Abstract
Context
Idiopathic type 1 diabetes is characterized by the absence of autoantibodies and the underlying mechanisms are not clear.
Objective
We aimed to study the epidemiology, describe the clinical characteristics, and report results of genetic studies in pediatric patients with idiopathic type 1 diabetes.
Methods
This was a prospective study of type 1 diabetes patients attending Sidra Medicine from 2018 to 2020. Autoantibodies (GAD65, IAA, IA-2A, and ZnT8) were measured and genetic testing was undertaken in patients negative for autoantibodies to rule out monogenic diabetes. Demographic and clinical data of patients with idiopathic type 1 diabetes were compared with patients with autoimmune type 1 diabetes.
Results
Of 1157 patients with type 1 diabetes, 63 were antibody-negative. Upon genome sequencing, 4 had maturity onset diabetes of the young (MODY), 2 had Wolfram syndrome, 1 had H syndrome, and 3 had variants of uncertain significance in MODY genes; 53 patients had idiopathic type 1 diabetes. The most common age of diagnosis was 10 to 14 years. C-peptide level was low but detectable in 30 patients (56.6%) and normal in 23 patients (43.4%) The average body mass index was in the normal range and 33% of the patients had a history of diabetic ketoacidosis (DKA).
Conclusion
Four percent of the children had idiopathic type 1 diabetes. There were statistically significant differences in the C-peptide level and insulin requirement between the 2 groups. DKA was less common in the idiopathic group. Mutations in MODY genes suggest the importance of autoantibody testing and genetic screening for known causes of monogenic diabetes in idiopathic type 1 diabetes. The mechanism of idiopathic type 1 diabetes is unknown but could be due to defects in antibody production or due to autoantibodies that are not yet detectable or discovered.
Keywords: diabetes, insulin, glucose, pediatric, autoantibodies, pancreas
Type 1 diabetes is one of the most common chronic diseases of childhood, affecting millions of people worldwide and it is increasing in prevalence and incidence. In 1997, the American Diabetes Association proposed 2 subcategories for type 1 diabetes mellitus: type 1A or immune-mediated diabetes and idiopathic type 1 or idiopathic diabetes [1]. Immune-mediated diabetes accounts for 70% to 90% of type 1 diabetes and it results from cellular autoimmune destruction of the β-cells of the pancreas, mediated by T cells [2]. Markers of the immune destruction of the β-cell include islet cell autoantibodies (IA2), insulin autoantibodies (IAA), glutamic acid decarboxylase, 65 kDa isoform (GAD65) autoantibodies, and Zinc transporter 8 (ZnT8) autoantibodies. There is little or no insulin secretion with low or undetectable levels of plasma C-peptide and exogenous insulin is necessary to preserve life [3]. Idiopathic diabetes is characterized by the absence of β-cell autoimmune markers, with permanent insulinopenia, and it is prone to ketoacidosis.
Monogenic diabetes is uncommon, accounting for approximately 1% to 6% of pediatric patients with diabetes [4]. It results from one or more defects in a single gene important for the development or function of β-cells [5]. The disease may be inherited within families as a dominant or recessive trait or may present as a spontaneous case due to de novo mutations. In such cases, family history suggesting a monogenic condition is lacking. Maturity onset diabetes of the young (MODY) is the most common type of monogenic diabetes [4]. The majority of these patients are initially misdiagnosed as having type 1 or type 2 diabetes which leads to suboptimal management of the patients and their families. Hence the International Society of Pediatric and Adolescent Diabetes (ISPAD) recommends genetic testing to rule out causes of monogenic diabetes in type 1 diabetes patients who tested negative for islet cell autoantibodies [4].
In 2000, Imagawa et al described a novel subtype of idiopathic type 1 diabetes which is a nonautoimmune, fulminant type 1 diabetes characterized by a rapid onset and absence of diabetes-related antibodies [6]. The study by Imagawa et al analyzed 56 patients with type 1 diabetes recruited within 1 year after receiving the diagnosis. Eleven patients were negative for GAD, IA2, and IAA antibodies; ZnT8 was not tested then. Pancreatic biopsies revealed neither insulitis nor hyperexpression of major histocompatibility (MHC) class I molecules in islets. The onset of overt diabetes in these 11 patients was rapid, with a short duration of symptoms before the diagnosis and diabetic ketoacidosis (DKA) occurred soon after the onset of hyperglycemic symptoms. Moreover, the patients had markedly elevated serum pancreatic enzyme concentrations, a finding in accordance with the lymphocytic infiltration of the exocrine pancreas seen in the biopsy specimens.
Despite ongoing research, the pathogenesis of diabetes is still unclear. Hameed et al [7] measured the autoantibodies (GAD, IA2, and IAA antibody) at diagnosis for children with newly diagnosed diabetes mellitus (DM). Those who were autoantibody-negative were tested again later for (ZnT8, IA2, and GAD). Data analysis showed that persistently autoantibody-negative patients could not be distinguished from autoantibody-positive patients by clinical characteristics (age at diagnosis, duration of diabetes, insulin dose/kg/d, pH at diagnosis, glycated hemoglobin A1c [HbA1c], body mass index [BMI], or family history of type 1 diabetes or autoimmune disorders) [7]. A study from India reported that nearly 30% of the patients in their cohort of patients with type 1 diabetes had all islet autoantibodies negative [8]
In this study, we describe the demographics, epidemiology, clinical features, and genetic findings of a cohort of patients with idiopathic type 1 diabetes attending our pediatric diabetes clinic in Qatar. We also aimed to compare the demographic and clinical characteristics of autoimmune and nonautoimmune type 1 diabetic patients.
Methods
Ethics Approval
This study was approved by the Institutional Review Board (IRB) for the protection of human subjects in Sidra Medicine, Qatar (IRB reference number 1702007592). Informed consent was taken from the parents as required.
Subject Recruitment
We conducted a prospective study of children and adolescents with type 1 diabetes attending the pediatric endocrinology clinic in Sidra Medicine from 2018 to 2020. Sidra Medicine is the only childhood diabetes center in the state of Qatar and all children with DM are referred here, thus allowing us to capture all children diagnosed with DM for this study. The diagnosis of DM was made as per the American Diabetes Association (ADA) Guidelines and was further classified into different types of diabetes with the help of clinical history and examination as well as autoantibody assays, biochemical tests, and genetic testing. All patients had an onset of DM at ≤18 years of age. Patients diagnosed as type 1 diabetes were classified as autoantibody-positive and autoantibody-negative diabetes, based on their autoantibody status.
Laboratory Investigations
Peripheral blood samples were collected from subjects and type 1 diabetes autoantibodies (GAD65, IAA, IA-2A, ZnT8) were measured and titers recorded. C-peptide, thyroid peroxidase (TPO), and celiac disease antibodies were also measured.
Genetic Testing
DNA samples were extracted from blood specimens as per the standard method. Samples were sent to Prevention Genetics laboratories for genetic testing to rule out a panel of monogenic diabetes causative genes for all patients who had 4 autoantibodies negative. The genes analyzed were ABCC8, APPL1, BLK, GCK, HNF1A, HNF1B, HNF4A, KCNJ11, KLF11, NEUROD1, PAX4, PDX1, WFS1, INS, and CEL.
Antibody Assay Methodology
Complete autoantibody profiling was performed at the time of recruitment for all known patients with DM at Mayo Clinic Laboratories. Newly diagnosed cases were tested at the time of first presentation before starting insulin treatment.
GAD65 radioimmunoassay
(125)I-labeled recombinant human glutamic acid decarboxylase (GAD65) was incubated with the patient’s diluted serum. Antihuman IgG and IgM were then added to form an immunoprecipitate. After washing the precipitated immune complexes, the specific antibodies were detected by counting gamma emission from the pellet’s bound (125)I-GAD65 [9].
Insulin autoantibody radioimmunoassay
For the insulin autoantibody radioimmunoassay, (125)I-labeled recombinant human insulin is added to the test serum; if antibody is present in the sample, it forms a soluble complex with the labeled insulin. Subsequent addition of goat antihuman IgG and IgM precipitates the complex. The amount of radioactivity in the precipitate is proportional to the level of antibody in the serum.
IA-2 autoantibody radioimmunoassay
For the IA-2 autoantibody radioimmunoassay, (125) I-labeled recombinant human IA-2 is added to the test serum; if antibody is present, it forms a soluble complex with the (125) I-labeled IA-2. Subsequent addition of goat antihuman IgG and IgM precipitates the complex. The amount of radioactivity in the precipitate is proportional to the level of antibody in the serum [10].
Zinc Transporter 8 autoantibody enzyme immunoassay
ZnT8 antibodies are principally directed against the C terminal domain of ZnT8. The ZnT8 autoantibody enzyme-linked immunosorbent assay (ELISA) is based on the bridging principle that employs the ability of divalent ZnT8 autoantibodies to bind to ZnT8 coated on to the plate well with 1 arm and to liquid ZnT8-biotin with the other arm. Calibrators or undiluted serum samples in duplicate were added to ZnT8-coated plate wells and incubated overnight. ZnT8-biotin was added to each well and plate. After another incubation, aspiration, and wash, streptavidin-peroxidase was added to each well. Another incubation, aspiration, and wash were performed and peroxidase substrate was added. After a final incubation, 0.5 mol/L H2SO4 stop solution was added to each well. Absorbance was measured at 450 nm, blanked against wells containing peroxidase substrate and H2SO4 only.
Statistical Analysis
The means ± SD were calculated and compared for the autoimmune and idiopathic type 1 diabetic patient groups. The Student t test and Chi-square test were performed as required to assess the significance of some of the clinical and biochemical variables. A P value of 0.05 was used as a cutoff to accept or reject the null hypothesis and assess significance.
Results
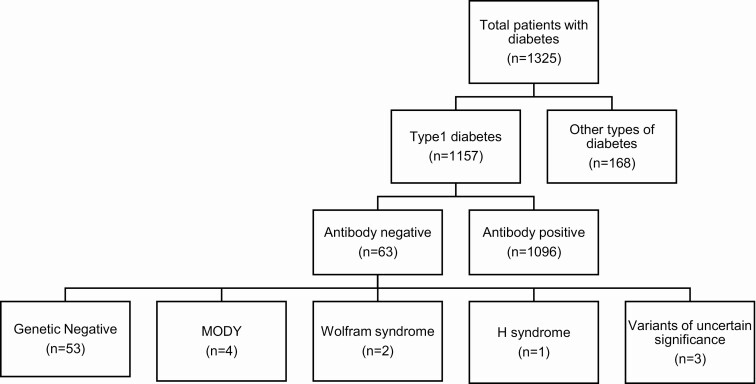
We identified 1325 patients younger than 18 years with diabetes mellitus who received care in our clinics. Of these, 1157 were thought to be type 1 diabetes; however, upon measurement of autoantibodies, 63 were negative. Genetic screening to rule out MODY was conducted in all of these autoantibody-negative patients and 4 patients were found to have MODY; we identified an INS mutation in 1 patient and HNF1A mutations in 3 patients. Additionally, 2 patients were found to have a mutation in the WFS1 gene causing Wolfram syndrome while 1 patient was found to have a mutation in the SLC29A3 gene causing H syndrome. Three patients also had mutations of uncertain significance in HNF1B, KLF11, PDX1 genes.
The total number of children with idiopathic type 1 diabetes was found to be 53, which constitutes 4% of total cohort of children with DM in Qatar.
The autoantibody test was done at the time of diagnosis in 23 patients (42.6%), the remaining patients (57.4%) were tested at an average duration of 5.9 years from diagnosis. Figure 1 shows a breakdown of the different categories studied. Table 1 shows all the mutations found in monogenic diabetes causative genes.
Figure 1.
Results of autoantibody and genetic testing done in our cohort.
Table 1.
Mutations found in monogenic diabetes causative genes
| Gene | Variant | Clinical Significance | |
|---|---|---|---|
| Patient 1 | INS | c0.152 C > G | MODY10 |
| Patient 2 | HNF1A | c0.527-6_527-3delCTGC | MODY3 |
| Patients 3, 4 | HNF1A | c0.955 + 1G > A | MODY3 |
| Patient 5 | WFS1 | c0.2643_2646delCTTT | Wolfram syndrome |
| Patient 6 | WFS1 | c0.1433G > A, | Wolfram syndrome |
| Patient 7 | SLC29A3 | c0.1228C > T | H syndrome |
| Patient 8 | KLF11 | c0.1298A > G | Variant of uncertain significance |
| Patient 9 | KLF11 | c0.1298 A > G | Variant of uncertain significance |
| Patient 10 | PDX1 | c0.98C > A | Variant of uncertain significance |
Clinical Features
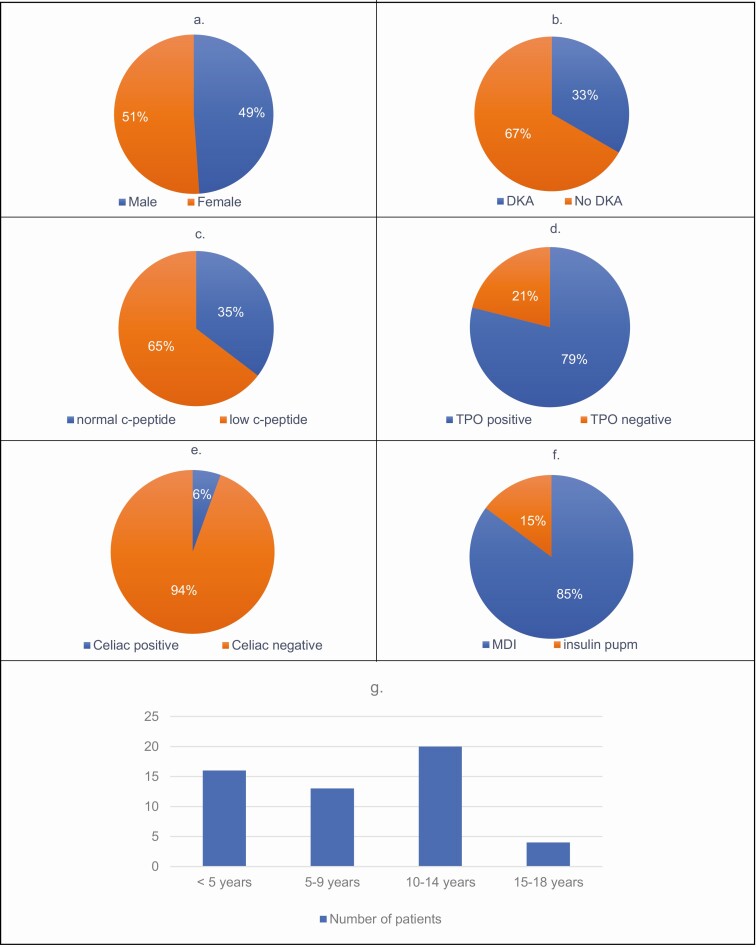
We analyzed the data of the 53 idiopathic type 1 diabetes patients followed in our clinic and the results are summarized in Fig. 2. The average current age is 13 years and the most common age of onset was 10 to 14 years of age. Fourteen patients (25.9%) have fairly good control of their diabetes with HbA1c of <8%, almost half have levels of 8% to 10%, and the rest have levels above 10% in our idiopathic type1 cohort. C-peptide was detectable in these patients, with a mean level of 0.87 ng/mL.
Figure 2.
Clinical features observed in idiopathic type 1 diabetes cohort. 2a, Gender distribution. 2b, History of diabetic ketoacidosis (DKA). 2c, C-peptide level. 2d, Thyroid peroxidase (TPO) status. 2e, Celiac disease status. 2f, Insulin therapy. 2g, Age of onset.
The average BMI of our patients was 21.8 kg/m2 and the average insulin requirement was 0.71 IU/kg/day. Insulin was delivered in most of the patients using insulin multiple daily injection, while 8 patients (14.8%) used insulin pumps. While the majority of the patients tested negative for celiac disease and TPO antibodies, 3 patients (5.6%) tested positive for celiac autoantibodies and 12 patients tested positive for TPO antibodies (22.6%).
There were statistically significant differences observed in the C-peptide level and insulin requirement between idiopathic type 1 diabetes and type 1 diabetes. DKA was less common in the idiopathic group. Refer to Table 2 to see a detailed comparison between the 2 groups.
Table 2.
Comparison of idiopathic type 1 DM with antibody-positive DM
| Antibody-negative DM | Antibody-positive DM | P value | |
|---|---|---|---|
| Current age, years | 13 (± 4.1 SD) | 11 (± 3.6 SD) | - |
| Gender (female:male) | 51:49 | 51:49 | - |
| Age at onset, years | 10-14 | 5-9 | 0.39 |
| Current HbA1c | 9.2% (± 2.5 SD) | 9.9% (± 2.2 SD) | 0.3 |
| C-peptide, ng/mL | 0.87 (± 0.85 SD) | 0.16 (± 0.17 SD) | <0.001 |
| Insulin requirement, U/kg/day | 0.71 (± 0.28 SD) | 0.93 (± 0.26 SD) | 0.002 |
| BMI | Normal | Normal | - |
| Family history of DM | More common (22%) | Less common (9%) | 0.06 |
| Ethnicity | 53% Qataris | 49% Qataris | 0.69 |
| TPO-positive | 22.6% | 19.6% | 0.63 |
| Celiac-positive | 5.5% | 3.7% | 0.64 |
| DKA at presentation | Less common (33%) | More common (62%) | <0.003 |
Abbreviations: BMI, body mass index; DKA, diabetic ketoacidosis; DM, diabetes mellitus; HbA1c, glycated hemoglobin A1c; TPO, thyroid peroxidase.
Discussion
Our study was able to identify all patients with idiopathic type 1 DM in Qatar. Despite presenting initially with a clinical picture like type 1 diabetes, we found that 4% of newly diagnosed children and adolescents initially diagnosed as type 1 diabetes were negative for all 4 major islet antibodies. We found statistically significant differences in the insulin requirement and C-peptide levels when comparing idiopathic autoimmune type 1 diabetes. Higher C-peptide levels and lower insulin doses were required in our cohort of idiopathic diabetes subjects. A study of 303 autoantibody-negative Swedish children revealed MODY in 46 children [11]. They reported a higher C-peptide level suggesting slower beta cell destruction, less severe symptoms, and higher BMI in their cohort of children with idiopathic type 1 diabetes [11]. Another study from the UK found autoantibody-negative type 1 diabetes in 268 subjects of their cohort of 1778 adults with type 1 diabetes [12]. They reported higher incidence with increasing age and male gender as well as higher BMI in the idiopathic group [12]. Aguilera et al also reported higher BMI, milder symptoms, lower insulin requirement, as well as higher C-peptide (basal and stimulated) in idiopathic type 1 diabetes [1]. However, Hameed et al did not find any significant differences in clinical features in their comparison of idiopathic and autoimmune type 1 diabetes [7]. Celiac disease and TPO antibodies were found positive in only 5.6% and 22.6% of patients. Contrary to previous reports we noticed a slight female preponderance in our cohort. The mean age of onset was also slightly higher than immune-mediated DM.
We examined all 14 MODY genes in our cohort and identified 4 patients with MODY—3 with HNF1A mutations and 1 with an INS gene mutation. Two patients with a WFS1 gene mutation causing Wolfram syndrome and 1 with SLC29A3 mutation that causes H syndrome were also identified. There have been some previous reports of MODY genes being responsible for idiopathic diabetes [13, 14]; however, no concrete evidence has been reported for these variants. Some reports also suggest this idiopathic type 1 diabetes is more likely related to type 2 diabetes than type 1 diabetes, which is an area that needs to be explored further [1, 15].
The pathophysiological mechanism of idiopathic type 1 DM is not well understood and data regarding this type of DM is scarce especially in children of Arab ethnicity. A review by Patel et al [16] suggests immune-mediated destruction of pancreatic islet by T cells as a mechanism for idiopathic type 1 diabetes. They also suggest that CD20+ cells, in particular, were of importance since CD20Hi/hyperimmune patients were associated with antibody-positive diabetes while CD20Lo subjects had autoantibody-negative diabetes [17]. Another study proposes defects in antibody production as the cause for autoantibody negativity [18].
Currently, the known autoantibodies associated with DM are GAD65, IAA, IA-2, and ZNT8; however, there could very well be some autoantibodies involved in the mechanism that are not yet detectable by existing assays. Ethnicity of patients is also suggested to be associated with changes in autoimmunity, with Asian and African ethnicity having a higher frequency of autoantibody negativity. They present with episodic DKA and fluctuating degree of insulinopenia between episodes [16, 19]. The treatment of diabetes in such patients is mainly insulin replacement; however, some combination therapies with immunomodulators, incretin-based agents, as well proton pump inhibitors can be beneficial in β-cell regeneration and may lead to better clinical outcomes [20].
One limitation of our study is the relatively small sample size which prevents further genetic and human leukocyte antigen (HLA) analysis for drawing conclusions. Our results highlight the importance of routine measurement of all 4 autoantibodies at the time of diagnosis of type 1 diabetes, and the role of genetic testing of those patients who were found to be autoantibody-negative. This type of accurate classification is important for disease management and prognosis since some reports also mention these patients having a higher cardiovascular risk in the long term [3]. Additional research needs to be done on idiopathic type 1 diabetes to understand the underlying molecular mechanisms.
Glossary
Abbreviations
- BMI
body mass index
- DKA
diabetic ketoacidosis
- DM
diabetes mellitus
- GAD65
glutamic acid decarboxylase (65 kDa)
- HbA1c
glycated hemoglobin A1c
- IA2
islet cell autoantibodies
- IAA
insulin autoantibodies
- MODY
maturity onset diabetes of the young
- TPO
thyroid peroxidase
- ZnT8
zinc transporter 8
Financial Support
This research was supported by the Qatar National Research Fund (QNRF-NPRP 10-6100017-AXX) awarded to Professor Khalid Hussain.
Author Contributions
T.A. and B.H. collected patient information, recruited the patients, analyzed and interpreted the data, and drafted the manuscript. K.H. designed the study, obtained funding, and reviewed and edited the manuscript. H.A., S.M., A.K., M.A., M.Z., A.E., F.A., and G.P. collected patient information and reviewed the manuscript.
Additional Information
Disclosures: All authors claim nothing to disclose.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Aguilera E, Casamitjana R, Ercilla G, Oriola J, Gomis R, Conget I. Adult-onset atypical (type 1) diabetes: additional insights and differences with type 1A diabetes in a European Mediterranean population. Diabetes Care. 2004;27(5):1108-1114. [DOI] [PubMed] [Google Scholar]
- 2. Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383(9911):69-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Catarino D, Silva D, Guiomar J, et al. Non-immune-mediated versus immune-mediated type 1 diabetes: diagnosis and long-term differences-retrospective analysis. Diabetol Metab Syndr. 2020;12:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hattersley AT, Greeley SAW, Polak M, et al. ISPAD clinical practice consensus guidelines 2018: the diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes. 2018;19(Suppl 27):47-63. [DOI] [PubMed] [Google Scholar]
- 5. Sanyoura M, Philipson LH, Naylor R. Monogenic diabetes in children and adolescents: recognition and treatment options. Curr Diab Rep. 2018;18(8):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Imagawa A, Hanafusa T, Miyagawa J, Matsuzawa Y. A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes-related antibodies. Osaka IDDM study group. N Engl J Med. 2000;342(5):301-307. [DOI] [PubMed] [Google Scholar]
- 7. Hameed S, Ellard S, Woodhead HJ, et al. Persistently autoantibody negative (PAN) type 1 diabetes mellitus in children. Pediatr Diabetes. 2011;12(3 Pt 1):142-149. [DOI] [PubMed] [Google Scholar]
- 8. Vipin VP, Zaidi G, Watson K, et al. High prevalence of idiopathic (islet antibody-negative) type 1 diabetes among Indian children and adolescents. Pediatr Diabetes. 2021;22(1):47-51. [DOI] [PubMed] [Google Scholar]
- 9. Walikonis JE, Lennon VA. Radioimmunoassay for glutamic acid decarboxylase (GAD65) autoantibodies as a diagnostic aid for stiff-man syndrome and a correlate of susceptibility to type 1 diabetes mellitus. Mayo Clin Proc. 1998;73(12):1161-1166. [DOI] [PubMed] [Google Scholar]
- 10. Masuda M, Powell M, Chen S, et al. Autoantibodies to IA-2 in insulin-dependent diabetes mellitus. Measurements with a new immunoprecipitation assay. Clin Chim Acta. 2000;291(1):53-66. [DOI] [PubMed] [Google Scholar]
- 11. Carlsson A, Shepherd M, Ellard S, et al. Absence of islet autoantibodies and modestly raised glucose values at diabetes diagnosis should lead to testing for MODY: lessons from a 5-year pediatric Swedish National Cohort Study. Diabetes Care. 2020;43(1):82-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bravis V, Kaur A, Walkey HC, et al. ; ADDRESS-2 Management Committee, Patient Advocate Group and Investigators . Relationship between islet autoantibody status and the clinical characteristics of children and adults with incident type 1 diabetes in a UK cohort. BMJ Open. 2018;8(4):e020904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Møller AM, Dalgaard LT, Pociot F, Nerup J, Hansen T, Pedersen O. Mutations in the hepatocyte nuclear factor-1alpha gene in Caucasian families originally classified as having Type I diabetes. Diabetologia. 1998;41(12):1528-1531. [DOI] [PubMed] [Google Scholar]
- 14. Kawasaki E, Sera Y, Yamakawa K, et al. Identification and functional analysis of mutations in the hepatocyte nuclear factor-1alpha gene in anti-islet autoantibody-negative Japanese patients with type 1 diabetes. J Clin Endocrinol Metab. 2000;85(1):331-335. [DOI] [PubMed] [Google Scholar]
- 15. Sobngwi E, Gautier JF. Adult-onset idiopathic Type I or ketosis-prone Type II diabetes: evidence to revisit diabetes classification. Diabetologia. 2002;45(2):283-285. [DOI] [PubMed] [Google Scholar]
- 16. Patel SK, Ma CS, Fourlanos S, Greenfield JR. Autoantibody-negative type 1 diabetes: a neglected subtype. Trends Endocrinol Metab. 2021;32(5):295-305. [DOI] [PubMed] [Google Scholar]
- 17. Arif S, Leete P, Nguyen V, et al. Blood and islet phenotypes indicate immunological heterogeneity in type 1 diabetes [published correction appears in Diabetes. 2015 Sep;64(9):3334]. Diabetes. 2014;63(11):3835-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kumar V, Jarzabek-Chorzelska M, Sulej J, Karnewska K, Farrell T, Jablonska S. Celiac disease and immunoglobulin a deficiency: how effective are the serological methods of diagnosis? Clin Diagn Lab Immunol. 2002;9(6):1295-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S14-S31. [DOI] [PubMed] [Google Scholar]
- 20. Pozzilli P, Maddaloni E, Buzzetti R. Combination immunotherapies for type 1 diabetes mellitus. Nat Rev Endocrinol. 2015;11(5):289-297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.