Abstract
Simple Summary
Electrical activity in the brain dynamically changes throughout the day. Abnormalities in brain activity have been associated with various brain disorders, including Alzheimer’s disease (AD). While brain disorders stem from complex pathological processes, resulting in abnormalities in neural activity and cognitive deficits, recent studies have demonstrated that controlling brain activity can modify disease pathologies as well as cognitive functions. In particular, studies in mouse models of AD have provided promising results regarding the amelioration of AD pathology by invasive and non-invasive brain stimulations. In this review article, by focusing on AD, we provide an overview of this emerging field. We summarise how brain activity changes in humans and mouse models, and how different artificial manipulations of brain activity can modify AD pathology. Although further investigations are essential, this research direction will provide insight into non-pharmacological intervention strategies for dementia.
Abstract
Brain state varies from moment to moment. While brain state can be defined by ongoing neuronal population activity, such as neuronal oscillations, this is tightly coupled with certain behavioural or vigilant states. In recent decades, abnormalities in brain state have been recognised as biomarkers of various brain diseases and disorders. Intriguingly, accumulating evidence also demonstrates mutual interactions between brain states and disease pathologies: while abnormalities in brain state arise during disease progression, manipulations of brain state can modify disease pathology, suggesting a therapeutic potential. In this review, by focusing on Alzheimer’s disease (AD), the most common form of dementia, we provide an overview of how brain states change in AD patients and mouse models, and how controlling brain states can modify AD pathology. Specifically, we summarise the relationship between AD and changes in gamma and slow oscillations. As pathological changes in these oscillations correlate with AD pathology, manipulations of either gamma or slow oscillations can modify AD pathology in mouse models. We argue that neuromodulation approaches to target brain states are a promising non-pharmacological intervention for neurodegenerative diseases.
Keywords: dementia, Alzheimer’s disease, neuromodulation, neural oscillations, optogenetics
1. Introduction
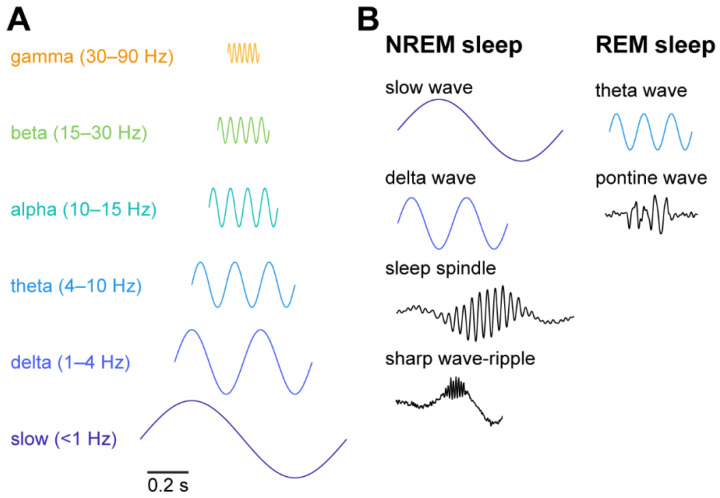
The brain is never at rest. The activity state of the brain, called the brain state, varies from moment to moment. While brain state can be defined as a collective action of the neural population at a given moment, it spans over multiple spatiotemporal scales (Figure 1) [1,2,3]. Hans Berger first described the 8–12 Hz rhythm, called alpha waves, in a human scalp electroencephalogram (EEG) recording [4]. Since then, intensive research has discovered a wide range of activity patterns or brain states (Figure 1A). For example, gamma (30–90 Hz) oscillations are a fast activity state and appear locally, compared to slower frequency oscillations. Gamma oscillations are related to various cognitive functions, such as attention, conscious perception and memory [5,6,7,8,9,10,11,12]. The sleep–wake cycle can be considered as slower state changes and consists of multiple neural events and oscillations (Figure 1). Rapid eye movement (REM) sleep is characterised by theta oscillations and ponto-geniculo-occipital (PGO) waves, whereas non-REM (NREM) sleep is characterised by slow oscillations, sleep spindles, and sharp wave-ripples [13,14,15,16,17,18,19,20,21] (Figure 1B). These sleep-related neural events or oscillations have also been implicated in various homeostatic and cognitive functions, including waste clearance [22,23] and memory consolidation [24,25,26,27,28,29].
Figure 1.
Neural oscillations and events across brain states. (A) Neural oscillations and their frequency band. (B) Characteristic neural oscillations and events during sleep states.
Given the prominence of these neural oscillations and events, it is not surprising that consistent associations can be seen between various brain disorders and abnormalities in neural oscillations or brain states [30,31,32,33,34]. For example, abnormalities in gamma oscillations have been recognised as a neurophysiological marker for various neuropsychiatric disorders and neurodegenerative diseases, such as schizophrenia [34], autism spectrum disorder (ASD) [35,36], depression [37,38], and Alzheimer’s disease (AD) [31]: more specifically, a reduction in sensory-evoked gamma power can be seen in schizophrenia and ASD patients, whereas varied changes in gamma oscillations have been reported in depressive disorders and AD patients [34,36,38,39,40,41]. Additionally, abnormalities in sleep patterns and sleep-related oscillations have been linked with depression [42], schizophrenia [43,44], addiction [45] and AD [46,47,48,49].
Although it remains to be determined how abnormalities in brain states can be causally linked to disease pathogenesis, an emerging approach, called “neuromodulation”, aims to alter neural activity to modify disease state [50]. For example, while deep brain stimulation (DBS) is an invasive approach, chronically implanting a depth electrode into the patient brain to electrically stimulate a target brain region, it can alleviate the symptoms of Parkinson’s disease [51,52] and has also been examined in treatment-resistant depression [53,54], obsessive-compulsive disorder [55,56] and AD [57,58]. In addition to invasive treatment, non-invasive neuromodulation approaches, such as transcranial magnetic stimulation (TMS), have been explored in various brain disorders, such as schizophrenia [59,60,61], depression [62], addiction [63,64], and AD [65,66,67]. Despite many clinical trials being conducted, it remains unclear how neuromodulation approaches can act on neural circuits to result in cellular and molecular responses that modify disease state. To tackle this challenge, preclinical studies in animal models could offer insight into better neuromodulation approaches. Thus, it is crucial to understand how brain state is regulated, how brain state is changed during disease pathogenesis, and how neuromodulation approaches can alter neural activity, resulting in a modification of disease state.
In this literature review, we focus on AD, the most common form of dementia. Although AD is one of the most intensively studied neurodegenerative diseases [68,69,70,71,72,73,74,75,76,77,78], intervention and treatment options remain limited. In AD, amyloid plaques and tauopathy are major pathological hallmarks, with other pathological features including inflammation and lipid metabolism [68,70,71,73,74,75,77,78,79,80]. Although molecules associated with these features are promising targets for pharmacological treatments [81,82,83,84,85], neuromodulation-based interventions are now being considered, given the multifaceted pathologies of AD.
Abnormalities in EEG patterns have been recognised since as early as the 1930s [30]. Since then, EEG abnormalities have been described in terms of the following three features [31]: (1) slower neural oscillations, (2) decreased complexity of EEG, and (3) reduced degrees of functional connectivity. Hence, these hallmarks of EEG abnormalities can be recognised as either a biomarker of or target for neuromodulation-based intervention. Indeed, accumulating evidence indicates that neuromodulation approaches have the potential to modify Alzheimer’s disease states [47,86]. In particular, targeting gamma oscillations and slow (<1 Hz) oscillations has provided encouraging results in AD mouse models [87,88,89,90,91,92]. Since these oscillations have been well characterised with respect to their induction mechanisms, gamma and slow oscillations would make good targets for neuromodulation-based treatment.
In this review, we first summarise the mechanisms of these two neural oscillations, gamma and slow, followed by a brief overview of the relationship between these oscillations and AD in both human patients and mouse models. Then, we review recent animal studies that examined the effect of invasive and non-invasive neuromodulation approaches on AD pathology. Finally, we discuss future directions in this field. Readers may also refer to other recent reviews relevant to this field [33,46,47,48,49,67,86,93].
2. Gamma Oscillations and AD
Jasper and his colleague first described gamma waves [94]. The investigation of gamma (30–90 Hz) oscillations has gained popularity following a series of studies by Freeman [95,96] and by Singer and his colleagues [9]. Gamma oscillations have been observed across many brain regions, not just in the neocortex, but also in the entorhinal cortex [97,98,99], amygdala [100], hippocampus [101,102,103], striatum [104,105], olfactory bulb [106,107], basal forebrain [108,109] and developing thalamus [110]. They have been associated with various cognitive functions, including attentional selection [8,111], working memory [12,112], perceptual binding [6,9], and memory encoding [111,113]. Abnormalities in gamma oscillations have links to various neuropsychiatric disorders [34] and neurodegenerative diseases [32]. Here, after describing the induction mechanisms of gamma oscillations, we summarise the relationship between gamma oscillations and AD in both humans and mouse models. Then, we discuss emerging therapeutic approaches based on gamma oscillations.
2.1. Mechanisms of Gamma Oscillations
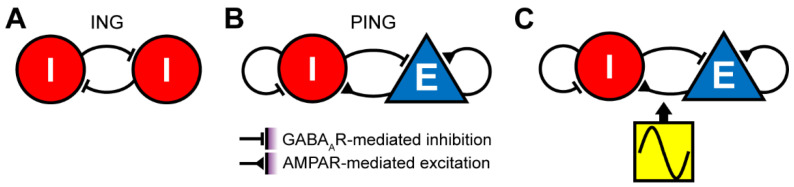
The induction mechanisms of gamma oscillations in cortical circuits have been investigated by a wide range of approaches, including computational models [5,103,114,115,116,117], brain slice experiments [118,119] and in vivo optogenetic experiments [120,121]. Computational studies have suggested several mechanisms that are potentially involved in generating gamma oscillations (Figure 2) [5,114,122]. In the interneuron gamma (ING) mechanism (Figure 2A), mutual inhibition between GABAergic neurons is sufficient to generate gamma oscillations. Two distinct regimes can be considered: in the high-firing, noise-free condition, individual GABAergic neurons elicit spikes at around 40 Hz. Mutual inhibition via GABAA receptors quickly leads to synchronous firing [103]. In the more realistic, noisy condition, individual GABAergic neurons fire sparsely and stochastically. When the inhibitory feedback is strong enough, gamma oscillations arise. Thus, gamma oscillations are an emerging property of the mutual inhibition network. In both conditions, GABAA receptor-mediated inhibition plays a role in the generation of gamma oscillations in the absence of excitatory inputs [103,117].
Figure 2.
Mechanisms of gamma oscillations. (A) Interneuron gamma (ING) mechanism. Gamma oscillations arise from mutually connected GABAergic neurons. Two regimes can be considered: in one regime, each interneuron fires rhythmically with a frequency determined by the kinetics of the GABAergic feedback (~40 Hz). In the second regime, although each interneuron sparsely and stochastically fires at an average rate below 40 Hz, recurrent inhibitory interactions lead to gamma oscillations. (B) Pyramidal-interneuron network gamma (PING) mechanism. Pyramidal cells first activate interneurons via AMPA receptors (AMPARs). This leads to recurrent inhibition via GABAA receptors (GABAARs), resulting in rhythmic firing of excitatory and inhibitory populations at the gamma range. (C) Gamma oscillations are inherited by oscillatory activity from upstream areas.
In the pyramidal-interneuron gamma (PING) mechanism (Figure 2B), the alternation between the fast excitation and delayed feedback inhibition can generate gamma oscillations [5,107,114,122,123]. The fast excitation is mediated by AMPA receptors, whereas the feedback inhibition is mediated by GABAA receptors. The third, simple mechanism is the inheritance of gamma rhythm from upstream areas (Figure 2C) [124]. In this mechanism, the downstream network can reliably and precisely respond to rhythmic inputs from their upstream. In addition to these, other mechanisms can also be taken into consideration, such as neuromodulators [125,126] and pace-making chattering cells [127].
Experimentally, optogenetic activation of cortical parvalbumin-positive (PV+) GABAergic neurons is sufficient to produce gamma oscillations, whereas αCaMKII+ neurons (pyramidal neurons) cannot entrain optogenetic stimulation at the gamma-frequency range [120,121]. Although these results apparently support the ING mechanism, the determination of a precise mechanism is complicated. Although the computational models described above predict how the depolarization of excitatory or inhibitory neurons could affect the oscillations, they do not predict how each network configuration could respond to periodic optogenetic stimulations. Rather, models incorporating physiological data suggested that these optogenetic experiments cannot conclusively distinguish between the ING and PING mechanisms, since the PING model can explain the experimental observations [114]. Thus, further studies with different stimulation protocols are required to determine the mechanisms of gamma oscillations. As gamma oscillations may have therapeutic potential for neurodegenerative diseases, it is important to investigate whether different induction mechanisms of gamma oscillations could lead to distinct molecular and cellular responses and what types of induction mechanisms have beneficial or detrimental effects on AD pathology.
2.2. Gamma Oscillations and AD in Humans
While gamma oscillations in human subjects have been assessed by either EEG or magnetoencephalography (MEG), the relationship between AD pathology and changes in gamma oscillations is inconclusive: a number of studies reported a reduction in gamma power or coherence across cortical regions in AD patients [40,128,129], whereas some reported opposite results [39,41,130,131,132]. This inconsistency may stem from various experimental parameters. For example, gamma oscillations were assessed in an eye-closed, resting state condition [40,41,131,133,134] or during sensory stimulus presentation [39,41,129,130,132]. As expected, cortical regions which showed significant effects also varied depending on conditions and studies. Additionally, while many studies compared gamma oscillations between AD patients and healthy subjects, several studies also compared AD and mild-cognitive impairment (MCI) patients [41,130,131,134]. A consistent approach, recruiting a large number of subjects, would be ideal to address this issue.
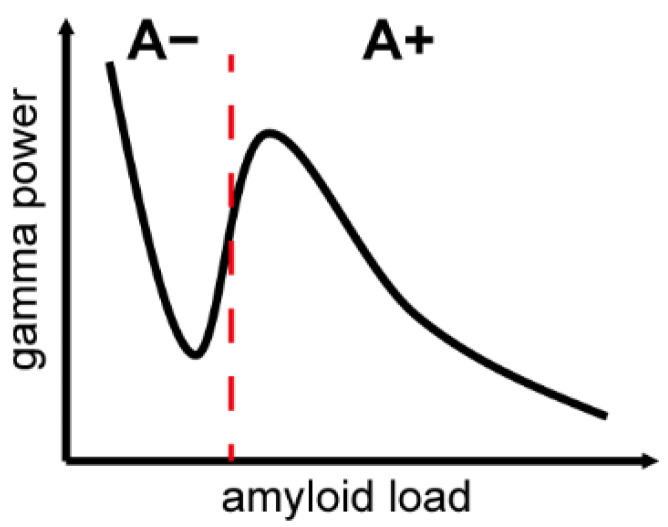
A recent study of >300 individuals provides valuable insight into changes in gamma oscillations during AD pathogenesis [135]. This study revealed the inverted U-shape relationship between amyloid depositions and gamma power (Figure 3): as the amyloid deposition reaches a supra-threshold level, gamma power increases. On the other hand, as the amyloid deposition increases further to an enhanced pathological state, gamma power decreases. These results imply a compensatory mechanism at an early phase of AD pathogenesis, which may be overwhelmed by a higher amyloid load, leading to a breakdown of neural circuits [135,136,137,138]. These results may also be reconciled with the contradictory observations mentioned above, as changes in gamma oscillations may vary depending on the stage of pathogenesis. In the future, it is important to correlate changes in gamma oscillations with AD pathology in large-scale clinical studies. In addition, it is crucial to find out whether animal models can replicate this inverted U-shape relationship to investigate the underlying mechanisms at the molecular, cellular and neural circuit levels.
Figure 3.
Inverted U-shape relationship between amyloid load and gamma power. This inverted U-shape relationship is evident in the neurodegeneration-positive group assessed by 18F-FDG PET. A+ and A−, amyloid-positive and -negative groups based on 18F-florbetapir PET, respectively. Please note that although these two groups were dichotomized with a threshold (red dotted line), amyloid load is a continuous value. Modified from Gaubert et al. (2019).
2.3. Gamma Oscillations and AD in Mouse Models
A reduction in gamma power is consistently observed in various mouse models, including APP-PS1 [139], J20 [140,141], 5xFAD [89], CRND8 [142,143], APOE4 [144,145] and tau models [146] (Table 1). Multiple brain regions have been investigated, such as the hippocampus [89,143,145], entorhinal cortex [139] and prefrontal cortex [140,141]. In the hippocampus, abnormalities in the coupling of gamma oscillations with sharp wave-ripples or theta oscillations have been consistently observed [89,142,145]. While these results imply that the overexpression of amyloid-β impairs hippocampal ensembles, amyloid precursor protein (APP) also plays a critical role in theta-gamma coupling in the hippocampus, as mice lacking APP exhibit a reduction in theta-gamma coupling without statistically significant changes in gamma and theta power [147].
Table 1.
Gamma oscillations in AD mouse models.
| Mouse Model | Age (Months) | Sex | Preparation | Frequency Band (Hz) | Changes in γ Oscillations | Reference |
|---|---|---|---|---|---|---|
| APP/PS1 | 4–5 | NA | EC slices | 20–60 | Reduced γ frequency in LEC No effect in MEC |
[139] |
| J20 | 4–7 | M/F | in vivo cEEG | 20–80 | Reduced γ power | [140] |
| 7–8 | M/F | in vivo cEEG | 30–90 | Reduced γ power | [141] | |
| 5xFAD | 3 | M | in vivo LFP in CA1 | 20–50 | Reduced γ power during SWRs | [89] |
| TgCRND8 | 1 | NA | HC slices | θ: 3–12 low γ: 25–85 high γ: 120–250 |
No change in γ power Disrupted θ–γ coupling |
[142] |
| 1 | M | in vivo HC LFP | low γ: 25–45 high γ: 60–100 |
Reduced γ power | [143] | |
| APOE4 | 5–17 | F | in vivo HC LFP | 30–50 | Reduced γ power | [144] |
| 4–5 | F | in vivo HC LFP | 30–50 | Reduced γ power during SWRs | [145] | |
| 3R tau overexpression | 7 | M | HC slices | 50–90 | Reduced γ power and peak frequency | [146] |
cEEG, cortical EEG. EC, entorhinal cortex. HC, hippocampus. LFP, local field potential. LEC, lateral EC. MEC, medial EC. SWR, sharp wave-ripple.
Additionally, an association between deficits in PV+ neurons and abnormal gamma oscillations has been shown [140,141]. More specifically, this is caused by a reduction in voltage-gated sodium channel subunit Nav1.1 expression in PV+ neurons of J20 mice, with experimental studies illustrating that genetic modifications to increase Nav1.1 expression lead to a restoration of gamma oscillations and a beneficial effect on cognitive decline [141]. Given the mechanisms of gamma oscillations described above (Figure 2), it is important to examine how the deficits in PV+ neurons can affect activity in pyramidal cells and interactions between PV+ and pyramidal neurons.
Intriguingly, a subset of interneurons (such as PV+, somatostatin-positive, and cholecystokinin-positive GABAergic neurons) are vulnerable to amyloid pathology [140,141,148], whereas pyramidal cells are more vulnerable to tauopathy [149]. These results suggest selective vulnerability depending on AD pathogenesis and pathologies. As efforts have been made to comprehensively characterise molecular mechanisms of such selective vulnerability in both humans and mouse models [149,150,151,152,153], deficits at the neural circuit level will also become clear in coming years.
Compared to human studies, the following aspects have been less explored in mouse models: firstly, although several studies have investigated multiple age points to show modifications in gamma oscillations during AD pathogenesis [136,145,154,155], none of them have reported the inverted U-shape change, that is, a transient increase in gamma power at an early phase of AD pathogenesis, as reported in a human clinical study (Figure 3). A longitudinal assessment of mouse models correlating with amyloid burden and other pathological features may address this issue. Secondly, commonly used mouse models are familial AD models. Thus, the relation to late-onset AD (LOAD) remains unclear. A recent effort to develop LOAD mouse models [156] may bridge the gap between human and animal studies. Additionally, the effect of tauopathy in gamma oscillations needs to be explored further. Finally, the electrophysiological approach is markedly different between human and mouse studies. For example, very few studies in mice have assessed sensory-evoked, task event-related or sensory steady-state responses. Additionally, cortex-wide gamma coherence has not been assessed in mice, in contrast to human EEG and MEG studies. Filling these methodological gaps will be crucial in the future.
2.4. Neuromodulation of Gamma Oscillations for AD
As summarised above, it is clear that a reduction in gamma power is associated with AD pathology, at least in mouse models. Leveraging this knowledge, various invasive and non-invasive neuromodulation approaches have been adopted to modify AD pathology (Table 2) [88,89,90,91,157,158,159,160]. For example, Tsai and her colleagues have elegantly demonstrated that both invasive and non-invasive gamma stimulations can ameliorate AD pathology [86,89,90,91]: optogenetic induction of gamma oscillations in the hippocampus can reduce amyloid load by activating microglia [89]. More surprisingly, non-invasive 40 Hz sensory stimulation (either auditory or visual) has similar effects [89,90]. The same approach can also reduce tau phosphorylation and seeding in the T301S model [89,90]. These effects are associated with modifications to microglia-associated transcripts, as well as synaptic signaling and plasticity-related proteins [89,91]. Although the effect of this non-invasive approach remains to be confirmed in humans, multisensory 40 Hz stimulation can affect wider brain regions, including hippocampal areas, as well as sensory cortices [90]. Another group showed that optogenetic stimulation of PV+ neurons in the medial septum can induce gamma oscillations in the hippocampus, resulting in an improvement in cognitive function [88]. Although it remains to be determined whether this approach could also reduce amyloid load in the hippocampus, these studies have illustrated the potential for certain induction mechanisms of gamma oscillations to modify AD pathology in a beneficial manner. However, further investigation of these is required before its potential as a non-pharmaceutical therapy is considered.
Table 2.
Summary of invasive and non-invasive neuromodulation of gamma oscillations in AD mouse models.
| Induction Method | Stimulation Protocol | Duration | Model | Sex | Age (Months) | Modulated AD Phenotype | Reference | |
|---|---|---|---|---|---|---|---|---|
| Invasive | Optogenetic | 1 ms pulses, 40 Hz, CA1 | 1 h | 5xFAD::PV-Cre, AAV5-EF1α-DIO-ChR2-eYFP | M | 3 | Reduced Aβ Reduced inflammation |
[89] |
| 12 ms pulses, 40 Hz, Medial Septum | 10 min | PVJ20, AAVdj-EF1α-DIO-ChETA-eYFP | M/F | NA | Improved spatial memory | [88] | ||
| 40 Hz, Basal Forebrain | 1 h/d for 3 days | 5xFAD::PV-Cre::Ai32 | M/F | 4–6 | Increased Aβ | [157] | ||
| Non-Invasive | Visual | 12.5 ms on, 12.5 ms off, 40 Hz flicker | 1 h/day for 7 days | 5xFAD | M | 6 | Reduced Aβ | [89] |
| APP/PS1 | M/F | 5 | Reduced Aβ | |||||
| TauP301S | M | 4 | Reduced tauopathy | |||||
| 40 Hz flicker | 1 h/day for 30 days | APP/PS1 | F | 8 | Reduced Aβ Reduced tauopathy Increased sleep regulation |
[160] | ||
| 12.5 ms on, 12.5 ms off, 40 Hz flicker | 1 h/day for 22 days | TauP301S | M | 7.5–8 | Reduced neuronal damage Reduced inflammation Reduced tauopathy Improved spatial memory |
[91] | ||
| 1 h/day for 6 weeks | CK-p25 | M/F | 6–9 | Reduced neuronal damage Reduced inflammation Improved spatial memory |
||||
| Auditory | 1 ms 10 kHz tones, 40 Hz, 60 dB | 1 h/day for 7 days | 5xFAD | NA | 6 | Reduced Aβ Reduced inflammation Improved memory |
[90] | |
| APP/PS1 | NA | 6–9 | Reduced Aβ Reduced inflammation |
|||||
| TauP301S | NA | 2 | Reduced tauopathy | |||||
| Combined Auditory and Visual | 10 s on, 10 s off | 1 h/day for 7 days | 5xFAD | NA | 6 | Reduced Aβ Reduced inflammation |
||
| Visual and Exercise | 40 Hz light flicker and 30–50 min exercise | Daily, 6 days a week for 12 weeks | 3xTg | M | 12–15 | Reduced Aβ Reduced tauopathy Reduced neuronal damage Improved spatial memory |
[159] | |
| Transcranial Focused Ultrasound | 400 μs pulses, 5 s on 5 s off, 40 Hz, Hippocampus | 1 h/day for 5 days | 5xFAD | M | 6 | Increased microglia/Aβ Co-localisation | [158] |
Interestingly, a recent alternative optogenetic approach to induce cortical gamma oscillations showed opposing effects on AD pathology [157]: although optogenetic activation of basal forebrain PV+ neurons could induce cortical gamma oscillations, amyloid load increased in the medial prefrontal cortex and septum. As basal forebrain PV+ neurons preferentially innervate cortical GABAergic neurons [161], the optogenetic activation of basal forebrain PV+ neurons could suppress cortical PV+ neurons, rather than activating them. Thus, the induction mechanism of cortical gamma oscillations in this study differs from that of Iaccarino et al. (2016) [89]. These results suggest that the beneficial effect of gamma oscillations may depend on the induction method, rather than the frequency of local field potentials itself. As there are multiple mechanisms to induce gamma oscillations (Figure 2), it is important to investigate how different approaches can activate different components of neural circuits as well as non-neuronal cells. This type of effort will refine this therapeutic option. As several parameters (duration, frequency, age, etc.) must be explored, real-time monitoring of AD pathology in vivo [162] will accelerate this field.
Regarding clinical applications, since a current major limitation is that most studies have focused on amyloid pathology (Table 2), it is important to investigate how the induction of gamma oscillations affects other pathological features, especially tauopathy [89,90]. In addition, because changes in gamma oscillations in humans can vary depending on the stage of AD pathogenesis [135], it is also critical to determine whether this neuromodulation approach could be beneficial even for patients who exhibit higher gamma power. Again, developing and examining better animal models will benefit this exciting research direction.
3. Slow Oscillations and AD
Slow (<1 Hz) oscillations are another well-characterised type of neural oscillation, since the series of landmark studies by Steriade and colleagues [20,163,164]. Slow oscillations are comprised of cycles of global silence (DOWN state) and synchronous firing (UP state) across neuronal populations [2,163,165,166,167]. When they appear during NREM sleep and under anaesthesia, they can predominantly be observed in the cerebral cortex [2,20,166,167,168,169,170], but also in other brain regions, including the thalamus [171,172], thalamic reticular nucleus [173], hippocampus [170], striatum [174,175], brainstem [176] and claustrum [177]. Slow oscillations play a role in sleep-dependent memory consolidation [26,29,178].
The sleep–wake cycle regulates the concentration of amyloid-β and tau in the cerebrospinal fluid (CSF) and interstitial fluid (ISF), with a higher level of amyloid-β and tau occurring due to prolonged wakefulness or sleep deprivation [179,180]. Slow oscillations are also linked to the activity of the glymphatic system, a highly organised CSF transport system, to clear protein waste products including amyloid-β [23]. Indeed, abnormalities in slow oscillations have been associated with AD [47,49]. Thus, these results suggest a close relationship between the glymphatic system degradation, sleep disturbance and disease progression in dementias [181].
Here, we summarise how slow oscillations are generated and how the reduction in slow wave activity correlates with AD pathology in human patients and mouse models. Finally, we discuss a therapeutic opportunity based on slow oscillations. Although slow oscillations are closely related to sleep, especially NREM sleep, we focus primarily on the oscillation itself and slow wave activity. Readers may refer to recent comprehensive reviews on sleep and AD elsewhere [46,47,48,49,182,183]. Readers can also refer to an up-to-date review of the detailed mechanisms of slow oscillations [184]. Although covering the detailed molecular mechanism is beyond the scope of this review article, transcriptomic and synaptic phosphorylation profiles related to sleep–wake cycles have recently been characterised [185,186].
Regarding terminologies, slow oscillations refer to oscillations at less than 1 Hz, whereas delta oscillations refer to oscillations at 1–4 Hz. However, delta oscillations are often described as 0.5–4 Hz oscillations in literature; hence, they may include slow oscillations. Slow wave activity (SWA) typically refers to spectral power around the 0.5–4 Hz range. SWA is closely associated with sleep homeostasis [187]: it increases proportionally to time spent awake and peaks in slow-wave sleep, whereas it decreases as sleep propensity is reduced.
3.1. Mechanisms of Slow Oscillations
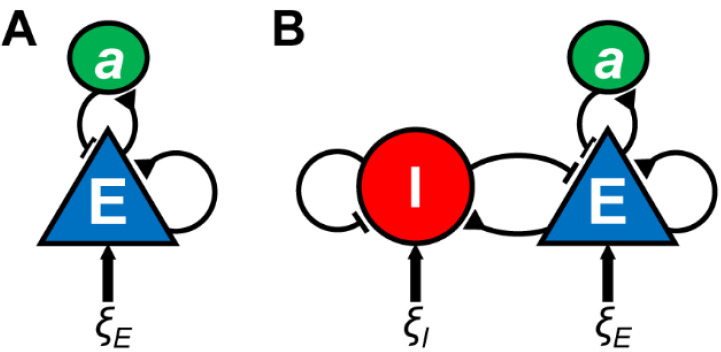
Earlier studies showed that slow oscillations can be generated in isolated cortical gyrus [188], a cortical slab [169] and cortical slice [189], suggesting that cortical circuits are sufficient for the generation of slow oscillations. Subsequent studies have consistently demonstrated that recurrent excitation of layer (L) 5 pyramidal cells is a source of slow oscillations [166,168,189,190]. This notion has been confirmed by computational studies, in which UP and DOWN states can be reproduced by models of neural populations with recurrent excitation and slow adaptation (e.g., activity-dependent K+ current or synaptic depression) (Figure 4A) [191,192,193,194,195].
Figure 4.
Models of slow oscillations. Two types of neural population (rate) models for UP and DOWN dynamics are illustrated. (A) A neural population model with recurrent excitation, slow adaptive process a and noisy fluctuations ξE. (B) A neural population model with recurrent excitation and inhibition, slow adaptive process and independent noisy inputs. The physiological implementation of the adaptive process can be activity-dependent K+ current or synaptic depression.
Multiple receptors and ion channels contribute to shaping UP and DOWN states. For example, both NMDA and non-NMDA receptors are involved in the excitatory drive of UP states [189]. While both excitatory and inhibitory neurons are active during UP states (see below for more details), GABAA receptors play a critical role in UP state duration [196]. The termination mechanism of UP states remains to be fully determined, as various processes have been proposed (for a review, see [184]).
Although recurrent excitation of L5 pyramidal cells plays a dominant role in slow oscillations, accumulating evidence has demonstrated a complex picture: while recurrent excitatory activity during UP states is balanced by inhibition [197], two major GABAergic cell classes, PV+ and somatostatin-positive neurons, regulate the transitions of UP and DOWN states [198]. A recent study also showed that deep-layer neurogliaform cells contribute to slow oscillations by preferentially firing during DOWN states [199]. These experimental results may favour the computational models, which implement active contributions from inhibitory neurons to the UP–DOWN dynamics (Figure 4B) [200,201].
With respect to subcortical areas, thalamic neurons play a critical role in the full manifestation of slow oscillations via T-type calcium channels in thalamocortical cells [171,172]. Thalamic neurons drive PV+ neurons during DOWN states [202]. PV+ neurons can be also activated by claustral neurons to induce DOWN states across cortical regions [177]. Moreover, it has been suggested that astrocytes play a role in slow oscillations and NREM sleep [203,204,205,206,207]. Thus, the exact mechanisms of slow oscillations remain to be fully determined [208]. While the detailed biophysical models of cortical columns [209,210] could provide valuable insight into the mechanisms of slow oscillations, implementing subcortical inputs and the non-neuronal components are still challenging.
Although slow oscillations can arise from various cortical areas as “slow waves”, they often start from the lateral and medial frontal cortical regions and propagate as travelling waves to posterior cortical areas in the human brain [211,212,213]. While cortex-wide spontaneous activity can be examined by various means, correlating different signals (e.g., electrical, hemodynamic, intracellular calcium signals) is still an open issue; this would help gain insight into the mechanisms of cortex-wide slow waves.
3.2. Slow Oscillations and AD in Humans
Sleep disturbance is a common symptom of AD pathogenesis, with sleep fragmentation, increased nocturnal activity and excessive daytime napping contributing to the disruptions to daily life [46,214,215,216,217,218,219,220]. Thus, it is not surprising to see the robust association between abnormalities in slow-wave sleep and AD pathology in humans [46,221,222,223,224,225,226]. Additionally, earlier studies in the 1980s and 1990s demonstrated an association between abnormalities in slow wave activity and AD pathology, including cognitive functions [227,228,229]. Specifically, studies show that impairments in slow-wave sleep are associated with impaired cognition. In recent decades, it has become evident that these associations are underpinned by structural changes and AD pathology in the brain: age-related prefrontal atrophy is associated with reduced slow-wave activity during NREM sleep [230]. Additionally, a bi-directional relationship between slow-wave sleep and AD pathology exists, as slow-wave activity during NREM sleep decreases as amyloid-β deposition and tau accumulation increase [231,232]. The reduction in slow-wave activity is also associated with the impairment in sleep-dependent memory consolidation [231]. Thus, changes in slow oscillations are a robust biomarker of AD pathogenesis in humans although underlying cellular and circuit mechanisms remain unclear.
3.3. Slow Oscillations and AD in Mouse Models
The sleep-wake cycle has been examined across different pathological stages in various mouse models, including 3xTg-AD [233,234], APP/PS1 [234,235], Tg2576 [234,236], P301S Tau [237], rTG4510 [238], PLB1Triple [239], and PLB2tau [240] models (Table 3). Slow (<1 Hz) oscillations have been analysed together with delta oscillations (1–4 Hz), which can be used to determine NREM sleep. In several AD mouse models, NREM sleep is reduced and fragmented [233,234,237,238], which implies that changes also occur in the patterns of slow oscillations. It has been suggested that the reduction in GABAergic tone impairs long-range synchronous firing in an amyloid mouse model [92]. Intriguingly, P301S Tau model exhibited the inverted U-shape profile at the delta frequency, meaning that delta power increases at an early disease stage, whereas it decreases at a later stage [237]. Longitudinal studies in AD mouse models may provide valuable insight into the mechanisms of age-related changes in slow oscillations.
Table 3.
Slow and delta oscillations in AD mouse models.
| Mouse Model | Age (Months) | Sex | Frequency Band (Hz) | Changes in Oscillations | Reference |
|---|---|---|---|---|---|
| 3xTg-AD | 7, 20 | M/F | <1 | Increased frequency at 7 months Decreased frequency at 20 months More irregular at 20 months |
[233] |
| 3xTg-AD | 18 | M/F | 0.1–4 | No change | [234] |
| APP/PS1 | 8–10 | M/F | 0.1–4 | Decreased power during NREM | |
| Tg2576 | 12 | M/F | 0.1–4 | Decreased power during W | |
| Tg2576 | 2, 6, 12 | NA | 0.5–4 | Decreased power during NREM at 6–12 months | [236] |
| APP/PS1 | 3, 6, 9 | NA | >1 | Shorter NREM at 9 months | [235] |
| P301S | 3–12 | M | 1–4 | Increased power during NREM at 6–9 months Decreased power during W and NREM at 11 months |
[237] |
| rTg4510 | 5–10 | M | 0.1–4 | Decreased power during NREM from 6 months | [238] |
| PLB1triple | 5–21 | M/F | 0.5–5 | Decreased power during REM at 9 months Decreased power during W at 21 months |
[239] |
| PLB2tau | 6 | F | 1.5–5 | Increased power during REM Decreased power during NREM |
[240] |
REM, rapid eye movement sleep. NREM, non-REM sleep. W, wakefulness.
3.4. Neuromodulation of Slow Oscillations for AD
Pharmacological and optogenetic intervention approaches can modify abnormalities in slow oscillations, hence the AD disease state in mouse models (Table 4) [87,92,241,242]. For example, a breakdown of long-range coherence of slow oscillations in an AD mouse model can be rescued by enhancing GABAergic inhibition with a GABAA receptor agonist [92]. This is consistent with the notion that aberrant somatic GABAergic tone plays a critical role in the hyperactivity of cortical neurons [140,243]. Kastanenka and his colleagues demonstrated frequency-specific effects of optogenetically induced cortical slow oscillations on AD pathology [87,241]: slow-wave-specific 0.6 Hz optogenetic stimulation of αCaMKII+ neurons in the anterior cortical area can reduce amyloid-β and increase GABAA and GABAB receptor expression. On the other hand, 1.2 Hz stimulation, a slight offset from a slow-wave-specific frequency band, shows an opposing effect without altering GABAA and GABAB receptor expression. These results imply that increasing inhibitory tone may play a role in reducing amyloid burden. One potential caveat is that, because increased and decreased firing rate can also modify AD pathology [244,245,246,247], optogenetic stimulation at higher frequencies may also induce a higher firing rate, leading to the promotion of amyloid deposition. Future studies are needed to determine whether and how the temporal structure of neural population activity, rather than simple firing rates, can modify AD pathology. Additionally, although abnormalities in slow and delta oscillations have been reported in tau models (Table 3), it remains to be explored whether artificial manipulations of slow oscillations can modify tauopathy as well as other pathological features.
Table 4.
Summary of invasive and non-invasive neuromodulation of slow oscillations in AD mouse models.
| Induction Method | Protocol | Duration | Model | Sex | Age (Months) | Modulated AD Phenotype | Reference | |
|---|---|---|---|---|---|---|---|---|
| Invasive | Optogenetic | 400 ms pulses, 0.6 Hz, Anterior Cortex | 24 h/day for 1 month | APP/PS1, AAV5-CamKIIα-hChR2(H134R)-mCherry | M/F | 4–7 | Reduced Aβ Reduced calcium overload Restored GABA levels |
[87] |
| 400 ms pulses, 1.2 Hz, Anterior Cortex | 24 h/day for 1 month | APP/PS1, AAV5-CamKIIα-hChR2(H134R)-mCherry | M/F | 3–9 | Increased Aβ Increased calcium overload Decreased spine densityNo change in GABA levels |
[241] | ||
| Non-Invasive | BACE Inhibitor (oral) | Administration of 0.25 g/kg NB-360 in food pellets | 6 weeks ad lib | APP23xPS45 | F | 6–8 | Reduced Aβ Reduced calcium overload Improved spatial memory |
[242] |
| GABA-A Agonist (i.p.) | Administration of 0.05 mg/kg clonazepam | Once/day for 5 days | APP23xPS45 | M/F | 6–8 | Improved spatial memory | [92] |
Nevertheless, these studies suggest that pharmacological and non-pharmacological interventions for slow oscillations have therapeutic potential for AD. Indeed, accumulating evidence shows bidirectional relationships between sleep and AD pathogenesis [48,248]. It is important to investigate whether artificially enhanced slow oscillations can also trigger other non-neuronal events, such as glymphatic waste clearance, which can be seen in natural slow-wave sleep and even under anaesthesia [23,249]. As discussed above, because multiple components contribute to slow oscillations, various approaches could be explored to modify these and, hence, AD pathology. It is by taking advantage of these various approaches for the neuromodulation of slow oscillations that evidence for potential different modes of action on AD pathology will be unearthed.
4. Conclusions and Future Directions
By focusing on gamma and slow oscillations, we summarised how these oscillations can change during AD pathogenesis in both humans and mouse models. We also reviewed emerging invasive and non-invasive neuromodulation approaches to modify AD pathology in mouse models based on gamma and slow oscillations. Although further studies are essential to uncover the underlying mechanisms before clinical applications, these neuromodulation-based interventions are promising frontiers for AD and beyond.
To further explore this emerging field, the following four areas are important for investigation. Firstly, it is essential to comprehensively characterise electrophysiological biomarkers in AD animal models, with respect to neural oscillations. As well as offering potential biomarkers for early diagnosis, this will aid in the understanding of neural oscillations in AD and abnormalities throughout its progression. As we discussed above, although reduced gamma power has consistently been reported in mouse models (Table 1), the available evidence in human patients is conflicting [39,40,41,128,129,130,131,132]. The gap between mouse models and humans may be due to discrepancies in methodologies between studies, a lack of proper longitudinal studies in mouse models, or the limitations of animal models [250]. Since better mouse models which reflect human pathological features are under development [156], it will become important to conduct detailed in vivo electrophysiological investigations correlating with various molecular and cellular AD pathologies—not just amyloid pathology, but also tauopathy and other pathological features.
Secondly, it is worth exploring other brain states, not just gamma and slow oscillations, because a wide range of neural oscillations or events have been studied in general neuroscience [1,13,33,251]. With respect to sleep, the reduction in REM sleep duration is an early biomarker during AD pathogenesis [221,227,228,252]. During REM sleep, hippocampal theta rhythms and ponto-geniculo-occipital (PGO) waves are prominent electrophysiological markers [14,21,253,254]. Intriguingly, PGO waves and theta rhythms are temporally coupled across animal models [21,255,256,257]. Therefore, it is interesting to investigate how this functional coupling between several sleep-related neural events is affected during AD pathogenesis. PGO waves are originated from mesopontine cholinergic areas [17,19,258] and the neurodegeneration of mesopontine cholinergic neurons has been associated with AD [259,260], as well as Lewy body dementia [261]. Additionally, abnormalities in sleep spindles and sharp wave-ripples during NREM sleep have also been associated with AD [16,49,252,262]. Therefore, these sleep-related neural oscillations may be alternative targets for non-pharmacological interventions.
Thirdly, the means of neuromodulation needs to be explored further. Although optogenetic approaches can achieve cell type-specific manipulations with a high spatiotemporal resolution, non-invasive approaches are ideal for clinical applications. A chemogenetic approach, along with a detailed characterization of brain state, could be a promising direction, since a recent study demonstrated that the chemogenetic attenuation of hyperactivity in the entorhinal cortex can ameliorate AD pathology, including the spread of pathological tau [245]. At present, repetitive transcranial magnetic stimulation (rTMS) and transcranial direct or alternating current stimulation (tDCS/ACS) have shown promising results [65,66]. In addition to these brain stimulation methods, sensory stimulation is also an attractive approach to modulate gamma oscillations across brain regions [86,89,90]. Several neural oscillations or neural events during sleep are also known to be induced or modulated by sensory inputs [263,264,265]: for example, PGO waves during REM sleep can be triggered by sounds [265]. In addition, slow oscillations can be modulated by sounds to promote memory consolidation [263]. Thus, neuromodulations based on auditory stimulus during sleep may be an attractive option.
Finally, the most important research direction is to uncover the mechanisms of how the manipulation of neural oscillations can modify pathological features across multiple spatial levels, from molecular to neural circuit levels. Supposing that neurons are a key driver for a certain neural oscillation, how can subsequent molecular responses trigger non-neuronal events, such as microglial and astrocytic activation and the modification of neurovascular coupling? In addition, neural oscillations are typically induced by multiple neural circuit motifs [5,208,266]. A fundamental issue is to uncover the direct link among electrophysiological signatures, cell-type-specific neuronal activity, non-neuronal activity and molecular responses. Given the complexity of such interactions over multiple spatiotemporal scales, computational approaches will play a crucial role in better understanding the effects of neuromodulation approaches on AD pathology. Thus, we predict that integrative, systems-level approaches will become increasingly important in the coming years.
In conclusion, bi-directional relationships between AD pathology and brain states have become evident. While gamma oscillations and slow oscillations are promising targets, many issues remain to be explored. For future clinical applications, it is crucial to establish a causal relationship between AD pathology and neuromodulations at various levels, from molecules to neural circuits.
Author Contributions
N.B. and S.S. wrote the manuscript with the input from A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Horizon2020-RIA (DEEPER, 101016787) and MRC (MR/V033964/1) to SS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are included in this published article as figures and tables.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Buzsáki G., Draguhn A. Neuronal Oscillations in Cortical Networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 2.Harris K., Thiele A. Cortical state and attention. Nat. Rev. Neurosci. 2011;12:509–523. doi: 10.1038/nrn3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buzsáki G., Logothetis N., Singer W. Scaling Brain Size, Keeping Timing: Evolutionary Preservation of Brain Rhythms. Neuron. 2013;80:751–764. doi: 10.1016/j.neuron.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger H. Über das Elektrenkephalogramm des Menschen. Eur. Arch. Psychiatry Clin. Neurosci. 1929;87:527–570. doi: 10.1007/BF01797193. [DOI] [Google Scholar]
- 5.Buzsáki G., Wang X.-J. Mechanisms of Gamma Oscillations. Annu. Rev. Neurosci. 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engel A.K., Fries P., Singer W. Dynamic predictions: Oscillations and synchrony in top–down processing. Nat. Rev. Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- 7.Fries P. Neuronal Gamma-Band Synchronization as a Fundamental Process in Cortical Computation. Annu. Rev. Neurosci. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- 8.Fries P. Rhythms for Cognition: Communication through Coherence. Neuron. 2015;88:220–235. doi: 10.1016/j.neuron.2015.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singer W., Gray C.M. Visual Feature Integration and the Temporal Correlation Hypothesis. Annu. Rev. Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- 10.Varela F., Lachaux J.-P., Rodriguez E., Martinerie J. The brainweb: Phase synchronization and large-scale integration. Nat. Rev. Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 11.Wang X.-J. Neurophysiological and Computational Principles of Cortical Rhythms in Cognition. Physiol. Rev. 2010;90:1195–1268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller E.K., Lundqvist M., Bastos A.M. Working Memory 2.0. Neuron. 2018;100:463–475. doi: 10.1016/j.neuron.2018.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adamantidis A.R., Herrera C.G., Gent T.C. Oscillating circuitries in the sleeping brain. Nat. Rev. Neurosci. 2019;20:746–762. doi: 10.1038/s41583-019-0223-4. [DOI] [PubMed] [Google Scholar]
- 14.Brown R., Basheer R., McKenna J., Strecker R.E., McCarley R. Control of Sleep and Wakefulness. Physiol. Rev. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buzsáki G. Theta Oscillations in the Hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/S0896-6273(02)00586-X. [DOI] [PubMed] [Google Scholar]
- 16.Buzsáki G. Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus. 2015;25:1073–1188. doi: 10.1002/hipo.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Callaway C., Lydic R., Baghdoyan H.A., Hobson J.A. Pontogeniculooccipital waves: Spontaneous visual system activity during rapid eye movement sleep. Cell. Mol. Neurobiol. 1987;7:105–149. doi: 10.1007/BF00711551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colgin L.L. Rhythms of the hippocampal network. Nat. Rev. Neurosci. 2016;17:239–249. doi: 10.1038/nrn.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Datta S. Cellular basis of pontine ponto-geniculo-occipital wave generation and modulation. Cell. Mol. Neurobiol. 1997;17:341–365. doi: 10.1023/A:1026398402985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steriade M., Nunez A., Amzica F. A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: Depolarizing and hyperpolarizing components. J. Neurosci. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsunematsu T., Patel A.A., Onken A., Sakata S. State-dependent brainstem ensemble dynamics and their interactions with hippocampus across sleep states. eLife. 2020;9:9. doi: 10.7554/eLife.52244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mestre H., Mori Y., Nedergaard M. The Brain’s Glymphatic System: Current Controversies. Trends Neurosci. 2020;43:458–466. doi: 10.1016/j.tins.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie L., Kang H., Xu Q., Chen M.J., Liao Y., Thiyagarajan M., O’Donnell J., Christensen D.J., Nicholson C., Iliff J.J., et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buzsáki G. Two-stage model of memory trace formation: A role for “noisy” brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- 25.Fernández-Ruiz A., Oliva A., De Oliveira E.F., Rocha-Almeida F., Tingley D., Buzsáki G. Long-duration hippocampal sharp wave ripples improve memory. Science. 2019;364:1082–1086. doi: 10.1126/science.aax0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J., Gulati T., Ganguly K. Competing Roles of Slow Oscillations and Delta Waves in Memory Consolidation versus Forgetting. Cell. 2019;179:514–526. doi: 10.1016/j.cell.2019.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Born J., Rasch B., Gais S. Sleep to Remember. Neuroscience. 2006;12:410–424. doi: 10.1177/1073858406292647. [DOI] [PubMed] [Google Scholar]
- 28.Stickgold R., Hobson J.A., Fosse R., Fosse M. Sleep, Learning, and Dreams: Off-line Memory Reprocessing. Science. 2001;294:1052–1057. doi: 10.1126/science.1063530. [DOI] [PubMed] [Google Scholar]
- 29.Todorova R., Zugaro M. Isolated cortical computations during delta waves support memory consolidation. Science. 2019;366:377–381. doi: 10.1126/science.aay0616. [DOI] [PubMed] [Google Scholar]
- 30.Berger H. Über das Elektrenkephalogramm des Menschen. Dritte Mitteilung. Eur. Arch. Psychiatry Clin. Neurosci. 1931;94:16–60. doi: 10.1007/bf01835097. [DOI] [Google Scholar]
- 31.Jeong J. EEG dynamics in patients with Alzheimer’s disease. Clin. Neurophysiol. 2004;115:1490–1505. doi: 10.1016/j.clinph.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Mably A.J., Colgin L.L. Gamma oscillations in cognitive disorders. Curr. Opin. Neurobiol. 2018;52:182–187. doi: 10.1016/j.conb.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeuchi Y., Berényi A. Oscillotherapeutics—Time-targeted interventions in epilepsy and beyond. Neurosci. Res. 2020;152:87–107. doi: 10.1016/j.neures.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Uhlhaas P.J., Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 35.Rojas D.C., Wilson L.B. γ-band abnormalities as markers of autism spectrum disorders. Biomark. Med. 2014;8:353–368. doi: 10.2217/bmm.14.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simon D.M., Wallace M.T. Dysfunction of sensory oscillations in Autism Spectrum Disorder. Neurosci. Biobehav. Rev. 2016;68:848–861. doi: 10.1016/j.neubiorev.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baskaran A., Milev R., McIntyre R.S. The neurobiology of the EEG biomarker as a predictor of treatment response in depression. Neuropharmacology. 2012;63:507–513. doi: 10.1016/j.neuropharm.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 38.Fitzgerald P.J., Watson B.O. Gamma oscillations as a biomarker for major depression: An emerging topic. Transl. Psychiatry. 2018;8:1–7. doi: 10.1038/s41398-018-0239-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osipova D., Pekkonen E., Ahveninen J. Enhanced magnetic auditory steady-state response in early Alzheimer’s disease. Clin. Neurophysiol. 2006;117:1990–1995. doi: 10.1016/j.clinph.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 40.Stam C.J., van Cappellen van Walsum A.M., Pijnenburg Y.A.L., Berendse H.W., de Munck J.C., Scheltens P., Van Dijk B.W. Generalized Synchronization of MEG Recordings in Alzheimer’s Disease: Evidence for Involvement of the Gamma Band. J. Clin. Neurophysiol. 2002;19:562–574. doi: 10.1097/00004691-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Van Deursen J.A., Vuurman E.F.P.M., Verhey F.R.J., Van Kranen-Mastenbroek V.H.J.M., Riedel W. Increased EEG gamma band activity in Alzheimer’s disease and mild cognitive impairment. J. Neural Transm. 2008;115:1301–1311. doi: 10.1007/s00702-008-0083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ford D.E., Kamerow D.B. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262:1479–1484. doi: 10.1001/jama.1989.03430110069030. [DOI] [PubMed] [Google Scholar]
- 43.Kaskie E.R., Graziano B., Ferrarelli F. Schizophrenia and sleep disorders: Links, risks, and management challenges. Nat. Sci. Sleep. 2017;9:227–239. doi: 10.2147/NSS.S121076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manoach D.S., Stickgold R. Abnormal Sleep Spindles, Memory Consolidation, and Schizophrenia. Annu. Rev. Clin. Psychol. 2019;15:451–479. doi: 10.1146/annurev-clinpsy-050718-095754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dolsen M.R., Harvey A.G. Life-time history of insomnia and hypersomnia symptoms as correlates of alcohol, cocaine and heroin use and relapse among adults seeking substance use treatment in the United States from 1991 to 1994. Addiction. 2017;112:1104–1111. doi: 10.1111/add.13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kent B.A., Feldman H.H., Nygaard H.B. Sleep and its regulation: An emerging pathogenic and treatment frontier in Alzheimer’s disease. Prog. Neurobiol. 2021;197:101902. doi: 10.1016/j.pneurobio.2020.101902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee Y.F., Gerashchenko D., Timofeev I., Bacskai B.J., Kastanenka K.V. Slow Wave Sleep Is a Promising Intervention Target for Alzheimer’s Disease. Front. Neurosci. 2020;14:705. doi: 10.3389/fnins.2020.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang C., Holtzman D.M. Bidirectional relationship between sleep and Alzheimer’s disease: Role of amyloid, tau, and other factors. Neuropsychopharmacology. 2020;45:104–120. doi: 10.1038/s41386-019-0478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mander B.A. Local Sleep and Alzheimer’s Disease Pathophysiology. Front. Neurosci. 2020;14:525970. doi: 10.3389/fnins.2020.525970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krames E., Peckham P.H., Rezai A. Neuromodulation: Comprehensive Textbook of Principles, Technologies, and Terapies. 2nd ed. Academic Press; Cambridge, MA, USA: 2018. [Google Scholar]
- 51.Poewe W., Seppi K., Tanner C.M., Halliday G.M., Brundin P., Volkmann J., Schrag A.E., Lang A.E. Parkinson disease. Nat. Rev. Dis. Prim. 2017;3:17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 52.Benabid A., Pollak P., Hoffmann D., Gervason C., Hommel M., Perret J., De Rougemont J., Gao D. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337:403–406. doi: 10.1016/0140-6736(91)91175-T. [DOI] [PubMed] [Google Scholar]
- 53.Scangos K.W., Makhoul G.S., Sugrue L.P., Chang E.F., Krystal A.D. State-dependent responses to intracranial brain stimulation in a patient with depression. Nat. Med. 2021;27:229–231. doi: 10.1038/s41591-020-01175-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mayberg H.S., Lozano A.M., Voon V., McNeely H.E., Seminowicz D., Hamani C., Schwalb J.M., Kennedy S. Deep Brain Stimulation for Treatment-Resistant Depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 55.Grover S., Nguyen J.A., Viswanathan V., Reinhart R.M.G. High-frequency neuromodulation improves obsessive–compulsive behavior. Nat. Med. 2021;27:232–238. doi: 10.1038/s41591-020-01173-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Greenberg B.D., Malone D.A., Friehs G.M., Rezai A.R., Kubu C.S., Malloy P.F., Salloway S.P., Okun M., Goodman W.K., Rasmussen S.A. Three-Year Outcomes in Deep Brain Stimulation for Highly Resistant Obsessive–Compulsive Disorder. Neuropsychopharmacology. 2006;31:2384–2393. doi: 10.1038/sj.npp.1301165. [DOI] [PubMed] [Google Scholar]
- 57.Mirzadeh Z., Bari A., Lozano A.M. The rationale for deep brain stimulation in Alzheimer’s disease. J. Neural Transm. 2015;123:775–783. doi: 10.1007/s00702-015-1462-9. [DOI] [PubMed] [Google Scholar]
- 58.Kuhn J., Hardenacke K., Lenartz D., Gruendler T., Ullsperger M., Bartsch C., Mai J.K., Zilles K., Bauer A., Matusch A., et al. Deep brain stimulation of the nucleus basalis of Meynert in Alzheimer’s dementia. Mol. Psychiatry. 2014;20:353–360. doi: 10.1038/mp.2014.32. [DOI] [PubMed] [Google Scholar]
- 59.Ahn S., Mellin J.M., Alagapan S., Alexander M.L., Gilmore J.H., Jarskog L.F., Fröhlich F. Targeting reduced neural oscillations in patients with schizophrenia by transcranial alternating current stimulation. NeuroImage. 2019;186:126–136. doi: 10.1016/j.neuroimage.2018.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brunelin J., Mondino M., Gassab L., Haesebaert F., Gaha L., Suaud-Chagny M.-F., Saoud M., Mechri A., Poulet E. Examining Transcranial Direct-Current Stimulation (tDCS) as a Treatment for Hallucinations in Schizophrenia. Am. J. Psychiatry. 2012;169:719–724. doi: 10.1176/appi.ajp.2012.11071091. [DOI] [PubMed] [Google Scholar]
- 61.Farzan F., Barr M.S., Sun Y., Fitzgerald P.B., Daskalakis Z.J. Transcranial magnetic stimulation on the modulation of gamma oscillations in schizophrenia. Ann. New York Acad. Sci. 2012;1265:25–35. doi: 10.1111/j.1749-6632.2012.06543.x. [DOI] [PubMed] [Google Scholar]
- 62.Fox M.D., Buckner R.L., White M.P., Greicius M.D., Pascual-Leone A. Efficacy of Transcranial Magnetic Stimulation Targets for Depression Is Related to Intrinsic Functional Connectivity with the Subgenual Cingulate. Biol. Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Terraneo A., Leggio L., Saladini M., Ermani M., Bonci A., Gallimberti L. Transcranial magnetic stimulation of dorsolateral prefrontal cortex reduces cocaine use: A pilot study. Eur. Neuropsychopharmacol. 2016;26:37–44. doi: 10.1016/j.euroneuro.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diana M., Raij T., Melis M., Nummenmaa A., Leggio L., Bonci A. Rehabilitating the addicted brain with transcranial magnetic stimulation. Nat. Rev. Neurosci. 2017;18:685–693. doi: 10.1038/nrn.2017.113. [DOI] [PubMed] [Google Scholar]
- 65.Holczer A., Németh V.L., Vékony T., Vécsei L., Klivenyi P., Must A. Non-invasive Brain Stimulation in Alzheimer’s Disease and Mild Cognitive Impairment—A State-of-the-Art Review on Methodological Characteristics and Stimulation Parameters. Front. Hum. Neurosci. 2020;14:179. doi: 10.3389/fnhum.2020.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Freitas C., Mondragón-Llorca H., Pascual-Leone A. Noninvasive brain stimulation in Alzheimer’s disease: Systematic review and perspectives for the future. Exp. Gerontol. 2011;46:611–627. doi: 10.1016/j.exger.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bréchet L., Michel C.M., Schacter D.L., Pascual-Leone A. Improving autobiographical memory in Alzheimer’s disease by transcranial alternating current stimulation. Curr. Opin. Behav. Sci. 2021;40:64–71. doi: 10.1016/j.cobeha.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 69.Henstridge C.M., Hyman B.T., Spires-Jones T.L. Beyond the neuron–cellular interactions early in Alzheimer disease pathogenesis. Nat. Rev. Neurosci. 2019;20:94–108. doi: 10.1038/s41583-018-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herrup K. The case for rejecting the amyloid cascade hypothesis. Nat. Neurosci. 2015;18:794–799. doi: 10.1038/nn.4017. [DOI] [PubMed] [Google Scholar]
- 71.Long J., Holtzman D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell. 2019;179:312–339. doi: 10.1016/j.cell.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Musiek E., Holtzman D.M. Three dimensions of the amyloid hypothesis: Time, space and ’wingmen’. Nat. Neurosci. 2015;18:800–806. doi: 10.1038/nn.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ballatore C., Lee V.M.-Y., Trojanowski J.Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 74.Butterfield D.A., Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019;20:148–160. doi: 10.1038/s41583-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hickman S., Izzy S., Sen P., Morsett L., El Khoury J. Microglia in neurodegeneration. Nat. Neurosci. 2018;21:1359–1369. doi: 10.1038/s41593-018-0242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee V.M.-Y., Goedert M., Trojanowski J.Q. Neurodegenerative Tauopathies. Annu. Rev. Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 77.Saez-Atienzar S., Masliah E. Cellular senescence and Alzheimer disease: The egg and the chicken scenario. Nat. Rev. Neurosci. 2020;21:433–444. doi: 10.1038/s41583-020-0325-z. [DOI] [PubMed] [Google Scholar]
- 78.Sweeney M., Kisler K., Montagne A., Toga A.W., Zlokovic B.V. The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci. 2018;21:1318–1331. doi: 10.1038/s41593-018-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Belloy M., Napolioni V., Greicius M.D. A Quarter Century of APOE and Alzheimer’s Disease: Progress to Date and the Path Forward. Neuron. 2019;101:820–838. doi: 10.1016/j.neuron.2019.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lewcock J.W., Schlepckow K., Di Paolo G., Tahirovic S., Monroe K.M., Haass C. Emerging Microglia Biology Defines Novel Therapeutic Approaches for Alzheimer’s Disease. Neuron. 2020;108:801–821. doi: 10.1016/j.neuron.2020.09.029. [DOI] [PubMed] [Google Scholar]
- 81.Golde T.E., DeKosky S.T., Galasko D. Alzheimer’s disease: The right drug, the right time. Science. 2018;362:1250–1251. doi: 10.1126/science.aau0437. [DOI] [PubMed] [Google Scholar]
- 82.Cummings J., Lee G., Ritter A., Sabbagh M., Zhong K. Alzheimer’s disease drug development pipeline: 2019. Alzheimer’s Dementia: Transl. Res. Clin. Interv. 2019;5:272–293. doi: 10.1016/j.trci.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sevigny J., Chiao P., Bussière T., Weinreb P.H., Williams L., Maier M., Dunstan R., Salloway S., Chen T., Ling Y., et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 84.Sierksma A., Escott-Price V., De Strooper B. Translating genetic risk of Alzheimer’s disease into mechanistic insight and drug targets. Science. 2020;370:61–66. doi: 10.1126/science.abb8575. [DOI] [PubMed] [Google Scholar]
- 85.Pérez M., Hernández F., Avila J. Protein Biomarkers for the Diagnosis of Alzheimer’s Disease at Different Stages of Neurodegeneration. Int. J. Mol. Sci. 2020;21:6749. doi: 10.3390/ijms21186749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Adaikkan C., Tsai L.-H. Gamma Entrainment: Impact on Neurocircuits, Glia, and Therapeutic Opportunities. Trends Neurosci. 2020;43:24–41. doi: 10.1016/j.tins.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 87.Kastanenka K.V., Hou S.S., Shakerdge N., Logan R., Feng D., Wegmann S., Chopra V., Hawkes J.M., Chen X., Bacskai B.J. Optogenetic Restoration of Disrupted Slow Oscillations Halts Amyloid Deposition and Restores Calcium Homeostasis in an Animal Model of Alzheimer’s Disease. PLoS ONE. 2017;12:e0170275. doi: 10.1371/journal.pone.0170275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Etter G., Van Der Veldt S., Manseau F., Zarrinkoub I., Trillaud-Doppia E., Williams S. Optogenetic gamma stimulation rescues memory impairments in an Alzheimer’s disease mouse model. Nat. Commun. 2019;10:1–11. doi: 10.1038/s41467-019-13260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iaccarino H.F., Singer A.C., Martorell A.J., Rudenko A., Gao F., Gillingham T.Z., Mathys H., Seo J., Kritskiy O., Abdurrob F., et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nat. Cell Biol. 2016;540:230–235. doi: 10.1038/nature20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Martorell A.J., Paulson A., Suk H.-J., Abdurrob F., Drummond G.T., Guan W., Young J.Z., Kim D.N.-W., Kritskiy O., Barker S.J., et al. Multi-sensory Gamma Stimulation Ameliorates Alzheimer’s-Associated Pathology and Improves Cognition. Cell. 2019;177:256–271.e22. doi: 10.1016/j.cell.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Adaikkan C., Middleton S., Marco A., Pao P.-C., Mathys H., Kim D.N.-W., Gao F., Young J.Z., Suk H.-J., Boyden E.S., et al. Gamma Entrainment Binds Higher-Order Brain Regions and Offers Neuroprotection. Neuron. 2019;102:929–943.e8. doi: 10.1016/j.neuron.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Busche M.A., Kekuš M., Adelsberger H., Noda T., Förstl H., Nelken I., Konnerth A. Rescue of long-range circuit dysfunction in Alzheimer’s disease models. Nat. Neurosci. 2015;18:1623–1630. doi: 10.1038/nn.4137. [DOI] [PubMed] [Google Scholar]
- 93.Isla A.G., Balleza-Tapia H., Fisahn A. Efficacy of preclinical pharmacological interventions against alterations of neuronal network oscillations in Alzheimer’s disease: A systematic review. Exp. Neurol. 2021;343:113743. doi: 10.1016/j.expneurol.2021.113743. [DOI] [PubMed] [Google Scholar]
- 94.Jasper H.H., Andrews H.L. Brain potentials and voluntary muscle activity in man. J. Neurophysiol. 1938;1:87–100. doi: 10.1152/jn.1938.1.2.87. [DOI] [Google Scholar]
- 95.Bressler S.L., Freeman W.J. Frequency analysis of olfactory system EEG in cat, rabbit, and rat. Electroencephalogr. Clin. Neurophysiol. 1980;50:19–24. doi: 10.1016/0013-4694(80)90319-3. [DOI] [PubMed] [Google Scholar]
- 96.Freeman W.J. Mass Action in the Nervous System. Academic Press; New York, NY, USA: 1975. [Google Scholar]
- 97.Nakazono T., Lam T.N., Patel A.Y., Kitazawa M., Saito T., Saido T.C., Igarashi K.M. Impaired In Vivo Gamma Oscillations in the Medial Entorhinal Cortex of Knock-in Alzheimer Model. Front. Syst. Neurosci. 2017;11:48. doi: 10.3389/fnsys.2017.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chrobak J.J., Buzsaki G. Gamma Oscillations in the Entorhinal Cortex of the Freely Behaving Rat. J. Neurosci. 1998;18:388–398. doi: 10.1523/JNEUROSCI.18-01-00388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Charpak S., Paré D., Llinás R. The Entorhinal Cortex Entrains Fast CA1 Hippocampal Oscillations in the Anaesthetized Guinea-pig: Role of the Monosynaptic Component of the Perforant Path. Eur. J. Neurosci. 1995;7:1548–1557. doi: 10.1111/j.1460-9568.1995.tb01150.x. [DOI] [PubMed] [Google Scholar]
- 100.Bauer E.P., Paz R., Paré D. Gamma Oscillations Coordinate Amygdalo-Rhinal Interactions during Learning. J. Neurosci. 2007;27:9369–9379. doi: 10.1523/JNEUROSCI.2153-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Harris K., Csicsvari J., Hirase H., Dragoi G., Buzsaki G. Organization of cell assemblies in the hippocampus. Nat. Cell Biol. 2003;424:552–556. doi: 10.1038/nature01834. [DOI] [PubMed] [Google Scholar]
- 102.Buzsaki G., Leung L.-W.S., Vanderwolf C.H. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. Rev. 1983;6:139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- 103.Wang X.-J., Buzsaki G. Gamma Oscillation by Synaptic Inhibition in a Hippocampal Interneuronal Network Model. J. Neurosci. 1996;16:6402–6413. doi: 10.1523/JNEUROSCI.16-20-06402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cohen M.X., Axmacher N., Lenartz D., Elger C.E., Sturm V., Schlaepfer T. Good Vibrations: Cross-frequency Coupling in the Human Nucleus Accumbens during Reward Processing. J. Cogn. Neurosci. 2009;21:875–889. doi: 10.1162/jocn.2009.21062. [DOI] [PubMed] [Google Scholar]
- 105.Popescu A.T., Popa D., Pare D. Coherent gamma oscillations couple the amygdala and striatum during learning. Nat. Neurosci. 2009;12:801–807. doi: 10.1038/nn.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Beshel J., Kopell N., Kay L.M. Olfactory Bulb Gamma Oscillations Are Enhanced with Task Demands. J. Neurosci. 2007;27:8358–8365. doi: 10.1523/JNEUROSCI.1199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Eeckman F.H., Freeman W.J. Correlations between unit firing and EEG in the rat olfactory system. Brain Res. 1990;528:238–244. doi: 10.1016/0006-8993(90)91663-2. [DOI] [PubMed] [Google Scholar]
- 108.Yague J.G., Tsunematsu T., Sakata S. Distinct Temporal Coordination of Spontaneous Population Activity between Basal Forebrain and Auditory Cortex. Front. Neural Circuits. 2017;11:64. doi: 10.3389/fncir.2017.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nair J., Klaassen A.-L., Arato J., Vyssotski A.L., Harvey M., Rainer G. Basal forebrain contributes to default mode network regulation. Proc. Natl. Acad. Sci. USA. 2018;115:1352–1357. doi: 10.1073/pnas.1712431115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Minlebaev M., Colonnese M., Tsintsadze T., Sirota A., Khazipov R. Early Gamma Oscillations Synchronize Developing Thalamus and Cortex. Science. 2011;334:226–229. doi: 10.1126/science.1210574. [DOI] [PubMed] [Google Scholar]
- 111.Jensen O., Kaiser J., Lachaux J.-P. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007;30:317–324. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 112.Howard M.W., Rizzuto D.S., Caplan J.B., Madsen J.R., Lisman J., Aschenbrenner-Scheibe R., Schulze-Bonhage A., Kahana M.J. Gamma Oscillations Correlate with Working Memory Load in Humans. Cereb. Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- 113.Osipova D., Takashima A., Oostenveld R., Fernandez G., Maris E., Jensen O. Theta and Gamma Oscillations Predict Encoding and Retrieval of Declarative Memory. J. Neurosci. 2006;26:7523–7531. doi: 10.1523/JNEUROSCI.1948-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tiesinga P., Sejnowski T.J. Cortical Enlightenment: Are Attentional Gamma Oscillations Driven by ING or PING? Neuron. 2009;63:727–732. doi: 10.1016/j.neuron.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Whittington M., Traub R., Kopell N., Ermentrout B., Buhl E. Inhibition-based rhythms: Experimental and mathematical observations on network dynamics. Int. J. Psychophysiol. 2000;38:315–336. doi: 10.1016/S0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- 116.Traub R.D., Whittington M.A., Stanford I.M., Jefferys J.G.R. A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nat. Cell Biol. 1996;383:621–624. doi: 10.1038/383621a0. [DOI] [PubMed] [Google Scholar]
- 117.Lytton W.W., Sejnowski T.J. Simulations of cortical pyramidal neurons synchronized by inhibitory interneurons. J. Neurophysiol. 1991;66:1059–1079. doi: 10.1152/jn.1991.66.3.1059. [DOI] [PubMed] [Google Scholar]
- 118.Traub R.D., Whittington M.A., Colling S.B., Buzsaki G., Jefferys J.G. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. J. Physiol. 1996;493:471–484. doi: 10.1113/jphysiol.1996.sp021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Whittington M.A., Traub R.D., Jefferys J. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nat. Cell Biol. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- 120.Cardin J.A., Carlen M., Meletis K., Knoblich U., Zhang F., Deisseroth K., Tsai L.H., Moore C.I. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sohal V., Zhang F., Yizhar O., Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nat. Cell Biol. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jefferys J.G., Traub R.D., Whittington M.A. Neuronal networks for induced ‘40 Hz’ rhythms. Trends Neurosci. 1996;19:202–208. doi: 10.1016/S0166-2236(96)10023-0. [DOI] [PubMed] [Google Scholar]
- 123.Wilson H.R., Cowan J.D. Excitatory and Inhibitory Interactions in Localized Populations of Model Neurons. Biophys. J. 1972;12:1–24. doi: 10.1016/S0006-3495(72)86068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tiesinga P., Fellous J.-M., Sejnowski T.J. Regulation of spike timing in visual cortical circuits. Nat. Rev. Neurosci. 2008;9:97–107. doi: 10.1038/nrn2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fisahn A., Pike F.G., Buhl E.H., Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nat. Cell Biol. 1998;394:186–189. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- 126.Andersson R., Galter D., Papadia D., Fisahn A. Histamine induces KCNQ channel-dependent gamma oscillations in rat hippocampus via activation of the H1 receptor. Neuropharmacology. 2017;118:13–25. doi: 10.1016/j.neuropharm.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 127.Gray C.M., McCormick D.A. Chattering Cells: Superficial Pyramidal Neurons Contributing to the Generation of Synchronous Oscillations in the Visual Cortex. Science. 1996;274:109–113. doi: 10.1126/science.274.5284.109. [DOI] [PubMed] [Google Scholar]
- 128.Ribary U., Ioannides A.A., Singh K., Hasson R., Bolton J.P., Lado F., Mogilner A., Llinas R. Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proc. Natl. Acad. Sci. USA. 1991;88:11037–11041. doi: 10.1073/pnas.88.24.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Başar E., Emek-Savaş D.D., Güntekin B., Yener G.G. Delay of cognitive gamma responses in Alzheimer’s disease. NeuroImage: Clin. 2016;11:106–115. doi: 10.1016/j.nicl.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Van Deursen J., Vuurman E., van Kranen-Mastenbroek V., Verhey F., Riedel W. 40-Hz steady state response in Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging. 2011;32:24–30. doi: 10.1016/j.neurobiolaging.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 131.Rossini P., Del Percio C., Pasqualetti P., Cassetta E., Binetti G., Forno G.D., Ferreri F., Frisoni G.B., Chiovenda P., Miniussi C., et al. Conversion from mild cognitive impairment to Alzheimer’s disease is predicted by sources and coherence of brain electroencephalography rhythms. Neuroscience. 2006;143:793–803. doi: 10.1016/j.neuroscience.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 132.Başar E., Femir B., Emek-Savaş D.D., Güntekin B., Yener G.G. Increased long distance event-related gamma band connectivity in Alzheimer’s disease. NeuroImage: Clin. 2017;14:580–590. doi: 10.1016/j.nicl.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang J., Fang Y., Wang X., Yang H., Yu X., Wang H. Enhanced Gamma Activity and Cross-Frequency Interaction of Resting-State Electroencephalographic Oscillations in Patients with Alzheimer’s Disease. Front. Aging Neurosci. 2017;9:243. doi: 10.3389/fnagi.2017.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Koenig T., Prichep L., Dierks T., Hubl D., Wahlund L., John E., Jelic V. Decreased EEG synchronization in Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging. 2005;26:165–171. doi: 10.1016/j.neurobiolaging.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 135.Gaubert S., Raimondo F., Houot M., Corsi M.-C., Naccache L., Sitt J.D., Hermann B., Oudiette D., Gagliardi G., Habert M.-O., et al. EEG evidence of compensatory mechanisms in preclinical Alzheimer’s disease. Brain. 2019;142:2096–2112. doi: 10.1093/brain/awz150. [DOI] [PubMed] [Google Scholar]
- 136.Jones D.T., Knopman D.S., Gunter J.L., Graff-Radford J., Vemuri P., Boeve B.F., Petersen R.C., Weiner M.W., Jack C.R. Cascading network failure across the Alzheimer’s disease spectrum. Brain. 2016;139:547–562. doi: 10.1093/brain/awv338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lim H.K., Nebes R., Snitz B., Cohen A., Mathis C., Price J., Weissfeld L., Klunk W., Aizenstein H.J. Regional amyloid burden and intrinsic connectivity networks in cognitively normal elderly subjects. Brain. 2014;137:3327–3338. doi: 10.1093/brain/awu271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mormino E.C., Smiljic A., Hayenga A.O., Onami S.H., Greicius M.D., Rabinovici G.D., Janabi M., Baker S.L., Yen I.V., Madison C.M., et al. Relationships between Beta-Amyloid and Functional Connectivity in Different Components of the Default Mode Network in Aging. Cereb. Cortex. 2011;21:2399–2407. doi: 10.1093/cercor/bhr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Klein A.S., Donoso J.R., Kempter R., Schmitz D., Beed P. Early Cortical Changes in Gamma Oscillations in Alzheimer’s Disease. Front. Syst. Neurosci. 2016;10:83. doi: 10.3389/fnsys.2016.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Verret L., Mann E., Hang G.B., Barth A.M., Cobos I., Ho K., Devidze N., Masliah E., Kreitzer A.C., Mody I., et al. Inhibitory Interneuron Deficit Links Altered Network Activity and Cognitive Dysfunction in Alzheimer Model. Cell. 2012;149:708–721. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Martinez-Losa M., Tracy T.E., Ma K., Verret L., Clemente-Perez A., Khan A.S., Cobos I., Ho K., Gan L., Mucke L., et al. Nav1.1-Overexpressing Interneuron Transplants Restore Brain Rhythms and Cognition in a Mouse Model of Alzheimer’s Disease. Neuron. 2018;98:75–89. doi: 10.1016/j.neuron.2018.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Goutagny R., Gu N., Cavanagh C., Jackson J., Chabot J.-G., Quirion R., Krantic S., Williams S. Alterations in hippocampal network oscillations and theta-gamma coupling arise before Aβ overproduction in a mouse model of Alzheimer’s disease. Eur. J. Neurosci. 2013;37:1896–1902. doi: 10.1111/ejn.12233. [DOI] [PubMed] [Google Scholar]
- 143.Hamm V., Héraud C., Bott J.-B., Herbeaux K., Strittmatter C., Mathis C., Goutagny R. Differential contribution of APP metabolites to early cognitive deficits in a TgCRND8 mouse model of Alzheimer’s disease. Sci. Adv. 2017;3:e1601068. doi: 10.1126/sciadv.1601068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jones E.A., Gillespie A.K., Yoon S.Y., Frank L., Huang Y. Early Hippocampal Sharp-Wave Ripple Deficits Predict Later Learning and Memory Impairments in an Alzheimer’s Disease Mouse Model. Cell Rep. 2019;29:2123–2133.e4. doi: 10.1016/j.celrep.2019.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gillespie A.K., Jones E.A., Lin Y.-H., Karlsson M.P., Kay K., Yoon S.Y., Tong L.M., Nova P., Carr J.S., Frank L., et al. Apolipoprotein E4 Causes Age-Dependent Disruption of Slow Gamma Oscillations during Hippocampal Sharp-Wave Ripples. Neuron. 2016;90:740–751. doi: 10.1016/j.neuron.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Richetin K., Steullet P., Pachoud M., Perbet R., Parietti E., Maheswaran M., Eddarkaoui S., Bégard S., Pythoud C., Rey M., et al. Tau accumulation in astrocytes of the dentate gyrus induces neuronal dysfunction and memory deficits in Alzheimer’s disease. Nat. Neurosci. 2020;23:1567–1579. doi: 10.1038/s41593-020-00728-x. [DOI] [PubMed] [Google Scholar]
- 147.Zhang X., Zhong W., Brankačk J., Weyer S.W., Müller U.C., Tort A.B.L., Draguhn A. Impaired theta-gamma coupling in APP-deficient mice. Sci. Rep. 2016;6:21948. doi: 10.1038/srep21948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shi A., Petrache A.L., Shi J., Ali A.B. Preserved Calretinin Interneurons in an App Model of Alzheimer’s Disease Disrupt Hippocampal Inhibition via Upregulated P2Y1 Purinoreceptors. Cereb. Cortex. 2019;30:1272–1290. doi: 10.1093/cercor/bhz165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fu H., Possenti A., Freer R., Nakano Y., Villegas N.C.H., Tang M., Cauhy P.V.M., Lassus B.A., Chen S., Fowler S.L., et al. A tau homeostasis signature is linked with the cellular and regional vulnerability of excitatory neurons to tau pathology. Nat. Neurosci. 2018;22:47–56. doi: 10.1038/s41593-018-0298-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mathys H., Davila-Velderrain J., Peng Z., Gao F., Mohammadi S., Young J.Z., Menon M., He L., Abdurrob F., Jiang X., et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature. 2019;570:332–337. doi: 10.1038/s41586-019-1195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zalocusky K.A., Najm R., Taubes A.L., Hao Y., Yoon S.Y., Koutsodendris N., Nelson M.R., Rao A., Bennett D.A., Bant J., et al. Neuronal ApoE upregulates MHC-I expression to drive selective neurodegeneration in Alzheimer’s disease. Nat. Neurosci. 2021;24:786–798. doi: 10.1038/s41593-021-00851-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Leng K., Li E., Eser R., Piergies A., Sit R., Tan M., Neff N., Li S.H., Rodriguez R.D., Suemoto C.K., et al. Molecular characterization of selectively vulnerable neurons in Alzheimer’s disease. Nat. Neurosci. 2021;24:276–287. doi: 10.1038/s41593-020-00764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Roussarie J.-P., Yao V., Rodriguez P.R., Oughtred R., Rust J., Plautz Z., Kasturia S., Albornoz C., Wang W., Schmidt E.F., et al. Selective Neuronal Vulnerability in Alzheimer’s Disease: A Network-Based Analysis. Neuron. 2020;107:821–835.e12. doi: 10.1016/j.neuron.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ahnaou A., Moechars D., Raeymaekers L., Biermans R., Manyakov N.V., Bottelbergs A., Wintmolders C., Van Kolen K., Van De Casteele T., Kemp J.A., et al. Emergence of early alterations in network oscillations and functional connectivity in a tau seeding mouse model of Alzheimer’s disease pathology. Sci. Rep. 2017;7:14189. doi: 10.1038/s41598-017-13839-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hermann D., Both M., Ebert U., Gross G., Schoemaker H., Draguhn A., Wicke K., Nimmrich V. Synaptic transmission is impaired prior to plaque formation in amyloid precursor protein–overexpressing mice without altering behaviorally-correlated sharp wave–ripple complexes. Neuroscience. 2009;162:1081–1090. doi: 10.1016/j.neuroscience.2009.05.044. [DOI] [PubMed] [Google Scholar]
- 156.Oblak A.L., Forner S., Territo P.R., Sasner M., Carter G.W., Howell G.R., Sukoff-Rizzo S.J., Logsdon B.A., Mangravite L.M., Mortazavi A., et al. Model organism development and evaluation for late-onset Alzheimer’s disease: MODEL-AD. Alzheimer’s Dementia Transl. Res. Clin. Interv. 2020;6 doi: 10.1002/trc2.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wilson C.A., Fouda S., Sakata S. Effects of optogenetic stimulation of basal forebrain parvalbumin neurons on Alzheimer’s disease pathology. Sci. Rep. 2020;10:1–9. doi: 10.1038/s41598-020-72421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bobola M., Chen L., Ezeokeke C., Olmstead T., Nguyen C., Sahota A., Williams R., Mourad P. Transcranial focused ultrasound, pulsed at 40 Hz, activates microglia acutely and reduces Aβ load chronically, as demonstrated in vivo. Brain Stimul. 2020;13:1014–1023. doi: 10.1016/j.brs.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Park S.-S., Park H.-S., Kim C.-J., Kang H.-S., Kim D.-H., Baek S.-S., Kim T.-W. Physical exercise during exposure to 40-Hz light flicker improves cognitive functions in the 3xTg mouse model of Alzheimer’s disease. Alzheimer’s Res. Ther. 2020;12:1–15. doi: 10.1186/s13195-020-00631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yao Y., Ying Y., Deng Q., Zhang W., Zhu H., Lin Z., Zhang S., Ma J., Zhao Y. Non-invasive 40-Hz Light Flicker Ameliorates Alzheimer’s-Associated Rhythm Disorder via Regulating Central Circadian Clock in Mice. Front. Physiol. 2020;11:294. doi: 10.3389/fphys.2020.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Freund T.F., Meskenaite V. gamma-Aminobutyric acid-containing basal forebrain neurons innervate inhibitory interneurons in the neocortex. Proc. Natl. Acad. Sci. USA. 1992;89:738–742. doi: 10.1073/pnas.89.2.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Klunk W., Bacskai B.J., Mathis C.A., Kajdasz S.T., McLellan M.E., Frosch M.P., Debnath M.L., Holt D.P., Wang Y., Hyman B.T. Imaging Aβ Plaques in Living Transgenic Mice with Multiphoton Microscopy and Methoxy-X04, a Systemically Administered Congo Red Derivative. J. Neuropathol. Exp. Neurol. 2002;61:797–805. doi: 10.1093/jnen/61.9.797. [DOI] [PubMed] [Google Scholar]
- 163.Steriade M., Nuñez A., Amzica F. Intracellular analysis of relations between the slow (<1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J. Neurosci. 1993;13:3266–3283. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Steriade M., Contreras D., Dossi R.C., Nunez A. The slow (<1 Hz) oscillation in reticular thalamic and thalamocortical neurons: Scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J. Neurosci. 1993;13:3284–3299. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sakata S., Harris K.D. Laminar-dependent effects of cortical state on auditory cortical spontaneous activity. Front. Neural Circuits. 2012;6:109. doi: 10.3389/fncir.2012.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sakata S., Harris K.D. Laminar Structure of Spontaneous and Sensory-Evoked Population Activity in Auditory Cortex. Neuron. 2009;64:404–418. doi: 10.1016/j.neuron.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Luczak A., Bartho P., Marguet S.L., Buzsaki G., Harris K.D. Sequential structure of neocortical spontaneous activity in vivo. Proc. Natl. Acad. Sci. USA. 2007;104:347–352. doi: 10.1073/pnas.0605643104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chauvette S., Volgushev M., Timofeev I. Origin of Active States in Local Neocortical Networks during Slow Sleep Oscillation. Cereb. Cortex. 2010;20:2660–2674. doi: 10.1093/cercor/bhq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Timofeev I., Grenier F., Bazhenov M., Sejnowski T., Steriade M. Origin of Slow Cortical Oscillations in Deafferented Cortical Slabs. Cereb. Cortex. 2000;10:1185–1199. doi: 10.1093/cercor/10.12.1185. [DOI] [PubMed] [Google Scholar]
- 170.Isomura Y., Sirota A., Özen S., Montgomery S., Mizuseki K., Henze D.A., Buzsáki G. Integration and Segregation of Activity in Entorhinal-Hippocampal Subregions by Neocortical Slow Oscillations. Neuron. 2006;52:871–882. doi: 10.1016/j.neuron.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 171.Hughes S.W., Cope D.W., Blethyn K.L., Crunelli V. Cellular Mechanisms of the Slow (<1 Hz) oscillation in thalamocortical neurons in vitro. Neuron. 2002;33:947–958. doi: 10.1016/S0896-6273(02)00623-2. [DOI] [PubMed] [Google Scholar]
- 172.David F., Schmiedt J.T., Taylor H.L., Orban G., Di Giovanni G., Uebele V.N., Renger J.J., Lambert R.C., LeResche N., Crunelli V. Essential thalamic contribution to slow waves of natural sleep. J. Neurosci. 2013;33:19599–19610. doi: 10.1523/JNEUROSCI.3169-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Blethyn K.L., Hughes S.W., Tóth T.I., Cope D.W., Crunelli V. Neuronal Basis of the Slow (<1 Hz) oscillation in neurons of the nucleus reticularis thalami in vitro. J. Neurosci. 2006;26:2474–2486. doi: 10.1523/JNEUROSCI.3607-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wilson C.J. Chapter 18 The generation of natural firing patterns in neostriatal neurons. Prog. Brain Res. 1993;99:277–297. doi: 10.1016/s0079-6123(08)61352-7. [DOI] [PubMed] [Google Scholar]
- 175.Wilson C.J., Groves P.M. Spontaneous firing patterns of identified spiny neurons in the rat neostriatum. Brain Res. 1981;220:67–80. doi: 10.1016/0006-8993(81)90211-0. [DOI] [PubMed] [Google Scholar]
- 176.Mena-Segovia J., Sims H.M., Magill P.J., Bolam J.P. Cholinergic brainstem neurons modulate cortical gamma activity during slow oscillations. J. Physiol. 2008;586:2947–2960. doi: 10.1113/jphysiol.2008.153874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Narikiyo K., Mizuguchi R., Ajima A., Shiozaki M., Hamanaka H., Johansen J.P., Mori K., Yoshihara Y. The claustrum coordinates cortical slow-wave activity. Nat. Neurosci. 2020;23:741–753. doi: 10.1038/s41593-020-0625-7. [DOI] [PubMed] [Google Scholar]
- 178.Timofeev I., Chauvette S. Sleep slow oscillation and plasticity. Curr. Opin. Neurobiol. 2017;44:116–126. doi: 10.1016/j.conb.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 179.Kang J.-E., Lim M.M., Bateman R.J., Lee J.J., Smyth L.P., Cirrito J.R., Fujiki N., Nishino S., Holtzman D.M. Amyloid-β Dynamics Are Regulated by Orexin and the Sleep-Wake Cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Holth J.K., Fritschi S.K., Wang C., Pedersen N.P., Cirrito J.R., Mahan T.E., Finn M.B., Manis M., Geerling J.C., Fuller P.M., et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science. 2019;363:880–884. doi: 10.1126/science.aav2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nedergaard M., Goldman S.A. Glymphatic failure as a final common pathway to dementia. Science. 2020;370:50–56. doi: 10.1126/science.abb8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gagnon J., Lafreniere A., Rauchs G., Petit D., Carrier J. Sleep in normal aging, Alzheimer’s disease, and mild cognitive impairment. In: Dringenberg H.C., editor. Handbook of Sleep Research. Vol. 30. Elsevier; Amsterdam, The Netherlands: 2019. pp. 677–692. [Google Scholar]
- 183.Ning S., Jorfi M. Beyond the sleep-amyloid interactions in Alzheimer’s disease pathogenesis. J. Neurophysiol. 2019;122:1–4. doi: 10.1152/jn.00118.2019. [DOI] [PubMed] [Google Scholar]
- 184.Sanchez-Vives M.V. Origin and dynamics of cortical slow oscillations. Curr. Opin. Physiol. 2020;15:217–223. doi: 10.1016/j.cophys.2020.04.005. [DOI] [Google Scholar]
- 185.Noya S.B., Colameo D., Brüning F., Spinnler A., Mircsof D., Opitz L., Mann M., Tyagarajan S.K., Robles M.S., Brown S.A. The forebrain synaptic transcriptome is organized by clocks but its proteome is driven by sleep. Science. 2019;366:eaav2642. doi: 10.1126/science.aav2642. [DOI] [PubMed] [Google Scholar]
- 186.Brüning F., Noya S.B., Bange T., Koutsouli S., Rudolph J.D., Tyagarajan S.K., Cox J., Mann M., Brown S.A., Robles M.S. Sleep-wake cycles drive daily dynamics of synaptic phosphorylation. Science. 2019;366:eaav3617. doi: 10.1126/science.aav3617. [DOI] [PubMed] [Google Scholar]
- 187.Borbély A.A. A two process model of sleep regulation. Hum. Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 188.Timofeev I., Steriade M. Low-frequency rhythms in the thalamus of intact-cortex and decorticated cats. J. Neurophysiol. 1996;76:4152–4168. doi: 10.1152/jn.1996.76.6.4152. [DOI] [PubMed] [Google Scholar]
- 189.Sanchez-Vives M., McCormick D.A. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat. Neurosci. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- 190.Beltramo R., D’Urso G., Maschio M.D., Farisello P., Bovetti S., Clovis Y., Lassi G., Tucci V., Tonelli D.D.P., Fellin T. Layer-specific excitatory circuits differentially control recurrent network dynamics in the neocortex. Nat. Neurosci. 2013;16:227–234. doi: 10.1038/nn.3306. [DOI] [PubMed] [Google Scholar]
- 191.Levenstein D., Buzsáki G., Rinzel J. NREM sleep in the rodent neocortex and hippocampus reflects excitable dynamics. Nat. Commun. 2019;10:1–12. doi: 10.1038/s41467-019-10327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hill S., Tononi G. Modeling Sleep and Wakefulness in the Thalamocortical System. J. Neurophysiol. 2005;93:1671–1698. doi: 10.1152/jn.00915.2004. [DOI] [PubMed] [Google Scholar]
- 193.Destexhe A. Self-sustained asynchronous irregular states and Up–Down states in thalamic, cortical and thalamocortical networks of nonlinear integrate-and-fire neurons. J. Comput. Neurosci. 2009;27:493–506. doi: 10.1007/s10827-009-0164-4. [DOI] [PubMed] [Google Scholar]
- 194.Bazhenov M., Timofeev I., Steriade M., Sejnowski T.J. Model of Thalamocortical Slow-Wave Sleep Oscillations and Transitions to Activated States. J. Neurosci. 2002;22:8691–8704. doi: 10.1523/JNEUROSCI.22-19-08691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Compte A., Sanchez-Vives M., McCormick D.A., Wang X.-J. Cellular and Network Mechanisms of Slow Oscillatory Activity (<1 Hz) and wave propagations in a cortical network model. J. Neurophysiol. 2003;89:2707–2725. doi: 10.1152/jn.00845.2002. [DOI] [PubMed] [Google Scholar]
- 196.Fröhlich F., Bazhenov M., Timofeev I., Steriade M., Sejnowski T.J. Slow State Transitions of Sustained Neural Oscillations by Activity-Dependent Modulation of Intrinsic Excitability. J. Neurosci. 2006;26:6153–6162. doi: 10.1523/JNEUROSCI.5509-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shu Y., Hasenstaub A., McCormick D.A. Turning on and off recurrent balanced cortical activity. Nat. Cell Biol. 2003;423:288–293. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- 198.Zucca S., D’Urso G., Pasquale V., Vecchia D., Pica G., Bovetti S., Moretti C., Varani S., Mazon M.M., Chiappalone M., et al. An inhibitory gate for state transition in cortex. eLife. 2017;6:e26177. doi: 10.7554/eLife.26177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Valero M., Viney T.J., Machold R., Mederos S., Zutshi I., Schuman B., Senzai Y., Rudy B., Buzsáki G. Sleep down state-active ID2/Nkx2.1 interneurons in the neocortex. Nat. Neurosci. 2021;24:401–411. doi: 10.1038/s41593-021-00797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Parga N., Abbott L.F. Network model of spontaneous activity exhibiting synchronous transitions between up and down states. Front. Behav. Neurosci. 2007;1:57–66. doi: 10.3389/neuro.01.1.1.004.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jercog D., Roxin A., Barthó P., Luczak A., Compte A., De La Rocha J. UP-DOWN cortical dynamics reflect state transitions in a bistable network. eLife. 2017;6:e22425. doi: 10.7554/eLife.22425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zucca S., Pasquale V., Roig P.L.D.L., Panzeri S., Fellin T. Thalamic Drive of Cortical Parvalbumin-Positive Interneurons during Down States in Anesthetized Mice. Curr. Biol. 2019;29:1481–1490.e6. doi: 10.1016/j.cub.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bojarskaite L., Bjørnstad D.M., Pettersen K.H., Cunen C., Hermansen G.H., Åbjørsbråten K.S., Chambers A.R., Sprengel R., Vervaeke K., Tang W., et al. Astrocytic Ca2+ signaling is reduced during sleep and is involved in the regulation of slow wave sleep. Nat. Commun. 2020;11:1–16. doi: 10.1038/s41467-020-17062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Buskila Y., Bellot-Saez A., Morley J.W. Generating Brain Waves, the Power of Astrocytes. Front. Neurosci. 2019;13:1125. doi: 10.3389/fnins.2019.01125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Szabó Z., Héja L., Szalay G., Kékesi O., Füredi A., Szebényi K., Dobolyi Á., Orbán T.I., Kolacsek O., Tompa T., et al. Extensive astrocyte synchronization advances neuronal coupling in slow wave activity in vivo. Sci. Rep. 2017;7:1–18. doi: 10.1038/s41598-017-06073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hirase H., Qian L., Barthó P., Buzsaki G. Calcium Dynamics of Cortical Astrocytic Networks In Vivo. PLoS Biol. 2004;2:e96. doi: 10.1371/journal.pbio.0020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Amzica F., Steriade M. Neuronal and Glial Membrane Potentials during Sleep and Paroxysmal Oscillations in the Neocortex. J. Neurosci. 2000;20:6648–6665. doi: 10.1523/JNEUROSCI.20-17-06648.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Timofeev I., Schoch S., LeBourgeois M.K., Huber R., Riedner B.A., Kurth S. Spatio-temporal properties of sleep slow waves and implications for development. Curr. Opin. Physiol. 2020;15:172–182. doi: 10.1016/j.cophys.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Billeh Y.N., Cai B., Gratiy S.L., Dai K., Iyer R., Gouwens N.W., Abbasi-Asl R., Jia X., Siegle J.H., Olsen S.R., et al. Systematic Integration of Structural and Functional Data into Multi-scale Models of Mouse Primary Visual Cortex. Neuron. 2020;106:388–403.e18. doi: 10.1016/j.neuron.2020.01.040. [DOI] [PubMed] [Google Scholar]
- 210.Markram H., Muller E., Ramaswamy S., Reimann M.W., Abdellah M., Sanchez C.A., Ailamaki A., Alonso-Nanclares L., Antille N., Arsever S., et al. Reconstruction and Simulation of Neocortical Microcircuitry. Cell. 2015;163:456–492. doi: 10.1016/j.cell.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 211.Nir Y., Staba R.J., Andrillon T., Vyazovskiy V., Cirelli C., Fried I., Tononi G. Regional Slow Waves and Spindles in Human Sleep. Neuron. 2011;70:153–169. doi: 10.1016/j.neuron.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Murphy M., Riedner B.A., Huber R., Massimini M., Ferrarelli F., Tononi G. Source modeling sleep slow waves. Proc. Natl. Acad. Sci. USA. 2009;106:1608–1613. doi: 10.1073/pnas.0807933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Massimini M., Huber R., Ferrarelli F., Hill S., Tononi G. The Sleep Slow Oscillation as a Traveling Wave. J. Neurosci. 2004;24:6862–6870. doi: 10.1523/JNEUROSCI.1318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Burke S.L., Hu T., Spadola C., Burgess A., Li T., Cadet T. Treatment of Sleep Disturbance May Reduce the Risk of Future Probable Alzheimer’s Disease. J. Aging Health. 2019;31:322–342. doi: 10.1177/0898264318795567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Keage H.A.D., Banks S., Yang K.L., Morgan K., Brayne C., Matthews F. What sleep characteristics predict cognitive decline in the elderly? Sleep Med. 2012;13:886–892. doi: 10.1016/j.sleep.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 216.Lim A.S.P., Kowgier M., Yu L., Buchman A.S., Bennett D.A. Sleep Fragmentation and the Risk of Incident Alzheimer’s Disease and Cognitive Decline in Older Persons. Sleep. 2013;36:1027–1032. doi: 10.5665/sleep.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Petrovsky D.V., McPhillips M.V., Li J., Brody A., Caffeé L., Hodgson N.A. Sleep disruption and quality of life in persons with dementia: A state-of-the-art review. Geriatr. Nurs. 2018;39:640–645. doi: 10.1016/j.gerinurse.2018.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Potvin O., Lorrain D., Forget H., Dubé M., Grenier S., Préville M., Hudon C. Sleep Quality and 1-Year Incident Cognitive Impairment in Community-Dwelling Older Adults. Sleep. 2012;35:491–499. doi: 10.5665/sleep.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sindi S., Kåreholt I., Johansson L., Skoog J., Sjöberg L., Wang H.-X., Johansson B., Fratiglioni L., Soininen H., Solomon A., et al. Sleep disturbances and dementia risk: A multicenter study. Alzheimer’s Dement. 2018;14:1235–1242. doi: 10.1016/j.jalz.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 220.Yaffe K., Nettiksimmons J., Yesavage J., Byers A. Sleep Quality and Risk of Dementia Among Older Male Veterans. Am. J. Geriatr. Psychiatry. 2015;23:651–654. doi: 10.1016/j.jagp.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 221.Bliwise D., Tinklenberg J., Yesavage J., Davies H., Pursley A., Petta D., Widrow L., Guilleminault C., Zarcone V., Dement W. REM latency in Alzheimer’s disease. Biol. Psychiatry. 1989;25:320–328. doi: 10.1016/0006-3223(89)90179-0. [DOI] [PubMed] [Google Scholar]
- 222.Liguori C., Placidi F., Izzi F., Spanetta M., Mercuri N.B., Di Pucchio A. Sleep dysregulation, memory impairment, and CSF biomarkers during different levels of neurocognitive functioning in Alzheimer’s disease course. Alzheimer’s Res. Ther. 2020;12:1–13. doi: 10.1186/s13195-019-0571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Liguori C., Romigi A., Nuccetelli M., Zannino S., Sancesario G., Martorana A., Albanese M., Mercuri N.B., Izzi F., Bernardini S., et al. Orexinergic System Dysregulation, Sleep Impairment, and Cognitive Decline in Alzheimer Disease. JAMA Neurol. 2014;71:1498–1505. doi: 10.1001/jamaneurol.2014.2510. [DOI] [PubMed] [Google Scholar]
- 224.Liu S., Pan J., Tang K., Lei Q., He L., Meng Y., Cai X., Li Z. Sleep spindles, K-complexes, limb movements and sleep stage proportions may be biomarkers for amnestic mild cognitive impairment and Alzheimer’s disease. Sleep Breath. 2019;24:637–651. doi: 10.1007/s11325-019-01970-9. [DOI] [PubMed] [Google Scholar]
- 225.Loewenstein R.J., Weingartner H., Gillin J., Kaye W., Ebert M., Mendelson W.B. Disturbances of sleep and cognitive functioning in patients with dementia. Neurobiol. Aging. 1982;3:371–377. doi: 10.1016/0197-4580(82)90025-2. [DOI] [PubMed] [Google Scholar]
- 226.Vitiello M.V., Prinz P.N., Williams D.E., Frommlet M.S., Ries R.K. Sleep Disturbances in Patients With Mild-Stage Alzheimer’s Disease. J. Gerontol. 1990;45:M131–M138. doi: 10.1093/geronj/45.4.M131. [DOI] [PubMed] [Google Scholar]
- 227.Prinz P.N., Vitaliano P.P., Vitiello M.V., Bokan J., Raskind M., Peskind E., Gerber C. Sleep, EEG and mental function changes in senile dementia of the Alzheimer’s type. Neurobiol. Aging. 1982;3:361–370. doi: 10.1016/0197-4580(82)90024-0. [DOI] [PubMed] [Google Scholar]
- 228.Prinz P.N., Peskind E.R., Vitaliano P.P., Raskind M.A., Eisdorfer C., Zemcuznikov H.N., Gerber C.J. Changes in the Sleep and Waking EEGs of Nondemented and Demented Elderly Subjects. J. Am. Geriatr. Soc. 1982;30:86–92. doi: 10.1111/j.1532-5415.1982.tb01279.x. [DOI] [PubMed] [Google Scholar]
- 229.Moe K.E., Vitiello M.V., Larsen L.H., Prinz P.N. Sleep/wake patterns In Alzheimer’s disease: Relationships with cognition and function. J. Sleep Res. 1995;4:15–20. doi: 10.1111/j.1365-2869.1995.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 230.Mander A.B., Rao V., Lu B., Saletin J., Lindquist J.R., Ancoli-Israel S., Jagust W., Walker M.P. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat. Neurosci. 2013;16:357–364. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Mander B.A., Marks S.M., Vogel J.W., Rao V., Lu B., Saletin J.M., Ancoli-Israel S., Jagust W.J., Walker M.P. β-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat. Neurosci. 2015;18:1051–1057. doi: 10.1038/nn.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Lucey B.P., McCullough A., Landsness E.C., Toedebusch C.D., McLeland J.S., Zaza A.M., Fagan A.M., McCue L., Xiong C., Morris J.C., et al. Reduced non–rapid eye movement sleep is associated with tau pathology in early Alzheimer’s disease. Sci. Transl. Med. 2019;11:eaau6550. doi: 10.1126/scitranslmed.aau6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Castano-Prat P., Perez-Mendez L., Perez-Zabalza M., Sanfeliu C., Giménez-Llort L., Sanchez-Vives M.V. Altered slow (<1 Hz) and fast (beta and gamma) neocortical oscillations in the 3xTg-AD mouse model of Alzheimer's disease under anes-thesia. Neurobiol. Aging. 2019;79:142–151. doi: 10.1016/j.neurobiolaging.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 234.Kent B., Strittmatter S., Nygaard H.B. Sleep and EEG Power Spectral Analysis in Three Transgenic Mouse Models of Alzheimer’s Disease: APP/PS1, 3xTgAD, and Tg2576. J. Alzheimer’s Dis. 2018;64:1325–1336. doi: 10.3233/JAD-180260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Roh J.H., Huang Y., Bero A.W., Kasten T., Stewart F.R., Bateman R., Holtzman D.M. Disruption of the Sleep-Wake Cycle and Diurnal Fluctuation of -Amyloid in Mice with Alzheimer’s Disease Pathology. Sci. Transl. Med. 2012;4:150ra122. doi: 10.1126/scitranslmed.3004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zhang B., Veasey S.C., Wood M.A., Leng L.Z., Kaminski C., Leight S., Abel T., Lee V.M.-Y., Trojanowski J.Q. Impaired Rapid Eye Movement Sleep in the Tg2576 APP Murine Model of Alzheimer’s Disease with Injury to Pedunculopontine Cholinergic Neurons. Am. J. Pathol. 2005;167:1361–1369. doi: 10.1016/S0002-9440(10)61223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Holth J.K., Mahan T.E., Robinson G.O., da Rocha A.S., Holtzman D.M. Altered sleep and EEG power in the P301S Tau transgenic mouse model. Ann. Clin. Transl. Neurol. 2017;4:180–190. doi: 10.1002/acn3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Holton C.M., Hanley N., Shanks E., Oxley P., McCarthy A., Eastwood B.J., Murray T.K., Nickerson A., Wafford K.A. Longitudinal changes in EEG power, sleep cycles and behaviour in a tau model of neurodegeneration. Alzheimer’s Res. Ther. 2020;12:1–15. doi: 10.1186/s13195-020-00651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Jyoti A., Plano A., Riedel G., Platt B. Progressive age-related changes in sleep and EEG profiles in the PLB1Triple mouse model of Alzheimer’s disease. Neurobiol. Aging. 2015;36:2768–2784. doi: 10.1016/j.neurobiolaging.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 240.Koss D.J., Robinson L., Drever B.D., Plucinska K., Stoppelkamp S., Veselcic P., Riedel G., Platt B. Mutant Tau knock-in mice display frontotemporal dementia relevant behaviour and histopathology. Neurobiol. Dis. 2016;91:105–123. doi: 10.1016/j.nbd.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 241.Kastanenka K.V., Calvo-Rodriguez M., Hou S.S., Zhou H., Takeda S., Arbel-Ornath M., Lariviere A., Lee Y.F., Kim A., Hawkes J.M., et al. Frequency-dependent exacerbation of Alzheimer’s disease neuropathophysiology. Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-019-44964-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Keskin A.D., Kekuš M., Adelsberger H., Neumann U., Shimshek D.R., Song B., Nelken I., Nelken I., Sakmann B., Konnerth A., et al. BACE inhibition-dependent repair of Alzheimer’s pathophysiology. Proc. Natl. Acad. Sci. USA. 2017;114:8631–8636. doi: 10.1073/pnas.1708106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Busche M.A., Eichhoff G., Adelsberger H., Abramowski D., Wiederhold K.-H., Haass C., Staufenbiel M., Konnerth A., Garaschuk O. Clusters of Hyperactive Neurons Near Amyloid Plaques in a Mouse Model of Alzheimer’s Disease. Science. 2008;321:1686–1689. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- 244.Bero A.W., Yan P., Roh J.H., Cirrito J.R., Stewart F.R., Raichle M.E., Lee J.-M., Holtzman D.M. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat. Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Rodriguez G.A., Barrett G.M., Duff K.E., Hussaini S.A. Chemogenetic attenuation of neuronal activity in the entorhinal cortex reduces Aβ and tau pathology in the hippocampus. PLoS Biol. 2020;18:e3000851. doi: 10.1371/journal.pbio.3000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Yamamoto K., Tanei Z.-I., Hashimoto T., Wakabayashi T., Okuno H., Naka Y., Yizhar O., Fenno L.E., Fukayama M., Bito H., et al. Chronic Optogenetic Activation Augments Aβ Pathology in a Mouse Model of Alzheimer Disease. Cell Rep. 2015;11:859–865. doi: 10.1016/j.celrep.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 247.Yuan P., Grutzendler J. Attenuation of β-Amyloid Deposition and Neurotoxicity by Chemogenetic Modulation of Neural Activity. J. Neurosci. 2016;36:632–641. doi: 10.1523/JNEUROSCI.2531-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ju Y.-E., Lucey B., Holtzman D.M. Sleep and Alzheimer disease pathology—A bidirectional relationship. Nat. Rev. Neurol. 2014;10:115–119. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Iliff J.J., Wang M., Liao Y., Plogg B.A., Peng W., Gundersen G.A., Benveniste H., Vates G.E., Deane R., Goldman S.A., et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci. Transl. Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Götz J., Bodea L.-G., Goedert M. Rodent models for Alzheimer disease. Nat. Rev. Neurosci. 2018;19:583–598. doi: 10.1038/s41583-018-0054-8. [DOI] [PubMed] [Google Scholar]
- 251.Steriade M., McCormick D., Sejnowski T.J. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 252.Romanella S., Roe D., Tatti E., Cappon D., Paciorek R., Testani E., Rossi A., Rossi S., Santarnecchi E. The Sleep Side of Aging and Alzheimer’s Disease. Sleep Med. 2021;77:209–225. doi: 10.1016/j.sleep.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Rasch B., Born J. About Sleep’s Role in Memory. Physiol. Rev. 2013;93:681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Héricé C., Patel A.A., Sakata S. Circuit mechanisms and computational models of REM sleep. Neurosci. Res. 2019;140:77–92. doi: 10.1016/j.neures.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Karashima A., Nakao M., Honda K., Iwasaki N., Katayama N., Yamamoto M. Theta wave amplitude and frequency are differentially correlated with pontine waves and rapid eye movements during REM sleep in rats. Neurosci. Res. 2004;50:283–289. doi: 10.1016/j.neures.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 256.Karashima A., Nakamura K., Sato N., Nakao M., Katayama N., Yamamoto M. Phase-locking of spontaneous and elicited ponto–geniculo–occipital waves is associated with acceleration of hippocampal theta waves during rapid eye movement sleep in cats. Brain Res. 2002;958:347–358. doi: 10.1016/S0006-8993(02)03673-9. [DOI] [PubMed] [Google Scholar]
- 257.Ramirez-Villegas J.F., Besserve M., Murayama Y., Evrard H.C., Oeltermann A., Logothetis N.K. Coupling of hippocampal theta and ripples with pontogeniculooccipital waves. Nat. Cell Biol. 2021;589:96–102. doi: 10.1038/s41586-020-2914-4. [DOI] [PubMed] [Google Scholar]
- 258.Patel A.A., McAlinden N., Mathieson K., Sakata S. Simultaneous Electrophysiology and Fiber Photometry in Freely Behaving Mice. Front. Neurosci. 2020;14:148. doi: 10.3389/fnins.2020.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Mufson E.J., Mash D.C., Hersh L.B. Neurofibrillary tangles in cholinergic pedunculopontine neurons in Alzheimer’s disease. Ann. Neurol. 1988;24:623–629. doi: 10.1002/ana.410240506. [DOI] [PubMed] [Google Scholar]
- 260.Parvizi J., Van Hoesen G.W., Damasio A. The selective vulnerability of brainstem nuclei to Alzheimer’s disease. Ann. Neurol. 2001;49:53–66. doi: 10.1002/1531-8249(200101)49:1<53::AID-ANA30>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 261.Bohnen N.I., Albin R.L. The cholinergic system and Parkinson disease. Behav. Brain Res. 2011;221:564–573. doi: 10.1016/j.bbr.2009.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Weng Y.-Y., Lei X., Yu J. Sleep spindle abnormalities related to Alzheimer’s disease: A systematic mini-review. Sleep Med. 2020;75:37–44. doi: 10.1016/j.sleep.2020.07.044. [DOI] [PubMed] [Google Scholar]
- 263.Ngo H.-V.V., Martinetz T., Born J., Mölle M. Auditory Closed-Loop Stimulation of the Sleep Slow Oscillation Enhances Memory. Neuron. 2013;78:545–553. doi: 10.1016/j.neuron.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 264.Arzi A., Shedlesky L., Ben-Shaul M., Nasser K., Oksenberg A., Hairston I.S., Sobel N. Humans can learn new information during sleep. Nat. Neurosci. 2012;15:1460–1465. doi: 10.1038/nn.3193. [DOI] [PubMed] [Google Scholar]
- 265.Ball W.A., Morrison A.R., Ross R.J. The effects of tones on PGO waves in slow wave sleep and paradoxical sleep. Exp. Neurol. 1989;104:251–256. doi: 10.1016/0014-4886(89)90037-X. [DOI] [PubMed] [Google Scholar]
- 266.Buzsáki G. Rhythms of the Brain. Oxford University Press (OUP); New York, NY, USA: 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included in this published article as figures and tables.