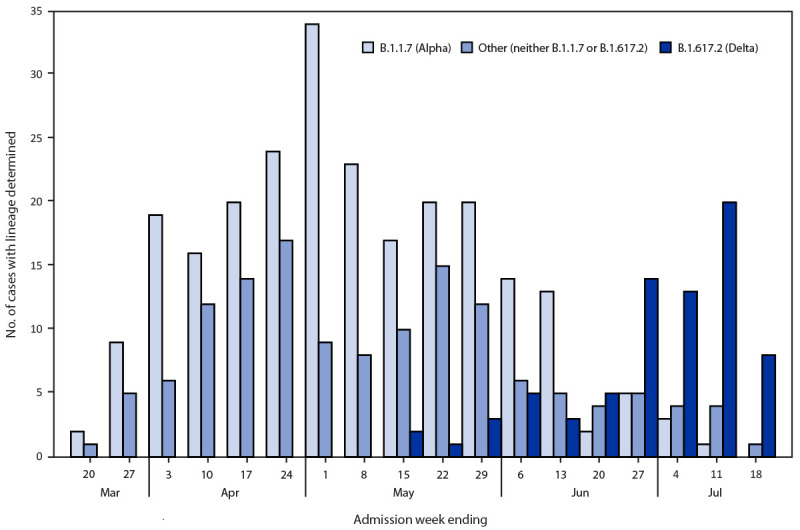
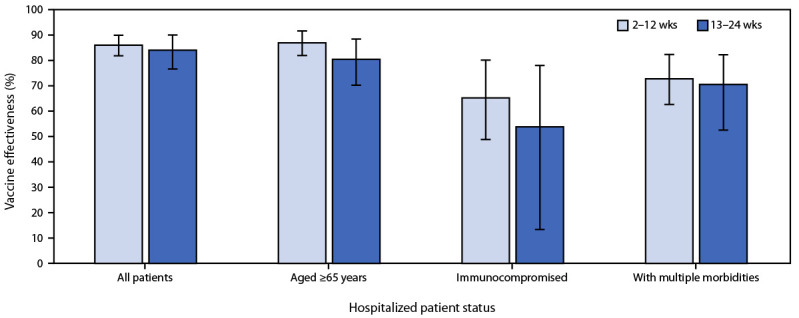
Mark W Tenforde
Mark W Tenforde, MD, PhD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
1,✉,
Wesley H Self
Wesley H Self, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
2,
Eric A Naioti
Eric A Naioti, MPH
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
1,
Adit A Ginde
Adit A Ginde, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
3,
David J Douin
David J Douin, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
3,
Samantha M Olson
Samantha M Olson, MPH
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
1,
H Keipp Talbot
H Keipp Talbot, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
2,
Jonathan D Casey
Jonathan D Casey, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
2,
Nicholas M Mohr
Nicholas M Mohr, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
4,
Anne Zepeski
Anne Zepeski, PharmD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
4,
Manjusha Gaglani
Manjusha Gaglani, MBBS
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
5,6,
Tresa McNeal
Tresa McNeal, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
5,
Shekhar Ghamande
Shekhar Ghamande, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
5,
Nathan I Shapiro
Nathan I Shapiro, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
7,
Kevin W Gibbs
Kevin W Gibbs, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
8,
D Clark Files
D Clark Files, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
8,
David N Hager
David N Hager, MD, PhD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
9,
Arber Shehu
Arber Shehu, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
9,
Matthew E Prekker
Matthew E Prekker, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
10,
Heidi L Erickson
Heidi L Erickson, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
10,
Michelle N Gong
Michelle N Gong, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
11,
Amira Mohamed
Amira Mohamed, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
11,
Daniel J Henning
Daniel J Henning, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
12,
Jay S Steingrub
Jay S Steingrub, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
13,
Ithan D Peltan
Ithan D Peltan, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
14,
Samuel M Brown
Samuel M Brown, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
14,
Emily T Martin
Emily T Martin, PhD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
15,
Arnold S Monto
Arnold S Monto, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
15,
Akram Khan
Akram Khan, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
16,
Catherine L Hough
Catherine L Hough, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
16,
Laurence W Busse
Laurence W Busse, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
17,
Caitlin C ten Lohuis
Caitlin C ten Lohuis, ACNP-BC
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
17,
Abhijit Duggal
Abhijit Duggal, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
18,
Jennifer G Wilson
Jennifer G Wilson, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
19,
Alexandra June Gordon
Alexandra June Gordon, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
19,
Nida Qadir
Nida Qadir, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
20,
Steven Y Chang
Steven Y Chang, MD, PhD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
20,
Christopher Mallow
Christopher Mallow, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
21,
Carolina Rivas
Carolina Rivas
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
21,
Hilary M Babcock
Hilary M Babcock, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
22,
Jennie H Kwon
Jennie H Kwon, DO
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
22,
Matthew C Exline
Matthew C Exline, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
23,
Natasha Halasa
Natasha Halasa, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
2,
James D Chappell
James D Chappell, MD, PhD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
2,
Adam S Lauring
Adam S Lauring, MD, PhD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
24,
Carlos G Grijalva
Carlos G Grijalva, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
2,
Todd W Rice
Todd W Rice, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
2,
Ian D Jones
Ian D Jones, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
2,
William B Stubblefield
William B Stubblefield, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
2,
Adrienne Baughman
Adrienne Baughman
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
2,
Kelsey N Womack
Kelsey N Womack, PhD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
2,
Christopher J Lindsell
Christopher J Lindsell, PhD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
2,
Kimberly W Hart
Kimberly W Hart, MA
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
2,
Yuwei Zhu
Yuwei Zhu, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
2,
Meagan Stephenson
Meagan Stephenson, MPH
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
1,
Stephanie J Schrag
Stephanie J Schrag, DPhil
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
1,
Miwako Kobayashi
Miwako Kobayashi, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
1,
Jennifer R Verani
Jennifer R Verani, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
1,
Manish M Patel
Manish M Patel, MD
11CDC COVID-19 Response Team; 2Vanderbilt University Medical Center, Nashville, Tennessee; 3University of Colorado School of Medicine, Aurora, Colorado; 4University of Iowa, Iowa City, Iowa; 5Baylor Scott & White Health, Temple, Texas; 6Texas A&M University College of Medicine, Temple, Texas; 7Beth Israel Deaconess Medical Center, Boston, Massachusetts; 8Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina; 9Johns Hopkins Hospital, Baltimore, Maryland; 10Hennepin County Medical Center, Minneapolis, Minnesota; 11Montefiore Healthcare Center, Albert Einstein College of Medicine, Bronx, New York; 12University of Washington School of Medicine, Seattle, Washington; 13Baystate Medical Center, Springfield, Massachusetts; 14Intermountain Medical Center and University of Utah, Salt Lake City, Utah; 15University of Michigan School of Public Health, Ann Arbor, Michigan; 16Oregon Health & Science University Hospital, Portland, Oregon; 17Emory University School of Medicine, Atlanta, Georgia; 18Cleveland Clinic, Cleveland, Ohio; 19Stanford University School of Medicine, Palo Alto, California; 20Ronald Reagan-UCLA Medical Center, Los Angeles, California; 21University of Miami, Miami, Florida; 22Washington University, St. Louis, Missouri; 23Ohio State University Wexner Medical Center, Columbus, Ohio; 24University of Michigan School of Medicine, Ann Arbor, Michigan.
1;
IVY Network Investigators1;
IVY Network,
Nicole Calhoun
Nicole Calhoun
2Baylor Scott & White Health
2,
Kempapura Murthy
Kempapura Murthy
3Baylor Scott & White Health
3,
Judy Herrick
Judy Herrick
4Baylor Scott & White Health
4,
Amanda McKillop
Amanda McKillop
5Baylor Scott & White Health
5,
Eric Hoffman
Eric Hoffman
6Baylor Scott & White Health
6,
Martha Zayed
Martha Zayed
7Baylor Scott & White Health
7,
Michael Smith
Michael Smith
8Baylor Scott & White Health
8,
Natalie Settele
Natalie Settele
9Baylor Scott & White Health
9,
Jason Ettlinger
Jason Ettlinger
10Baylor Scott & White Health
10,
Elisa Priest
Elisa Priest
11Baylor Scott & White Health
11,
Jennifer Thomas
Jennifer Thomas
12Baylor Scott & White Health
12,
Alejandro Arroliga
Alejandro Arroliga
13Baylor Scott & White Health
13,
Madhava Beeram
Madhava Beeram
14Baylor Scott & White Health
14,
Ryan Kindle
Ryan Kindle
15Baystate Medical Center
15,
Lori-Ann Kozikowski
Lori-Ann Kozikowski
16Baystate Medical Center
16,
Lesley De Souza
Lesley De Souza
17Baystate Medical Center
17,
Scott Ouellette
Scott Ouellette
18Baystate Medical Center
18,
Sherell Thornton-Thompson
Sherell Thornton-Thompson
19Baystate Medical Center
19,
Patrick Tyler
Patrick Tyler
20Beth Israel Deaconess Medical Center
20,
Omar Mehkri
Omar Mehkri
21Cleveland Clinic
21,
Kiran Ashok
Kiran Ashok
22Cleveland Clinic
22,
Susan Gole
Susan Gole
23Cleveland Clinic
23,
Alexander King
Alexander King
24Cleveland Clinic
24,
Bryan Poynter
Bryan Poynter
25Cleveland Clinic
25,
Nicholas Stanley
Nicholas Stanley
26Emory University
26,
Audrey Hendrickson
Audrey Hendrickson
27Hennepin County Medical Center
27,
Ellen Maruggi
Ellen Maruggi
28Hennepin County Medical Center
28,
Tyler Scharber
Tyler Scharber
29Hennepin County Medical Center
29,
Jeffrey Jorgensen
Jeffrey Jorgensen
30Intermountain Medical Center
30,
Robert Bowers
Robert Bowers
31Intermountain Medical Center
31,
Jennifer King
Jennifer King
32Intermountain Medical Center
32,
Valerie Aston
Valerie Aston
33Intermountain Medical Center
33,
Brent Armbruster
Brent Armbruster
34Intermountain Medical Center
34,
Richard E Rothman
Richard E Rothman
35Johns Hopkins University
35,
Rahul Nair
Rahul Nair
36Montefiore Medical Center
36,
Jen-Ting (Tina) Chen
Jen-Ting (Tina) Chen
37Montefiore Medical Center
37,
Sarah Karow
Sarah Karow
38Ohio State University
38,
Emily Robart
Emily Robart
39Ohio State University
39,
Paulo Nunes Maldonado
Paulo Nunes Maldonado
40Ohio State University
40,
Maryiam Khan
Maryiam Khan
41Ohio State University
41,
Preston So
Preston So
42Ohio State University
42,
Joe Levitt
Joe Levitt
43Stanford University
43,
Cynthia Perez
Cynthia Perez
44Stanford University
44,
Anita Visweswaran
Anita Visweswaran
45Stanford University
45,
Jonasel Roque
Jonasel Roque
46Stanford University
46,
Adreanne Rivera
Adreanne Rivera
47University of California, Los Angeles
47,
Trevor Frankel
Trevor Frankel
48University of California, Los Angeles
48,
Michelle Howell
Michelle Howell
49UCHealth University of Colorado Hospital
49,
Jennifer Friedel
Jennifer Friedel
50UCHealth University of Colorado Hospital
50,
Jennifer Goff
Jennifer Goff
51UCHealth University of Colorado Hospital
51,
David Huynh
David Huynh
52UCHealth University of Colorado Hospital
52,
Michael Tozier
Michael Tozier
53UCHealth University of Colorado Hospital
53,
Conner Driver
Conner Driver
54UCHealth University of Colorado Hospital
54,
Michael Carricato
Michael Carricato
55UCHealth University of Colorado Hospital
55,
Alexandra Foster
Alexandra Foster
56UCHealth University of Colorado Hospital
56,
Paul Nassar
Paul Nassar
57University of Iowa
57,
Lori Stout
Lori Stout
58University of Iowa
58,
Zita Sibenaller
Zita Sibenaller
59University of Iowa
59,
Alicia Walter
Alicia Walter
60University of Iowa
60,
Jasmine Mares
Jasmine Mares
61University of Iowa
61,
Logan Olson
Logan Olson
62University of Iowa
62,
Bradley Clinansmith
Bradley Clinansmith
63University of Iowa
63,
Carolina Rivas
Carolina Rivas
64University of Miami
64,
Hayley Gershengorn
Hayley Gershengorn
65University of Miami
65,
EJ McSpadden
EJ McSpadden
66University of Michigan
66,
Rachel Truscon
Rachel Truscon
67University of Michigan
67,
Anne Kaniclides
Anne Kaniclides
68University of Michigan
68,
Lara Thomas
Lara Thomas
69University of Michigan
69,
Ramsay Bielak
Ramsay Bielak
70University of Michigan
70,
Weronika Damek Valvano
Weronika Damek Valvano
71University of Michigan
71,
Rebecca Fong
Rebecca Fong
72University of Michigan
72,
William J Fitzsimmons
William J Fitzsimmons
73University of Michigan
73,
Christopher Blair
Christopher Blair
74University of Michigan
74,
Andrew L Valesano
Andrew L Valesano
75University of Michigan
75,
Julie Gilbert
Julie Gilbert
76University of Michigan
76,
Christine D Crider
Christine D Crider
77University of Washington
77,
Kyle A Steinbock
Kyle A Steinbock
78University of Washington
78,
Thomas C Paulson
Thomas C Paulson
79University of Washington
79,
Layla A Anderson
Layla A Anderson
80University of Washington
80,
Christy Kampe
Christy Kampe
81Vanderbilt University Medical Center
81,
Jakea Johnson
Jakea Johnson
82Vanderbilt University Medical Center
82,
Rendie McHenry
Rendie McHenry
83Vanderbilt University Medical Center
83,
Marcia Blair
Marcia Blair
84Vanderbilt University Medical Center
84,
Douglas Conway
Douglas Conway
85Vanderbilt University Medical Center
85,
Mary LaRose
Mary LaRose
86Wake Forest University
86,
Leigha Landreth
Leigha Landreth
87Wake Forest University
87,
Madeline Hicks
Madeline Hicks
88Wake Forest University
88,
Lisa Parks
Lisa Parks
89Wake Forest University
89,
Jahnavi Bongu
Jahnavi Bongu
90Washington University
90,
David McDonald
David McDonald
91Washington University
91,
Candice Cass
Candice Cass
92Washington University
92,
Sondra Seiler
Sondra Seiler
93Washington University
93,
David Park
David Park
94Washington University
94,
Tiffany Hink
Tiffany Hink
95Washington University
95,
Meghan Wallace
Meghan Wallace
96Washington University
96,
Carey-Ann Burnham
Carey-Ann Burnham
97Washington University
97,
Olivia G Arter
Olivia G Arter
98Washington University
98