Abstract
In Rat-1 fibroblasts nonmitogenic doses of lysophosphatidic acid (LPA) stimulate a transient activation of mitogen-activated protein kinase (MAPK), whereas mitogenic doses elicit a sustained response. This sustained phase of MAPK activation regulates cell fate decisions such as proliferation or differentiation, presumably by inducing a program of gene expression which is not observed in response to transient MAPK activation. We have examined the expression of members of the AP-1 transcription factor complex in response to stimulation with different doses of LPA. c-Fos, c-Jun, and JunB are induced rapidly in response to LPA stimulation, whereas Fra-1 and Fra-2 are induced after a significant lag. The expression of c-Fos is transient, whereas the expression of c-Jun, JunB, Fra-1, and Fra-2 is sustained. The early expression of c-Fos can be reconstituted with nonmitogenic doses of LPA, but the response is transient compared to that observed with mitogenic doses. In contrast, expression of Fra-1, Fra-2, and JunB and optimal expression of c-Jun are observed only with doses of LPA which induce sustained MAPK activation and DNA synthesis. LPA-stimulated expression of c-Fos, Fra-1, Fra-2, c-Jun, and JunB is inhibited by the MEK1 inhibitor PD098059, indicating that the Raf-MEK-MAPK cascade is required for their expression. In cells expressing a conditionally active form of Raf-1 (ΔRaf-1:ER), we observed that selective, sustained activation of Raf-MEK-MAPK was sufficient to induce expression of Fra-1, Fra-2, and JunB but, interestingly, induced little or no c-Fos or c-Jun. The induction of c-Fos observed in response to LPA was strongly inhibited by buffering the intracellular [Ca2+]. Moreover, although Raf activation or calcium ionophores induced little c-Fos expression, we observed a synergistic induction in response to the combination of ΔRaf-1:ER and ionomycin. These results suggest that kinetically distinct phases of MAPK activation serve to regulate the expression of distinct AP-1 components such that sustained MAPK activation is required for the induced expression of Fra-1, Fra-2, c-Jun, and JunB. However, in contrast to the case for Fra-1, Fra-2, and JunB, activation of the MAPK cascade alone is not sufficient to induce c-Fos expression, which rather requires cooperation with other signals such as Ca2+ mobilization. Finally, the identification of the Fra-1, Fra-2, c-Jun, and JunB genes as genes which are selectively regulated by sustained MAPK activation or in response to activated Raf suggests that they are candidates to mediate certain of the effects of Ras proteins in oncogenic transformation.
Growth factors and oncogenes exert their effects on cells by activating intracellular signal pathways which elicit changes in gene expression leading to cell cycle progression or cellular transformation. The dimeric transcription factor AP-1 is a major target of cell growth, differentiation, and stress signalling pathways (7, 50, 101). AP-1 consists of various combinations of Fos and Jun family members that dimerize via a leucine zipper domain and bind to DNA via an adjacent basic region (32, 51, 58). The Fos family consists of four gene products (c-Fos, FosB, Fra-1, and Fra-2), while the Jun family is made up of three gene products (c-Jun, JunB, and JunD). Fos proteins form heterodimers with Jun and ATF family proteins (37), whereas Jun proteins can form heterodimers with ATF2 (98) and can form functional homodimers, albeit with reduced stability (38, 79).
AP-1 binds to a specific target DNA sequence, TGAC/GTCA, named the TRE (for tetradecanoyl phorbol acetate-responsive element) (6), which is found in the promoters of many genes, including those for cell cycle regulators such as cyclin D1 (3, 41) and autocrine growth factors such as heparin-binding epidermal growth factor (HB-EGF) (70) and vascular endothelial growth factor (45). The binding affinity for a given TRE is determined by the different AP-1 dimer combinations and the context of the surrounding sequences (27, 38, 39, 81). In addition, some Fos and Jun proteins possess transcriptional activation domains which are regulated by phosphorylation, with the result that different dimer combinations may exhibit different transactivation properties (50, 101). Finally, the expression of Fos and Jun proteins is temporally coordinated in response to various stimuli, with the result that the composition of AP-1 may change in the cell as a function of time and stimulus (18, 26, 52, 53, 57, 74, 85). All of these factors contribute to make AP-1 a versatile and dynamic transcriptional complex able to respond to differing environmental cues.
Cell transformation by Ras oncoproteins is intimately linked to increases in AP-1-mediated gene expression. Microinjection of Ras proteins induces c-Fos expression (87), and chronic Ras transformation leads to an increase in AP-1 binding activity and up-regulation of at least four AP-1 components (Fra-1, Fra-2, c-Jun, and JunB) (71, 96). In addition, dominant interfering mutants of c-Jun and c-Fos can revert the phenotype of Ras-transformed cells (63, 89), c-Jun is required for Ras-induced malignancy (48), and c-Fos is required for Ras-driven malignant progression in a multistep skin carcinogenesis model in mice (82).
The Ras-dependent Raf–MEK–mitogen-activated protein kinase (Raf-MEK-MAPK) cascade is one of the key signalling pathways responsible for transmitting signals from growth factor receptors to the nucleus (15, 42, 47, 50, 66, 95, 101). Signals from growth factor receptors lead to activation of the membrane-tethered Ras proteins, which recruit the serine/threonine kinase Raf to the plasma membrane where it in turn is activated (61, 65, 88, 90, 97, 100). Raf phosphorylates and activates the dual-specificity MAPK kinase, MEK (28, 44, 55), which in turn phosphorylates MAPK at adjacent Thr and Tyr residues, thereby reactivating it (24). Activated MAPKs accumulate in the nucleus (13, 62, 91), where they can phosphorylate and activate the transcription factors Elk-1 and Sap1a, leading to the enhanced expression of genes such as that for c-Fos (34, 42, 43, 64, 94, 101). In this way the Raf-MEK-MAPK cascade serves to amplify low-level extracellular signals into intracellular messages which are transmitted into the nucleus, thereby connecting growth factor receptors to changes in gene expression and cell fate. Expression of c-Jun is also stimulated by Ras proteins, but the mechanism for this is less clear. c-Jun expression can be stimulated by activation of preexisting ATF2–c-Jun dimers at the c-Jun TRE/AP-1 site (30, 98). The transcriptional activity of ATF2 and c-Jun is stimulated by phosphorylation of sites in their transactivation domains by the p38 and Jun N-terminal kinases (JNK/stress-activated protein kinase) (50, 101), but these kinases are only weakly activated by Ras proteins (29) and growth factors (29, 56). In addition, there is evidence to suggest that additional sites outside the transactivation domain may also be important (1).
While the role of MAPK in regulating the c-Fos serum response element is well recognized (42, 95, 101), this is an early and transient event and yet serum and growth factors are required to be present for 8 to 10 h to ensure cell cycle reentry, during which they stimulate a sustained activation of MAPK (19, 20, 49, 99). Activation of MEK and MAPK is sufficient to cause differentiation and transformation (23), but it is the duration of MAPK activation which appears to be a key determinant of cell fate signalling decisions. In PC12 cells, nerve growth factor stimulates a sustained activation of MAPK which is required for cell cycle arrest and terminal differentiation, whereas factors which elicit transient activation of MAPK do not promote differentiation (40, 66, 91). In CCL39 fibroblasts, thrombin requires sustained MAPK activation to stimulate DNA synthesis (49, 99), while in Rat-1 cells, sustained MAPK activity is observed only in response to doses of lysophosphatidic acid (LPA) which stimulate DNA synthesis (20). Finally, low-level activation of the Raf-MEK-MAPK pathway promotes cell proliferation, whereas persistent high-level activation promotes cell cycle arrest and a quasidifferentiated state in NIH 3T3 cells (84, 103). Since sustained MAPK activation is associated with nuclear accumulation of MAPKs (13, 60, 91), it has been proposed that the quantitative differences in the duration of MAPK activation may be reflected in qualitative changes in gene expression, thereby determining cellular responses (66). The logical conclusion from this model is that some genes will be expressed only under conditions of sustained MAPK activation. One example of such a gene is that for the cyclin-dependent kinase inhibitor p21Cip1 (84, 103); however, the links between the MAPK pathway and the cell cycle apparatus are not yet fully understood.
Here we have examined the role of sustained MAPK activation in the expression of different components of the AP-1 transcription factor complex in response to LPA stimulation. We demonstrate that the duration of MAPK activation determines the repertoire of Fos and Jun proteins expressed during cell cycle reentry. Specifically, we demonstrate that Fra-1, Fra-2, c-Jun, and JunB are targets for sustained MAPK activation and therefore are likely to mediate the longer-term effects of Ras and Raf in transformed cells.
MATERIALS AND METHODS
Materials.
LPA was purchased from Avanti Polar Lipids or Sigma, 4-hydroxytamoxifen (4-HT) and β-estradiol (β-E2) were from Sigma, and ionomycin and BAPTA-AM [1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetra(acetoxymethyl)ester] were from Calbiochem. Fetal bovine serum (FBS) and all other cell culture reagents were from Gibco Life Technologies. Rabbit antipeptide antisera to c-Fos, Fra-1, Fra-2, and JunB were from Santa Cruz Biotechnology. Antipeptide antiserum to c-Jun was kindly provided by David Gillespie, CRC Beatson Laboratories, Glasgow, United Kingdom. Horseradish peroxidase-conjugated secondary antibodies were from Bio-Rad, and detection was with the enhanced chemiluminescence (ECL) system (Amersham). Protease inhibitors were from Boehringer Mannheim. All other reagents were of the highest grade commercially available.
Cells and cell culture.
Rat-1 and NIH 3T3 cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with glutamine, penicillin-streptomycin, and 7% (vol/vol) FBS. R1ΔRaf-1:ER-4 cells (21) and NIH 3T3 c4 or c9 cells (70, 83) expressing ΔRaf-1:ER were grown in the same medium without phenol red and maintained in the presence of 400 μg of G418 ml−1.
Stimulations and preparation of cell lysates.
For Rat-1 cells and their transfected derivatives, cells were grown to confluence and prepared for stimulation by replacing the medium with serum-free Dulbecco’s modified Eagle’s medium for 24 h to induce a quiescent state (G0 arrest). In the case of NIH 3T3 cells, this medium was modified to 0.5% (vol/vol) FBS with 500 mg of fatty acid-free bovine serum albumin and 10 ml of insulin-transferrin-selenium supplement (ITS-X; Gibco-BRL) per liter, as we observed loss of attachment to the substratum if NIH 3T3 cells were incubated overnight under serum-free conditions. Cells were stimulated with the appropriate agonists by adding them as 10× solutions to the culture dish, thereby causing minimal disturbance. Incubations proceeded at 37°C with 5% CO2 for the indicated times and were terminated by aspiration of the medium and addition of ice-cold TG lysis buffer (20, 21). Cell extracts were harvested and clarified by centrifugation at 14,000 × g at 4°C for 10 min, and the supernatants were boiled in Laemmli sample buffer and stored at −20°C.
Western blot analysis.
Equal amounts of cell lysates, typically 50 μg, were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with 10% gels (Hoefer Mighty Small system) and transferred to methanol-soaked polyvinylidene difluoride (PVDF) membranes at 300 mA for 1 h in a Bio-Rad mini-Transblot apparatus. After staining to confirm equal loading, filters were incubated in phosphate-buffered saline plus 0.1% (vol/vol) Tween 20 (TPBS) supplemented with 5% (wt/vol) powdered milk (TPBS-milk) for at least 1 h before probing with antisera. First- and second-antibody incubations were performed in TPBS-milk at room temperature for 1 h with six intervening washes during 20 min. Antibody-antigen complexes were detected by using the ECL system according to the manufacturer’s instructions. Exposures for c-Fos, Fra-1, and Fra-2 were typically 5 to 15 s, and those for c-Jun and JunB were 30 s to 5 min. Results were recorded on a charge-coupled device video camera, and integrated optical densities were derived by using the Solitaire Plus 512 Seescan Imaging System. Quantification was performed with multiple exposures for each blot, and results were independently confirmed by scanning densitometry.
RNase protection.
The Rat c-fos riboprobe was prepared by using a Bluescript plasmid containing bases 303 to 453 of rat c-fos cDNA and protected a fragment of 150 bp. A riboprobe prepared from a plasmid containing a 120-bp fragment of the human GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene was included in all hybridizations and used as a loading control. Riboprobes were generated from linearized cDNAs by reverse transcription in the presence of [32P]CTP, and the labelled riboprobes were purified from unincorporated nucleotides by using G50 Sephadex columns (Boehringer Mannheim) or by gel purification. Ten micrograms of total RNA (RNeasy kit; Qiagen) or tRNA (as a negative control) was hybridized overnight at 45°C with purified c-fos-specific and GAPDH riboprobes. Unhybridized RNA was digested with RNase A and RNase T1, extracted with phenol, ethanol precipitated, and resolved by electrophoresis on 6% acrylamide sequencing gels. Results were quantitated with a Molecular Dynamics PhosphorImager.
RESULTS
Kinetics of LPA-stimulated expression of Fos and Jun proteins in Rat-1 cells.
Initially we defined the kinetics of induction of AP-1 proteins in quiescent, serum-starved Rat-1 cells stimulated to reenter the cell cycle by LPA. In these studies we examined the expression of c-Fos and c-Jun, the classical immediate-early gene products, and the Fos-related proteins Fra-1 and Fra-2, which are reported to exhibit delayed kinetics of induction in response to serum. In addition, we also examined the expression of JunB, which, together with Fra-1, Fra-2, and c-Jun, is reported to be expressed in Ras-transformed cells (71, 96). Quiescent Rat-1 cells were either untreated or stimulated with LPA for various times from 30 min to 10 h. Under these conditions, Rat-1 cells start to enter S phase 12 h after stimulation and pass the restriction point approximately 10 h after LPA addition (20, 22). The kinetics of expression of Fos and Jun proteins was analyzed by Western immunoblotting of whole-cell lysates (Fig. 1A and B) as described in Materials and Methods.
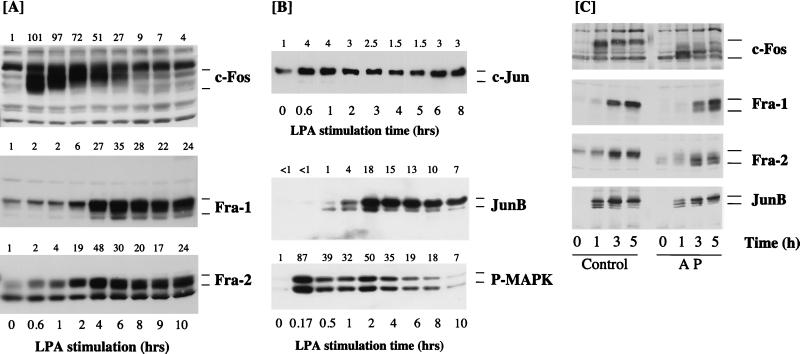
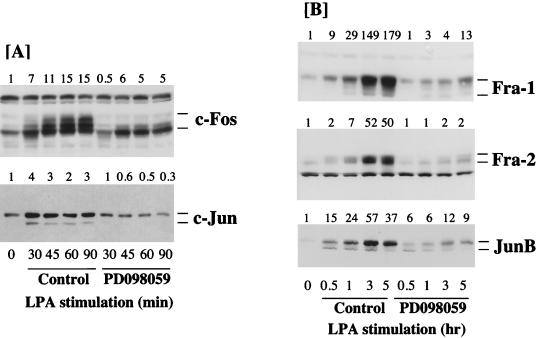
FIG. 1.
Kinetics of expression of Fos and Jun proteins in Rat-1 cells stimulated with LPA. (A and B) Confluent, serum-starved Rat-1 cells were stimulated with 100 μM LPA for the times indicated. Whole-cell detergent lysates were fractionated by SDS-PAGE, transferred to PVDF membranes, and subjected to Western immunoblotting with antibodies specific for c-Fos, Fra-1, Fra-2 (A) or c-Jun, JunB, or phospho-MAPK (P-MAPK) (B). Fold increases in protein expression or MAPK activation are shown above each lane for each blot. Note that the basal level of JunB was not detectable; the basal level of 1 is set at the lowest detectable value, making the fold increases an underestimate. (C) Quiescent Rat-1 cells were stimulated for the indicated times with 50 μM LPA. Whole-cell detergent lysates were prepared, divided into two equal portions, and incubated in the absence (Control) or presence (AP) of 20 U of alkaline phosphatase at 37°C for 2 h. Both samples were then fractionated by SDS-PAGE, transferred to PVDF membranes, and subjected to Western immunoblotting with antibodies specific for c-Fos, Fra-1, Fra-2, or JunB. Note that the apparent molecular weights of all four proteins are reduced to a greater or lesser extent by AP treatment. In the case of JunB, this is most apparent at the 1-h time point, when the protein exists as a triplet with the uppermost band being the major protein in control samples; AP treatment causes dephosphorylation of the upper band so that JunB now appears as three bands of approximately equal intensity. The results shown are from a single experiment typical of at least three others giving identical results.
c-Fos expression was rapidly and strongly induced within 40 min after LPA addition (Fig. 1A). Relative to that of the other AP-1 proteins studied, c-Fos expression in response to LPA was transient, peaking between 40 min and 1 h and returning to basal levels 6 to 8 h after LPA addition; this result is consistent with the rapid and transient induction of c-Fos mRNA (35, 43, 54, 75, 93). The c-Fos protein was resolved as a broad band of 55 to 65 kDa, and previous studies indicate that this is due to differential phosphorylation of the c-Fos protein (25, 57). Indeed, the pattern of c-Fos-immunoreactive bands observed is virtually identical to that observed in Sf9 cells when c-Fos is coexpressed with v-Raf and MAPK (2), and the hyperphosphorylated forms of c-Fos can be reduced to a lower-molecular-mass band by treatment of cell lysates with alkaline phosphatase (Fig. 1C) (57). The c-Fos protein is reported to be phosphorylated on at least two C-terminal regulatory sites (Ser362 and Ser374 in the rat sequence) by MAPK and the MAPK-activated p90RSK in vitro and in response to serum stimulation in vivo (14). Phosphorylation of these two sites is implicated in increasing the half-life of the c-Fos protein, and we note that the most hyperphosphorylated forms of c-Fos persisted for the longest time in response to growth factor stimulation (Fig. 1A). Indeed, cell transformation by c-Fos correlates with persistent expression of a partially phosphorylated form of the c-Fos protein (60).
Relative to c-Fos, Fra-1 was induced with delayed kinetics but in a sustained manner in response to LPA stimulation. Fra-1 expression was unchanged until 2 h after LPA stimulation, at which time Fra-1 was strongly induced, and it was maintained at elevated levels for at least a further 8 h (Fig. 1A). Compared to Fra-1, Fra-2 was more rapidly induced but, unlike c-Fos, was maintained at high levels for up to 10 h after addition of LPA. In the case of Fra-2, we observed a reduction in electrophoretic mobility of the preexisting Fra-2 protein which was most pronounced as early as 10 min after addition of LPA (see also Fig. 2B). We also observed some heterogeneity in the molecular weight of Fra-1 which was apparent only after prolonged stimulation, when the Fra-1 protein was detectable as a ladder with a major band uppermost and two or three fainter bands beneath. In both cases this reduction in mobility is most likely to be due to phosphorylation of the proteins, since it can be reversed by treating the lysates with alkaline phosphatase (Fig. 1C) (57).
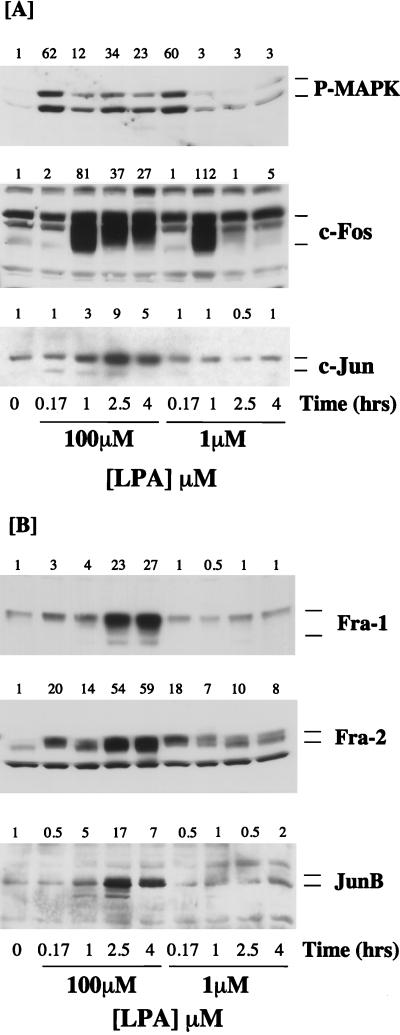
FIG. 2.
Expression of Fos and Jun proteins in response to mitogenic and nonmitogenic doses of LPA. Confluent, serum-starved Rat-1 cells were stimulated with 100 or 1 μM LPA for the times indicated. Whole-cell detergent lysates were fractionated by SDS-PAGE, transferred to PVDF membranes, and subjected to Western immunoblotting with antibodies specific for phospho-MAPK (P-MAPK), c-Fos, or c-Jun (A) or Fra-1, Fra-2, or JunB (B). Fold increases in protein expression or MAPK activation are shown above each lane for each blot. The results shown are from a single experiment typical of at least three giving identical results.
c-Jun protein expression was induced after 30 to 40 min of LPA stimulation, but the response differed from that for c-Fos in three ways. First, there was a higher basal level of c-Jun expression detectable in virtually all experiments (except with NIH 3T3 cells [see Fig. 6]), whereas c-Fos expression was rarely detected in serum-starved cells. Second, in all experiments the magnitude of the increase in c-Jun in response to LPA was modest compared to the robust accumulation observed for c-Fos. Finally, the increase in c-Jun expression was generally sustained for a longer period of time than that for c-Fos, such that elevated levels of c-Jun were still observed late in G1 (see also reference 57). In these experiments we observed some heterogeneity in the electrophoretic mobility of c-Jun in response to LPA, but this differed in degree between experiments (see, for example, Fig. 1 versus Fig. 2 and 4). In most cases c-Jun was detected as a minor protein of approximately 39 kDa and a major protein of approximately 41 kDa. The phosphorylation of c-Jun at Ser63, Ser73, Thr91, and Thr95 is reported to induce a similar reduction in mobility of c-Jun (68, 78). However, the SAPK/JNKs, which are responsible for phosphorylation of Ser63 and Ser73, are activated only weakly in response to LPA stimulation (12). Under conditions in which alkaline phosphatase reduced the apparent mobilities of c-Fos, Fra-1, Fra-2, and JunB, it had no effect on that of c-Jun (22), consistent with previous reports (57). There may be several reasons for this. It is possible that candidate phosphorylation sites are not accessible to the phosphatase under these conditions. In addition, it is known that c-Jun undergoes a complex pattern of phosphorylation and dephosphorylation upon cell stimulation which can adequately be monitored only by 2-dimensional phosphopeptide mapping (68, 78). Finally, c-Jun is known to be targeted for ubiquitination (77), and this pattern may represent such modification.
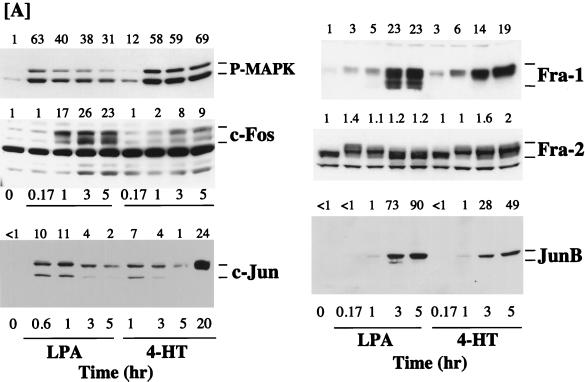
FIG. 6.
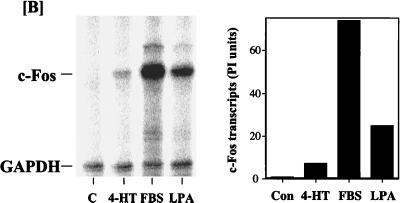
Expression of Fos and Jun proteins in NIH 3T3 cells in response to activation of ΔRaf-1:ER. (A) Confluent, serum-starved c4 cells (NIH 3T3 cells expressing ΔRaf-1:ER) were stimulated with either 50 μM LPA or 1 μM 4-HT for the times indicated. Whole-cell detergent lysates were fractionated by SDS-PAGE, transferred to PVDF membranes, and subjected to Western immunoblotting with antibodies specific for phospho-MAPK (P-MAPK), c-Fos, c-Jun, Fra-1, Fra-2, or JunB. Fold increases in protein expression or MAPK activation are shown above each lane for each blot. Note that the basal levels of c-Jun and JunB were not detectable; the basal level of 1 is set at the lowest detectable value, making the fold increases an underestimate. The results shown are from a single experiment typical of three giving identical results. (B) c4 cells were stimulated with 1 μM 4-HT, 50 μM LPA, or 20% FBS for the times indicated. Cell extracts were prepared, and c-Fos transcripts were analyzed by RNase protection as described in Materials and Methods. The graph represents a quantitative analysis of the data shown in the autoradiograph. Con, control; PI, phosphorimager units.
FIG. 4.
Effect of PD098059 on LPA-stimulated expression of Fos and Jun proteins. Confluent, serum-starved Rat-1 cells were pretreated for 30 min with 40 μM PD098059 or a vehicle control as indicated before being stimulated with 50 μM LPA for the times indicated. Whole-cell detergent lysates were fractionated by SDS-PAGE, transferred to PVDF membranes, and subjected to Western immunoblotting with antibodies specific for c-Fos or c-Jun (A) or Fra-1, Fra-2, or JunB (B). Fold increases in protein expression are shown above each lane for each blot. The results shown are from a single experiment typical of at least three giving identical results.
The kinetics of expression of the JunB protein were similar to those for Fra-2 except that the basal level of JunB was so low as to be undetectable in some experiments (Fig. 1B; see Fig. 6A). Increased expression of JunB was detected 1 h after LPA stimulation and appeared as a doublet and in some cases a triplet of approximately 40 to 42 kDa. JunB expression was maximal between 1 and 2 h after LPA stimulation, at which time the upper band of the doublet was the predominant form. Strong expression of JunB persisted for up to 10 h after addition of LPA. The form of JunB with the lowest electrophoretic mobility most likely reflects a phosphorylation event, since the apparent molecular mass of this protein was reduced upon treatment with alkaline phosphatase (Fig. 1C) (57).
We have previously observed that the activity of p44 MAPK persists for up to 8 h in Rat-1 cells stimulated with a maximal mitogenic dose of LPA. Activation of both p42 and p44 MAPKs in these experiments was confirmed by Western immunoblot analysis of cell extracts with an antibody which specifically recognizes the activated forms of MAPK. LPA stimulated a pronounced peak of MAPK activation at 10 min, which persisted above basal levels in a sustained second phase for at least 8 h (Fig. 1B). These data confirm those from our previous experiments in which sustained activation of p44 MAPK was assessed by immunocomplex kinase assay (20).
In a parallel series of experiments we observed that EGF stimulated the expression of c-Fos, Fra-1, Fra-2, c-Jun, and JunB in quiescent Rat-1 cells with kinetics similar to those with LPA, although in general EGF induced c-Fos expression to a level lower than that observed in response to LPA (22).
Although we did not address the expression of FosB in this study, it has recently been reported that FosB exhibits a kinetic profile of induced expression similar to that observed for c-Fos in serum-stimulated NIH 3T3 cells (57). In addition, our preliminary experiments indicated that JunD was readily detectable in unstimulated Rat-1 cells and that its expression was not elevated in response to LPA (22, 57).
The expression of Fra-1 and Fra-2 requires mitogenic doses of LPA and sustained MAPK activation.
Optimally mitogenic doses of LPA (e.g., 100 μM) stimulate a biphasic activation of MAPK in Rat-1 cells in which the sustained phase persists for several hours (Fig. 1). In contrast, nonmitogenic doses of LPA (e.g., 1 μM) can fully reconstitute the early peak of MAPK activation but fail to elicit the sustained phase of MAPK activation (Fig. 2). Accordingly, the 50% effective concentration (EC50) for activation of the peak of MAPK activity at 10 min is approximately 100 nM, whereas the EC50 for the sustained phase of the response is approximately 10 μM, which is similar to that for induction of DNA synthesis (20). Given the distinct temporal pattern of expression of AP-1 components described above, we examined the expression of Fos and Jun proteins in response to mitogenic (100 μM) and nonmitogenic (1 μM) doses of LPA.
Consistent with our previous observations, 100 μM LPA elicited a robust activation of MAPK followed by a sustained phase, whereas 1 μM LPA fully reconstituted the early peak of MAPK activation at 10 min but did not cause sustained activation of MAPK (Fig. 2A). Stimulation of quiescent Rat-1 cells with 100 μM LPA resulted in the normal profile of c-Fos and c-Jun expression that is shown in Fig. 1. c-Fos and c-Jun expression peaked at 1 h (Fig. 2A), whereas Fra-1 and Fra-2 were induced after a short lag period but were strongly expressed after 4 h of LPA stimulation (Fig. 2B). Stimulation of cells with 1 μM LPA elicited the same early peak of c-Fos, but the response was more transient, while expression of c-Jun was greatly reduced (Fig. 2A). Treatment of Rat-1 cells with 1 μM LPA failed to induce the expression of Fra-1 and Fra-2. Furthermore, in these experiments we observed that JunB was significantly induced only at the mitogenic doses of LPA (Fig. 2B). These results indicate that LPA-induced expression of Fra-1, Fra-2, c-Jun, and JunB correlates with sustained MAPK activation in Rat-1 cells.
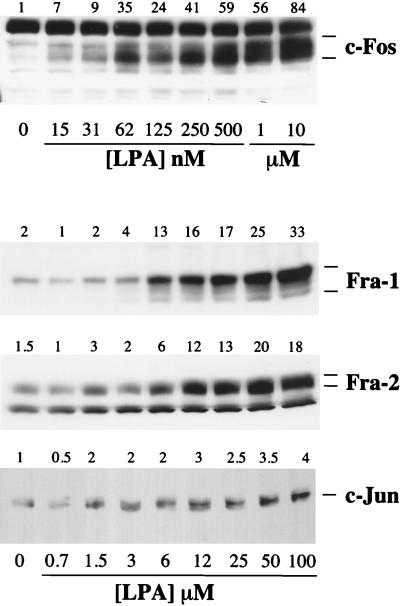
The correlation between the MAPK signal duration and the expression of c-Fos, Fra-1, Fra-2, and c-Jun was strengthened by analyzing the dose-response curves for the expression of these proteins in response to LPA (Fig. 3). LPA-stimulated c-Fos expression, measured after 1 h, was saturable with an EC50 of approximately 100 to 200 nM, similar to that required for the early peak of MAPK activation (20). In contrast, stimulated expression of Fra-1, Fra-2, or c-Jun, assayed 4 h after LPA stimulation, was observed only at micromolar doses of LPA, with EC50 values of approximately 10 μM (Fig. 3). These are the same doses of LPA required to elicit a sustained phase of MAPK activation and to stimulate DNA synthesis.
FIG. 3.
Dose-response curves for expression of Fos and Jun proteins in response to LPA. Confluent, serum-starved Rat-1 cells were stimulated with increasing doses of LPA for 1 h (c-Fos) or 4 h (Fra-1, Fra-2, or c-Jun) as indicated. Whole-cell detergent lysates were fractionated by SDS-PAGE, transferred to PVDF membranes, and subjected to Western immunoblotting with antibodies specific for c-Fos, c-Jun, Fra-1, or Fra-2. Fold increases in protein expression are shown above each lane for each blot. The results shown are from a single experiment typical of at least three giving identical results.
PD098059 inhibits LPA-stimulated expression of all AP-1 components.
Although the kinetic and pharmacological correlations between sustained MAPK activation and the induced expression of Fra-1, Fra-2, c-Jun, and JunB were strong, we sought direct evidence of a role for the Raf-MEK-MAPK pathway in the LPA-stimulated expression of these genes. To address this, we utilized PD098059, a highly selective inhibitor of MEK1 (5), to prevent activation of MEK1 and MAPK in response to LPA stimulation. We have previously demonstrated that PD098059 completely inhibits both LPA- and EGF-stimulated MAPK activation in Rat-1 cells with a 50% inhibitory concentration of approximately 5 to 10 μM, with complete inhibition observed at 40 μM (21).
Pretreatment of Rat-1 cells with 40 μM PD098059 resulted in a pronounced, though not complete, inhibition of LPA-stimulated c-Fos expression which was apparent at all times tested (Fig. 4A). These data suggest that induction of c-Fos exhibits a strong dependency on the activity of the MEK-MAPK cascade. In addition, the remaining c-Fos protein was predominantly in the hypophosphorylated state, suggesting that the Raf-MEK-MAPK cascade plays a role in the observed hyperphosphorylation of c-Fos in LPA-stimulated cells. These results support the proposal that phosphorylation of c-Fos is catalyzed by MAPK or the MAPK-dependent p90RSK pathway in whole cells (14). Similarly, pretreatment of Rat-1 cells with PD098059 also inhibited the LPA-induced increased expression of c-Jun (Fig. 4).
In the same series of experiments, pretreatment of Rat-1 cells with PD098059 resulted in complete inhibition of the LPA-stimulated expression of Fra-1, Fra-2, and JunB, strongly suggesting that the expression of these proteins requires the activity of MEK and MAPK (Fig. 4B). In addition, we also noted that the reduced mobility of Fra-2 which was observed after 10 or 15 min of LPA stimulation was prevented by treatment with PD098059. Indeed, the time of maximal MAPK activation (10 min) (Fig. 1) correlates well with the kinetics of appearance of the reduced-mobility forms of Fra-2, suggesting that MAPK may be responsible for phosphorylation of preexisting Fra-2. It was not possible to reliably confirm if Fra-1 hyperphosphorylation was blocked by PD098059, since PD098059 strongly inhibited the stimulated expression of Fra-1, making it difficult to detect any form of Fra-1. However, it has been reported that Fra-1 and Fra-2 are phosphorylated in vitro by MAPK (36, 76).
Taken together, these results suggest that activation of the MAPK cascade is required for the expression of the AP-1 proteins studied here but that expression of Fra-1, Fra-2, c-Jun, and JunB in particular requires sustained activation of this pathway. This proposal is not based solely on studies with LPA in Rat-1 cells, as we have recently made similar observations by studying a variety of growth factors in the CCL39 cell line (9).
Activation of ΔRaf-1:ER is sufficient to induce expression of Fra-1, Fra-2, and JunB but not c-Fos or c-Jun.
The preceding experiments demonstrated a strong requirement for sustained MAPK activation in regulating the expression of Fra-1, Fra-2, c-Jun, and JunB, whereas the early phase of MAPK activation is associated with expression of c-Fos and a modest increase in c-Jun. We wished to determine if activation of the MAPK cascade alone would be sufficient to induce expression of these genes. It has been shown that Fra-1, Fra-2, c-Jun, and JunB are expressed at elevated levels in Ras-transformed cells (71); however, Ras regulates at least three different effector pathways (Raf, phosphatidylinositol 3′-kinase, and RalGDS/Rlf) (67), and it is unclear what roles the different pathways play in the regulation of gene expression (102). For these studies we used Rat-1 (R1ΔRafER-4) and 3T3 (NIH 3T3 c4 or c9) cells expressing a conditionally active form of oncogenic human Raf-1 (ΔRaf-1:ER). Activation of ΔRaf-1:ER in these cells, by β-E2 or 4-HT, leads to rapid, protein synthesis-independent activation of MEK and MAPK without the activation of other parallel growth factor-stimulated signal pathways (83) until 15 to 20 h later (69, 73).
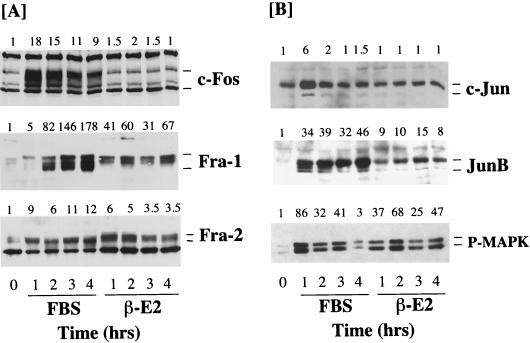
Treatment of quiescent R1ΔRafER-4 cells with serum resulted in increased expression of c-Fos, c-Jun, Fra-1, Fra-2, and JunB (Fig. 5A), indicating that the signalling pathways regulated by serum were not compromised by expression of ΔRaf-1:ER. Activation of ΔRaf-1:ER for up to 4 h had no effect on the expression of c-Fos and c-Jun but significantly induced the expression of Fra-1, Fra-2, and JunB in R1ΔRafER-4 cells (Fig. 5A). The expression of Fra-1, Fra-2, and JunB observed in response to activation of ΔRaf-1:ER was slightly less than that observed in response to serum but was still much stronger than that seen for c-Fos or c-Jun. Analysis of MAPK activation under these conditions, using the phosphospecific MAPK antibody, revealed that serum stimulation and activation of ΔRaf-1:ER induced similar levels of MAPK activation.
FIG. 5.
Expression of Fos and Jun proteins in Rat-1 cells in response to activation of ΔRaf-1:ER. Confluent, serum-starved R1ΔRaf-1:ER-4 cells were stimulated with either 20% FBS or 1 μM β-E2 for 1 to 4 h. Whole-cell detergent lysates were fractionated by SDS-PAGE, transferred to PVDF membranes, and subjected to Western immunoblotting with antibodies specific for c-Fos, Fra-1, or Fra-2 (A) or c-Jun, JunB, or phospho-MAPK (P-MAPK) (B). Fold increases in protein expression or MAPK activation are shown above each lane for each blot. The results shown are from a single experiment typical of three giving identical results.
A similar analysis was performed with NIH 3T3 c4 and c9 cells expressing ΔRaf-1:ER. Again, ΔRaf-1:ER activation caused only a weak activation of c-Fos expression in these cells, although the response to LPA proceeded normally (Fig. 6A). The basal level of c-Jun in NIH 3T3 cells was considerably lower than that in Rat-1 cells and was not detectable, making the fold increase in c-Jun an underestimate. Increased expression of c-Jun was observed in response to LPA and in response to activation of ΔRaf-1:ER at early times (1 to 5 h), but a much more pronounced induction was observed 20 h after activation of ΔRaf-1:ER, which has also been observed by others (92). Under these conditions, it has previously been demonstrated that activation of ΔRaf-1:ER leads to the expression and release of growth factors such as HB-EGF, which may act in an autocrine manner to promote c-Jun expression (69, 73). In the same experiments we observed strong expression of Fra-1 and JunB in response to both LPA and ΔRaf-1:ER activation. Even after prolonged serum withdrawal, NIH 3T3 c4 cells exhibited high levels of Fra-2, so both LPA and ΔRaf-1:ER activation elicited only modest increases in Fra-2 expression. However, we again observed that both LPA and ΔRaf-1:ER caused a similar reduction in electrophoretic mobility of the Fra-2 protein (Fig. 6). Indeed, the electrophoretic mobility of Fra-2 was reduced to its greatest extent at the time of maximal MAPK activation by LPA (10 min) or ΔRaf-1:ER (1 h). Since the mobility shift of Fra-2 is abolished by treatment of cells with PD098059 or treatment of lysates with alkaline phosphatase, these results suggest that the Raf-MEK-MAPK cascade is both necessary and sufficient for hyperphosphorylation of Fra-2.
Since many studies have suggested a role for the MAPK cascade in regulating c-Fos expression (34, 42, 43, 50, 64, 95, 101), we were intrigued by the fact that ΔRaf-1:ER activation caused such a small increase in c-Fos protein levels. We investigated this further by RNase protection analysis of c-Fos mRNA induction following ΔRaf-1:ER activation. Under these conditions, we observed a very modest and transient expression of c-Fos transcripts in response to activation of ΔRaf-1:ER, whereas serum stimulation elicited a robust response (Fig. 6B). For example, in a side-by-side comparison, ΔRaf-1:ER stimulated the expression of c-Fos protected transcripts by 7-fold, LPA stimulated the accumulation of c-Fos transcripts by 25-fold, and serum did so by 73-fold. In these experiments the maximal activations of MAPK seen in response to serum, LPA, and ΔRaf-1:ER were similar, although the kinetics of the response to ΔRaf-1:ER activation was slightly slower than that for serum or LPA (Fig. 5 and 6A). It is therefore unlikely that the lack of inducibility of c-Fos by ΔRaf-1:ER is due to insufficient activation of MAPK. Thus, while activation of MAPK is required for c-Fos expression, activation of that pathway alone is not sufficient to induce c-Fos.
In summary, selective and sustained activation of the MAPK cascade alone is sufficient to induce expression of Fra-1, Fra-2, and JunB, whereas induced expression of c-Fos is very weak. c-Jun expression is induced weakly as a result of acute activation of the MAPK pathway but strongly after a prolonged activation (15 to 20 h), which may reflect the action of an autocrine factor.
Synergistic expression of c-Fos by ΔRaf-1:ER activation and Ca2+.
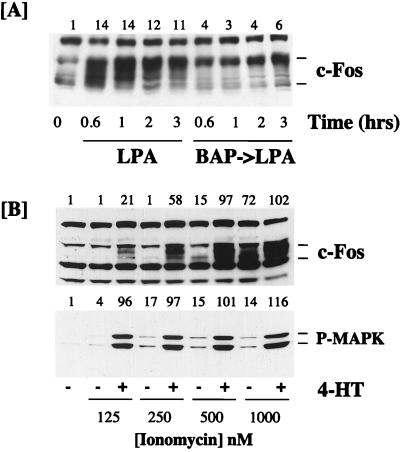
In addition to activating the Ras-Raf-MAPK cascade, LPA stimulates the release of Ca2+ from intracellular stores, resulting in a transient increase in the intracellular [Ca2+] ([Ca2+]i). We have recently shown that an immediately-early gene, that for MAPK phosphatase-1 (MKP-1), is unresponsive to the activation of ΔRaf-1:ER in Rat-1 cells (21). In this case the expression of MKP-1 is dependent upon both the MAPK cascade and Ca2+ mobilization and can be reconstituted by a combination of ionomycin and ΔRaf-1:ER activation. These results prompted us to examine a possible role for Ca2+ in the regulation of c-Fos expression. Pretreatment of Rat-1 cells with BAPTA-AM to buffer increases in [Ca2+]i prior to stimulation with LPA resulted in a strong, but not complete, inhibition of subsequent c-Fos expression (Fig. 7A). These data suggest that LPA-induced c-Fos expression requires an increase in [Ca2+]i. In these experiments we noted that the small amount of c-Fos protein which was still induced in response to LPA plus BAPTA-AM was predominantly in the hyperphosphorylated form (Fig. 7A). This may be due to enhanced MAPK-dependent phosphorylation (14), since under these conditions, LPA-stimulated MKP-1 expression is inhibited and MAPK activation is greatly enhanced (21).
FIG. 7.
Effect of Ca2+ on c-Fos expression in response to LPA and ΔRaf-1:ER. (A) R1ΔRaf-1:ER-4 cells were pretreated with 30 μM BAPTA-AM (BAP) or a vehicle control before being stimulated with 50 μM LPA for the times indicated. Whole-cell detergent lysates were fractionated by SDS-PAGE, transferred to PVDF membranes, and subjected to Western immunoblotting with antibodies specific for c-Fos. (B) R1ΔRaf-1:ER-4 cells were stimulated with increasing doses of ionomycin in the absence or presence of 1 μM 4-HT for 60 min. Whole-cell detergent lysates were fractionated by SDS-PAGE, transferred to PVDF membranes, and subjected to Western immunoblotting with antibodies specific for c-Fos or phospho-MAPK (P-MAPK). Fold increases in protein expression or MAPK activation are shown above each lane for each blot. Similar results were obtained in additional experiments with R1ΔRaf-1:ER-4 and NIH 3T3 c4 cells.
To examine possible cooperation between Ca2+ and the MAPK pathway, we stimulated serum-starved R1ΔRaf-1:ER-4 cells with increasing concentrations of ionomycin in the absence or presence of 4-HT to activate ΔRaf-1:ER and analyzed c-Fos expression by immunoblotting. In these experiments we observed that activation of ΔRaf-1:ER in the presence of a low dose of ionomycin (125 or 250 nM) resulted in synergistic induction of c-Fos expression (Fig. 7B). Even at 500 nM ionomycin, a dose sufficient to weakly induce c-Fos on its own, we observed synergy with ΔRaf-1:ER activation (Fig. 7B). The c-Fos protein induced by ionomycin alone (e.g., at 500 nM) exhibited a lower apparent molecular weight than that induced in response to the combination of ΔRaf-1:ER and ionomycin. Since c-Fos is phosphorylated by MAPK and MAPK-dependent kinases (14), this may be related to the weak and very transient activation of MAPK which is observed in response to Ca2+ ionophores in these cells (Fig. 7B) (20). In order to determine if under these conditions ΔRaf-1:ER and Ca2+ were not simply synergizing to hyperactivate the MAPK cascade, the same samples were immunoblotted with the phosphospecific MAPK antibody. In these experiments it was evident that ionomycin alone elicited little activation of MAPK and did not amplify the activation of MAPK observed in response to ΔRaf-1:ER activation. Similar results were obtained when we examined the synergy between ΔRaf-1:ER and ionomycin in NIH 3T3 c4 cells (data not shown). These results suggest that ionomycin and ΔRaf-1:ER activation cooperate to induce c-Fos expression by activating separate pathways.
DISCUSSION
The duration of MAPK activation determines the expression of distinct Fos and Jun proteins.
Considerable interest has been focused on the regulation of gene expression following growth factor stimulation or oncogenic transformation and how such patterns of gene expression relate to the biological effects observed. It is well documented that the strength and duration of signal pathway activation can have profound effects on the biological responses of cells. For example, Raf-activated signalling pathways can elicit either a mitogenic response or cell cycle arrest in NIH 3T3 cells, depending on the level of pathway activation (103). Other studies have demonstrated that growth and/or differentiation factors can stimulate the sustained activation of MAPK and that this correlates with cell proliferation or differentiation, depending on the magnitude of the response and the cell type (19, 20, 40, 49, 66, 91). Here we have demonstrated that the duration of MAPK activation determines the pattern of expression of different Fos and Jun proteins, allowing the MAPK cascade to impart significant variation to the repertoire of AP-1 complexes assembled during cell cycle reentry.
The LPA-stimulated expression of Fos and Jun proteins described here is strongly dependent on the Raf-MEK-MAPK pathway (Fig. 4), but the temporal pattern of expression is highly coordinated (Fig. 1) and is determined by the duration of MAPK activation (Fig. 2 and 3). In response to a maximal proliferative dose of LPA, c-Fos expression peaks within 1 h and then declines to basal levels by 6 h, whereas Fra-1, Fra-2, c-Jun, and JunB are expressed in a sustained manner, persisting throughout the G1 phase of the cell cycle. In contrast, a nonmitogenic dose of LPA, which fails to induce the sustained phase of MAPK activation, still induces c-Fos but fails to induce the expression of Fra-1 and Fra-2 and elicits a greatly reduced expression of c-Jun and JunB.
These results suggest that in Rat-1 cells one of the functions of the sustained phase of MAPK activity is to determine the pool of AP-1 proteins available to form dimers. For example, other studies have demonstrated that c-Fos makes only a transient contribution to AP-1 complexes early in G1, and the assembly of heterodimers containing Fra-1, Fra-2, c-Jun, and JunB in the mid-to-late G1 phase has been documented (52, 53). Based on these observations, it seems reasonable to speculate that dimers containing Fra-1, Fra-2, c-Jun, and JunB exhibit distinct properties (such as recognition of a subset of TREs and/or unique transcriptional properties) such that they activate or repress distinct AP-1-regulated genes (38, 39, 81). In this way the changing pattern of Fos and Jun protein expression during cell cycle reentry could provide the link between sustained MAPK activation and the cell cycle regulatory apparatus.
Given their different kinetics of expression and dependency on sustained MAPK activation, it seems pertinent to consider the biological functions of c-Fos, Fra-1, Fra-2, c-Jun, and JunB in relation to cell cycle reentry. In Rat-1 cells the maximal induction of c-Fos at 1 h is the same at both doses of LPA, but the response is clearly more transient in response to 1 μM LPA. These data might suggest a correlation between the more prolonged gradual decline in c-Fos expression, sustained MAPK activation, and DNA synthesis. However, in pharmacological terms there seems to be a poor correlation between LPA-stimulated c-Fos expression (EC50 of 0.1 to 0.2 μM) and DNA synthesis (EC50 of 10 to 20 μM) (20). In this regard it is interesting that c-Fos−/− mice are viable and that c-Fos−/− fibroblasts proliferate normally (11, 45). Furthermore, enforced expression of c-Fos can cause morphological transformation without affecting cell cycle progression (72), and ΔRaf-1:ER can induce DNA synthesis (103) without promoting significant c-Fos expression (Fig. 5 and 6). While our results do not address the role of c-Fos in cell cycle reentry directly, they are consistent with many studies in the literature which argue against a role for c-Fos in driving cell cycle reentry in most cell types.
The kinetic and pharmacological correlation between sustained MAPK activation and cell cycle reentry in response to LPA (20) certainly suggests that the expression of Fra-1, Fra-2, c-Jun, and JunB correlates well with proliferative efficacy. Overexpression of Fra-1 leads to morphological transformation and anchorage-independent growth of Rat-1 cells (10) and can cooperate with c-Jun in transforming NIH 3T3 cells (71). Furthermore, Fra-1, Fra-2, c-Jun, and JunB are found to be overexpressed in Ras-transformed thyroid cells (96) and NIH 3T3 cells (71), and antisense-mediated inhibition of Fra-1 expression causes a partial reversion of the Ras-transformed phenotype (96). Microinjection of neutralizing antisera specific for the individual Fos or Jun proteins has revealed that Fra-1, Fra-2, c-Jun, and JunB serve an important function during the G1-to-S transition and in normal asynchronous cell proliferation (52).
Our results have implications for the role of AP-1 in Ras signalling. The major AP-1 components up-regulated in Ras-transformed cells are Fra-1, Fra-2, c-Jun, and JunB (NIH 3T3 cells) (71) and Fra-1 and JunB (thyroid cells) (96). Since Ras regulates at least three different effector pathways (Raf, phosphatidylinositol 3′-kinase, and RalGDS) (67), it is conceivable that all three pathways may contribute to changes in gene expression. Our analysis here shows that reconstitution of just one of these pathways, Raf-MEK-MAPK, is sufficient to account for the effects of Ras on Fra-1, Fra-2, c-Jun, and JunB, and these results have been independently confirmed (92).
Recent studies have demonstrated a link between the Ras-Raf-MEK-MAPK pathway and the cell cycle apparatus. Inducible expression of RasV12 or conditional activation of Raf is sufficient to drive the expression of cyclin D1 (84, 103), and MEK is required for activation of the cyclin D1 promoter (59). The cyclin D1 promoter contains an AP-1 site (3, 41) which binds to c-Fos and the three Jun proteins (3), but it seems unlikely that c-Fos is required for cyclin D1 expression, since activation of ΔRaf-1:ER induces cyclin D1 (103) without significant levels of c-Fos expression (Fig. 5 and 6). Based on these observations, it will be interesting to study the role of Fra-1, Fra-2, and JunB in the expression of cell cycle-regulatory genes such as those for cyclin D1 and p21Cip1, which are linked to sustained MAPK activation. However, we emphasize that AP-1 is unlikely to be the sole target of sustained MAPK activity. Indeed, cyclin D1 has also been proposed to be a direct transcriptional target of Ets-2 (3); this may provide a direct link between the MAPK pathway and cyclin D1 expression, since Ets-2 is phosphorylated and regulated by MAPK (70).
How does the duration of MAPK activation determine the expression of these individual proteins? Given the temporal pattern of Fos and Jun protein expression, our results are consistent with a model in which the early peak of c-Fos expression directs the subsequent expression of Fra-1 and Fra-2 by forming functional AP-1 complexes at AP-1 sites in their promoter enhancer regions. In this way the MAPK-dependent expression of c-Fos would be reflected in the expression of c-Fos target genes such as that for Fra-1, and such a model would be consistent with the ability of c-Fos:ER to induce Fra-1 expression (10). However, ΔRaf-1:ER induces robust expression of Fra-1 and JunB but little, if any, expression of c-Fos (Fig. 5 and 6), and serum-stimulated transcription of Fra-1 is insensitive to cycloheximide (17). Indeed, in c-Fos−/− fibroblasts serum-induced expression of Fra-1 is reduced and delayed, but not abolished, and the expression of other AP-1 components is normal (11, 45). This suggests that c-Fos is not required for the expression of Fra-1, Fra-2, and JunB and may reflect redundancy between c-Fos and other Fos family members or indeed other transcription factors. Fra-2 expression may be regulated directly by the MAPK cascade via an SRE site or indirectly via the AP-1 site (86). JunB expression is regulated by Ras via tandem inverted Ets binding sites in the promoter (16, 31). The recent demonstration that ΔRaf-1:ER activation is sufficient to promote Ets-2 phosphorylation and the activation of both AP-1/Ets and Ets/Ets cis-acting elements (70, 104) suggests a direct, AP-1-independent pathway between the MAPK cascade and expression of JunB. Our demonstration that JunB expression is blocked by PD098059 and can be reconstituted by ΔRaf-1:ER alone suggests that such a pathway may indeed operate for JunB expression.
c-Jun is reported to be up-regulated in Ras-transformed cells but exhibits weak inducibility by ΔRaf-1:ER. This may suggest that c-Jun expression requires integration of two signals, both of which can be provided by Ras, whereas Raf can only provide one. Alternatively, the enhanced expression of c-Jun in constitutively Ras-transformed cells (71) may reflect activation of an autocrine loop. Indeed, activation of the MAPK cascade leads to the expression and release of HB-EGF, which can act as an autocrine factor to promote activation of JNK (69, 73); in this way expression of c-Jun could result from autoregulation of its own promoter (30, 98). The delayed expression of c-Jun in response to activation of ΔRaf-1:ER (Fig. 6) or MEK (92) may be due to such an autocrine loop.
It is important to stress that the changes in steady-state protein levels reported here may not simply reflect changes in the transcription rate. The duration of MAPK activation may regulate mRNA stability, and there is certainly precedent for MAPK or SAPK regulating the stability of Fos and Jun proteins. For example, MAPK phosphorylates c-Fos, thereby increasing its half-life and transforming potential (14), while MAPK- or SAPK-catalyzed phosphorylation of c-Jun reduces its ubiquitination, thereby stabilizing the c-Jun protein (77). Future studies will aim to address the mechanism by which the duration of MAPK activation determines the pattern of protein expression reported here.
Activation of the MAPK cascade by ΔRaf-1:ER is sufficient for expression of Fra-1, Fra-2, and JunB but not c-Fos.
One of the central paradigms to have emerged in signal transduction is that activation of the MAPK cascade regulates c-Fos expression (34, 42, 43, 50, 64, 95, 101). However, our results, together with those of others, suggest that c-Fos regulation is more complex than this. In R1ΔRaf:ER-4 cells we did not observe expression of c-Fos in response to activation of ΔRaf-1:ER, while in NIH 3T3 cells RNase protection assays confirmed that c-Fos transcripts were only weakly induced by activation of ΔRaf-1:ER. In both cells serum- and LPA-stimulated c-Fos expression was normal. In addition, it is clear that the inability to induce c-Fos does not reflect a generalized failure of ΔRaf-1:ER to activate gene expression, since other genes, such as those for Fra-1, Fra-2, and JunB (Fig. 5 and 6), cyclin D1 (103), and c-Myc (8), are induced strongly by activation of ΔRaf-1:ER.
The results with PD098059 (Fig. 4) and ΔRaf-1:ER (Fig. 5) suggest that the MAPK cascade is necessary but not sufficient for c-Fos expression. One possibility is that expression of c-Fos requires activation of multiple signals and it is the synergistic integration of these signals which leads to induced c-Fos expression. This model is supported by experiments in which ΔRaf-1:ER activation and ionomycin synergized to induce expression of c-Fos without amplifying activation of the MAPK cascade. Since buffering of increased [Ca2+]i inhibited LPA-induced c-Fos expression (Fig. 7A) without blocking MAPK activation (21), these results are most consistent with induced c-Fos expression requiring the integration of MAPK- and Ca2+-driven signals. This could occur at the level of the c-Fos promoter itself, which contains both MAPK-responsive elements (ternary complex factor/serum response element) and Ca2+-responsive elements (33, 46). Robertson et al. have shown that both the Ca2+-responsive element and SRE sites are required for optimal expression of c-Fos in response to growth factors (80). In addition to the possible integration of signalling pathways at different cis-acting promoter elements, a recent study has demonstrated that dynamic regulation of histone H4 hyperacetylation may also provide an additional level of signal cooperation to regulate c-Fos expression (4). Future studies will aim to address the mechanism of the observed cooperation between ΔRaf-1:ER and Ca2+ in regulating c-Fos expression.
Conclusion.
In summary, these results demonstrate that the magnitude and duration of MAPK activation determine the repertoire of Fos and Jun family members available to form AP-1 complexes at different stages during the G1 phase of the cell cycle. This is likely to lead to the formation of discrete AP-1 complexes with distinct properties which could, in principle, allow for changes in AP-1-regulated gene expression as the cell proceeds through G1. These results provide strong support for the proposal that quantitative differences in duration of MAPK activation will lead to qualitative changes in gene expression (66) and suggest that the temporal changes in AP-1 composition may represent a suitable experimental paradigm to test this model further.
ACKNOWLEDGMENTS
We thank Kathryn Balmanno, Allan Balmain, Jerlyn Beltman, Gideon Bollag, Michelle Garrett, Frank McCormick, John Pascall, and Doug Woods for stimulating discussions and particularly Iris Treines, Hugh Paterson, and Chris Marshall for discussion of their unpublished results.
The DNAX Research Institute is supported by Schering Plough Corporation. This work was initiated at ONYX Pharmaceuticals as part of a project supported by a collaborative research agreement with Bayer AG and was continued at The Babraham Institute, where it was supported by a competitive strategic grant from the BBSRC.
REFERENCES
- 1.Abate C, Luk D, Curran T. Transcriptional regulation by Fos and Jun in vitro: interaction among multiple activator and regulatory domains. Mol Cell Biol. 1991;11:3624–3632. doi: 10.1128/mcb.11.7.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal S, Corbley M J, Roberts T M. Reconstitution of signal transduction from the membrane to the nucleus in a baculovirus expression system: activation of Raf-1 leads to hypermodification of c-jun and c-fos via multiple pathways. Oncogene. 1995;11:427–438. [PubMed] [Google Scholar]
- 3.Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, Pestell R G. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. doi: 10.1074/jbc.270.40.23589. [DOI] [PubMed] [Google Scholar]
- 4.Alberts A S, Geneste O, Treisman R. Activation of SRF-regulated chromosomal templates by Rho-family GTPases requires a signal that also induces H4 hyperacetylation. Cell. 1998;92:475–487. doi: 10.1016/s0092-8674(00)80941-1. [DOI] [PubMed] [Google Scholar]
- 5.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 6.Angel P, Baumann I, Stein B, Delius H, Rahmsdorf H J, Herrlich P. 12-O-Tetradecanoyl-phorbol-13-acetate induction of the human collagenase gene is mediated by an inducible enhancer element in the 5′-flanking region. Mol Cell Biol. 1987;7:2256–2266. doi: 10.1128/mcb.7.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 8.Aziz, N., H. Cherwinski, and M. McMahon. Unpublished data.
- 9.Balmanno, K., and S. J. Cook. Unpublished data.
- 10.Bergers G, Graninger P, Braselmann S, Wrighton C, Busslinger M. Transcriptional activation of the fra-1 gene by AP-1 is mediated by regulatory sequences in the first intron. Mol Cell Biol. 1995;15:3748–3758. doi: 10.1128/mcb.15.7.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brusselbach S, Mohle-Steinlein U, Wang Z Q, Schreiber M, Lucibello F C, Muller R, Wagner E F. Cell proliferation and cell cycle progression are not impaired in fibroblasts and ES cells lacking c-Fos. Oncogene. 1995;10:79–86. [PubMed] [Google Scholar]
- 12.Cadwallader K, Beltman J, McCormick F, Cook S J. Differential regulation of extracellular signal-regulated protein kinase 1 and Jun N-terminal kinase 1 by Ca2+ and protein kinase C in endothelin-stimulated Rat-1 cells. Biochem J. 1997;321:795–804. doi: 10.1042/bj3210795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen R-H, Sarnecki C, Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992;12:915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen R-H, Juo P C-H, Curran T, Blenis J. Phosphorylation of c-Fos at the C-terminus enhances its transforming activity. Oncogene. 1996;12:1493–1502. [PubMed] [Google Scholar]
- 15.Cobb M H, Hepler J E, Cheng M, Robbins D. The mitogen-activated protein kinases, ERK1 and ERK2. Semin Cancer Biol. 1994;5:261–268. [PubMed] [Google Scholar]
- 16.Coffer P, de Jonge M, Mettouchi A, Binetruy B, Ghysdael J, Kruijer W. junB promoter regulation: Ras mediated transactivation by c-Ets-1 and c-Ets-2. Oncogene. 1994;9:911–921. [PubMed] [Google Scholar]
- 17.Cohen D R, Curran T. fra-1: a serum-inducible, cellular immediate-early gene that encodes a Fos-related antigen. Mol Cell Biol. 1988;8:2063–2069. doi: 10.1128/mcb.8.5.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen D R, Ferreira P C, Gentz R, Franza B R, Jr, Curran T. The product of a fos-related gene, fra-1, binds cooperatively to the AP-1 site with Jun: transcription factor AP-1 is comprised of multiple protein complexes. Genes Dev. 1989;3:173–184. doi: 10.1101/gad.3.2.173. [DOI] [PubMed] [Google Scholar]
- 19.Cook S J, Rubinfeld B, Albert I, McCormick F. RapV12 antagonizes Ras-dependent activation of ERK1 and ERK2 by LPA and EGF in Rat-1 fibroblasts. EMBO J. 1993;12:3475–3485. doi: 10.1002/j.1460-2075.1993.tb06022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cook S J, McCormick F. Kinetic and biochemical correlation between sustained p44ERK1 (44 kDa extracellular signal-regulated kinase 1) activation and lysophosphatidic acid-stimulated DNA synthesis in Rat-1 cells. Biochem J. 1996;320:237–245. doi: 10.1042/bj3200237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cook S J, Beltman J, Cadwallader K A, McMahon M, McCormick F. Regulation of mitogen-activated protein kinase phosphatase-1 expression by extracellular signal-related kinase-dependent and Ca2+-dependent signal pathways in Rat-1 cells. J Biol Chem. 1997;272:13309–13319. doi: 10.1074/jbc.272.20.13309. [DOI] [PubMed] [Google Scholar]
- 22.Cook, S. J. Unpublished data.
- 23.Cowley S, Paterson H, Kemp P, Marshall C J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 24.Crews C M, Alessandrini A, Erickson R L. The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science. 1992;258:478–480. doi: 10.1126/science.1411546. [DOI] [PubMed] [Google Scholar]
- 25.Curran T, Miller A D, Zokas L, Verma I M. Viral and cellular fos proteins: a comparative analysis. Cell. 1984;36:259–268. doi: 10.1016/0092-8674(84)90219-8. [DOI] [PubMed] [Google Scholar]
- 26.Curran T, Morgan J I. Memories of fos. Bioessays. 1987;7:255–258. doi: 10.1002/bies.950070606. [DOI] [PubMed] [Google Scholar]
- 27.De Cesare D, Vallone D, Caracciolo A, Sassone-Corsi P, Nerlov C, Verde P. Heterodimerization of c-Jun with ATF-2 and c-Fos is required for positive and negative regulation of the human urokinase enhancer. Oncogene. 1995;11:365–376. [PubMed] [Google Scholar]
- 28.Dent P, Haser W, Haystead T A J, Vincent L A, Roberts T M, Sturgill T W. Activation of mitogen-activated protein kinase by v-Raf in NIH3T3 cells and in vitro. Science. 1992;257:1404–1407. doi: 10.1126/science.1326789. [DOI] [PubMed] [Google Scholar]
- 29.Derijard B, Hibi M, Wu I H, Barrett T, Su B, Deng T, Karin M, Davis R J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 30.Devary Y, Gottlieb R A, Lau L F, Karin M. Rapid and preferential activation of the c-jun gene during the mammalian UV response. Mol Cell Biol. 1991;11:2804–2811. doi: 10.1128/mcb.11.5.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galang C K, Der C J, Hauser C A. Oncogenic Ras can induce transcriptional activation through a variety of promoter elements including tandem c-Ets-2 binding sites. Oncogene. 1994;9:2913–2921. [PubMed] [Google Scholar]
- 32.Gentz R, Rausher F, Abate C, Curran T. Parallel association of Fos and Jun leucine zippers juxtaposes DNA binding domains. Science. 1989;243:1695–1699. doi: 10.1126/science.2494702. [DOI] [PubMed] [Google Scholar]
- 33.Ghosh A, Greenberg M E. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995;268:239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- 34.Gille H, Kortenjann M, Thomae O, Moomaw C, Slaughter C, Cobb M H, Shaw P E. ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO J. 1995;14:951–962. doi: 10.1002/j.1460-2075.1995.tb07076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenberg M E, Ziff E B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984;311:433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- 36.Gruda M C, Kovary K, Metz R, Bravo R. Regulation of Fra-1 and Fra-2 phosphorylation differs during the cell cycle of fibroblasts and phosphorylation in vitro by MAP kinase affects DNA binding activity. Oncogene. 1994;9:2537–2547. [PubMed] [Google Scholar]
- 37.Hai T, Curran T. Cross family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci USA. 1991;88:3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halazonetis T D, Georgeopoulos K, Greenberg M E, Leder P. c-Jun dimerizes with itself and with c-Fos forming complexes with different binding affinities. Cell. 1988;55:917–924. doi: 10.1016/0092-8674(88)90147-x. [DOI] [PubMed] [Google Scholar]
- 39.Hawker K L, Vass J K, Ozanne B W. Isolation of novel, transcriptionally active AP-1 binding sites: implications for cellular transformation. Oncogene. 1996;13:283–292. [PubMed] [Google Scholar]
- 40.Heasley L E, Johnson G L. The beta-PDGF receptor induces neuronal differentiation of PC12 cells. Mol Biol Cell. 1992;3:545–553. doi: 10.1091/mbc.3.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herber B, Truss M, Beato M, Müller R. Inducible regulatory elements in the human cyclin D1 promoter. Oncogene. 1994;9:1295–1304. [PubMed] [Google Scholar]
- 42.Hill C S, Treisman R. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell. 1995;80:199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- 43.Hipskind R A, Baccarini M, Nordheim A. Transient activation of RAF-1, MEK, and ERK2 coincides kinetically with ternary complex factor phosphorylation and immediate-early gene promoter activity in vivo. Mol Cell Biol. 1994;14:6219–6231. doi: 10.1128/mcb.14.9.6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howe L R, Leevers S J, Gómez N, Nakielny S, Cohen P, Marshall C J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992;71:335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- 45.Hu E, Mueller E, Oliviero S, Papaioannou V E, Johnson R, Spiegelman B M. Targeted disruption of the c-fos gene demonstrates c-fos-dependent and -independent pathways for gene expression stimulated by growth factors or oncogenes. EMBO J. 1994;13:3094–3103. doi: 10.1002/j.1460-2075.1994.tb06608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson C M, Hill C S, Chawla S, Treisman R, Bading H. Calcium controls gene expression via three distinct pathways that can function independently of the Ras/mitogen-activated protein kinases (ERKs) signaling cascade. J Neurosci. 1997;17:6189–6202. doi: 10.1523/JNEUROSCI.17-16-06189.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson G L, Vaillancourt R R. Sequential protein kinase reactions controlling cell growth and differentiation. Curr Opin Cell Biol. 1994;6:230–238. doi: 10.1016/0955-0674(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 48.Johnson R, Spiegelman B, Hanahan D, Wisdom R. Cellular transformation and malignancy induced by Ras require c-Jun. Mol Cell Biol. 1996;16:4504–4511. doi: 10.1128/mcb.16.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kahan C, Seuwen K, Meloch S, Pouyssegur J. Coordinate, biphasic activation of p44 mitogen-activated protein kinase and S6 kinase by growth factors in hamster fibroblasts. Evidence for thrombin-induced signals different from phosphoinositide turnover and adenylylcyclase inhibition. J Biol Chem. 1992;267:13369–13375. [PubMed] [Google Scholar]
- 50.Karin M, Hunter T. Transcriptional control by protein phosphorylation: signal transmission from the cell surface to the nucleus. Curr Biol. 1995;5:747–757. doi: 10.1016/s0960-9822(95)00151-5. [DOI] [PubMed] [Google Scholar]
- 51.Kouzarides T, Ziff E. Leucine zippers of fos, jun and GCN4 dictate dimerization specificity and thereby control DNA binding. Nature. 1989;340:568–571. doi: 10.1038/340568a0. [DOI] [PubMed] [Google Scholar]
- 52.Kovary K, Bravo R. Existence of different Fos/Jun complexes during the G0-to-G1 transition and during exponential growth in mouse fibroblasts: differential role of Fos proteins. Mol Cell Biol. 1992;12:5015–5023. doi: 10.1128/mcb.12.11.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kovary K, Bravo R. Expression of different Jun and Fos proteins during the G0-to-G1 transition in mouse fibroblasts: in vitro and in vivo associations. Mol Cell Biol. 1991;11:2451–2459. doi: 10.1128/mcb.11.5.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kruijer W, Cooper J A, Hunter T, Verma I M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984;312:711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- 55.Kyriakis J M, App H, Zhang X F, Banerjee P, Brautigan D L, Rapp U R, Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992;358:417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- 56.Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubie E A, Ahmad M F, Avruch J, Woodgett J R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 57.Lallemand D, Spyrou G, Yaniv M, Pfarr C M. Variations in Jun and Fos protein expression and AP-1 activity in cycling, resting and stimulated fibroblasts. Oncogene. 1997;14:819–830. doi: 10.1038/sj.onc.1200901. [DOI] [PubMed] [Google Scholar]
- 58.Landschulz W H, Johnson P F, McKnight S L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988;240:1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- 59.Lavoie J N, L’Allemain G, Brunet A, Muller R, Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 60.Lee W M, Lin C, Curran T. Activation of the transforming potential of the human fos proto-oncogene requires message stabilization and results in increased amounts of partially modified Fos protein. Mol Cell Biol. 1988;8:5521–5527. doi: 10.1128/mcb.8.12.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leevers S J, Paterson H F, Marshall C J. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature. 1994;369:411–414. doi: 10.1038/369411a0. [DOI] [PubMed] [Google Scholar]
- 62.Lenormand P, Sardet C, Pagés G, L’Allemain G, Brunet A, Pouysségur J. Growth factors induce nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45mapkk) in fibroblasts. J Cell Biol. 1993;122:1079–1088. doi: 10.1083/jcb.122.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lloyd A, Yancheva N, Wasylyk B. Transformation suppressor activity of a Jun transcription factor lacking its activation domain. Nature. 1991;352:635–638. doi: 10.1038/352635a0. [DOI] [PubMed] [Google Scholar]
- 64.Marais R, Wynne J, Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993;73:381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- 65.Marais R, Light Y, Paterson H, Marshall C J. Ras recruits Raf-1 to the plasma membrane for activation by tyrosine phosphorylation. EMBO J. 1995;14:101–110. doi: 10.1002/j.1460-2075.1995.tb07316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marshall C J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 67.Marshall C J. Ras effectors. Curr Opin Cell Biol. 1996;8:197–204. doi: 10.1016/s0955-0674(96)80066-4. [DOI] [PubMed] [Google Scholar]
- 68.May G H, Funk M, Black E J, Clark W, Hussain S, Woodgett J R, Gillespie D A. An oncogenic mutation uncouples the v-Jun oncoprotein from positive regulation by the SAPK/JNK pathway in vivo. Curr Biol. 1998;8:117–120. doi: 10.1016/s0960-9822(98)70043-0. [DOI] [PubMed] [Google Scholar]
- 69.McCarthy S A, Samuels M L, Pritchard C A, Abraham J A, McMahon M. Rapid induction of heparin-binding epidermal growth factor/diphtheria toxin receptor expression by Raf and Ras oncogenes. Genes Dev. 1995;9:1953–1964. doi: 10.1101/gad.9.16.1953. [DOI] [PubMed] [Google Scholar]
- 70.McCarthy S A, Chen D, Yang B S, Garcia R J, Cherwinski H, Chen X R, Klagsburg M, Hauser C A, Ostrowski M C, McMahon M. Rapid phosphorylation of Ets-2 accompanies mitogen-activated protein kinase activation and the induction of heparin-binding epidermal growth factor gene expression by oncogenic Raf-1. Mol Cell Biol. 1997;17:2401–2412. doi: 10.1128/mcb.17.5.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mechta F, Lallemand D, Pfarr C M, Yaniv M. Transformation by ras modifies AP1 composition and activity. Oncogene. 1997;14:837–847. doi: 10.1038/sj.onc.1200900. [DOI] [PubMed] [Google Scholar]
- 72.Miao G G, Curran T. Cell transformation by c-Fos requires an extended period of expression and is independent of the cell cycle. Mol Cell Biol. 1994;14:4295–4310. doi: 10.1128/mcb.14.6.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis R J, Johnson G L, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 74.Morgan J I, Curran T. Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends Neurosci. 1989;12:459–462. doi: 10.1016/0166-2236(89)90096-9. [DOI] [PubMed] [Google Scholar]
- 75.Muller R, Bravo R, Burckhardt J, Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984;312:716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- 76.Murakami M, Sonobe M H, Ui M, Kabuyama Y, Watanabe H, Wada T, Handa H, Iba H. Phosphorylation and high level expression of Fra-2 in v-src transformed cells: a pathway of activation of endogenous AP-1. Oncogene. 1997;14:2435–2444. doi: 10.1038/sj.onc.1201077. [DOI] [PubMed] [Google Scholar]
- 77.Musti A M, Treier M, Bohmann D. Reduced ubiquitin-dependent degradation of c-Jun after phosphorylation by MAP kinases. Science. 1997;275:400–402. doi: 10.1126/science.275.5298.400. [DOI] [PubMed] [Google Scholar]
- 78.Papavassiliou A G, Treier M, Bohmann D. Intramolecular signal transduction in c-Jun. EMBO J. 1995;14:2014–2019. doi: 10.1002/j.1460-2075.1995.tb07193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rauscher F J, III, Voulalas P J, Franza B R, Jr, Curran T. Fos and Jun bind cooperatively to the AP-1 site: reconstitution in vitro. Genes Dev. 1988;2:1687–1699. doi: 10.1101/gad.2.12b.1687. [DOI] [PubMed] [Google Scholar]
- 80.Robertson L M, Kerppola T K, Vendrell M, Luk D, Smeyne R J, Bocchiaro C, Morgan J I, Curran T. Regulation of c-fos expression in transgenic mice requires multiple interdependent transcription control elements. Neuron. 1995;14:241–252. doi: 10.1016/0896-6273(95)90282-1. [DOI] [PubMed] [Google Scholar]
- 81.Ryseck R P, Bravo R. c-Jun, JunB and JunD differ in their binding affinities to AP-1 and CRE consensus sequences; effect of Fos proteins. Oncogene. 1991;6:533–542. [PubMed] [Google Scholar]
- 82.Saez E, Rutberg S E, Mueller E, Oppenheim H, Smoluk J, Yuspa S H, Spiegelman B M. c-fos is required for malignant progression of skin tumors. Cell. 1995;82:721–732. doi: 10.1016/0092-8674(95)90469-7. [DOI] [PubMed] [Google Scholar]
- 83.Samuels M L, Weber M J, Bishop M, McMahon M. Conditional transformation of cells and rapid activation of the mitogen-activated protein kinase cascade by an estradiol-dependent human Raf-1 protein kinase. Mol Cell Biol. 1993;13:6241–6252. doi: 10.1128/mcb.13.10.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sewing A, Wiseman B, Lloyd A C, Land H. High-intensity Raf signal causes cell cycle arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5588–5597. doi: 10.1128/mcb.17.9.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sonnenberg J L, Macgregor-Leon P F, Curran T, Morgan J I. Dynamic alterations in the levels and composition of transcription factor AP-1 complexes after seizure. Neuron. 1989;3:359–365. doi: 10.1016/0896-6273(89)90260-2. [DOI] [PubMed] [Google Scholar]
- 86.Sonobe M H, Yoshida T, Murakami M, Kameda T, Iba H. fra-2 promoter can respond to serum-stimulation through AP-1 complexes. Oncogene. 1995;10:689–696. [PubMed] [Google Scholar]
- 87.Stacey D W, Watson T, Kung H F, Curran T. Microinjection of transforming Ras proteins induces c-fos expression. Mol Cell Biol. 1987;7:523–527. doi: 10.1128/mcb.7.1.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stokoe D, Macdonald S G, Cadwallader K, Symons M, Hancock J F. Activation of Raf as a result of recruitment to the plasma membrane. Science. 1994;264:1463–1467. doi: 10.1126/science.7811320. [DOI] [PubMed] [Google Scholar]
- 89.Suzuki T, Murakami M, Onai N, Fukuda E, Hashimoto Y, Sonobe M H, Kameda T, Ichinose M, Miki K, Iba H. Analysis of AP-1 function in cellular transformation pathways. J Virol. 1994;68:3527–3535. doi: 10.1128/jvi.68.6.3527-3535.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Traverse S, Cohen P, Paterson H, Marshall C, Rapp U, Grand R J A. Specific association of activated MAP kinase kinase kinase (Raf) with the plasma membranes of ras-transformed retinal cells. Oncogene. 1993;8:3175–3181. [PubMed] [Google Scholar]
- 91.Traverse S, Seedorf K, Paterson H, Marshall C J, Cohen P, Ullrich A. EGF triggers neuronal differentiation of PC12 cells that overexpress the EGF receptor. Curr Biol. 1994;4:694–701. doi: 10.1016/s0960-9822(00)00154-8. [DOI] [PubMed] [Google Scholar]
- 92.Treinies I, Paterson H F, Hooper S, Wilson R, Marshall C J. Activated MEK stimulates expression of AP-1 components independently of phosphatidylinositol 3-kinase (PI3-kinase) but requires a PI3-kinase signal to stimulate DNA synthesis. Mol Cell Biol. 1999;19:321–329. doi: 10.1128/mcb.19.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5′ element and c-fos 3′ sequences. Cell. 1985;42:889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- 94.Treisman R. Ternary complex factors: growth factor regulated transcriptional activators. Curr Opin Genet Dev. 1994;4:96–101. doi: 10.1016/0959-437x(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 95.Treisman R. Regulation of transcription by MAP kinase cascades. Curr Opin Cell Biol. 1996;8:205–215. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- 96.Vallone D, Battista S, Pierantoni G M, Fedele M, Casalino L, Santoro M, Viglietto G, Fusco A, Verde P. Neoplastic transformation of rat thyroid cells requires the junB and fra-1 gene induction which is dependent on the HMGI-C gene product. EMBO J. 1997;16:5310–5321. doi: 10.1093/emboj/16.17.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van Aelst L, Barr M, Marcus S, Polverino A, Wigler M. Complex formation between RAS and RAF and other protein kinases. Proc Natl Acad Sci USA. 1993;90:6213–6217. doi: 10.1073/pnas.90.13.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Van Dam H, Wilhelm D, Herr I, Steffen A, Herrlich P, Angel P. ATF-2 is preferentially activated by stress-activated protein kinases to mediate c-jun induction in response to genotoxic agents. EMBO J. 1995;14:1798–1811. doi: 10.1002/j.1460-2075.1995.tb07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vouret-Craviari V, Van Obberghen-Schilling E, Scimeca J C, Van Obberghen E, Pouysségur J. Differential activation of p44mapk (ERK1) by α-thrombin and thrombin-receptor peptide agonist. Biochem J. 1993;289:209–214. doi: 10.1042/bj2890209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Warne P H, Viciana P R, Downward J. Direct interaction of Ras and the amino terminal region of Raf-1 in vitro. Nature. 1993;364:352–355. doi: 10.1038/364352a0. [DOI] [PubMed] [Google Scholar]
- 101.Whitmarsh A J, Davis R J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 102.Wolthuis R M, de Ruiter N D, Cool R H, Bos J L. Stimulation of gene induction and cell growth by the Ras effector Rlf. EMBO J. 1997;16:6748–6761. doi: 10.1093/emboj/16.22.6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Woods D, Parry D, Cherwinski H, Bosch E, Lees E, McMahon M. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5598–5611. doi: 10.1128/mcb.17.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang B S, Hauser C A, Henkel G, Colman M S, Van Beveren C, Stacey K J, Hume D A, Maki R A, Ostrowski M C. Ras-mediated phosphorylation of a conserved threonine residue enhances the transactivation activities of c-Ets1 and c-Ets2. Mol Cell Biol. 1996;16:538–547. doi: 10.1128/mcb.16.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]