Abstract
This study examines in vivo the role and functional interrelationships of components regulating exit from the G1 resting phase into the DNA synthetic (S) phase of the cell cycle. Our approach made use of several key experimental attributes of the developing mouse lens, namely its strong dependence on pRb in maintenance of the postmitotic state, the down-regulation of cyclins D and E and up-regulation of the p57KIP2 inhibitor in the postmitotic lens fiber cell compartment, and the ability to target transgene expression to this compartment. These attributes provide an ideal in vivo context in which to examine the consequences of forced cyclin expression and/or of loss of p57KIP2 inhibitor function in a cellular compartment that permits an accurate quantitation of cellular proliferation and apoptosis rates in situ. Here, we demonstrate that, despite substantial overlap in cyclin transgene expression levels, D-type and E cyclins exhibited clear functional differences in promoting entry into S phase. In general, forced expression of the D-type cyclins was more efficient than cyclin E in driving lens fiber cells into S phase. In the case of cyclins D1 and D2, ectopic proliferation required their enhanced nuclear localization through CDK4 coexpression. High nuclear levels of cyclin E and CDK2, while not sufficient to promote efficient exit from G1, did act synergistically with ectopic cyclin D/CDK4. The functional differences between D-type and E cyclins was most evident in the p57KIP2-deficient lens wherein cyclin D overexpression induced a rate of proliferation equivalent to that of the pRb null lens, while overexpression of cyclin E did not increase the rate of proliferation over that induced by the loss of p57KIP2 function. These in vivo analyses provide strong biological support for the prevailing view that the antecedent actions of cyclin D/CDK4 act cooperatively with cyclin E/CDK2 and antagonistically with p57KIP2 to regulate the G1/S transition in a cell type highly dependent upon pRb.
Progression into the DNA synthetic (S) phase of the mammalian cell cycle requires inactivation of the retinoblastoma protein (pRb) via its phosphorylation by cyclin-dependent kinases. This phosphorylation cancels pRb-mediated repression of the transactivation of genes whose activities are necessary for S-phase entry (52, 61). During the G1 phase, pRb phosphorylation is initially triggered by the cyclin D-dependent kinases CDK4 and CDK6 and then followed by cyclin E-dependent CDK2 (23). The cyclin D- and E-dependent kinases have a propensity to phosphorylate distinct serine and threonine residues of pRb (10), and under normal conditions where both kinases are sequentially expressed at physiologic levels, pRb phosphorylation by cyclin E-CDK2 may depend upon the previous action of cyclin D-dependent kinases (10, 23). Inhibition of cyclin D-dependent kinases in cells containing a functional pRb protein prevents pRb phosphorylation and leads to G1 phase arrest (4, 46), whereas cells lacking pRb function are refractory to such signals and continue to enter S phase (22, 26, 29, 33). In contrast, inhibition of cyclin E-dependent kinase activity in pRb-negative cells prevents S-phase entry (41), implying that cyclin E-CDK2 targets also non-pRb substrates whose phosphorylation is essential for G1 exit. Overexpression of either cyclin D1 or E leads to a decrease in the duration of G1 phase in rodent fibroblasts (40, 46) with additive effects when ectopic expression of both is enforced (49), but only D1 induction leads to rapid and immediate pRb hyperphosphorylation (48). Because the induction and assembly of the cyclin D-dependent kinases are controlled by extracellular mitogenic and integrin-dependent matrix signals (3), the ability of these enzymes to modulate pRb function ultimately helps to place the cell’s commitment to enter S phase under non-cell-autonomous controls.
The stimulatory actions of the G1 cyclins are countered by those of the CDK inhibitors (CKIs). There are two classes of CKIs, the INK4 proteins (INK4a to -d), which act specifically on cyclin D-dependent kinases, and the CIP/KIP family (p21CIP1, p27KIP1, and p57KIP2), which functions more broadly to inhibit cyclin E-, A-, and B-dependent kinases as well (13, 54). The levels of p27KIP1 in quiescent (G0) T cells and fibroblasts are relatively high and greatly exceed that of the G1 cyclins, but once these cells are stimulated to reenter the cycle and progress into late G1 phase, much of the p27KIP1 is degraded (25, 39, 43). Nonetheless, residual levels of p27KIP1 and p21CIP1 in continuously proliferating cells are believed to set an inhibitory threshold which active cyclin-CDK complexes are forced to overcome (54).
The three D-type cyclins, D1, D2, and D3, share many structural features and biochemical properties but exhibit distinct patterns of expression with respect to cell type and developmental stage (52). Skeletal myoblasts induced to differentiate under low mitogen conditions exhibit a marked decrease in cyclin D1 and a reciprocal rise in cyclin D3 expression, with a reversal of this pattern occurring upon exposure to the antidifferentiation agents bFGF and TGF-β (47). Such observations suggest different modes of regulation for cyclins D3 and D1 but do not resolve whether the two play distinct roles in muscle differentiation. A comparative analysis of each D-type cyclin in a granulocyte differentiation system also suggested functional differences, in that cells overexpressing cyclins D2 and D3 but not D1 were unable to differentiate in granulocyte colony-stimulating factor (24). These observations could relate to the fact that cyclins D2 and D3, unlike D1, can interact with CDK2 (in addition to CDK4/6) to form active complexes (14). Although their differential regulation and unique biological effects in these cell culture-based systems imply that there may be distinct roles for each of the D-type cyclins in cellular growth and differentiation, such biological distinctions are not supported by the finding that, in D1- or D2-deficient mice, the vast majority of tissues which express more than one D-type cyclin appear to grow and develop normally, suggesting functional redundancy (15, 55, 56).
Forced expression of D-type and E cyclins in several cell types in transgenic mice has established an oncogenic or growth-promoting role for these G1 cyclins (5, 6, 27, 50, 60). Nonetheless, a direct functional comparison of each D-type cyclin and cyclin E in a well-defined pRb-dependent system in vivo has yet to be conducted. We have relied upon the developing mouse lens to study cyclin function, as this organ system is (i) extremely simple and comprised of a single cell type, (ii) organized anatomically into an anterior layer of proliferating epithelial cells and a posterior compartment of postmitotic differentiated lens fiber cells, (iii) a stable developmental chronometer enabling a traceable account of experimentally induced alterations in growth, differentiation, and apoptosis, and (iv) dispensable, thus tolerating the genetic manipulation of pathways essential for viability. The cell cycle components operating in the lens have also been well defined by previous studies. pRb plays an exclusive role in controlling the mitotic arrest of lens fiber cells (36), and loss of pRb alone is associated with a dramatic increase of proliferation, accompanied by impaired expression of late-stage differentiation markers in these cells. In addition, the deregulation of lens cell proliferation by the loss of pRb activates a p53-dependent apoptotic program that leads to the efficient elimination of abnormally growing cells (36). Furthermore, lenses rendered null for both pRb-related proteins, p107 and p130, exhibit normal patterns of growth (unpublished observations).
G1 cyclins and CKIs exhibit contrasting patterns of expression in the developing lens. In situ hybridization studies performed on embryonic lenses have revealed that endogenous cyclin D1 and D2 mRNAs are present in both the proliferative epithelial cells and in the postmitotic equatorial lens fiber cells, whereas cyclin E transcripts are not readily detected in normal lenses but are up-regulated in pRb-deficient lenses (17). Among the known CKIs, p57KIP2 appears to play an important role as evidenced by the observations that its transcripts and protein levels are high in postmitotic lens fiber cells and its elimination by gene targeting results in inappropriate proliferation and apoptosis of lens fiber cells (62). The null p57KIP2 phenotype is reminiscent of, but less dramatic than, the deregulated growth and apoptosis observed in the pRb-deficient lens (36). While these findings suggest a functional link between p57KIP2 and pRb, the more attenuated consequences of p57KIP2 deficiency point to additional regulators of pRb inactivation and S-phase entry in lens fiber cells in vivo. In this study, we examined the consequences of forced expression of each G1 cyclin and its partner CDK in the mouse lens and have attempted to ascertain whether these G1 cyclin-CDK activities are modulated by p57KIP2.
MATERIALS AND METHODS
Production of transgenic mice.
To generate the various transgenic constructs, full-length cDNAs of the mouse cyclins D1 to D3, cyclin E, CDK2, and CDK4 genes were inserted into the CPV-1 expression cassette (a kind gift from Paul Overbeek) between the αA-crystallin promoter and the simian virus 40 splice and polyadenylation sequences (9, 21). By using standard transgenic mouse methodology, gel-purified transgenic inserts were microinjected into the pronucleus of fertilized eggs derived from B6/CBA F1 intercrosses. B6/CBA F1 mice (Jackson Laboratories) were also used to propagate the transgenic lines. Tail-derived genomic DNAs were assayed for the presence of the transgene by standard Southern and/or DNA slot blot procedures (51) using specific probes directed to the open reading frames of each transgene.
Protein isolation and Western immunoblot analyses.
Lenses were homogenized in ice-cold 0.1 M Tris (pH 7.4), the water-soluble lens proteins were separated from water-insoluble lens membrane proteins by centrifugation at 4°C for 20 min (57), and the protein concentration was determined by the Bradford assay (Bio-Rad). The membrane pellets were resuspended in a mixture of ice-cold 0.1 M Tris (pH 8.0), 7 M urea, and 5 mM EDTA (8). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12.5% polyacrylamide) and electroblotted to nitrocellulose. Blots were blocked for 1 h at room temperature in phosphate-buffered saline (PBS) containing 5% milk and 0.1% Tween 20. The primary antibody incubations were carried out for 1 h with affinity-purified polyclonal antisera against either cyclin D1 or E (Santa Cruz Biotechnology, Inc.) or MIP26 or α-crystallins (gifts from Joe Horwitz and Sam Zigler) or with a rat monoclonal antibody against cyclins D2 and D3 described previously (59), diluted in blocking solution, followed by either anti-rabbit or anti-rat immunoglobulin G horseradish peroxidase-conjugated antibody as the secondary antibody. Peroxidase activity was detected with enhanced chemiluminescence (Amersham).
Quantification of transgenic cyclin proteins.
To generate recombinant cyclin proteins, cDNAs which included the coding region of cyclins D1, D3, or E subcloned into the pGEX vector (Pharmacia Biotech, Inc.) were transfected into Escherichia coli BL 21 DE3 cells (Novagene). After 4 h of IPTG (isopropyl-β-d-thiogalactopyranoside) induction, overnight cultures were sonicated, and the fusion proteins were purified by using glutathione-Sepharose 4B RediPack columns (Pharmacia Biotech, Inc.). The concentration of recombinant glutathione S-transferase (GST)-cyclin fusion proteins was determined by quantification of the intensity of the bands by Coomassie-stained SDS-PAGE using a Gel Doc 1000 apparatus (Bio-Rad) and employing recombinant cyclin D1 (gift from N. Pavletich) as the concentration standard. Fifty micrograms of total lens proteins, homogenized as described above, and a range of different amounts of recombinant cyclins were separated by SDS–12% PAGE and electroblotted to nitrocellulose. Blots were treated as described above. The intensity of the bands generated by chemiluminescence reaction developed by using ECL + Plus (Amersham Life Science) exposed in a film was determined with a Gel Doc 1000 apparatus (Bio-Rad), and the formula that fit the standard curve best was determined by using the CA-Cricket Graph III program. This formula was applied to estimate the concentrations of the different transgenic cyclin proteins.
Histological analysis and indirect IF.
Samples were fixed in 10% buffered formalin and processed through paraffin embedding using standard procedures. Sections measuring 3 μm in thickness were cut parallel to the optic nerve, affixed to poly-l-lysine-coated slides, dewaxed in xylene, rehydrated through an ethanol series, and stained with hematoxylin and eosin. For indirect immunofluorescence (IF), paraffin-embedded sections were rehydrated, rinsed in PBS, and blocked in 3% bovine serum albumin in PBS at room temperature for 20 min. Affinity-purified polyclonal antisera against either cyclins D1, E, CDK4, or CDK2 (Santa Cruz Biotechnology, Inc.); p57KIP2 (62); MIP26; α-, β-, or γ-crystallins (gifts from Sam Zigler and Joe Horwitz); or a rat monoclonal antibody against cyclin D2 or D3 (59) were diluted in 3% BSA–PBS, incubated on sections overnight at 4°C, and washed in PBS. The secondary antibody, fluorescein isothiocyanate-linked anti-rabbit or anti-rat immunoglobulin G antibody, was diluted, incubated for 1 h at room temperature, and washed in PBS. The amount of primary and secondary antibody used was titrated in order to yield quantitative information.
BrdU incorporation and TUNEL assays.
Pregnant mice were injected intraperitoneally with bromodeoxyuridine (BrdU) dissolved in PBS at a dose of 100 μg/g of body weight (35). After 2 h, the embryos were processed to detect in situ BrdU incorporation into newly synthesized DNA as described previously (37). For detection of apoptosis in situ, the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay was performed as described previously (20) with minor modifications (37). To obtain a quantitative estimate of the rates of S-phase entry and apoptosis in the lens fiber region, BrdU- or TUNEL-positive nuclei were scored in a minimum of 20 different lens sections derived from two independent embryos. Nontransgenic lens fiber cells are always postmitotic and do not exhibit apoptotic features. The statistical analysis of the data was carried out using the Microsoft SPSS program.
RESULTS
Formation of the ocular lens results from a series of developmental events whose well-defined nature greatly enhances the use of this organ as a model system for cell cycle analysis in vivo. Lens development begins on embryonic day 9.5 (E9.5) with the creation of the lens placode followed by its invagination and eventual formation of a hollow lens vesicle by E11.5. Next, lens vesicle cells positioned on the posterior wall withdraw from the cell cycle and actively differentiate into elongating primary lens fiber cells. By E14.5, the elongation process is complete, and the resultant structure is composed of an anterior layer of proliferating epithelial cells and a posterior compartment of postmitotic differentiated lens fibers. The continued growth of the lens ensues from the recruitment of proliferating epithelial cells to the lateral equatorial region where their growth is arrested and they differentiate into secondary lens fiber cells. For purposes of this study, it is important to note that growth is restricted to the anterior epithelial layer and that apoptosis, while occasionally observed in this anterior region, does not take place in the lens fiber cell compartment (45). Previous studies have shown that endogenous cyclin D1 and D2 mRNAs are expressed in both proliferative epithelial cells and in differentiating equatorial lens fiber cells (17). On the protein level, endogenous cyclin D2 protein expression is below the level of detection, while cyclin D1 immunoreactivity is readily detected in the sensory retina, the proliferative anterior epithelial layer of the lens, and to a lesser degree, in differentiating lens fiber cells of the equatorial region. Most importantly, cyclin D1 is not detected in the central-most postmitotic, fully differentiated lens fiber region (see below). Cyclin E, although detectable by reverse transcription-PCR, is not detected via RNA in situ hybridization or indirect IF analyses (data not shown).
Production of cyclin transgenic mice and analysis of lens fiber cell transgene expression.
Several independent transgenic lines that ectopically express one of the three D-type cyclins or cyclin E in the postmitotic differentiated lens fiber cell compartment were generated with the aid of the αA-crystallin promoter. This promoter has proven effective in directing the expression of many different transgenes to the lens fiber cell region exclusively, commencing on E12.5 and continuing thereafter (42). The level and regional pattern of expression of each transgene in fully formed E16.5 lenses or in lens-derived extracts were examined and compared with nontransgenic, age-matched controls. The transgenic lines selected for further study were found to express detectable levels of the transgene-encoded proteins by Western blot analysis (Fig. 1), and these levels were higher than those in their nontransgenic littermate controls (data not shown). To determine the amount of ectopic cyclins present in the lenses of transgenic mice, the intensity of Western blot-positive bands of several lines per construct was compared to that of bands generated by known amounts of recombinant cyclins (Fig. 1). This analysis allowed us to estimate that αA-cyclin D1 line 9 and αA-cyclin D1 line 23 had amounts of cyclin D1 of 76 and 46 ng per 50 μg of total protein extract, respectively; αA-cyclin D3 line 25 and αA-cyclin D3 line 20 had 100 and 400 ng of cyclin D3 per 50 μg of total protein extract, respectively, while αA-cyclin E line 36 and αA-cyclin E line 40 had 27 and 44 ng of cyclin E per 50 μg of total protein extract, respectively. To compare the phenotypes of cyclin D1 and cyclin E transgenic mice, the studies below were performed using αA-cyclin D1 line 23 and αA-cyclin E line 40, which had very similar levels of transgenic proteins (46 ng and 45 ng per 50 μg of total protein extract, respectively). Since cyclins are also expressed endogenously in the anterior epithelial layer (see above), IF was used to verify that abundant levels of each transgene-encoded product were properly directed to the lens fiber cell compartment. The results of representative IF studies of the various cyclin transgene-expressing lenses are presented in Fig. 2 (and see below). These studies showed that cyclin D3 transgene-encoded protein was evenly distributed throughout the nucleus and cytoplasm of lens fiber cells (Fig. 2E), whereas transgene-encoded cyclins D1, D2, and E exhibited more complex subcellular distribution patterns. Anti-D1 signals were detected in nuclei of the equatorial region with less abundant nuclear staining in the central lens fiber cells (Fig. 2B and C). In contrast, the intervening region showed predominantly cytoplasmic staining with only a few nuclei staining positive (Fig. 2). An identical staining pattern for the transgene-encoded cyclin D2 protein was observed (data not shown). Cyclin E was detected in nuclei within the equatorial region and in the cytoplasm of the remainder of the lens fiber cell compartment (Fig. 2I). Since all of the cyclin transgene constructs were driven by identical promoter and enhancer elements, these complex and distinct expression patterns likely reflect differences in posttranscriptional regulation.
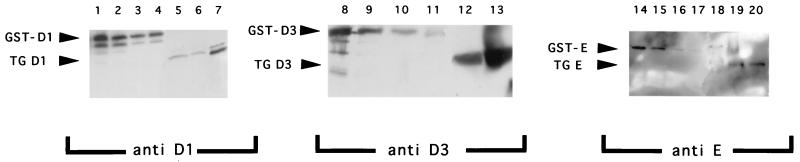
FIG. 1.
Western blot analysis of recombinant GST-cyclin fusion proteins and total lens lysates derived from αA-cyclin D1 and D3 and αA-cyclin E transgenic mice as assayed with anti-cyclin D1 (lanes 1 to 7), anti-cyclin D3 (lanes 8 to 13) and anti-cyclin E (lanes 14 to 20) antibodies. The bands indicated with arrowheads correspond either to the previously reported sizes for cyclin D1 (35 kDa), cyclin D3 (33 kDa), or cyclin E (50 kDa) proteins or to the GST-cyclin fusion proteins. Lanes 1 to 4, decreasing amounts of GST-cyclin D1 fusion protein used as standard curve (200 ng [lane 1], 100 ng [lane 2], 50 ng [lane 3], and 25 ng [lane 4]). Lanes 5 to 7, transgenic cyclin D1 protein from lenses of αA-cyclin D1 line 23 (lane 5), a transgenic mouse hemizygous for αA-cyclin D1 line 23 and αA-cdk4 line 14 transgenes (lane 6), and αA-cyclin D1 line 9 (lane 7). Lanes 8 to 13, decreasing amounts of GST-cyclin D3 fusion protein used as standard curve (100 ng [lane 8], 50 ng [lane 9], 25 ng [lane 10], and 10 ng [lane 11]). Lanes 12 and 13, transgenic cyclin D3 protein from lenses of αA-cyclin D3 line 25 (lane 12) and αA-cyclin D3 line 20 (lane 13). Lanes 14 to 17, decreasing amounts of GST-cyclin E fusion protein used as standard curve (100 ng [lane 14], 50 ng [lane 15], 25 ng [lane 16], and 10 ng [lane 17]). Lanes 18 to 20, transgenic cyclin E protein from lenses of αA-cyclin E line 36 (lane 18), αA-cyclin E line 40 (lane 19), and a transgenic mouse hemizygous for αA-cyclin E line 40 and αA-cdk2 line 31 transgenes (lane 20).
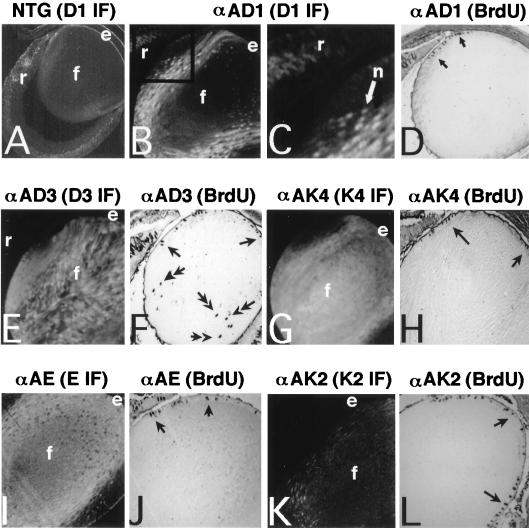
FIG. 2.
In situ immunofluorescence analysis of transgene-derived protein expression in the embryonic (E16.5) lenses of the various αA-cyclin and αA-cdk transgenic mice and their pattern of cellular proliferation as assayed by an indirect immunoperoxidase method for the detection of BrdU incorporation into newly synthesized DNA in situ. (A) Nontransgenic lens stained with anti-cyclin D1 antibody. Cyclin D1 is the only endogenous cyclin detected with the available antisera. (B) αA-cyclin D1 line 23 stained with anti-cyclin D1 antibody. (C) Higher magnification of the region framed in the upper left corner of panel B. A white arrow points to a positive stained nucleus, and a black arrow indicates a negative stained nucleus. (D) BrdU incorporation in αA-cyclin D1 line 23. Arrows point to positive nuclear-associated staining indicating physiological S-phase entry in the anterior epithelial layer. (E) αA-cyclin D3 line 25 stained with anti-cyclin D3 antibody. (F) BrdU incorporation pattern in αA-cyclin D3 line 25. Single arrows point to normal S-phase activity in the anterior epithelial layer, while double-headed arrows point to a subset of ectopic cycling in the lens fiber compartment. (G) αA-cdk4 line 14 stained with anti-CDK4 antibody. (H) BrdU incorporation pattern in αA-cdk4 line 14. (I) αA-cyclin E line 40 stained with anti-cyclin E antibody. (J) BrdU incorporation pattern in αA-cyclin E line 40. (K) αA-cdk2 line 31 stained with anti-CDK2 antibody. (L) BrdU incorporation pattern in αA-cdk2 line 31. Abbreviations: e, anterior epithelial layer; f, lens fiber cells; r, retina.
Analysis of forced expression of D-type cyclins and cyclin E in the lens and its effect on cell morphology, S-phase entry, and apoptosis.
Unlike nontransgenic lenses in which the lens fiber cells are arrayed along a well-defined axis, E16.5 lenses from all three αA-D3 transgenic lines exhibited a severely disorganized pattern. These morphological abnormalities (apparent even in the IF studies [compare Fig. 2E with the more organized lens shown in panel G]) were consistent with altered patterns of proliferation and apoptosis (see below). In contrast, the other αA-cyclin transgenic lenses (D1, D2, and E) presented with lens fiber cell compartments that were morphologically indistinguishable from nontransgenic controls (data not shown). These findings prompted a thorough analysis of patterns of growth, apoptosis, and differentiation markers in each of the transgenic lines.
For analysis of differentiation status, the temporal and spatial expression patterns of the family of lens crystallins and the membrane intrinsic protein 26 (MIP26) was examined by Western blot analysis and IF methods. These specific markers were selected since normal lens fiber cell differentiation is highly dependent upon the proper expression of these proteins. In this regard, α-crystallins are considered markers of early-stage differentiation, while γ-crystallins and MIP26 are classical late-stage markers (8, 32, 45). Despite the D3-induced morphological defects, analysis of the ratio of early- to late-stage differentiation markers as measured by Western blotting (Fig. 3) or of their pattern of distribution by IF (data not shown) failed to uncover significant differences in the levels and regional distribution of these markers between age-matched wild-type and transgenic lenses.
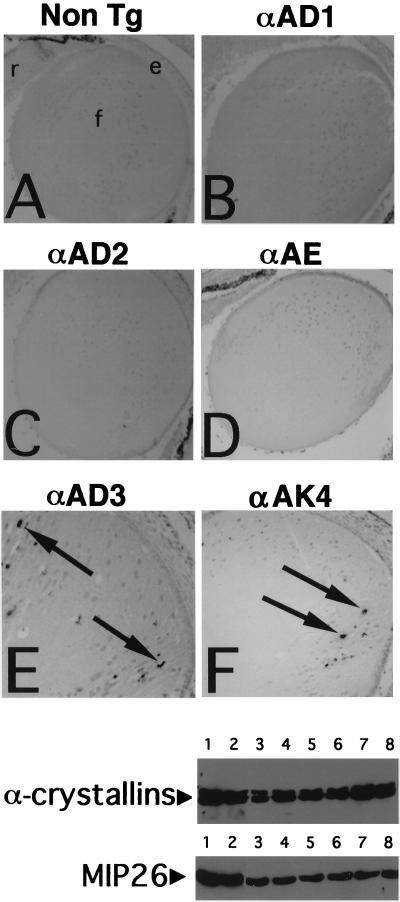
FIG. 3.
Analysis of apoptosis and stage-specific differentiation markers in the embryonic lenses of G1 cyclin transgenic and control mice. Lettered panels, results of TUNEL assay of E16.5 eye sections from nontransgenic mice (A) and from αA-cyclin D1, D2, E, or D3 and αA-cdk4 transgenic lines (B to F, respectively). e, anterior epithelial layer; f, lens fiber cells; r, retina. Arrows indicate TUNEL-positive nuclear staining consistent with apoptotic internucleosomal cleavage. Bottom panels, Western blot analyses of early (α-crystallin)- and late (MIP26)-stage differentiation markers. Each lane contains soluble lysate fraction (α-crystallin) or membrane fraction derived from a lens equivalent which permits an internal comparison between α-crystallin and MIP26 levels as described previously. The upper blot was probed with a rabbit polyclonal anti-α-crystallin antibody and the lower blot was probed with anti-MIP26 antibody. Lanes: 1, nontransgenic lens; 2, αA-cyclin D1 line 23; 3, αA-cyclin D2 line 2; 4, 5, and 6, αA-cyclin D3 lines 20, 25, and 42, respectively; 7 and 8, αA-cyclin E lines 40 and 36, respectively.
Since overexpression of G1 cyclins has been correlated with inappropriate S-phase entry, we next examined whether enforced expression of a D-type cyclin or cyclin E is sufficient to promote S-phase entry in normally postmitotic lens fiber cells. Lenses from αA-D3 mice showed ectopic nuclear-associated BrdU staining in the lens fiber cell compartment (Fig. 2F and Table 1), indicating that forced expression of cyclin D3 alone was sufficient to drive some lens fiber cells into S phase. This experimental result was obtained in three separate studies performed with three independent transgenic lines. In contrast, a normal pattern of cell proliferation (restricted to the anterior epithelial layer) was observed in all αA-D1 (Fig. 2D), αA-D2 (not shown), and αA-E (Fig. 2J) transgenic lenses.
TABLE 1.
Average number of positive nuclei per lens ± standard deviation in E16.5 transgenic embryos
| Transgene | No. of BrdU-positive nuclei | No. of TUNEL-positive nuclei |
|---|---|---|
| NTG | 0 | 0 |
| D1 | 0 | 0 |
| D2 | 0 | 0 |
| D3 | 10.4 ± 1.6 | 5.7 ± 0.9 |
| E | 0 | 0 |
| CDK4 | 0 | 3.0 ± 0.5 |
| CDK2 | 0 | 0 |
| D1/CDK4 | 5.2 ± 0.7 | 4.8 ± 0.7 |
| E/CDK2 | 1.6 ± 0.6 | 0 |
| E/CDK4 | 6.1 ± 1.7 | 3.0 ± 0.7 |
| D1/E/CDK2 | 3.4 ± 0.7 | 0 |
| E/CDK4/CDK2 | 12.0 ± 1.6 | 2.5 ± 0.6 |
| D1/E/CDK4/CDK2 | 23.0 ± 3.2 | 6.3 ± 1.5 |
To assess whether lens fiber cell expression of the G1 cyclins also leads to an apoptotic response, E16.5 lenses from each transgenic line were analyzed by TUNEL. Apoptotic nuclei were not detected in the lens fiber region of nontransgenic lenses or in αA-D1, αA-D2, and αA-E transgenic lenses (Fig. 3A to D). In contrast, several apoptotic nuclei were observed in αA-D3 lens fiber cells (Fig. 3E and Table 1). D3-induced proliferation and apoptosis, albeit modest, are reminiscent of those obtained in previous studies using the pRb-deficient lens that showed that inappropriate lens fiber cell proliferation activates an apoptotic response (reference 36 and see Fig. 7I).
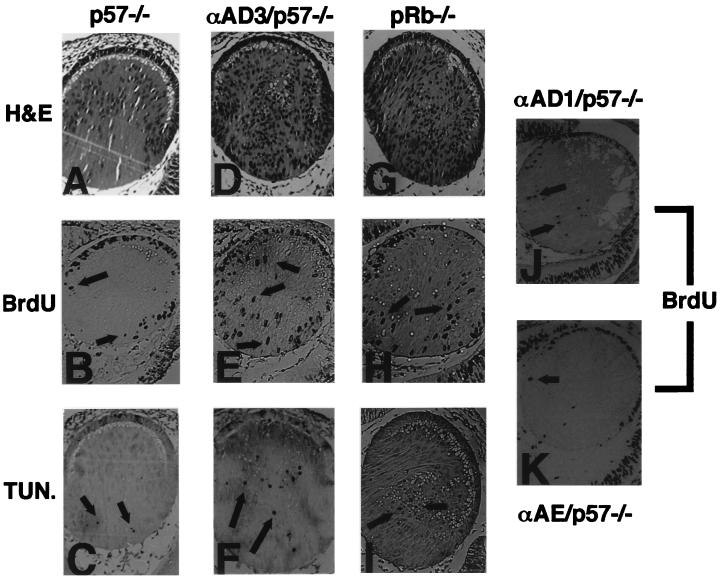
FIG. 7.
Functional interactions between D-type cyclins and p57KIP2. Hematoxylin and eosin (H&E) staining of E13.5 lens sections of a p57KIP2 null mouse (A), a αA-cyclin D3 line 25/p57KIP2 null mouse (D), and a pRb null mouse (G). BrdU incorporation pattern in lenses of a p57KIP2 null mouse (B), a αA-cyclin D3 line 25/p57KIP2 null mouse (E), a pRb null mouse (H), a αA-cyclin D1 line 23/p57KIP2 null mouse (J), and a αA-cyclin E line 40/p57KIP2 null mouse (K). TUNEL (TUN.) assay in lenses of a p57KIP2 null mouse (C), a αA-cyclin D3 line 25/p57KIP2 null mouse (F), and a pRb null mouse (I). Arrows point to nuclei testing positive by either BrdU incorporation or TUNEL assay in the lens fiber compartment.
Forced expression of CDK4 or CDK2 does not promote S-phase entry.
Since the G1 cyclins form active holenzymes by assembling with CDK subunits, it was important to address whether abundant levels of these catalytic units alone, as opposed to the regulatory cyclin subunits, could promote S-phase entry. To this end, high-levels of CDK4 or CDK2 were directed to the lens fiber cell compartment with the aid of the αA-crystallin promoter. IF analyses revealed that transgenic CDK4 was evenly distributed in the cytoplasm and nuclei of all lens fiber cells (Fig. 2G). In contrast, transgene-encoded CDK2 localized primarily to the cytoplasm with only a subset of equatorial and posterior cell nuclei staining positive (Fig. 2K). In the nontransgenic lens, endogenous CDK4 exhibited a diffuse pattern of subcellular distribution similar to that of the transgene-encoded protein, albeit at much lower levels, whereas endogenous CDK2 was localized to the cytoplasm of the lens fiber cells only (data not shown). Despite a clear increase in the level of transgene-encoded CDK4 and CDK2 above endogenous levels, these transgenic lenses failed to show BrdU incorporation in the lens fiber cells, indicating that a high level of CDK4 or CDK2 expression is not sufficient to promote S-phase entry (Fig. 2H and L). Unexpectedly, despite a lack of ectopic proliferation, CDK4 transgenic lines exhibited lens fiber cell apoptosis (Fig. 3F), indicating that inappropriate proliferation is not a requirement for activation of an apoptotic response. This response appears to be specific to CDK4, since apoptosis was not detected in the αA-CDK2 transgenic lines even under circumstances where CDK2 was directed to the nucleus (see below).
CDK-induced nuclear translocation of cyclins D1, D2, and E promotes S-phase entry with D1/CDK4 and D2/CDK4 but not E/CDK2.
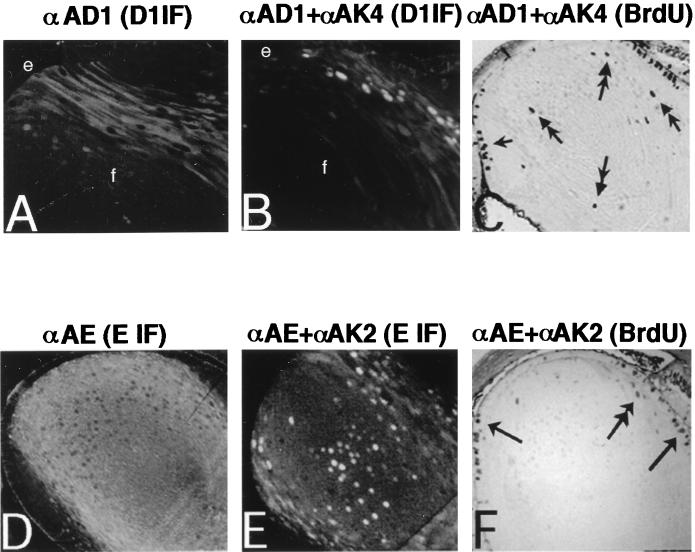
The failure of transgenic cyclins D1, D2, and E to induce S-phase entry could relate, in part, to low nuclear cyclin levels that are insufficient to counter suppression by CDK inhibitors or to low partner CDK levels (see below). To address the latter, each cyclin was coexpressed along with its partner CDK through the production of double transgenics by crossing mice carrying single transgenes. Western blot analysis of lenses from transgenic mice hemizygous for αA-cyclin D1 line 23 and αA-cdk4 line 14 transgenes or αA-cyclin E line 40 and αA-cdk2 line 31 transgenes showed no significant difference in the levels of transgenic cyclin proteins compared to the respective single transgenic lines (Fig. 1). Upon quantification, these levels were 46 and 44 ng per 50 μg of protein for αA-cyclin D1 line 23 and the hemizygous D1/CDK4, respectively. In the case of αA-cyclin E line 40 and the hemizygous E/CDK2, the levels were 44 and 46 ng of cyclin E per 50 μg of total protein extract, respectively. Strikingly, lenses derived from mice hemizygous for both αA-D1 and αA-CDK4 transgenes showed a significant shift in D1 levels from the cytoplasm to nucleus in many lens fiber cells (Fig. 4A and B). To monitor the actual increase in nuclear D1 levels, the endogenous cyclin D1 levels present in the sensory retina nuclei served as an internal control (not shown). Most significantly, the increase in nuclear cyclin D1 levels was associated with S-phase entry as evidenced by the presence of BrdU-positive nuclei (Fig. 4C; Table 1). Similar results were obtained with the combination D2/CDK4 (not shown). In sharp contrast, despite an equally marked cytoplasmic-to-nuclear shift in the E/CDK2 double transgenics (Fig. 4D and E), only a few fiber nuclei stained weakly positive for BrdU incorporation. Moreover, these BrdU-positive nuclei were unusual in that they were all located in very close physical proximity to the major proliferative zone of the anterior epithelial layer (Fig. 4F; see also Discussion).
FIG. 4.
Functional interactions among cyclins D1 or E and their respective catalytic subunits, CDK4 or CDK2, and their impact on growth in E16.5 lens sections. (A) Same as panel B in Fig. 2 (αA-cyclin D1 line 23 stained with anti-cyclin D1 antibody). (B and C) Lens derived from a transgenic mouse hemizygous for αA-cyclin D1 line 23 and αA-cdk4 line 14 transgenes stained with anti-cyclin D1 antibody (B) and its pattern of BrdU incorporation (C). Abbreviations: e, anterior epithelial layer; f, lens fiber cells. (D) Same as panel I in Fig. 2 (αA-cyclin E line 40 stained with anti-cyclin E antibody). (E and F) Lens derived from a transgenic mouse hemizygous for αA-cyclin E line 40 and αA-cdk2 line 31 transgenes stained with anti-cyclin E antibody (E) and its pattern of BrdU incorporation (F). Single arrows point to appropriate S-phase activity in the anterior epithelial layer, while double-headed arrows point to ectopic cycling in the lens fiber compartment.
Functional cooperation between D1/CDK4 and E/CDK2.
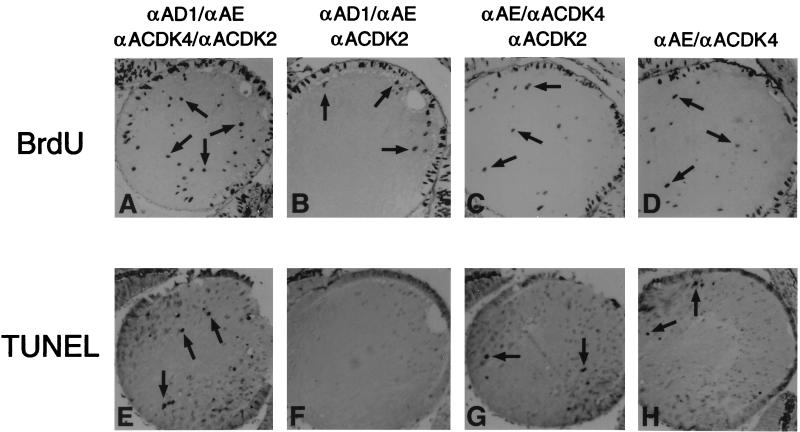
Cell culture experiments have shown that cyclins D1 and E act synergistically to decrease the duration of G1 in rodent fibroblasts when both are co-overexpressed (49). In order to assess whether functional cooperation exists in vivo, the degrees of proliferation and apoptosis in lenses expressing various combinations of transgene-encoded cyclin D1, cyclin E, CDK4 and CDK2 were compared. Coexpression of all four transgenes (D1/CDK4/E/CDK2) promoted the highest degree of cellular proliferation (Fig. 5A and Table 1). Most significant was the fact that the D1/CDK4/E/CDK2 proliferative response exceeded that obtained when one or more of these four transgenes was not present (Table 1). For example, transgenic lenses expressing E/CDK4/CDK2 showed an average of 12 ± 1.6 positive nuclei per lens section versus 23 ± 3.2 for all four transgenes (P < 0.0001) (Fig. 5C and Table 1). Such a result underscores the importance of ectopic D1 but also suggests that low levels of endogenous cyclin D1 (present in the equatorial region) may be sufficient to activate CDK4 in these transgenic lenses. When all of the various transgenic combinations are compared, CDK4 also appears to play a very important role, since, in its absence, D1/E transgenic lenses show no S-phase entry (data not shown), and D1/E/CDK2 transgenic lenses had only 3.4 ± 0.7 BrdU-positive nuclei versus 23 ± 3.2 for all four transgenes (P < 0.0001) (Fig. 5B and Table 1). On the surface, this result suggests that cyclin D1 does not play a key role. However, it is worth noting that all of the BrdU-positive nuclei in the E/CDK2/CDK4 lens fiber region are again located in very close physical proximity to the major proliferative zone of the anterior epithelial layer, and we speculate that the anterior position of these BrdU-positive nuclei could be due to the presence of diffusible growth factors that normally serve to stimulate proliferation in the anterior epithelial compartment (Fig. 5B). Furthermore, CDK4/E compound transgenic lenses also possessed an average of 6.1 ± 1.7 positive nuclei/lens section (Fig. 5D and Table 1). This degree of S-phase entry likely reflects cooperative interactions with low endogenous levels of D1 and CDK2. In fact, some nuclear cyclin D1 expression was detected in E/CDK2/CDK4 and CDK4/E lenses in the region where the BrdU incorporation occurred (data not shown).
FIG. 5.
Functional cooperation between D1/CDK4 and E/CDK2 in the developing lens. (A to D) BrdU incorporation pattern in lenses derived from transgenic mouse αA-cyclin D1 line 23, αA-cdk4 line 14, αA-cyclin E line 40, and αA-cdk2 line 31 (A); αA-cyclin D1 line 23, αA-cyclin E line 40, and αA-cdk2 line 31 (B); αA-cdk4 line 14, αA-cyclin E line 40, and αA-cdk2 line 31 (C); and αA-cdk4 line 14 and αA-cyclin E line 40 (D). (E to H) Results of TUNEL assay in lenses derived from transgenic mouse αA-cyclin D1 line 23, αA-cdk4 line 14, αA-cyclin E line 40, and αA-cdk2 line 31 (E); αA-cyclin D1 line 23, αA-cyclin E line 40, and αA-cdk2 line 31 (F); αA-cdk4 line 14, αA-cyclin E line 40, and αA-cdk2 line 31 (G); and αA-cdk4 line 14 and αA-cyclin E line 40 (H). Arrows point to nuclei testing positive by either BrdU incorporation or TUNEL assay in the lens fiber compartment.
The compound transgenic lenses were also assayed for apoptosis. These studies revealed that ectopic CDK4 expression appears to be essential for the induction of apoptosis in proliferating lens fiber cells (Table 1). As mentioned above, CDK4 can induce apoptosis in the absence of S-phase entry (Fig. 3F). Strikingly, we observed the converse as well. In particular, despite the presence of BrdU-positive nuclei in the D1/E/CDK2 lenses, no apoptotic nuclei were detected in many sections examined by TUNEL assay (Fig. 5F), a finding that is consistent with the notion that apoptosis is not an obligate consequence of ectopic proliferation in the lens.
Role of the p57KIP2 inhibitor in early lens formation and its functional relationship to the G1 cyclins in the fully formed lens.
We have demonstrated that p57KIP2 levels are dramatically up-regulated in the equatorial region of the E14.5 lens, a region where epithelial cells undergo cell cycle arrest and differentiate into secondary lens fiber cells (62). Through gene targeting, we also established that high p57KIP2 levels facilitate secondary lens fiber cell growth arrest since loss of p57KIP2 function resulted in inappropriate S-phase entry (62). Here, we investigated two additional aspects of p57KIP2 function: (i) its role in regulating lens cell growth arrest and initiation of primary lens fiber cell differentiation in early lens development and (ii) its functional relationship to G1 cyclin/CDK activity in the fully formed lens.
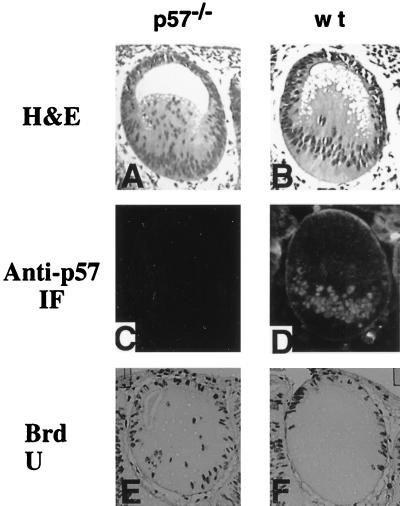
Anti-p57KIP2 antibodies detected strong nuclear staining in extending primary lens fiber cells and a complete absence of staining in the future anterior lens epithelial cells (Fig. 6D). When age-matched p57KIP2-deficient lenses were examined, the complete absence of p57KIP2 signal was associated with many BrdU-positive nuclei and retarded primary lens fiber cell extension (Fig. 6A, C, and E). These results establish that p57KIP2 plays an essential role in the key developmental transition when primary lens fiber cells arrest growth and initiate differentiation.
FIG. 6.
Impaired lens fiber cells elongation in early development in the p57KIP2 null mouse. Hematoxylin and eosin (H&E) staining of E12.5 lens sections of a p57KIP2 null mouse (A) and of a wild-type mouse (B). In situ IF analysis of p57KIP2 protein expression in the embryonic (E12.5) lenses of a p57KIP2 null mouse (C) and of a wild-type mouse (D). BrdU incorporation pattern in lenses of a p57KIP2 null mouse (E) and of a wild-type mouse (F).
By E13.5, the p57KIP2-deficient lens attains a grossly normal lens structure and organization. However, a high rate of proliferation persists in the equatorial region, and considerably less proliferation is observed in the more central lens fiber cells (Fig. 7). This regional pattern of proliferation matches well with cyclin D1 expression in these regions (17) (Fig. 2), suggesting that the high levels of p57KIP2 may serve to suppress the actions of cyclin D1 in the lens equatorial cells in vivo. To investigate this possible functional interrelationship further, we examined the impact of p57KIP2 deficiency on the growth and apoptosis of lens fiber cells expressing one of the αA-driven D-type cyclins or cyclin E. We carried out these studies at E13.5 to enable comparison with the pRb−/− lenses, since pRb−/− embryos die by E14.5. Regardless, similar results for the cyclins in the presence or absence of p57KIP2 were obtained with E16.5 lenses (data not shown). In the p57KIP2-deficient background, each of the D-type cyclins showed a significant increase in the number of BrdU-positive lens fiber nuclei both at E13.5 (Table 2) and E16.5 (data not shown); as above, cyclin D3 appeared to be more potent than D1 in the absence of coexpressed CDK4 (25.9 ± 1.1 BrdU-positive nuclei versus 10.5 ± 1.3, respectively [P < 0.0001]). The combination of p57KIP2 deficiency and forced cyclin D3 expression yielded a proliferative profile that was equivalent to that of the pRb-deficient lens (25.9 ± 1.1 BrdU-positive nuclei versus 25.5 ± 1.3, respectively [P = 0.341]) (Fig. 7H and I; Table 2). Similar levels of apoptosis were also observed between the two groups (9.1 ± 0.7 nuclei versus 9.3 ± 0.7; Table 2). In contrast, overexpression of cyclin E in the p57KIP2-deficient lens yielded a phenotype that was similar to p57KIP2 deficiency alone (2.5 ± 0.5 nuclei versus 2.0 ± 0.6, respectively [P = 0.4]) (Fig. 7K). Together, these findings reinforce the biological differences observed above for the D-type versus E cyclins and further suggest that, in the normal lens, limiting amounts of the D-type cyclins combined with abundant levels of p57KIP2 are among the major determinants controlling G1 exit in this pRb-dependent cell type.
TABLE 2.
Average number of positive nuclei per lens ± standard deviation in E13.5 transgenic, pRb−, and/or p57KIP2-deficient embryos
| Transgene | No. of BrdU-positive nuclei | No. of TUNEL-positive nuclei |
|---|---|---|
| p57−/− | 2.5 ± 0.5 | 3.9 ± 0.7 |
| D3/p57−/− | 25.9 ± 1.1 | 9.1 ± 0.7 |
| pRb−/− | 25.5 ± 1.3 | 9.3 ± 0.7 |
| D1/p57−/− | 10.5 ± 1.3 | 4.2 ± 0.6 |
| E/p57−/− | 2.0 ± 0.6 | 4.0 ± 0.7 |
DISCUSSION
Functional cooperation between cyclin D- and E-dependent kinases in vivo.
This study provides a comprehensive in vivo analysis of the growth, differentiation, and apoptotic properties of each of the G1 cyclins, their CDKs, and p57KIP2 inhibitor in a cell type whose G1/S transition is dependent upon pRb. Through the production and characterization of compound transgenic/knockout mice, this study also provides insight into the functional interrelationships among these regulatory molecules. Ectopic expression of the D-type cyclins, but not cyclin E, was capable of efficiently promoting G1 exit in normally postmitotic lens fiber cells. Because we compared cyclin D1 and cyclin E transgenic lines with very similar amounts of ectopic protein (46 and 45 ng per 50 μg of total protein extract, respectively), it is unlikely that the differential phenotype was merely due to levels of expression rather than to distinct biological characteristics of these two cyclins in this setting. Among the three D-type cyclins, cyclin D3 promoted S-phase entry by itself while cyclins D1 and D2 required coexpression of ectopic CDK4. In this case, there was a significant difference in the overall levels of transgenic proteins between cyclin D1 and D3 (46 to 76 ng versus 400 to 900 ng per 50 μg of total protein extract, respectively). However, based on IF studies of these transgenic lenses, we believe that the distinction between D1 and D2 versus D3 relates more to the poor nuclear localization of the former two in the absence of CDK4. This impression is based on the observation that once adequate nuclear levels of cyclin D1 or D2 are achieved through CDK4 coexpression, the rate of proliferation appears similar to that achieved with ectopic D3 expression. In addition, upon elimination of p57KIP2, cyclin D1 expression is capable of promoting entry into S phase. However, it remains possible that cyclin D3 may have distinct functional properties as well (e.g., the ability to more efficiently activate CDK2). This issue is difficult to address more rigorously on the biochemical level due to the small size of the embryonic mouse lens.
Given the pRb-dependent nature of lens fiber cell growth (36), cyclin D/CDK4 and E/CDK2 complexes do not appear to be functionally equivalent in neutralizing pRb’s inhibitory function in this setting, suggesting either that pRb is not a critical substrate for the cyclin E/CDK2 holoenzyme or, more likely, that this complex alone cannot functionally inactivate pRb. The finding that D/CDK4 and E/CDK2 cooperate strongly to promote S-phase entry is consistent with the model that proposes the involvement of these kinases in two independent limiting steps that control transit through the G1/S boundary. Other studies have established that cyclin E is still required for S-phase entry in pRb-negative cells (41) and that cyclin E likely possesses E2F-independent functions, suggested by the capacity of this cyclin to rescue E2F activity by an alternative route in the presence of p16 and active pRb (2, 30). Studies in Drosophila have shown that the dependence of cyclin E on E2F is tissue or stage specific, and this finding may explain the inability of cyclin E to promote S-phase entry in the postmitotic lens (11, 12). This line of evidence implies that E/CDK2 phosphorylates other key substrates, although it may well contribute to pRb inactivation as well (53, 61). Indeed, the actions of the cyclin D- and E-dependent kinases on pRb may be sequential in nature, with the cyclin E/CDK2 complex acting to further abolish the function of that subset of pRb molecules that have previously been phosphorylated at preferred sites by D/CDK4 kinases (10, 23). The findings of our lens study parallel those concerning the Drosophila eye, suggesting conservation of these principles over a large phylogenetic distance. During Drosophila eye development, cells progressing through particular cell cycle intervals occupy precise anatomical positions in relation to the morphogenetic furrow. In this setting, increased cyclin D expression is evident in the G1 compartment, while cyclin E takes place as cells enter the S-phase zone (16).
Subcellular distribution of the G1 cyclins in relation to CDK coexpression.
An unanticipated finding in the lens studies was the dramatic shift in subcellular distribution for D1, D2, or E upon coexpression of its partner CDK. This observed shift indicates that alterations in the levels of the G1 CDK subunits can influence the subcellular localization of their cyclin partners in vivo. The mechanisms underlying these effects remain unclear, but coexpression of cyclins and CDKs is not likely to be sufficient for their nuclear localization as evidenced by the finding that cyclin D1 was excluded from a subset of lens fiber nuclei despite high levels of ectopic CDK4 expression in these cells in the D1/CDK4 lenses. Previous reports have described cell-cycle-dependent changes in the subcellular localization of cyclin D1 in proliferating fibroblasts, with D1 progressively accumulating in the nuclei during G1 phase and excluded during S phase (4, 28), even though high levels of enzyme activity persist (31, 34). Cyclin E is also excluded from the nucleus in late S phase unless it is overexpressed (41), and this subcellular compartmentalization of the E/CDK2 complex appears to be an important determinant of its kinase activity (7). Hence, the nuclear targeting of cyclins D1 and D2 or cyclin E must involve regulatory mechanisms that extend beyond abundant CDK4 or CDK2 levels.
CDK4-induced apoptosis does not require S-phase progression.
The results presented here show that overexpression of CDK4 can induce apoptosis in the absence of proliferation. Moreover, despite the presence of BrdU-positive nuclei in the D1/E/CDK2 lenses, no apoptotic nuclei were detected in many lenses examined. Together, these two results indicate that ectopic CDK4 plays a critical proapoptotic role. The activation of apoptosis under G0/G1 block is not without precedent and has been reported following Wilms tumor suppressor gene (WT1) overexpression in myeloblastic leukemia M1 cells, calcium ionophore treatment in TSU-pr1 androgen-independent prostatic cancer cells, and induction of differentiation in HL-60 cells (18, 38, 58). To place into context the activation of apoptosis by ectopic CDK4, we need to consider the status of endogenous CDKs in the embryonic lens; among these kinases, CDK5 might be highly relevant. First, CDK5 and its activating subunit p35 are highly expressed in lens fiber cells of rats at E16.5, and a shift to nuclear staining is observed immediately prior to nuclear degradation (19). Moreover, immunoprecipitates of CDK5 from lens fibers showed kinase activity in vitro with histone H1 as a substrate, suggesting a role of p35/CDK5 in differentiating lens fiber cells in addition to its reported function in neurons (19). Second, both CDK4 and p35/CDK5 have each been associated with apoptosis previously; a dominant negative of CDK4 promotes survival of nerve growth factor-deprived sympathetic neurons (44), and double labeling of p35/CDK5 and confocal microscopy detected this kinase complex in both adult and embryonic dying cells (1). In the lens system, one is tempted to speculate that the ectopic expression of CDK4 titrates away endogenous cyclin D1 which normally binds to and inhibits the p35/CDK5 complex, resulting in an indirect activation of CDK5 triggering apoptosis with no effect in proliferation.
Functional relationship between the G1 cyclins and p57KIP2 in vivo.
The loss of pRb function results in a high rate of ectopic mitotic activity and apoptosis in the lens fiber compartment of the developing mouse (36). We have reported that loss of p57KIP2 also leads to lens cell fiber proliferation and apoptosis, albeit to a degree that is far less pronounced than that observed in the pRb null lens (62). The more attenuated phenotype of the p57KIP2 null lens is very similar to that of transgenic lenses overexpressing cyclin D described here. Nonetheless, in the p57KIP2 null lens, a very high rate of proliferation (equivalent to the pRb null condition) is observed in the equatorial region where endogenous cyclin D1 and CDK4 normally persist (62). Here, D-type cyclin overexpression in a p57KIP2 null background resulted in a degree of abnormal proliferation and apoptosis that was equivalent to that brought about by the loss of pRb. In contrast, overexpression of cyclin E in this setting had no effect on either proliferation or apoptosis. Together, these observations indicate that a multilevel regulatory circuit exists to govern the pRb-regulated G1/S transition in lens fiber cells in vivo and that a balance between proliferation and cell cycle exit is achieved primarily through the opposing forces of activating D-type cyclin kinases and inhibitory p57KIP2.
Our findings suggest that in the anterior epithelial layer of the lens, high-cyclin D-CDK4 and only sporadic p57KIP2 expression account for the normally high rate of proliferation of undifferentiated epithelial cells. As cells reach the equatorial region of the lens fiber compartment, despite continued D1, D2, and CDK4 expression, the abundant nuclear levels of p57KIP2 correlate with proliferative arrest and the onset of lens fiber differentiation. Throughout the latter process, CDK4 expression continues but is accompanied by diminished cyclin D1 and persistent p57KIP2 levels. Together, these results provide in vivo support for the view that cyclins D1 and D2, CDK4, and p57KIP2 are the critical components regulating pRb-mediated growth arrest in the developing lens.
ACKNOWLEDGMENTS
We thank Nicole Schreiber-Agus and Andrew Koff for critical reading of the manuscript. We also thank Sam Zigler and Joe Horwitz for antibodies against MIP26 and the crystallins; Paul Overbeek for the CPV-1 cassette; and the Analytical Imaging Facility at Albert Einstein College of Medicine.
This work was supported by the following NIH grants: RO1HD28317, RO1EY09300, RO1EY11267, and the Cancer Center Core grant T2P30CA13330; the DAMD Breast Cancer Grant (to S.J.E.); NIH training grant 2T32AG00194 (to E.G.L.); and NIH training grant T32GM07288 (to N.J.L.). R.A.D. is a recipient of the Irma T. Hirschl Award.
REFERENCES
- 1.Ahuja H S, Zhu Y, Zakeri Z. Association of cyclin-dependent kinase 5 and its activator p35 with apoptotic cell death. Dev Genet. 1997;21:258–267. doi: 10.1002/(SICI)1520-6408(1997)21:4<258::AID-DVG3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 2.Alevizopoulos K, Vlach J, Hennecke S, Amati B. Cyclin E and c-Myc promote cell proliferation in the presence of p16INK4a and of hypophosphorylated retinoblastoma family proteins. EMBO J. 1997;16:5322–5333. doi: 10.1093/emboj/16.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assoian R K. Control of the G1 phase cyclin-dependent kinases by mitogenic growth factors and the extracellular matrix. Cytokine Growth Factor Rev. 1997;8:165–170. doi: 10.1016/s1359-6101(97)00011-7. [DOI] [PubMed] [Google Scholar]
- 4.Baldin V, Lukas J, Marcote M J, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 5.Bodrug S E, Warner B J, Bath M L, Lindeman G J, Harris A W, Adams J M. Cyclin D1 transgene impedes lymphocyte maturation and collaborates in lymphomagenesis with the myc gene. EMBO J. 1994;13:2124–2130. doi: 10.1002/j.1460-2075.1994.tb06488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bortner D M, Rosenberg M P. Induction of mammary gland hyperplasia and carcinomas in transgenic mice expressing human cyclin E. Mol Cell Biol. 1997;17:453–459. doi: 10.1128/mcb.17.1.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bresnahan W A, Boldogh I, Ma T, Albrecht T, Thompson E A. Cyclin E/dk2 activity is controlled by different mechanisms in the G0 and G1 phases of the cell cycle. Cell Growth Differ. 1996;7:1283–1290. [PubMed] [Google Scholar]
- 8.Broekhuyse R M, Kuhlmann E D, Stols A L. Lens membranes. II. Isolation and characterization of the main intrinsic polypeptide (MIP) of bovine lens fiber membranes. Exp Eye Res. 1976;23:365–371. doi: 10.1016/0014-4835(76)90135-4. [DOI] [PubMed] [Google Scholar]
- 9.Chepelinsky A B, King C R, Zelenka P S, Piatigorsky J. Lens-specific expression of the chloramphenicol acetyltransferase gene promoted by 5′ flanking sequences of the murine alpha A-crystallin gene in explanted chicken lens epithelia. Proc Natl Acad Sci USA. 1985;82:2334–2338. doi: 10.1073/pnas.82.8.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connell-Crowley L, Harper J W, Goodrich D W. Cyclin D1/CDK4 regulates retinoblastoma protein-mediated cell cycle arrest by site-specific phosphorylation. Mol Biol Cell. 1997;8:287–301. doi: 10.1091/mbc.8.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duronio R J, O’Farrell P H. Developmental control of the G1 to S transition in Drosophila: cyclin E is a limiting downstream target of E2F. Genes Dev. 1995;9:1456–1468. doi: 10.1101/gad.9.12.1456. [DOI] [PubMed] [Google Scholar]
- 12.Duronio R J, Brook A, Dyson N, O’Farrell P H. E2F-induced S phase requires cyclin E. Genes Dev. 1996;10:2513–2525. doi: 10.1101/gad.10.19.2505. [DOI] [PubMed] [Google Scholar]
- 13.Elledge S J, Harper J W. CDK inhibitors: on the threshold of checkpoints and development. Curr Opin Cell Biol. 1994;6:847–852. doi: 10.1016/0955-0674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 14.Ewen M E, Sluss H K, Sherr C J, Matsushime H, Kato J, Livingston D M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993;73:487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- 15.Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 1995;9:2364–2372. doi: 10.1101/gad.9.19.2364. [DOI] [PubMed] [Google Scholar]
- 16.Finley R L, Jr, Thomas B J, Zipursky S L, Brent R. Isolation of Drosophila cyclin D, a protein expressed in the morphogenetic furrow before entry into S phase. Proc Natl Acad Sci USA. 1996;93:3011–3015. doi: 10.1073/pnas.93.7.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fromm L, Overbeek P A. Regulation of cyclin and cyclin-dependent kinase gene expression during lens differentiation requires the retinoblastoma protein. Oncogene. 1996;12:69–75. [PubMed] [Google Scholar]
- 18.Furuya Y, Ohta S, Yasuda K, Ito H. Enhanced expression of cyclin-dependent kinase apoinhibitor in apoptosis of androgen-independent prostatic cancer cell line induced by calcium ionophore. Anticancer Res. 1997;17:2391–2394. [PubMed] [Google Scholar]
- 19.Gao C Y, Zakeri Z, Zhu Y, He H, Zelenka P S. Expression of Cdk5, p35, and Cdk5-associated kinase activity in the developing rat lens. Dev Genet. 1997;20:267–275. doi: 10.1002/(SICI)1520-6408(1997)20:3<267::AID-DVG9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Gavrieli Y, Sherman Y, Ben-Sasson S A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorman C M, Moffat L F, Howard B H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982;2:1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guan K L, Jenkins C W, Li Y, Nichols M A, Wu X, O’Keefe C L, Matera A G, Xiong Y. Growth suppression by p18, p16INK4a/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor correlates with wild-type pRb function. Genes Dev. 1994;8:2939–2952. doi: 10.1101/gad.8.24.2939. [DOI] [PubMed] [Google Scholar]
- 23.Hatakeyama M, Brill J A, Fink G R, Weinberg R A. Collaboration of G1 cyclins in the functional inactivation of the retinoblastoma protein. Genes Dev. 1994;8:1759–1771. doi: 10.1101/gad.8.15.1759. [DOI] [PubMed] [Google Scholar]
- 24.Kato J, Sherr C J. Inhibition of granulocyte differentiation by G1 cyclins D2 and D3 but not D1. Proc Natl Acad Sci USA. 1993;90:11513–11517. doi: 10.1073/pnas.90.24.11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato J, Matsuoka M, Polyak K, Massague J, Sherr C J. Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase-4 activation. Cell. 1994;79:487–496. doi: 10.1016/0092-8674(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 26.Koh J, Enders G H, Dynlacht B D, Harlow E. Tumor-derived p16 alleles encoding proteins defective in cell-cycle inhibition. Nature. 1995;375:506–510. doi: 10.1038/375506a0. [DOI] [PubMed] [Google Scholar]
- 27.Lovec H, Grzeschiczek A, Kowalski M B, Moroy T. Cyclin D1/bcl-1 cooperates with myc genes in the generation of B-cell lymphoma in transgenic mice. EMBO J. 1994;13:3487–3495. doi: 10.1002/j.1460-2075.1994.tb06655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lukas J, Pagano M, Staskova Z, Draetta G, Bartek J. Cyclin D1 protein oscillates and is essential for cell cycle progression in human tumour cell lines. Oncogene. 1994;9:707–718. [PubMed] [Google Scholar]
- 29.Lukas J, Bartkova J, Rohde M, Strauss M, Bartek J. Cyclin D1 is dispensable for G1 control in retinoblastoma gene-deficient cells independently of cdk4 activity. Mol Cell Biol. 1995;15:2600–2611. doi: 10.1128/mcb.15.5.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lukas J, Herzinger T, Hansen K, Moroni M C, Resnitzky D, Helin K, Reed S I, Bartek J. Cyclin E-induced S phase without activation of the pRb/E2F pathway. Genes Dev. 1997;11:1479–1492. doi: 10.1101/gad.11.11.1479. [DOI] [PubMed] [Google Scholar]
- 31.Matsushime H, Quelle D E, Shurtleff S A, Shibuya M, Sherr C J, Kato J. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McAvoy J W. Beta- and gamma-crystallin synthesis in rat lens epithelium explanted with neural retina. Differentiation. 1980;17:85–91. doi: 10.1111/j.1432-0436.1980.tb01084.x. [DOI] [PubMed] [Google Scholar]
- 33.Medema R H, Herrera R E, Lam F, Weinberg R A. Growth suppression by p16ink4a requires functional retinoblastoma protein. Proc Natl Acad Sci USA. 1995;92:6289–6293. doi: 10.1073/pnas.92.14.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyerson M, Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol. 1994;14:2077–2086. doi: 10.1128/mcb.14.3.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller M W, Nowakowski R S. Use of bromodeoxyuridine immunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Res. 1988;457:44–52. doi: 10.1016/0006-8993(88)90055-8. [DOI] [PubMed] [Google Scholar]
- 36.Morgenbesser S D, Williams B O, Jacks T, DePinho R A. p53-dependent apoptosis produced by Rb-deficiency in the developing mouse lens. Nature. 1994;371:72–74. doi: 10.1038/371072a0. [DOI] [PubMed] [Google Scholar]
- 37.Morgenbesser S D, Schreiber-Agus N, Bidder M, Mahon K A, Overbeek P A, Horner J, DePinho R A. Contrasting roles for c-Myc and L-Myc in the regulation of cellular growth and differentiation in vivo. EMBO J. 1995;14:743–756. doi: 10.1002/j.1460-2075.1995.tb07053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murata Y, Kudoh T, Sugiyama H, Toyoshima K, Akiyama T. The Wilms tumor suppressor gene WT1 induces G1 arrest and apoptosis in myeloblastic leukemia M1 cells. FEBS Lett. 1997;409:41–45. doi: 10.1016/s0014-5793(97)00477-8. [DOI] [PubMed] [Google Scholar]
- 39.Nourse J, Firpo E, Flanagan W M, Coats S, Polyak K, Lee M H, Massague J, Crabtree G, Roberts J M. Interleukin-2-mediated elimination of p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–573. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- 40.Ohtsubo M, Roberts J M. Cyclin-dependent regulation of G1 in mammalian fibroblasts. Science. 1993;259:1908–1912. doi: 10.1126/science.8384376. [DOI] [PubMed] [Google Scholar]
- 41.Ohtsubo M, Theodoras A M, Schumacher J, Roberts J M, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Overbeek P A, Chepelinsky A B, Khillan J S, Piatigorsky J, Westphal H. Lens-specific expression and developmental regulation of the bacterial chloramphenicol acetyltransferase gene driven by the murine alpha A-crystallin promoter in transgenic mice. Proc Natl Acad Sci USA. 1985;82:7815–7819. doi: 10.1073/pnas.82.23.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pagano M, Tam S W, Theodoras A M, Beer-Romano P, DalSal G, Chau V, Yew P R, Draetta G F, Rolfe M. Role of the ubiquitin-proteosome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 44.Park D S, Levine B, Ferrari G, Greene L A. Cyclin dependent kinase inhibitors and dominant negative cyclin dependent kinase 4 and 6 promote survival of NGF-deprived sympathetic neurons. J Neurosci. 1997;17:8975–8983. doi: 10.1523/JNEUROSCI.17-23-08975.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19:134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- 46.Quelle D E, Ashmun R A, Shurtleff S A, Kato J, Bar-Sagi D, Roussel M F, Sherr C J. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- 47.Rao S S, Kohtz D S. Positive and negative regulation of D-type cyclin expression in skeletal myoblasts by basic fibroblast growth factor and transforming growth factor beta. A role for cyclin D1 in control of myoblast differentiation. J Biol Chem. 1995;270:4093–4100. doi: 10.1074/jbc.270.8.4093. [DOI] [PubMed] [Google Scholar]
- 48.Resnitzky D, Reed S I. Different roles for cyclins D1 and E in regulation of the G1-to-S transition. Mol Cell Biol. 1995;15:3463–3469. doi: 10.1128/mcb.15.7.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Resnitzky D, Gossen M, Bujard H, Reed S I. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robles A I, Larcher F, Whalin R B, Murillas R, Richie E, Gimenez-Conti I B, Jorcano J L, Conti C J. Expression of cyclin D1 in epithelial tissues of transgenic mice results in epidermal hyperproliferation and severe thymic hyperplasia. Proc Natl Acad Sci USA. 1996;93:7634–7638. doi: 10.1073/pnas.93.15.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 52.Sherr C J. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 53.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 54.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 55.Sicinski P, Donaher J L, Parker S B, Li T, Fazeli A, Gardner H, Haslam S Z, Bronson R T, Elledge S J, Weinberg R A. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82:621–630. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 56.Sicinski P, Donaher J L, Geng Y, Parker S B, Gardner H, Park M Y, Robker R L, Richards J S, McGinnis L K, Biggers J D, Eppig J J, Bronson R T, Elledge S J, Weinberg R A. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature. 1996;384:470–474. doi: 10.1038/384470a0. [DOI] [PubMed] [Google Scholar]
- 57.Takemoto L, Kuck J, Kuck K. Changes in the major intrinsic polypeptide (MIP26K) during opacification of the Emory mouse lens. Exp Eye Res. 1988;47:329–336. doi: 10.1016/0014-4835(88)90015-2. [DOI] [PubMed] [Google Scholar]
- 58.Terui Y, Furukawa Y, Kikuchi J, Saito M. Apoptosis during HL-60 cell differentiation is closely related to a G0/G1 cell cycle arrest. J Cell Physiol. 1995;164:74–84. doi: 10.1002/jcp.1041640110. [DOI] [PubMed] [Google Scholar]
- 59.Vallance S J, Lee H M, Roussel M F, Shurtleff S A, Kato J, Strom D K, Sherr C J. Monoclonal antibodies to mammalian D-type G1 cyclins. Hybridoma. 1994;13:37–44. doi: 10.1089/hyb.1994.13.37. [DOI] [PubMed] [Google Scholar]
- 60.Wang T C, Cardiff R D, Zukerberg L, Lees E, Arnold A, Schmidt E V. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669–671. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- 61.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 62.Zhang P, Liegeois N J, Wong C, Finegold M, Hou H, Thompson J C, Silverman A, Harper J W, DePinho R A, Elledge S J. Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith-Wiedemenn syndrome. Nature. 1997;387:151–158. doi: 10.1038/387151a0. [DOI] [PubMed] [Google Scholar]