) Efficacy of endoscopic interventions for the management of obesity: a meta-analysis to compare endoscopic sleeve gastroplasty, AspireAssist, and primary obesity surgery endolumenal. Obes Surg 29(7):2287–2298. 10.1007/s11695-019-03865-w [DOI] [PubMed] [Google Scholar]
- 7.Gys B, Plaeke P, Lamme B et al. (2019) Endoscopic gastric plication for morbid obesity: a systematic review and meta-analysis of published data over time. Obes Surg. 10.1007/s11695-019-04010-3 [DOI] [PubMed] [Google Scholar]
- 8.Ginsberg GG, Chand B, Cote GA et al. (2011) A pathway to endoscopic bariatric therapies. Gastrointest Endosc. 10.1016/j.gie.2011.08.053 [DOI] [PubMed] [Google Scholar]
- 9.Chand B (2011) A pathway to endoscopic bariatric therapies: ASGE/ASMBS task force on endoscopic bariatric therapy. Surg Obes Relat Dis. 10.1016/j.soard.2011.09.008 [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 10.1016/j.jclinepi.2009.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinós JC, Turró R, Mata A et al. (2013) Early experience with the incisionless operating platform™ (iop) for the treatment of obesity: the primary obesity surgery endolumenal (pose) procedure. Obes Surg. 10.1007/s11695-013-0937-8 [DOI] [PubMed] [Google Scholar]
- 12.García RG, Velázquez JV (2019) Reinforced POSE: the 18-plication solution. Obes Surg 10.1007/s11695-019-04014-z [DOI] [PubMed] [Google Scholar]
- 13.López-Nava G, Bautista-Castaño I, Jimenez A, De Grado T, Fernandez-Corbelle JP (2015) The primary obesity surgery endolumenal (POSE) procedure: one-year patient weight loss and safety outcomes. Surg Obes Relat Dis. 10.1016/j.soard.2014.09.026 [DOI] [PubMed] [Google Scholar]
- 14.Abeid M, Miller KA, Kaddah T, Zaitoun N (2019) Outcome of primary obesity surgery endolumenal procedure as obesity treatment in private practice setting: an intervention study. Obes Surg 29(4):1364–1366. 10.1007/s11695-018-03698-z [DOI] [PubMed] [Google Scholar]
- 15.Sullivan S, Swain JM, Woodman G et al. (2017) Randomized sham-controlled trial evaluating efficacy and safety of endoscopic gastric plication for primary obesity: the ESSENTIAL trial. Obesity 25(2):294–301. 10.1002/oby.21702 [DOI] [PubMed] [Google Scholar]
- 16.Espinós JC, Turró R, Moragas G et al. (2016) Gastrointestinal physiological changes and their relationship to weight loss following the POSE procedure. Obes Surg 26(5):1081–1089. 10.1007/s11695-015-1863-8 [DOI] [PubMed] [Google Scholar]
- 17.Miller K, Turró R, Greve JW, Bakker CM, Buchwald JN, Espinós JC (2017) MILEPOST multicenter randomized controlled trial: 12-month weight loss and satiety outcomes after pose SM vs. medical therapy. Obes Surg 27(2):310–322. 10.1007/s11695-016-2295-9 [DOI] [PubMed] [Google Scholar]
- 18.Jirapinyo P, Thompson CC (2018) Gastric plications for weight loss: distal primary obesity surgery endoluminal through a belt-and-suspenders approach. VideoGIE. 10.1016/j.vgie.2018.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fayad L, Adam A, Schweitzer M et al. (2019) Endoscopic sleeve gastroplasty versus laparoscopic sleeve gastrectomy: a case-matched study. Gastrointest Endosc. 10.1016/j.gie.2018.08.030 [DOI] [PubMed] [Google Scholar]
- 20.O’Brien PE, Hindle A, Brennan L et al. (2019) Long-term outcomes after bariatric surgery: a systematic review and meta-analysis of weight loss at 10 or more years for all bariatric procedures and a single-centre review of 20-year outcomes after adjustable gastric banding. Obes Surg. 10.1007/s11695-018-3525-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flum DR, Belle SH, King WC et al. (2009) Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med 361:445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hedjoudje A, Dayyeh BA, Cheskin LJ et al. (2019) Efficacy and safety of endoscopic sleeve gastroplasty: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 10.1016/J.CGH.2019.08.022 [DOI] [PubMed] [Google Scholar]
- 23.Cotton PB, Eisen GM, Aabakken L et al. (2010) A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 10.1016/j.gie.2009.10.027 [DOI] [PubMed] [Google Scholar]
- 24.Shikora SA, Bergenstal R, Bessler M et al. (2009) Implantable gastric stimulation for the treatment of clinically severe obesity: results of the SHAPE trial. Surg Obes Relat Dis. 10.1016/j.soard.2008.09.012 [DOI] [PubMed] [Google Scholar]
- 25.Gersin KS, Rothstein RI, Rosenthal RJ et al. (2010) Open-label, sham-controlled trial of an endoscopic duodenojejunal bypass liner for preoperative weight loss in bariatric surgery candidates. Gastrointest Endosc. 10.1016/j.gie.2009.11.051 [DOI] [PubMed] [Google Scholar]
- 26.Kotinda APST, de Moura DTH, Ribeiro IB et al. (2020) Efficacy of intragastric balloons for weight loss in overweight and obese adults: a systematic review and meta-analysis of randomized controlled trials. Obes Surg. 10.1007/s11695-020-04558-5 [DOI] [PubMed] [Google Scholar]
- 27.Tate CM, Geliebter A (2017) Intragastric balloon treatment for obesity: review of recent studies. Adv Ther. 10.1007/s12325-017-0562-3 [DOI] [PubMed] [Google Scholar]
- 28.Genco A, López-Nava G, Wahlen C et al. (2013) Multi-Centre European experience with intragastric balloon in overweight populations: 13 years of experience. Obes Surg. 10.1007/s11695-012-0829-3 [DOI] [PubMed] [Google Scholar]
- 29.Fayad L, Cheskin LJ, Adam A et al. (2019) Endoscopic sleeve gastroplasty versus intragastric balloon insertion: efficacy, durability, and safety. Endoscopy. 10.1055/a-0852-3441 [DOI] [PubMed] [Google Scholar]
- 30.Singh S, de Moura DTH, Khan A et al. (2020) Intragastric balloon versus endoscopic sleeve gastroplasty for the treatment of obesity: a systematic review and meta-analysis. Obes Surg. 10.1007/s11695-020-04644-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh S, Hourneaux de Moura DT, Khan A, Bilal M, Ryan MB, Thompson CC (2019) Safety and efficacy of endoscopic sleeve gastroplasty worldwide for treatment of obesity: a systematic review and meta-analysis. Surg Obes Relat Dis. 10.1016/J.SOARD.2019.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Miranda Neto AA, de Moura DTH, Ribeiro IB et al. (2020) Efficacy and safety of endoscopic sleeve gastroplasty at mid term in the management of overweight and obese patients: a systematic review and meta-analysis. Obes Surg. 10.1007/s11695-020-04449-9 [DOI] [PubMed] [Google Scholar]
- 33.Cheskin LJ, Hill C, Adam A et al. (2019) Endoscopic sleeve gastroplasty versus high-intensity diet and lifestyle therapy: a case-matched study. Gastrointest Endosc. 10.1016/j.gie.2019.09.029 [DOI] [PubMed] [Google Scholar]
- 34.Saumoy M, Schneider Y, Zhou XK et al. (2018) A single-operator learning curve analysis for the endoscopic sleeve gastroplasty. Gastrointest Endosc 87(2):442–447. 10.1016/j.gie.2017.08.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.