Abstract
Aflatoxin B1 (AFB1), a threatening mycotoxin, usually provokes oxidative stress and causes hepatotoxicity in animals and humans. Luteolin (LUTN), well-known as an active phytochemical agent, acts as a strong antioxidant. This research was designed to investigate whether LUTN exerts protective effects against AFB1-induced hepatotoxicity and explore the possible molecular mechanism in mice. A total of forty-eight mice were randomly allocated following four treatment groups (n = 12): Group 1, physiological saline (CON). Group 2, treated with 0.75 mg/kg BW aflatoxin B1 (AFB1). Group 3, treated with 50 mg/kg BW luteolin (LUTN), and Group 4, treated with 0.75 mg/kg BW aflatoxin B1 + 50 mg/kg BW luteolin (AFB1 + LUTN). Our findings revealed that LUTN treatment significantly alleviated growth retardation and rescued liver injury by relieving the pathological and serum biochemical alterations (ALT, AST, ALP, and GGT) under AFB1 exposure. LUTN ameliorated AFB1-induced oxidative stress by scavenging ROS and MDA accumulation and boosting the capacity of the antioxidant enzyme (CAT, T-SOD, GSH-Px and T-AOC). Moreover, LUTN treatment considerably attenuates the AFB1-induced apoptosis in mouse liver, as demonstrated by declined apoptotic cells percentage, decreased Bax, Cyt-c, caspase-3 and caspase-9 transcription and protein with increased Bcl-2 expression. Notably, administration of LUTN up-regulated the Nrf2 and its associated downstream molecules (HO-1, NQO1, GCLC, SOD1) at mRNA and protein levels under AFB1 exposure. Our results indicated that LUTN effectively alleviated AFB1-induced liver injury, and the underlying mechanisms were associated with the activation of the Nrf2 signaling pathway. Taken together, LUTN may serve as a potential mitigator against AFB1-induced liver injury and could be helpful for the development of novel treatment to combat liver diseases in humans and/or animals.
Keywords: aflatoxin B1, luteolin, oxidative stress, apoptosis, liver injury, Nrf2 signaling pathway
1. Introduction
Aflatoxins are one of the most dangerous mycotoxins, produced mainly by Aspergillus flavus and Aspergillus parasiticus and usually found in agricultural environments and food commodities [1]. According to studies, even low levels of aflatoxins in the diet can be harmful to human health [2,3]. Currently, approximately 4.5 billion people are in danger of being exposed to aflatoxins, which cause 4.6–28.2% of all occurrences of hepatocellular carcinoma worldwide [4,5]. Aflatoxin B1 (AFB1) is the most potent liver carcinogen among the aflatoxins, and it has been categorized as a Class I carcinogen by the International Agency for Research on Cancer (IARC) [6]. Additionally, AFB1 is reported to cause severe health issues, including growth retardation, hepatotoxicity, neurotoxicity, teratogenicity, mutagenicity and immunotoxicity in humans and animals [7,8,9]. The liver is believed to be the primary target organ for AFB1 toxicity, a major metabolizing and detoxifying organ in the body [10]. Previous research has shown that reactive oxygen species (ROS) formation appears to be a significant contributor to the toxicity caused by AFB1 in the liver [11]. ROS overproduction could lead to mitochondrial oxidative stress, resulting in lipid peroxidation and decreased antioxidant enzyme activity, causing cellular and organismal hepatic damage [5,12]. Therefore, it is imperative to find an effective antioxidant to protect the liver and alleviate the AFB1 toxicity. Natural active biological compounds derived from plants have recently received much attention due to their low toxicity. Herbal metabolites are effective alternatives for tackling the hazardous effects of AFB1 [13,14].
Flavonoids are bioactive compounds primarily present in plants with a wide range of pharmacological and health-promoting properties [15]. Luteolin (3,4,5,7-tetrahydroxyflavone) is a type of natural flavonoid found in various plants worldwide, such as fruits, vegetables, and some herbal medicines [16]. Luteolin possesses a diverse range of biological properties, including antioxidant [17], antimicrobial [18], anti-inflammatory [19], anticancer [20], and neuroprotective capabilities [21]. The nuclear factor erythroid 2-related factor (Nrf2) plays a central role in the activation of cytoprotective genes in response to xenobiotics and oxidative stress by binding to the antioxidant response element (ARE) [22,23]. Moreover, the Nrf2 gene is typically expressed in metabolically active tissues such as the liver [10]. A recent study revealed that AFB1-induced liver damage in broiler chicks has been associated with dysregulating the Nrf2 signaling pathway [13]. Therefore, Nrf2 signaling is regarded as the most significant therapeutic target for preventing and treating oxidative stress-induced liver damage [24,25]. Although, a study reported that luteolin has a therapeutic impact on ochratoxin A-induced oxidative injury in NRK-52E kidney cells [17]. Moreover, LUTN has been reported to exert antifibrogenic effects against carbon tetrachloride-(CCL4) on hepatic satellite cells and liver fibrosis via multiple mechanisms [26,27]. However, the preventative actions of LUTN against AFB1-induced liver damage and underlying mechanisms have still not been explored. Therefore, the current research was designed to investigate whether LUTN exerts protective effects against AFB1-induced hepatotoxicity and explore the possible molecular mechanism in mice. Presumably, this is the first study to highlight the protective role of luteolin against AFB1-induced hepatic damage in mice.
2. Materials and Methods
2.1. Chemicals and Antibodies
The luteolin (LUTN, #41753–43-9, purity ≥ 98%) was bought from (Shanghai Yuanye Bio-Technology Co., Ltd.). Aflatoxin B1 (AFB1, #1162-65-8) used in the present study was supplied by Sigma-Aldrich (St. Louis, MO, USA). The ELISA assay kits for malondialdehyde (MDA, #A003-1), reactive oxygen species (ROS, #E004), glutathione peroxidase (GSH-Px, #A005), total antioxidant capacity (T-AOC, #A015), catalase (CAT, #A007-1) and total superoxide dismutase (T-SOD, #A001) were from (Jiancheng Bioengineering Institute, Nanjing China). Apoptosis detection kit annexin V-FITC/PI (#KGA-108) was supplied by (Jiangsu KGI, Biotech, CO., Ltd. China). A test kit for the bicinchoninic acid assay was provided by Thermo Fisher Scientific (Waltham, MA, USA). Chemiluminescence WesternBright ECL substrate kit (# ab65623) was supplied by (Abcam, Shanghai, Trading, Co., Ltd. China). The primary antibodies, heme oxygenase-1 (HO-1, #A1346), glutamate-cysteine ligase catalytic subunit (GCLC, #A1038), quinone oxidoreductase 1 (NQO1, #A1518), nuclear factor erythroid 2-related factor 2 (Nrf2, #A0674), Bcl-2-associated X protein (Bax, #A19684), caspase-3 (#A2156) and B-cell lymphoma 2 (Bcl-2, A0208) were procured from (Abclonal Tech, Woburn, MA, USA). Secondary antibodies, anti-mouse IgG (#4410), anti-rabbit IgG (#4414), and β-actin (#3700), were obtained from (Cell Signaling Technology, Boston, MA, USA).
2.2. Animals
Four weeks old male C57BL/6 mice were bought from Wuhan University (Wuhan, China). As an adoption period, mice were housed in separate cages and given an appropriate environment one week before the start of the experiment. Standard feed pellets and freshwater were accessible to the animal’s ad libitum. All mice were housed under laboratory conditions, light-dark period (12 h light/12 h dark), relative humidity of 45–60%, and the temperature of 22 ± 2 °C. Huazhong Agricultural University permitted the animal experiments under the Laboratory Animals Care and Ethics Committee (Permit No. HZAUMO-2018-07). Besides this, the health of experimental mice was closely monitored, and necessary measures were taken to assure the maximum welfare of the animals.
2.3. Experimental Design and Treatment
The 48 mice were randomly assigned into four groups as follows: (n = 12):
Group 1, (CON) received physiological saline.
Group 2, (AFB1), treated with 0.75 mg/kg BW aflatoxin B1.
Group 3, (LUTN), treated with 50 mg/kg BW luteolin.
Group 4, (AFB1 + Luteolin), treated with 0.75 mg/kg BW aflatoxin B1 + 50 mg/kg BW luteolin.
LUTN and AFB1 were dissolved in phosphate buffer saline. Following our preliminary experiment and the results of previous researchers, we chose a dose of 0.75 mg/kg BW for AFB1 as reported this dose could induce hepatotoxicity [28], and oral gavage of 50 mg kg BW LUTN could ameliorate liver damage in mice [29]. The experiment lasted for 15 days, and all groups received oral administration once a day at 9.00 a.m. During the whole experiment, feed intake and body gain were recorded.
2.4. Sample Collection
The mice were individually weighed and euthanized at the end of the experiment to collect the blood and liver samples. The serum was separated from blood samples following the centrifugation and was kept at −20 °C for serum biochemical assays. The liver tissues were removed and rinsed in ice-cold isotonic saline. Afterward, the liver samples were weighed and fixed in 4% fresh paraformaldehyde for histopathological analysis or quickly frozen in liquid nitrogen and kept at −80 °C for further assessment. The remaining liver tissues were utilized to prepare single-cell suspension for flow cytometry investigations. The following formula was used to calculate the liver coefficient:
| (1) |
2.5. Determination of Serum Biochemical Indicators
An automatic biochemistry analyzer (Beckman Synchron CX4 PRO, Fullerton, CA, USA) was used to quantify the amounts of globulin and albumin, as well as GGT, ALP, AST, and ALT in serum samples following the manufacturer’s suggested protocol.
2.6. Hematoxylin and Eosin (H&E) Staining
The H&E staining was conducted as reported in our prior study [30]. Liver specimens were fixed for 24 h in 4% fresh paraformaldehyde solution, dried with alcohol solvent, and then embedded. The 5 µm fragments were sectioned and processed for H&E staining to assess the pathological observation in the liver of mice under a microscope (Nikon, Tokyo, Japan).
2.7. Determination of Oxidative Stress Indices
The 10% tissue homogenates were prepared from collected liver samples following our previously reported procedure [6]. The bicinchoninic acid determination kit was used to measure the protein concentration of tissue homogenate. ROS, MDA, T-SOD, GSH-Px, CAT and T-AOC were detected using commercially available ELISA kits. The measurements were carried out following the kit’s protocols.
2.8. Apoptosis Assay by Flow Cytometry
Single-cell suspensions were prepared to detect the apoptosis rate in the liver of mice following our previously described procedure [31,32]. Briefly, an annexin V-FITC/PI apoptosis detection kit was used to determine the apoptosis rate in the liver following the manufacturer’s recommended instructions. The cells were stained with annexin V-FITC (5 µL) and PI (5 µL) in the dark for 30 min at room temperature. Finally, apoptosis rates were determined using flow cytometry (Beckman-CytoFLEX Coulter, CA, USA). The data were examined by using FlowJo (BD Biosciences, NJ, USA).
2.9. Quantitative Real-Time PCR (qRT-PCR) and Western Blotting Analysis
The transcription levels of pertaining genes used in the present study were determined by qRT-PCR following the method previously mentioned in our study [1]. The primers tested in the current research are presented in Supplementary Table S1. Relative mRNA expression was normalized to the CON group. The 2−ΔΔCt formula was used to quantify with the β-actin as a reference gene [33]. The protein expression of Nrf2 signaling and mitochondrial apoptotic pathways in mouse liver was evaluated by Western blot according to the previously described procedure [34,35]. The bicinchoninic acid assay kit was used to quantify the protein contents of samples. Chemiluminescence WesternBrightTM ECL substrate kit was used to identify the blots, and then FluroChem FC2 Imaging System was used to visualize and quantify the results.
2.10. Statistical Analysis
The experimental data were analyzed for significance by using SPSS (version 22., IBM Corporation, Armonk, NY, USA) software. A one-way analysis of variance (ANOVA) was used for statistical analysis, followed by the least significant difference (LSD) test. The results are presented as mean ± SD. The significance level of data was set at p-value < 0.05.
3. Results
3.1. Luteolin Alleviates Growth Retardation of Mice Induced by AFB1
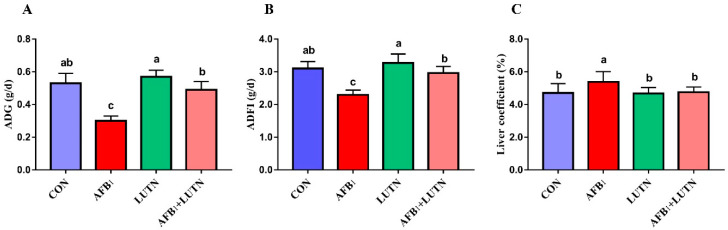
The protective effects of luteolin (LUTN) on the growth of mice exposed to aflatoxin B1 (AFB1) are depicted in Figure 1. During the entire experimental period, the group exposed to AFB1 recorded the (p < 0.05) lowest ADG and ADFI in the comparison of CON, LUTN and AFB1 + LUTN groups, respectively. Contrastingly, LUTN therapy considerably improved growth performance in mice, as evidenced by increased ADG and ADFI (p < 0.05) compared to the AFB1 group. Furthermore, LUTN treatment significantly reduced liver coefficient (p < 0.05) increased by AFB1 (Figure 1C). These findings indicated that LUTN could eliminate the harmful effect of AFB1 on the growth of mice.
Figure 1.
Luteolin treatment alleviates growth retardation of mice induced by aflatoxin B1. (A) Average daily gain (ADG), (B) average daily feed intake (ADFI) and (C) liver coefficients (%). The results are presented as mean ± SD (n = 12). a–c Columns with different lowercase letters indicated significant differences between the compared groups (p < 0.05).
3.2. Luteolin Protects AFB1-Induced Liver Damages in Mice
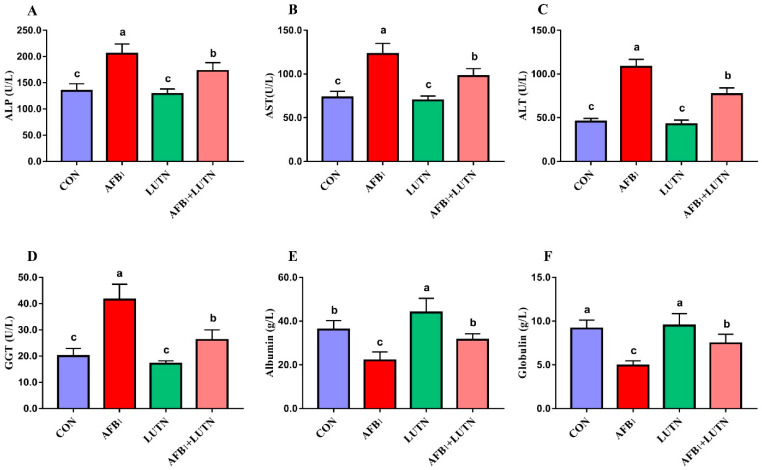
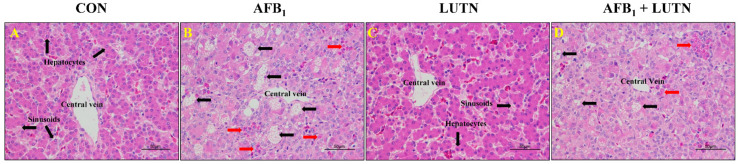
The effects of LUTN treatment on the biochemical profile of mice exposed to AFB1 are summarized in Figure 2A–F. Compared with the CON group, AFB1 exposure considerably (p < 0.05) increased serum liver enzymes activities such as ALP, ALT, AST, and GGT, while decreased globulin and albumin content. On the other hand, LUTN treatment considerably reversed AFB1-induced alterations on the biochemical profile of mice. Moreover, histological analysis revealed that AFB1 exposure damaged the liver structure of mice, as evident by microvesicular appearance of the lipid droplets with small and large area of blood infiltration were observed in the AFB1 treated group (Figure 3B). Strikingly, LUTN treatment evidently (p < 0.05) ameliorated and restored liver injury induced by AFB1, indicating that LUTN could protect the liver from AFB1-induced hepatotoxicity.
Figure 2.
Luteolin treatment prevents AFB1-induced liver damages in mice. (A) alkaline phosphate (ALP), (B) aspartate aminotransferase (AST), (C) alanine aminotransferase (ALT) (D) gamma-glutamyl transferase (GGT), (E) albumin and (F) globulin. The results are presented as mean ± SD (n = 6). a–c Columns with different lowercase letters indicated significant differences between the compared groups (p < 0.05).
Figure 3.
The histopathology of liver sections was stained with H&E staining. (A,C) Histological section of the liver from CON and LUTN group showed a normal histoarchitecture consisting of central vein surrounded by hepatocytes possessed sinusoids spaces. (B) The liver section from the AFB1 group showed microvesicular (black arrow) appearance of the abundant fatty droplets with small and large area of blood infiltration (red arrow) showed a hepatotoxicity. (D) Liver tissue from the group of mice treated with LUTN and challenged with AFB1 manifested recovered status of the liver from hepatotoxicity, as depicted small and rare patches of the lipids (black arrow) and the blood infiltration (red arrow) as compared to the AFB1 alone challenged group.
3.3. Luteolin Ameliorates Oxidative Damage in the Liver of Mice Induced by AFB1
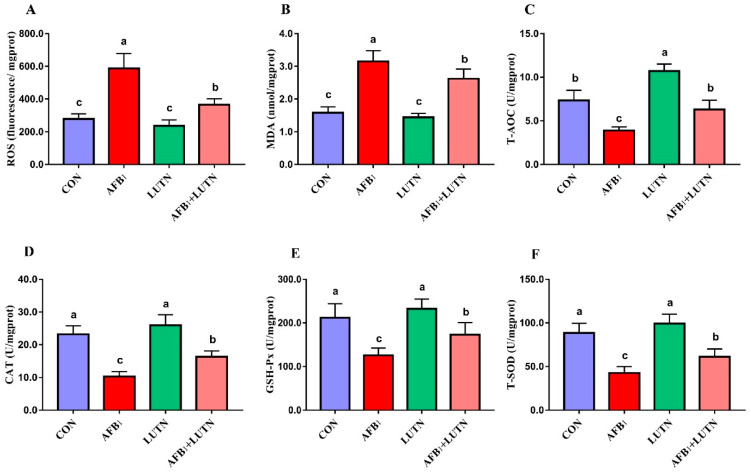
To detect the redox status in the liver of experimental mice, ROS, MDA, CAT, T-SOD, GSH-Px, and T-AOC were detected. As shown in Figure 4A,B, mice exposed to AFB1 revealed a significant (p < 0.05) increase in ROS and MDA levels. At the same time, the activities of T-AOC, CAT, GSH-Px and T-SOD were significantly reduced as compared to the CON, LUTN and AFB1 + LUTN groups, respectively. Contrastingly, LUTN treatment significantly (p < 0.05) reversed AFB1-induced alterations in the oxidative stress markers and antioxidant variables in the liver of mice, as evident by decreased ROS and MDA levels by 38% and 20%, respectively, while increased antioxidant enzyme activities of CAT, T-SOD, GSH-Px, and T-AOC by 36.85, 30.27, 27.26, and 40% respectively, as compared to the AFB1 treated group (Figure 4A–F).
Figure 4.
Luteolin treatment ameliorates oxidative damage in the liver of mice induced by AFB1. (A) Reactive oxygen species (ROS), (B) malondialdehyde (MDA) (C) total antioxidant capacity (T-AOC), (D) catalase (CAT), (E) glutathione peroxidase (GSH-Px) and (F) total superoxide dismutase (T-SOD). The results are presented as mean ± SD (n = 6). a–c Columns with different lowercase letters indicated significant differences between the compared groups (p < 0.05).
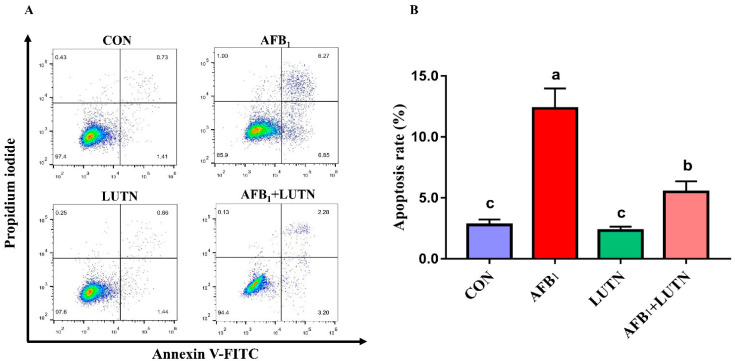
3.4. Luteolin Treatment Prevents AFB1-Induced Apoptosis in Mice Hepatocytes
Apoptosis rate in the hepatocytes was measured by flow cytometry (Figure 5). Apoptosis analysis revealed that AFB1 exposure considerably increased (p < 0.05) the proportion of apoptotic cells relative to the CON, LUTN, and AFB1 + LUTN groups, respectively. However, LUTN administration dramatically reduced (p < 0.05) the proportion of apoptotic cells in comparison to the AFB1 group.
Figure 5.
Luteolin treatment prevents AFB1-induced apoptosis in mice hepatocytes. (A) The apoptosis rates of hepatocytes were measured using flow cytometry. (B) Statistical analysis of apoptosis rate. The results are presented as mean ± SD (n = 3). a–c Columns with different lowercase letters indicated significant differences between the compared groups (p < 0.05).
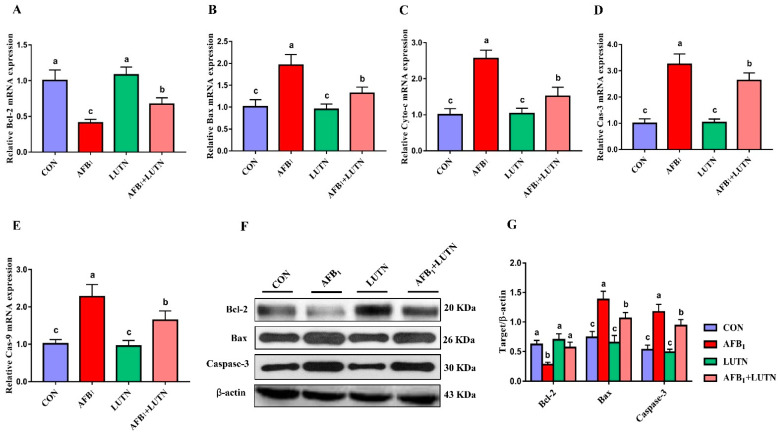
3.5. Luteolin Restrains AFB1-Induced Mitochondrial Apoptosis Pathway
Mitochondrial apoptosis-associated transcription and protein expressions were detected by qRT-PCR and western blotting. As depicted in Figure 6A–E, the transcripts levels of Bax, cytochrome-c, caspase-3, and caspase-9 were significantly (p < 0.05) increased, while Bcl-2 was decreased under AFB1 exposure. Contrastingly, LUTN administration significantly reversed the transcripts levels of these genes as comparative to the AFB1 exposed group. Moreover, we investigated the protein expression of Bcl-2, Bax and caspase-3 in the liver tissue of mice by western blotting (Figure 6F,G). Interestingly, the results of western blotting for Bcl-2, Bax and caspase-3 were consistent with the qRT-PCR results. Similarly, in the AFB1 exposed group, Bcl-2 protein expression was (p < 0.05) down-regulated, while Bax and caspase-3 protein expression was (p < 0.05) up-regulated as compared to the CON group. Conversely, LUTN treatment significantly alleviated the alterations in the Bcl-2, Bax and caspase-3 protein levels induced by AFB1.
Figure 6.
Luteolin treatment restrains AFB1-induced mitochondrial-mediated apoptosis pathway. The relative expression of mitochondrial-mediated apoptosis pathway transcripts was analyzed by qRT-PCR. (A–E) The relative mRNA expression of Bcl-2, Bax, Cyto-c, Cas-3 and Cas-9. The results are presented as mean ± SD (n = 6). Western blotting was used to detect the expression of mitochondrial apoptosis-associated proteins. (F) Western blotting analysis of Bcl-2, Bax and Cas-3. (G) Quantitative analysis of western blotting for Bcl-2, Bax and Cas-3. The results are presented as mean ± SD (n = 3). a–c Columns with different lowercase letters indicated significant differences between the compared groups (p < 0.05). B-cell lymphoma 2 (Bcl-2); Bcl-2-associated X protein (Bax); cytochrome-c (Cyt-c); caspase-3 (Cas-3); caspase-9 (Cas-9).
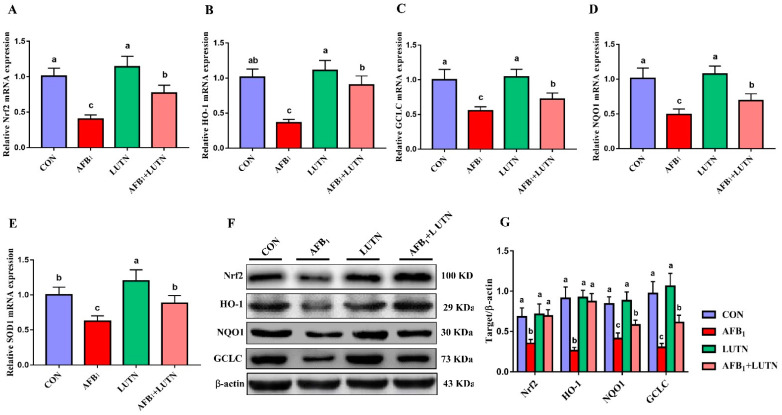
3.6. Luteolin Treatment Activates Nrf2 Signaling Pathway in the Liver of AFB1 Exposed Mice
To confirm our hypothesis that LUTN promotes the antioxidant capacity and alleviates hepatotoxicity in mice induced by AFB1 is associated with Nrf2 signaling pathway activation, the transcript levels and protein expression of Nrf2 and downstream targets were detected. As depicted in Figure 7A–E, AFB1 exposed group showed a significant (p < 0.05) decrease in the gene expression of Nrf2, HO-1, GCLC, NQO1 and SOD1 compared to the CON and other experimental groups. In contrast, LUTN treatment considerably improved the transcript expressions of Nrf2, HO-1, GCLC, NQO1 and SOD1 altered by AFB1 (p < 0.05). Further, the protein expression of Nrf2 and its target genes were detected by western blotting and revealed the same tendency (Figure 7F,G). Similarly, protein expression of Nrf2 and its downstream targets, including HO-1, GCLC, and NQO1, were (p < 0.05) down-regulated in the AFB1 challenged group. However, LUTN treatment considerably up-regulated the Nrf2, NQO1, HO-1 and GCLC protein expressions as down-regulated by AFB1 (p < 0.05).
Figure 7.
Luteolin treatment activates the Nrf2 signaling pathway in the liver of AFB1 exposed mice. The relative expression of Nrf2-mediated antioxidant signaling transcripts was analyzed by qRT-PCR. (A–E) The relative gene expression of Nrf2 and its downstream molecules, HO-1, GCLC, NQO1 and SOD1. The results are presented as mean ± SD (n = 6). The expression of Nrf2 and its associated proteins was detected by western blotting. (F) Western blots for Nrf2, HO-1, GCLC, and NQO1. (G) Quantification of western blots for Nrf2, HO-1, GCLC, and NQO1. The results are presented as mean ± SD (n = 3). a–c Columns with different lowercase letters indicated significant differences between the compared groups (p < 0.05). Nuclear erythroid-2-related factor (Nrf2); glutamate-cysteine ligase catalytic subunit (GCLC), heme oxygenase-1 (HO-1); superoxide dismutase 1 (SOD1); quinone oxidoreductase 1 (NQO1).
4. Discussion
Aflatoxins, threatening mycotoxins, are commonly found in cereals and animal forages and pose major health and economic risks to humans and animals [36]. Growth retardation is one of the most important symptoms of aflatoxins poisoning. In the present study, AFB1 exposure decreased ADG and ADFI in mice. The observed growth retardation may have resulted from anorexia, suppression of lipogenesis, and protein synthesis induced by AFB1 [37]. The liver is regarded as the main target organ of AFB1 poisoning. We found that AFB1 generated clinical and pathological symptoms of liver injury in mice, as evident from the increased haptic enzymes AST, ALT, ALP, GGT, and decreased globulin and albumin content as well as fatty droplets and hepatocytes infiltration with macro vesicles in the liver of mice. These findings imply that AFB1 can cause hepatotoxicity, consistent with previous findings [28,38,39]. Interestingly, luteolin (LUTN) treatment attenuated growth retardation and alleviated toxic effects on serum biochemical profile and pathological changes in the liver of mice induced by AFB1. The current results align with a previous study, which has reported that LUTN supplementation prevented acetaminophen-induced hepatic injury in rats [16]. The present findings strongly suggested that LUTN treatment can mitigate AFB1-induced hepatic damage in mice. However, special clinical studies are warranted to know the hepatoprotective effects of LUTN on ongoing or established AFB1 toxicity in humans or animals.
Oxidative stress is considered to be critical molecular process underlying cell damage [40]. Oxidative stress is associated with significant increase in the generation of reactive oxygen species (ROS) while decreased antioxidant capacity in the body [41]. Previous studies reported that excessive ROS production is a significant cause of AFB1-induced hepatotoxicity and apoptosis in mice [5,30,42]. Similarly, in the present study, AFB1 exposure generated ROS and MDA accumulation and inhibited antioxidant enzyme activities such as T-SOD, CAT, GAH-Px and T-AOC, and induced apoptosis in the liver of mice. LUTN is considered a potent ROS scavenger, protecting cells from ROS accumulation and apoptosis induced by oxidative stress [43]. We found that LUTN treatment substantially suppressed oxidative stress induced by AFB1 as evidenced by decreased ROS and MDA accumulation, strengthened antioxidant defense system (T-SOD, CAT, GAH-Px and T-AOC), and reduced apoptosis rate in the liver of mice.
Mitochondria have been identified as the primary targets for AFB1 harmful effects in causing apoptosis [44]. The mitochondrial apoptosis pathway is regulated by anti-apoptotic and pro-apoptotic members of the Bcl-2 family [45]. Bcl-2 is an anti-apoptotic protein that impedes the release of apoptogenic molecules (Cyt-c), while Bax is a pro-apoptotic protein that promotes the release of Cyt-c into the cytoplasm by competing with Bcl-2, thereby causing cell death [46,47]. The release of Cyt-c into the cytoplasm, resulted in caspase-3 and caspase-9 activation and the induction of apoptosis [48,49]. Previously, studies reported that AFB1 exposure could cause apoptosis in various tissues and cells and the mechanism was linked with the mitochondrial apoptosis pathway [5,50,51,52]. Our results showed that expressions of Bax, Cyt-c caspase-3 and caspase-9 were considerably up-regulated, while Bcl-2 was down-regulated by AFB1. These findings demonstrated that AFB1-induced excessive apoptosis in the liver of mice is linked with the mitochondrial apoptosis pathway. Notably, LUTN treatment increased the Bcl-2 expression, inhibited mitochondrial Cyt-c release and reduced the activated caspase-3 and caspase-9 expression in the liver of mice under AFB1 exposure. Furthermore, mitochondria are the primary target of ROS attacks, and superfluous ROS production can lead to oxidative stress and mitochondrial malfunctioning [31]. We observed that LUTN suppressed ROS generation induced by AFB1. As a result, we hypothesized that LUTN reduces AFB1-induced excessive apoptosis in mouse liver either directly or through the suppression of oxidative stress.
Nrf2 is a transcription factor that plays a significant role in the process of AFB1-induced cytotoxicity [53,54]. Under normal conditions, Nrf2 stays in the cytosol by its specific antagonist, Keap1, while under stimulation, it dissociates from Keap1 and translocates to the nucleus, where it binds to ARE and regulates the transcripts of downstream antioxidant genes (HO-1, NQO1, SOD, GCLC) [55]. Nrf2 and its targeted antioxidative genes, HO-1, NQO1, SOD, and GCLC, are critical components to maintain the redox system and have been shown to exhibit cytoprotective resistance against oxidative stress [56]. The present study revealed that AFB1 exposure inhibited the expression of Nrf2 and its associated-target genes such as HO-1, NQO1, SOD1, and GCLC in the liver of mice. However, LUTN dramatically rescued these effects induced by AFB1. Previously, LUTN prevents the progression of liver fibrosis induced by carbon tetrachloride-(CCL4) through targeting AKT/mTOR/p70S6K and TGFβ/Smad signaling pathways [26]. The current results agree with previous investigations that AFB1 exposure suppressed Nrf2 nuclear translocation [28,54], and LUTN could rescue cells from oxidative damage by activating Nrf2 and up-regulating the cellular antioxidant genes expression [57,58,59]. However, further research is needed to address the preventive effects of LUTN against ongoing and/or established AFB1-induced toxicity in human and/or animal.
5. Conclusions
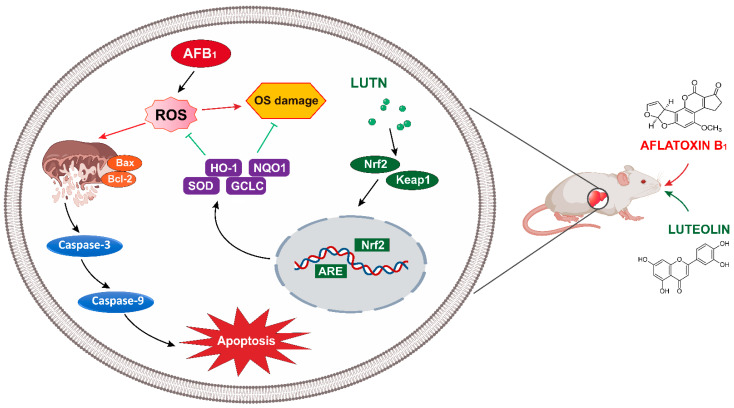
The current study provides significant evidence on the potential protective effects of luteolin (LUTN) against AFB1-induced hepatotoxicity in mice. LUTN effectively rescued liver injury, as evident by the amelioration of toxic effects on serum biochemical profile and pathological alterations induced by AFB1. LUTN attenuates AFB1-induced excessive apoptosis by inhibiting the mitochondria-dependent apoptosis pathway. Additionally, LUTN suppressed AFB1-induced oxidative stress by scavenging ROS accumulation and enhancing antioxidant enzymes capacity via regulation of Nrf2 signaling. The current study suggested that the key mechanisms underlying the LUTN hepatoprotective effects were associated with the activation of the Nrf2 signaling pathway (Figure 8). The present study suggested that LUTN may serve as a potential mitigator against AFB1-induced liver injury and could be helpful for the development of novel treatment to combat liver diseases in humans and/or animals.
Figure 8.
Schematic diagram representing hepatoprotective mechanism of luteolin against AFB1-induced apoptosis and oxidative stress via the activation of Nrf2 signaling pathway in the liver of mice.
Acknowledgments
This work was supported by the Taif University Researchers Supporting Program (Project number: TURSP-2020/151), Taif University, Saudi Arabia. The authors would like to thank the Deanship of Scientific Research at King Khalid University, Abha, KSA for funding this work under Grant number (R.G.P.2/47/42).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antiox10081268/s1, Table S1: List of primers used in the present study for qRT-PCR analysis.
Author Contributions
S.A.R., A.S., I.R.R., M.M.A.-D., K.W.: Conceptualization, Methodology, Formal analysis, Drafted manuscript, Writing-original draft, Writing—review & editing. D.M.B., R.W.A., M.A.R.: Data analysis, Statistical guidance, Writing—review & editing. A.N., P.R., A.A., A.F.E.-K.: Guided, Software, Data curation, Resources, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The present research was approved by Scientific Ethic Committee of Huazhong Agricultural University on 12 June 2018, (Permit No. HZAUMO-2018-07).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and supplementary materials.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhao L., Feng Y., Deng J., Zhang N.-Y., Zhang W.-P., Liu X.-L., Rajput S.A., Qi D.-S., Sun L.-H. Selenium deficiency aggravates aflatoxin B1–induced immunotoxicity in chick spleen by regulating 6 selenoprotein genes and redox/inflammation/apoptotic signaling. J. Nutr. 2019;149:894–901. doi: 10.1093/jn/nxz019. [DOI] [PubMed] [Google Scholar]
- 2.Kensler T.W., Roebuck B.D., Wogan G.N., Groopman J.D. Aflatoxin: A 50-year odyssey of mechanistic and translational toxicology. Toxicol. Sci. 2010;120:S28–S48. doi: 10.1093/toxsci/kfq283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Min L., Fink-Gremmels J., Li D., Tong X., Tang J., Nan X., Yu Z., Chen W., Wang G. An overview of aflatoxin B1 biotransformation and aflatoxin M1 secretion in lactating dairy cows. Anim. Nutr. 2021;7:42–48. doi: 10.1016/j.aninu.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abrar M., Anjum F.M., Butt M.S., Pasha I., Randhawa M.A., Saeed F., Waqas K. Aflatoxins: Biosynthesis, occurrence, toxicity, and remedies. Crit. Rev. Food Sci. Nutr. 2013;53:862–874. doi: 10.1080/10408398.2011.563154. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y., Wang T., Li P., Chen J., Nepovimova E., Long M., Wu W., Kuca K. Bacillus amyloliquefaciens B10 can alleviate aflatoxin B1-induced kidney oxidative stress and apoptosis in mice. Ecotoxicol. Environ. Saf. 2021;218:112286. doi: 10.1016/j.ecoenv.2021.112286. [DOI] [PubMed] [Google Scholar]
- 6.Rajput S.A., Zhang C., Feng Y., Wei X.T., Khalil M.M., Rajput I.R., Baloch D.M., Shaukat A., Rajput N., Qamar H., et al. Proanthocyanidins alleviates aflatoxinB1-induced oxidative stress and apoptosis through mitochondrial pathway in the bursa of fabricius of broilers. Toxins. 2019;11:157. doi: 10.3390/toxins11030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Limaye A., Yu R.-C., Chou C.-C., Liu J.-R., Cheng K.-C. Protective and detoxifying effects conferred by dietary selenium and curcumin against AFB1-mediated toxicity in livestock: A review. Toxins. 2018;10:25. doi: 10.3390/toxins10010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CONTAM. Schrenk D., Bignami M., Bodin L., Chipman J.K., del Mazo J., Grasl-Kraupp B., Hogstrand C., Hoogenboom L., Leblanc J.C., et al. Risk assessment of aflatoxins in food. EFSA J. 2020;18:e06040. doi: 10.2903/j.efsa.2020.6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rushing B.R., Selim M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019;124:81–100. doi: 10.1016/j.fct.2018.11.047. [DOI] [PubMed] [Google Scholar]
- 10.Rajput S.A., Sun L., Zhang N.-Y., Khalil M.M., Ling Z., Chong L., Wang S., Rajput I.R., Bloch D.M., Khan F.A. Grape seed proanthocyanidin extract alleviates aflatoxinB1-induced immunotoxicity and oxidative stress via modulation of NF-κB and Nrf2 signaling pathways in broilers. Toxins. 2019;11:23. doi: 10.3390/toxins11010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eftekhari A., Ahmadian E., Panahi-Azar V., Hosseini H., Tabibiazar M., Dizaj S.M. Hepatoprotective and free radical scavenging actions of quercetin nanoparticles on aflatoxin B1-induced liver damage: In vitro/in vivo studies. Artif. Cells Nanomed. Biotechnol. 2018;46:411–420. doi: 10.1080/21691401.2017.1315427. [DOI] [PubMed] [Google Scholar]
- 12.Wang W.J., Xu Z.L., Yu C., Xu X.H. Effects of aflatoxin B1 on mitochondrial respiration, ROS generation and apoptosis in broiler cardiomyocytes. Anim. Sci. J. 2017;88:1561–1568. doi: 10.1111/asj.12796. [DOI] [PubMed] [Google Scholar]
- 13.Zhao L., Deng J., Xu Z.-J., Zhang W.-P., Khalil M.M., Karrow N.A., Sun L.-H. Mitigation of aflatoxin B1 hepatoxicity by dietary hedyotis diffusa is associated with activation of NRF2/ARE signaling in chicks. Antioxidants. 2021;10:878. doi: 10.3390/antiox10060878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pauletto M., Giantin M., Tolosi R., Bassan I., Barbarossa A., Zaghini A., Dacasto M. Curcumin mitigates AFB1-induced hepatic toxicity by triggering cattle antioxidant and anti-inflammatory pathways: A whole transcriptomic in vitro study. Antioxidants. 2020;9:1059. doi: 10.3390/antiox9111059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panche A., Diwan A., Chandra S. Flavonoids: An overview. J. Nutr. Sci. 2016;5:E47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tai M., Zhang J., Song S., Miao R., Liu S., Pang Q., Wu Q., Liu C. Protective effects of luteolin against acetaminophen-induced acute liver failure in mouse. Int. Immunopharmacol. 2015;27:164–170. doi: 10.1016/j.intimp.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Liu M., Cheng C., Li X., Zhou S., Hua J., Huang J., Li Y., Yang K., Zhang P., Zhang Y., et al. Luteolin alleviates ochratoxin A induced oxidative stress by regulating Nrf2 and HIF-1α pathways in NRK-52E rat kidney cells. Food Chem. Toxicol. 2020;141:111436. doi: 10.1016/j.fct.2020.111436. [DOI] [PubMed] [Google Scholar]
- 18.Qian W., Liu M., Fu Y., Zhang J., Liu W., Li J., Li X., Li Y., Wang T. Antimicrobial mechanism of luteolin against Staphylococcus aureus and Listeria monocytogenes and its antibiofilm properties. Microb. Pathog. 2020;142:104056. doi: 10.1016/j.micpath.2020.104056. [DOI] [PubMed] [Google Scholar]
- 19.Ziyan L., Yongmei Z., Nan Z., Ning T., Baolin L. Evaluation of the anti-inflammatory activity of luteolin in experimental animal models. Planta Med. 2007;73:221–226. doi: 10.1055/s-2007-967122. [DOI] [PubMed] [Google Scholar]
- 20.Seelinger G., Merfort I., Wölfle U., Schempp C.M. Anti-carcinogenic effects of the flavonoid luteolin. Molecules. 2008;13:2628–2651. doi: 10.3390/molecules13102628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kempuraj D., Thangavel R., Kempuraj D.D., Ahmed M.E., Selvakumar G.P., Raikwar S.P., Zaheer S.A., Iyer S.S., Govindarajan R., Chandrasekaran P.N., et al. Neuroprotective effects of flavone luteolin in neuroinflammation and neurotrauma. Biofactors. 2021;47:190–197. doi: 10.1002/biof.1687. [DOI] [PubMed] [Google Scholar]
- 22.Bataille A., Manautou J. Nrf2: A potential target for new therapeutics in liver disease. Clin. Pharmacol. Ther. 2012;92:340–348. doi: 10.1038/clpt.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw P., Chattopadhyay A. Nrf2–ARE signaling in cellular protection: Mechanism of action and the regulatory mechanisms. J. Cell. Physiol. 2020;235:3119–3130. doi: 10.1002/jcp.29219. [DOI] [PubMed] [Google Scholar]
- 24.Li P., Li K., Zou C., Tong C., Sun L., Cao Z., Yang S., Lyu Q. Selenium yeast alleviates ochratoxin A-induced hepatotoxicity via modulation of the PI3K/AKT and Nrf2/Keap1 signaling pathways in chickens. Toxins. 2020;12:143. doi: 10.3390/toxins12030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu J., Wang H., Chen F., Fu J., Xu Y., Hou Y., Kou H.H., Zhai C., Nelson M.B., Zhang Q., et al. An overview of chemical inhibitors of the Nrf2-ARE signaling pathway and their potential applications in cancer therapy. Free. Radic. Biol. Med. 2016;99:544–556. doi: 10.1016/j.freeradbiomed.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Li J., Li X., Xu W., Wang S., Hu Z., Zhang Q., Deng X., Wang J., Zhang J., Guo C. Antifibrotic effects of luteolin on hepatic stellate cells and liver fibrosis by targeting AKT/mTOR/p70S6K and TGFβ/Smad signalling pathways. Liver Int. 2015;35:1222–1233. doi: 10.1111/liv.12638. [DOI] [PubMed] [Google Scholar]
- 27.Domitrović R., Jakovac H., Tomac J., Šain I. Liver fibrosis in mice induced by carbon tetrachloride and its reversion by luteolin. Toxicol. Appl. Pharmacol. 2009;241:311–321. doi: 10.1016/j.taap.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Xu F., Yu K., Yu H., Wang P., Song M., Xiu C., Li Y. Lycopene relieves AFB1-induced liver injury through enhancing hepatic antioxidation and detoxification potential with Nrf2 activation. J. Funct. Foods. 2017;39:215–224. doi: 10.1016/j.jff.2017.10.027. [DOI] [Google Scholar]
- 29.Liu G., Zhang Y., Liu C., Xu D., Zhang R., Cheng Y., Pan Y., Huang C., Chen Y. Luteolin alleviates alcoholic liver disease induced by chronic and binge ethanol feeding in mice. J. Nutr. 2014;144:1009–1015. doi: 10.3945/jn.114.193128. [DOI] [PubMed] [Google Scholar]
- 30.Rajput S.A., Sun L., Zhang N., Khalil M.M., Gao X., Ling Z., Zhu L., Khan F.A., Zhang J., Qi D. Ameliorative effects of grape seed proanthocyanidin extract on growth performance, immune function, antioxidant capacity, biochemical constituents, liver histopathology and aflatoxin residues in broilers exposed to aflatoxin B1. Toxins. 2017;9:371. doi: 10.3390/toxins9110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajput S.A., Shaukat A., Rajput I.R., Kamboh A.A., Iqbal Z., Saeed M., Akhtar R.W., Shah S.A.H., Raza M.A., El Askary A., et al. Ginsenoside Rb1 prevents deoxynivalenol-induced immune injury via alleviating oxidative stress and apoptosis in mice. Ecotoxicol. Environ. Saf. 2021;220:112333. doi: 10.1016/j.ecoenv.2021.112333. [DOI] [PubMed] [Google Scholar]
- 32.Wu K., Jia S., Zhang J., Zhang C., Wang S., Rajput S.A., Sun L., Qi D. Transcriptomics and flow cytometry reveals the cytotoxicity of aflatoxin B1 and aflatoxin M1 in bovine mammary epithelial cells. Ecotoxicol. Environ. Saf. 2020;209:111823. doi: 10.1016/j.ecoenv.2020.111823. [DOI] [PubMed] [Google Scholar]
- 33.Gao X., Xiao Z., Li C., Zhang J., Zhu L., Sun L., Zhang N., Khalil M.M., Rajput S.A., Qi D. Prenatal exposure to zearalenone disrupts reproductive potential and development via hormone-related genes in male rats. Food Chem. Toxicol. 2018;116:11–19. doi: 10.1016/j.fct.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Wang S., Zhang C., Yang J., Wang X., Wu K., Zhang B., Zhang J., Yang A., Rajput S.A., Qi D. Sodium butyrate protects the intestinal barrier by modulating intestinal host defense peptide expression and gut microbiota after a challenge with deoxynivalenol in weaned piglets. J. Agric. Food Chem. 2020;68:4515–4527. doi: 10.1021/acs.jafc.0c00791. [DOI] [PubMed] [Google Scholar]
- 35.Wang S., Yang J., Zhang B., Wu K., Yang A., Li C., Zhang J., Zhang C., Rajput S.A., Zhang N., et al. Deoxynivalenol impairs porcine intestinal host defense peptide expression in weaned piglets and IPEC-J2 cells. Toxins. 2018;10:541. doi: 10.3390/toxins10120541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan Y., Zhao L., Ma Q., Li X., Shi H., Zhou T., Zhang J., Ji C. Effects of Bacillus subtilis ANSB060 on growth performance, meat quality and aflatoxin residues in broilers fed moldy peanut meal naturally contaminated with aflatoxins. Food Chem. Toxicol. 2013;59:748–753. doi: 10.1016/j.fct.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Long M., Zhang Y., Li P., Yang S.-H., Zhang W.-K., Han J.-X., Wang Y., He J.-B. Intervention of grape seed proanthocyanidin extract on the subchronic immune injury in mice induced by aflatoxin B1. Int. J. Mol. Sci. 2016;17:516. doi: 10.3390/ijms17040516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang N.-Y., Qi M., Zhao L., Zhu M.-K., Guo J., Liu J., Gu C.-Q., Rajput S.A., Krumm C.S., Qi D.-S., et al. Curcumin prevents aflatoxin B1 hepatoxicity by inhibition of cytochrome P450 isozymes in chick liver. Toxins. 2016;8:327. doi: 10.3390/toxins8110327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balogh K., Kövesi B., Zándoki E., Kulcsár S., Ancsin Z., Erdélyi M., Dobolyi C., Bata-Vidács I., Inotai K., Szekeres A., et al. Effect of sterigmatocystin or aflatoxin contaminated feed on lipid peroxidation and glutathione redox system and expression of glutathione redox system regulatory genes in broiler chicken. Antioxidants. 2019;8:201. doi: 10.3390/antiox8070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang B., Chen Q., Wang L., Gao X., Zhu W., Mu P., Deng Y. Aflatoxin b1 induces neurotoxicity through reactive oxygen species generation, DNA damage, apoptosis, and S-phase cell cycle arrest. Int. J. Mol. Sci. 2020;21:6517. doi: 10.3390/ijms21186517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen H.-M., Shi C.-Y., Shen Y., Ong C.-N. Detection of elevated reactive oxygen species level in cultured rat hepatocytes treated with aflatoxin B1. Free. Radic. Biol. Med. 1996;21:139–146. doi: 10.1016/0891-5849(96)00019-6. [DOI] [PubMed] [Google Scholar]
- 42.Wu J., Gan Z., Zhuo R., Zhang L., Wang T., Zhong X. Resveratrol attenuates aflatoxin B1-induced ROS formation and increase of m6A RNA Methylation. Animals. 2020;10:677. doi: 10.3390/ani10040677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krishnaswamy R., Devaraj S.N., Padma V.V. Lutein protects HT-29 cells against Deoxynivalenol-induced oxidative stress and apoptosis: Prevention of NF-κB nuclear localization and down regulation of NF-κB and cyclo-Oxygenase–2 expression. Free. Radic. Biol. Med. 2010;49:50–60. doi: 10.1016/j.freeradbiomed.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Xu F., Wang P., Yao Q., Shao B., Yu H., Yu K., Li Y. Lycopene alleviates AFB 1-induced immunosuppression by inhibiting oxidative stress and apoptosis in the spleen of mice. Food Funct. 2019;10:3868–3879. doi: 10.1039/C8FO02300J. [DOI] [PubMed] [Google Scholar]
- 45.Antonsson B. Mitochondria and the Bcl-2 family proteins in apoptosis signaling pathways. Mol. Cell. Biochem. 2004;256:141–155. doi: 10.1023/B:MCBI.0000009865.70898.36. [DOI] [PubMed] [Google Scholar]
- 46.Siddiqui W.A., Ahad A., Ahsan H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update. Arch. Toxicol. 2015;89:289–317. doi: 10.1007/s00204-014-1448-7. [DOI] [PubMed] [Google Scholar]
- 47.Gross A., McDonnell J.M., Korsmeyer S.J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 48.Green D.R., Reed J.C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 49.Ly J.D., Grubb D.R., Lawen A. The mitochondrial membrane potential (Δψ m) in apoptosis: An update. Apoptosis. 2003;8:115–128. doi: 10.1023/A:1022945107762. [DOI] [PubMed] [Google Scholar]
- 50.Peng X., Chen K., Chen J., Fang J., Cui H., Zuo Z., Deng J., Chen Z., Geng Y., Lai W. Aflatoxin B 1 affects apoptosis and expression of B ax, B cl-2, and C aspase-3 in thymus and bursa of fabricius in broiler chickens. Environ. Toxicol. 2016;31:1113–1120. doi: 10.1002/tox.22120. [DOI] [PubMed] [Google Scholar]
- 51.Raj H.G., Kohli E., Rohil V., Dwarakanath B., Parmar V.S., Malik S., Adhikari J., Tyagi Y.K., Goel S., Gupta K., et al. Acetoxy-4-methylcoumarins confer differential protection from aflatoxin B1-induced micronuclei and apoptosis in lung and bone marrow cells. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis. 2001;494:31–40. doi: 10.1016/S1383-5718(01)00176-0. [DOI] [PubMed] [Google Scholar]
- 52.Li H., Xing L., Zhang M., Wang J., Zheng N. The toxic effects of aflatoxin B1 and aflatoxin M1 on kidney through regulating L-proline and downstream apoptosis. BioMed Res. Int. 2018;2018:9074861. doi: 10.1155/2018/9074861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin S., Yang H., Jiao Y., Pang Q., Wang Y., Wang M., Shan A., Feng X. Dietary curcumin alleviated acute ileum damage of ducks (Anas platyrhynchos) induced by AFB1 through regulating Nrf2-ARE and NF-κB signaling pathways. Foods. 2021;10:1370. doi: 10.3390/foods10061370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y., Wang W. Aflatoxin B1 impairs mitochondrial functions, activates ROS generation, induces apoptosis and involves Nrf2 signal pathway in primary broiler hepatocytes. Anim. Sci. J. 2016;87:1490–1500. doi: 10.1111/asj.12550. [DOI] [PubMed] [Google Scholar]
- 55.Jang J., Wang Y., Kim H.S., Lalli M.A., Kosik K.S. Nrf2, a regulator of the proteasome, controls self-renewal and pluripotency in human embryonic stem cells. Stem Cells. 2014;32:2616–2625. doi: 10.1002/stem.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suraweera T.L., Rupasinghe H., Dellaire G., Xu Z. Regulation of Nrf2/ARE pathway by dietary flavonoids: A friend or foe for cancer management? Antioxidants. 2020;9:973. doi: 10.3390/antiox9100973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tan X., Yang Y., Xu J., Zhang P., Deng R., Mao Y., He J., Chen Y., Zhang Y., Ding J. Luteolin exerts neuroprotection via modulation of the p62/Keap1/Nrf2 pathway in intracerebral hemorrhage. Front. Pharmacol. 2020;10:1551. doi: 10.3389/fphar.2019.01551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cho Y.-C., Park J., Cho S. Anti-inflammatory and anti-oxidative effects of luteolin-7-O-glucuronide in LPS-stimulated murine macrophages through TAK1 inhibition and Nrf2 activation. Int. J. Mol. Sci. 2020;21:2007. doi: 10.3390/ijms21062007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article and supplementary materials.