Abstract
Background
Sarcopenia and obesity are reportedly associated with risk of cognitive decline, and sarcopenic obesity (SO) heightens the risk, but the evidence is sparse and inconclusive. This study aimed to examine the association between SO and cognitive impairment.
Methods
A total of 542 community-dwelling adults aged between 21 and 90 years were recruited. All participants were assessed for body composition (dual X-ray absorptiometry), handgrip strength (HGS), gait speed (GS), and cognitive function (Repeatable Battery for the Assessment of Neuropsychological Status). Sarcopenia was defined by the presence of low appendicular lean mass index (ALMI) and low HGS or low GS according to the 2019 Asian Working Group for Sarcopenia criteria, and obesity was defined based on the upper two quintiles of fat mass index (FMI).
Results
Sarcopenia alone or in combination with obesity were not significantly associated with cognitive impairment after controlling for confounding variables. Obesity on its own was significantly associated with greater odds of impaired attention (OR: 2.05, 95%CI 1.12–3.82). Low ALMI was not associated, but low HGS, slow GS, and high FMI were individually associated with cognitive impairment: low HGS and immediate memory (OR: 1.91, 95% CI 1.04–3.49); low GS and immediate memory (OR: 2.17, 95% CI 1.26–3.72); high FMI and attention (OR: 2.06, 95% CI 1.22–3.51). Co-occurring high FMI with either low HGS or slow GS exacerbated the observed odds of global and domain-specific (attention, visuospatial) cognitive impairment.
Conclusions
Lean mass is not relevant, whereas muscle strength and physical performance or adiposity are relevant in defining sarcopenia or sarcopenic obesity in terms of their cognitive impacts.
Introduction
Around 50 million people live with dementia worldwide, and this number is projected to increase to 152 million by 2050 [1]. While there is currently no cure for dementia, there is good evidence in support of the prevention or delaying dementia in at-risk individuals in the pre-dementia stage [2]. Mild cognitive impairment is a pre-dementia stage that can be identified years before onset of dementia.
Sarcopenia is a geriatric syndrome, characterised by the progressive loss of skeletal muscle mass and muscle strength with age [3]. With its increasing prevalence in Asia [4], sarcopenia is a public health challenge given its association with functional decline and physical disability [5]. It is also associated with a >2-fold increased risk of cognitive impairment [6,7], plausibly attributed to shared pathophysiology between the two conditions [8,9].
Concurrent with reduction in muscle mass, increase in total body fat and abdominal adiposity is another age-associated phenomenon [10]. Studies have reported mixed findings of a positive relationship between obesity and cognitive impairment [11,12], or a negative relationship [13] depending on age and sex of subjects [14–17]. While obesity in mid-life (45-65y) is an established risk factor for future dementia [2], being obese (body mass index (BMI) ≥30) in late-life (>65y) reduced the risk of dementia compared to normal BMI [18]. This “obesity paradox” in dementia risk across time may be explained by the age-related body composition changes in the trajectory toward sarcopenia and disability [18]. The conflicting evidence and paradox are likely due to the use of BMI to measure obesity because BMI does not differentiate between fat and fat-free mass [19]. Furthermore, most studies only assessed global cognition functioning and did not distinguish between different domains of cognition [14,15].
The coexistence of sarcopenia and obesity—sarcopenic obesity (SO) [20], has been purported to have additive exacerbating effects on cognitive performance [9,21]. However, the evidence on this relationship remained inconclusive. Two studies on community-dwelling older adults found that SO was associated with poorer cognitive performance than either sarcopenia or obesity alone [21,22], while another study reported similar results only among older adults aged 70 and above but not among those aged 60–69 years [9].
The lack of an age appropriate and standardised obesity definition poses a challenge in studying the negative health consequences associated with SO [23]. The aforementioned studies have used percentage body fat [21,22], and waist circumference [9] to determine obesity. Recently, we presented data to show that fat mass index (FMI) may be the most optimal criterion for SO among the obesity measures, as it accounts for body size differences and improves the association between adiposity and physical function [24]. To date, no studies have examined the relationship between cognition and SO based on FMI.
Given the global pandemic of obesity and that sarcopenia could also occur in middle-aged adults [25,26], it is paramount to understand the association between SO and cognitive impairment in a general adult population inclusive of all age groups. Hence, this study aimed to examine the association between SO and global as well as domain-specific aspects of cognition among healthy community-dwelling adults. To better understand this association, this study also examined the association between individual components of SO and cognitive impairment.
Materials and methods
Participants
This is part of the Yishun Study, a cross-sectional study that determined the normative values and prevalence of sarcopenia among a representative sample of 542 community-dwelling adults aged 21–90 years in Singapore. The sampling method has been detailed elsewhere [25]. The final analysis sample consists of 535 participants (308 women, 227 men) who had complete body composition data. All participants provided written informed consent prior to participation and ethics approval was obtained from the National Healthcare Group Domain Specific Review Board (2017/00212).
Anthropometric and body composition measurements
Body weight and height were measured using a digital balance and stadiometer (Seca, GmbH & Co. KG, Hamburg, Germany). BMI was calculated as weight (kg) divided by height (m) squared. Bone mineral density, total body fat percentage, fat mass and appendicular lean mass were measured using dual X-ray absorptiometry (DXA; Discovery WI, Hologic, Inc., Marlborough, USA). FMI and appendicular lean mass index (ALMI) were used as measures of fat mass and muscle mass respectively in the present study to account for height-associated differences in body composition [4,27]. Both FMI and ALMI were calculated as fat mass (kg) and appendicular lean mass (kg) divided by height (m) squared, where fat mass equals to total body fat mass and appendicular lean mass equals to the sum of lean mass in the upper and lower limbs.
Muscle strength and physical function assessment
Handgrip strength (HGS) was used as indication of muscular strength of participants [28] and physical function was measured using usual gait speed (GS) [29]. HGS was assessed using a dynamometer (Jamar Plus+ Dynamometer; Patterson Medical, Evergreen Boulevard, Cedarburg, USA), and the highest of four readings (two trials per arm) recorded. GS was measured using the 6 m GAITRite Walkway (CIR Systems Inc., Sparta. New Jersey, USA) with a 2 m lead in and out phase, and the average speed (three trials) recorded.
Sarcopenia, obesity and sarcopenic obesity assessment
Sarcopenia was assessed using the latest Asian Working Group for Sarcopenia (AWGS) algorithm [4]. Poor physical function was defined as GS <1.0 m/s, low muscle mass as ALMI <7.0 and <5.4 kg/m2, and low muscle strength as HGS <28 and <18 kg for men and women respectively. Sarcopenia was defined as the presence of low muscle mass and poor muscle strength and/or physical performance [4]. Given that there is presently no consensus on the definition of obesity [30], obesity was defined in this study based on the top two quintiles of FMI. The cut-off points adjusted for gender were ≥ 7.63 kg/m2 and ≥ 9.93 kg/m2 for men and women respectively. Participants were categorised into four non-overlapping groups based on presence and absence of sarcopenia and obesity: SO (sarcopenic and obese), sarcopenic (sarcopenic and non-obese), obese (non-sarcopenic and obese) and normal (non-sarcopenic and non-obese).
Cognitive function assessment
Cognitive function was measured using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), which encompassed 12 subtests to assess five cognitive domains: immediate memory, visuospatial/constructional abilities, language, attention, and delayed memory [31]. Total scale index and domain-specific index scores for all participants were scored according to the RBANS manual [32], whereby each score was expressed as a standardised score with a mean of 100 and standard deviation of 15. Cognitive impairment was defined using a cut-off index score <80, which corresponds to the 9th percentile [32].
Other measures
Demographic characteristics such as age, gender, education levels, housing type, smoking history and medical history were collected through a questionnaire. Participants also completed the global physical activity questionnaire to report their typical weekly physical activity levels [33].
Statistical analysis
Differences in variables of interest among the four groups defined by sarcopenia and obesity status were examined with one-way analysis of variance for continuous variables and chi-squared test for categorical variables. Multivariate logistic regressions were performed to examine the associations between SO and impairment in global cognition as well as different domains of cognitive function. Odds ratios (OR) and 95% confidence intervals (CI) were estimated to compare the sarcopenic, obese and SO groups with the normal group. In order to examine the relationship between individual components of SO (muscle strength, muscle mass, physical function, and obesity) and cognitive impairment, participants were dichotomised based on each component and separate logistic regressions were conducted to determine the odds of impairment in different domains of cognitive function. Models were adjusted for age groups, gender, physical activity levels, medical history, and years of education. Statistical significance level was set at p < 0.05 and all analyses were performed using R statistical software, version 3.6.3.
Results
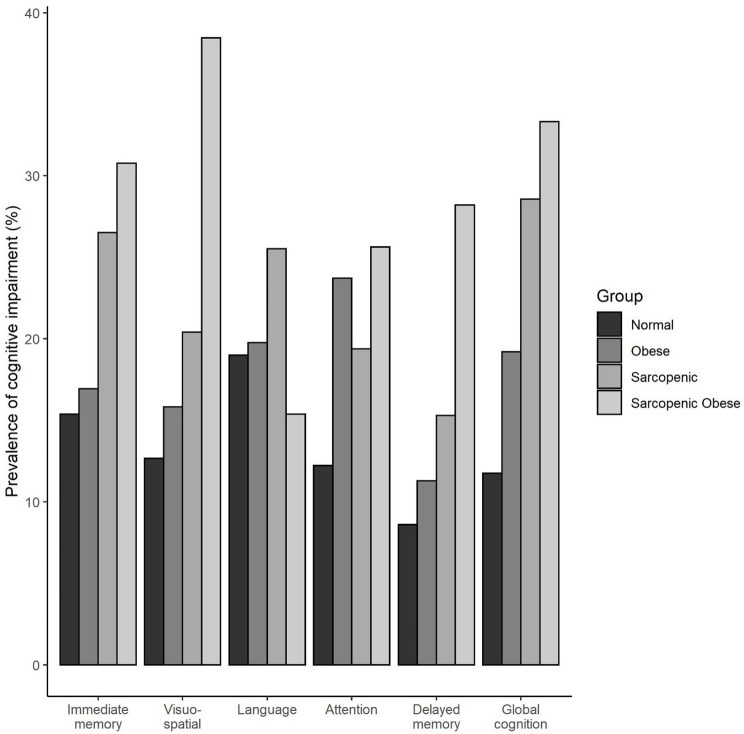
The prevalence of SO, sarcopenia, obesity and normal were 7.3%, 18.3%, 33.1% and 41.3% respectively. Table 1 provides the descriptive characteristics of participants across the four groups. The groups were found to differ in prevalence of medical conditions such as diabetes, hypertension, cardiovascular disease, and high cholesterol. HGS and GS were found to differ significantly between groups, whereby the SO group had the lowest HGS and slowest GS. Both the SO and sarcopenic groups were found to be significantly older and had significantly less education as compared to the obese and normal group. The SO group was also found to be significantly less physically active than the other three groups. The prevalence of cognitive impairment in respective domains across the four groups is shown in Fig 1.
Table 1. Descriptive characteristics of participants by sarcopenia and obesity status.
| Sarcopenic Obese (n = 39) | Sarcopenic (n = 98) | Obese (n = 177) | Normal (n = 221) | p-value | |
|---|---|---|---|---|---|
| Age (y) | 75.59 ± 9.00 | 71.11 ± 14.63 | 56.12 ± 17.86 | 51.97 ± 17.95 | <0.01* |
| Age groups | <0.01* | ||||
| 21–39 years | 0 (0.0%) | 6 (6.1%) | 41 (23.2%) | 64 (29.0%) | |
| 40–59 years | 1 (2.6%) | 8 (8.2%) | 47 (26.6%) | 65 (29.4%) | |
| 60–74 years | 13 (33.3%) | 32 (32.7%) | 59 (33.3%) | 63 (28.5%) | |
| ≥75 years | 25 (64.1%) | 52 (53.0%) | 30 (16.9%) | 29 (13.1%) | |
| Female (n, %) | 25 (64.1%) | 52 (53.1%) | 100 (56.5%) | 131 (59.3%) | 0.61 |
| Smoker (n, %) | 2 (5.1%) | 8 (8.2%) | 20 (11.3%) | 29 (13.1%) | 0.36 |
| Education (y) | 6.79 ± 4.93 | 7.30 ± 5.18 | 9.95 ± 4.97 | 10.89 ± 4.55 | <0.01* |
| PA (MET-min/wk) | 31.69 ± 27.40 | 49.75 ± 46.98 | 75.11 ± 105.26 | 72.74 ± 73.53 | <0.01* |
| Medical history | |||||
| Diabetes | 9 (23.1%) | 27 (27.6%) | 24 (13.6%) | 20 (9.0%) | < 0.01* |
| Hypertension | 29 (74.4%) | 49 (50.0%) | 65 (36.7%) | 53 (24.0%) | < 0.01* |
| Cardiovascular disease | 4 (10.3%) | 12 (12.2%) | 7 (4.0%) | 9 (4.1%) | 0.01* |
| High cholesterol | 27 (69.2%) | 44 (44.9%) | 73 (41.2%) | 58 (26.2%) | <0.01* |
| Stroke | 1 (2.6%) | 2 (2.0%) | 1 (0.6%) | 1 (0.5%) | 0.36 |
| Depression | 2 (5.1%) | 1 (1.0%) | 4 (2.3%) | 1 (0.5%) | 0.11 |
| Body composition | |||||
| BMI (kg/m2) | 25.72 ± 1.78 | 21.48 ± 2.00 | 29.21 ± 4.43 | 22.56 ± 2.55 | <0.01* |
| FMI (kg/m2) | 10.68 ± 1.58 | 7.03 ± 1.55 | 11.42 ± 2.89 | 7.08 ± 1.72 | <0.01* |
| ALMI (kg/m2) | 5.45 ± 0.71 | 5.35 ± 0.78 | 7.04 ± 1.33 | 6.04 ± 1.13 | <0.01* |
| Physical function | |||||
| HGS (kg) | 20.85 ± 6.10 | 22.27 ± 6.26 | 30.50 ± 9.96 | 30.54 ± 8.98 | <0.01* |
| GS (m/s) | 0.84 ± 0.15 | 0.90 ± 0.15 | 1.07 ± 0.18 | 1.15 ± 0.17 | <0.01* |
| Cognitive function | |||||
| RBANS index score | 84.82 ± 14.83 | 86.41 ± 15.41 | 93.65 ± 17.19 | 96.28 ± 15.58 | <0.01* |
| Immediate memory domain | 84.69 ± 14.14 | 88.73 ± 16.31 | 93.77 ± 16.16 | 95.10 ± 15.64 | <0.01* |
| Visuospatial domain | 89.46 ± 19.38 | 90.13 ± 15.12 | 95.98 ± 16.88 | 99.03 ± 16.43 | <0.01* |
| Language domain | 89.56 ± 11.84 | 88.70 ± 15.30 | 91.20 ± 15.02 | 91.28 ± 16.12 | 0.50 |
| Attention domain | 88.05 ± 15.10 | 91.52 ± 15.23 | 96.11 ± 19.32 | 100.47 ± 16.95 | <0.01* |
| Delayed memory domain | 90.49 ± 17.01 | 94.41 ± 16.52 | 98.55 ± 15.89 | 100.35 ± 14.30 | <0.01* |
PA, physical activity levels; BMI, body mass index; FMI, fat mass index; ALMI, appendicular lean mass index; HGS, handgrip strength; GS, gait speed; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status.
*p < 0.05.
All data are presented as mean ± SD or number (%).
Fig 1. Prevalence of cognitive impairment by sarcopenia and obesity status.
Association between cognitive impairment and sarcopenia and obesity status
Table 2 presents the logistic regression analysis results for the association between SO and risk of impairment in different domains of cognitive function. In the unadjusted models, the SO group was found to have significantly higher odds of impairment as compared to the normal group in global cognitive function (OR: 3.75, 95% confidence interval (CI) 1.69–8.13, p < .01), as well as domains of immediate memory (OR: 2.44, 95% CI 1.10–5.21, p = .023), visuospatial/constructional abilities (OR: 4.31, 95% CI 2.00–9.17, p < .01), attention (OR: 2.48, 95% CI 1.05–5.53, p = .031), and delayed memory (OR: 4.18, 95% CI 1.76–9.61, p < .01). Significant association was also found for the sarcopenic group in the unadjusted models for global cognition (OR: 3.00, 95% CI 1.65–5.49, p < .01), and immediate memory (OR: 1.99, 95% CI 1.11–3.54, p = .020). However, the risk of cognitive impairment for the SO and sarcopenic groups were not significant in the adjusted models. The obese group was found to have significantly higher odds of association with impaired global cognition (OR: 1.78, 95% CI 1.03–3.13, p = .041), and attention (OR: 2.24, 95% CI 1.32–3.84, p < .01) in the unadjusted models. After adjusting for confounders, the OR of association between obesity and attention remains significant (OR: 2.08, 95% CI 1.15–3.81, p = .021).
Table 2. Association between sarcopenic obesity and risk of impairment in different domains of cognitive function.
| Sarcopenic Obese (n = 39) | Sarcopenic (n = 98) | Obese (n = 177) | Normal (n = 221) | ||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | ||
| Global cognitive function | |||||||
| model 1 | 3.75 (1.69–8.13) | < .01** | 3.00 (1.65–5.49) | < .01** | 1.78 (1.03–3.13) | .041* | 1.0 |
| model 2 | 1.55 (0.62–3.77) | .335 | 1.46 (0.74–2.89) | .274 | 1.59 (0.88–2.89) | .125 | 1.0 |
| model 3 | 1.62 (0.58–4.47) | .350 | 1.16 (0.53–2.50) | .706 | 1.55 (0.80–3.02) | .192 | 1.0 |
| Immediate memory | |||||||
| model 1 | 2.44 (1.10–5.21) | .023* | 1.99 (1.11–3.54) | .020* | 1.12 (0.65–1.92) | .673 | 1.0 |
| model 2 | 1.38 (0.56–3.27) | .468 | 1.25 (0.64–2.40) | .504 | 0.96 (0.54–1.68) | .875 | 1.0 |
| model 3 | 1.34 (0.52–3.32) | .534 | 1.10 (0.55–2.18) | .777 | 0.87 (0.48–1.55) | .631 | 1.0 |
| Visuospatial/constructional | |||||||
| model 1 | 4.31 (2.00–9.17) | < .01** | 1.77 (0.93–3.31) | .077 | 1.30 (0.73–2.29) | .370 | 1.0 |
| model 2 | 2.15 (0.87–5.22) | .092 | 0.85 (0.40–1.74) | .651 | 1.10 (0.60–2.02) | .757 | 1.0 |
| model 3 | 2.09 (0.80–5.44) | .132 | 0.68 (0.31–1.46) | .334 | 1.02 (0.53–1.93) | .963 | 1.0 |
| Language | |||||||
| model 1 | 0.77 (0.28–1.85) | .592 | 1.46 (0.82–2.56) | .190 | 1.05 (0.63–1.73) | .847 | 1.0 |
| model 2 | 0.44 (0.14–1.18) | .123 | 0.98 (0.50–1.86) | .943 | 0.94 (0.55–1.59) | .822 | 1.0 |
| model 3 | 0.40 (0.12–1.12) | .097 | 0.85 (0.43–1.67) | .644 | 0.86 (0.49–1.48) | .586 | 1.0 |
| Attention | |||||||
| model 1 | 2.48 (1.05–5.53) | .031* | 1.73 (0.90–3.27) | .095 | 2.24 (1.32–3.84) | < .01** | 1.0 |
| model 2 | 1.17 (0.45–2.88) | .737 | 0.96 (0.46–1.96) | .909 | 2.05 (1.18–3.60) | .012* | 1.0 |
| model 3 | 1.07 (0.37–2.92) | .904 | 0.68 (0.30–1.49) | .340 | 2.05 (1.12–3.82) | .021* | 1.0 |
| Delayed memory | |||||||
| model 1 | 4.18 (1.76–9.61) | < .01** | 1.92 (0.92–3.95) | .077 | 1.35 (0.70–2.64) | .369 | 1.0 |
| model 2 | 1.68 (0.63–4.32) | .289 | 0.79 (0.35–1.77) | .573 | 1.14 (0.56–2.31) | .713 | 1.0 |
| model 3 | 1.67 (0.61–4.45) | .312 | 0.70 (0.30–1.60) | .405 | 1.08 (0.52–2.26) | .830 | 1.0 |
Model 1: Unadjusted; model 2: Adjusted for age groups, gender, physical activity levels, diabetes, hypertension, cardiovascular disease, high cholesterol, stroke, and depression; model 3: Model 2 and years of education. OR, odds ratio; CI, confidence interval.
*p < .05,
** p < .01.
Table 3 presents the results on the odds of association between individual components of SO and cognitive impairment. After accounting for age groups, gender, physical activity levels, medical history and education, cognitive impairment was found to be significantly associated with low muscle strength, low physical function, and obesity. First, significant association was found between low HGS and impairment in immediate memory (OR: 1.91, 95% CI 1.04–3.49, p = .035). Second, low GS was also significantly associated with greater odds of impairment in the immediate memory (OR: 2.17, 95% CI 1.26–3.72, p < .01. Last, significant relationship was found between high FMI and impairment in attention (OR: 2.06, 95% CI 1.22–3.51, p < .01).
Table 3. Association between components of sarcopenic obesity and risk of impairment in different domains of cognitive function.
| Low ALMI (n = 276) | Low HGS (n = 99) | Low GS (n = 189) | High FMI (n = 216) | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Global cognitive function | 0.92 (0.51–1.63) | .768 | 1.71 (0.88–3.31) | .113 | 1.67 (0.91–3.03) | .096 | 1.48 (0.85–2.58) | .164 |
| Immediate memory | 0.87 (0.52–1.45) | .600 | 1.91 (1.04–3.49) | .035* | 2.17 (1.26–3.72) | < .01** | 0.92 (0.56–1.50) | .742 |
| Visuospatial/constructive | 0.80 (0.45–1.38) | .419 | 1.72 (0.91–3.23) | .094 | 1.40 (0.77–2.51) | .261 | 1.40 (0.82–2.39) | .214 |
| Language | 0.66 (0.40–1.09) | .106 | 0.88 (0.45–1.65) | .687 | 1.07 (0.61–1.83) | .811 | 0.79 (0.49–1.28) | .349 |
| Attention | 0.60 (0.34–1.04) | .069 | 1.21 (0.62–2.32) | .576 | 1.64 (0.91–2.92) | .095 | 2.06 (1.22–3.51) | < .01** |
| Delayed memory | 0.88 (0.47–1.63) | .675 | 1.57 (0.81–3.02) | .180 | 1.65 (0.85–3.19) | .138 | 1.42 (0.79–2.58) | .240 |
Model adjusted for age groups, gender, physical activity levels, diabetes, hypertension, cardiovascular disease, high cholesterol, stroke, depression, and years of education.
ALMI, appendicular lean mass index; HGS, handgrip strength; GS, gait speed; FMI, fat mass index; OR, odds ratio; CI, confidence interval.
*p < .05,
** p < .01.
We performed additional analyses and found that obesity combined with low HGS or slow GS indeed showed significantly greater odds of cognitive impairment (Tables 4 and 5). After adjusting for confounding variables, co-occurrence of low HGS and high FMI was found to be associated with significantly higher odds of impairment in global cognition (OR: 2.98, 95% CI 1.08–8.19, p = .034), visuospatial/constructional abilities (OR: 3.70, 95% CI 1.45–9.52, p < .01), and attention (OR: 3.11, 95% CI 1.17–8.20, p = .022). Similarly, significant association was found between impairment in the attention domain and co-occurrence of slow gait speed and high FMI (OR: 3.02, 95% CI 1.41–6.51, p < .01).
Table 4. Association between handgrip strength, obesity, and risk of impairment in different domains of cognitive function.
| Low HGS and Obese (n = 35) | Low HGS (n = 64) | Obese (n = 181) | Normal (n = 255) | ||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | ||
| Global cognitive function | 2.98 (1.08–8.19) | .034* | 1.58 (0.69–3.57) | .275 | 1.42 (0.75–2.69) | .279 | 1.0 |
| Immediate memory | 1.95 (0.78–4.76) | .145 | 1.76 (0.84–3.64) | .128 | 0.89 (0.50–1.57) | .695 | 1.0 |
| Visuospatial/constructive | 3.70 (1.45–9.52) | < .01** | 1.12 (0.49–2.49) | .782 | 1.07 (0.57–1.99) | .837 | 1.0 |
| Language | 0.46 (0.14–1.31) | .166 | 1.08 (0.51–2.26) | .830 | 0.90 (0.53–1.53) | .708 | 1.0 |
| Attention | 3.11 (1.17–8.20) | .022* | 1.03 (0.43–2.39) | .940 | 1.87 (1.03–3.41) | .039* | 1.0 |
| Delayed memory | 2.37 (0.88–6.24) | .083 | 1.58 (0.67–3.66) | .284 | 1.44 (0.71–2.96) | .313 | 1.0 |
Model adjusted for age groups, gender, physical activity levels, diabetes, hypertension, cardiovascular disease, high cholesterol, stroke, depression, and years of education.
HGS, handgrip strength; OR, odds ratio; CI, confidence interval.
*p < .05,
** p < .01.
Table 5. Association between gait speed, obesity, and risk of impairment in different domains of cognitive function.
| Low GS and Obese (n = 88) | Low GS (n = 101) | Obese (n = 128) | Normal (n = 218) | ||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | ||
| Global cognitive function | 2.20 (0.99–4.91) | .054 | 1.94 (0.89–4.21) | .092 | 1.74 (0.81–3.73) | .151 | 1.0 |
| Immediate memory | 1.87 (0.92–3.78) | .083 | 2.29 (1.17–4.47) | .015* | 0.90 (0.43–1.79) | .761 | 1.0 |
| Visuospatial/constructive | 1.86 (0.87–3.98) | .107 | 1.26 (0.59–2.65) | .547 | 1.26 (0.59–2.63) | .544 | 1.0 |
| Language | 0.86 (0.41–1.75) | .681 | 1.10 (0.56–2.12) | .767 | 0.80 (0.42–1.48) | .477 | 1.0 |
| Attention | 3.02 (1.41–6.51) | < .01** | 1.54 (0.70–3.34) | .279 | 2.01 (0.98–4.11) | .056 | 1.0 |
| Delayed memory | 2.17 (0.93–5.10) | .073 | 1.59 (0.69–3.68) | .276 | 1.36 (0.55–3.28) | .491 | 1.0 |
Model adjusted for age groups, gender, physical activity levels, diabetes, hypertension, cardiovascular disease, high cholesterol, stroke, depression, and years of education.
GS, gait speed; OR, odds ratio; CI, confidence interval.
*p < .05,
** p < .01.
Discussion
This study examined the association between SO and cognitive impairment among healthy community-dwelling adults. We found that sarcopenia with and without obesity were not associated with global or domain-specific cognitive impairment after adjusting for potential confounders. However, obesity on its own was significantly associated with greater odds of impairment in the attention domain. Furthermore, we identified that low muscle strength, low physical function and high fat mass were individually associated with impaired cognitive function.
Sarcopenia alone [6,7] and concurrent with obesity [9,21,22] have been purported to be associated with cognitive impairment. While we did observe that sarcopenia and SO were related to cognitive impairment in the unadjusted models, these associations were not significant after accounting for age, gender, and education. Increasing age and lower education levels are well established strong risk factors for Alzheimer’s disease [34], and strongly confounded the observed higher prevalence of cognitive impairment in SO and sarcopenic individuals, who were significantly much older and less educated as compared to the other groups. The diagnosis of sarcopenia requires the presence of low muscle mass as the core criterion [4]. The salient lack of independent association between low ALMI and all domains of cognitive function critically explains the insignificant association between sarcopenia and cognitive impairment.
On the other hand, we found that other functional components of sarcopenia, i.e. low HGS and GS, were individually associated with cognitive impairment even after accounting for confounding variables. These findings corroborate previous studies that suggested muscle quality is more relevant to cognitive function than muscle quantity [8,35–38]. Such results are also consistent with previous evidence of association between GS, HGS, and cognitive impairment [39,40]. Although the underlying mechanisms remain unclear, mutual pathological factors such as chronic inflammation, oxidative stress, hormonal changes, and insulin resistance have been postulated to explain the association between poor physical function and cognitive decline [41,42]. Neurological mechanisms are also plausible given that both HGS and GS performance involve multiple brain regions [43,44]. The present study findings revealed that low HGS and slow GS were associated with impairment in immediate memory domain. Future studies are warranted to elucidate the associated pathophysiological pathways.
Interestingly, we found that obesity on its own was associated with significantly greater risk of cognitive impairment. While obesity has been posited to have links with reduced cognitive function [45,46], there is criticism that the relationship between adiposity and cognitive impairment is obscured by the use of BMI to define obesity [47], since it is a measure of excess weight instead of body fat [48]. The present study is confirmatory in demonstrating that greater FMI was associated with cognitive impairment after accounting for confounding variables. Previous studies have reported poorer executive function among obese individuals [49,50]. Our results extend current body of knowledge by showing that greater adiposity was specifically associated with deficit in attention domain. This association is plausibly explained through systemic inflammation and insulin resistance [19]. Increased adipose tissues have been reported to increase secretion of proinflammatory cytokines [51] and impair insulin sensitivity [52], in which both conditions were associated with cognitive impairment among older adults [53,54].
SO was postulated to have stronger detrimental effects on cognitive function than either sarcopenia or obesity alone [9,21]. It has been demonstrated to affect multiple cognitive domains among community-dwelling adults [21] and type 2 diabetes patients [55]. In this study, we found that SO was not associated with cognitive impairment in adjusted models. This was due to the lack of independent effects of ALMI (as the core criterion of sarcopenia) on cognitive function. Our results indicate that in the absence of low ALMI, the co-occurrence of obesity with either low muscle strength or poor physical performance (as alternative definitions of sarcopenic obesity) were significantly associated with increased odds of global and domain-specific cognitive impairment. Thus, our observations strongly suggest that the impacts of sarcopenia and SO on cognitive outcome is stronger if based on muscle strength and physical performance parameters without the requisite core criterion of low ALMI.
Insulin resistance-induced endothelial dysfunction may mediate the relationship between sarcopenic obesity and cognitive function [9,56]. Beyond its catabolic effect on skeletal muscle tissues, insulin resistance has been suggested to correlate with poorer muscle function independently [57]. Thus, obese individuals with greater insulin resistance may be at greater risk of decline in muscle strength and physical performance. Since insulin resistance is not measured in present study, further research is required to examine the suggested mechanism behind the relationship between SO and cognitive impairment.
The strengths of the present study are the recruitment of a representative sample across different adult age groups and the use of a gold standard instrument to measure body composition. However, we have to acknowledge some limitations in the present study. First, as the study sample consisted of relatively healthy community-dwelling adults, caution is advised in generalising the results to individuals with more severe physical and cognitive impairments. Second, due to its cross-sectional nature, our study is not able to determine the temporality and direction of the associations identified. Future longitudinal studies are necessary to establish the specific relationship between components of SO and cognitive impairment.
In conclusion, we found no association between sarcopenic obesity based on AWGS’s definition of sarcopenia and cognitive impairment after adjusting for confounders. Low lean mass was notably not associated with cognitive impairment, but low muscle strength, low physical performance and obesity defined by high FMI were independently associated. The co-occurrence of obesity with either low muscle strength or poor physical performance was associated with exacerbated odds of observing cognitive impairment.
Supporting information
(CSV)
Acknowledgments
The authors gratefully acknowledge the support of Queenie Tan, Daniella Ng, Dr. Lilian Chye, Sylvia Ngu Siew Ching, Aizuriah Mohamed Ali, Mary Ng Pei Ern, Chua Xing Ying and Shermaine Thein in this study. The authors also gratefully acknowledge the strong support of Prof. Pang Weng Sun for us to initiate this Yishun Study.
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
This research was supported as part of a core funding from the Ministry of Health of Singapore to Geriatric Education and Research Institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Patterson C. World Alzheimer report 2018: the state of the art of dementia research: new frontiers. London, UK: Alzheimer’s Disease International; 2018. [Google Scholar]
- 2.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5):990S–991S. doi: 10.1093/jn/127.5.990S [DOI] [PubMed] [Google Scholar]
- 4.Chen L-K, Woo J, Assantachai P, Auyeung T-W, Chou M-Y, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300–307. doi: 10.1016/j.jamda.2019.12.012 [DOI] [PubMed] [Google Scholar]
- 5.Beaudart C, Rizzoli R, Bruyère O, Reginster J-Y, Biver E. Sarcopenia: burden and challenges for public health. Arch Public Health. 2014;72(1):45. doi: 10.1186/2049-3258-72-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng T-C, Chen W-L, Wu L-W, Chang Y-W, Kao T-W. Sarcopenia and cognitive impairment: A systematic review and meta-analysis. Clin Nutr. 2019;39(9):2695–2701. doi: 10.1016/j.clnu.2019.12.014 [DOI] [PubMed] [Google Scholar]
- 7.Chang K-V, Hsu T-H, Wu W-T, Huang K-C, Han D-S. Association between sarcopenia and cognitive impairment: a systematic review and meta-analysis. J Am Med Dir Assoc. 2016;17(12):1164.e7–1164.e15. doi: 10.1016/j.jamda.2016.09.013 [DOI] [PubMed] [Google Scholar]
- 8.Huang C-Y, Hwang A-C, Liu L-K, Lee W-J, Chen L-Y, Peng L-N, et al. Association of dynapenia, sarcopenia, and cognitive impairment among community-dwelling older Taiwanese. Rejuvenation Res. 2016;19(1):71–78. doi: 10.1089/rej.2015.1710 [DOI] [PubMed] [Google Scholar]
- 9.Levine M, Crimmins E. Sarcopenic obesity and cognitive functioning: the mediating roles of insulin resistance and inflammation? Curr Gerontol Geriatr Res. 2012;2012:1–7. doi: 10.1155/2012/826398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes VA, Roubenoff R, Wood M, Frontera WR, Evans WJ, Fiatarone Singh MA. Anthropometric assessment of 10-y changes in body composition in the elderly. Am J Clin Nutr. 2004;80(2):475–482. doi: 10.1093/ajcn/80.2.475 [DOI] [PubMed] [Google Scholar]
- 11.Elias M, Elias P, Sullivan L, Wolf P, D’agostino R. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int J Obes. 2003;27(2):260–268. doi: 10.1038/sj.ijo.802225 [DOI] [PubMed] [Google Scholar]
- 12.Walther K, Birdsill AC, Glisky EL, Ryan L. Structural brain differences and cognitive functioning related to body mass index in older females. Hum Brain Mapp. 2010;31(7):1052–1064. doi: 10.1002/hbm.20916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo HK, Jones RN, Milberg WP, Tennstedt S, Talbot L, Morris JN, et al. Cognitive function in normal-weight, overweight, and obese older adults: an analysis of the advanced cognitive training for independent and vital elderly cohort. J Am Geriatr Soc. 2006;54(1):97–103. doi: 10.1111/j.1532-5415.2005.00522.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noh H-M, Han J, Kim YJ, Jung J-H, Roh YK, Song HJ. Sex differences in the relationship between cognitive impairment and overweight or obesity in late life: A 3-year prospective study. Medicine. 2019;98(9):e14736. doi: 10.1097/MD.0000000000014736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vidyanti AN, Hardhantyo M, Wiratama BS, Prodjohardjono A, Hu C-J. Obesity is less frequently associated with cognitive impairment in elderly individuals: A cross-sectional study in Yogyakarta, Indonesia. Nutrients. 2020;12(2):367. doi: 10.3390/nu12020367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noh H-M, Oh S, Song HJ, Lee EY, Jeong J-Y, Ryu O-H, et al. Relationships between cognitive function and body composition among community-dwelling older adults: a cross-sectional study. BMC Geriatr. 2017;17(1):259. doi: 10.1186/s12877-017-0651-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waki T, Tanaka-Mizuno S, Takashima N, Takechi H, Hayakawa T, Miura K, et al. Waist Circumference and domain-specific cognitive function among non-demented Japanese older adults stratified by sex: Results from the Takashima cognition study. J Alzheimer’s Dis. 2020;73(3):887–896. doi: 10.3233/JAD-190395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O’Meara ES, Longstreth W, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66(3):336–342. doi: 10.1001/archneurol.2008.582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith E, Hay P, Campbell L, Trollor JN. A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment. Obes Rev. 2011;12(9):740–755. doi: 10.1111/j.1467-789X.2011.00920.x [DOI] [PubMed] [Google Scholar]
- 20.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904(1):437–448. doi: 10.1111/j.1749-6632.2000.tb06498.x [DOI] [PubMed] [Google Scholar]
- 21.Tolea MI, Chrisphonte S, Galvin JE. Sarcopenic obesity and cognitive performance. Clin Interv Aging. 2018;13:1111–1119. doi: 10.2147/CIA.S164113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Hai S, Liu Y, Cao L, Liu Y, Liu P, et al. Associations between sarcopenic obesity and cognitive impairment in elderly Chinese community-dwelling individuals. J Nutr Health Aging. 2019;23(1):14–20. doi: 10.1007/s12603-018-1088-3 [DOI] [PubMed] [Google Scholar]
- 23.Khor EQ-E, Lim J, Tay L, Yeo A, Yew S, Ding Y, et al. Obesity definitions in sarcopenic obesity: Differences in prevalence, agreement and association with muscle function. J Frailty Aging. 2020;9(1):37–43. doi: 10.14283/jfa.2019.28 [DOI] [PubMed] [Google Scholar]
- 24.Pang BWJ, Wee SL, Lau LK, Jabbar KA, Seah WT, Ng DHM, et al. Obesity Measures and Definitions of Sarcopenic Obesity in Singaporean Adults–the Yishun Study. J Frailty Aging. Forthcoming 2020. doi: 10.14283/jfa.2020.65 [DOI] [PubMed] [Google Scholar]
- 25.Pang BWJ, Wee SL, Lau LK, Jabbar KA, Seah WT, Ng DHM, et al. Prevalence and associated factors of sarcopenia in Singaporean adults–the Yishun Study. J Am Med Dir Assoc. Forthcoming 2020. doi: 10.1016/j.jamda.2020.05.029 [DOI] [PubMed] [Google Scholar]
- 26.Kim TN, Yang S, Yoo H-J, Lim K, Kang H, Song W, et al. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: the Korean sarcopenic obesity study. Int J Obes. 2009;33(8):885–892. doi: 10.1038/ijo.2009.130 [DOI] [PubMed] [Google Scholar]
- 27.Peltz G, Aguirre MT, Sanderson M, Fadden MK. The role of fat mass index in determining obesity. Am J Hum Biol. 2010;22(5):639–647. doi: 10.1002/ajhb.21056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, et al. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281(6):558–560. doi: 10.1001/jama.281.6.558 [DOI] [PubMed] [Google Scholar]
- 29.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–8. doi: 10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalinkovich A, Livshits G. Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev. 2017;35:200–221. doi: 10.1016/j.arr.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 31.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310–319. doi: 10.1076/jcen.20.3.310.823 [DOI] [PubMed] [Google Scholar]
- 32.Randolph C. Repeatable Battery for the Assessment of Neuropsychological Status manual. San Antonio, TX: Psychological Corporation; 1998. [Google Scholar]
- 33.Armstrong T, Bull F. Development of the world health organization global physical activity questionnaire (GPAQ). J Public Health. 2006;14(2):66–70. doi: 10.1007/s10389-006-0024-x [DOI] [Google Scholar]
- 34.Lindsay J, Laurin D, Verreault R, Hébert R, Helliwell B, Hill GB, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156(5):445–453. doi: 10.1093/aje/kwf074 [DOI] [PubMed] [Google Scholar]
- 35.Abellan van Kan G, Cesari M, Gillette-Guyonnet S, Dupuy C, Nourhashémi F, Schott A-M, et al. Sarcopenia and cognitive impairment in elderly women: results from the EPIDOS cohort. Age Ageing. 2013;42(2):196–202. doi: 10.1093/ageing/afs173 [DOI] [PubMed] [Google Scholar]
- 36.Auyeung TW, Kwok T, Lee J, Leung PC, Leung J, Woo J. Functional decline in cognitive impairment–the relationship between physical and cognitive function. Neuroepidemiology. 2008;31(3):167–173. doi: 10.1159/000154929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Auyeung TW, Lee J, Kwok T, Woo J. Physical frailty predicts future cognitive decline—a four-year prospective study in 2737 cognitively normal older adults. J Nutr Health Aging. 2011;15(8):690–694. doi: 10.1007/s12603-011-0110-9 [DOI] [PubMed] [Google Scholar]
- 38.Sui SX, Holloway-Kew KL, Hyde NK, Williams LJ, Leach S, Pasco JA. Muscle strength and gait speed rather than lean mass are better indicators for poor cognitive function in older men. Sci Rep. 2020;10, 10367. doi: 10.1038/s41598-020-67251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beauchet O, Annweiler C, Callisaya ML, De Cock A-M, Helbostad JL, Kressig RW, et al. Poor gait performance and prediction of dementia: results from a meta-analysis. J Am Med Dir Assoc. 2016;17(6):482–490. doi: 10.1016/j.jamda.2015.12.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobayashi-Cuya KE, Sakurai R, Suzuki H, Ogawa S, Takebayashi T, Fujiwara Y. Observational evidence of the association between handgrip strength, hand dexterity, and cognitive performance in community-dwelling older adults: a systematic review. J Epidemiol. 2018;28(9):373–381. doi: 10.2188/jea.JE20170041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment—a review of the evidence and causal mechanisms. Ageing Res Rev. 2013;12(4):840–851. doi: 10.1016/j.arr.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 42.Chou M-Y, Nishita Y, Nakagawa T, Tange C, Tomida M, Shimokata H, et al. Role of gait speed and grip strength in predicting 10-year cognitive decline among community-dwelling older people. BMC Geriatr. 2019;19,186. doi: 10.1186/s12877-019-1199-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carson RG. Get a grip: individual variations in grip strength are a marker of brain health. Neurobiol Aging. 2018;71:189–222. doi: 10.1016/j.neurobiolaging.2018.07.023 [DOI] [PubMed] [Google Scholar]
- 44.Harada T, Miyai I, Suzuki M, Kubota K. Gait capacity affects cortical activation patterns related to speed control in the elderly. Exp Brain Res. 2009;193(3):445–454. doi: 10.1007/s00221-008-1643-y [DOI] [PubMed] [Google Scholar]
- 45.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788–794. doi: 10.1016/S1474-4422(14)70136-X [DOI] [PubMed] [Google Scholar]
- 46.Prickett C, Brennan L, Stolwyk R. Examining the relationship between obesity and cognitive function: a systematic literature review. Obes Res Clin Pract. 2015;9(2):93–113. doi: 10.1016/j.orcp.2014.05.001 [DOI] [PubMed] [Google Scholar]
- 47.Papachristou E, Ramsay SE, Lennon LT, Papacosta O, Iliffe S, Whincup PH, et al. The relationships between body composition characteristics and cognitive functioning in a population-based sample of older British men. BMC Geriatr. 2015;15,172. doi: 10.1186/s12877-015-0169-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001;2(3):141–147. doi: 10.1046/j.1467-789x.2001.00031.x [DOI] [PubMed] [Google Scholar]
- 49.Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Gordon E. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Compr Psychiatry. 2007;48(1):57–61. doi: 10.1016/j.comppsych.2006.05.001 [DOI] [PubMed] [Google Scholar]
- 50.Sabia S, Kivimaki M, Shipley MJ, Marmot MG, Singh-Manoux A. Body mass index over the adult life course and cognition in late midlife: the Whitehall II Cohort Study. Am J Clin Nutr. 2009;89(2):601–607. doi: 10.3945/ajcn.2008.26482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balistreri CR, Caruso C, Candore G. The role of adipose tissue and adipokines in obesity-related inflammatory diseases. Mediators Inflamm. 2010;2010,802078. doi: 10.1155/2010/802078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 2007;21(12):1443–1455. doi: 10.1101/gad.1550907 [DOI] [PubMed] [Google Scholar]
- 53.Trollor JN, Smith E, Baune BT, Kochan NA, Campbell L, Samaras K, et al. Systemic inflammation is associated with MCI and its subtypes: the Sydney Memory and Aging Study. Dement Geriatr Cogn Disord. 2010;30(6):569–578. doi: 10.1159/000322092 [DOI] [PubMed] [Google Scholar]
- 54.Abbatecola AM, Paolisso G, Lamponi M, Bandinelli S, Lauretani F, Launer L, et al. Insulin resistance and executive dysfunction in older persons. J Am Geriatr Soc. 2004;52(10):1713–1718. doi: 10.1111/j.1532-5415.2004.52466.x [DOI] [PubMed] [Google Scholar]
- 55.Low S, Goh KS, Ng TP, Ang SF, Moh A, Wang J, et al. The prevalence of sarcopenic obesity and its association with cognitive performance in type 2 diabetes in Singapore. Clin Nutr. 2020;39(7):2274–2281. doi: 10.1016/j.clnu.2019.10.019 [DOI] [PubMed] [Google Scholar]
- 56.Buie JJ, Watson LS, Smith CJ, Sims-Robinson C. Obesity-related cognitive impairment: The role of endothelial dysfunction. Neurobiol Dis. 2019;132,104580. doi: 10.1016/j.nbd.2019.104580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity-definition, etiology and consequences. Curr Opin Clin Nutr Metab Care. 2008;11(6):693–700. doi: 10.1097/MCO.0b013e328312c37d [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(CSV)
Data Availability Statement
All relevant data are within the manuscript and its Supporting information files.